Abstract
Assembly of the synaptonemal complex (SC) in Drosophila depends on two independent pathways defined by the chromosome axis proteins C(2)M and ORD. Because C(2)M encodes a Kleisin-like protein and ORD is required for sister chromatid cohesion, we tested the hypothesis that these two SC assembly pathways depend on two cohesin complexes. Through single and double mutant analysis to study the mitotic cohesion proteins Stromalin (SA) and Nipped-B (SCC2) in meiosis, we provide evidence that there are at least two meiosis-specific cohesin complexes. One complex depends on C(2)M, SA and Nipped-B. Despite the presence of mitotic cohesins SA and Nipped-B, this pathway has only a minor role in meiotic sister centromere cohesion and is primarily required for homolog interactions. C(2)M is continuously incorporated into pachytene chromosomes even though SC assembly is complete. In contrast, the second complex, which depends on meiosis-specific proteins SOLO, SUNN and ORD is required for sister chromatid cohesion, localizes to the centromeres, and is not incorporated during prophase. Our results show that the two cohesin complexes have unique functions and are regulated differently. Multiple cohesin complexes may provide the diversity of activities required by the meiotic cell. For example, a dynamic complex may allow the chromosomes to regulate meiotic recombination, and a stable complex required may be required for sister-chromatid cohesion.
Keywords: cohesin, synaptonemal complex, meiosis, chromosome segregation, cohesion
Introduction
Meiosis I begins with the pairing of homologous chromosomes that are held together by the synaptonemal complex (SC) [1, 2]. The SC may regulate meiotic recombination through interactions with the chromosome axis, which interacts directly with the chromatin [3–5]. A crucial component of the axis is cohesin-related proteins. Mitotic cohesin is made up of four subunits: SMC1, SMC3, Stromalin (SA) and the Kleisin Rad21. However, multiple meiosis-specific cohesin complexes have been described in a variety of organisms [6, 7]. The function and properties of these meiosis-specific cohesin complexes, however, are poorly understood. Our mutant analysis in Drosophila has shown that SC initiation depends on two independent pathways defined by two sets of cohesin-related genes [8]. One pathway depends on C(2)M, a Kleisin family protein [9] that physically interacts with the cohesin SMC3 [10]. The second pathway depends on ORD, a cohesion protein that is not conserved [11]. SC assembly is absent in a c(2)M ord double mutant or oocytes lacking SMC1 and SMC3 [8], suggesting that cohesin complexes dependent on either C(2)M or ORD are required for all pathways of SC assembly.
Besides SMC1 and SMC3, the Drosophila cohesin subunits that associate with C(2)M are not known. Recent studies have suggested Stromalin has a role in maintaining the SC and cohesion late in meiotic prophase, but its role in SC assembly is not known [12]. We have examined the function of all known Drosophila cohesin subunits in SC assembly. Our data are consistent with a model that SA and the SCC2 homolog Nipped-B function in a pathway with C(2)M, while ORD functions in a distinct meiosis-specific cohesin pathway with two other proteins, SOLO and SUNN [13, 14].
These two groups of proteins differ not only in their function, but also their loading properties. We have discovered that C(2)M is exchanged during prophase, with subunits being added to and dissociating from the chromosomes throughout pachytene. In contrast, SOLO and SUNN at the centromeres are probably loaded only during premeiotic S-phase. The dissociation of cohesin complexes from meiotic chromosomes, and the failure to replace them, may be a contributing factor to the maternal age effect [15–17]. Our results modify this model by demonstrating that each cohesin complex is regulated differently.
Results
Stromalin is required for SC assembly
To test the hypothesis that C(2)M is part of a cohesin complex required for SC assembly, we investigated the role of other cohesin proteins in SC assembly. In addition to SMC1 and SMC3, the Drosophila mitotic cohesin complex includes the Kleisin, Rad21 (SCC1), encoded by the verthandi (vtd) gene, Stromalin (SCC3), encoded by the SA gene and the cohesin loader Nipped-B (SCC2). All of these proteins are essential; therefore, to generate oocytes lacking each protein, shRNAs were expressed using P{GAL4∷VP16-nos.UTR}CG6325MVD1 (herein referred to as MVD1) for germline-specific RNAi [18] (Figure S1A). The effect on SC assembly was assayed by examining transverse filament protein C(3)G localization in the germarium of the ovary [19]. Within a wild-type germarium, zygotene and early pachytene are in region 2a, pachytene then progresses as the oocytes move into region 2b and region 3. Knockdown of vtd (Rad21) did not have any effects on SC assembly (Figure S2), consistent with another report using a different method [20]. In contrast, in SA or Nipped-B RNAi oocytes, SC assembly was incomplete (Figure 1B,C). C(3)G was observed at the centromere, as shown by colocalization with the centromere histone H3 CID, and at several sites in the euchromatin, but there was an absence of C(3)G threads, showing that SC assembly in oocytes lacking SA or Nipped-B did not progress beyond zygotene.
Figure 1. Stromalin and Nipped-B are required for synapsis.
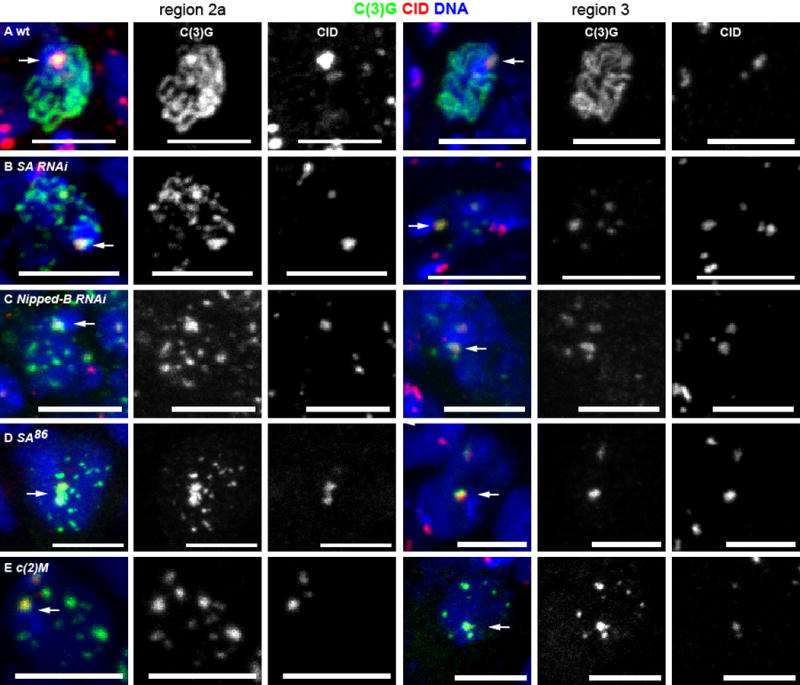
SC assembly in A) wild-type, B) SA RNAi, C) Nipped-B RNAi, D) SA86 germline clone and E) c(2)M mutant oocytes. Meiotic prophase begins in region 2a, with zygotene and early pachytene oocytes, with later pachytene stages in regions 2b and 3. Shown are representative oocytes in region 2a and region 3, with C(3)G (green) and centromere histone CID (red) and DNA (blue). An arrow indicates synapsis at the centromeres as shown by colocalization of C(3)G and CID. The scale bars = 5μm. See also Figure S1 and Figure S3.
C(2)M localization was absent in SA or Nipped-B RNAi oocytes (Figure 2B, Figure S2C), suggesting that the SC assembly defects could be related to a lack of C(2)M. In fact, the SC assembly defect in SA or Nipped-B RNAi was similar to the phenotype we previously observed with c(2)M mutants, where there were about 6–8 C(3)G patches per oocyte [8] (Figure 1B,C,E, Figure S3). Among all SA RNAi oocytes in the germarium, there was an average of 5.8 euchromatic SC patches per oocyte. However, between region 2a and 3 of the germarium, there was a significant decrease in the number of C(3)G patches in SA and Nipped-B RNAi oocytes (Figure 1B,C, Figure 2G,H, Figure S3). The average number of C(3)G patches in SA RNAi oocytes decreased from 10.5 (n=257) in region 2a to 2.4 (n=16) in region 3, and 43% had only one C(3)G patch that was always at the centromeres. These results suggest that SC assembled along the arms in the absence of SA or Nipped-B is unstable. In c(2)M mutants, the number of C(3)G patches did not decrease ([8], Figure 1E, Figure S3). These results show that the phenotype of SA and Nipped-B RNAi oocytes is similar to c(2)M mutants, but with some minor quantitative differences.
Figure 2. Cohesin localization in cohesin mutants and RNAi knockdowns.
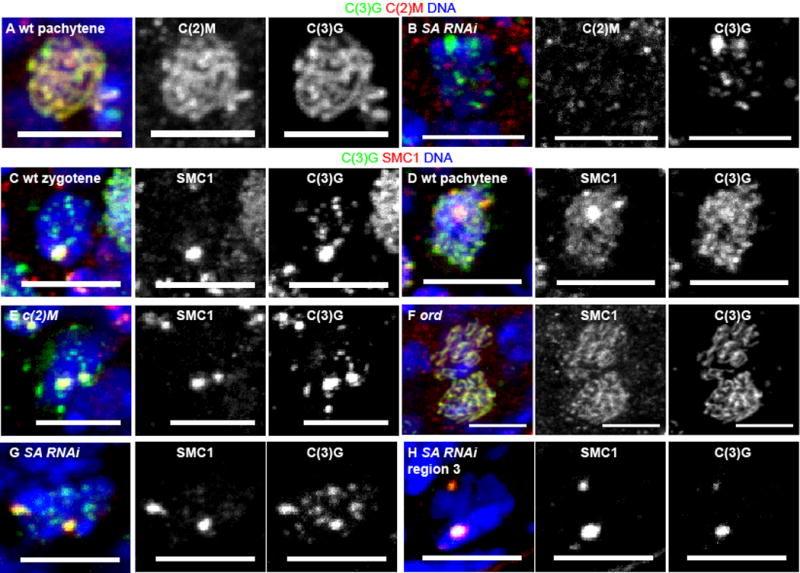
C(2)M (red) localization in (A) wild-type and (B) SA RNAi oocytes with C(3)G in green and DNA in blue. C-H) SMC1 (red) localization and SC assembly with C(3)G (green). The regions of brightest C(3)G and SMC1 are the centromeres (Figure 1) [8, 21, 24]. Zygotene (C) in wild-type is observed in some germaria in the most anterior (earliest) region 2a oocytes. Most of the germarium, region 2a, 2b and 3, contains pachytene (A,D) oocytes. The c(2)M (E) and ord (F) mutant oocytes and SA RNAi (G) oocytes are from the region 2a–2b where pachytene is expected in wild-type. The SA RNAi oocyte in H is from region 3. In all images, the scale bar = 5 μm. See also Figure S2.
The SC phenotype of SA RNAi was compared to a null mutant of SA (Figure S1B). The SA86 germline clone null mutant oocytes had patches of C(3)G in region 2a (avg. = 5.6, n=27) that was similar to the RNAi of SA. This decreased in region 3 to an average of 1.6 patches, which always included the centromeres (n=11) (Figure 1D). The results show that the RNAi closely resembles that of the SA null mutant phenotype.
SMC1 on the chromosome arms but not the centromeres depends on SA
To determine if SA and Nipped-B are required for loading of cohesins, we examined SMC1 localization. In wild type pachytene cells with complete SC formation, SMC1 was found at the centromeres and along the chromosome arms in threads (Figure 2D). In c(2)M mutants, where SC assembly was incomplete, SMC1 was observed at the centromere, and not in a threadlike pattern along the chromosome arms (Figure 2E). Conversely, in ord mutants, SMC1 was observed on the chromosome arms (Figure 2F). These observations are similar to a previous study by Khetani and Bickel [21]. In the c(2)M ord double mutant, in which all C(3)G is absent, SMC1 was also absent (Figure S2F). In SA or Nipped-B RNAi oocytes, SMC1 localization to the arms was almost absent (Figure 2G,H, Figure S2G), which was similar to c(2)M mutants and not ord. Based on C(3)G localization at the centromeres (Figure 1), it is likely that the SMC1 remaining in SA or Nipped-B RNAi oocytes is primarily at the centromeres. These results show that SMC1 at the centromere is dependent on ORD while SMC1 on the arms is dependent on C(2)M and SA. SMC1 localization was not affected by vtd RNAi (Figure S2H).
SA functions in the same pathway as C(2)M but not ORD or SUNN
To determine if the SC observed in SA or Nipped-B knockdown oocytes depends on either ORD or C(2)M, we expressed SA or Nipped-B shRNA in ord or c(2)M mutants. Oocytes mutant for c(2)M and with RNAi to either SA or Nipped-B had patches of C(3)G at the centromere and in the euchromatin that was similar to the c(2)M single mutant (Figure 3, compare B–D to G and I). Like SA RNAi, however, the number of C(3)G patches in SA RNAi c(2)M oocytes significantly decreased between region 2a and region 3 (Figure 3H, Figure S3). A similar but not significant decrease was observed in Nipped-B RNAi c(2)M (Figure 3J, Figure S3). The absence of a more severe phenotype in these experiments suggests that SA, Nipped-B and C(2)M function in the same pathway to promote sites of SC initiation in late zygotene and the completion of synapsis.
Figure 3. SA and C(2)M function in the same SC assembly pathway while ORD and SUNN function in germline mitosis.
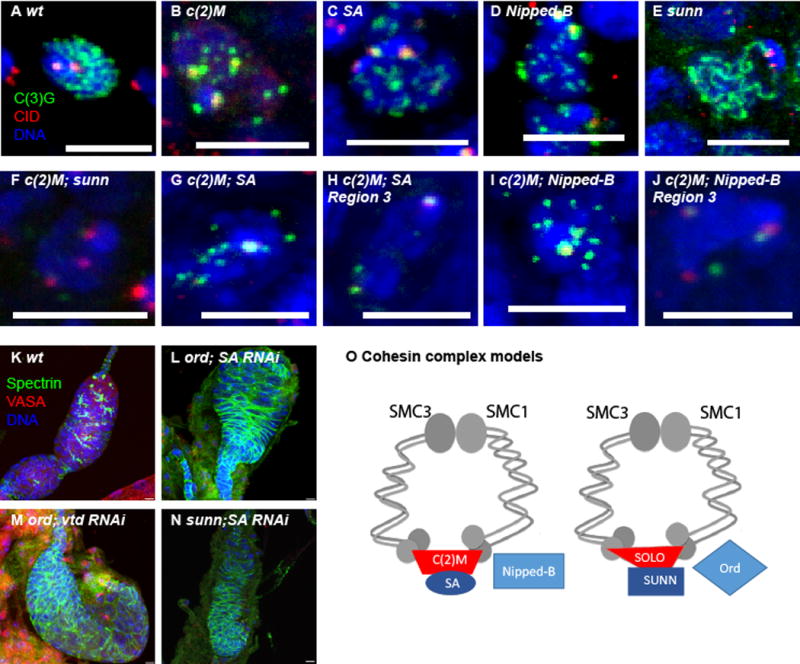
A) SC assembly in wild-type region 2a oocyte. B–E) SC assembly in representative region 2a of single mutant or RNAi oocytes. F–J) SC assembly in c(2)M; sunn double mutant or a c(2)M mutant also expressing RNAi for SA or Nipped-B. Each image shows a representative oocyte from region 2a (F,G,I) or region 3 (H,J). SC protein C(3)G is in green and centromere protein CID is in red. K–N) Lower magnification images showing ovary development in wild-type or females lacking two cohesins. Females are ord or sunn mutants also expressing RNAi for SA or vtd. The germline cells are shown by VASA (red) while the somatic follicle cells are shown with Spectrin (green). The absence of VASA expressing cells shows a failure of germline mitosis. In all images, the DNA is in blue and the scale bars = 5μm. O) Model for the two meiotic cohesin complexes that function in SC assembly and crossing over. See also Figure S3.
When SA shRNA was expressed in the germline of an ord mutant, the result was a surprisingly more severe phenotype: the ovaries were rudimentary in size and lacked development of 16 cell cysts with oocytes. The same result was observed when vtd shRNA was expressed in the germline of ord mutants. These small ovaries were examined for VASA and Spectrin expression, which mark germline and somatic cells, respectively [22]. The small ovaries had no VASA expressing cells, showing they lacked germ cells (Figure 3L, M). These results suggest that ORD has a function that is independent of other cohesin subunits such as vtd and SA. They also show that ORD has a redundant function in the germline mitotic divisions, as has been suggested previously [23].
We repeated these experiments with sunn mutants, which has a meiotic phenotype similar to ord [13]. The c(2)M; sunn double mutant had no evidence of SC assembly, similar to the c(2)M ord results (Figure 3F). However, when SA shRNA was expressed in a sunn mutant, we observed the same absent germ line phenotype as SA RNAi ord mutant oocytes (Figure 3N). Thus, like ORD, SUNN has a role in germline mitosis that is redundant with SA. The synergism between the two groups of genes is consistent with a two cohesin pathway model in the germline. In germline mitosis, SUNN/SOLO/ORD and the canonical SA/RAD21/Nipped-B cohesin pathways function redundantly in cohesion. In meiosis, this changes to SUNN/SOLO/ORD and SA/C(2)M/Nipped-B that are the active cohesin pathways and they are not redundant (Figure 3O).
SA depletion does not result in sister centromere cohesion defects at meiosis I
Mutations in ord or sunn have cohesion defects in female meiosis [13, 14]. To determine if SA is required for cohesion in meiosis, we used two cytological assays in SA RNAi oocytes. First, we looked for defects in sister centromere cohesion at metaphase I in stage 14 oocytes using the centromere marker CENP-C. In wild-type, eight CENP-C foci are expected, one for each chromosome, and we observed an average of 7.1 (Figure 4A,E). In sunn mutant oocytes, which is required for sister chromatid cohesion [13], there was a significantly elevated number of CENP-C foci relative to wild-type or SA-knockdown oocytes (avg. = 11.1, Figure 4D, E). In SA RNAi oocytes, the number of CENP-C foci was not significantly greater than wild-type, suggesting that SA is not required for sister centromere cohesion (avg = 7.2, Figure 4B, E). Similarly, the number of CENP-C foci was not significantly increased in SA germline clone oocytes (avg. = 6.9, Figure 4C, E). These results suggest that SUNN, but not SA, is required for sister centromere cohesion at meiosis I.
Figure 4. Oocytes lacking SA maintain sister-centromere cohesion.
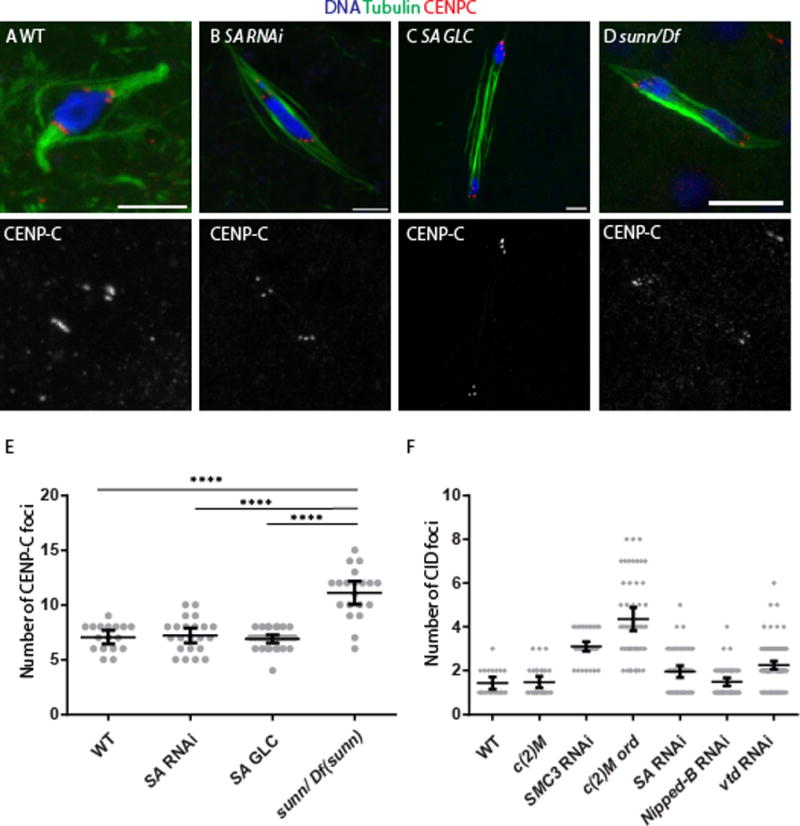
Mature Stage 14 oocytes, which is where metaphase I occurs, from (A) wild type, (B) SA RNAi, (C) SA germ line clone and (D) a sunn mutant are shown with tubulin in green and CENP-C in red to label the centromeres and the DNA in blue. In some cases, precocious anaphase was observed, which is when the karyosome has separated into two groups of chromosomes that appear to be moving towards the poles. Precocious anaphase was elevated in SA RNAi (60%, n=33), SA germ line clones (15%, n=27) and the sunn mutant (33%, n=30). This phenotype can be caused by a failure in arm cohesion or reduced crossing [29, 47], and because all these mutants or RNAi are expected to reduce crossing over, this phenotype is not necessarily indicative of a cohesion defect. The scale bars are 5 μm. (E) A dot plot showing the number of CENP-C foci in each stage 14 oocyte. Wild-type, and the two SA genotypes are significantly different than sunn, as shown by a Mann-Whitney test. The horizontal and vertical lines show the mean and 95% confidence limits. The number of CENP-C foci expected for wild-type is eight, although less could be observed due to overlap of signals. (F) A dot plot showing the number of CID foci in each pachytene oocyte. The SMC3 RNAi and the c(2)M ord double mutant are significantly different than all the other genotypes (p<0.001) by a Mann-Whitney test. The horizontal and vertical lines show the mean and 95% confidence limits.
Second, centromere clustering was examined during pachytene of meiosis. During pachytene, all 8 centromeres are usually found in one or two clusters. We and others previously demonstrated that ord, sunn and solo, but not c(2)M, mutants, have defects in centromere clustering [8, 13, 14, 24]. Similarly, SMC3 RNAi and c(2)M ord oocytes had clustering defects, with a significant number of oocytes with more than 2 CID foci (Figure 4F). While there may be a slight increase in the number of CID foci in SA or Nipped-B RNAi oocytes compared to wild-type or c(2)M, they were significantly less (p < 0.001) than SMC3 RNAi or ord c(2)M (Figure 4F).
C(2)M is integrated into the SC throughout pachytene
To determine if the two cohesin pathways are under different temporal regulation, we expressed cohesin subunits with a pulse of heat shock and determined when they could be incorporated into the meiotic chromosomes. C(2)M fused to an HA epitope tag (UASp-c(2)M3XHA) was expressed under the control of a heat shock promoter. If C(2)M can only be incorporated at a specific time in meiosis, and because oocytes are arranged in temporal order, then localization of heat-shock induced C(2)M would be observed in a restricted region of the germarium (e.g. region 2a). If C(2)M can be incorporated at multiple stages of meiosis, then localization of heat-shock induced C(2)M would be observed at many or all regions of the germarium (e.g. regions 2a, 2b and 3).
With fixation one hour after heat shock, no C(2)M-HA was detected (Figure S4). With fixation at 6 hours after heat shock, however, C(2)M-HA localization was detected as threads in almost 100% of region 2a, 2b and 3 oocytes, consistent with loading occurring at all stages of meiotic pachytene (Figure 5A, Figure S5A). Since six hours is not enough time for a cyst to change position within the germarium, most of the oocytes were in pachytene at the time of heat shock and incorporated C(2)M-HA. At 24 hours after heat shock, some early region 2a oocytes did not incorporate C(2)M-HA (Figure 5B, Figure S5B). These oocytes could have been premeiotic at the time of heat shock. Consistent with this conclusion, the number of oocytes that did not incorporate C(2)M-HA increased as the time between heat shock and fixation was increased (Figure S4). These results were used to estimate the duration of each stage in the germarium.
Figure 5. Meiotic cohesin protein C(2)M is incorporated throughout pachytene.
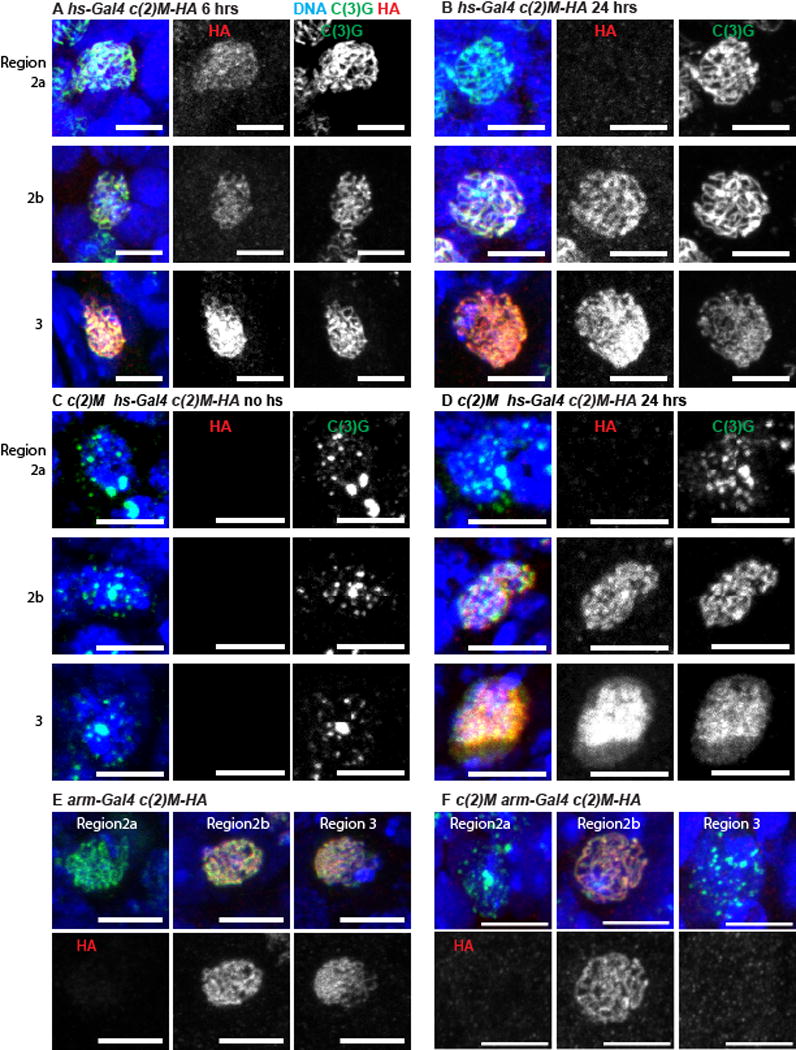
(A–D) C2M-HA was induced with a 1 hour heat shock and then fixed 6 or 24 hours later. In all images, the DNA is in blue, C(3)G is in green, C(2)M-HA is in red and the scale bar = 5 μm. Interpreting these experiments takes advantage of the germarium organization, which contains approximately seven 16-cell cysts, each containing an oocyte. The cysts are arranged in temporal order, where the more posterior oocytes (e.g region 3) have been in pachytene longer than more anterior ones (e.g. region 2a) and it takes about 12–24 hours for a cyst to move down one position (Figure S4). All oocytes carried the UASp-c(2)M3XHA transgene either in a wild-type (A,B) or in a c(2)M mutant (C,D) background. C(2)M-HA was induced with a 1 hour heat shock and then fixed 6 (A) or 24 (B,D) hours later, or without heat shock (C). (E,F) Oocytes from the indicated region with C(2)M-HA induced by arm-GAL4 in either wild type (E) or a c(2)M mutant (F) background. See also Figure S4 and Figure S5.
Heat shock induced expression of C(2)M was repeated in a c(2)M mutant background to determine if cohesins could be loaded and the SC assembled late. In the absence of heat shock, the typical patchy SC phenotype of c(2)M mutants was observed (Figure 5C, Figure S5C). Following heat shock, full length SC (with C(2)M and C(3)G in threads) was observed in region 2a, 2b and 3 oocytes (Figure 5D, Figure S5D). This suggests that, even if the SC is not assembled early in prophase due to the absence of C(2)M, assembly can resume if C(2)M becomes available later. The newly assembled SC in region 3 of the germarium appeared diffuse and disorganized, suggesting that late SC assembly was abnormal (Figure 5D).
To determine if heat shock induced c(2)M is functional, nondisjunction was measured. Because oocytes are laid in the order they develop, the effect of heat shock at successive stages of oogenesis could be measured. Heat shock induced expression partially reduced the nondisjunction frequency in the eggs laid 7–10 days after heat shock (Figure 6, Table S1). These oocytes were in the germarium, likely region 2a, at the time of heat shock [25]. These results can be explained by proposing that C(2)M must be loaded during a narrow window of time during early prophase to properly organize the SC and support crossing over. Rescue of the nondisjunction phenotype was not complete, suggesting that a single pulse of C(2)M is not sufficient, or there is a narrow window within the 7–10 day time point where C(2)M is required.
Figure 6. Rescue of nondisjunction defect by heat shock-induced expression of C(2)M.
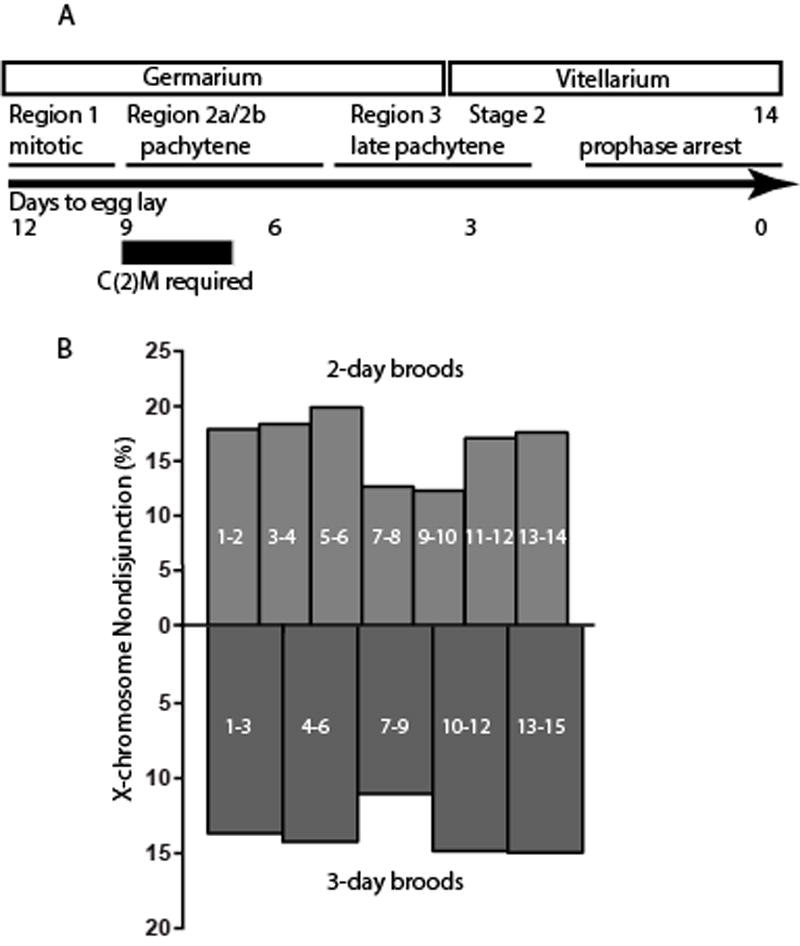
A) Schematic showing the timeline of oocyte development [48, 49]. B) Nondisjunction in c(2)M−; UASp-c(2)M3XHA/hs-Gal4 females measured following 1 hour of heat shock and the number of days indicated within each bar. Significant reductions in nondisjunction were observed in the 6–9 (p=0.003) day period of the 3-day broods and the 7–8 (p=0.003) and 9–10 (p=0.02) periods of the 2-day broods. Nondisjunction was calculated and statistical significance determined as described [50]. The data is in Table S1. See also Figure S5.
To directly test the effect of adding C(2)M late, we used the armadillo (arm) promoter to express C(2)M [18]. In females carrying the combination of arm- GAL4 and UASp-c(2)M3XHA, C(2)M-HA protein expression was delayed in region 2a (Figure 5E, Figure S5E). While C(3)G and C(2)M normally appear simultaneously, with arm-GAL4, C(2)M appeared later than the first C(3)G. Furthermore, arm-GAL4 controlled expression of the c(2)M transgene did not rescue the nondisjunction phenotype of a c(2)M mutant, in contrast to MVD1 controlled expression (Table S1) [9]. Indeed, when C(2)M was expressed using arm-GAL4; UASp-c(2)M3XHA in c(2)M mutant females, there was a delay in the appearance of SC threads, with more zygotene nuclei in region 2a than typical for wild type (Figure 5F, Figure S5F). These results suggest the timing of C(2)M expression and SC assembly is critical; the SC must assemble early in region 2a in order to promote crossing over.
C(2)M is also removed from the SC throughout pachytene
When c(2)M was expressed using arm-GAL4, C(2)M was present in region 2b and absent in some (9/17, Figure 5F, Figure S5E) but present in other (8/17, Figure 5) region 3 oocytes. The loss of C(2)M in region 3 oocytes corresponds to the known decrease in arm-GAL4 expression in region 2b and 3 [18]. Thus, these results show that there was complete turnover of SC-associated C(2)M between region 2b and region 3 oocytes, which is approximately 12–24 hours. Another promoter that expresses for a limited time is bam-GAL4:VP16, which peaks in 8-cell cysts of region 1 but usually extends in early region 2a [26]. When combined with the UASp-c(2)M3XHA transgene, C(2)M-HA expression was observed early in region 2a but absent in all region 2b or 3 cysts (n=7 germaria) (Figure S5G). These experiments show that C(2)M is unloaded from chromosomes during pachytene.
Centromere cohesins SUNN and SOLO are not integrated during pachytene
The heat shock protocol was repeated with UASp transgenes encoding Venus-tagged SUNN [13]. When UASp-sunn.Venus was expressed with MVD1, foci of SUNN at the centromeres were observed at all stages (Figure 7A, n=3, Figure S6A). In contrast to C(2)M, however, SUNN was not detectable in most pachytene oocytes 24 hours after heat shock induced expression of UASp-sunn.Venus (Figure S6B, n=10). In four of the 10 germaria, however, SUNN foci were visible in early region 2a (Figure 7B). The absence of foci in most pachytene oocytes, including all oocytes in late region 2a, region 2b and 3, suggests that centromeric SUNN is not loaded during pachytene. Similar results were found with SOLO [14] (Figure S6C,D). Furthermore, when we dissected and fixed ovaries 5 days following heat shock to allow premeiotic cells to enter meiosis, region 2b centromeric SUNN was observed in in 5 out of 9 germaria (Figure 7C, D). These results show that SUNN at the centromeres can only be loaded during a narrow window of meiosis, which is at or earlier than when foci were observed (region 2a) at the 24 hour time point. In germaria where no SUNN was observed, it is likely there were no oocytes that could load SUNN at the time of heat shock.
Figure 7. Centromeric cohesin protein SUNN is not incorporated during meiotic pachytene.
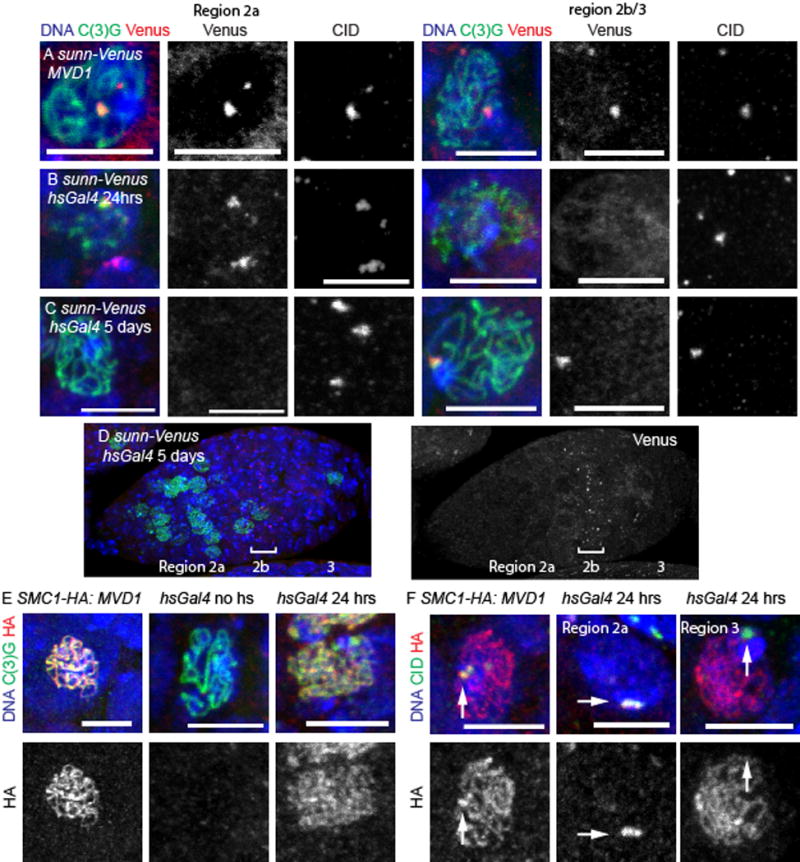
(A–D) Oocytes are shown in merged image with SUNN-Venus in red, C(3)G in green and DNA in blue. CID is shown only in a single channel image. SUNN-Venus was expressed constitutively with using MVD1 (A) or was induced with a 1 hour heat shock and then fixed 24 (B) or 5 days later (C,D). The whole germarium in panel D shows a band of SUNN-Venus foci in region 2b (indicated by a bracket). (E) SMC1-HA (red) induced with MVD1 or expressed with 1 hour of heat shock and fixed 24 hours later. C(3)G is in green. (F) SMC1-HA (red) induced with MVD1 or expressed with 1 hour of heat shock and fixed 24 hours later. CID marks the centromeres in green (arrows). In all images, the DNA is in blue and the scale bar = 5 μm. Whole germarium images of each genotype is in Figure S6.
If these observations reflect the loading properties of cohesin complexes, then SMC1 or SMC3 should follow the patterns of both C(2)M and SUNN. Using a UASp-SMC13XHA transgene, SMC1 that colocalizes with C(3)G at the centromeres and along the chromosome arms was observed throughout pachytene when expression was controlled by MVD1 (Figure 7E, Figure S6E). When SMC1 expression was induced with heat shock and fixed 24 hours later, both foci and threads were observed, although at different times (Figure 7E, Figure S6F,G). In 8/16 germaria there was thread-like SMC1 in regions 2b and 3, and all of these oocytes lacked SMC1 centromere foci (Figure 7F). The thread-like localization of SMC1 in some region 2b and 3 oocytes indicates that SMC1 is loaded during pachytene. It is unclear why the thread-like SMC1 is not observed in all germaria. Because the SMC1 signal in immunofluorescence experiments is weaker than C(2)M, it is possible there is a detection problem. Alternatively, SMC1 may not be loaded onto the arms during early pachytene. In 12/16 germaria, there was SMC1 foci in region 2a that colocalized with CID (Figure 7F). This restricted appearance is similar to heat-shock induced SUNN and may reflect loading that only occurs early, such as during meiotic S-phase. These region 2a cells with bright heat shock induced SMC1 foci always lacked threads, showing that the centromeric and euchromatic SMC1 proteins are loaded at different times in meiosis.
Discussion
The two complexes are directed towards either sister or homolog interactions
Based on similar mutant phenotypes and double mutant analysis, we propose that SC assembly in Drosophila depends on two meiotic cohesin complexes. The first includes C(2)M, SA and Nipped-B. The most important function of C(2)M/SA/Nipped-B is SC assembly, which is demonstrated by the more significant SC assembly defects observed in c(2)M mutants and SA or Nipped-B knockdowns compared to sunn, solo or ord mutants. Our cytological results suggest that, like c(2)M [9], SA has only a minor role in meiotic sister-centromere cohesion. Correlating with this difference is that C(2)M, SA or Nipped-B are required for the accumulation of SMC proteins on the chromosome arms but not the centromeres [21]. Furthermore, Nipped-B, like C(2)M, localizes to the chromosome arms but not the centromeres [8, 27]. These observations indicate a significant change in cohesin regulation. While SA and Nipped-B are required for sister chromatid cohesion in mitotic cells, they have a new partner, C(2)M, for a non-cohesion function in meiosis. There are minor differences in the c(2)M and SA phenotypes, which has also been observed with solo and sunn [13], suggesting there could be additional minor complexes. SA and Nipped-B could be required to maintain sister chromatid cohesion on the chromosome arms in late prophase, a function that C(2)M likely does not have [12].
The second proposed meiotic cohesin complex includes SOLO, SUNN and ORD, which are also highly diverged, making homology assignments difficult. Based on sequence features, SUNN may be a SA homolog [13] while SOLO has been shown to interact with SMC1 and to have sequence motifs similar to the SMC1 interaction domains of Kleisins [14, 28]. The role of ORD in this context is unclear. It is possible that ORD is a positive regulator like Nipped-B. Genetic evidence shows that ord, sunn and solo are required for sister chromatid cohesion, which correlates with SMC1/3 and SC accumulation at the centromeres [13, 14, 29]. In addition, there are elevated levels of sister chromatid exchange and abnormal SC structure in ord and solo mutants [11, 14].
Surprisingly, we found an important role for the meiotic cohesins SUNN and ORD in mitotic germline cells, which is consistent with prior observations that ORD localizes to centromeric foci in premeiotic cells [21] and ord mutants have defects in mitotically dividing germline cells [23]. Since Rec8 in C. elegans is also observed in premeiotic cells [30, 31], it may be a conserved feature of meiotic cohesins required for sister chromatid cohesion that they accumulate and function in premeiotic mitotic germline cells.
Differential regulation and dynamics of meiotic cohesins
A striking difference between C(2)M/SA and SOLO/SUNN is that C(2)M incorporation is continuous during pachytene even after the SC is fully assembled. This may result in a dynamic SC, which has been observed in budding yeast [32]. Paradoxically, we have also found that C(2)M must load during a narrow window of early prophase in order to support crossover formation. Cohesins have been shown to be loaded during prophase in a number of systems [7] including Drosophila [33–35]. In contrast, centromeric SOLO/SUNN can only be loaded prior to meiotic prophase. Sister-chromatid cohesion in mitotic cells is established during S-phase [36] and in mammals, Rec8 cohesin cannot be replenished and dissociates with age [15, 16, 37]. Whereas in mitotic cells the dynamic and stable cohesin complexes involve the same four core subunits, in meiosis, there may be separate cohesin complexes that differ in their regulation and capacity to be replaced or replenished. These observations complement Weng et al. [12] who showed there was cohesin replenishment during meiotic prophase, although after pachytene and possibly not at the centromeres. Interestingly, mouse Nipbl (Nipped-B) [38] and the meiosis specific SMC1β [39] show pronounced accumulation starting at leptotene, indicating that, as in Drosophila, some mouse cohesins are loaded during pachytene while other cohesins are stable.
Evolutionary conservation and function of multiple cohesin complexes
Meiosis-specific cohesin complexes appear to be a highly conserved feature of meiosis [7, 40–43]. While there is some variation in the constituents of each cohesin complex, our results suggesting two major pathways contributing to SC assembly help explain the results with coh-3/coh-4 and rec-8 in C. elegans [31] or rad21l and rec8 in mouse [44]. Only the double mutants in each case eliminate all SC. The role of the respective Kleisins could also be conserved. Like C(2)M, Rad21L has been proposed to be primarily responsible for inter-homolog chromosome interactions [45, 46]. Multiple cohesin complexes may be required because some cohesins need to be loaded at a specific time (S phase for cohesion) while others need to be exchanged during pachytene. A dynamic cohesin complex may be important to provide plasticity to the meiotic chromosomes and allow them to respond to DSBs and regulate crossover formation, crossover interference and chromosome segregation. Alternatively, different cohesin complexes may accumulate at different locations. If meiotic cohesins, directly or indirectly, interact with SC proteins, they may have a strong influence on the pattern of SC assembly and influence the frequency and distribution of DSBs and crossovers.
Supplementary Material
Acknowledgments
We thank Li Nguyen for technical assistance; Scott Hawley, Gary Karpen, Christian Lehner and Claudio Sunkel for providing antibodies; Oren Schuldiner for an SMC1 clone; Sarah Radford for helpful comments on the manuscript. We thank the TRiP at Harvard Medical School for providing transgenic RNAi fly stocks used in this study. Fly stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were also used in this study. M.G. was funded by a Busch Predoctoral Fellowship and V.A. was funded by an Aresty Foundation Summer Research Fellowship. This work was supported by NIH grant GM101955 to K.S.M.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization, K.S.M. and B.D.M.; Investigation, M.RG., K.B.M., V.A., B.K. and E.J.; Writing – Original Draft, K.S.M; Writing – Review & Editing, K.S.M., M.R.G, B.K. and B.D.M.; Funding Acquisition, K.S.M. and B.D.M.; Supervision, K.S.M. and B.D.M..
References
- 1.Lake CM, Hawley RS. The molecular control of meiotic chromosomal behavior: events in early meiotic prophase in Drosophila oocytes. Annu Rev Physiol. 2012;74:425–451. doi: 10.1146/annurev-physiol-020911-153342. [DOI] [PubMed] [Google Scholar]
- 2.Bhalla N, Dernburg AF. Prelude to a division. Annu Rev Cell Dev Biol. 2008;24:397–424. doi: 10.1146/annurev.cellbio.23.090506.123245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehrotra S, McKim KS. Temporal analysis of meiotic DNA double-strand break formation and repair in Drosophila females. PLoS Genet. 2006;2:e200. doi: 10.1371/journal.pgen.0020200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler KR, Gutierrez-Velasco S, Martin-Castellanos C, Smith GR. Protein determinants of meiotic DNA break hot spots. Mol Cell. 2013;49:983–996. doi: 10.1016/j.molcel.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zickler D, Kleckner N. Recombination, Pairing, and Synapsis of Homologs during Meiosis. Cold Spring Harb Perspect Biol. 2015;7 doi: 10.1101/cshperspect.a016626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jessberger R. Cohesin complexes get more complex: the novel kleisin RAD21L. Cell Cycle. 2011;10:2053–2054. doi: 10.4161/cc.10.13.15802. [DOI] [PubMed] [Google Scholar]
- 7.Rankin S. Complex elaboration: making sense of meiotic cohesin dynamics. FEBS J. 2015 doi: 10.1111/febs.13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanneti NS, Landy K, Joyce EF, McKim KS. A Pathway for Synapsis Initiation during Zygotene in Drosophila Oocytes. Curr Biol. 2011;21:1852–1857. doi: 10.1016/j.cub.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Manheim EA, McKim KS. The Synaptonemal Complex Component C(2)M Regulates Meiotic Crossing over in Drosophila. Curr Biol. 2003;13:276–285. doi: 10.1016/s0960-9822(03)00050-2. [DOI] [PubMed] [Google Scholar]
- 10.Heidmann D, Horn S, Heidmann S, Schleiffer A, Nasmyth K, Lehner CF. The Drosophila meiotic kleisin C(2)M functions before the meiotic divisions. Chromosoma. 2004;113:177–187. doi: 10.1007/s00412-004-0305-5. [DOI] [PubMed] [Google Scholar]
- 11.Webber HA, Howard L, Bickel SE. The cohesion protein ORD is required for homologue bias during meiotic recombination. J Cell Biol. 2004;164:819–829. doi: 10.1083/jcb.200310077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng KA, Jeffreys CA, Bickel SE. Rejuvenation of Meiotic Cohesion in Oocytes during Prophase I Is Required for Chiasma Maintenance and Accurate Chromosome Segregation. PLoS Genet. 2014;10:e1004607. doi: 10.1371/journal.pgen.1004607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krishnan B, Thomas SE, Yan R, Yamada H, Zhulin IB, McKee BD. Sisters Unbound Is Required for Meiotic Centromeric Cohesion in Drosophila melanogaster. Genetics. 2014 doi: 10.1534/genetics.114.166009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan R, McKee BD. The Cohesion Protein SOLO Associates with SMC1 and Is Required for Synapsis, Recombination, Homolog Bias and Cohesion and Pairing of Centromeres in Drosophila Meiosis. PLoS Genet. 2013;9:e1003637. doi: 10.1371/journal.pgen.1003637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiang T, Duncan FE, Schindler K, Schultz RM, Lampson MA. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr Biol. 2010;20:1522–1528. doi: 10.1016/j.cub.2010.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tachibana-Konwalski K, Godwin J, van der Weyden L, Champion L, Kudo NR, Adams DJ, Nasmyth K. Rec8-containing cohesin maintains bivalents without turnover during the growing phase of mouse oocytes. Genes Dev. 2010;24:2505–2516. doi: 10.1101/gad.605910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jessberger R. Age-related aneuploidy through cohesion exhaustion. EMBO Rep. 2012;13:539–546. doi: 10.1038/embor.2012.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rorth P. Gal4 in the Drosophila female germline. Mech Dev. 1998;78:113–118. doi: 10.1016/s0925-4773(98)00157-9. [DOI] [PubMed] [Google Scholar]
- 19.Page SL, Hawley RS. c(3)G encodes a Drosophila synaptonemal complex protein. Genes Dev. 2001;15:3130–3143. doi: 10.1101/gad.935001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Urban E, Nagarkar-Jaiswal S, Lehner CF, Heidmann SK. The cohesin subunit Rad21 is required for synaptonemal complex maintenance, but not sister chromatid cohesion, during Drosophila female meiosis. PLoS Genet. 2014;10:e1004540. doi: 10.1371/journal.pgen.1004540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khetani RS, Bickel SE. Regulation of meiotic cohesion and chromosome core morphogenesis during pachytene in Drosophila oocytes. J Cell Sci. 2007;120:3123–3137. doi: 10.1242/jcs.009977. [DOI] [PubMed] [Google Scholar]
- 22.Santos AC, Lehmann R. Germ cell specification and migration in Drosophila and beyond. Curr Biol. 2004;14:R578–589. doi: 10.1016/j.cub.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki WY, Orr-Weaver TL. Sister-chromatid misbehavior in Drosophila ord mutants. Genetics. 1992;132:1047–1061. doi: 10.1093/genetics/132.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeo S, Lake CM, Morais-de-Sa E, Sunkel CE, Hawley RS. Synaptonemal complex-dependent centromeric clustering and the initiation of synapsis in Drosophila oocytes. Curr Biol. 2011;21:1845–1851. doi: 10.1016/j.cub.2011.09.044. [DOI] [PubMed] [Google Scholar]
- 25.Bhagat R, Manheim EA, Sherizen DE, McKim KS. Studies on crossover specific mutants and the distribution of crossing over in Drosophila females. Cytogenetic and Genome Res. 2004;107:160–171. doi: 10.1159/000080594. [DOI] [PubMed] [Google Scholar]
- 26.Mathieu J, Cauvin C, Moch C, Radford SJ, Sampaio P, Perdigoto CN, Schweisguth F, Bardin AJ, Sunkel CE, McKim K, et al. Aurora B and cyclin B have opposite effects on the timing of cytokinesis abscission in Drosophila germ cells and in vertebrate somatic cells. Dev Cell. 2013;26:250–265. doi: 10.1016/j.devcel.2013.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gause M, Webber HA, Misulovin Z, Haller G, Rollins RA, Eissenberg JC, Bickel SE, Dorsett D. Functional links between Drosophila Nipped-B and cohesin in somatic and meiotic cells. Chromosoma. 2008;117:51–66. doi: 10.1007/s00412-007-0125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan R, Thomas SE, Tsai JH, Yamada Y, McKee BD. SOLO: a meiotic protein required for centromere cohesion, coorientation, and SMC1 localization in Drosophila melanogaster. J Cell Biol. 2010;188:335–349. doi: 10.1083/jcb.200904040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bickel SE, Orr-Weaver TL, Balicky EM. The sister-chromatid cohesion protein ORD is required for chiasma maintenance in Drosophila oocytes. Curr Biol. 2002;12:925–929. doi: 10.1016/s0960-9822(02)00846-1. [DOI] [PubMed] [Google Scholar]
- 30.Pasierbek P, Jantsch M, Melcher M, Schleiffer A, Schweizer D, Loidl J. A Caenorhabditis elegans cohesion protein with functions in meiotic chromosome pairing and disjunction. Genes Dev. 2001;15:1349–1360. doi: 10.1101/gad.192701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Severson AF, Ling L, van Zuylen V, Meyer BJ. The axial element protein HTP-3 promotes cohesin loading and meiotic axis assembly in C. elegans to implement the meiotic program of chromosome segregation. Genes Dev. 2009;23:1763–1778. doi: 10.1101/gad.1808809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voelkel-Meiman K, Moustafa SS, Lefrancois P, Villeneuve AM, MacQueen AJ. Full-length synaptonemal complex grows continuously during meiotic prophase in budding yeast. PLoS Genet. 2012;8:e1002993. doi: 10.1371/journal.pgen.1002993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gause M, Misulovin Z, Bilyeu A, Dorsett D. Dosage-sensitive regulation of cohesin chromosome binding and dynamics by Nipped-B, Pds5, and Wapl. Mol Cell Biol. 2010;30:4940–4951. doi: 10.1128/MCB.00642-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham MD, Gause M, Cheng Y, Noyes A, Dorsett D, Kennison JA, Kassis JA. Wapl antagonizes cohesin binding and promotes Polycomb-group silencing in Drosophila. Development. 2012;139:4172–4179. doi: 10.1242/dev.084566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eichinger CS, Kurze A, Oliveira RA, Nasmyth K. Disengaging the Smc3/kleisin interface releases cohesin from Drosophila chromosomes during interphase and mitosis. EMBO J. 2013;32:656–665. doi: 10.1038/emboj.2012.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peters JM, Nishiyama T. Sister chromatid cohesion. Cold Spring Harb Perspect Biol. 2012;4 doi: 10.1101/cshperspect.a011130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burkhardt S, Borsos M, Szydlowska A, Godwin J, Williams SA, Cohen PE, Hirota T, Saitou M, Tachibana-Konwalski K. Chromosome Cohesion Established by Rec8-Cohesin in Fetal Oocytes Is Maintained without Detectable Turnover in Oocytes Arrested for Months in Mice. Curr Biol. 2016;26:678–685. doi: 10.1016/j.cub.2015.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuleszewicz K, Fu X, Kudo NR. Cohesin loading factor Nipbl localizes to chromosome axes during mammalian meiotic prophase. Cell Div. 2013;8:12. doi: 10.1186/1747-1028-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biswas U, Wetzker C, Lange J, Christodoulou EG, Seifert M, Beyer A, Jessberger R. Meiotic cohesin SMC1β provides prophase I centromeric cohesion and is required for multiple synapsis-associated functions. PLoS Genet. 2013;9:e1003985. doi: 10.1371/journal.pgen.1003985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fukuda T, Fukuda N, Agostinho A, Hernández-Hernández A, Kouznetsova A, Höög C. STAG3-mediated stabilization of REC8 cohesin complexes promotes chromosome synapsis during meiosis. EMBO J. 2014;33:1243–1255. doi: 10.1002/embj.201387329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hopkins J, Hwang G, Jacob J, Sapp N, Bedigian R, Oka K, Overbeek P, Murray S, Jordan PW. Meiosis-specific cohesin component, Stag3 is essential for maintaining centromere chromatid cohesion, and required for DNA repair and synapsis between homologous chromosomes. PLoS Genet. 2014;10:e1004413. doi: 10.1371/journal.pgen.1004413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Winters T, McNicoll F, Jessberger R. Meiotic cohesin STAG3 is required for chromosome axis formation and sister chromatid cohesion. EMBO J. 2014;33:1256–1270. doi: 10.1002/embj.201387330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitajima TS, Yokobayashi S, Yamamoto M, Watanabe Y. Distinct cohesin complexes organize meiotic chromosome domains. Science. 2003;300:1152–1155. doi: 10.1126/science.1083634. [DOI] [PubMed] [Google Scholar]
- 44.Llano E, Herrán Y, García-Tuñón I, Gutiérrez-Caballero C, de Álava E, Barbero JL, Schimenti J, de Rooij DG, Sánchez-Martín M, Pendás AM. Meiotic cohesin complexes are essential for the formation of the axial element in mice. J Cell Biol. 2012;197:877–885. doi: 10.1083/jcb.201201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ishiguro K, Kim J, Shibuya H, Hernández-Hernández A, Suzuki A, Fukagawa T, Shioi G, Kiyonari H, Li XC, Schimenti J, et al. Meiosis-specific cohesin mediates homolog recognition in mouse spermatocytes. Genes Dev. 2014;28:594–607. doi: 10.1101/gad.237313.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J, Hirano T. RAD21L, a novel cohesin subunit implicated in linking homologous chromosomes in mammalian meiosis. J Cell Biol. 2011;192:263–276. doi: 10.1083/jcb.201008005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McKim KS, Jang JK, Theurkauf WE, Hawley RS. Mechanical basis of meiotic metaphase arrest. Nature. 1993;362:364–366. doi: 10.1038/362364a0. [DOI] [PubMed] [Google Scholar]
- 48.Spradling AC. In: Developmental genetics of oogenesis In The Development of Drosophila melanogaster. Bate M, Arias AM, editors. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1993. pp. 1–70. [Google Scholar]
- 49.Jia D, Xu Q, Xie Q, Mio W, Deng WM. Automatic stage identification of Drosophila egg chamber based on DAPI images. Sci Rep. 2016;6:18850. doi: 10.1038/srep18850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeng Y, Li H, Schweppe NM, Hawley RS, Gilliland WD. Statistical analysis of nondisjunction assays in Drosophila. Genetics. 2010;186:505–513. doi: 10.1534/genetics.110.118778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


