Abstract
Objective
Polyunsaturated fatty acids (PUFAs) regulate fatty acid desaturase (FADS1, FADS2) expression in the liver; however, it is unknown whether PUFAs regulate FADS in adipocytes. This is important to study considering reports that link altered desaturase activity with adipose tissue PUFA profiles, body weight, and whole-body glucose homeostasis. Therefore, the present study aimed to determine the direct effects of PUFAs on FADS expression in differentiated 3T3-L1 adipocytes.
Methods
Differentiated 3T3-L1 adipocytes were treated with either α-linolenic (ALA), linoleic (LA), eicosapentaenoic (EPA), or arachidonic acid (AA). Gene expression, protein abundance, and cellular PUFA content were analyzed by real-time RT-PCR, Western blotting, and gas chromatography, respectively.
Results
Fads1 and Fads2 gene expression was reduced by EPA and AA, but not ALA or LA. Reductions in gene expression were reflected in FADS2 protein levels, but not FADS1. Treating cells with ALA and LA led to significant increases in the cellular content of downstream PUFAs. Neither ALA nor EPA changed docosahexaenoic acid content.
Conclusions
Differentiated 3T3-L1 adipocytes have a functional FADS pathway that can be regulated by PUFA. Therefore, this common adipocyte model is suitable to study dietary regulation of the FADS pathway.
Introduction
The Fads1 and Fads2 genes code for fatty acid desaturases, which are enzymes responsible for the introduction of cis double bonds at the Δ-5 and Δ-6 positions in polyunsaturated fatty acids (PUFAs), respectively (1,2). These enzymes have important roles in fatty acid (FA) biosynthesis by converting the essential dietary FAs, α-linolenic acid (ALA; 18:3n-3) and linoleic acid (LA; 18:2n-6), into their corresponding long-chain counterparts, eicosapentaenoic acid (EPA; 20:5n-3), docosahexaenoic acid (DHA; 22:6n-3), and arachidonic acid (AA; 20:4n-6) (1). Changes in desaturase activity alter cellular FA content, which consequently influences numerous biological processes including membrane transport, ion channel modulation, eicosanoid signaling, and gene expression (2).
Dietary FAs have been shown to regulate desaturase activity (2,3). Currently, most research has examined FA regulation of desaturase expression and/or activity in the liver. Both Fads1 and Fads2 gene expression are reduced by PUFAs in several hepatic models, ranging from human HepG2 cells treated with distinct PUFAs (4), to mice (5,6) and baboons (4) fed PUFA-enriched diets. Moreover, PUFA-mediated reductions in Fads expression are reflected in liver FA content (4). While PUFAs are known to regulate global lipogenic gene expression during adipogenesis (7), their regulation of Fads expression and activity in differentiated adipocytes remains poorly studied. This is critical to investigate given that PUFAs affect a number of adipocyte processes, including gene expression, adipokine secretion, macrophage recruitment, and insulin signaling (8–11). Examining whether PUFAs can regulate adipocyte desaturases will generate novel insights regarding the role of these enzymes as determinants of adipocyte FA content and, ultimately, reveal whether these enzymes act as mediators of PUFA bioactivity. This is particularly relevant considering recent reports have linked altered desaturase activity with adipose tissue FA profiles, body weight, and whole-body glucose homeostasis (12–14).
The current study aimed to examine whether PUFAs regulate the expression of Fads1 and Fads2 genes and the abundance of their respective desaturase proteins, as well as FA content, in differentiated 3T3-L1 adipocytes. We found that the FADS pathway is both functional in adipocytes and regulated by PUFAs, thus supporting the need for future investigations regarding the role of desaturase enzymes as mediators of PUFA bioactivity in adipose tissue.
Methods
Chemicals and cell culture reagents
Cell culture reagents including fetal bovine serum, 3-isobutyl-1-methylxanthine, dexamethasone, bovine serum albumin (BSA), and human insulin were purchased from Sigma Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium, penicillin streptomycin, and trypsin-ethylenediamine tetra-acetic acid were purchased from Hyclone laboratories (Logan, UT, USA). FAs were purchased from Cayman Chemical (Ann Arbor, MI, USA). Murine 3T3-L1 pre-adipocytes were purchased from ATCC (Rockville, MD, USA).
Cell culture methods
Pre-adipocytes were cultured and differentiated using established protocols, as previously described (15). Once pre-adipocytes were differentiated, cells were switched to serum-free media conditions for FA treatments. FA stock solutions were made by solubilizing FAs in 100% EtOH. 2% BSA was prepared directly in serum-free media. FA stock solutions were diluted in the 2% BSA serum-free media to give a FA:BSA molar ratio of 1:3, as previously described (7). Cells were treated with a final concentration of 100 μM FAs for 48 hours, starting on day 8 of our differentiation protocol. An equivalent volume of 100% EtOH diluted in 2% BSA serum-free media was used as the control. FA treatments did not cause cell toxicity, as confirmed using the Promega Cytotoxicity Assay (Madison, WI, USA). After the treatment period, total RNA, protein, and lipids were extracted. All experiments were replicated (n = 3) using different passage numbers.
RNA and protein extraction
Total RNA and protein were extracted using a dual-extraction protocol, as described previously (15).
Real-time RT-PCR analysis
Total RNA (1 μg) was used for real-time RT-PCR, as previously described (15). The online Roche Universal Probe Library and Assay Design Center was used to design all primer sequences. Rplp0 was used as the housekeeping gene and data were analyzed using the ΔΔCt method.
Western blot analysis
Protein samples were analyzed for the abundance of FADS1, FADS2, and GAPDH (Abcam, Cambridge, MA, USA), as previously described (16). Specific primary antibody dilutions were as follows: FADS1 - 1:2000; FADS2 - 1:300; and GAPDH - 1:3000.
Lipid extraction and quantification
A modified Bligh and Dyer lipid extraction protocol was used for the analysis of total FA content, as described previously (15). Peaks were identified by comparison to FA methyl ester standard peaks. FA peaks were quantified by comparison to a C19:0 internal standard. All FA data are reported as fold-changes relative to control.
Statistical analysis
All data sets were analyzed using a nonparametric Mann-Whitney U test. Data are reported as mean ± SEM, and statistical significance was considered at a P < 0.05.
Results
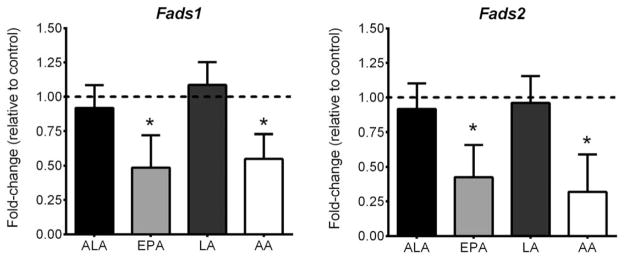
We first examined whether FAs could regulate Fads1 and Fads2 gene expression in differentiated 3T3-L1 adipocytes. Differentiation was confirmed by strong increases in the expression of Ppar-γ (~2000-fold), Srebp-1c (~1500-fold), and Fabp4 (~4500-fold). Both EPA and AA significantly down-regulated Fads1 and Fads2 gene expression, while neither ALA nor LA had an effect (Figure 1).
Figure 1.
Polyunsaturated fatty acid regulation of Fads1 and Fads2 gene expression. Data are expressed as fold-changes relative to the control condition (which is depicted as a dotted line at 1.00). An n = 3 was used for each treatment. * indicates statistical significance compared to control condition (P < 0.05).
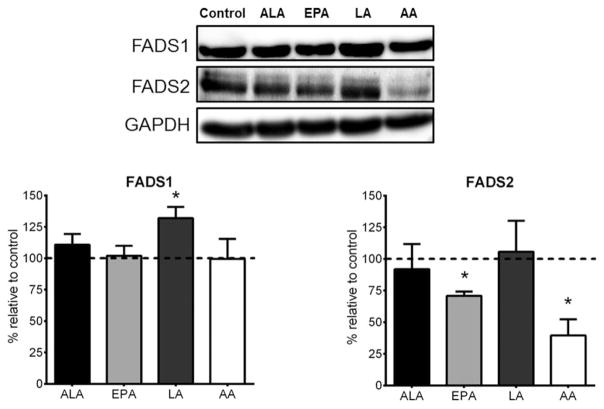
We next determined whether the changes seen in gene expression were reflected in protein content. As illustrated in Figure 2, we found that only EPA and AA significantly reduced FADS2 protein levels by 25–50% in differentiated adipocytes. Interestingly, ALA, EPA, and AA had no effect on FADS1 protein levels, while LA caused a significant 25% increase in FADS1 protein.
Figure 2.
Polyunsaturated fatty acid regulation of FADS1 and FADS2 protein content. Data are expressed as fold-changes relative to control condition (which is depicted as a dotted line at 100%). Representative blots shown for FADS1, FADS2, and GAPDH, where GAPDH was used to control for loading. Transfer efficiency was confirmed by Ponceau staining membranes. An n = 3 was used for each treatment. * indicates statistical significance compared to control condition (P < 0.05).
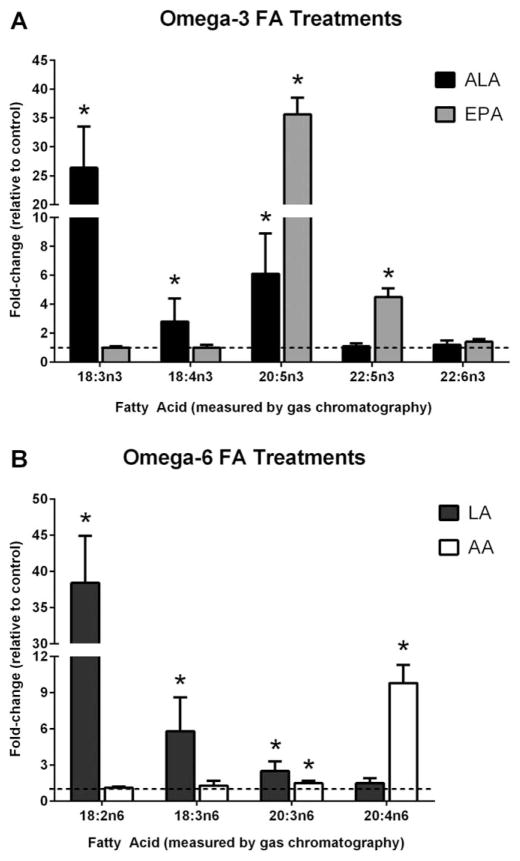
We next measured FA content in differentiated adipocytes treated with the various FAs using gas chromatography (Figure 3A, B). Treating cells with ALA led to a ~26-fold increase in cellular ALA content (P = 3.3 × 10−6). However, we also observed significant increases in cellular levels of several downstream omega-3 FAs. Both stearidonic acid (SDA; 18:4n-3) and EPA levels were increased by ~3-fold (P = 0.06) and ~6-fold (P = 1.0 × 10−4), respectively. ALA treatment did not alter docosapentaenoic acid (DPA; 22:5n-3) or DHA content (Figure 3A). EPA treatment caused significant increases in EPA (~36-fold; P = 4.0 × 10−4) and DPA (~4.5-fold; P = 2.0 × 10−3) levels, but did not alter ALA or SDA. No changes in DHA content were seen following EPA treatment. Neither ALA nor EPA treatments changed omega-6 FA content (data not shown).
Figure 3.
Cellular fatty acid levels following polyunsaturated fatty acid treatment. Adipocytes were treated for 48 hours with either (A) omega-3 fatty acids (ALA or EPA) or (B) omega-6 fatty acids (LA or AA). Subsequently, cellular fatty acid content was measured by gas chromatography. Fatty acid data are expressed as fold-changes relative to control condition (which is depicted as a dotted line at 1). An n = 3 was used for each treatment. * indicates statistical significance compared to control condition (P < 0.05).
Treating cells with LA caused a ~38-fold increase (P = 7.5 × 10−6) in cellular LA levels (Figure 3B). We also observed significant increases in the cellular levels of several downstream omega-6 FAs. Both γ-linoleic acid (GLA; 18:3n-6) and di-homo-γ -linoleic acid (DGLA; 20:3n-6) were significantly increased by ~6-fold (P = 1.0 × 10−4) and ~2.5-fold (P = 7.0 × 10−4); however, LA treatment did not alter AA levels. Differentiated adipocytes treated with AA experienced a significant increase of ~10-fold (P = 1.5 × 10−6) in AA content, as well as a small ~1.5-fold increase in DGLA levels (P = 0.02). Neither LA nor AA treatments affected omega-3 FA content (data not shown).
Discussion
The current study has demonstrated that EPA and AA down-regulate gene expression and protein content of adipocyte FADS. Moreover, treating differentiated 3T3-L1 adipocytes with ALA and LA showed that the FADS pathway is functional in this commonly used cell culture model, as evidenced by increases in downstream PUFAs. We also report that DHA levels in adipocytes are held steady following omega-3 FA treatments. Given that previous studies suggest that desaturase enzymes in adipose tissue are associated with various metabolic disturbances, such as insulin resistance and dyslipidemia (14), our results highlight the relevance of studying this pathway in adipocytes and the role of these enzymes as mediators of PUFA bioactivity.
We found that EPA decreased the expression of both desaturases at the gene and protein level; thereby agreeing with previous reports from the liver (4,5). However, despite a reduction in FADS2 protein content, EPA treatment did not alter ALA or SDA levels. Further, EPA treatment caused a significant increase in cellular DPA content, but no change in DHA levels; thereby aligning with previous reports (17,18). Similarly, treating adipocytes with ALA did not alter DHA levels. The lack of changes in DHA content is not surprising given the low rate of conversion between ALA and EPA, and the extremely low rate of conversion between EPA and DHA (19). This suggests that the endogenous production of DHA in adipocytes is tightly regulated.
Treating differentiated adipocytes with AA caused a significant reduction in FADS2 gene and protein content. Despite these reductions, AA treatments did not alter cellular levels of LA or GLA. However, AA treatment caused a slight, but significant increase in DGLA content. These results lend themselves to an intriguing hypothesis. AA is a precursor for pro-inflammatory eicosanoids, while DGLA is converted into eicosanoids with anti-inflammatory properties (20). As such, it is plausible that adipocytes control cellular AA content in order to limit its availability for the production of pro-inflammatory eicosanoids. However, when adipocyte AA content increases, then adipocytes may compensate by increasing DGLA levels to offset any potential changes in eicosanoid metabolism. This hypothesis is further supported by the fact that while LA treatment increased GLA and DGLA levels, no changes in AA content were observed.
The current study validated the use of 3T3-L1 adipocytes for studying diet regulation of the FADS enzymes and further showed that adipocyte FA content is influenced by the FADS pathway. Continuing this line of investigation is relevant given that the FADS enzymes are expressed in human adipose tissue (14) and recent research in a Fads2−/− mouse suggests a role for desaturases in the development of obesity-related complications (13). Future investigations whereby desaturase expression is altered (i.e., over-expressed or inhibited) in 3T3-L1 cells will generate new insights regarding the role of these enzymes as mediators of PUFA content and bioactivity in adipocytes. Additional studies examining different FA doses, various time-points during differentiation, eicosanoid production, and the impact of regulating FADS activity on FA composition of specific lipid fractions (e.g., triglycerides, phospholipids, etc.) are also warranted. Together, the crucial role of desaturases in adipocyte PUFA metabolism positions these enzymes as attractive candidates for future nutrigenomics research.
Acknowledgments
Funding agencies: This work was supported by an Operating Grant from the CIHR (Mutch) and an NSERC doctoral award (Ralston).
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Merino DM, Ma DW, Mutch DM. Genetic variation in lipid desaturases and its impact on the development of human disease. Lipids Health Dis. 2010;9:63. doi: 10.1186/1476-511X-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakamura MT, Nara TY. Structure, function, and dietary regulation of delta6, delta5, and delta9 desaturases. Annu Rev Nutr. 2004;24:345–376. doi: 10.1146/annurev.nutr.24.121803.063211. [DOI] [PubMed] [Google Scholar]
- 3.Slagsvold JE, Thorstensen K, Kvitland M, Mack M, Bjerve KS. Regulation of desaturase expression in HL60 cells. Scand J Clin Lab Invest. 2007;67:632–642. doi: 10.1080/00365510601175463. [DOI] [PubMed] [Google Scholar]
- 4.Reardon HT, Hsieh AT, Park WJ, et al. Dietary long-chain polyunsaturated fatty acids upregulate expression of FADS3 transcripts. Prostaglandins Leukot Essent Fatty Acids. 2013;88:15–19. doi: 10.1016/j.plefa.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho HP, Nakamura MT, Clarke SD. Cloning, expression, and nutritional regulation of the mammalian Delta-6 desaturase. J Biol Chem. 1999;274:471–477. doi: 10.1074/jbc.274.1.471. [DOI] [PubMed] [Google Scholar]
- 6.Cho HP, Nakamura M, Clarke SD. Cloning, expression, and fatty acid regulation of the human delta-5 desaturase. J Biol Chem. 1999;274:37335–37339. doi: 10.1074/jbc.274.52.37335. [DOI] [PubMed] [Google Scholar]
- 7.Barber E, Sinclair AJ, Cameron-Smith D. Comparative actions of omega-3 fatty acids on in-vitro lipid droplet formation. Prostaglandins Leukot Essent Fatty Acids. 2013;89:359–366. doi: 10.1016/j.plefa.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 8.Kalupahana NS, Claycombe KJ, Moustaid-Moussa N. (n-3) Fatty acids alleviate adipose tissue inflammation and insulin resistance: Mechanistic insights. Adv Nutr. 2011;2:304–316. doi: 10.3945/an.111.000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lottenberg AM, da Afonso MS, Lavrador MS, Machado RM, Nakandakare ER. The role of dietary fatty acids in the pathology of metabolic syndrome. J Nutr Biochem. 2012;23:1027–1040. doi: 10.1016/j.jnutbio.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 10.Oliver E, McGillicuddy FC, Harford KA, et al. Docosahexaenoic acid attenuates macrophage-induced inflammation and improves insulin sensitivity in adipocytes-specific differential effects between LC n-3 PUFA. J Nutr Biochem. 2012;23:1192–1200. doi: 10.1016/j.jnutbio.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 11.Siriwardhana N, Kalupahana NS, Fletcher S, et al. n-3 and n-6 polyunsaturated fatty acids differentially regulate adipose angiotensinogen and other inflammatory adipokines in part via NF-kappaB-dependent mechanisms. J Nutr Biochem. 2012;23:1661–1667. doi: 10.1016/j.jnutbio.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Truong H, DiBello JR, Ruiz-Narvaez E, Kraft P, Campos H, Baylin A. Does genetic variation in the Delta6-desaturase promoter modify the association between alpha-linolenic acid and the prevalence of metabolic syndrome? Am J Clin Nutr. 2009;89:920–925. doi: 10.3945/ajcn.2008.27107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stoffel W, Hammels I, Jenke B, et al. Obesity resistance and deregulation of lipogenesis in Delta6-fatty acid desaturase (FADS2) deficiency. EMBO Rep. 2014;15:110–120. doi: 10.1002/embr.201338041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sjogren P, Sierra-Johnson J, Gertow K, et al. Fatty acid desaturases in human adipose tissue: Relationships between gene expression, desaturation indexes and insulin resistance. Diabetologia. 2008;51:328–335. doi: 10.1007/s00125-007-0876-9. [DOI] [PubMed] [Google Scholar]
- 15.Ralston JC, Badoud F, Cattrysse B, McNicholas PD, Mutch DM. Inhibition of stearoyl-CoA desaturase-1 in differentiating 3T3-L1 pre-adipocytes up-regulates elongase 6 and down-regulates genes affecting triacylglycerol synthesis. Int J Obes. 2014;38:1449–1456. doi: 10.1038/ijo.2014.35. [DOI] [PubMed] [Google Scholar]
- 16.Holloway GP, Perry CG, Thrush AB, et al. PGC-1alpha’s relationship with skeletal muscle palmitate oxidation is not present with obesity despite maintained PGC-1alpha and PGC-1beta protein. Am J Physiol Endocrinol Metab. 2008;294:E1060–E1069. doi: 10.1152/ajpendo.00726.2007. [DOI] [PubMed] [Google Scholar]
- 17.Qin X, Park HG, Zhang J, et al. Fatty acid profiles of undifferentiated and differentiated white and brown adipose cell lines supplemented with alpha-linolenic acid. Faseb J. 2014;28:821.5. [Google Scholar]
- 18.Murali G, Desouza CV, Clevenger ME, Ramalingam R, Saraswathi V. Differential effects of eicosapentaenoic acid and docosahexaenoic acid in promoting the differentiation of 3T3-L1 preadipocytes. Prostaglandins Leukot Essent Fatty Acids. 2014;90:13–21. doi: 10.1016/j.plefa.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Burdge GC, Finnegan YE, Minihane AM, Williams CM, Wootton SA. Effect of altered dietary n-3 fatty acid intake upon plasma lipid fatty acid composition, conversion of [13C]alpha-linolenic acid to longer-chain fatty acids and partitioning towards beta-oxidation in older men. Br J Nutr. 2003;90:311–321. doi: 10.1079/bjn2003901. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Lin H, Gu Y. Multiple roles of dihomo-gamma-linolenic acid against proliferation diseases. Lipids Health Dis. 2012;11:25. doi: 10.1186/1476-511X-11-25. [DOI] [PMC free article] [PubMed] [Google Scholar]