Abstract
BACKGROUND
Monoamine oxidase-A (MAO-A) is a treatment target in neurodegenerative illness and mood disorders that increases oxidative stress and predisposition toward apoptosis. Increased MAO-A levels in prefrontal cortex (PFC) and anterior cingulate cortex (ACC) occur in rodent models of depressive behavior and human studies of depressed moods. Extreme dysphoria is common in borderline personality disorder (BPD), especially when severe, and the molecular underpinnings of severe BPD are largely unknown. We hypothesized that MAO-A levels in PFC and ACC would be highest in severe BPD and would correlate with symptom magnitude.
METHODS
[11C] Harmine positron emission tomography measured MAO-A total distribution volume (MAO-A VT), an index of MAO-A density, in severe BPD subjects (n = 14), moderate BPD subjects (n = 14), subjects with a major depressive episode (MDE) only (n = 14), and healthy control subjects (n = 14). All subjects were female.
RESULTS
Severe BPD was associated with greater PFC and ACC MAO-A VT compared with moderate BPD, MDE, and healthy control subjects (multivariate analysis of variance group effect: F6,102 = 5.6, p < .001). In BPD, PFC and ACC MAO-A VT were positively correlated with mood symptoms (PFC: r = .52, p = .005; ACC: r = .53, p = .004) and suicidality (PFC: r = .40, p = .037; ACC: r = .38, p = .046), while hippocampus MAO-A VT was negatively correlated with verbal memory (r = −.44, p = .023).
CONCLUSIONS
These results suggest that elevated MAO-A VT is associated with multiple indicators of BPD severity, including BPD symptomatology, mood symptoms, suicidality, and neurocognitive impairment.
Keywords: Borderline personality disorder, Harmine, Imaging, Monoamine oxidase-A, Positron emission tomography, Suicidal behavior
Psychiatric illnesses are often conceptualized as comprising multiple clusters of target pathologies that co-segregate across different disorders (1). Consistent with this perspective, monoamine oxidase-A (MAO-A) is an important target in neurodegenerative illness, due to its role in apoptosis (2,3), and in affect dysregulation, due to the association of elevated MAO-A levels in the prefrontal cortex (PFC) and anterior cingulate cortex (ACC) with depressive symptoms (4–7). MAO-A is an enzyme located on outer mitochondrial membranes in glia and monoamine-releasing neurons that increases oxidative stress, influences predisposition toward intrinsic apoptosis, and metabolizes monoamines (3). In brain tissue, greater MAO-A levels are correlated with elevated MAO-A activity (8), and an index of MAO-A density, the MAO-A distribution volume (MAO-A VT), can be measured with [11C] harmine positron emission tomography (PET) (9). [11C] Harmine is a selective, reversible PET radiotracer that binds to the center of the active pocket of the MAO-A enzyme with high affinity (10).
Borderline personality disorder (BPD), especially when severe, is a major burden on public health and health care systems due to its high prevalence, occurring in 10% of outpatient psychiatric cases and 20% of psychiatric inpatient cases (11). A core feature of BPD is affect dysregulation with accompanying episodes of severe dysphoria that drive expression of other BPD symptoms, including intense anger, dysfunctional interpersonal interactions, and recurrent suicidal behavior (12,13). Severe dysphoria in BPD is highly clinically relevant because it is associated with need for inpatient care (14), although other dimensions of the illness, such as neurocognitive impairment, also contribute to BPD severity (15). Severe BPD is associated with greater functional impairment (16,17), dysregulated emotional responses to negative stimuli (18), and increased suicidality (17), which together contribute to the clinical burden of BPD.
The principal aim of the present investigation was to measure MAO-A VT in BPD with moderate or severe symptoms. One model of emotional dysregulation in BPD highlights a deficient regulatory control system localized to the PFC and ACC (19) that is based on PET studies showing reduced engagement of these regions (20–24). In support of this model, meta-analysis of functional neuroimaging studies pinpoints hypoactivity of the ACC and dorsal regions of the PFC as central to the abnormal neurocircuitry underlying negative emotionality in BPD, although specific pathologies therein have not been identified (25). Depressive behaviors in rodents, such as those observed in chronic social defeat or chronic restraint stress paradigms, are associated with greater MAO-A messenger RNA and activity in the PFC (26,27). Moreover, depressive and anxious behaviors in rodents, particularly in the context of altered social environments, are associated with increased gene expression and reduced degradation of brain MAO-A (28,29). Since affect dysregulation emerges from impaired social interaction and is core to worsened presentations of BPD (30,31), the primary study hypothesis was that PFC and ACC MAO-A VT would be most elevated in severe BPD, particularly in relation to mood symptoms and suicidality, and elevated to a lesser extent in moderate BPD. Given that several of the functions attributed to MAO-A, including generation of oxidative stress and induction of apoptosis, are processes implicated in neurocognitive impairment (32), a secondary aim was to investigate MAO-A VT in functional regions linked to verbal memory ability in BPD. Therefore, the secondary hypothesis was that MAO-A VT would be elevated in the hippocampus when verbal memory impairment is more severe.
METHODS AND MATERIALS
Participants
Fifty-six female subjects completed the study protocol: 14 with severe BPD, 14 with moderate BPD, 14 with a major depressive episode (MDE) and no BPD, and 14 healthy control subjects. In clinical settings, female subjects comprise up to 74% of BPD cases (33). Hence, we restricted our analysis to women to focus on the type of BPD presentation most likely to come to clinical attention. Healthy and MDE control subjects were selected based on matching age and sex to the BPD samples. All subjects in the study were female and age-matching between control subjects and BPD samples was within 3 years. Samples had a similar proportion of women aged 41 to 51 years in each group to control for the effect of perimenopause on MAO-A VT (χ23 = .6, p = .63) (34). Each participant provided written consent after study procedures had been explained. All study components were approved by the Research Ethics Board for Human Subjects at the Centre for Addiction and Mental Health, Toronto, Canada.
BPD Subjects
BPD participants were recruited from out-patient dialectical behavior therapy clinics (39.3%), inpatient psychiatric wards (17.9%), outpatient tertiary care (17.9%), and outpatient primary/secondary care (25.0%). BPD was diagnosed following clinical assessment and use of the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) (35) by a psychiatrist experienced in the assessment and treatment of personality disorders (N.J.K.), who also administered the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (36). The BPD diagnosis was consistent with the diagnosis given to subjects by their tertiary care center treatment providers. BPD participants also met diagnostic criteria for current MDE. We included current MDE as an inclusion criterion to maximize generalizability of results, given that approximately 90% of female BPD cases have comorbid major depressive disorder (37). To rule out confounds of other major mood or psychotic disorders, exclusion criteria for BPD subjects included past history of mania, hypomania, or psychotic illness. Exclusion criteria also included use of medications in the past 3 months that are known or hypothesized to interfere with MAO-A levels, such as MAO-A inhibitors, mood stabilizers (e.g., lithium, valproic acid, carbamazepine), and psychostimulants (38). In contrast to some imaging markers, MAO-A levels are insensitive to monoamine reuptake inhibition; for example, selective serotonin reuptake inhibitors (SSRIs) do not affect MAO-A VT (5). Therefore, use of recent antidepressants that do not target MAO-A was permitted. As BPD is frequently associated with inpatient care, eight BPD participants, mostly recruited from inpatient settings, had been taking prescription psychotropic medications during the month before PET scanning, which included SSRIs (eight subjects), selective serotonin/norepinephrine reuptake inhibitors (one subject), atypical antipsychotics (three subjects), and benzodiazepines (three subjects).
MDE Subjects
MDE subjects were screened with the SCID-I and SCID-II, which was verified by subsequent consultation with a psychiatrist (JHM). MDE subjects were sex- and age-matched within 2 years to the BPD participants. MDE subjects had no lifetime history of additional psychiatric illness and had not taken antidepressant medication within the previous 2 weeks. Forty-three percent had never taken antidepressant treatment.
Healthy Subjects
Healthy subjects were also screened with the SCID-I and SCID-II by an experienced rater and verified by review with a psychiatrist (JHM). Healthy subjects were sex- and age-matched within 3 years to the BPD participants. Healthy subjects had no history of psychiatric illness or psychotropic medication use.
Healthy and MDE subjects were a subset of participants from previously published studies in our laboratory (5,39). All study participants were nonsmoking and provided negative urine drug screen tests on assessment and PET scanning days. Additionally, all study subjects refrained from the use of alcohol the night before PET scanning and drinking tea, coffee, or caffeinated beverages on the day of PET scanning.
Image Acquisition
Each participant underwent a single [11C] harmine PET scan. Intravenous [11C] harmine (370 MBq [10 MCi[) was administered as a bolus at the start of each PET scan. An automatic blood sampling system measured arterial blood radioactivity continuously for the first 22 minutes. Manual samples were obtained at 2.5, 7.5, 15.0, 20.0, 30.0, 45.0, 60.0, and approximately 90.0 minutes after injection. Whole blood and plasma radioactivity were measured as previously described (9). Fifteen frames lasting 1 minute each were acquired, followed by 15 frames of 5 minutes each. [11C] Harmine was of very high radiochemical purity (99.0 ± .9%) and high specific activity (109.5 ± 67.0 GBq [2958.6 ± 1811.1 MCi]/μmol) at the time of injection. PET images were obtained using a high-resolution research tomograph PET camera (in-plane resolution; full-width at half maximum, 3.1 mm; 207 axial sections of 1.2 mm) (Siemens Molecular Imaging, Knoxville, Tennessee) as described elsewhere (5).
Image Analysis
Each participant also underwent magnetic resonance imaging (MRI) on a 1.5T GE scanner (fast spoiled gradient echo T1-weighted image; x, y, z voxel dimensions: .78, .78, and 1.5 mm) (GE Medical Systems, Milwaukee, Wisconsin) for the region of interest (ROI) analysis. The ROIs were determined utilizing a semi-automated method, where regions of a template MRI were transformed onto the individual MRI based on a series of transformations and deformations that matched the template image to the individual co-registered MRI followed by segmentation of the individual MRI to select the gray matter voxels, as previously reported (5,40).
The PFC and ACC were chosen as the primary regions of interest, because these regions participate in affective regulation (41), comprise the network of frontal control processes regulating emotional activity in subcortical limbic structures (19), and show hypoactivity during low mood states in BPD (25). In addition, several subregions of the PFC were sampled, including dorsolateral prefrontal cortex, ventrolateral prefrontal cortex, medial prefrontal cortex, and orbitofrontal cortex (OFC). The borders of these subregions were defined based on their cytoarchitectural differences from adjacent cortex (42). Secondary ROIs included regions implicated in BPD and/or those known to have moderate to high MAO-A density and included hippocampus, dorsal putamen, thalamus, and midbrain.
MAO-A VT represents the total tissue binding of [11C] harmine at equilibrium and is highly correlated with MAO-A level (9,43), as would be expected since MAO-A affinity is similar across regions in primates in vivo (44). Both the unconstrained two-tissue compartment model and Logan model (45) with arterial sampling, for which the underestimate of VT is negligible, measure VT with high reliability and validity. We applied the latter technique, which has been described in detail previously (9).
Measures of Symptom Severity for BPD Subjects
BPD severity was defined as the number of DSM-IV BPD symptoms present in clinical assessment with the SCID-II. This method of defining BPD severity has been employed by other research groups (16–18,31). The severe BPD group was defined as having seven or more BPD symptoms and moderate BPD was defined as having five or six BPD symptoms; this cutoff was chosen based on the sample median. Measures of mood symptoms, suicidality, and cognition were administered on the day of the [11C] harmine PET scan. Overall severity of mood symptoms was assessed with the 17-item Hamilton Depression Rating Scale (HDRS) (46) and suicidal ideation was assessed with the suicide subscale of the Overt Aggression Scale-Modified for Outpatients (OAS-M) (47). A set of cognitive tests were also administered with the prioritized measures being the Wechsler Test of Adult Reading-Revised (48) and Hopkins Verbal Learning Test-Revised (49). In addition, the Suicide Ideation Scale (50), Barratt Impulsiveness Scale Version 11 (51), Anger Questionnaire (52), and State-Trait Anger Expression Inventory-2 (53) were administered.
Statistical Analysis
To compare MAO-A VT in the PFC and ACC across groups (severe BPD, moderate BPD, MDE, healthy), the primary analysis employed multivariate analysis of variance (MAN-OVA). To specifically assess whether regional MAO-A VT was elevated in the severe BPD group compared with the moderate BPD group, MDE participants, and healthy subjects, additional comparisons were conducted using the protected least significant difference (LSD) procedure. To assess the relationship between PFC and ACC MAO-A VT and severity of mood symptoms and suicidal ideation in BPD, Pearson’s correlation coefficients were assessed. To assess the relationship between hippocampal MAO-A VT and verbal memory, the semi-partial correlation coefficient was calculated, which controlled for the effect of IQ on verbal memory ability. Tests of significance for correlational analyses were all two-tailed.
RESULTS
Subject Characteristics
Participants were aged 18 to 51 years. Groups did not differ in age. There was no significant group difference in HDRS scores between the three patient groups (Table 1).
Table 1.
Demographic and Clinical Characteristics of All Study Participantsa
| Healthy (n = 14) | Major Depressive Episode (n = 14) | Moderate Severity BPD (n = 14) | High Severity BPD (n = 14) | p Value | |
|---|---|---|---|---|---|
| Age, Years | 32.9 (8.2) | 30.9 (6.6) | 32.9 (10.6) | 34.2 (10.2) | .65b |
| HDRSc | .9 (1.4) | 20.1 (4.5) | 21.3 (4.2) | 23.6 (4.1) | .10d |
BPD, borderline personality disorder; HDRS, Hamilton Depression Rating Scale.
Values are expressed as mean (standard deviation).
Analysis of variance of participant age for the four study groups (moderate BPD, severe BPD, major depressive episode only, and healthy).
17-item Hamilton Depression Rating Scale score on day of positron emission tomography scanning.
Analysis of variance of Hamilton Depression Rating Scale scores for the three patient groups (moderate BPD, severe BPD, and major depressive episode only).
The severe BPD group had significantly greater BPD symptoms, suicidal ideation, and trait anger compared with the moderate BPD group (Table 2).
Table 2.
Clinical Characteristics of Borderline Personality Disorder Groupsa
| Characteristics | Moderate BPD (n = 14) | Severe BPD (n = 14) |
|---|---|---|
| Education, Yearsb | 14.9 (1.5) | 14.5 (2.1) |
| Estimated Full-Scale IQc | 103.8 (9.2) | 109.6 (8.9) |
| Diagnosis | ||
| Number of BPD symptomsd | 5.4 (.5) | 7.8 (1.0)e |
| Comorbid conditions | ||
| % with anxiety disorderd | 64.3 | 71.4 |
| % with eating disorderd | 57.1 | 50.0 |
| % with lifetime substance use disorderd | 14.3 | 7.1 |
| % with somatoform disorderd | 7.1 | 7.1 |
| % with other DSM-IV-TR personality disorderd | 71.4 | 57.1 |
| % Taking Psychotropic Medicationd | 28.6 | 28.6 |
| Number of Psychiatric Hospitalizationsb | 2.3 (2.9) | 3.2 (2.9) |
| Suicidality Measures | ||
| Suicide Ideation Scaleb | 9.6 (9.5) | 17.0 (9.0)f |
| OAS-M Suicidality Scaleb | 1.4 (1.2) | 2.1 (1.1) |
| % endorsing moderate or severe suicidality on OAS-M Suicidality scaleg | 14.3 | 57.1f |
| Anger/Aggression Measures | ||
| STAXI-2 state anger (T-score)b | 56.3 (11.6) | 56.9 (15.0) |
| STAXI-2 trait anger (T-score)b | 54.0 (11.9) | 67.6 (14.5)f |
| Buss-Perry angerc | 17.2 (6.9) | 23.9 (7.4)f |
| Buss-Perry physical aggressionc | 18.4 (9.4) | 24.4 (9.8) |
| Buss-Perry verbal aggressionc | 13.4 (5.3) | 16.1 (5.4) |
| Barratt Impulsiveness Scale 11 | ||
| Attentional impulsivenessc | 20.7 (4.8) | 23.9 (3.5) |
| Motor impulsivenessc | 27.2 (6.6) | 28.5 (5.5) |
| Nonplanning impulsivenessc | 30.5 (5.9) | 31.3 (4.6) |
BPD, borderline personality disorder; OAS-M, Overt Aggression Scale-Modified for Outpatients; STAXI-2, State Trait Anger Expression Inventory-2.
Values are expressed as mean (standard deviation), except where indicated.
Mann-Whitney U test.
Independent samples t test.
Fisher’s exact test.
p < .001, two-tailed.
p < .05, two-tailed.
Chi-square test.
Comparison of MAO-A VT in Severe BPD, Moderate BPD, MDE, and Healthy Groups
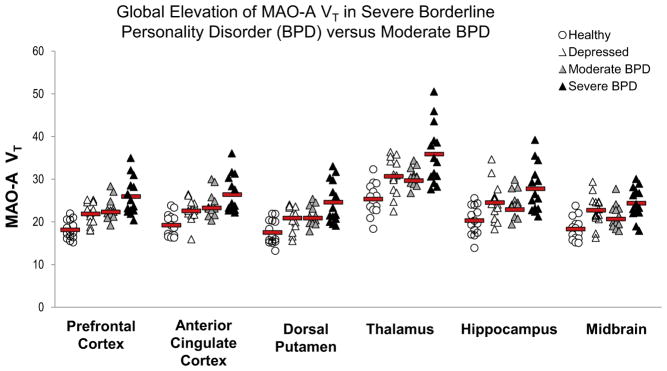
As depicted in Figure 1, MAO-A VT was substantially elevated in severe BPD, on average by 43% and 42% in the PFC and ACC, respectively, compared with the healthy group; by 16% and 13% in the PFC and ACC, respectively, compared with moderate BPD; and by 19% and 17% in the PFC and ACC, respectively, compared with MDE only (MANOVA group effect: F6,102 = 5.6, p < .001). Significant univariate effects were also detected in both PFC and ACC (F3,52 = 9.4 to 12.6, p values all <.001). Comparisons based on the LSD test revealed that PFC and ACC MAO-A VT were significantly elevated in severe BPD compared with moderate BPD (p values = .006 to .024), MDE only (p values = .002 to .007), and the healthy group (p values all ≤ .001). In addition, moderate BPD showed greater PFC and ACC MAO-A VT compared with the healthy group (p values = .002 to .005), as did the MDE group compared with the healthy group (p values = .005 to .017). No difference was observed in PFC and ACC MAO-A VT between MDE and moderate BPD groups (p values = .62 to .73). When the analyses were repeated with only the unmedicated BPD subjects (n = 20), all significant relationships persisted. A post hoc analysis found that MAO-A VT was increased in all BPD subjects versus MDE in the PFC (t40 = 1.9, p = .035, one-tailed) and ACC (t40 = 1.8, p = .037, one-tailed).
Figure 1.
Multivariate analysis of variance (MANOVA) indicates that severe BPD was associated with greater monoamine oxidase-A distribution volume (MAO-A VT) in both prefrontal and anterior cingulate cortex compared with the moderate BPD, depressed, and healthy groups (MANOVA group effect: F6,102 = 5.6, p < .001; least significant difference for severe BPD vs. other groups, p value range: <.001 to .024). Mean MAO-A VT was greater in severe vs. moderate BPD for each brain region sampled (p value range: .003 to .024). When the effect of group on MAO-A VT was evaluated across all regions, similar results were found (MANOVA group effect: F30,127 = 2.8, p = .002). Red bars indicate mean MAO-A VT values.
Multivariate analysis of covariance (MANCOVA) was used to test the group effect with HDRS score (HDRS ≥ 20 or HDRS < 20) as the covariate. Results indicated that the main group effect remained significant (MANCOVA group effect: F6,100 = 3.4, p = .005). All main contrasts were significant with inclusion of depressive symptoms as the covariate of interest (MANCOVA: severe BPD vs. moderate BPD: F2,24 = 4.1, p = .029; MANCOVA: severe BPD vs. healthy group: F2,24 = 6.0, p = .008; MANCOVA: severe BPD vs. MDE: F2,24 = 3.6, p = .041).
There was also a significant main group effect across all brain regions sampled (MANOVA group effect: F30,127 = 2.8, p = .002). In addition, comparisons using the LSD test revealed that besides the PFC and ACC, MAO-A VT values for all other regions were significantly greater in severe BPD versus the healthy group (p values all < .001), severe BPD versus moderate BPD (p values = .001 to .012), and MDE versus the healthy group (p values ≤ .001 to .027). MAO-A VT values were significantly greater in severe BPD versus MDE for the additional regions, except the hippocampus and midbrain (p values = .002 to .21), and were significantly greater in moderate BPD versus the healthy group, save the hippocampus and OFC (p values = .004 to .13). MAO-A VT values did not differ between moderate BPD and MDE for any of the additional regions (p values = .11 to .99). Results were unchanged when only the unmedicated subjects were included in the analyses.
Relationship of MAO-A VT With Severity of Mood Symptoms, Suicidality, and Cognition in BPD
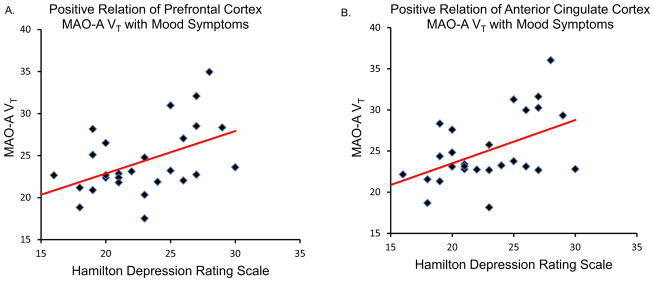
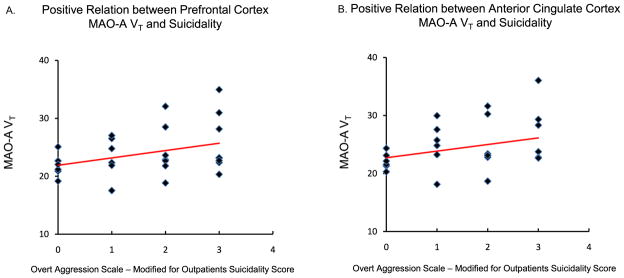
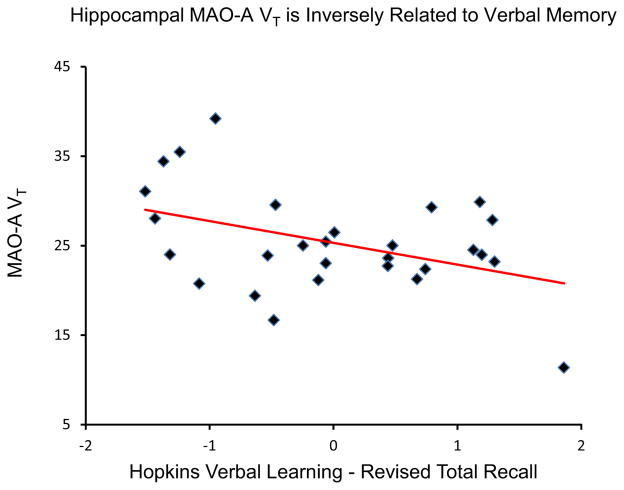
In the 28 BPD subjects, PFC MAO-A VT was correlated with both total HDRS score (r = .52, p = .005) (Figure 2) and suicidality, assessed using the OAS-M (r = .40, p = .037) (Figure 3), while ACC MAO-A VT was similarly correlated with mood symptoms (r = .53, p = .004) (Figure 2) and suicidality (r = .38, p = .046) (Figure 3). Conversely, a negative correlation was observed between hippocampus MAO-A VT in the BPD participants and total verbal recall, controlling for IQ on verbal recall (semi-partial correlation coefficient = −.44, p = .023) (Figure 4).
Figure 2.
(A) Prefrontal cortex monoamine oxidase-A total distribution volume (MAO-A VT) is positively correlated with Hamilton Depression Rating Scale score (Pearson’s r = .52, p = .005, two-tailed). (B) Anterior cingulate cortex MAO-A VT is positively correlated with Hamilton Depression Rating Scale score (Pearson’s r = .53, p = .004, two-tailed).
Figure 3.
(A) Prefrontal cortex monoamine oxidase-A total distribution volume (MAO-A VT) is positively correlated with the Suicidality subscale score of the Overt Aggression Scale-Modified for Outpatients (Pearson’s r = .40, p = .037, two-tailed). (B) Anterior cingulate cortex MAO-A VT is positively correlated with the Suicidality subscale score of the Overt Aggression Scale-Modified for Outpatients (Pearson’s r = .38, p = .046, two-tailed).
Figure 4.
Hippocampal monoamine oxidase-A total distribution volume (MAO-A VT) is negatively correlated with Total Recall score of the Hopkins Verbal Learning Test-Revised (HVLT-R), controlling for IQ on HVLT-R (semi-partial correlation coefficient = −.41, p = .023, two-tailed). Note: x axis depicts residuals of HVLT-R total recall independent of IQ.
Relationship of MAO-A VT With Severity of Mood Symptoms in Clinical Subjects
In a combined sample of the 42 clinical participants (28 BPD and 14 MDE), PFC MAO-A VT and ACC MAO-A VT were both strongly correlated with HDRS (r = .47, p = .002; and r = .43, p = .005, respectively).
DISCUSSION
This is the first imaging study of MAO-A in BPD, and its key findings are that PFC and ACC MAO-A VT were significantly elevated in severe BPD compared with moderate BPD, MDE, and the healthy group. The difference in PFC and ACC MAO-A VT between severe BPD and the healthy group was particularly robust: PFC and ACC MAO-A VT were increased 43% and 42%, respectively, which is the largest magnitude reported for any psychiatric condition. Consistent with the relationship between overall severity of BPD and MAO-A VT in these regions, greater MAO-A VT in the PFC and ACC was also associated with severity of mood symptoms and suicidality. Additionally, an inverse relationship between verbal memory and MAO-A VT in a region for which intact function is necessary for optimal cognitive performance (e.g., hippocampus) was detected. These findings have key implications for understanding the pathophysiology of BPD, especially when severe; for developing biomarkers of suicidality; and for identifying targets of cognitive impairment in BPD.
Since severe BPD is associated with greater functional impairment, suicidality, and consumption of health care resources (16,17), distinguishing and treating the underlying processes associated with illness severity is essential to reduce burden. Given that MAO-A increases production of hydrogen peroxide and ammonia through enhanced monoamine metabolism and heightens predisposition toward intrinsic apoptosis through mitochondrial membrane perturbation (3), it has been suggested that increased MAO-A protein and/or activity may contribute to neurodegeneration in Parkinson’s disease (54), Huntington’s disease (55), and Alzheimer’s disease (56–58). Highly elevated MAO-A VT in severe BPD may signal the involvement of similar patterns of ongoing neuronal and glial injury, since a high symptom burden typically persists in BPD for at least 5 to 10 years (59). Traditionally, therapeutics with antioxidant or antiapoptotic properties have not been conceptualized as relevant for BPD, although evidence suggests that riluzole, a treatment indicated for amyotrophic lateral sclerosis that has antiapoptotic properties, can reduce suicidal behavior in BPD (60). Moreover, the newest MAO-A inhibitors that achieve a high brain-to-gut ratio to circumvent side effects have been studied in neurodegenerative conditions (3). Based on our finding of greater MAO-A expression in BPD, there is reason to consider such treatments for the illness, since classical MAO-A inhibitors are effective treatments for BPD, especially when severe (61,62).
The molecular abnormalities underlying severe BPD are largely unknown, since only a few studies have examined the relationship of symptom severity to neurochemical indices. In some illnesses, the severity of the pathology may relate to illness-specific expression of symptomatology that is common across disorders, such as dysphoria. However, it is also possible that biomarkers of severity relate to symptoms specific to the disorder. Because increased MAO-A VT is associated with depressive symptoms in BPD and MDE, the relationship between MAO-A VT and depressive symptoms in BPD could reflect a common biological mechanism operating in BPD and MDE. Alternatively, it may also relate to the influence of co-existing MDE on differential expression of BPD symptomatology. To date, the investigated markers of severity in BPD have mainly related to the domain of impulsivity. In a combined sample of healthy and BPD subjects, a correlation between an index related to total glutamate levels in the dorsal ACC and impulsivity, particularly cognitively oriented impulsivity, was found after controlling for group effects (63). Initial investigations of PFC serotonin 2A receptor binding in BPD reported no relationship to measures of severity (64,65), but a more recent sample found an association of lifetime aggression with reduced serotonin 2A receptor binding in the medial OFC of female subjects with BPD (66). Another study reported greater mu-opioid receptor binding in the OFC, caudate, and ventral striatum in BPD, which did not reach a statistically significant threshold when correlated with measures of impulsivity, neuroticism, or dissociation (67). Finally, reduced α[11C]-methyl-L-tryptophan uptake in PFC and ACC was found to be correlated with measures of impulsivity and suicidal intent in samples of BPD patients (23,68). Interestingly, the findings of the present study are consistent with several reports of abnormal markers implicated in proapoptotic signaling from postmortem investigations that predominantly sampled the PFC of suicide victims with mood dysregulation: reduced phosphoinositide 3-kinase activity (69), reduced R1 levels (70), increased caspase-8 levels (71), and reduced Bcl-2 (72).
To the best of our knowledge, this is the first study of BPD to demonstrate a correlation between present suicidal ideation and an in vivo brain marker. There is a need for biomarkers associated with suicide risk, because 60% to 75% of people with BPD make suicide attempts and 8% to 10% commit suicide (73). Although empirically determined risk factors improve suicide risk assessment, these factors are mostly longstanding and cannot reliably predict when suicide is imminent. Biomarker development in psychiatric conditions involves creation of a panel of low-cost markers for which each marker specifically relates to an implicated pathology (74). We are unaware of any peripheral markers in development for the pathologies associated with apoptosis that have been identified in suicide victims with mood dysregulation. However, measurement of plasma MAO-A levels (75) and detection of MAO-A activity using magnetic resonance spectroscopy (76) represent possibilities of low-cost surrogate markers for MAO-A levels in the brain.
The correlation between hippocampus MAO-A VT and verbal memory in BPD is consistent with interpreting elevated MAO-A level as a pathological influence on this neural substrate of verbal memory function. Pathologies associated with apoptosis and/or oxidative stress in functionally critical regions are common in diseases presenting with memory impairment and are usually assumed to be etiological if correlated with severity of cognitive impairment (32). For example, decreased verbal memory performance is associated with microstructural alterations of hippocampal gray matter in Parkinson’s disease (77) and increased hippocampal binding of radioligand selective for amyloid plaques and neurofibrillary tangles in Alzheimer’s disease (78).
Our results indicated that group differences in MAO-A VT were present beyond the hypothesized regions of PFC and ACC. In general, MAO-A VT tends to be highly intercorrelated among brain regions. Although our results support the hypothesis that negative mood states in BPD are related to elevated PFC and ACC MAO-A VT, the pathological mechanisms responsible for raising MAO-A levels may have a global effect. However, we suggest that the functional impact of increased MAO-A VT may be most important in the PFC and ACC, given their role in affect regulation.
We note several limitations of the present investigation. We chose to assess BPD severity based on the number of DSM-IV symptoms present, an approach used in some laboratories (16–18,31). Although there is no universally accepted gold standard for measuring BPD severity (79), other options would have been the Zanarini Rating Scale for Borderline Personality Disorder (80) or the Borderline Personality Disorder Severity Index (81). These other scales are designed to capture DSM-IV BPD criteria (80,81) and are highly correlated with number of BPD criteria (81). Even so we found a consistent relationship between PFC and ACC MAO-A VT and several additional measures of severity. Second, a small subset of our BPD participants had been taking psychotropic medication, mainly SSRIs, at the time of scanning, although our main findings did not change when we excluded these individuals from the analysis. Third, to decrease sample heterogeneity, we only sampled women; therefore, our findings may not be generalizable to men. Fourth, we applied MAO-A VT as our primary measure, because it is the most stable and least variable measure of [11C] harmine binding. However, the measure of MAO-A used in this study reflects total binding, and approximately 15% of MAO-A VT represents free and nonspecific binding. Hence, our interpretations assumed that substantial changes in free and nonspecific binding (150% to 300%) were not occurring, an assumption consistent with previous evaluations observed in MAO-A occupancy studies (9).
In summary, we found that elevated MAO-A in PFC and ACC was related to several markers of severity in BPD, including more borderline and mood symptoms and greater suicidality. Increased hippocampal MAO-A levels were additionally associated with decreased verbal memory performance. Understanding severe BPD is paramount given its high morbidity and mortality and high healthcare burden. These results offer a novel perspective on the pathophysiological mechanisms of BPD and suggest that therapeutics that inhibit MAO-A, reduce cellular oxidative stress levels, and/or oppose apoptotic processes should be tested in severe presentations of BPD.
Acknowledgments
This research received project support from the Canadian Institutes of Health Research, Physicians’ Services Incorporated Foundation, and American Psychiatric Institute for Research and Education. These organizations did not participate in the design or execution of this study or the writing of the manuscript. Dr. Meyer takes responsibility for the integrity of the data and the accuracy of the data analysis; all authors had full access to all data in the study.
We thank the Campbell Family Mental Health Research Institute; technicians Alvina Ng and Laura Nguyen; chemistry staff Jun Parkes, Armando Garcia, Winston Stableford, and Min Wong; engineers Terry Bell and Ted Harris-Brandts; and students Malcolm Blagrove and Amanda Brijmohan for their assistance with this project.
Footnotes
DISCLOSURES
Drs. Meyer, Wilson, and Houle have received operating grant funds for other studies from Eli-Lilly, GlaxoSmithKline, Bristol Myers Squibb, Lundbeck, and SK Life Sciences in the past 5 years. Dr. Meyer has consulted to several of these companies, as well as Takeda, Sepracor, Trius, Mylan, and Teva. Dr. Links received an honorarium from Lundbeck within the past 5 years. None of these companies participated in the design or execution of this study or in writing the manuscript. All other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: The seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ou XM, Chen K, Shih JC. Monoamine oxidase A and repressor R1 are involved in apoptotic signaling pathway. Proc Natl Acad Sci U S A. 2006;103:10923–10928. doi: 10.1073/pnas.0601515103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Youdim MB, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci. 2006;7:295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 4.Meyer JH, Ginovart N, Boovariwala A, Sagrati S, Hussey D, Garcia A, et al. Elevated monoamine oxidase A levels in the brain: An explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry. 2006;63:1209–1216. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- 5.Meyer JH, Wilson AA, Sagrati S, Miler L, Rusjan P, Bloomfield PM, et al. Brain monoamine oxidase A binding in major depressive disorder: Relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch Gen Psychiatry. 2009;66:1304–1312. doi: 10.1001/archgenpsychiatry.2009.156. [DOI] [PubMed] [Google Scholar]
- 6.Bacher I, Houle S, Xu X, Zawertailo L, Soliman A, Wilson AA, et al. Monoamine oxidase A binding in the prefrontal and anterior cingulate cortices during acute withdrawal from heavy cigarette smoking. Arch Gen Psychiatry. 2011;68:817–826. doi: 10.1001/archgenpsychiatry.2011.82. [DOI] [PubMed] [Google Scholar]
- 7.Sacher J, Wilson AA, Houle S, Rusjan P, Hassan S, Bloomfield PM, et al. Elevated brain monoamine oxidase A binding in the early postpartum period. Arch Gen Psychiatry. 2010;67:468–474. doi: 10.1001/archgenpsychiatry.2010.32. [DOI] [PubMed] [Google Scholar]
- 8.Saura J, Kettler R, Da Prada M, Richards JG. Quantitative enzyme radioautography with 3H-Ro 41-1049 and 3H-Ro 19-6327 in vitro: Localization and abundance of MAO-A and MAO-B in rat CNS, peripheral organs, and human brain. J Neurosci. 1992;12:1977–1999. doi: 10.1523/JNEUROSCI.12-05-01977.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ginovart N, Meyer JH, Boovariwala A, Hussey D, Rabiner EA, Houle S, Wilson AA. Positron emission tomography quantification of [11C]-harmine binding to monoamine oxidase-A in the human brain. J Cereb Blood Flow Metab. 2006;26:330–344. doi: 10.1038/sj.jcbfm.9600197. [DOI] [PubMed] [Google Scholar]
- 10.Son SY, Ma J, Kondou Y, Yoshimura M, Yamashita E, Tsukihara T. Structure of human monoamine oxidase A at 2.2-A resolution: The control of opening the entry for substrates/inhibitors. Proc Natl Acad Sci U S A. 2008;105:5739–5744. doi: 10.1073/pnas.0710626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torgersen S, Kringlen E, Cramer V. The prevalence of personality disorders in a community sample. Arch Gen Psychiatry. 2001;58:590–596. doi: 10.1001/archpsyc.58.6.590. [DOI] [PubMed] [Google Scholar]
- 12.Koenigsberg HW, Harvey PD, Mitropoulou V, New AS, Goodman M, Silverman J, et al. Are the interpersonal and identity disturbances in the borderline personality disorder criteria linked to the traits of affective instability and impulsivity? J Pers Disord. 2001;15:358–370. doi: 10.1521/pedi.15.4.358.19181. [DOI] [PubMed] [Google Scholar]
- 13.Tragesser SL, Solhan M, Schwartz-Mette R, Trull TJ. The role of affective instability and impulsivity in predicting future BPD features. J Pers Disord. 2007;21:603–614. doi: 10.1521/pedi.2007.21.6.603. [DOI] [PubMed] [Google Scholar]
- 14.Zanarini MC, Frankenburg FR, DeLuca CJ, Hennen J, Khera GS, Gunderson JG. The pain of being borderline: Dysphoric states specific to borderline personality disorder. Harv Rev Psychiatry. 1998;6:201–207. doi: 10.3109/10673229809000330. [DOI] [PubMed] [Google Scholar]
- 15.Bazanis E, Rogers RD, Dowson JH, Taylor P, Meux C, Staley C, et al. Neurocognitive deficits in decision-making and planning of patients with DSM-III-R borderline personality disorder. Psychol Med. 2002;32:1395–1405. doi: 10.1017/s0033291702006657. [DOI] [PubMed] [Google Scholar]
- 16.Gunderson JG, Daversa MT, Grilo CM, McGlashan TH, Zanarini MC, Shea MT, et al. Predictors of 2-year outcome for patients with borderline personality disorder. Am J Psychiatry. 2006;163:822–826. doi: 10.1176/ajp.2006.163.5.822. [DOI] [PubMed] [Google Scholar]
- 17.Black DW, Blum N, Letuchy E, Carney Doebbeling C, Forman-Hoffman VL, Doebbeling BN. Borderline personality disorder and traits in veterans: Psychiatric comorbidity, healthcare utilization, and quality of life along a continuum of severity. CNS Spectr. 2006;11:680–689. doi: 10.1017/s1092852900014772. [DOI] [PubMed] [Google Scholar]
- 18.Hazlett EA, Speiser LJ, Goodman M, Roy M, Carrizal M, Wynn JK, et al. Exaggerated affect-modulated startle during unpleasant stimuli in borderline personality disorder. Biol Psychiatry. 2007;62:250–255. doi: 10.1016/j.biopsych.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 19.New AS, Triebwasser J, Charney DS. The case for shifting borderline personality disorder to Axis I. Biol Psychiatry. 2008;64:653–659. doi: 10.1016/j.biopsych.2008.04.020. [DOI] [PubMed] [Google Scholar]
- 20.Siever LJ, Buchsbaum MS, New AS, Spiegel-Cohen J, Wei T, Hazlett EA, et al. d,l-fenfluramine response in impulsive personality disorder assessed with [18F]fluorodeoxyglucose positron emission tomography. Neuropsychopharmacology. 1999;20:413–423. doi: 10.1016/S0893-133X(98)00111-0. [DOI] [PubMed] [Google Scholar]
- 21.New AS, Hazlett EA, Buchsbaum MS, Goodman M, Reynolds D, Mitropoulou V, et al. Blunted prefrontal cortical 18fluorodeox-yglucose positron emission tomography response to metachlorophenylpiperazine in impulsive aggression. Arch Gen Psychiatry. 2002;59:621–629. doi: 10.1001/archpsyc.59.7.621. [DOI] [PubMed] [Google Scholar]
- 22.Soloff PH, Meltzer CC, Greer PJ, Constantine D, Kelly TM. A fenfluramine-activated FDG-PET study of borderline personality disorder. Biol Psychiatry. 2000;47:540–547. doi: 10.1016/s0006-3223(99)00202-4. [DOI] [PubMed] [Google Scholar]
- 23.Leyton M, Okazawa H, Diksic M, Paris J, Rosa P, Mzengeza S, et al. Brain regional alpha-[11C]methyl-L-tryptophan trapping in impulsive subjects with borderline personality disorder. Am J Psychiatry. 2001;158:775–782. doi: 10.1176/appi.ajp.158.5.775. [DOI] [PubMed] [Google Scholar]
- 24.Goyer PF, Andreason PJ, Semple WE, Clayton AH, King AC, Compton-Toth BA, et al. Positron-emission tomography and personality disorders. Neuropsychopharmacology. 1994;10:21–28. doi: 10.1038/npp.1994.3. [DOI] [PubMed] [Google Scholar]
- 25.Ruocco AC, Amirthavasagam S, Choi-Kain LW, McMain SF. Neural correlates of negative emotionality in borderline personality disorder: An activation-likelihood-estimation meta-analysis. Biol Psychiatry. 2013;73:153–160. doi: 10.1016/j.biopsych.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Grunewald M, Johnson S, Lu D, Wang Z, Lomberk G, Albert PR, et al. Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression. J Biol Chem. 2012;287:24195–24206. doi: 10.1074/jbc.M112.373936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Filipenko ML, Beilina AG, Alekseyenko OV, Dolgov VV, Kudryavtseva NN. Repeated experience of social defeats increases serotonin transporter and monoamine oxidase A mRNA levels in raphe nuclei of male mice. Neurosci Lett. 2002;321:25–28. doi: 10.1016/s0304-3940(01)02495-8. [DOI] [PubMed] [Google Scholar]
- 28.Libert S, Pointer K, Bell EL, Das A, Cohen DE, Asara JM, et al. SIRT1 activates MAO-A in the brain to mediate anxiety and exploratory drive. Cell. 2011;147:1459–1472. doi: 10.1016/j.cell.2011.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kabayama M, Sakoori K, Yamada K, Ornthanalai VG, Ota M, Morimura N, et al. Rines E3 ubiquitin ligase regulates MAO-A levels and emotional responses. J Neurosci. 2013;33:12940–12953. doi: 10.1523/JNEUROSCI.5717-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sadikaj G, Russell JJ, Moskowitz DS, Paris J. Affect dysregulation in individuals with borderline personality disorder: Persistence and interpersonal triggers. J Pers Assess. 2010;92:490–500. doi: 10.1080/00223891.2010.513287. [DOI] [PubMed] [Google Scholar]
- 31.Yen S, Zlotnick C, Costello E. Affect regulation in women with borderline personality disorder traits. J Nerv Ment Dis. 2002;190:693–696. doi: 10.1097/00005053-200210000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- 33.Korzekwa MI, Dell PF, Links PS, Thabane L, Webb SP. Estimating the prevalence of borderline personality disorder in psychiatric outpatients using a two-phase procedure. Compr Psychiatry. 2008;49:380–386. doi: 10.1016/j.comppsych.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 34.Rekkas PV, Wilson AA, Lee VW, Yogalingam P, Sacher J, Rusjan P, et al. Greater monoamine oxidase a binding in perimenopausal age as measured with carbon 11-labeled harmine positron emission tomography. JAMA Psychiatry. 2014;71:873–879. doi: 10.1001/jamapsychiatry.2014.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) Washington, DC: American Psychiatric Press, Inc; 1997. [Google Scholar]
- 36.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition (SCID-I/P), Version 2. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 37.Zanarini MC, Frankenburg FR, Dubo ED, Sickel AE, Trikha A, Levin A, Reynolds V. Axis I comorbidity of borderline personality disorder. Am J Psychiatry. 1998;155:1733–1739. doi: 10.1176/ajp.155.12.1733. [DOI] [PubMed] [Google Scholar]
- 38.Arai Y, Se K, Kinemuchi H, Tadano T, Satoh S, Satoh N, et al. Selective inhibition of MAO-A in serotonergic synaptosomes by two amphetamine metabolites, p-hydroxyamphetamine and p-hydroxynorephedrine. Neurochem Int. 1990;17:587–592. doi: 10.1016/0197-0186(90)90046-v. [DOI] [PubMed] [Google Scholar]
- 39.Chiuccariello L, Houle S, Miler L, Cooke RG, Rusjan PM, Rajkowska G, et al. Elevated monoamine oxidase A binding during major depressive episodes is associated with greater severity and reversed neurovegetative symptoms. Neuropsychopharmacology. 2014;39:973–980. doi: 10.1038/npp.2013.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rusjan P, Mamo D, Ginovart N, Hussey D, Vitcu I, Yasuno F, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89. doi: 10.1016/j.pscychresns.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 41.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nat Neurosci. 2007;10:1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uylings HB, Sanz-Arigita EJ, de Vos K, Pool CW, Evers P, Rajkowska G. 3-D cytoarchitectonic parcellation of human orbitofrontal cortex correlation with postmortem MRI. Psychiatry Res. 2010;183:1–20. doi: 10.1016/j.pscychresns.2010.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tong J, Meyer JH, Furukawa Y, Boileau I, Chang LJ, Wilson AA, et al. Distribution of monoamine oxidase proteins in human brain: Implications for brain imaging studies. J Cereb Blood Flow Metab. 2013;33:863–871. doi: 10.1038/jcbfm.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bottlaender M, Dolle F, Guenther I, Roumenov D, Fuseau C, Bramoulle Y, et al. Mapping the cerebral monoamine oxidase type A: Positron emission tomography characterization of the reversible selective inhibitor [11C]befloxatone. J Pharmacol Exp Ther. 2003;305:467–473. doi: 10.1124/jpet.102.046953. [DOI] [PubMed] [Google Scholar]
- 45.Logan J, Fowler JS, Volkow ND, Wolf AP, Dewey SL, Schlyer DJ, et al. Graphical analysis of reversible radioligand binding from time-activity measurements applied to [N-11C-methyl]-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coccaro EF, Harvey PD, Kupsaw-Lawrence E, Herbert JL, Bernstein DP. Development of neuropharmacologically based behavioral assessments of impulsive aggressive behavior. J Neuropsychiatry Clin Neurosci. 1991;3:S44–S51. [PubMed] [Google Scholar]
- 48.Wechsler D. WAIS-R Manual. New York: Psychological Corp; 1981. [Google Scholar]
- 49.Benedict RH, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test–Revised: Normative data and analysis of interform and test-retest reliability. Clin Neuropsychol. 1998;12:43–55. [Google Scholar]
- 50.Beck AT, Kovacs M, Weissman A. Assessment of suicidal intention: The Scale for Suicide Ideation. J Consult Clin Psychol. 1979;47:343–352. doi: 10.1037//0022-006x.47.2.343. [DOI] [PubMed] [Google Scholar]
- 51.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol. 1995;51:768–774. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 52.Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- 53.Spielberger CD. Professional Manual for the State-Trait Anger Expression Inventory-2 (STAXI-2) Odessa, FL: Psychological Assessment Resources; 1999. [Google Scholar]
- 54.Jiang H, Jiang Q, Liu W, Feng J. Parkin suppresses the expression of monoamine oxidases. J Biol Chem. 2006;281:8591–8599. doi: 10.1074/jbc.M510926200. [DOI] [PubMed] [Google Scholar]
- 55.Richards G, Messer J, Waldvogel HJ, Gibbons HM, Dragunow M, Faull RL, Saura J. Up-regulation of the isoenzymes MAO-A and MAO-B in the human basal ganglia and pons in Huntington’s disease revealed by quantitative enzyme radioautography. Brain Res. 2011;1370:204–214. doi: 10.1016/j.brainres.2010.11.020. [DOI] [PubMed] [Google Scholar]
- 56.Sherif F, Gottfries CG, Alafuzoff I, Oreland L. Brain gamma-aminobutyrate aminotransferase (GABA-T) and monoamine oxidase (MAO) in patients with Alzheimer’s disease. J Neural Transm Park Dis Dement Sect. 1992;4:227–240. doi: 10.1007/BF02260906. [DOI] [PubMed] [Google Scholar]
- 57.Sparks DL, Woeltz VM, Markesbery WR. Alterations in brain monoamine oxidase activity in aging, Alzheimer’s disease, and Pick’s disease. Arch Neurol. 1991;48:718–721. doi: 10.1001/archneur.1991.00530190064017. [DOI] [PubMed] [Google Scholar]
- 58.Emilsson L, Saetre P, Balciuniene J, Castensson A, Cairns N, Jazin EE. Increased monoamine oxidase messenger RNA expression levels in frontal cortex of Alzheimer’s disease patients. Neurosci Lett. 2002;326:56–60. doi: 10.1016/s0304-3940(02)00307-5. [DOI] [PubMed] [Google Scholar]
- 59.Gunderson JG, Stout RL, McGlashan TH, Shea MT, Morey LC, Grilo CM, et al. Ten-year course of borderline personality disorder: Psychopathology and function from the Collaborative Longitudinal Personality Disorders study. Arch Gen Psychiatry. 2011;68:827–837. doi: 10.1001/archgenpsychiatry.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pittenger C, Krystal JH, Coric V. Initial evidence of the beneficial effects of glutamate-modulating agents in the treatment of self-injurious behavior associated with borderline personality disorder. J Clin Psychiatry. 2005;66:1492–1493. doi: 10.4088/jcp.v66n1121d. [DOI] [PubMed] [Google Scholar]
- 61.Cowdry RW, Gardner DL. Pharmacotherapy of borderline personality disorder. Alprazolam, carbamazepine, trifluoperazine, and tranylcypromine. Arch Gen Psychiatry. 1988;45:111–119. doi: 10.1001/archpsyc.1988.01800260015002. [DOI] [PubMed] [Google Scholar]
- 62.Soloff PH, Cornelius J, George A, Nathan S, Perel JM, Ulrich RF. Efficacy of phenelzine and haloperidol in borderline personality disorder. Arch Gen Psychiatry. 1993;50:377–385. doi: 10.1001/archpsyc.1993.01820170055007. [DOI] [PubMed] [Google Scholar]
- 63.Hoerst M, Weber-Fahr W, Tunc-Skarka N, Ruf M, Bohus M, Schmahl C, Ende G. Correlation of glutamate levels in the anterior cingulate cortex with self-reported impulsivity in patients with borderline personality disorder and healthy controls. Arch Gen Psychiatry. 2010;67:946–954. doi: 10.1001/archgenpsychiatry.2010.93. [DOI] [PubMed] [Google Scholar]
- 64.Soloff PH, Price JC, Meltzer CC, Fabio A, Frank GK, Kaye WH. 5HT2A receptor binding is increased in borderline personality disorder. Biol Psychiatry. 2007;62:580–587. doi: 10.1016/j.biopsych.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 65.Meyer JH, McMain S, Kennedy SH, Korman L, Brown GM, DaSilva JN, et al. Dysfunctional attitudes and 5-HT2 receptors during depression and self-harm. Am J Psychiatry. 2003;160:90–99. doi: 10.1176/appi.ajp.160.1.90. [DOI] [PubMed] [Google Scholar]
- 66.Soloff PH, Chiappetta L, Mason NS, Becker C, Price JC. Effects of serotonin-2A receptor binding and gender on personality traits and suicidal behavior in borderline personality disorder. Psychiatry Res. 2014;222:140–148. doi: 10.1016/j.pscychresns.2014.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Prossin AR, Love TM, Koeppe RA, Zubieta JK, Silk KR. Dysregulation of regional endogenous opioid function in borderline personality disorder. Am J Psychiatry. 2010;167:925–933. doi: 10.1176/appi.ajp.2010.09091348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Leyton M, Paquette V, Gravel P, Rosa-Neto P, Weston F, Diksic M, Benkelfat C. alpha-[11C]Methyl-L-tryptophan trapping in the orbital and ventral medial prefrontal cortex of suicide attempters. Eur Neuropsychopharmacol. 2006;16:220–223. doi: 10.1016/j.euroneuro.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 69.Dwivedi Y, Rizavi HS, Teppen T, Zhang H, Mondal A, Roberts RC, et al. Lower phosphoinositide 3-kinase (PI 3-kinase) activity and differential expression levels of selective catalytic and regulatory PI 3-kinase subunit isoforms in prefrontal cortex and hippocampus of suicide subjects. Neuropsychopharmacology. 2008;33:2324–2340. doi: 10.1038/sj.npp.1301641. [DOI] [PubMed] [Google Scholar]
- 70.Johnson S, Stockmeier CA, Meyer JH, Austin MC, Albert PR, Wang J, et al. The reduction of R1, a novel repressor protein for monoamine oxidase A, in major depressive disorder. Neuropsychopharmacology. 2011;36:2139–2148. doi: 10.1038/npp.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miguel-Hidalgo JJ, Whittom A, Villarreal A, Soni M, Meshram A, Pickett JC, et al. Apoptosis-related proteins and proliferation markers in the orbitofrontal cortex in major depressive disorder. J Affect Disord. 2014;158:62–70. doi: 10.1016/j.jad.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim HW, Rapoport SI, Rao JS. Altered expression of apoptotic factors and synaptic markers in postmortem brain from bipolar disorder patients. Neurobiol Dis. 2010;37:596–603. doi: 10.1016/j.nbd.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oldham JM. Borderline personality disorder and suicidality. Am J Psychiatry. 2006;163:20–26. doi: 10.1176/appi.ajp.163.1.20. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt HD, Shelton RC, Duman RS. Functional biomarkers of depression: Diagnosis, treatment, and pathophysiology. Neuropsychopharmacology. 2011;36:2375–2394. doi: 10.1038/npp.2011.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meyer JH. Targeting MAO-A as a Biomarker for MDD. Hollywood, FL: American College of Neuropsychopharmacology; 2012. [Google Scholar]
- 76.Yamaguchi K, Ueki R, Nonaka H, Sugihara F, Matsuda T, Sando S. Design of chemical shift-switching 19F magnetic resonance imaging probe for specific detection of human monoamine oxidase A. J Am Chem Soc. 2011;133:14208–14211. doi: 10.1021/ja2057506. [DOI] [PubMed] [Google Scholar]
- 77.Carlesimo GA, Piras F, Assogna F, Pontieri FE, Caltagirone C, Spalletta G. Hippocampal abnormalities and memory deficits in Parkinson disease: A multimodal imaging study. Neurology. 2012;78:1939–1945. doi: 10.1212/WNL.0b013e318259e1c5. [DOI] [PubMed] [Google Scholar]
- 78.Shin J, Lee SY, Kim SH, Kim YB, Cho SJ. Multitracer PET imaging of amyloid plaques and neurofibrillary tangles in Alzheimer’s disease. Neuroimage. 2008;43:236–244. doi: 10.1016/j.neuroimage.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 79.Zanarini MC, Stanley B, Black DW, Markowitz JC, Goodman M, Pilkonis P, et al. Methodological considerations treatment trials for persons personality disorder. Ann Clin Psychiatry. 2010;22:75–83. [PMC free article] [PubMed] [Google Scholar]
- 80.Zanarini MC, Vujanovic AA, Parachini EA, Boulanger JL, Frankenburg FR, Hennen J. Zanarini Rating Scale for Borderline Personality Disorder (ZAN-BPD) A continuous measure of DSM-IV borderline psychopathology. J Pers Disord. 2003;17:233–242. doi: 10.1521/pedi.17.3.233.22147. [DOI] [PubMed] [Google Scholar]
- 81.Arntz A, van den Hoorn M, Cornelis J, Verheul R, van den Bosch WM, de Bie AJ. Reliability and validity of the borderline personality disorder severity index. J Pers Disord. 2003;17:45–59. doi: 10.1521/pedi.17.1.45.24053. [DOI] [PubMed] [Google Scholar]