Abstract
Introduction
Transoral laser microsurgery (TLM) has won territory in larynx oncology, establishing itself as an effective option in treatment of glottic, supraglottic, and hypopharynx tumors. Its advantages include limited resections, a reduction in number of tracheostomies, and the use of nasogastric tubes. Moreover, its oncological benefits are similar to those from open surgery in patients with early or advanced stages, when correctly selected.
Objective
The objective of this study is to review oncologic outcomes obtained with the treatment of a series of glottic tumors, treated by TLM.
Methods
Retrospective analysis of patients previously untreated, diagnosed with squamous cell carcinoma of the glottis (T1a, T1b, T2) in a tertiary university hospital. Endpoints for analysis were local control, overall and disease-specific survival, and larynx preservation rate.
Results
The study group included 58 patients that met the inclusion criteria: 57 (98.3%) men and 1 (1.7%) woman. Mean age was 65.5 ± 10.7 years (Min: 46/Max: 88). The tumor stages of the patients included were 30 T1a, 11 (19%) T1b, and 17 (29.3%) T2. Three-year overall survival rate was 89.7% (Fig. 1), and three-year disease-specific survival rate was 96.5%, three-year local control rate was 98.3%, and three-year organ preservation rate was 98.3%.
Conclusion
TLM is a safe and effective option in the treatment of glottis carcinomas, associated with less morbidity and a high percentage of local control, overall survival, specific survival, and organ preservation.
Keywords: larynx, CO2 laser, carcinoma
Introduction
Since Strong and Jako1 2 proposed laser endoscopic surgery for the treatment of larynx cancer in the seventies, transoral laser microsurgery (TLM) has gained territory in larynx oncology and established itself as an effective option in the treatment of glottic, supraglottic, and hypopharynx tumors.
Advantages such as the magnification that the microscope generates limits the number of resections by allowing the differentiation of healthy tissue from affected, thus, preserving disease free areas, reducing the number of tracheostomies and the use of nasogastric tubes.3 4 5 There are oncological benefits as well, similar to those in open surgery in patients with early stages and correctly selected, in addition to the good results in terms of quality voice after TLM comparable with radiotherapy itself in the treatment of early glottis tumors (T1). In different studies published on the treatment of laryngeal tumors, the 5-year local control ranges from 78–94% for T16 and a 47–91% for T2, using TLM, open partial laryngectomy, or radiotherapy.7 8
For this reason, the aim of this study was to review oncologic outcomes obtained by the treatment of a series of glottic tumors treated by TLM for T1a, T1b, and T2 glottic squamous cell carcinomas (SCC) in a tertiary university hospital. Endpoints for analysis in this study were locoregional control, overall and disease-specific survival, and larynx preservation rate.
Materials and Methods
We performed a retrospective analysis of patients previously untreated, diagnosed with squamous cell carcinoma of the glottis (T1a, T1b, T2), N - / +, M0 according to criteria of the Union Internationale Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) in a tertiary university hospital. We included patients treated with curative intent by TLM between January 2009 and January 2012. We identified through informative research of the records of our service, using the International Classification of Diseases (ICD-9). This study received approval from the ethics committee of our center.
We obtained demographic data (Age, Sex), comorbidities, tumor stage, additional test information, histological results, complications, survival, type of surgery, among others, by reviewing medical history. We excluded exclusive anterior commissure SCĆs.
Prior to surgery all the patients received information on treatment options (TLM versus RT versus Open surgery). However, we actually try to avoid the use of open surgery in early tumor stages because we prefer a less invasive treatment option at first; we only use this approach in patients for which it is not possible to achieve a correct exposure of the larynx during the suspension laryngoscopy. In addition, we usually perform a CT scan in all patients to confirm the disease local extension and to assess the neck.
Next, all cases were discussed in an interdisciplinary committee of head and neck tumors. Patients with lesions suspicious for malignancy were scheduled for laryngeal microsurgery with biopsy followed by laryngeal laser resection in cases that were positive. After surgery, we presented TNM in the committee, which assessed the need for reoperation or additional radiotherapy (RT).
To perform the surgical procedure, we used general anesthesia with orotracheal intubation in all patients, and performed resection using a Lumenis CO2 laser device (Yokneam, Israel) with a power setting of 4–8 W, in super pulsed mode and continuous setting, varying size and shape of the spot according to the moment of the surgery by using the micro-manipulator Acuspot-Acublade (Lumenis, Yokneam, Israel). We classified type of cordectomy according to the European Laryngological Society (ELSOC)9 proposal. In the case of small tumors (T1a, T1b), whenever possible, we attempted en bloc resection and, after resection, we pinned and oriented the piece on a corkboard. Sometimes we added a picture or a diagram on the orientation of the surgical specimen in the larynx, as it is very difficult for the pathologist to have a three-dimensional idea of the orientation of the piece. For bulky tumors (T2), most cases required piecemeal resections. Laser vestibulectomy was performed when the lateral or anterior portion of the tumor was hidden by a ventricular fold. In all cases the surgeons tried to achieve a margin of healthy tissue of 2–3mm, trying to preserve the functions without affecting the oncological radicality of the procedure.
During surgery, we did not perform intraoperative biopsies if resection was satisfactory; on the contrary case, we conducted examination of frozen section when the depth of cancer infiltration was difficult to estimate. Subsequently, we performed vaporization of the surgical area to prevent tumor recurrence. Resection margins were classified as free, uncertain, or affected. In case of having affected margins, we performed a re-intervention. As for patients with uncertain margins, only if there was evidence a suspicious lesion suggestive of persistence or recurrence during follow-up did we perform a new surgery.
In patients with positive neck ganglion (N1, N2), we performed a functional neck dissection in the same surgical act, while in N0 patients, we followed the necks. However, if during follow-up metastatic disease was evident, we performed a functional neck dissection, which was done in a second look surgery accompanied by microlaryngoscopy exploration.
In our service, patients treated for head and neck malignant tumors are followed during at least 5 years at the department of Head and Neck Oncology. However, for this study, we considered a group of patients that have been followed for a minimum of 36 months.
Statistical analysis was performed with the SPSS program for Windows, Version 20.0 (SPSS, Inc. Illinois, EE.UU). Quantitative variables in the study are expressed as media ± typical deviation. We calculated overall survival, specific survival, local control with laser, final local control with laser and organ preservation using the Kaplan-Meier survival analysis. For overall survival, we used as reference survival time from the surgery until death from all causes or last revision, while specific survival was considered from the time of surgery until death by tumor cause or until the performance of a total laryngectomy. We calculated the influence of various factors on survival using the log-rank method and we correlated the influence of the margins on the need for re-intervention as well as the influence of T stadium on the need to place a nasogastric tube or dysphagia by the Pearson Chi-square test.
Results
Fifty-eight patients met the inclusion criteria: 57 (98.3%) were men and 1 (1.7%) were women. The mean age was 65.5 ± 10.7 years (Min: 46/Max: 88). Of these, 15 (25.9%) were diabetic, 31 (53.4%) were hypertensive, 53 (91.4%) were smokers, and 12 (20.7%) consumed alcoholic beverages. The tumor stage of the patients included 30 T1a, 11(19%) T1b, and 17 (29.3%) T2 (Table 1). We classified 55 (94.8%) patients as N0, 2 (3.4%) as N1 (T1a, T2), and 1 (1.7%) as N2A (pT2). There were no cases of distant metastases. The mean follow-up was 43.1 ± 11 months (Min: 19 / Max: 72). Regarding the type of cordectomy, the most common was type 4 (27 = 46.55%) and type 5a (21 = 36.2%) (Table 1). Surgical margins were free in 41 (70.7%) patients, uncertain in 12 (20.7%), and affected in 5 (8.6%) cases (Table 2).
Table 1. Cordectomy according to ELSOC classification.
| Type of cordectomy | Groups | |||
|---|---|---|---|---|
| pT1a | pT1b | pT2 | Total (%) | |
| Type 3 | 2 | 0 | 0 | 2 (3.44%) |
| Type 4 | 26 | 0 | 1 | 27 (46.55%) |
| Type 5a | 2 | 11 | 8 | 21 (36.2%) |
| Type 5b | 0 | 0 | 3 | 3 (5.17%) |
| Type 5c | 0 | 0 | 5 | 5 (8.62%) |
| Total | 30 | 11 | 17 | 58 (100%) |
Abbreviations: ELSOC, European Laryngological Society.
Table 2. Relationship between tumor stage and surgical margin.
| Margins | Groups | |||
|---|---|---|---|---|
| pT1a | pT1b | pT2 | Total | |
| Free | 23 | 10 | 8 | 41 |
| Uncertain | 3 | 1 | 8 | 12 |
| Affected | 4 | 0 | 1 | 5 |
| Total | 30 | 11 | 17 | 58 |
The mean hospital stay was 2.1 days (Min: 1/Max: 14); however, in our service, most patients are discharged the day after surgery. Regarding immediate post-surgical complications, 1 (1.7%) patient had postoperative bleeding, which required the placement of a clip in the superior laryngeal pedicle. Other patients presented an episode of atrial fibrillation and needed to be in anesthetic resuscitation for 24 hours. Another patient suffered a falling tooth after surgery. No patient in this study needed an urgent tracheotomy. Nine (15.5%) patients required nasogastric (NG) in the immediate postoperative period with an average of 2.06 days (Min: 1 / Max: 6), being more frequent in patients with the most advanced tumors (p = 0.009). In none of the patients was it necessary to place a percutaneous gastrostomy tube (PEG). During follow-up, 6 (10.3%) patients had some degree of postoperative dysphagia, which improved with swallowing rehabilitation. In the late postoperative period, no patients presented complications associated with surgical technique, such as aspiration pneumonia, chondritis of the thyroid cartilage, among others.
Regarding associated metastasis to the glottis SCC, 3 (5.2%) patients had palpable metastasis at the moment of diagnosis. In two cases (N1), we performed bilateral functional neck dissection and, in one case (N2a), we performed a modified radical neck dissection.
We achieved local control in the first surgery in 46 (79.3%) patients, in that 80.48% were T1 (32/41) and 76.47% were T2 (13/17). In 9 (15.5%) patients, we performed a second look operation (4 T1a, 4 T2, 1 T1b), and in another three patients, 2 re-interventions (3 T1a) were necessary to achieve local control. In one case (T2), a total laryngectomy was necessary, achieving 98.3% laser local control as well as functional laryngeal preservation. In terms of margins, 41 (70.6%) specimens were free of tumor involvement, 12 (20.6%) were uncertain, and 5 (8.6%) were affected. In the group of uncertain margins were where most re-interventions were needed: up to 8 of the 12 patients (Table 2) (p < 0.001). The mean time elapsed until reoperation was 6.7 months (Min: 2 / Max: 11). Regional control was possible in 56 (96.6%) patients, while we observed cervical nodal disease in two (3.4%) patients during follow-up. Median time to onset of positive lymph was 11 months (Min: 10/Max: 12) and a functional neck dissection was performed in both. Distant metastases were not evident in either case during follow-up.
The use of complementary therapies in our study was limited because most patients were in early stages. Three patients received additional RT, having positive metastases after making the neck dissection, two (3.4%) of these patients belong to the free margins group, and another patient to the affected margins group. In another two patients in the uncertain margins groups, which also had regional recurrence during follow-up, we performed a cervical neck dissection and subsequent treatment with RT. For the N+ patient, RT courses were scheduled within 4–6 weeks postoperatively and delivered weekly over a period of 6 to 12 weeks to reach a total dose of 45 to 60 Gy.
Regarding the occurrence of second primary, 4 (6.8%) patients were affected, 2 of them for lung cancer, one bladder cancer, and another for malignant melanoma. The mean time to onset of the second primary was 20.25 months (Min: 15 / Max: 31).
Three years overall survival was 89.7% (Fig. 1), and three years disease-specific survival was 96.5% (Fig. 2), reaching 98.3% when including salvage surgery (Table 3). Overall survival according to T stage was 90% (T1a), 90.9% (T1b), and 82.4% (T2), with no statistically significant difference between them (p = 0.590). As for N, the three-year survival of the 3 patients with metastases at diagnosis and 2 developed metastases during follow-up was 60%. We were unable to obtain statistical significance probably due to the limited sample N+ (p = 0.625).
Fig. 1.
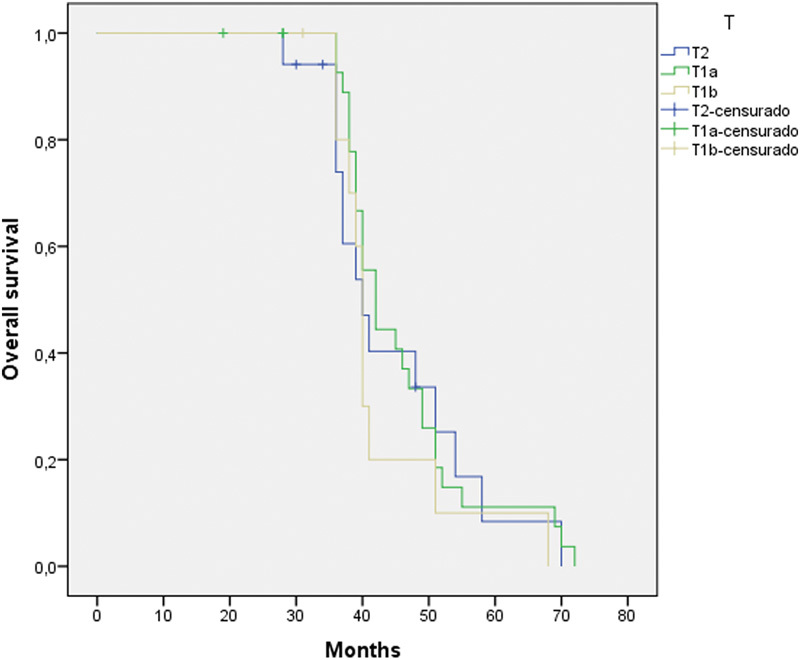
Kaplan-Meier three-year overall survival according to tumor stage and for all causes of death (87.9%).
Fig. 2.
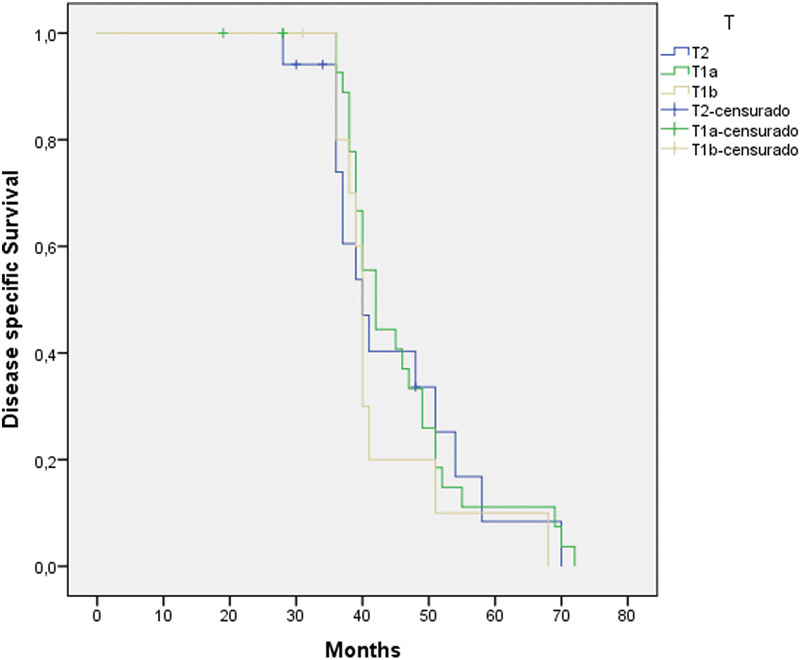
Kaplan-Meier three-year specific survival according to tumor stage (98.3%).
Table 3. Results according to tumor stage.
| Results | Groups | |||
|---|---|---|---|---|
| pT1a | pT1b | pT2 | p | |
| 3Y Overall survival | 90% | 90.9% | 82.4% | 0.590 |
| 3Y Disease free specific survival | 100% | 100% | 88.2% | 0.765 |
| Local control with TLM (1 intervention) |
76.6% | 91% | 47% | 0.017 |
| Ultimate local control only with TLM | 100% | 100% | 94.1% | 0.706 |
| 3Y laryngectomy free survival | 100% | 100% | 94.1% | 0.622 |
During follow-up, 6 (10.3%) patients died, one patient (1/58 = 1.7%) by regional disease progression, 4 patients by second synchronous or metachronous neoplasms, and another one because of a stroke.
Discussion
In this retrospective study, we analyzed the results of a group of 58 patients with early laryngeal carcinomas (T1a, T1b and T2), treated by first intention with TLM. However, we must remember that when we are treating these kind of tumors, in addition to endoscopic techniques, we have open surgery and radiotherapy (RT) - three tools with similar effectiveness that do not allow us to favor one over the others in the treatment of these tumors based on oncologic outcomes.10
In different published studies of the treatment of laryngeal tumors, the 5-year local control ranged from 78–94% for T16 and a 47–91% for T2, using the 3 modes previously described.7 8 Regarding our study, 79.3% local control was achieved with one surgery alone (T1: 80.48%; T2: 76.47%), reaching a 98.3% local control after a second look intervention, similar results to those described in the literature by Lee et al, who obtained exclusive local control with laser in 94.2% of patients.11 In the whole group, a total laryngectomy was required only in one case, achieving a rate of organ preservation of 98.3%, similar to those obtained by Lester et al, who got 98.1%12 and Lee et al, who got 96.2%11 of organ preservation.
Several authors suggest that in those patients in whom the anterior commissure, arytenoid, subglottic, or vocal muscle are affected will have a higher risk of local recurrence.11 13 14 While other factors may directly influence the outcome of surgery and the likelihood of tumor recurrence will be an adequate laryngeal exposure during surgery. To accomplish this, we had to mobilize the laryngoscopy blade during surgery looking for the best exposure and resect the ventricular fold when the exposure of the anterior commissure or laryngeal ventricle was not possible.11
At the glottal level, resection margins over 1–2mm will be considered adequate,11 although some authors have suggested the effectiveness of margins less than 0.5mm for in situ or T1 carcinomas.15 A different concept from the average of 5mm was suggested for open surgery in head and neck cancer.16 Ansarin et al17 concluded there was a need for additional treatment for patients with positive margins (reoperation or RT), since the presence of positive margins was associated with a greater tendency to recurrence. These results correlate with those obtained by Crespo et al, who showed 37.5% local recurrence rates in patients who had positive margins against 0% recurrence in the group of patients with negative margins.18 However, both Lee et al and Hartl et al showed no statistically direct relationship between the presence of positive margins and the tendency to recurrence, suggesting the possibility of reoperation for these patients based on the findings during follow-up.11 19 This is precisely where follow-up plays a key role in the success of surgery, because, despite being able to get uncertain or positive margins, the phenomenon of vaporization after tumor resection will allow a safety margin of ∼2–3mm20 and, for this reason, in our center as well as in others, the presence of uncertain or positive margins in all cases is not associated to reoperation.
To classify the degree of involvement of surgical margins, we decided to use the classification proposed by Blanch et al, who aside from setting margins as positive or negative, also included the presence of uncertain margins.20 In our sample, 70.6% of patients had clear margins, 20.6% presented uncertain margins, and 8.6% presented affected margins. It was the uncertain margins group that required the most second-look interventions, being necessary in up to 66.6% (8/12) of patients.
In our study, the three-year overall survival rate was 89.7% for all causes of death, the ultimate local control only with laser was 96.5%. The three-year specific survival rate including salvage surgery was 98.3%, while the laryngeal preservation rate was 98.3%, comparable with other results reported in the literature. Lester et al achieved a five-year overall survival rate of 88.8%, a five-year disease-specific survival rate of 96.6%, and 98.1% of laryngeal preservation.12 Lee et al achieved a five-year overall survival rate of 87.9%, an ultimate local control only with laser of 94.2%, a five-year specific survival rate of 99%, and 96.2% of laryngeal preservation.11 Lucioni et al, in turn, reported an overall survival rate of 90.8%, an ultimate local control only with laser of 94.3%, a disease specific survival rate of 98.8%, and 97.7% of laryngeal preservation with a minimum follow-up of 24 months. All these results were based on studies in T1-T2 stages.13
In our study, only three cases (5.2%) presented complications in the immediate postoperative period, none of them mortal. We must remember, however, that the percentage of tumor complications in all stages operated by experimental surgeons oscillates between 5–6%, a hypothesis which we were able to confirm when comparing tumor size, tumor location, and degree of surgeon experience with the appearance of complications. In any case, of all of the complications described in the literature, the postoperative hemorrhage is most fearful due to the vital risk it brings to a patient without tracheostomy.13 21 22
Other important factors to consider when contextualizing this surgery (MTL) is quality of life. Vilaseca et al, in a prospective study that included patients treated for carcinoma in early and moderately advanced stages, obtained favorable results regarding the improvement in quality of life after surgery for most of their subjects, speech preservation being again the factor assessed.23 Voice is undoubtedly one of the main factors to take into consideration when treating laryngeal tumors in early stages. Van Gogh et al, in a study that prospectively evaluated the voice in patients treated with RT or laser surgery in T1a carcinomas, showed that vocal recovery was faster in the group treated with laser. Three months after surgery they did not find differences from normal voice, except for the fundamental frequency and at the highest pitch. They concluded that the MTL should be the treatment of choice for T1a tumors.24 However, Remmelts et al25 found evidence against the use of laser in patients with deeply infiltrative T1a lesions and T1b lesions, since voice quality might become worse when a laser resection is performed in such patients. More recently, a meta-analysis conducted by Greulich et al about voice outcomes following radiation versus laser microsurgery for T1 glottic carcinoma concluded that VHI scores were comparable following XRT and TLM for T1 glottic carcinoma, according to reports from current literature, suggesting no clinically significant difference in functional voice outcomes between treatment types.26 Unfortunately, we have not included the results regarding vocal quality of patients in our study, because, despite having a voice unit, patients were not assessed systematically in the postoperative period.
Finally, we must note the limitations of our study. Remembering that it is a retrospective study, in which we did not have a control group to compare our results (Open surgery and/or RT) and we did not evaluate the voice or the quality of life in our patients after surgery.
Conclusion
Several studies support the use of TLM in the treatment of laryngeal tumors T1a, T1b, and T2, as well as for well-selected advanced tumor stages. Our results coincide with those given in the literature, showing that TLM is a safe and effective option in the treatment of glottic carcinomas associated with less morbidity and a high percentage of local control, overall survival, specific survival, and organ preservation.
References
- 1.Strong M S, Jako G J. Laser surgery in the larynx. Early clinical experience with continuous CO 2 laser. Ann Otol Rhinol Laryngol. 1972;81(6):791–798. doi: 10.1177/000348947208100606. [DOI] [PubMed] [Google Scholar]
- 2.Strong M S. Laser excision of carcinoma of the larynx. Laryngoscope. 1975;85(8):1286–1289. doi: 10.1288/00005537-197508000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Vilaseca-González I, Bernal-Sprekelsen M, Blanch-Alejandro J L, Moragas-Lluis M. Complications in transoral CO2 laser surgery for carcinoma of the larynx and hypopharynx. Head Neck. 2003;25(5):382–388. doi: 10.1002/hed.10207. [DOI] [PubMed] [Google Scholar]
- 4.Stoeckli S J, Schnieper I, Huguenin P, Schmid S. Early glottic carcinoma: treatment according patient's preference? Head Neck. 2003;25(12):1051–1056. doi: 10.1002/hed.10323. [DOI] [PubMed] [Google Scholar]
- 5.Steiner W, Ambrosch P. Stuttgart: Georg Thieme Verlag; 2000. Advantages of transoral laser microsurgery over standard therapy; pp. 44–45. [Google Scholar]
- 6.Bradley P J Mackenzie K Wight R Pracy P Paleri V; ENT-UK Head & Neck Group. Consensus statement on management in the UK: transoral laser assisted microsurgical resection of early glottic cancer Clin Otolaryngol 2009344367–373. [DOI] [PubMed] [Google Scholar]
- 7.McCoul E D, Har-El G. Meta-analysis of impaired vocal cord mobility as a prognostic factor in T2 glottic carcinoma. Arch Otolaryngol Head Neck Surg. 2009;135(5):479–486. doi: 10.1001/archoto.2009.47. [DOI] [PubMed] [Google Scholar]
- 8.Mendenhall W M, Werning J W, Hinerman R W, Amdur R J, Villaret D B. Management of T1-T2 glottic carcinomas. Cancer. 2004;100(9):1786–1792. doi: 10.1002/cncr.20181. [DOI] [PubMed] [Google Scholar]
- 9.Remacle M, Van Haverbeke C, Eckel H. et al. Proposal for revision of the European Laryngological Society classification of endoscopic cordectomies. Eur Arch Otorhinolaryngol. 2007;264(5):499–504. doi: 10.1007/s00405-007-0279-z. [DOI] [PubMed] [Google Scholar]
- 10.Dey P, Arnold D, Wight R, MacKenzie K, Kelly C, Wilson J. Radiotherapy versus open surgery versus endolaryngeal surgery (with or without laser) for early laryngeal squamous cell cancer. Cochrane Database Syst Rev. 2002;(2):CD002027. doi: 10.1002/14651858.CD002027. [DOI] [PubMed] [Google Scholar]
- 11.Lee H S, Chun B G, Kim S W. et al. Transoral laser microsurgery for early glottic cancer as one-stage single-modality therapy. Laryngoscope. 2013;123(11):2670–2674. doi: 10.1002/lary.24080. [DOI] [PubMed] [Google Scholar]
- 12.Lester S E, Rigby M H, Taylor S M. Transoral laser microsurgery outcomes with early glottic cancer: the Dalhousie University experience. J Laryngol Otol. 2011;125(5):509–512. doi: 10.1017/S002221511000304X. [DOI] [PubMed] [Google Scholar]
- 13.Lucioni M, Marioni G, Bertolin A, Giacomelli L, Rizzotto G. Glottic laser surgery: outcomes according to 2007 ELS classification. Eur Arch Otorhinolaryngol. 2011;268(12):1771–1778. doi: 10.1007/s00405-011-1695-7. [DOI] [PubMed] [Google Scholar]
- 14.Mortuaire G, Francois J, Wiel E, Chevalier D. Local recurrence after CO2 laser cordectomy for early glottic carcinoma. Laryngoscope. 2006;116(1):101–105. doi: 10.1097/01.mlg.0000184524.23282.74. [DOI] [PubMed] [Google Scholar]
- 15.Sigston E, de Mones E, Babin E. et al. Early-stage glottic cancer: oncological results and margins in laser cordectomy. Arch Otolaryngol Head Neck Surg. 2006;132(2):147–152. doi: 10.1001/archotol.132.2.147. [DOI] [PubMed] [Google Scholar]
- 16.Hinni M L, Ferlito A, Brandwein-Gensler M S. et al. Surgical margins in head and neck cancer: a contemporary review. Head Neck. 2013;35(9):1362–1370. doi: 10.1002/hed.23110. [DOI] [PubMed] [Google Scholar]
- 17.Ansarin M, Cattaneo A, Santoro L. et al. Laser surgery of early glottic cancer in elderly. Acta Otorhinolaryngol Ital. 2010;30(4):169. [PMC free article] [PubMed] [Google Scholar]
- 18.Crespo A N, Chone C T, Gripp F M, Spina A L, Altemani A. Role of margin status in recurrence after CO2 laser endoscopic resection of early glottic cancer. Acta Otolaryngol. 2006;126(3):306–310. doi: 10.1080/00016480500316985. [DOI] [PubMed] [Google Scholar]
- 19.Hartl D M, de Monès E, Hans S, Janot F, Brasnu D. Treatment of early-stage glottic cancer by transoral laser resection. Ann Otol Rhinol Laryngol. 2007;116(11):832–836. doi: 10.1177/000348940711601107. [DOI] [PubMed] [Google Scholar]
- 20.Blanch J L, Vilaseca I, Bernal-Sprekelsen M. et al. Prognostic significance of surgical margins in transoral CO2 laser microsurgery for T1-T4 pharyngo-laryngeal cancers. Eur Arch Otorhinolaryngol. 2007;264(9):1045–1051. doi: 10.1007/s00405-007-0320-2. [DOI] [PubMed] [Google Scholar]
- 21.Olthoff A, Ewen A, Wolff H A. et al. Organ function and quality of life after transoral laser microsurgery and adjuvant radiotherapy for locally advanced laryngeal cancer. Strahlenther Onkol. 2009;185(5):303–309. doi: 10.1007/s00066-009-1967-y. [DOI] [PubMed] [Google Scholar]
- 22.Vilaseca I, Bernal-Sprekelsen M. Transoral laser microsurgery for locally advanced laryngeal cancer. Acta Otorrinolaringol Esp. 2013;64(2):140–149. doi: 10.1016/j.otorri.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 23.Vilaseca I, Ballesteros F, Martínez-Vidal B M, Lehrer E, Bernal-Sprekelsen M, Blanch J L. Quality of life after transoral laser microresection of laryngeal cancer: a longitudinal study. J Surg Oncol. 2013;108(1):52–56. doi: 10.1002/jso.23348. [DOI] [PubMed] [Google Scholar]
- 24.van Gogh C D, Verdonck-de Leeuw I M, Wedler-Peeters J, Langendijk J A, Mahieu H F. Prospective evaluation of voice outcome during the first two years in male patients treated by radiotherapy or laser surgery for T1a glottic carcinoma. Eur Arch Otorhinolaryngol. 2012;269(6):1647–1652. doi: 10.1007/s00405-012-1947-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Remmelts A J, Hoebers F J, Klop W M, Balm A J, Hamming-Vrieze O, van den Brekel M W. Evaluation of lasersurgery and radiotherapy as treatment modalities in early stage laryngeal carcinoma: tumour outcome and quality of voice. Eur Arch Otorhinolaryngol. 2013;270(7):2079–2087. doi: 10.1007/s00405-013-2460-x. [DOI] [PubMed] [Google Scholar]
- 26.Greulich M T, Parker N P, Lee P, Merati A L, Misono S. Voice outcomes following radiation versus laser microsurgery for T1 glottic carcinoma: systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2015;152(5):811–819. doi: 10.1177/0194599815577103. [DOI] [PMC free article] [PubMed] [Google Scholar]


