Abstract
IMPORTANCE
Perimenopause is a period of high risk for mood disorders, and it has been proposed that perimenopause is also a window of risk for processes linked to later dementia. However, in human perimenopause, the neurobiological changes implicated in the genesis of mood disorders or dementia have not been identified. Monoamine oxidase A (MAO-A) is an important brain enzyme that creates oxidative stress, influences apoptosis, and metabolizes monoamines. After declines in estrogen level, MAO-A density may be elevated for a month or longer, and repeated declines in estrogen level occur with greater magnitude during perimenopause.
OBJECTIVE
To investigate whether MAO-A total distribution volume (VT), an index of MAO-A density, is elevated in women of perimenopausal age (41–51 years).
DESIGN, SETTING, AND PARTICIPANTS
In a cross-sectional study at a tertiary care psychiatric hospital, 58 women underwent carbon 11–labeled harmine positron emission tomography. These included 19 young women of reproductive age (mean [SD], 28.26 [5.05] years), 27 women of perimenopausal age (mean [SD] age, 45.21 [3.41] years; including 14 women with change in menstrual cycle length with a mean [SD] age of 45.50 [4.00] years and 13 women with no change in menstrual cycle length with a mean [SD] age of 44.92 [2.81] years), and 12 women in menopause (mean [SD] age, 56.25 [3.19] years).
MAIN OUTCOMES AND MEASURES
Values of MAO-A VT in the prefrontal cortex, anterior cingulate cortex, dorsal striatum, ventral striatum, thalamus, hippocampus, and midbrain.
RESULTS
On average, MAO-A VT in perimenopausal age was elevated by 34% compared with reproductive age and by 16% compared with menopause (multivariate analysis of variance, group effect, F16,94 = 3.03; P < .001). Within the perimenopausal age group, meeting Stages of Reproductive Aging Workshop criteria for perimenopause, which is mainly based on menstrual cycle length, was not associated with MAO-A VT (F8,18 = 0.548; P = .81) but tendency to cry was positively correlated with MAO-A VT in the prefrontal cortex (r = 0.54; P = .008).
CONCLUSIONS AND RELEVANCE
To our knowledge, this is the first report of a change in a central biomarker during perimenopausal age that is also present during major depressive episodes and high-risk states for major depressive episodes. The functions of MAO-A influence oxidative stress and apoptosis, 2 processes implicated as excessive in both mood disorders and dementia. Hence, greater MAO-A VT during perimenopause may represent a new target for assessing novel interventions to prevent mood disorders and reduce longer-term risk of neurodegenerative disease.
The transition of perimenopause has an important influence on the long-term health of women.1 Most health investigations of perimenopause focus on physical symptoms and the influence of hormone replacement therapy (HRT) on risk for dementia. However, recent studies2,3 demonstrate a high rate of new-onset major depressive episodes (MDEs) and minor depression (sustained depressed mood and anhedonia for several weeks) during perimenopause, with respective rates of 17% and 16%.2 Unfortunately, the neurobiological changes in perimenopause that explain the predisposition to mood disorders and/or dementia are not established in humans.
Menstrual cycles continue for most of perimenopause, although cycles are less regular, with peaks and declines that are substantially greater and more erratic than those in the main reproductive years; plasma estrogen levels reach a stable but lower plateau in the last year of perimenopause (eAppendix in Supplement).1 It has been suggested that estrogen fluctuations in perimenopause influence cognition and mood through several processes, including neuronal survival in the dentate gyrus,4 changes in signal transduction factors regulating synaptic plasticity and connectivity,5 clearance of extracellular serotonin,6 and altered levels of proteins that influence cell survival such as Bcl27 and monoamine oxidase A (MAO-A).8–14 To our knowledge, none of these markers have been examined in perimenopause in any species. Our study focuses on the latter marker, MAO-A, an important brain enzyme that creates oxidative stress, influences apoptosis, and metabolizes monoamines. In regions with high MAO-A density in animal models and in cell lines, changes in 17β-estradiol levels typically have an inverse influence on later MAO-A levels, messenger RNA (mRNA), and activity10–14 (ie, reduced estrogen agonism or effect is associated with higher MAO-A levels, mRNA, and activity, which may be sustained even longer than a month past the initial shift in estrogen level13).
In this study, carbon 11 ([11C])–labeled harmine positron emission tomography (PET) is applied to investigate MAO-A total distribution volume (VT), an index of MAO-A density in the brain, in women of perimenopausal age, young reproductive age, and menopausal age. In brain tissue during health, MAO-A levels are highly correlated with MAO-A activity, and hormonal influences on MAO-A level parallel changes in MAO-A activity.10–14 Harmine itself binds to the center of the functional cavity in MAO-A.15 Thus, [11C]harmine is an excellent PET radiotracer with high affinity and selectivity for the MAO-A enzyme as well as reversible kinetics, and it has been modeled in humans.16,17 The resulting MAO-A VT values are highly correlated with known MAO-A density.16–18 Given that the strong fluctuations in estrogen level during perimenopause include episodes of large declines and given that large declines in estrogen level are associated with elevations in MAO-A density, mRNA, and activity,10–14 our main hypothesis is that women of perimenopausal age have increased MAO-A VT throughout the brain. Our second hypothesis is that this increase normalizes during menopause as episodic estrogen declines no longer occur. Although we hypothesize global changes in MAO-A VT during perimenopause, our third hypothesis is that elevated MAO-A VT in specific regions becomes more functionally relevant and that mood-associated symptoms, such as the psychological symptom of crying, are positively correlated with prefrontal and anterior cingulate cortices. Crying is a frequent symptom of perimenopause,19,20 and elevated MAO-A VT has been reported during postpartum blues,21 which is strongly associated with increased crying.22
Methods
Participants
Fifty-eight women underwent [11C]harmine PET imaging (Table 1). Twenty-seven women were in the perimenopausal age range (41–51 years); among these 27 women, 14 were experiencing clinical symptoms of perimenopause (mean [SD] age, 45.50 [4.00] years) as per the Stages of Reproductive Aging Workshop (STRAW) criteria,23 while 13 were not experiencing a change in cycle length (mean [SD] age, 44.92 [2.81] years). Women in the perimenopausal age range were scanned within the follicular phase of their menstrual cycle. Nineteen younger women were of reproductive age (mean [SD] age, 28.26 [5.05] years), and 12 women were in menopause (mean [SD] age, 56.25 [3.19] years; mean [SD] time since last menstrual period, 9.00 [3.95] years). The mean age at onset of perimenopause is known to be 46 years,1 with 95% entering perimenopause between 39 and 51 years; thus, the age range in this study is inclusive of most women.
Table 1.
Demographic Characteristics and 17-Item Hamilton Depression Rating Scale Scores of Study Participants
| Characteristic | Mean (SD) | ||
|---|---|---|---|
| Young Reproductive Age (n = 19) | Perimenopausal Age (n = 27) | Older Menopausal Age (n = 12) | |
| Age, y | 28.26 (5.05) | 45.21 (3.41) | 56.25 (3.19) |
| Education, y | 16.00 (2.94) | 16.31 (2.43) | 15.86 (1.13) |
| 17-Item Hamilton Depression Rating Scale scorea | 0.50 (0.83) | 1.12 (1.92) | 0.31 (0.61) |
Scores derived on the day of scanning.
For all participants, exclusion criteria included recent pregnancy (within 6 months), recent abortion (within 6 months), oral contraceptive use within 2 years, HRT, treatment with bioidentical hormones, and hysterectomy. Other exclusion criteria included cigarette smoking, herbal, drug, or medication use within 8 weeks, history of psychiatric or medical illness, suicide attempts, and substance abuse. Participants received a pregnancy test to ensure they were not pregnant at the time of testing, and they had a urine drug screen. Screening instruments to confirm the absence of past or current mental disorders included a score higher than 7 on the 17-item Hamilton Depression Rating Scale,24 the Structured Clinical Interview for DSM-IV for Axis I disorders25 and Axis II disorders,26 and the Adult Crying Inventory.27 The Adult Crying Inventory is a 54-item self-report with excellent internal consistency (Cronbach α = 0.87–0.95),28,29 and scores are highly correlated with crying frequency and proneness to crying.29,30
Women of perimenopausal age kept a 4-month diary to rate heaviness of bleeding and length of cycles. In addition, women of perimenopausal age completed the Menstrual Cycle Questionnaire31 and Greene Climacteric Scale.20 Onset of perimenopause (with physical symptoms) within the perimenopausal age group was based on the STRAW criteria,23 with a main weighting of a change in cycle length of 7 days or greater for consecutive cycles and a lesser weighting that is given to other criteria such as vasomotor symptoms and follicle-stimulating hormone levels.
This study was reviewed and approved by the Research Ethics Board for Human Subjects at the Centre for Addiction and Mental Health, University of Toronto, Toronto, Ontario, Canada. Written informed consent was obtained from each participant prior to participation in the study.
Image Acquisition and Analysis
Each participant underwent 1 [11C]harmine PET scan to determine regional MAO-A VT. For each PET scan, 370 MBq of [11C]harmine was administered as a bolus intravenously. An automatic blood sampling system was used to measure arterial blood radioactivity during the first 10 minutes of the scan. Manual samples were obtained at 2.5, 7.5, 15, 20, 30, 45, 60, and 90 minutes after injection. The method of measuring radioactivity in whole blood and parent compound in plasma has been previously described17 (eAppendix in Supplement).
Each participant also underwent magnetic resonance imaging (MRI) (Signa 1.5-T scanner; fast spoiled gradient-echo, T1-weighted image; voxel dimensions, x = 0.78, y = 0.78, and z = 1.5 mm; GE Medical Systems) for the region-of-interest (ROI) delineation. The ROIs were determined using a semiautomated method in which regions of a template MRI are transformed onto the individual MRI based on a series of transformations and deformations that match the template image to the individual coregistered MRI as well as segmentation of the individual MRI to select the gray matter voxels as previously described.32,33 Each participant’s MRI was coregistered to the summed PET image, and the resulting transformation was applied to the ROIs to create a mask for time activity curve extraction.
For the first 2 hypotheses, ROIs sampled were implicated in affect regulation and MDEs or have moderate to high MAO-A density and included the prefrontal cortex, anterior cingulate cortex, midbrain, thalamus, putamen, striatum, and hippocampus.18,33–38 For [11C]harmine PET, VT may be validly and reliably measured with either an unconstrained 2-tissue compartment model or with the Logan model with arterial sampling (for which the underestimate of VT is negligible at the noise level of time activity curves from the ROIs),17 and the latter was applied in this study.
Statistical Analysis
The main analysis consisted of a multivariate analysis of variance (MANOVA) investigating the effect of group (young reproductive age, perimenopausal age, menopause) on global regional MAO-A VT. To specifically assess whether regional MAO-A VT was elevated in the perimenopausal age group compared with the young reproductive age group and whether MAO-A VT was lower in the menopause group compared with the perimenopausal age group, additional comparisons were carried out using the protected least significant difference procedure.
To assess the relationship of MAO-A VT to physical symptoms in perimenopause, MANOVA was applied with MAO-A VT as the dependent variable and presence of menstrual cycle change as a predictor variable. Pearson correlation coefficients were applied to assess the correlation between the Adult Crying Inventory score and MAO-A VT in the prefrontal cortex and anterior cingulate cortex, 2 regions implicated in the generation of cognitive mood symptoms. These are also regions for which MAO-A VT has been associated with level of depressive symptoms in MDEs.33
Results
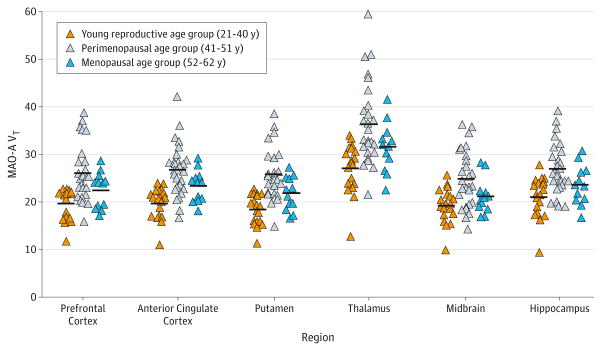
As shown in Figure 1, MAO-A VT was strongly elevated during perimenopausal age across all regions assessed, on average by 34% compared with reproductive age and 16% compared with menopause (MANOVA, group effect, F16,94 = 3.03; P < .001; regional comparisons, P ≤ .05, Fisher least significant difference test). Significant univariate effects were also found in all brain regions (F2,58 = 7.63–16.49; P ≤ .001). Additional comparisons (based on least significant difference test) showed greater MAO-A VT in women in the perimenopausal age group relative to the young reproductive age group in all regions (P < .001) and in the perimenopausal age group relative to the menopause group in all regions (P ≤ .05). In addition, MAO-A VT in menopausal women was significantly higher than in young reproductive age women in the putamen, anterior cingulate, and striatum (P < .05). Consistent with the overall results, independent samples t test comparing mean MAO-A VT showed significant whole-brain differences between groups (young reproductive vs perimenopausal age range, t44 = −4.7, P < .001; perimenopausal age range vs menopause, t37 = 2.1, P = .04; reproductive age vs menopause, t29 = −2.4, P = .02). Furthermore, MANOVA to assess group effect on regional MAO-A VT was rerun across the 3 groups, including only women in the reproductive age group within the follicular phase (n = 13) because all the women in perimenopause were in the follicular phase. Results were similar with a strong group effect (F16,82 = 2.073; P < .02) and similar significant univariate effects within each brain region (P = .01 to P < .001).
Figure 1. Monoamine Oxidase A (MAO-A) Total Distribution Volume (VT) in Women of Young Reproductive Age, Women of Perimenopausal Age (All Women Regardless of Cycle Length), and Older Women in Menopause.
On average, MAO-A VT in perimenopausal age was 34% greater compared with reproductive age and 16% greater compared with menopause (multivariate analysis of variance, group effect, F16,94 = 3.03; P < .001). Horizontal lines indicate mean MAO-A VT.
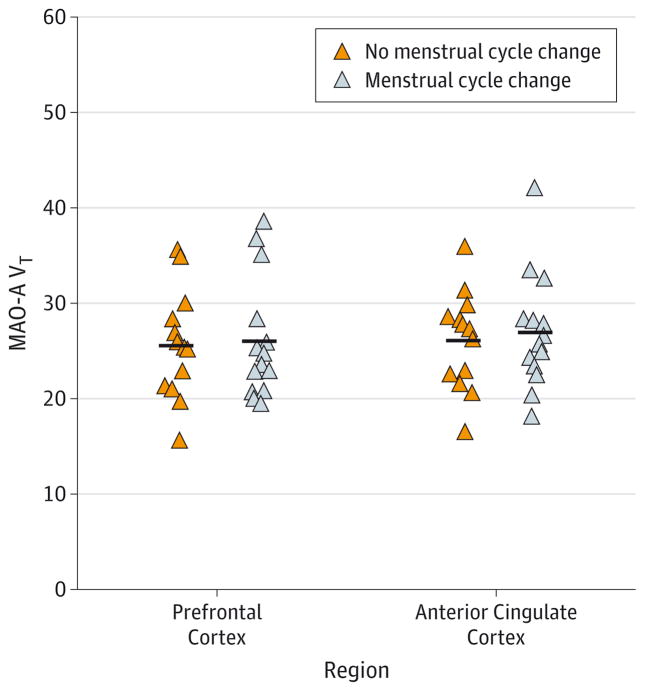
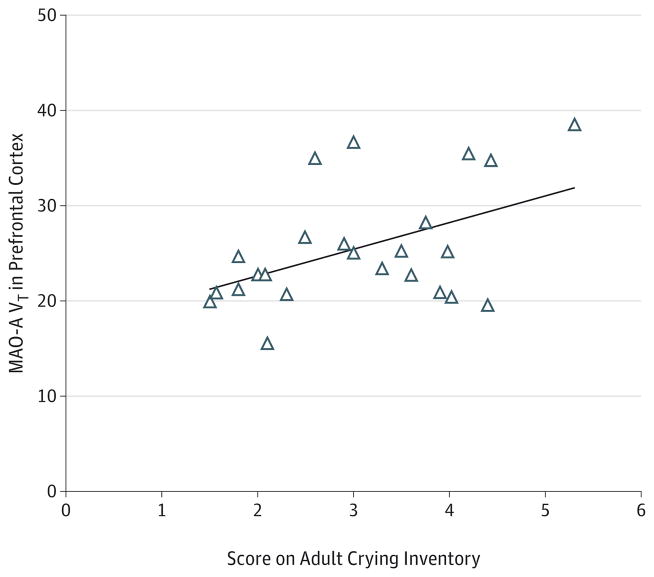
Within the perimenopausal age group, MAO-A VT levels were not associated with meeting STRAW criteria23 for perimenopause (F8,18 = 0.548; P = .81) (Figure 2). Comparisons of the demographic and clinical characteristics of the women who did or did not meet STRAW criteria are shown in Table 2 (for additional comparisons of supportive STRAW criteria with MAO-A VT, see eAppendix in Supplement). Within the perimenopausal age group, a significant positive correlation was found between tendency to cry, as measured with the Adult Crying Inventory, and MAO-A VT in the prefrontal cortex (r = 0.54; P = .008) (Figure 3). The correlation with crying was not significant in the anterior cingulate cortex (r = 0.35; P = .10), although it was in the same direction.
Figure 2. Comparison of Monoamine Oxidase A (MAO-A) Total Distribution Volume (VT) in the Prefrontal Cortex and Anterior Cingulate Cortex in Women in the Perimenopausal Age Group With No Change in Menstrual Cycle Length vs Those With a Significant Change in Menstrual Cycle Length.
Within the perimenopausal age group, MAO-A VT was not associated with meeting Stages of Reproductive Aging Workshop criteria for perimenopause (F8,18 = 0.548; P = .81). Horizontal lines indicate mean MAO-A VT.
Table 2.
Demographic and Clinical Characteristics of Subgroups in the Perimenopausal Age Group
| Characteristic | Mean (SD) | |
|---|---|---|
| No Change in Menstrual Cycle Length (n = 13) | Significant Change in Menstrual Cycle Lengtha (n = 14) | |
| Age, y | 44.92 (2.81) | 45.50 (4.00) |
| Education, y | 16.54 (2.74) | 16.07 (2.13) |
| 17-Item Hamilton Depression Rating Scale scoreb | 1.46 (2.57) | 1.07 (1.53) |
| Adult Crying Inventory score | 3.24 (0.93) | 2.81 (1.17) |
| Climacteric measure | ||
| Menopause Rating Scale score | ||
| Psychological symptoms | 1.41 (1.50) | 2.79 (2.61) |
| Somatovegetative symptoms | 1.50 (1.57) | 2.50 (2.14) |
| Urogenital symptoms | 0.33 (0.65) | 1.82 (2.02)c |
| Total | 3.25 (3.17) | 7.11 (5.52)c |
| Greene Climacteric Scale score | ||
| Psychological | 4.08 (3.40) | 5.87 (2.81) |
| Anxiety | 2.17 (1.91) | 2.74 (1.64) |
| Depression | 1.92 (1.89) | 3.27 (1.42)c |
| Somatic | 2.33 (1.84) | 2.91 (1.93) |
| Vasomotor | 0.50 (0.76) | 1.55 (1.50)c |
| Sexual dysfunction | 0.25 (0.60) | 0.82 (0.94) |
| Hormonal measure | ||
| Estradiol, pg/mL | 150.9 (102.8) | 150.6 (221.2) |
| Progesterone, ng/mL | 0.85 (1.13) | 0.31 (0.19) |
| Follicle-stimulating hormone, mIU/mL | 7.6 (4.9) | 21.5 (24.5)d |
SI conversion factors: To convert estradiol to picomoles per liter, multiply by 3.671; to convert progesterone to nanomoles per liter, multiply by 3.18; and to convert follicle-stimulating hormone to international units per liter, multiply by 1.0.
As defined by Stages of Reproductive Aging Workshop criteria.23
Scores derived on the day of scanning.
P < .05, 2-tailed t test with Welch correction for unequal variances.
P < .05, 1-tailed t test with Welch correction for unequal variances.
Figure 3. Positive Correlation Between Monoamine Oxidase A (MAO-A) Total Distribution Volume (VT) in the Prefrontal Cortex and Scores on the Adult Crying Inventory in the Perimenopausal Age Group.
For the correlation between MAO-A VT in the prefrontal cortex and scores on the Adult Crying Inventory, r = 0.54 (2-tailed P = .008).
Discussion
Our main findings are that MAO-A VT is strongly elevated throughout the brain during the typical age of perimenopause (41–51 years) compared with younger age and that MAO-A VT is lower in menopause than in perimenopause. This suggests a new mechanism for the exceptionally high prevalence of MDEs during perimenopause, has potential implications for the timing and approach of HRT, and raises new considerations for defining changes in the brain relative to other physical changes during perimenopause.
Given the high prevalence of depressive symptoms during perimenopause, it would be expected that some central biomarkers of MDE would be present during perimenopause; however, to our knowledge, this is the first demonstration of a change in a central biomarker during perimenopausal age that is similarly changed during MDEs. Elevated MAO-A levels, demonstrated most frequently in the prefrontal cortex, occur during MDEs in humans,33,36–38 and greater MAO-A activity and mRNA have also been demonstrated in cortical and subcortical regions in animal models of depression.39–41 To date, no other markers of change in major depressive disorder, including glial cell loss, lower γ-aminobutyric acid levels, abnormal glutamate/ glutamine levels, reduced N-acetyl aspartate levels, deficiencies in functional brain-derived neurotrophic factor and cyclic adenosine monophosphate response element–binding protein in hippocampus, and hippocampal volume loss, have been reported as abnormal during perimenopausal age. It is possible that this reflects a lack of focused investigation, although some markers such as volumetric MRI measurement of the hippocampus have been assessed in relation to age. One partial exception is the finding by Moses-Kolko et al42 of relatively increased serotonin 1A receptor binding in the prefrontal cortex as women age, which was interpreted as protective against MDEs beyond age 65 years. From the perspective that major depressive disorder is a complex psychiatric illness with multiple abnormalities, our study suggests that markers related to excessive MAO-A activity, such as indices of greater oxidative stress, or markers of greater predisposition toward intrinsic apoptosis may also be more likely to change during perimenopausal age. Also, given that an emerging strategy in complex illnesses with multiple phenotypes is to develop methods of prevention through reducing risk of individual phenotypes, we suggest that MAO-A VT might be a useful target for assessing the impact of novel strategies to prevent MDEs in perimenopause.
Our results suggest that the optimal timing for strategies to maintain neuropsychiatric health during perimenopause is between ages 41 and 51 years because oxidative stress and apoptosis are processes influenced by MAO-A that are enhanced in dementia,43,44 and it has also been proposed that greater activity of MAO-A itself may predispose to neurotoxic effects in Alzheimer disease.45 A recent concept in HRT for preventing cognitive aging and dementia is that HRT should be investigated for benefit prior to neurobiological changes that accompany menopause46,47 because late HRT increases likelihood of dementia, possibly due to strokes and brain atrophy.48,49 Our study suggests that late HRT overlaps poorly with the elevation in MAO-A VT but early HRT in a window of ages 41 to 51 years would overlap much more closely. Given that strong estrogen declines are implicated in elevating MAO-A levels, an interesting future direction would be to assess whether HRT, which should dampen estrogen fluctuation, specifically targets the increase in MAO-A VT observed in perimenopause.
Recent advances have been made in understanding the role of MAO-A in neurodegenerative disease,43 neurodevelopment,50,51 and mood disorders.33,36–38,52 However, MAO-A was identified more than 30 years ago, raising the question as to why a change in MAO-A levels or activity has not previously been reported in perimenopause. Based on the sex ratio and age distribution presented in previous studies, no study individually sampled more than 3 women aged 41 to 51 years.18,53–55 For example, within the 2 studies providing precise details of age and sex, among 87 participants aged 21 hours to 99 years, the number of women between ages 41 and 51 years sampled in each study was 1 and 0.18,53
Presently, the criteria for perimenopause, such as the STRAW classification,23 emphasize physical criteria such as menstrual cycle length, vasomotor symptoms, and plasma follicle-stimulating hormone levels. However, given the lack of correlation between these measures and brain MAO-A VT, this indicates that measures reflective of the state of other organs cannot be assumed to be optimally representative of changes in the brain. Our study suggests there is additional value for including psychological measures such as tendency to cry, which did relate to the brain measure of MAO-A VT, to gauge some aspects of perimenopause in addition to the main STRAW criteria.23
Our study has the advantage of measuring an index of MAO-A density in vivo but has disadvantages associated with PET imaging. The resolution of PET does not allow us to determine the cellular specificity of the changes in MAO-A VT, and MAO-A can be present in glia and neurons. A second limitation is that we selected MAO-A VT as a marker. The advantage of this marker is that it is robustly measured with [11C]harmine PET, but the disadvantage is that it represents both specific binding and free and nonspecific binding. Even so, the free and nonspecific binding for [11C]harmine represents only 15% of MAO-A VT, so the differences in this measure primarily reflect a change in specific MAO-A binding.17,56 A third limitation is that the prefrontal and anterior cingulate cortices were prioritized for the third hypothesis of a correlation between greater MAO-A VT with the psychological measurement of crying, but MAO-A VT is highly intercorrelated among different brain regions within individuals; hence, it cannot be concluded that MAO-A VT in the prefrontal cortex is preferentially related to crying vs the other regions assayed. In addition, the cross-sectional design is a limitation. However, the magnitude of change across the groups is quite substantive, particularly between women of reproductive age and women of perimenopausal age, so it is extremely unlikely that a bias in sampling could explain this difference.
Conclusions
To our knowledge, this is the first report of a change in a central biomarker during perimenopausal age that is also present during MDEs. The functions of MAO-A influence processes implicated in both mood disorders and dementia, and greater levels of MAO-A itself (particularly in the prefrontal cortex) are associated with MDEs and depressive symptoms.33,36–38,43 Hence, greater MAO-A levels during perimenopause may represent a new target for assessing novel interventions to prevent mood disorders and reduce longer-term risk of neurodegenerative disease, and the optimal timing of such interventions should be early. Finally, our study found that change in MAO-A VT during perimenopausal age was associated with crying, a psychological symptom, rather than the change in menstrual cycle length. This demonstrates that staging of perimenopause may not be uniform between the brain and the rest of the body and that future definitions of perimenopause should consider staging brain changes in perimenopause differently from alterations in menstrual cycle length.
Acknowledgments
Funding/Support: This work was supported by an operating grant and Canada Research Chair from the Canadian Institutes of Health Research, the NARSAD Young Investigator Grant from the Brain and Behavior Research Foundation, the Canadian Foundation for Innovation, and the Ministry for Research and Innovation.
Footnotes
Conflict of Interest Disclosures: Drs Wilson, Houle, and Meyer have received operating grant funds for other studies from Eli Lilly and Co, GlaxoSmithKline, Bristol-Myers Squibb, Lundbeck, and SK Life Science in the past 5 years. Dr Meyer has been a consultant to these companies as well as Takeda, Sepracor, Trius, Mylan, and Teva; is developing natural health products to treat high MAO-A states; is an inventor for patents to apply measures of MAO to diagnose or treat mood disorders; and is applying for additional similar patents. Dr Stewart is a scientific advisor to Eli Lilly and Co regarding the Cymbalta Pregnancy Registry and serves as an editor for the effects of SSRIs on the fetus in UpToDate.
Role of the Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Previous Presentation: This work was presented in part at the 68th Annual Meeting of the Society of Biological Psychiatry; May 17, 2013; San Francisco, California.
Supplemental content at jamapsychiatry.com
Author Contributions: Drs Rekkas and Meyer had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Rekkas, Sacher, Houle, Meyer.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Rekkas, Lee, Kish, Meyer.
Critical revision of the manuscript for important intellectual content: Rekkas, Wilson, Yogalingam, Sacher, Rusjan, Houle, Stewart, Kolla, Kish, Chiuccariello, Meyer.
Statistical analysis: Rekkas, Meyer.
Obtained funding: Rekkas, Sacher, Meyer.
Administrative, technical, or material support: Rekkas, Wilson, Lee, Sacher, Rusjan, Houle, Stewart, Kolla, Chiuccariello, Meyer.
Study supervision: Rekkas, Meyer.
Additional Contributions: From the Research Imaging Centre at the Centre for Addiction and Mental Health, Toronto, Ontario, Canada, Cynthia Xu, MD, and Laura Miler, BSc, served as research coordinators, Leslie Romano, SSW, served as a research analyst, Alvina Ng, BSc, and Laura Nguyen, BSc, served as technicians, Jun Parkes, MSc, Armando Garcia, BSc, Winston Stableford, BSc, and Min Wong, BSc, served as chemistry staff, and Terry Bell, BSc, and Ted Harris-Brandts, BSc, served as engineers. The Canadian Institutes of Health Research provides some overhead funding to institutions, which may have supported the salary of some of these staff located at the Centre for Addiction and Mental Health. Also, the Canadian Institutes of Health Research funded other projects for which some people would have received salary support for their contributions.
References
- 1.Speroff L, Fritz M. Clinical Gynecologic Endocrinology and Infertility. 7. Philadelphia, PA: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 2.Cohen LS, Soares CN, Vitonis AF, Otto MW, Harlow BL. Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry. 2006;63(4):385–390. doi: 10.1001/archpsyc.63.4.385. [DOI] [PubMed] [Google Scholar]
- 3.Freeman EW, Sammel MD, Lin H, Nelson DB. Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry. 2006;63(4):375–382. doi: 10.1001/archpsyc.63.4.375. [DOI] [PubMed] [Google Scholar]
- 4.McClure RE, Barha CK, Galea LA. 17β-Estradiol, but not estrone, increases the survival and activation of new neurons in the hippocampus in response to spatial memory in adult female rats. Horm Behav. 2013;63(1):144–157. doi: 10.1016/j.yhbeh.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 5.McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci. 2012;126(1):4–16. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benmansour S, Weaver RS, Barton AK, Adeniji OS, Frazer A. Comparison of the effects of estradiol and progesterone on serotonergic function. Biol Psychiatry. 2012;71(7):633–641. doi: 10.1016/j.biopsych.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morissette M, Al Sweidi S, Callier S, Di Paolo T. Estrogen and SERM neuroprotection in animal models of Parkinson’s disease. Mol Cell Endocrinol. 2008;290(1–2):60–69. doi: 10.1016/j.mce.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Ou XM, Chen K, Shih JC. Monoamine oxidase A and repressor R1 are involved in apoptotic signaling pathway. Proc Natl Acad Sci U S A. 2006;103(29):10923–10928. doi: 10.1073/pnas.0601515103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fitzgerald JC, Ufer C, De Girolamo LA, Kuhn H, Billett EE. Monoamine oxidase-A modulates apoptotic cell death induced by staurosporine in human neuroblastoma cells. J Neurochem. 2007;103(6):2189–2199. doi: 10.1111/j.1471-4159.2007.04921.x. [DOI] [PubMed] [Google Scholar]
- 10.Luine VN, McEwen BS. Effect of oestradiol on turnover of type A monoamine oxidase in brain. J Neurochem. 1977;28(6):1221–1227. doi: 10.1111/j.1471-4159.1977.tb12313.x. [DOI] [PubMed] [Google Scholar]
- 11.Holschneider DP, Kumazawa T, Chen K, Shih JC. Tissue-specific effects of estrogen on monoamine oxidase A and B in the rat. Life Sci. 1998;63(3):155–160. doi: 10.1016/s0024-3205(98)00255-0. [DOI] [PubMed] [Google Scholar]
- 12.Gundlah C, Lu NZ, Bethea CL. Ovarian steroid regulation of monoamine oxidase-A and -B mRNAs in the macaque dorsal raphe and hypothalamic nuclei. Psychopharmacology (Berl) 2002;160(3):271–282. doi: 10.1007/s00213-001-0959-0. [DOI] [PubMed] [Google Scholar]
- 13.Smith LJ, Henderson JA, Abell CW, Bethea CL. Effects of ovarian steroids and raloxifene on proteins that synthesize, transport, and degrade serotonin in the raphe region of macaques. Neuropsychopharmacology. 2004;29(11):2035–2045. doi: 10.1038/sj.npp.1300510. [DOI] [PubMed] [Google Scholar]
- 14.Ma ZQ, Violani E, Villa F, Picotti GB, Maggi A. Estrogenic control of monoamine oxidase A activity in human neuroblastoma cells expressing physiological concentrations of estrogen receptor. Eur J Pharmacol. 1995;284(1–2):171–176. doi: 10.1016/0014-2999(95)00387-z. [DOI] [PubMed] [Google Scholar]
- 15.Son SY, Ma J, Kondou Y, Yoshimura M, Yamashita E, Tsukihara T. Structure of human monoamine oxidase A at 2.2-A resolution: the control of opening the entry for substrates/ inhibitors. Proc Natl Acad Sci U S A. 2008;105(15):5739–5744. doi: 10.1073/pnas.0710626105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bergström M, Westerberg G, Långström B. 11C-harmine as a tracer for monoamine oxidase A (MAO-A): in vitro and in vivo studies. Nucl Med Biol. 1997;24(4):287–293. doi: 10.1016/s0969-8051(97)00013-9. [DOI] [PubMed] [Google Scholar]
- 17.Ginovart N, Meyer JH, Boovariwala A, et al. Positron emission tomography quantification of [11C]-harmine binding to monoamine oxidase-A in the human brain. J Cereb Blood Flow Metab. 2006;26(3):330–344. doi: 10.1038/sj.jcbfm.9600197. [DOI] [PubMed] [Google Scholar]
- 18.Tong J, Meyer JH, Furukawa Y, et al. Distribution of monoamine oxidase proteins in human brain: implications for brain imaging studies. J Cereb Blood Flow Metab. 2013;33(6):863–871. doi: 10.1038/jcbfm.2013.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grigoriou V, Augoulea A, Armeni E, et al. Prevalence of vasomotor, psychological, psychosomatic and sexual symptoms in perimenopausal and recently postmenopausal Greek women: association with demographic, life-style and hormonal factors. Gynecol Endocrinol. 2013;29(2):125–128. doi: 10.3109/09513590.2012.708801. [DOI] [PubMed] [Google Scholar]
- 20.Greene JG. Constructing a standard climacteric scale. Maturitas. 2008;61(1–2):78–84. doi: 10.1016/j.maturitas.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Sacher J, Wilson AA, Houle S, et al. Elevated brain monoamine oxidase A binding in the early postpartum period. Arch Gen Psychiatry. 2010;67(5):468–474. doi: 10.1001/archgenpsychiatry.2010.32. [DOI] [PubMed] [Google Scholar]
- 22.Gale S, Harlow BL. Postpartum mood disorders: a review of clinical and epidemiological factors. J Psychosom Obstet Gynaecol. 2003;24(4):257–266. doi: 10.3109/01674820309074690. [DOI] [PubMed] [Google Scholar]
- 23.Soules MR, Sherman S, Parrott E, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Fertil Steril. 2001;76(5):874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.First M, Spitzer R, Williams J, Gibbon M. Structured Clinical Interview for DSM-IV, Non-Patient Edition (SCID-NP, Version 1.0) Washington, DC: American Psychiatric Press; 1995. [Google Scholar]
- 26.First M, Gibbon M, Spitzer RL, Williams JBW, Benjamin LS. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. Washington, DC: American Psychiatric Publishing; 1997. [Google Scholar]
- 27.Vingerhoets AJJM. Adult Crying Questionnair. In: Vingerhoets AJJM, Cornelius RR, editors. Adult Crying: A Biopsychological Approach. New York, NY: Taylor & Francis; 2001. p. 303. [Google Scholar]
- 28.Scheirs GM, Sijtsma K. The study of crying: some methodological considerations and a comparison of methods for analyzing questionnaires. In: Vingerhoets AJJM, Cornelius RR, editors. Adult Crying: A Biopsychological Approach. New York, NY: Taylor & Francis; 2001. pp. 277–298. [Google Scholar]
- 29.Van Tilberg MAL, Unterberg ML, Vingerhoets AJJM. Crying during adolescence: the role of gender, menarche and empathy. Br J Dev Psychol. 2002;20(1):77–87. doi: 10.1348/026151002166334. [DOI] [Google Scholar]
- 30.Peter M, Vingerhoets AJJM, Van Heck GL. Personality, gender and crying. Eur J Pers. 2001;15(1):19–28. doi: 10.1002/per.386. [DOI] [Google Scholar]
- 31.Hauser GA, Huber IC, Keller PJ, Lauritzen C, Schneider HPG. Evaluation of climacteric symptoms (Menopause Rating Scale) [in German] Zentralbl Gynakol. 1994;116(1):16–23. [PubMed] [Google Scholar]
- 32.Rusjan P, Mamo D, Ginovart N, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147(1):79–89. doi: 10.1016/j.pscychresns.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Chiuccariello L, Houle S, Miler L, et al. Elevated monoamine oxidase a binding during major depressive episodes is associated with greater severity and reversed neurovegetative symptoms. Neuropsychopharmacology. 2014;39(4):973–980. doi: 10.1038/npp.2013.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ressler KJ, Mayberg HS. Targeting abnormal neural circuits in mood and anxiety disorders: from the laboratory to the clinic. Nat Neurosci. 2007;10(9):1116–1124. doi: 10.1038/nn1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saura J, Bleuel Z, Ulrich J, et al. Molecular neuroanatomy of human monoamine oxidases A and B revealed by quantitative enzyme radioautography and in situ hybridization histochemistry. Neuroscience. 1996;70(3):755–774. doi: 10.1016/s0306-4522(96)83013-2. [DOI] [PubMed] [Google Scholar]
- 36.Meyer JH, Ginovart N, Boovariwala A, et al. Elevated monoamine oxidase a levels in the brain: an explanation for the monoamine imbalance of major depression. Arch Gen Psychiatry. 2006;63(11):1209–1216. doi: 10.1001/archpsyc.63.11.1209. [DOI] [PubMed] [Google Scholar]
- 37.Meyer JH, Wilson AA, Sagrati S, et al. Brain monoamine oxidase A binding in major depressive disorder: relationship to selective serotonin reuptake inhibitor treatment, recovery, and recurrence. Arch Gen Psychiatry. 2009;66(12):1304–1312. doi: 10.1001/archgenpsychiatry.2009.156. [DOI] [PubMed] [Google Scholar]
- 38.Johnson S, Stockmeier CA, Meyer JH, et al. The reduction of R1, a novel repressor protein for monoamine oxidase A, in major depressive disorder. Neuropsychopharmacology. 2011;36(10):2139–2148. doi: 10.1038/npp.2011.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Filipenko ML, Beilina AG, Alekseyenko OV, Dolgov VV, Kudryavtseva NN. Increase in expression of brain serotonin transporter and monoamine oxidase a genes induced by repeated experience of social defeats in male mice. Biochemistry (Mosc) 2002;67(4):451–455. doi: 10.1023/a:1015238124000. [DOI] [PubMed] [Google Scholar]
- 40.Yu Y, Wang R, Chen C, et al. Antidepressant-like effect of trans-resveratrol in chronic stress model: behavioral and neurochemical evidences. J Psychiatr Res. 2013;47(3):315–322. doi: 10.1016/j.jpsychires.2012.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Grunewald M, Johnson S, Lu D, et al. Mechanistic role for a novel glucocorticoid-KLF11 (TIEG2) protein pathway in stress-induced monoamine oxidase A expression. J Biol Chem. 2012;287(29):24195–24206. doi: 10.1074/jbc.M112.373936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moses-Kolko EL, Price JC, Shah N, et al. Age, sex, and reproductive hormone effects on brain serotonin-1A and serotonin-2A receptor binding in a healthy population. Neuropsychopharmacology. 2011;36(13):2729–2740. doi: 10.1038/npp.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Youdim MBH, Edmondson D, Tipton KF. The therapeutic potential of monoamine oxidase inhibitors. Nat Rev Neurosci. 2006;7(4):295–309. doi: 10.1038/nrn1883. [DOI] [PubMed] [Google Scholar]
- 44.Yan MH, Wang X, Zhu X. Mitochondrial defects and oxidative stress in Alzheimer disease and Parkinson disease. Free Radic Biol Med. 2013;62:90–101. doi: 10.1016/j.freeradbiomed.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burke WJ, Li SW, Chung HD, et al. Neurotoxicity of MAO metabolites of catecholamine neurotransmitters: role in neurodegenerative diseases. Neurotoxicology. 2004;25(1–2):101–115. doi: 10.1016/S0161-813X(03)00090-1. [DOI] [PubMed] [Google Scholar]
- 46.Scott E, Zhang QG, Wang R, Vadlamudi R, Brann D. Estrogen neuroprotection and the critical period hypothesis. Front Neuroendocrinol. 2012;33(1):85–104. doi: 10.1016/j.yfrne.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brinton RD. The healthy cell bias of estrogen action: mitochondrial bioenergetics and neurological implications. Trends Neurosci. 2008;31(10):529–537. doi: 10.1016/j.tins.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Resnick SM, Espeland MA, Jaramillo SA, et al. Postmenopausal hormone therapy and regional brain volumes: the WHIMS-MRI Study. Neurology. 2009;72(2):135–142. doi: 10.1212/01.wnl.0000339037.76336.cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wassertheil-Smoller S, Hendrix SL, Limacher M, et al. WHI Investigators. Effect of estrogen plus progestin on stroke in postmenopausal women: the Women’s Health Initiative: a randomized trial. JAMA. 2003;289(20):2673–2684. doi: 10.1001/jama.289.20.2673. [DOI] [PubMed] [Google Scholar]
- 50.Holschneider DP, Chen K, Seif I, Shih JC. Biochemical, behavioral, physiologic, and neurodevelopmental changes in mice deficient in monoamine oxidase A or B. Brain Res Bull. 2001;56(5):453–462. doi: 10.1016/s0361-9230(01)00613-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bortolato M, Chen K, Shih JC. Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv Drug Deliv Rev. 2008;60(13–14):1527–1533. doi: 10.1016/j.addr.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meyer JH. Neuroimaging markers of cellular function in major depressive disorder: implications for therapeutics, personalized medicine, and prevention. Clin Pharmacol Ther. 2012;91(2):201–214. doi: 10.1038/clpt.2011.285. [DOI] [PubMed] [Google Scholar]
- 53.Fowler CJ, Wiberg A, Oreland L, Marcusson J, Winblad B. The effect of age on the activity and molecular properties of human brain monoamine oxidase. J Neural Transm. 1980;49(1–2):1–20. doi: 10.1007/BF01249185. [DOI] [PubMed] [Google Scholar]
- 54.Galva MD, Bondiolotti GP, Olasmaa M, Picotti GB. Effect of aging on lazabemide binding, monoamine oxidase activity and monoamine metabolites in human frontal cortex. J Neural Transm Gen Sect. 1995;101(1–3):83–94. doi: 10.1007/BF01271547. [DOI] [PubMed] [Google Scholar]
- 55.Kornhuber J, Konradi C, Mack-Burkhardt F, Riederer P, Heinsen H, Beckmann H. Ontogenesis of monoamine oxidase-A and -B in the human brain frontal cortex. Brain Res. 1989;499(1):81–86. doi: 10.1016/0006-8993(89)91136-0. [DOI] [PubMed] [Google Scholar]
- 56.Sacher J, Houle S, Parkes J, et al. Monoamine oxidase A inhibitor occupancy during treatment of major depressive episodes with moclobemide or St. John’s wort: an [11C]-harmine PET study. J Psychiatry Neurosci. 2011;36(6):375–382. doi: 10.1503/jpn.100117. [DOI] [PMC free article] [PubMed] [Google Scholar]