Abstract
Background/aims
The Y402H polymorphism in the complement factor H (CFH) gene is an important risk factor for age-related macular degeneration (AMD). Complement activation products and proinflammatory cytokines are associated with this polymorphism at the systemic level, but less is known of the associations in the outer retina of the genotyped eye. Here we investigate complement activation products and their role in nuclear factor (NF)-κB activation and gene expression of the NLRP3 inflammasome pathway.
Methods
Postmortem donor eyes were genotyped for the CFH Y402H polymorphism and assessed for complement C3a, C5a, interleukin (IL)-18 and tumour necrosis factor (TNF)-α. ARPE19 cells were stimulated basolaterally with C5a or TNF-α in polarised cultures. NF-κB activation was assessed with a reporter cell line. Gene expression of inflammasome-related (NLRP3, caspase-1, IL-1β and IL-18) and classic inflammatory (IL-6 and IL-8) genes was studied. The distribution of inflammasome products, IL-1β and IL-18, was studied in postmortem donor eyes with AMD pathologies.
Results
Eyes with the homozygous at-risk variant demonstrated higher levels of C5a, IL-18 and TNF-α in Bruch’s membrane and choroid. C5a promoted NF-κB activation and upregulation of IL-18 in polarised ARPE19. TNF-α promoted NF-κB activation and gene expression of caspase-1, IL-1β, IL-18, IL-6 and IL-8, but downregulated NLRP3. In eyes with geographic atrophy, strong immunoreactivity was observed for inflammasome products IL-1β and IL-18 compared with age-matched controls.
Conclusion
The at-risk polymorphism of the CFH Y402H may contribute to AMD disease process through increased complement and NF-κB activation, and the upregulation of IL-18, a product of inflammasome activation.
INTRODUCTION
Age-related macular degeneration (AMD) is a common neurodegenerative disease of the eye that can lead to irreversible central blindness among the elderly.1 Chronic inflammation is thought to play a central role in AMD development and progression to late-stage disease. One of the most salient, supportive examples is the association between AMD and the single nucleotide polymorphism Y402H (rs1061170, sequence: T1277C) in the gene coding complement factor H (CFH). Studies suggest that individuals with the homozygous at-risk CC variant of this polymorphism have an increased risk of AMD incidence, and probably progression, compared with those with the protective TT variant.2,3
The mechanism by which the at-risk variant confers an increased risk of AMD is still unclear. Our earlier study, and those of others, reported that the at-risk variant is associated with the elevated levels of complement activation products and classic proinflammatory cytokines systemically, in the blood of patients with AMD.4,5 The extent to which systemic levels of cytokines and complement proteins may affect the local retinal tissues is important to understand the AMD pathologies, particularly in light of a recent finding that there is an increase of membrane attack complex (MAC), the terminal end product of complement pathway in the choroidal tissues from donors with the at-risk variant.6 To further understand the role of the complement cascade in AMD, we assessed the complement activation products and classic proinflammatory cytokines in eye tissues genotyped for CFH Y402H polymorphism.
Complement activation products, C3a and C5a, are anaphylatoxins and mediate the expression of many inflammatory cytokines, including those associated with nuclear factor (NF)-κB pathway, a master switch for cytokine production.7,8 The NF-κB pathway mediates the expression of classic inflammatory cytokines (interleukin (IL)-6, IL-8) and those associated with the activation of the NLRP3 inflammasomes (IL-1β, IL-18), which play important roles in AMD pathology.9,10 The relationship between C3a, C5a and NF-κB activation is unclear in the retinal pigment epithelium (RPE), a cell that is in close proximity to the choroidal circulation in outer retina. Thus, in order to further understand the cellular mechanisms associated with complement cascade and cytokine secretion, we also assessed whether complement activation products promoted NF-κB activation in RPE in vitro.
MATERIALS AND METHODS
Human eye tissues and CFH Y402H genotyping
This study was approved by the Clinical Ethics Research Board of the University of British Columbia (UBC) and strictly adhered to the Declaration of Helsinki. Twenty pairs of non-diseased donor eyes, consented for research, were obtained from the Eye Bank of British Columbia (Vancouver, British Columbia, Canada). Donors with the following pathologies were excluded in this study: local or systemic infection, progressive brain pathologies, systemic diseases of unknown origin, lymphoproliferative or myeloproliferative disorders or any intrinsic eye disease. The right globes were fixed and paraffin-embedded to obtain 6 μm sections for immunohistochemistry. The left eyes were flash-frozen, and the neuroretinal tissues were used for CFH Y402H genotyping, as previously published.11
Eyes with geographic atrophy (GA) or choroidal neovascularisation (CNV) were obtained from the Department of Pathology, UBC. Multiple sections were prepared as above, through the macular area were stained by H&E and then screened to determine the ocular pathologies. In total, seven eyes with GA and six eyes with CNV were included in this study.
Immunohistochemistry
The immunohistochemical procedures and analysis followed those previously described.12 Briefly, sections were probed with primary antibodies against molecules of interest (see online supplementary appendix 1), followed by secondary antibodies and developed in ABC–AEC system (Vector Labs, Burlingame, California, USA). The immunoreactive RPE cells were counted and normalised to 1050 μm length Bruch’s membrane (BM). The immunoreactivity intensity in BM and choroid was scored semi-quantitatively with a score of 0 indicating background levels of labelling as determined by comparison with the negative control sections. The most intense immunoreactivity was classified as a score of 3. For intermediate intensity levels, a score of 1 was given to samples with the weakest labelling, while a score of 2 represented intermediate labelling. The immunoreactivity was compared among the at-risk CC, CT and the protective TT genotyped eyes.
In vitro RPE stimulation
ARPE19 and ARPE19/NF-κB-luciferase reporter cells were grown on Transwell inserts. ARPE19/NF-κB luciferase reporter cell line was established as previously described.12 Briefly, approximately 1.6×105/cm2 cells were seeded in a laminin-coated Transwell insert (0.4 μm pore size, 12 mm diameter, Fisher Scientific, Ottawa, Ontario, Canada) in 0.5 mL Dulbecco’s modified Eagle Medium/F12 medium (Life Technologies, Burlington, Ontario, Canada) containing 10% fetal bovine serum (FBS), 100 U/mL penicillin and 100 μg/mL streptomycin (Fisher Scientific). After overnight incubation, medium was changed to 1% FBS. Cells were maintained for at least 4 weeks before stimulation.13,14 The transepithelial resistance of ARPE19 or ARPE19/NF-κB-luciferase reporter cells was 24.4±4.03 Ω cm2 and demonstrated an immunofluorescence pattern of tight junctional protein, ZO-1 (see online supplementary appendix 2). After washing the cells, 5 μg/mL recombinant human complement C3a or C5a (R&D Systems, Minneapolis, Minnesota, USA), or 20 ng/mL recombinant human tumour necrosis factor α (TNF-α, R&D Systems) was introduced to the basal compartment of the Transwell inserts for 12 h. Cell lysates were then collected for quantitative PCR (q-PCR).
Quantitative PCR
Expression of selected genes was assessed by q-PCR, using primers listed in online supplementary appendix 3.12 A 7500 Fast SDS (Applied Biosystems, Carlsbad, California, USA) set with the following cycling conditions: 95°C for 30 s, 50°C for 30 s, 72°C for 45 s, 45 cycles was used for q-PCR, followed by melting curve analysis. The results were expressed in mRNA fold change normalised to the housekeeping gene, glyceraldehydes-3-phosphate dehydrogenase, using the 2−ΔΔCT method. The ΔCT values from stimulation and control groups were subjected to statistical analysis.
Statistical analysis
The Kruskal–Wallis and post-hoc Dunn’s multiple comparison tests were used to determine immunoreactivity intensity differences among groups. The Student’s t test was used to assess gene expression changes after exposure to C5a and TNF-α in vitro. One-way analysis of variance (ANOVA) and post-hoc Bonferroni multiple comparison tests were used to assess RPE labelling among eyes with GA, CNV and age-matched controls. The significance was set at p<0.05 using GraphPad Prism (V.5.0; GraphPad Prism 5 Software, La Jolla, California, USA).
RESULTS
Complement activation products are greater in outer retina of eyes with the at-risk variant in CFH gene
To determine whether there is increased complement activation in the outer retina associated with the risk variant of the CFH Y402H polymorphism, we compared the immunoreactivity levels of C3a and C5a, both activation products of the complement cascade, among eyes with the at-risk homozygous CC, the heterozygous CT and the protective TT variants. Of the 20 pairs of eyes used in this study, 5 pairs of eyes were CC, 9 were CT and 6 were TT. Totally, 9 were from female donors and 11 were from male donors. The mean age of the sample population was 59.1±3.7 years (mean±SEM and the time delay between death and eye harvest was 14.4±1.2 h. There is no significant difference in age, time delay and sex among eyes with various genotypes (one-way ANOVA and χ2, p>0.05).
The level of C5a was significantly different among donors with CC, CT or TT variants of the CFH Y402H polymorphism (Kruskal–Wallis test, p<0.05). The level of C5a in BM and choroid was increased in eyes with the at-risk CC variant compared with those with the protective TT variant (post-hoc Dunn’s test, p<0.05, figure 1). We found a similar trend for the C3a immunoreactivity among eyes with the CC, CT or TT variant; however, this did not reach significance (see online supplementary appendix 4).
Figure 1.
Increased C5a immunoreactivity in the Bruch’s membrane (BM)–choroid (Ch) complex is associated with the at-risk CC variant of the complement factor H (CFH) Y402H polymorphism in non-diseased postmortem eyes. (A) The level of C5a was significantly different among donors with CC, CT or TT variants of the CFH Y402H polymorphism (mean±SEM, Kruskal–Wallis test, p<0.05). The immunoreactivity was higher in the BM and Ch of the CC eyes compared with TT eyes (post-hoc Dunn’s test, *p<0.05). (B–D) The immunoreactivity for C5a was developed with AEC (red) and counterstained with Mayer’s haematoxylin (blue). Representative images of C5a immunoreactivity in the outer retina from a donor eye with the at-risk CC variant (B), the heterozygous CT variant (C) and the protective TT variant (D) are shown. Primary antibody omission and non-immune isotype antibodies were used as negative controls (E). Scale bar: 20 μm. RPE: retinal pigment epithelium.
Selected cytokines are highly expressed in outer retina of eyes with the at-risk variant in CFH gene
Previously, we reported that several cytokines were increased in the blood of patients with dry AMD having the at-risk CC variant compared with those with the CT or TT variant.4 In this study, we were interested in knowing whether the proinflammatory milieu, observed systemically in blood, also extended to include the local retinal tissues. Our results revealed a significant increase in IL-18 immunoreactivity in BM and choroid in tissues from CC compared with TT eyes (Kruskal–Wallis test and post-hoc Dunn’s test, p<0.05, figure 2A–D). Additionally, TNF-α immunoreactivity was also significantly higher in the choroid of CC donor eyes (Kruskal–Wallis test and post-hoc Dunn’s test, p<0.05, figure 2E–H). The immunoreactivity levels for IL-18 and TNF-α in CT eyes were either similar to TT or intermediate between CC and TT eyes.
Figure 2.
Increased interleukin (IL)-18 and tumour necrosis factor (TNF)-α in the Bruch’s membrane (BM)–choroid (Ch) complex is associated with the at-risk CC variant of complement factor H (CFH) Y402H polymorphism in non-diseased postmortem eyes. (A) The IL-18 immunoreactivity in BM and Ch was significantly different among donors with CC, CT or TT variants of the CFH Y402H polymorphism (mean±SEM, Kruskal–Wallis test, p<0.05). The immunoreactivity was higher in the BM and Ch of the CC eyes compared with TT eyes (post-hoc Dunn’s test, *p<0.05). (B–D) Representative pictures of IL-18 immunoreactivity in the outer retina from a donor eye with the at-risk CC variant (C), the heterozygous CT variant (C) and the protective TT variant (D) are shown. (E) TNF-α immunoreactivity in Ch was significantly different among donors with CC, CT or TT variants of the CFH Y402H polymorphism (mean±SEM, Kruskal–Wallis test, p<0.05). The immunoreactivity was higher in the Ch of the CC eyes compared with CT eyes (post-hoc Dunn’s test, *p<0.05). (F–H) Representative pictures of TNF-α immunoreactivity in the outer retina from a donor eye with the at-risk CC variant (F), the heterozygous CT variant (G) and the protective TT variant (H) are shown. The immunoreactivity for IL-18 and TNF-α was developed with AEC (red) and counterstained with Mayer’s haematoxylin (blue). Scale bar: 20 μm. RPE: retinal pigment epithelium.
Activation of the NF-κB pathway by proinflammatory mediators in RPE in vitro
Next, we investigated the effects of complement activation product, C5a, and cytokine TNF-α on ARPE19/NF-κB-luciferase reporter cells in polarised cultures. Basolateral stimulation with C5a (5 μg/mL) induced a 1.5-fold increase in NF-κB activation in ARPE19 reporter cells, while TNF-α (20 ng/mL) induced a 3.7-fold increase in NF-κB activation (n=3, Student’s t test, p<0.05, figure 3). The response pattern from polarised ARPE19 was similar to that from a non-polarised culture, but the magnitude of change was less. For example, in the non-polarised system, C5a stimulation induced a 4.6-fold increase in NF-κB activation, while TNF-α induced a 30-fold increase (n=3, Student’s t test, p<0.01, see online supplementary appendix 5). These responses demonstrate that C5a or TNF-α activates the NF-κB pathway in the ARPE19 cell.
Figure 3.
C5a and tumour necrosis factor (TNF)-α induced nuclear factor (NF)-κB pathway activation in polarised ARPE19/NF-κB luciferase reporter cells is shown. C5a stimulation at 5 μg/mL induced NF-κB activation in ARPE19 reporter cells, while TNF-α stimulation at 10 or 20 ng/mL induced NF-κB activation (n=3, Student’s t test, **p<0.01, *p<0.05).
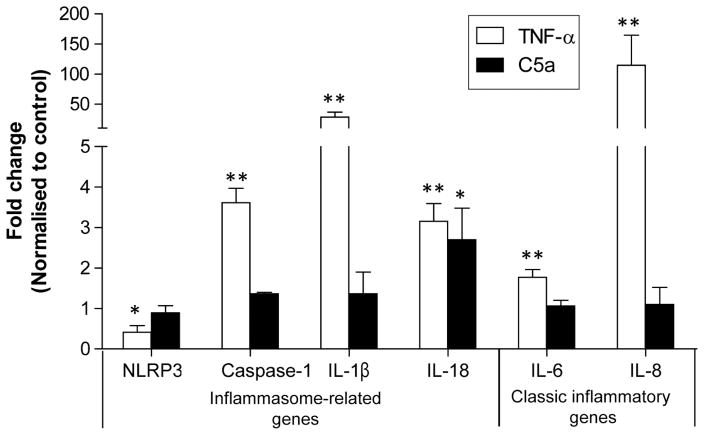
The NF-κB pathway mediates the expression of many cytokines, including classic ones such as IL-6, IL-8 and inflammasome genes.10 Therefore, we assessed the gene expression levels of NF-κB responsive genes in ARPE19 polarised cells. TNF-α stimulation caused upregulation of caspase-1 (~4 fold), IL-1β (~21 fold), IL-6 (~2 fold), IL-8 (~114 fold) and IL-18 (~3 fold), while it downregulated NLRP3 (~2 fold) at 12 h. Interestingly, C5a stimulation resulted in only upregulation of IL-18 (approximately threefold, n=3, Student’s t test, p<0.05, figure 4). The polarised cell culture did not demonstrate upregulation of IL-6 or IL-8 by C5a, while upregulation of these two cytokines was evident in the non-polarised ARPE19 cell (IL-6 ~1.5 fold; IL-8 ~5 fold, see online supplementary appendix 5).
Figure 4.
C5a or tumour necrosis factor (TNF)-α upregulated nuclear factor (NF)-κB-responsive genes in polarised ARPE19 cells. TNF-α (20 ng/ml, 12 h) upregulated the inflammasome genes caspase-1, interleukin (IL)-1β, IL-18 as well as proinflammatory genes IL-6, IL-8, but downregulated NLRP3. C5a (5 μg/mL, 12 h) upregulated IL-18 (n=3, Student’s t test, **p<0.01, *p<0.05).
Inflammasome products, IL-1β and IL-18, are increased in RPE of GA eyes
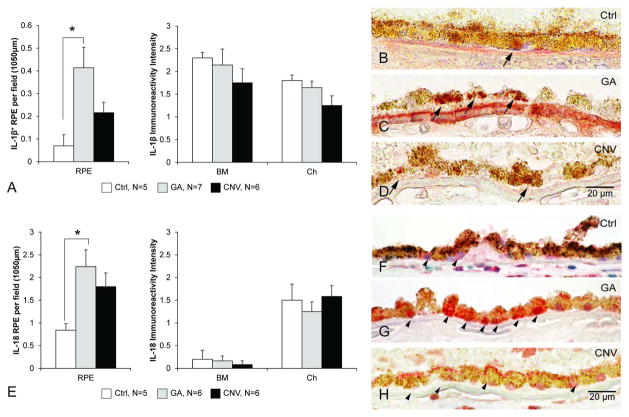
Our earlier work, and those of others, suggest that inflammasome activation is involved in the pathophysiology of AMD.10,15,16 To further support this idea, we assessed the expression of inflammasome activation products IL-1β and IL-18 in AMD eyes and compared with age-matched control eyes. The levels of both IL-1β and IL-18 were significantly higher in the RPE layer of GA eyes compared with controls (one-way ANOVA and post-hoc Bonferroni test, p<0.05, figure 5). Immunoreactivity in CNV eyes did not reach significance for either IL-1β or IL-18, but an increased trend compared with controls was observed (p>0.05, figure 5). Immunoreactivity in BM and choroid of GA and CNV eyes did not differ from age-matched control eyes (figure 5A, E).
Figure 5.
Increased interleukin (IL)-1β and IL-18 immunoreactivity in retinal pigment epithelium (RPE) cells in eyes with geographic atrophy (GA). (A) Eyes with GA (n=7) demonstrated more IL-1β-positive RPE cells than age-matched control eyes (n=5, one-way analysis of variance (ANOVA) and post-hoc Bonferroni test, *p<0.05). The IL-1β immunoreactivity in the Bruch’s membrane (BM)–choroid (Ch) complex did not differ among the three groups (Kruskal–Wallis test, p>0.05). Immunoreactivity in eyes with choroidal neovascularisation (CNV, n=6) did not differ from age-matched control (Ctrl) eyes. (B–D) Representative pictures of IL-1β immunoreactivity (AEC, red) from the central retina counterstained with Mayer’s haematoxylin (blue; scale bar: 20 μm) are shown. (E) Eyes with GA (n=7) had more IL-18-positive RPE cells than age-matched control eyes (n=5, one-way ANOVA and post-hoc Bonferroni test, *p<0.05). The eyes with CNV (n=6), while demonstrating a similar trend, did not reach significance. The IL-18 immunoreactivity in the BM–Ch complex did not differ among the three groups (Kruskal–Wallis test, p>0.05). (F–H) Representative pictures of IL-18 immunoreactivity (AEC, red) from the central retina counterstained with Mayer’s haematoxylin (blue, scale bar: 20 μm). IL-18 reactivity was found in RPE (arrowhead) from the Ctrl (F), as well as GA (G) and CNV (H) eyes are shown.
DISCUSSION
Complement activation product, C5a, is associated with the at-risk variant in the CFH gene and promotes inflammasome activation product IL-18
Here we showed that an elevated level of C5a in BM and choroid of the postmortem donor eye is associated with the at-risk homozygous variant of the CFH gene. This finding is consistent with a previous report demonstrating an association with the CFH at-risk variant and an elevated level of MAC, the terminal product of the complement cascade, in the choroid of postmortem donor eyes.6 The immunoreactivity level of C5a in CT eyes was intermediate between CC and TT eyes, but did not reach significance. This finding suggests that C5a may act synergistically with other factors to promote AMD, given that the heterozygous CT variant, like the homozygous CC variant also confers risk of AMD, but with a lower OR.2
Being juxtaposed to the choroid, the RPE cells are important gatekeepers associated with the exchange between the choroid and retina. With C5a receptors present on RPE,17 it is of importance to understand the effects of C5a on RPE function. We found that C5a stimulation activated the NF-κB pathway in RPE in vitro. The NF-κB pathway is known to mediate the translation of many proinflammatory mediators, including inflammasome products, and thereby contribute to the local proinflammatory status in outer retina. Thus, we further studied the expression patterns of several inflammatory cytokines that are relevant to AMD.18–20 We found that IL-18 is upregulated by C5a in RPE in vitro. While previous studies reported the role of C5a in angiogenesis and inflammation by upregulating vascular endothelial growth factor and intercellular adhesion molecule-1 (ICAM-1) and suppressing transforming growth factor-β2,21–23 our finding that IL-18, an inflammasome product, is upregulated in RPE in response to C5a is novel and supports the proposed relationship between complement cascade and inflammasome activation. Interestingly, recent studies reported that IL-18 may induce RPE degeneration, thus suggesting another cellular mechanism whereby complement activation may promote RPE degeneration via inflammasome activation and IL-18 overexpression.15,16
Our results demonstrated that the response to C5a may vary depending on the culture conditions. Stimulation of ARPE19 with C5a in non-polarised cultures yielded significant upregulation of IL-18, IL-6 and IL-8, but only IL-18 in the polarised cells. Other studies reported similar findings with the non-polarised ARPE19 culture.7 Similarly, TNF-α induced NF-κB activation in both culture systems, but was more robust in the non-polarised culture compared with the polarised culture method with basal stimulation. The polarised cell culture, originally proposed in 2006,13,14 has the advantage that cells can be stimulated from the basal or apical side, and thus may represent more physiological conditions. However, in our study, the non-polarised system did demonstrate a consistent, and more robust, trend in comparison with the polarised system—a finding that may in part be due to the resting state of the polarised cell cultures, which require over 4 weeks to mature.24
IL-18 and TNF-α immunoreactivity in outer retina is associated with the at-risk variant in the CFH gene
We also observed increased immunoreactivity of TNF-α and IL-18 in the choroidal tissues from donors with the CFH at-risk variant. These findings in outer retina support and extend our earlier finding of an association between systemic (plasma) levels of cytokines and the at-risk variant in the CFH gene and highlight the idea that individuals with the at-risk variant of CFH gene may have a high proinflammatory status in the outer retina.4 Presently, we do not know whether C5a, TNF-α or IL-18 is derived from the blood circulation or from the retinal microenvironment including RPE, or both. The subsequent elevation of caspase-1, IL-1β and IL-18 associated with the CFH at-risk variant may predispose these eyes to further inflammation-induced stress. In the AMD eye, we found IL-18 levels to be greater in GA compared with CNV and age-matched control eyes, supporting an involvement of IL-18 in GA. The general expression of IL-18 in RPE cells is higher than that of IL-1β, which is consistent with the finding in RPE in vitro by Shi et al.25
Our results suggest that CFH Y402H polymorphism, a known genetic risk factor of AMD, may contribute to the disease process through increased activation of the NF-κB pathways and upregulation of inflammasome genes. Further work is needed to understand the exact role of complement and inflammasome activation in the development of chronic inflammatory retinal diseases such as AMD.
Supplementary Material
Acknowledgments
The authors acknowledge Aikun Wang and Eleanor To for their technical assistance.
Funding This study was supported by Canadian Institute of Health Research (CIHR) grant number MOP-97806 and MOP-126195 to JAM.
Footnotes
Competing interests None declared.
Ethics approval Clinical Ethics Research Board of the University of British Columbia.
Provenance and peer review Not commissioned; externally peer reviewed.
Contributors SC, JCCW, JZC and JAM were involved in concept and design of study. VAW, JZC and JAM secured human specimens. SC, JCCW, JG, MW, ET, Aikun Wang and Eleanor To conducted the experiments in this study. SC, JCCW and JAM were involved in analysis and interpretation. JZC and JAM obtained funding. SC, JG and JAM were involved in preparing the manuscript.
References
- 1.Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358:2606–17. doi: 10.1056/NEJMra0801537. [DOI] [PubMed] [Google Scholar]
- 2.Hageman GS, Anderson DH, Johnson LV, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA. 2005;102:7227–32. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gangnon RE, Lee KE, Klein BE, et al. Effect of the Y402H variant in the complement factor H gene on the incidence and progression of age-related macular degeneration: results from multistate models applied to the Beaver Dam Eye Study. Arch Ophthalmol. 2012;130:1169–76. doi: 10.1001/archophthalmol.2012.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao S, Ko A, Partanen M, et al. Relationship between systemic cytokines and complement factor H Y402H polymorphism in patients with dry age-related macular degeneration. Am J Ophthalmol. 2013;156:1176–83. doi: 10.1016/j.ajo.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson PT, Betts KE, Radeke MJ, et al. Individuals homozygous for the age-related macular degeneration risk-conferring variant of complement factor H have elevated levels of CRP in the choroid. Proc Natl Acad Sci USA. 2006;103:17456–61. doi: 10.1073/pnas.0606234103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mullins RF, Dewald AD, Streb LM, et al. Elevated membrane attack complex in human choroid with high risk complement factor H genotypes. Exp Eye Res. 2011;93:565–7. doi: 10.1016/j.exer.2011.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukuoka Y, Strainic M, Medof ME. Differential cytokine expression of human retinal pigment epithelial cells in response to stimulation by C5a. Clin Exp Immunol. 2003;131:248–53. doi: 10.1046/j.1365-2249.2003.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang IH, Wong JH, Chang CM, et al. Involvement of intracellular calcium mobilization in IL-8 activation in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2015;56:761–9. doi: 10.1167/iovs.14-15299. [DOI] [PubMed] [Google Scholar]
- 9.Bauernfeind FG, Horvath G, Stutz A, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–91. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao J, Liu RT, Cao S, et al. NLRP3 inflammasome: activation and regulation in age-related macular degeneration. Mediators Inflamm. 2015;2015:690243. doi: 10.1155/2015/690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang JCC, Wang AK, Gao JY, et al. Technical Brief: Isolation of total DNA from postmortem human eye tissues and quality comparison between iris and retina. Mol Vis. 2012;18:3049–56. [PMC free article] [PubMed] [Google Scholar]
- 12.Liu RT, Wang A, To E, et al. Vinpocetine inhibits amyloid-beta induced activation of NF-kappaB, NLRP3 inflammasome and cytokine production in retinal pigment epithelial cells. Exp Eye Res. 2014;127:49–58. doi: 10.1016/j.exer.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannan R, Zhang N, Sreekumar PG, et al. Stimulation of apical and basolateral VEGF-A and VEGF-C secretion by oxidative stress in polarized retinal pigment epithelial cells. Mol Vis. 2006;12:1649–59. [PubMed] [Google Scholar]
- 14.Geisen P, McColm JR, King BM, et al. Characterization of barrier properties and inducible VEGF expression of several types of retinal pigment epithelium in medium-term culture. Curr Eye Res. 2006;31:739–48. doi: 10.1080/02713680600837408. [DOI] [PubMed] [Google Scholar]
- 15.Doyle SL, Ozaki E, Brennan K, et al. IL-18 attenuates experimental choroidal neovascularization as a potential therapy for wet age-related macular degeneration. Sci Transl Med. 2014;6:230ra44. doi: 10.1126/scitranslmed.3007616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ijima R, Kaneko H, Ye F, et al. Interleukin-18 induces retinal pigment epithelium degeneration in mice. Invest Ophthalmol Vis Sci. 2014;55:6673–8. doi: 10.1167/iovs.14-15367. [DOI] [PubMed] [Google Scholar]
- 17.Cortright DN, Meade R, Waters SM, et al. C5a, but not C3a, increases VEGF secretion in ARPE-19 human retinal pigment epithelial cells. Curr Eye Res. 2009;34:57–61. doi: 10.1080/02713680802546658. [DOI] [PubMed] [Google Scholar]
- 18.Seddon JM, George S, Rosner B, et al. Progression of age-related macular degeneration: prospective assessment of C-reactive protein, interleukin 6, and other cardiovascular biomarkers. Arch Ophthalmol. 2005;123:774–82. doi: 10.1001/archopht.123.6.774. [DOI] [PubMed] [Google Scholar]
- 19.Jonas JB, Tao Y, Neumaier M, et al. Cytokine concentration in aqueous humour of eyes with exudative age-related macular degeneration. Acta Ophthalmol. 2012;90:e381–8. doi: 10.1111/j.1755-3768.2012.02414.x. [DOI] [PubMed] [Google Scholar]
- 20.Tarallo V, Hirano Y, Gelfand BD, et al. DICER1 loss and Alu RNA induce age-related macular degeneration via the NLRP3 inflammasome and MyD88. Cell. 2012;149:847–59. doi: 10.1016/j.cell.2012.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nozaki M, Raisler BJ, Sakurai E, et al. Drusen complement components C3a and C5a promote choroidal neovascularization. Proc Natl Acad Sci USA. 2006;103:2328–33. doi: 10.1073/pnas.0408835103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu M, Liu B, Jawad S, et al. C5a contributes to intraocular inflammation by affecting retinal pigment epithelial cells and immune cells. Br J Ophthalmol. 2011;95:1738–44. doi: 10.1136/bjophthalmol-2011-300235. [DOI] [PubMed] [Google Scholar]
- 23.Skeie JM, Fingert JH, Russell SR, et al. Complement component C5a activates ICAM-1 expression on human choroidal endothelial cells. Invest Ophthalmol Vis Sci. 2010;51:5336–42. doi: 10.1167/iovs.10-5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonoda S, Sreekumar PG, Kase S, et al. Attainment of polarity promotes growth factor secretion by retinal pigment epithelial cells: relevance to age-related macular degeneration. Aging. 2010;2:28–42. doi: 10.18632/aging.100111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi G, Chen S, Wandu WS, et al. Inflammasomes induced by 7-Ketocholesterol and Other Stimuli in RPE and in Bone Marrow-Derived Cells Differ Markedly in Their Production of IL-1beta and IL-18. Invest Ophthalmol Vis Sci. 2015;56:1658–64. doi: 10.1167/iovs.14-14557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.