Abstract
Background
FRAX® is a validated, computer-based clinical fracture risk calculator that estimates 10-year risk of major osteoporotic (clinical spine, forearm, hip or shoulder) fracture, and hip fracture alone. It is widely used for decision-making in fracture prevention, but may underestimate risk in HIV-infected individuals. Some experts recommend considering HIV a cause of secondary osteoporosis when calculating FRAX in HIV-infected individuals.
Methods
From the Veterans Aging Study Virtual Cohort (VACS-VC), we included 24451 HIV-infected and uninfected 50-70 year old men with complete data in year 2000 to approximate all but two factors (i.e. history of secondary osteoporosis and parental hip fracture) for modified-FRAX calculation without bone density and 10-year observational data for incident fragility fracture. Accuracy of the modified-FRAX calculation was compared by observed/estimated (O/E) ratios of fracture by HIV status.
Results
Accuracy of modified-FRAX was less for HIV-infected (O/E=1.62, 95%CI: 1.45, 1.81) than uninfected men (O/E=1.29, 95%CI: 1.19, 1.40), but improved when HIV was included as a cause of secondary osteoporosis (O/E=1.20, 95%CI: 1.08, 1.34). However, only 3-6% of men with incident fractures were correctly identified by the modified-FRAX using accepted FRAX thresholds for pharmacologic therapy.
Conclusions
Modified-FRAX underestimated fracture rates more in older HIV-infected than otherwise similar uninfected men. Accuracy improved when HIV was included as a cause of secondary osteoporosis, but it still performed poorly for case-finding. Further studies are necessary to determine how to use FRAX or define an HIV-specific index to risk stratify for screening and treatment in older HIV-infected individuals.
Keywords: fracture incidence, HIV, men, FRAX
Introduction
With the aging of the HIV-infected population, identifying individuals at higher risk of fracture in order to address modifiable risk factors for fracture prevention is an increasingly important focus of long-term outpatient care. In unadjusted analyses, risk of fragility fracture is approximately 35% higher in HIV-infected than uninfected controls 1,2 and increases with age 3. HIV primary care guidelines recommend performance of dual energy x-ray absorptiometry (DXA) for bone mineral density (BMD) assessment in HIV-infected individuals over age 50 4,5 and risk stratification using FRAX starting at age 40 5. FRAX is an assessment tool for prediction of fractures (10-year probability of major osteoporotic and hip fracture) validated primarily in men and women over 50 years of age. FRAX includes 11 clinical risk factors for fractures, as well as femoral neck BMD, although the latter is not a necessary component 6.
In Europe, FRAX is commonly utilized for risk prognostication in the general population to identify individuals over age 40 who should undergo a screening DXA, and those at high enough risk of fracture to receive pharmacologic therapy without BMD evaluation 7. In the United States, where DXA is considered the preferred screening modality for older individuals, FRAX is utilized primarily in individuals who do not meet criteria for osteoporosis by DXA but have low bone density/osteopenia (T score<-1.0 but >-2.5) to determine appropriateness of pharmacologic therapy 8. There are no definitive data on similar use of FRAX for HIV-infected patients. Recent studies suggest that FRAX underestimates fracture rates in HIV-infected men9,10; however, both studies included men <50 years and compared the FRAX score to either BMD outcomes or prevalent rather than incident fractures.
The goal of this study was to compare the performance of a modified-FRAX among HIV-infected and uninfected men over age 50. We hypothesized that the modified-FRAX would underestimate actual fracture rates more in HIV-infected than in uninfected men. We evaluated whether inclusion of HIV as a cause of secondary osteoporosis in the FRAX calculation would improve the accuracy of FRAX estimates in HIV-infected men. Chronic liver disease is among conditions listed as a cause of secondary osteoporosis in the FRAX calculator; however, HCV-infection without hepatic decompensation is not usually included in the calculation. Since HCV-coinfection is fairly prevalent among HIV-infected individuals and has been associated with significant fracture risk 11,12, we also evaluated whether inclusion of HCV as a cause of secondary osteoporosis would improve the accuracy of FRAX estimates in HIV-infected men.
Methods
Study sample
The Veterans Aging Cohort Study Virtual Cohort (VACS-VC) 13 is a prospective observational cohort of HIV-infected Veterans matched with uninfected Veterans by age, sex, race-ethnicity, and geographic region who enrolled for care in the Veterans Health Administration (VHA) in the same calendar year. Our analysis included all male HIV-infected and uninfected Veterans in VACS-VC who were 50-70 years of age at year 2000 and have data available to approximate risk factors necessary for calculation of FRAX. In VACS-VC, 42924 males met these inclusion criteria, including 25720 with data to approximate all but two of the FRAX variables (parental history of hip fracture and secondary osteoporosis). Among these subjects, 1269 whose weight exceeded the 125 kg limit for the FRAX calculation were excluded, resulting in 24451 subjects with valid FRAX calculations. Each subject had at least one clinic visit recorded within the VHA database during the period of 2000-2010.
Estimated fracture risk by modified-FRAX
A “modified-FRAX” fracture risk estimate was calculated for each subject using the following nine variables available in VACS-VC to approximate FRAX calculator variables: age, race/ethnicity limited to categories utilized in FRAX (white, black, Asian, Hispanic), weight (kg), height (cm2), history of previous fragility fracture, ever glucocorticoid use, rheumatoid arthritis, and alcohol use. A subject was determined to be a current smoker if it was listed as a health factor in year 2000; therefore, prevalence is lower than previous VACS-VC analyses in which current smoking status was based upon listing as a health factor in any year within the study period3. The presence of the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code for alcohol use/abuse was used in order to approximate the 3 or more units of alcohol per day threshold utilized by FRAX. Rheumatoid arthritis was also determined by ICD-9-CM codes. Parental history of hip fracture and secondary osteoporosis (i.e., type-1 diabetes, osteogenesis imperfecta, untreated hyperthyroidism, hypogonadism or premature menopause <45 years, chronic malnutrition, malabsorption, and chronic liver disease) were not utilized in the calculation of the modified-FRAX as the data were either not collected or not validated in VACS-VC. By convention, parental history of hip fracture and secondary osteoporosis were entered as “no” for all subjects, but we also performed a sensitivity analysis for the test characteristics of the modified-FRAX by entering “Yes” for the parental history of hip fracture and secondary osteoporosis (see Methods below). HCV-infection status was also defined for subject by positive HCV antibody, HCV RNA, or ICD-9CM codes.
The estimated 10-year probability of major osteoporotic and hip fracture was calculated for each subject using a program that automated data entry and retrieval from the FRAX website calculator for the United States stratified by race/ethnicity (http://www.shef.ac.uk/FRAX/index.aspx).
Fracture ascertainment
Fracture outcomes were defined using codes from the ICD-9-CM to match FRAX-defined osteoporotic fractures of the hip, shoulder, forearm and clinical vertebral fractures. Hip: 820.0X, 820.1X, 820.2X, 820.3X, 820.8, 820.9; shoulder/upper arm: 812.X, 812.1X; radius and ulna: 813.0X, 813.1X, 813.2X, 813.3X, 813.4X, 813.5X, 813.8X, 813.9X and vertebrae: 805.2, 805.3, 805.4, 805.5, 805.6, 805.7; 805.8; 805.9. These codes were previously validated by chart review of 400 randomly selected radiology reports. Overall agreement between chart review and ICD-9-CM codes was 97 percent. Agreement beyond chance (kappa) was substantial (0.77, 95% CI: 0.62-0.90) 14. Analyses utilizing these fracture outcomes have been published previously 3,15. Only the first fractures occurring for each subject during the study period of 2001-2010 were included in analyses.
Statistical Analysis
Descriptive analyses
Descriptive analyses included means and standard deviations for continuous variables, and frequency and percentages for categorical variables. Variables were compared across groups using t-tests for continuous variables and chi-square or Fisher's exact tests for categorical variables. Incident fracture risk was calculated based upon the occurrence of a new fracture at a major osteoporotic fracture site (i.e. hip, upper arm, radius/ulna and vertebrae) and hip alone for each subject between 2001-2010 so as to fit the 10-year fracture estimate calculated by modified-FRAX. We also conducted a sensitivity analysis restricted to the 15876 subjects who had clinic data beyond 2010 to rule out the possibility that fractures did not occur because subjects had died during follow-up.
Assessment of Accuracy
Calibration was our measure of accuracy since thresholds for the numeric probability of fracture determined by FRAX have been established for BMD screening and pharmacologic treatment. Calibration assesses how well the observed fractures compare with estimated (predicted) probabilities of fracture across the entire spread of the data 16. The number of individuals observed to sustain a fracture versus the number of individuals expected to sustain a fracture by modified-FRAX, the observed/expected (O/E) ratio, at a major osteoporosis site or at the hip was calculated for HIV-infected and uninfected groups. Chi-square tests were used to test differences between O/E ratios between groups, and the Hosmer-Lemeshow chi-square statistic was used to compare O/E ratios over deciles of estimated risk.
To assess whether the accuracy of modified-FRAX improves when HIV is included as a cause of secondary osteoporosis, the modified-FRAX was recalculated for each subjects after entering “Yes” for secondary osteoporosis. Similarly, the accuracy of modified-FRAX was assessed when HCV-infection was considered a cause of secondary osteoporosis. Note that only one cause of secondary osteoporosis can be entered into the FRAX calculator; therefore, in this analysis, secondary osteoporosis was entered as “Yes” for all HCV-infected subjects without consideration of HIV status.
We evaluated the sensitivity/specificity of the modified-FRAX for prediction of incident fracture among HIV-infected men using FRAX thresholds for pharmacologic therapy endorsed by osteoporosis guidelines in the United States and Europe 7,17. In addition, we performed a sensitivity analysis for the test characteristics of the modified-FRAX for prediction of incident fracture by entering “Yes” for the two FRAX variables that were not available in the VACS dataset (parental history of hip fracture and secondary osteoporosis) and recalculating the modified-FRAX.
Analyses were performed using SAS version 9.4 (Cary, NC) and the R package ‘rmap’ 18. All tests were two-tailed, and a p-value <.05 was considered significant.
Results
Baseline characteristics
Compared to HIV-uninfected men, those with HIV infection were similar in age but differed in all other FRAX variables. HIV-infected men were more likely to be black, have previous fractures, glucocorticoid use and alcohol use/abuse than uninfected men, but had lower weight/body mass index (BMI) and were less likely to be current smokers or have rheumatoid arthritis than uninfected men (Table 1). HIV-infected men were also more likely to have HCV-infection than HIV-uninfected men (33.2% vs 7.9%, p<0.0001).
Table 1.
Baseline characteristics (year 2000) of male participants 50-70 years in the Veterans Aging Cohort Study Virtual Cohort (VACS-VC) by HIV status
| Characteristics | All (N=24451) | HIV-infected (N=7064) | HIV-uninfected (N=17387) | P-value |
|---|---|---|---|---|
| Modified FRAX variables | ||||
| Age (years), Mean ± SD | 55.6 ± 5.4 | 55.5 ± 5.3 | 55.7 ± 5.4 | 0.113 |
| Race/ethnicity, N (%) | ||||
| Asian | 65 (0.2) | 22 (0.3) | 43 (0.3) | 0.003 |
| Black | 11323 (46.3) | 3358 (47.5) | 7965 (45.8) | |
| Hispanic | 2117 (8.7) | 546 (7.7) | 1571 (9.0) | |
| White | 10946 (44.8) | 3138 (44.4) | 7808 (44.9) | |
| Weight (kg), Mean ± SD | 85.8 ± 16.3 | 79.2 ± 14.9 | 88.5 ± 16.0 | <0.0001 |
| Height (cm), Mean ± SD | 177.1 ± 7.3 | 177.1 ± 7.4 | 177.1 ± 7.3 | 0.577 |
| BMI (kg/m2), Mean ± SD | 27.3 ± 4.9 | 25.2 ± 4.4 | 28.2 ± 4.8 | <0.0001 |
| Previous fragility fracture, N (%) | 478 (2.0) | 167 (2.4) | 311 (1.8) | 0.003 |
| Current smoker, N (%) | 1464 (6.0) | 364 (5.2) | 1100 (6.3) | 0.0005 |
| Alcohol use, N (%) | 2602 (10.6) | 826 (11.7) | 1776 (10.2) | 0.0007 |
| Glucocorticoid use, N (%) | 40 (0.16) | 18 (0.25) | 22 (0.13) | 0.0245 |
| Rheumatoid arthritis, N (%) | 803 (3.3) | 188 (2.7) | 615 (3.5) | 0.0005 |
| Additional variables | ||||
| Hepatitis C (HCV) infection, N (%) | 3715 (15.2) | 2346 (33.2) | 1369 (7.9) | <0.0001 |
Ten year observed and estimated fracture risks by modified-FRAX
During an observation period of 10 years, mean estimated 10-year risk of fracture by modified-FRAX was higher for HIV-infected than uninfected men at major osteoporosis sites (2.9±1.5% versus 2.7±1.4%, p<0.0001) and at the hip (0.3±0.4% versus 0.3±0.2%, p<0.0001) (Figure 1). When HIV is included as a cause of secondary osteoporosis in the calculation of the modified-FRAX, the estimated rate of fracture in HIV-infected men increased 31% for major osteoporotic fractures, from 2.9±1.5% to 3.8±2.1%, and increased 67% for hip fractures, from 0.3±0.4% to 0.5±0.6 percent.
Figure 1.
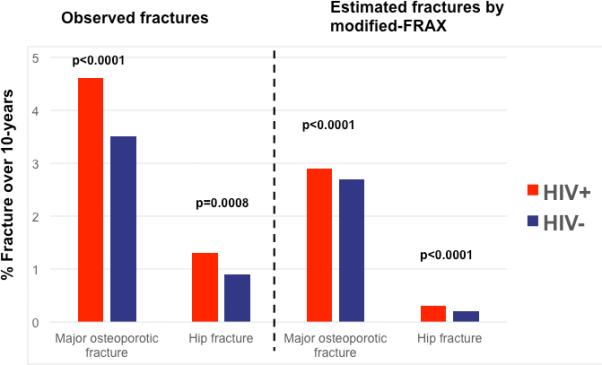
Observed and modified-FRAX estimated rates of fracture by HIV status
Accuracy of modified-FRAX in HIV-infected
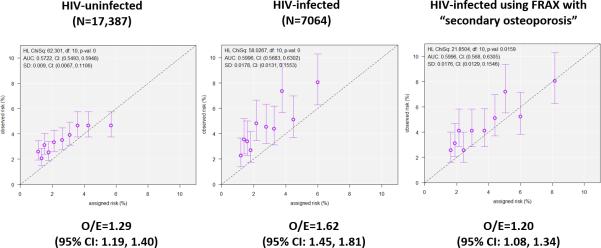
Among all participants, the modified-FRAX consistently underestimated the true fracture risk: the ratio of observed to expected fractures (O/E) was 1.39 (95% CI: 1.30, 1.48) (Table 2). The modified-FRAX was less accurate in HIV-infected than in uninfected men for major osteoporotic fractures (O/E: 1.62 vs. 1.29, p=0.03) but did not differ for hip fractures (O/E: 4.52 vs. 3.56, p=0.43). Figure 2 shows goodness-of-fit of observed and expected fractures in HIV-infected and uninfected men. Of note, the accuracy of the modified-FRAX for HIV-infected men appears to worsen with higher estimated risk for major osteoporotic fractures. In the sensitivity analysis that limited the sample to those men who had data after 2010 (N=15876), the O/E ratios were similarly higher in HIV-infected (O/E=1.81; 95% CI: 1.56, 2.09) than uninfected men (O/E=1.22; 95% CI: 1.11, 1.35).
Table 2.
Accuracy of modified-FRAX in HIV-infected and uninfected men
| Calibration | ||||
|---|---|---|---|---|
| N | Modified-FRAX estimated 10-yr risk of fracture | O/E | 95% CI | |
| Major osteoporotic fracture | ||||
| All | 24451 | 2.8 ± 1.5% | 1.39 | 1.30, 1.48 |
| HIV− | 17387 | 2.7 ± 1.4% | 1.29 | 1.19, 1.40 |
| HIV+ | 7064 | 2.9 ± 1.5% | 1.62 | 1.45, 1.81 |
| Modified-FRAX calculated with HIV as cause of secondary osteoporosis | ||||
| HIV− | 17387 | 2.7 ± 1.4% | 1.29 | 1.19, 1.40 |
| HIV+ | 7064 | 3.8 ± 2.1% | 1.20 | 1.08, 1.34 |
| Modified-FRAX calculated with HCV as cause of secondary osteoporosis | ||||
| HIV− | 17387 | 2.8 ± 1.5% | 1.27 | 1.17, 1.37 |
| HIV+ | 7064 | 3.1 ± 1.6% | 1.48 | 1.33, 1.65 |
| Hip fracture | ||||
| All | 24451 | 0.3 ± 0.3% | 3.87 | 3.42, 4.40 |
| HIV− | 17387 | 0.2 ± 0.3% | 3.56 | 3.03, 4.18 |
| HIV+ | 7064 | 0.3 ± 0.4% | 4.52 | 3.68, 5.53 |
| Modified-FRAX calculated with HIV as cause of secondary osteoporosis | ||||
| HIV− | 17387 | 0.2 ± 0.3% | 3.56 | 3.03, 4.18 |
| HIV+ | 7064 | 0.5 ± 0.6% | 2.66 | 2.17, 3.26 |
| Modified-FRAX calculated with HCV as cause of secondary osteoporosis | ||||
| HIV− | 17387 | 0.2 ± 0.3% | 3.44 | 2.93, 4.04 |
| HIV+ | 7064 | 0.3 ± 0.4% | 3.87 | 3.16, 4.75 |
Figure 2. Accuracy of modified-FRAX in HIV-infected men.
Each panel shows a plot of the observed probability for major osteoporotic facture (error bars indicate the 95% confidence interval) versus the mean estimated fracture probability for the subgroup divided by the decile of estimated probability. The dotted line represents a perfectly calibrated model and solid line represent the best fit for HIV-uninfected men (a), HIV-infected men (b), and HIV-infected men when ‘secondary osteoporosis’ is entered into the FRAX calculation (The p values indicate the goodness of fit using the Hosmer-Lemeshow test (p<0.05 indicates a significant difference from the perfectly calibrated model).
Accuracy of modified-FRAX with consideration of HIV or HCV as a cause of secondary osteoporosis
We recalculated the O/E ratios for HIV-infected men using the modified-FRAX and including HIV as a cause of secondary osteoporosis. As a result, the O/E ratio among HIV-infected men decreased from 1.62 (95% CI: 1.45, 1.81) to 1.20 (95% CI: 1.08, 1.34) for major osteoporotic fractures and from 4.52 (95% CI: 3.68, 5.53) to 2.66 (95% CI: 2.17, 3.26) at the hip (Table 2). With this adjustment, the O/E ratios were closer to the O/E ratios in uninfected men: 1.29 (95% CI: 1.19, 1.40) at major osteoporotic sites and 3.56 (95% CI: 3.03, 4.18) at the hip.
When we recalculated the O/E ratios including HCV as a cause of secondary osteoporosis without consideration for HIV, the O/E ratio among HIV-infected men decreased from 1.62 (95% CI: 1.45, 1.81) to 1.48 (95% CI: 1.33, 1.65) for major osteoporotic fractures and from 4.52 (95% CI: 3.68, 5.53) to 3.87 (95% CI: 3.16, 4.75) for fracture at the hip (Table 2). The O/E ratios were also closer to the O/E ratios in uninfected men: 1.27 (95% CI: 1.17, 1.37) at major osteoporotic site and 3.44 (95% CI 2.93, 4.04) at the hip. Overall, the accuracy improved more with consideration of HIV than HCV as a cause of secondary osteoporosis in the modified-FRAX calculation.
Modified FRAX and thresholds for pharmacologic treatment
Since the clinical utility of FRAX is based upon accepted thresholds for pharmacologic intervention, we compared the sensitivity/specificity of the modified-FRAX for fracture prediction using accepted FRAX thresholds for pharmacologic interventions in HIV-infected and uninfected groups. For HIV-infected men, the modified-FRAX calculated was with HIV as cause of secondary osteoporosis. Since none met the threshold (>20%) for major osteoporotic fracture endorsed by the National Osteoporosis Foundation (NOF) 17, we utilized the age-specific thresholds for major osteoporotic fractures endorsed by European osteoporosis societies (6.3% to 13.4% in 50-70 year olds) 7. At the hip, we used the threshold (>3%) endorsed by the NOF 17. Using these thresholds, only 21/326 (6.4%) HIV-infected men with fractures at major osteoporotic sites and 3/93 (3.2%) at the hip were correctly predicted (Table 3). The sensitivity was similarly poor among uninfected men: only 16/609 (2.6%) with fractures at major osteoporotic sites and 0/148 (0%) at the hip were correctly predicted. These analyses were meant only to illustrate the test characteristics of the modified-FRAX. Use of a FRAX score with complete risk factors and/or with BMD may greatly improve sensitivity/specificity at these thresholds.
Table 3.
Sensitivity and specificity of modified-FRAX using pharmacologic intervention by HIV status
| HIV-uninfected | HIV-infected | |||||
|---|---|---|---|---|---|---|
| Major osteoporotic site | Major osteoporotic site | |||||
| Fracture | No fracture | Total | Fracture | No fracture | Total | |
| Modified FRAX ≥ age-specific intervention threshold | 16 | 90 | 106 | 21 | 91 | 112 |
| Modified FRAX < age-specific intervention threshold | 593 | 16688 | 17281 | 305 | 6647 | 6952 |
| 609 | 16778 | 17387 | 326 | 6738 | 7064 | |
|
Sensitivity = 16 / 609 = 2.6% Specificity = 16688 / 16778 = 99.5% |
Sensitivity = 21 / 326 = 6.4% Specificity = 6647 / 6738 = 98.6% |
|||||
| Hip | Hip | |||||
|---|---|---|---|---|---|---|
| Fracture | No fracture | Total | Fracture | No fracture | Total | |
| Modified FRAX ≥ 3% | 0 | 9 | 9 | 3 | 71 | 74 |
| Modified FRAX < 3% | 148 | 17230 | 17378 | 90 | 6900 | 6990 |
| 148 | 17239 | 17387 | 93 | 6971 | 7064 | |
|
Sensitivity = 0 / 148 = 0% Specificity = 17230 / 17239 = 99.9% |
Sensitivity = 3 / 93 = 3.2% Specificity = 6900 / 6971 = 99.0% |
|||||
FRAX threshold for pharmacologic intervention based on age-specific European guidelines for major osteoporotic fracture and National Osteoporosis Foundation guidelines from the United States for the hip 7,17. Note: Among HIV-infected men, modified-FRAX was calculated with HIV as a cause of secondary osteoporosis.
Sensitivity analyses for missing FRAX variables
When we re-calculated the modified-FRAX by entering “Yes” for the two variables that were not available in the VACS (parental history of hip fracture and secondary osteoporosis), it overestimated incident fracture rates for both HIV-infected and uninfected individuals at major osteoporotic sites (7.3±3.7% versus 7.0±3.5%, p<0.0001) and at the hip (0.7±1.0% versus 0.6±0.8%, p<0.0001). As a result, O/E ratios were lower with this modified-FRAX calculation. Accuracy was better in HIV-infected than uninfected men for major osteoporotic fractures (O/E: 0.63 vs. 0.50, p=0.009), but did not differ for hip fractures (O/E: 1.86 vs. 1.48, p=0.29). Using the modified-FRAX calculation with “Yes” for the two variables that were not available in the VACS, fracture prediction using pharmacologic thresholds improved only slightly. Only 14/93 (15%) of HIV-infected men and 9/148 (6.1%) of uninfected men with fractures at the hip were correctly predicted.
Discussion
In this study of 50-70 year old men, 10-year fracture rates calculated using modified-FRAX without BMD underestimated true fracture rates at major osteoporosis sites and the hip in HIV-infected compared with uninfected men. The accuracy of the modified-FRAX for HIV-infected men was improved when HIV was considered a cause of secondary osteoporosis in the calculation; however, it still underestimates observed fracture rates. Similarly, the accuracy of the modified-FRAX improved when HCV-infection was considered a cause of secondary osteoporosis, but to a lesser degree than with HIV infection.
The greatest limitation of this study was the fact we did not have data on two predictors (secondary cause of osteoporosis and family history of hip fracture) used in the FRAX calculator and therefore, have been careful to present our outcomes in terms of the use of a “modified-FRAX”. Fracture risk from causes of secondary osteoporosis may vary. Given the lack of sufficient fracture risk data for specific secondary osteoporosis conditions, Kanis et al. assigned the same risk ratio for presence of secondary osteoporosis as history of rheumatoid arthritis in the FRAX algorithm 6. Family history of hip fracture had the least weight among all clinical risk variables for determination of hip fracture, but had a strong weight in the determination of major osteoporotic fracture 6. Also, our sensitivity analyses in which the modified-FRAX was recalculated with “Yes” entered for both missing predictors revealed that fracture prediction was improved only slightly. Aside from the two missing variables, the other FRAX variables were approximated based upon availability in the database of health factors and ICD-9 coding, which may not accurately reflect thresholds utilized by FRAX. For example the ICD-9 code for alcohol use/abuse and ever use of glucocorticoids may not accurately capture the thresholds for exposure (3 or more units of alcohol per day and current or > 3months at a dose of prednisolone 5mg daily or more, respectively) validated in FRAX. Given these limitations, the accuracy of the modified-FRAX results in this study cannot be readily compared with test performance characteristics in other studies utilizing complete data for FRAX calculation. Similarly, it would be inappropriate to compare the sensitivity/specificity of modified-FRAX using pharmacologic treatment thresholds to other studies. The objective of this analysis was to compare the test characteristics for FRAX within this cohort of subjects, and not to compare the test characteristics with an external population or sample; therefore, we are of the opinion that our primary comparisons between HIV-infected and uninfected groups are valid.
Other factors may have also contributed to the poor performance of the modified-FRAX in both the HIV-infected and uninfected groups. FRAX models are calibrated to the epidemiology of death and fracture for each country with available data19. Our study was based entirely on a United States (US) population, and similar results were found using all eligible subjects as well as when the sample was restricted to those who were alive and not lost to follow up beyond the 10-year observation period for fracture. However, FRAX has been criticized for not accurately reflecting the fracture incidence and mortality rate for non-white study populations, since the US database for development of FRAX were derived from the Rochester cohort in Olmsted County Minnesota, which is predominantly white 20,21. Since over half of the VACS is non-white, accuracy of FRAX may not be comparable to its performance in other predominantly white populations. In addition, there may be risk factors related to fragility fractures that are prevalent in male veterans that are not captured in FRAX. Addition of BMD to FRAX may have improved the accuracy; however, the improvement in other studies is usually modest and is not always predictable. In an analysis of FRAX performance in a cohort of ambulatory men (MrOS study), Ettinger et al. found that FRAX without BMD overestimated observed incidence of fracture (O/E ratios 0.7-0.9) and the addition of BMD did not improve the calibration (O/E ratios 0.7-1.1), nor did it consistently improve discrimination 22. Several smaller studies in HIV-infected individuals have found that FRAX calculated with the addition of BMD underestimated prevalent fractures9,10; however none have evaluated prediction of incident fracture.
In the majority of published cohort studies, HCV infection is associated with increased fracture risk in both HIV-infected and uninfected individuals, and HCV infection remains a significant predictor of fracture risk after adjustments in multivariate 23-26 or stratified analyses 11,27. From a meta-analysis, HIV/HCV-coinfection increases risk of fragility fracture by 77% over HIV-monoinfection 28. Therefore, it is not surprising that the modified-FRAX improves when HCV is considered a cause of secondary osteoporosis. Since the FRAX algorithm does not adjust for risk from multiple causes of secondary osteoporosis, the underestimation of risk in HIV/HCV-coinfected is likely to be much more than that of HIV or HCV monoinfection. For the modified FRAX, the O/E ratio in HIV/HCV-coinfected individuals was 2.20 (95% CI: 1.84, 2.63) for major osteoporotic sites and 6.63 (95% CI: 4.71, 9.33) at the hip. This finding may be unique to this cohort with such a high prevalence of HCV-coinfection, and will require corroboration in other populations.
Our analyses demonstrate that the modified-FRAX is a poor case-finding tool for HIV infected men using the pharmacologic therapy thresholds for HIV-infected men ages 50-70, even when considering HIV-infection as a cause of secondary osteoporosis in the calculation. The sensitivity of the modified-FRAX in this situation was only 3% at the hip using the NOF guidelines and did not improve with the age-specific thresholds endorsed by the European guidelines. Our findings cannot be generalized to suggest that the actual FRAX with all clinical factors available would be a poor case-finding tool in HIV-infected men. However, despite these caveats, our data suggest we that cannot assume that FRAX would be a good case-finding tool in the HIV-infected population, and additional studies are necessary to assure that widespread implementation is indicated.
Aside from lacking complete data on clinical risk factors for FRAX calculation, use of approximated variables for the clinical risk factors and lack of BMD data, there were other limitations to this analysis. Fracture events were identified by ICD-9 codes; however, fragility fracture codes have been validated previously in VACS, demonstrating excellent agreement between the administrative codes and chart review. In addition, our study was only conducted in men, which limits the generalizability of our results for women.
Conclusion
FRAX is a readily available calculator of fracture risk that can be utilized in HIV-infected individuals. In HIV-infected individuals, fracture estimates calculated using FRAX without BMD likely underestimates true fracture risk. The accuracy can be improved when HIV is considered a cause of secondary osteoporosis in the FRAX calculation; however, it still may be a poor case-finding tool for HIV-infected men between ages 50-70. Given the increased fracture risk among HIV-infected individuals, dietary and lifestyle modifications, antiretroviral modifications, and screening DXAs are indicated in higher-risk older individuals29. However, further studies are necessary to determine the role of FRAX in screening and risk-stratification for pharmacologic therapy in older HIV-infected individuals.
Acknowledgements
We would like to thank Melissa Skanderson for her help in data acquisition and Dr. Ethel Siris for reviewing the manuscript.
Funding
This work was supported by the National Institutes of Health: AHRQ (R01-HS018372), NIAAA (U24-AA020794, U01-AA020790, U01-AA020795, U01-AA020799, U24-AA022001, U24 AA022007, U10 AA013566-completed), NHLBI (R01-HL095136; R01-HL090342), NIAID (U01-A1069918), NIMH (P30-MH062294), NIDA (R01DA035616), NCI (R01 CA173754) and the Veterans Health Administration Office of Research and Development (VA REA 08-266, VA IRR Merit Award) and Office of Academic Affiliations (Medical Informatics Fellowship). Additional funding from the National Institutes of Health included: NIAID (R01 AI096089, R01 HD073977) and NINR (K01 NR013437)
Source of Funding: MTY has received honoraria from Gilead Sciences and Abbvie Pharmaceuticals for work as a consultant.
Veterans Aging Cohort Study funded by: National Institute on Alcohol Abuse and Alcoholism (U10 AA 13566) and VHA Public Health Strategic Health Core Group.
Footnotes
Conflicts of Interest: The other authors have no conflicts to declare.
References
- 1.Young B, Dao CN, Buchacz K, Baker R, Brooks JT. Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000-2006. Clin Infect Dis. 2011 Apr 15;52(8):1061–1068. doi: 10.1093/cid/ciq242. [DOI] [PubMed] [Google Scholar]
- 2.Hansen AB, Gerstoft J, Kronborg G, et al. Incidence of low- and high-energy fractures in persons with and without HIV-infection: a Danish population-based cohort study. Aids. Nov 16. 2011 doi: 10.1097/QAD.0b013e32834ed8a7. [DOI] [PubMed] [Google Scholar]
- 3.Womack JA, Goulet JL, Gibert C, et al. Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PloS one. 2011;6(2):e17217. doi: 10.1371/journal.pone.0017217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2014 Jan;58(1):1–10. doi: 10.1093/cid/cit757. [DOI] [PubMed] [Google Scholar]
- 5.European AIDS Clinical Society Treatment Guidelines. 2015; Version 8.0. 2015 http://www.eacsociety.org/guidelines/eacs-guidelines/eacs-guidelines.html.
- 6.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2008 Apr;19(4):385–397. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanis JA, McCloskey EV, Johansson H, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2013 Jan;24(1):23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.NOF. National Osteoporosis Foundation Clinician's Guide to Prevention and Treatment of Osteoporosis. 2014 doi: 10.1007/s00198-014-2794-2. http://www.nof.org/professionals/Clinicians_Guide.htm, 2014. [DOI] [PMC free article] [PubMed]
- 9.Calmy A, Fux CA, Norris R, et al. Low bone mineral density, renal dysfunction, and fracture risk in HIV infection: a cross-sectional study. The Journal of infectious diseases. 2009 Dec 1;200(11):1746–1754. doi: 10.1086/644785. [DOI] [PubMed] [Google Scholar]
- 10.Pepe J, Isidori AM, Falciano M, et al. The combination of FRAX and Ageing Male Symptoms scale better identifies treated HIV males at risk for major fracture. Clinical endocrinology. 2012 Nov;77(5):672–678. doi: 10.1111/j.1365-2265.2012.04452.x. [DOI] [PubMed] [Google Scholar]
- 11.Lo Re V, 3rd, Volk J, Newcomb CW, et al. Risk of hip fracture associated with hepatitis C virus infection and hepatitis C/human immunodeficiency virus coinfection. Hepatology. 2012 Nov;56(5):1688–1698. doi: 10.1002/hep.25866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong HV, Cortes YI, Shiau S, Yin MT. Osteoporosis and fractures in HIV/hepatitis C virus coinfection: a systematic review and meta-analysis. Aids. 2014 Jun 28; doi: 10.1097/QAD.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fultz SL, Skanderson M, Mole LA, et al. Development and verification of a “virtual” cohort using the National VA Health Information System. Medical care. 2006 Aug;44(8 Suppl 2):S25–30. doi: 10.1097/01.mlr.0000223670.00890.74. [DOI] [PubMed] [Google Scholar]
- 14.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977 Mar;33(1):159–174. [PubMed] [Google Scholar]
- 15.Womack JA, Goulet JL, Gibert C, et al. Physiologic frailty and fragility fracture in HIV- infected male veterans. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2013 May;56(10):1498–1504. doi: 10.1093/cid/cit056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007 Feb 20;115(7):928–935. doi: 10.1161/CIRCULATIONAHA.106.672402. [DOI] [PubMed] [Google Scholar]
- 17.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2014 Oct;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The 'rmap' package [computer program] 2013 [Google Scholar]
- 19.Kanis JA, Oden A, Johansson H, McCloskey E. Pitfalls in the external validation of FRAX. Osteoporosis international : a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2012 Feb;23(2):423–431. doi: 10.1007/s00198-011-1846-0. [DOI] [PubMed] [Google Scholar]
- 20.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clinic proceedings. 1996 Mar;71(3):266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 21.Donaldson MG, Palermo L, Schousboe JT, Ensrud KE, Hochberg MC, Cummings SR. FRAX and risk of vertebral fractures: the fracture intervention trial. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2009 Nov;24(11):1793–1799. doi: 10.1359/jbmr.090511. [DOI] [PubMed] [Google Scholar]
- 22.Ettinger B, Ensrud KE, Blackwell T, et al. Performance of FRAX in a cohort of community-dwelling, ambulatory older men: the Osteoporotic Fractures in Men (MrOS) study. Osteoporos Int. 2013 Apr;24(4):1185–1193. doi: 10.1007/s00198-012-2215-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin MT, Shi Q, Hoover DR, et al. Fracture incidence in HIV-infected women: results from the Women's Interagency HIV Study. Aids. 2010 Nov 13;24(17):2679–2686. doi: 10.1097/QAD.0b013e32833f6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young B, Dao CN, Buchacz K, Baker R, Brooks JT, Investigators HIVOS Increased rates of bone fracture among HIV-infected persons in the HIV Outpatient Study (HOPS) compared with the US general population, 2000-2006. Clinical Infectious Diseases. 2011;52(8):1061–1068. doi: 10.1093/cid/ciq242. [DOI] [PubMed] [Google Scholar]
- 25.Collin F, Duval X, Le Moing V, et al. Ten-year incidence and risk factors of bone fractures in a cohort of treated HIV1-infected adults. Aids. 2009 May 15;23(8):1021–1024. doi: 10.1097/QAD.0b013e3283292195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maalouf N, Zhang S, Drechsler H, Brown G, Tebas P, Bedimo R. Hepatitis C co-infection and severity of liver disease as risk factors for osteoporotic fractures among HIV-infected patients. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2013 May 15; doi: 10.1002/jbmr.1988. [DOI] [PubMed] [Google Scholar]
- 27.Hansen AB, Gerstoft J, Kronborg G, et al. Incidence of low and high-energy fractures in persons with and without HIV infection: a Danish population-based cohort study. Aids. 2012;26(3):285–293. doi: 10.1097/QAD.0b013e32834ed8a7. [DOI] [PubMed] [Google Scholar]
- 28.Dong HV, Cortes YI, Shiau S, Yin MT. Osteoporosis and fractures in HIV/hepatitis C virus coinfection: a systematic review and meta-analysis. Aids. 2014 Sep 10;28(14):2119–2131. doi: 10.1097/QAD.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown TT, Hoy J, Borderi M, et al. Recommendations for Evaluation and Management of Bone Disease in HIV. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015 Apr 15;60(8):1242–1251. doi: 10.1093/cid/civ010. [DOI] [PMC free article] [PubMed] [Google Scholar]