Abstract
MERTK-associated retinal degenerations are thought to have defects in phagocytosis of shed outer segment membranes by the retinal pigment epithelium (RPE), as do the rodent models of these diseases. We have subretinally injected an RPE-specific AAV2 vector, AAV2-VMD2-hMERTK, to determine whether this would provide long-term photoreceptor rescue in the RCS rat, which it did for up to 6.5 months, the longest time point examined. Moreover, we found phagosomes in the RPE in the rescued regions of RCS retinas soon after the onset of light. The same vector also had a major protective effect in Mertk-null mice, with a concomitant increase in ERG response amplitudes in the vector-injected eyes. These findings suggest that planned clinical trials with this vector will have a favorable outcome.
Keywords: Gene therapy, Retinal degeneration, MERTK, Phagocytosis, Treatment
65.1 Introduction
Retinitis pigmentosa is a family of diseases that affects approximately one in 3500 people worldwide and is a major cause of inherited blindness in the Western world. More than 50 genes have been identified in which mutations lead to retinitis pigmentosa (http://www.sph.uth.tmc.edu/retnet/). Vision loss results from the degeneration of rod and cone photoreceptors due to mutation of genes expressed either in these cells, or in the closely interacting retinal pigment epithelial (RPE) cells.
The RCS rat is a widely studied retinal degeneration (RD) model in which photoreceptor cells begin to degenerate at postnatal day (P) 20, with most disappearing by about P60 (Dowling and Sidman 1962). It has been known since the 1970s that this degeneration has a defect in the ability of the RPE to phagocytize rod outer segment tips, leading to an accumulation of outer segment debris in the subretinal space (Bok and Hall 1971; Mullen and LaVail 1976). The gene responsible for the defect in RCS rats was identified as the mer proto-oncogene tyrosine kinase (Mertk) (D’Cruz et al. 2000), which encodes a transmembrane receptor tyrosine kinase (Strick and Vollrath 2010).
Once the mutated gene was identified, proof of concept of gene replacement therapy was obtained in RCS rats using an adenovirus vector by Vollrath et al. (2001). Subsequently, a number of studies using different vectors, including adeno-associated virus (AAV) (Smith et al. 2003; Deng et al. 2012) and lentivirus (Tschernutter et al. 2005) were effective to different degrees, each showing improvement in photoreceptor survival, electroretinographic responses and RPE phagocytic function.
Numerous studies have described individuals with inherited RD due to MERTK mutations (Gal et al. 2000; Thompson et al. 2002; Tschernutter et al. 2006; Charbel Issa et al. 2009; Mackay et al. 2010; Shahzadi et al. 2010; Ostergaard et al. 2011), emphasizing the critical need for appropriate vectors for gene replacement therapy. Recombinant AAV (rAAV) in particular has gained prominence in the treatment of inherited retinal disorders in recent years (Boye et al. 2013). Three separate Phase I clinical trials for Leber congenital amaurosis type 2 have demonstrated the safety of AAV2 in human patients (Jacobson et al. 2006; Bainbridge et al. 2008; Cideciyan et al. 2008; Maguire et al. 2008).
A series of preclinical potency and safety evaluations of an AAV2 vector expressing human MERTK cDNA driven by an RPE-specific VMD2 (Bestrophin) promoter that was planned for human patients was recently carried out (Conlon et al. 2013). The −585/+38 bp version of the human VMD2 promoter had previously been shown to drive efficient and exclusive transgene expression in the RPE (Alexander and Hauswirth 2008). The effectiveness of the vector in RCS rats was demonstrated by electroretinogram (ERG) analysis done 60 days after injection at P9. The potential toxicity of the vector was assessed in Sprague–Dawley (SD) rats by electrophysiology, retinal morphology, and GLP-compliant experiments based on clinical observations and histopathology.
For the assessment of this RPE-specific vector on RDs for clinical trial application, it would be useful to know whether the vector is effective in long-term reversal of the defect in RPE phagocytosis and in rescue of photoreceptors in RCS rats. In addition, it would be important to demonstrate that the vector can rescue photoreceptors in a MERTK-associated RD in a different species with a different gene mutation. In this study, we have addressed both of these issues.
65.2 Materials and Methods
65.2.1 Animals
All studies were conducted in accordance with the ARVO Statement for the Use of Animals and the IACUC at UCSF. Inbred, pink-eyed RCS rats with inherited retinal dystrophy due to a deletion in the Mertk gene (D’Cruz et al. 2000) were characterized previously (Dowling and Sidman 1962; LaVail and Battelle 1975). Mertk knockout mice with an RCS-like retinal dystrophy phenotype were described earlier (Duncan et al. 2003).
65.2.2 Vector Injections, ERG Procedure and Histological Analysis
Subretinal injections of the AAV2-VMD2-hMERTK vector were made at P10 for RCS rats and at P4 for Mertk knockout mice using a previously described method (Lewin et al. 1998).
ERG analysis was carried out as previously described (Lewin et al. 1998).
For histologic studies to quantify the outer nuclear layer (ONL) thickness, methods previously described were used (LaVail and Battelle 1975; LaVail et al. 1987; Faktorovich et al. 1992).
65.3 Results
65.3.1 Long-Term Photoreceptor Rescue and Reversal of Phagocytosis Defect in RCS Rats
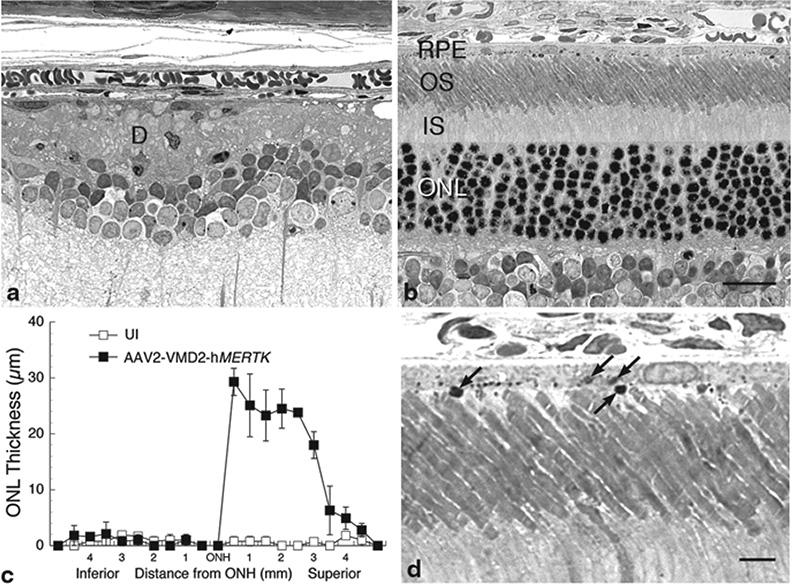
Comparison at P196 of the retinal structure of eyes of RCS rats injected subretinally with AAV2-VMD2-hMERTK and uninjected contralateral control eyes revealed a remarkable difference, equal to that seen by Conlon et al. (2013) for younger rats. In the uninjected eyes, most of the photoreceptor nuclei in the ONL had degenerated and disappeared, and an outer segment debris layer characteristic of retinal dystrophy in RCS rats was evident (Fig. 65.1a). By contrast, the vector-injected eyes appeared virtually normal in the areas of maximal rescue (Fig. 65.1b). The extent of photoreceptor rescue was typically about half of the full retinal length as shown in a retinal spidergram (Fig. 65.1c). When the RCS retinas were taken soon after the onset of light in the morning, large packets of outer segment disc membranes (phagosomes) were abundant in the RPE cell processes and internally within the RPE cell bodies (Fig. 65.1d).
Fig. 65.1.
Structural analysis of RCS rats injected subretinally into one eye with AAV2-VMD2-hMERTK compared with uninjected (UI) contralateral eyes of the same rats, a, b Light micrographs of 1-µm plastic sections of the posterior retina of the UI eye (a), where most photoreceptor nuclei in the ONL have degenerated and disappeared, and an outer segment debris (d) zone is present. The retina of the opposite eye from the eye injected with vector is shown (b), which is comparable in appearance to that of normal rat retinas. c Retinal spidergram showing the ONL thickness along the vertical meridian of UI and vector-injected eyes (each data point is the mean ± SD from 2 rats). d Higher magnification of a vector-injected eye showing phagosomes (arrows) at the apical surface and intracellularly in the RPE. IS inner segments. Scale bars: b = 20 µm; d = 5 µm
65.3.2 Photoreceptor Rescue in the MERTK-null Mouse
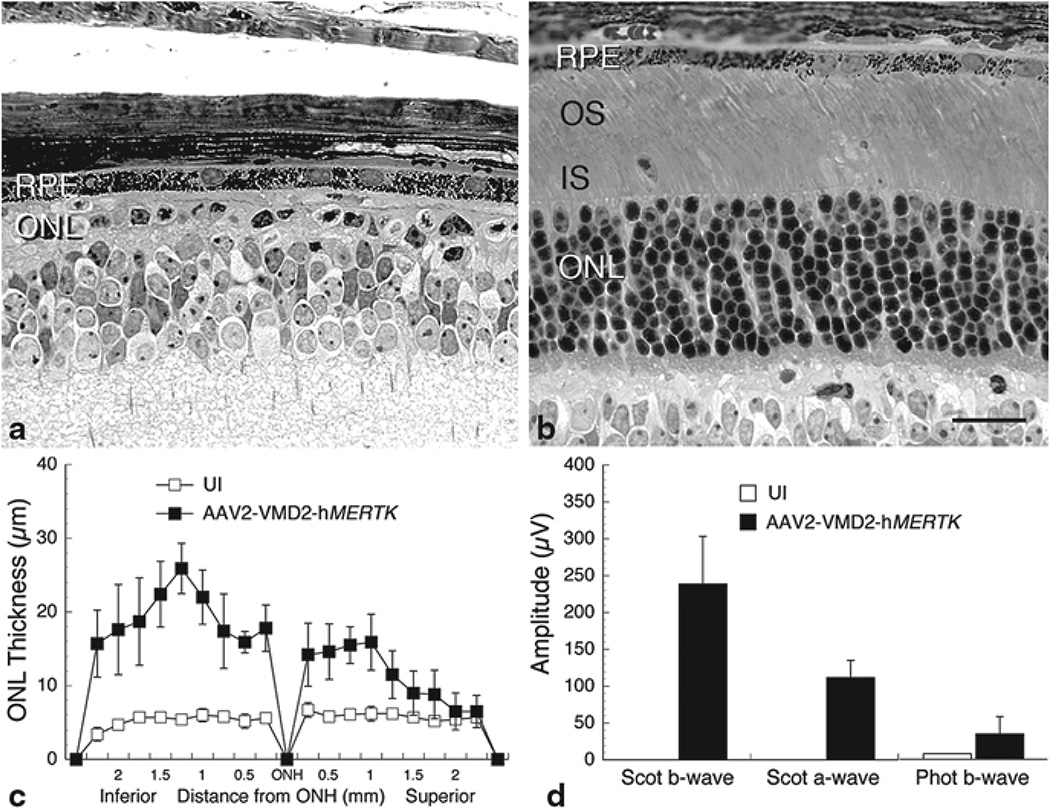
The differences at P52 in retinal structure between eyes of Mertk knockout mice injected subretinally with AAV2-VMD2-hMERTK at P4 and uninjected contralateral eyes were also remarkable. In the uninjected eyes, the ONL had been reduced to less than one complete row (Fig. 65.2a). By contrast, the vector-injected eyes appeared virtually normal in the areas of maximal rescue (Fig. 65.2b). The extent of photoreceptor rescue typically was most of the full retinal length, as viewed in a retinal spidergram of ONL thickness (Fig. 65.2c). The ERG responses were dramatically different for each of the waveforms; the uninjected eyes showed no scotopic a- or b-waves, and only minimal photopic b-waves, but the vector-injected eyes had responses that were 40–60% of normal (Fig. 65.2d).
Fig. 65.2.
Structural and functional analysis of Mertk knockout mice injected subretinally into one eye with AAV2-VMD2-hMERTK (b) compared with uninjected (UI) contralateral eyes of the same mice (a). Labeling as described in Fig. 65.1 and in the text. c Retinal spidergram showing the ONL thickness along the vertical meridian of UI and vector-injected eyes (each data point is the mean±SD from 5 mice). d Electroretinographic response amplitudes from the same mice as in c. Scale bar=20 µm
65.4 Discussion
In this study, we found that when the RPE-specific AAV2-VMD2-hMERTK vector was injected subretinally, it protected photoreceptors from degeneration in the RCS rat for up to 6.5 months of age, the oldest examined. Moreover, the absence in phagocytosis imparted by the Mertk gene defect in the RCS rats (Bok and Hall 1971) was clearly reversed, as large phagosomes were abundant when the eyes were taken soon after the onset of light, typical of circadian outer segment disc shedding in the rat (LaVail 1976, 1980).
We also found that in the Mertk knockout mouse, which exhibits rapid loss of most photoreceptors (Duncan et al. 2003), subretinal injection of the AAV2-VMD2-hMERTK vector protected a majority of photoreceptor cells from degenerating. As a consequence, the electrical activity of the photoreceptors in response to light was significantly increased over that in the uninjected control eyes, where the responses were almost abolished.
These findings strongly suggest that the RPE-specific AAV2-VMD2-hMERTK vector that is being used in a clinical trial of different forms of MERTK-associated RDs (FS Alkuraya, personal communation) will prove to be effective.
Acknowledgments
This study was supported by NIH grants EY001919, EY006842 and EY002162 (MML), The Foundation Fighting Blindness (MML, DV, WWH) and Unrestricted Awards to UCSF and the University of Florida from Research to Prevent Blindness.
Contributor Information
Matthew M. LaVail, Email: matthew.lavail@ucsf.edu, Beckman Vision Center, UCSF School of Medicine, 10 Koret Way, San Francisco, CA 941430730, USA.
Douglas Yasumura, Email: matthew.lavail@ucsf.edu, Beckman Vision Center, UCSF School of Medicine, 10 Koret Way, San Francisco, CA 941430730, USA.
Michael T. Matthes, Email: Michael.Matthes@ucsf.edu, Beckman Vision Center, UCSF School of Medicine, 10 Koret Way, San Francisco, CA 941430730, USA.
Haidong Yang, Email: yang.harvey@gmail.com, Beckman Vision Center, UCSF School of Medicine, 10 Koret Way, San Francisco, CA 941430730, USA.
William W. Hauswirth, Email: hauswrth@ufl.edu, Department of Ophthalmology, College of Medicine, University of Florida, Gainesville, FL 326100284, USA.
Wen-Tao Deng, Department of Ophthalmology, College of Medicine, University of Florida, Gainesville, FL 326100284, USA.
Douglas Vollrath, Email: Vollrath@stanford.edu, Department of Genetics, Stanford University School of Medicine, Stanford, CA 94305, USA.
References
- Alexander JJ, Hauswirth WW. Adeno-associated viral vectors and the retina. Adv Exp Med Biol. 2008;613:121–128. doi: 10.1007/978-0-387-74904-4_13. [DOI] [PubMed] [Google Scholar]
- Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’ congenital amaurosis. N Engl J Med. 2008;358:2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- Bok D, Hall MO. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J Cell Biol. 1971;49:664–682. doi: 10.1083/jcb.49.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boye SE, Boye SL, Lewin AS, et al. A comprehensive review of retinal gene therapy. Mol Ther. 2013;21:509–519. doi: 10.1038/mt.2012.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbel Issa P, Bolz HJ, Ebermann I, et al. Characterisation of severe rod-cone dystrophy in a consanguineous family with a splice site mutation in the MERTK gene. Br J Ophthalmol. 2009;93:920–925. doi: 10.1136/bjo.2008.147397. [DOI] [PubMed] [Google Scholar]
- Cideciyan AV, Aleman TS, Boye SL, et al. Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics. Proc Natl Acad Sci USA. 2008;105:15112–15117. doi: 10.1073/pnas.0807027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon TJ, Deng WT, Erger K, et al. Preclinical potency and safely studies of an AAV2-mediated gene therapy vector for the treatment of MERTK associated retinitis pigmentosa. Hum Gene Ther Clin Devt. 2013;24:23–28. doi: 10.1089/humc.2013.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Cruz PM, Yasumura D, Weir J, et al. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum Mol Genet. 2000;9:645–651. doi: 10.1093/hmg/9.4.645. [DOI] [PubMed] [Google Scholar]
- Deng WT, Dinculescu A, Li Q, et al. Tyrosine-mutant AAV8 delivery of human MERTK provides long-term retinal preservation in RCS rats. Invest Ophthalmol Vis Sci. 2012;53:1895–1904. doi: 10.1167/iovs.11-8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowling JE, Sidman RL. Inherited retinal dystrophy in the rat. J Cell Biol. 1962;14:73–109. doi: 10.1083/jcb.14.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JL, LaVail MM, Yasumura D, et al. An RCS-like retinal dystrophy phenotype in Mer knockout mice. Invest Ophthalmol Vis Sci. 2003;44:826–838. doi: 10.1167/iovs.02-0438. [DOI] [PubMed] [Google Scholar]
- Faktorovich EG, Steinberg RH, Yasumura D, et al. Basic fibroblast growth factor and local injury protect photoreceptors from light damage in the rat. J Neurosci. 1992;12:3554–3567. doi: 10.1523/JNEUROSCI.12-09-03554.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gal A, Li Y, Thompson DA, et al. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nat Genet. 2000;26:270–271. doi: 10.1038/81555. [DOI] [PubMed] [Google Scholar]
- Jacobson SG, Boye SL, Aleman TS, et al. Safety in nonhuman primates of ocular AAV2-RPE65, a candidate treatment for blindness in Leber congenital amaurosis. Hum Gene Ther. 2006;17:845–858. doi: 10.1089/hum.2006.17.845. [DOI] [PubMed] [Google Scholar]
- LaVail MM. Rod outer segment disc shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- LaVail MM. Circadian nature of rod outer segment disc shedding in the rat. Invest Ophthalmol Vis Sci. 1980;19:407–411. [PubMed] [Google Scholar]
- LaVail MM, Battelle BA. Influence of eye pigmentation and light deprivation on inherited retinal dystrophy in the rat. Exp Eye Res. 1975;21:167–192. doi: 10.1016/0014-4835(75)90080-9. [DOI] [PubMed] [Google Scholar]
- LaVail MM, Gorrin GM, Repaci MA, et al. Genetic regulation of light damage to photoreceptors. Invest Ophthalmol Vis Sci. 1987;28:1043–1048. [PubMed] [Google Scholar]
- Lewin AS, Drenser KA, Hauswirth WW, et al. Ribozyme rescue of photoreceptor cells in a transgenic rat model of autosomal dominant retinitis pigmentosa. Nat Med. 1998;4:967–971. doi: 10.1038/nm0898-967. [DOI] [PubMed] [Google Scholar]
- Mackay DS, Henderson RH, Sergouniotis PI, et al. Novel mutations in MERTK associated with childhood onset rod-cone dystrophy. Mol Vis. 2010;16:369–377. [PMC free article] [PubMed] [Google Scholar]
- Maguire AM, Simonelli F, Pierce EA, et al. Safely and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, LaVail MM. Inherited retinal dystrophy: primary defect in pigment epithelium determined with experimental rat chimeras. Science. 1976;192:799–801. doi: 10.1126/science.1265483. [DOI] [PubMed] [Google Scholar]
- Ostergaard E, Duno M, Batbayli M, et al. A novel MERTK deletion is a common founder mutation in the Faroe Islands and is responsible for a high proportion of retinitis pigmentosa cases. Mol Vis. 2011;17:1485–1492. [PMC free article] [PubMed] [Google Scholar]
- Shahzadi A, Riazuddin SA, Ali S, et al. Nonsense mutation in MERTK causes autosomal recessive retinitis pigmentosa in a consanguineous Pakistani family. Br J Ophthalmol. 2010;94:1094–1099. doi: 10.1136/bjo.2009.171892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AJ, Schlichtenbrede FC, Tschernutter M, et al. AAV-mediated gene transfer slows photoreceptor loss in the RCS rat model of retinitis pigmentosa. Mol Ther. 2003;8:188–195. doi: 10.1016/s1525-0016(03)00144-8. [DOI] [PubMed] [Google Scholar]
- Strick DJ, Vollrath D. Focus on molecules: MERTK. Exp Eye Res. 2010;91:786–787. doi: 10.1016/j.exer.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson DA, McHenry CL, Li Y, et al. Retinal dystrophy due to paternal isodisomy for chromosome 1 or chromosome 2, with homoallelism for mutations in RPE65 or MERTK, respectively. Am J Hum Genet. 2002;70:224–229. doi: 10.1086/338455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschernutter M, Schlichtenbrede FC, Howe S, et al. Long-term preservation of retinal function in the RCS rat model of retinitis pigmentosa following lentivirus-mediated gene therapy. Gene Ther. 2005;12:694–701. doi: 10.1038/sj.gt.3302460. [DOI] [PubMed] [Google Scholar]
- Tschernutter M, Jenkins SA, Waseem NH, et al. Clinical characterisation of a family with retinal dystrophy caused by mutation in the Mertk gene. Br J Ophthalmol. 2006;90:718–723. doi: 10.1136/bjo.2005.084897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollrath D, Feng W, Duncan JL, et al. Correction of the retinal dystrophy phenotype of the RCS rat by viral gene transfer of Mertk. Proc Natl Acad Sci U S A. 2001;98:12584–12589. doi: 10.1073/pnas.221364198. [DOI] [PMC free article] [PubMed] [Google Scholar]