Clinical History
A 77‐year‐old female presented with three months of headache, impulsivity, irritability and aggressive behavior. Neurological exam revealed a flattened affect, poor short‐term memory and urinary incontinence.
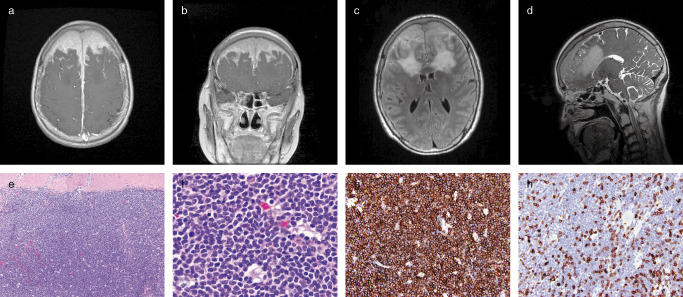
Magnetic Resonance Imaging (MRI) revealed an extra‐axial mass that extended over the convexities of the bilateral frontal lobes and invaded the superior sagittal sinus and the overlying calvarial diploic space (Figure 1a–d). The lesion infiltrated into the sub‐adjacent bilateral frontal lobe cortex and demonstrated homogenous contrast enhancement on T1‐weighted images (Figure 1a,b) and hyperintense signal on T2‐weighted images (Figure 1c,d). The patient underwent a bifrontal craniotomy that revealed a lesion which adhered to the undersurface of the bone flap. The left and right frontal lobe dura were opened and revealed an invasive lesion with an ill‐defined brain‐tumor interface. A subtotal resection was performed and multiple white‐tan tissue fragments were submitted for pathological evaluation.
Figure 1.
Microscopic Pathology
Microscopic examination demonstrated dural involvement by a dense cellular infiltrate, composed mainly of mature small lymphocytes and monocytoid cells with a low mitotic index. The lymphoid cells appeared in diffuse sheets with focal vague suggestions of follicular/nodular architecture (Figure 1e,f). Immunoperoxidase stains showed that the vast majority of mature small lymphocytes were positive for CD20 (Figure 1g). Stains for T‐cell markers CD3, CD5, and CD43 highlighted only a minority population of admixed small lymphocytes consistent with reactive T‐cells with no co‐expression of either CD5 (Figure 1h) or CD43 by the dominant B‐cell population. Stains for CD21 and CD23 highlighted scattered rounded aggregates of follicular dendritic cells, consistent with benign B‐cell follicles that have been colonized by the neoplastic B‐cell population. In addition, there were larger, more ill defined, and more elaborate meshworks of dendritic cells underlying the neoplastic B cells in other areas, which corresponded to the follicular areas in the H&E stain. The dominant B‐cell population was negative for CD10, CD23, BCL‐1 (cyclin D1), and BCL‐6. Additionally, immunohistochemistry for IgG highlighted numerous plasma cells, while the IgG4 was negative. Flow cytometry demonstrated a lambda‐restricted B‐cell lymphoid population lacking expression of CD5, CD10, and CD23. What is the diagnosis?
Diagnosis
Intracranial Marginal Zone B‐cell Lymphoma.
Discussion
Marginal Zone B‐cell Lymphoma (MZBCL) is a low‐grade lymphoma that was first described as a mucosa‐associated lymphoid tissue (MALT) of the gastrointestinal tract 2. It has since been characterized in the mucosa of other organs including lung, bladder, salivary gland, conjunctiva, and the lacrimal glands. In addition, it has been identified in tissue sites without mucosa, specifically the thyroid, thymus, breast, liver, orbit, and rarely the central nervous system (CNS). While the majority of primary CNS lymphomas (PCNSL) are aggressive diffuse large B‐cell lymphomas, low‐grade PCNSLs are a rare finding and appear in the literature as single case reports or case series 1. Of the low‐grade lymphomas however, MZBCL is the most common PCNSL 3. CNS MZBCL often presents in middle‐aged women (female‐to‐male ratio, 4:1) as an indolent, localized mass arising from the dura. Radiographically, these solitary dural‐based lesions are indistinguishable from a meningioma, which is the main differential diagnosis and was the preoperative diagnosis in our patient.
The MZBCL is thought to be derived from postgerminal center marginal zone B‐cells and are characterized by sheets of small to medium sized lymphocytes with moderate amounts of clear cytoplasm (“monocytoid B cells”) and irregular, centrally located nuclei with low mitotic rate. Occasional immunoblast‐like cells with large vesicular nuclei and prominent nucleoli have been described scattered among the neoplastic cells 6. Consistent with a B‐cell origin, these cells have been noted to express pan‐B‐lymphocyte markers (CD19, CD20, and CD79a), complement receptors, and surface immunoglobulin; however they do not express CD3, CD5, CD10, CD23, or cyclin D1, which help to exclude other differential considerations such as mantle cell lymphoma 4.
Multiple cytogenetic abnormalities have been described in MZBCL. The t(11,18) (q21;q21) or API2‐MALT1 translocation is observed in 20% to 30% of MALT type MZBCL; however it has not been reported in nodal, splenic, or CNS MZBCL. The most common numeric chromosomal abnormality in MZBCL is Trisomy 3. Either partial or complete trisomy 3 abnormalities have been detected by FISH in 60% to 80% of MZBCL outside of the CNS and recently been found to occur in 50% of CNS MZBCL 5, 6. IgH‐containing translocations, t(14;18) (q32;q21) (IgH‐MALT1 translocation) and t(3;14)(p14.1;q32) (IgH‐FOXP1 translocation) have been reported in a subset of patients with non gastrointestinal MZBCLs, but have not been identified in CNS MZBCL to date.
CNS MZBCL is characterized by an indolent clinical course and carries a far more favorable prognosis than parenchymal PCNSL. Long‐term disease control and overall 5‐year survival rates for both gastrointestinal and nongastrointestinal MZBCL is greater than 86% 3. Given the rarity of this tumor however, there is no current consensus on standard treatment. Current therapeutic options for clinically localized MZBCL include surgical resection or focal radiation therapy. Obtaining a complete resection of MZBCL in the dura is challenging due to the infiltrative behavior and frequency of multiple lesions; therefore, the majority of cases require adjuvant treatment with radiation or chemotherapy. While high‐dose Methotrexate is the most efficacious drug in parenchymal PCNSL, its role in CNS MZBCL is unknown. The anti‐CD20 monoclonal antibody, Rituximab, is effective in systemic MZL, but data is limited in dural‐based MZBCL. In the older population, combination chemoradiotherapy can lead to progressive leukoencephalopathy and extensive neurotoxicity. Therefore, following subtotal resection, our patient was started on combination chemotherapy alone with Rituximab and Bendamustine. Since completing two cycles she has had marked clinical response with improvement in her headaches, impulsive behavior and affect.
References
- 1. Bayraktar S, Stefanovic A, Montague N, Davis J, Murray T, Lossos IS (2010) Central nervous system manifestations of marginal zone B‐cell lymphoma. Ann Hematol 89:1003–1009. [DOI] [PubMed] [Google Scholar]
- 2. Issacson P, Wright DH (1983) Malignant lymphoma of mucosa associated lymphoid tissue. A distinctive type of B‐cell lymphoma. Cancer 52:1410–1416. [DOI] [PubMed] [Google Scholar]
- 3. Iwamoto FM, Abrey LE (2006) Primary dural lymphoma: a review. Neurosurg Focus 21:E5. [DOI] [PubMed] [Google Scholar]
- 4. Maes B, de Wolf‐Peeters C (2002) Marginal zone cell lymphoma: An update on recent advances. Histopathology 40:117–126. [DOI] [PubMed] [Google Scholar]
- 5. Muller‐Hermelink HK (2003) Genetic and molecular genetic studies in the diagnosis of B‐cell lymphomas: Marginal zone lymphomas. Hum Pathol 34:336–340. [DOI] [PubMed] [Google Scholar]
- 6. Tu PH, Giannini, C , Judkins AR, Schwalb JM, Burack R, O'Neill BP, Yachnis AT, Burger PC, Scheithauer BW, Perry A (2005) Clinicopathologic and Genetic Profile of Intracranial Marginal Zone Lymphoma: A Primary Low‐Grade CNS Lymphoma That Mimics Meningioma . J Clin Oncol 23:5718–5727. [DOI] [PubMed] [Google Scholar]


