Abstract
Background
Neutrophil gelatinase-associated lipocalin (NGAL) is expressed in neutrophils and involved in innate immunity by sequestering iron. NGAL's ability to complex with matrix metalloproteinase-9 (MMP-9) and extend its gelatinolytic activity led us to investigate its role in pregnancies complicated by preterm birth (PTB) and intra-amniotic infection/inflammation (IAI).
Methods
We assayed the amniotic fluid (AF) levels of NGAL and MMP-9 in 308 women that had a clinically indicated amniocentesis and a normal pregnancy outcome or PTB. qRT-PCR was employed to determine NGAL mRNA expression of placental villous trophoblast and amniochorion. Immunohistochemistry was used for cellular localization.
Results
AF NGAL levels were gestational age-regulated. Women with IAI and PTB had significantly higher levels of NGAL, MMP-9 and NGAL•MMP-9 complex.
Conclusion
The amniochorion is a source of NGAL and similarly to other inflammatory conditions this protein may augment the collagenolytic effect of MMP-9 and modulate host-microbe interactions in pregnancies complicated by IAI.
1. INTRODUCTION
Premature rupture of membranes (PPROM) and intra-amniotic infection are common causes of PTBs.1 Intra-amniotic infection and inflammation (IAI) provoke a cascade of events that initiate neutrophil chemotaxis, activation, and infiltration resulting in synthesis and release of metalloproteases.2,3
Matrix metallopeptidase-9 (MMP-9) belongs to the zinc-metalloproteinases family and plays a pivotal role in extracellular matrix degradation.4 Elevated expression and activity of MMP-9 has been identified in fetal membranes of women with PPROM and preterm labor. 5 Production of MMP-9 has been identified in amnion epithelial, placental trophoblast and chorion cells. MMP-9s ability to form a complex with neutrophil gelatinase-associated lipocain (NGAL), has been recently identified as an important modulator of MMP-9 preventing its auto-degradation thereby increasing its proteolytic activity.6,7
NGAL also known as lipocalin-2 is expressed by human neutrophils and various epithelial cells in response to inflammation, ischemia and neoplasia.8,9 It participates in innate immunity through an antimicrobial defensive mechanism, by sequestering catecholate-type bacterial siderophores and limiting bacterial growth.10 The role of NGAL in inflammation has been demonstrated by the numerous growth factors and cytokines that have been identified as positive regulators of NGAL.11 While the jury is still out as of how binding of MMP-9 to NGAL impacts on NGAL's bacteriostatic activity in vivo, data from cancer literature suggests that elevated levels of NGAL•MMP-9 complex associate with aggressive tumor phenotypes, drug resistance and poor survival.12
Existence and levels of NGAL in amniotic fluid (AF) in relationship with AF infection, inflammation and/or PPROM has been incompletely studied. The few reports that explored NGAL expression in reproductive tissues in vivo were limited to either term pregnancies13 or to small numbers of PPROM cases leading to inconclusive evidence with respect to AF NGAL levels.14 As far as we know, a direct assessment of NGAL•MMP-9 complex in AF of pregnancies complicated by PTB has not yet been conducted.
Here we evaluated the levels of NGAL, MMP-9, and NGAL•MMP-9 complex in pregnancies complicated by IAI leading to PTB along with their systematic regulation by gestational age (GA). We further provided direct insight into the source of AF NGAL by employing immunohistochemistry and quantitative polymerase chain reaction of placenta and fetal membranes.
2. MATERIALS AND METHODS
2.1. Patients and amniotic fluid collection
Using a prospective cohort study design, we investigated AF samples from 308 women pregnant with singletons who had a clinically indicated amniocentesis for the purpose of 2nd trimester genetic karyotyping (GA, median [range]: 19 [17-20] weeks, n=23); 3rd trimester fetal lung maturity testing (GA: 36 [35-37] weeks, n=25) or to rule-out AF infection in women who had preterm labor contractions refractory to tocolysis, preterm premature rupture of membranes (PPROM) or advanced cervical dilatation (3 cm) (GA: 29 [25-31] weeks, n=260).
GA was determined based on last menstrual period confirmed by an ultrasound examination prior to 20 weeks.15 Preterm labor was defined as documented cervical effacement and/or dilatation in patients <37 weeks of gestation with the presence of regular uterine contractions. PPROM was confirmed by an amniocentesis-dye positive test or collection of vaginal AF that was positive for “pooling”, “nitrazine”, “ferning”. Idiopatic PTB was established on women who delivered <37 weeks in the absence of intra-amniotic infection and histological chorioamnionitis. Exclusion criteria were presence of viral hepatitis infections or human immunodeficiency virus, anhydramnios, abnormal karyotype or congenital anomalies.
All women were recruited at Yale New Haven Hospital (YNHH) and were followed prospectively until delivery. The Human Investigation Committee of Yale University approved the study protocol. All patients provided written informed consent.
2.2. Chemical and microbiological studies of the amniotic fluid
Following retrieval, under sterile conditions, AF was analyzed by the YNHH clinical and microbiological laboratories for glucose concentration, white blood cell (WBC) count, lactate dehydrogenase (LDH) activity, Gram stain and cultured for aerobic and anaerobic bacteria, including Ureaplasma and Mycoplasma species. These results were available to the clinical team for management of the case and final cultures were reported 5 days after culturing. An AF glucose cut-off of ≤15 mg/dL, an LDH level ≥419 U/L, a positive Gram stain and/or culture result were considered suggestive of intra-amniotic infection. The remaining AF was transported to the research laboratory, spun at 3,000g at 4°C for 20 min., aliquoted in polypropylene cryotubes and stored at −80°C until analysis.
2.3. Enzyme-linked immunosorbent assays of human amniotic fluid NGAL, MMP-9, NGAL•MMP-9 complex and IL-6
NGAL, MMP-9 and NGAL/MMP-9 complex (R&D Systems, Minneapolis, MN), immunoassays were performed according to manufacturers’ instructions by investigators unaware of the clinical presentation. The minimal detectable concentration for NGAL, MMP-9 and NGAL•MMP-9 complex was 0.012ng/mL, 0.156ng/mL and 0.013ng/mL, respectively. The inter- and intra-assay coefficients of variation were <10%. The molar ratio of NGAL•MMP-9 was calculated based on 1:1 stoichiometry.
2.4. Western blotting for NGAL and MMP-9
We used AF samples of a select group of consecutively enrolled preterm women with (n=10) and without (n=10) severe IAI. Western blotting was performed on AF (5 μl/lane) mixed 1:2 with either reducing or non-reducing Laemmli sample buffer (Bio-Rad) and applied to 10% SDS-PAGE gels. After electrophoretic transfer, nitrocellulose membranes were blocked with 5% milk and then incubated overnight at 4°C with either rat monoclonal anti-NGAL or goat polyclonal anti-MMP-9 antibody (R&D Systems, 1:1000, Minneapolis, MN). Detection was performed using appropriate horseradish peroxidase-linked secondary antibodies and chemiluminescence (ECL-Plus, Amersham Biosystems). Optical density of the bands of interest (NGAL: ~25 kDa15, NGAL•MMP-9 complex: ~135 kDa15) was analyzed with Image J software (NIH, http:\rsb.info.nih.gov).
2.5. Histologic evaluation of the placenta and fetal membranes
Placental tissues were available for all patients included in this analysis. Hematoxylin and eosin-stained sections of the amniochorion membranes and umbilical cord were read by a perinatal pathologist unaware of the results of the MR score, fetal outcome or umbilical cord blood analyses. Each section was examined systematically for the presence or absence of inflammation and funisitis was diagnosed when neutrophils infiltrated the umbilical vessels walls or Wharton's jelly. Three histological stages of chorioamnionitis were complemented by the histological grading system devised by Salafia et. al., which includes four grades of inflammation of the amnion, chorion-decidua and umbilical cord.16,17
2.6. Mass spectrometry of amniotic fluid to assess for IAI severity
An AF proteomic fingerprint was generated using Surface Enhanced Laser Desorbtion Ionization Time-of-Flight (SELDI-TOF) mass spectrometry as previously described.18 The MR (Mass Restricted) score was generated based on 4 proteomic biomarkers: defensin-2, defensin-1, S100A12 (calgranulin C) and S100A8 (calgranulin A). The MR score ranges from 0 to 4, depending upon the presence or absence of each of the 4 protein biomarkers. A value of 1 was assigned if a biomarker peak was present and 0 if absent. The MR score represents the sum of the present biomarkers and was used to stratify the population as follows: MR=0 (absent IAI), MR=1-2 (mild IAI) and MR=3-4 (severe IAI). Due to previously demonstrated correlations between levels of IAI severity and adverse pregnancy outcomes, women with absent and mild IAI were analyzed together grouped as MR=0-2.19 Scorings of the AF SELDI-TOF tracings were performed without knowledge of the maternal outcome, results of the placental histological examination, or interleukin (IL)-6 levels. The results of the MR score were not used for patient clinical management.
2.7. Immunohistochemistry for NGAL and MMP-9
Five μm paraffin sections of fetal membranes and villous trophoblast were deparaffinized in xylene and rehydrated with graded ethanol to potassium-phosphate-buffered saline solution, pH 7.2. Following antigen retrieval with basic solution buffer pH 10, the sections were pretreated with 1% hydrogen peroxide for 15 minutes followed by one-hour incubation in 5% donkey serum. The sections were then incubated overnight at 4°C with rat monoclonal anti-NGAL and goat polyclonal anti-MMP-9 (R&D Systems, 1:100 dilution) antibodies. Detection was performed with biotinylated donkey anti-rat or donkey anti-goat IgG (Jackson ImmunoResearch, West Grove, PA, 1:600 dilution) as appropriate followed by avidin-biotin staining (Vectastain Elite ABC, Vector Laboratories, Burlingame, CA) and incubated with 3,3′-diaminobenzidine/nickel sulfate as chromogen solution. Specificity of staining was confirmed by omitting the primary antibodies. Specific staining was evaluated semi-quantitatively in a blinded fashion by examining six fields per slide and subjectively scoring on a scale from 0 (no staining) to 5 (intense blue-black staining) the intensity of the chromogen deposited in the amniochorion and placenta.
2.8. Quantitative real-time PCR procedures and primer sequences
Immediately after delivery, tissues (placental villous trophoblast, amniochorion membranes) from women who delivered preterm in the setting of absent IAI (MR=0, n=12) or severe IAI (MR=3-4, n=16) were frozen in liquid nitrogen and kept at −80°C for mRNA studies. Pathologic examination of the absent IAI tissues showed no evidence of histological chorioamnionitis. There was no significant difference in GA at delivery between the negative and the positive IAI groups. Additionally, tissues were obtained from a group of healthy, term, non-laboring women (n=7, GA: 38-40 weeks), undergoing scheduled elective cesarean delivery for indications such as fetal malpresentation or prior Cesarean birth. All term cases had reassuring fetal heart rate patterns prior to surgery.
Total RNA was isolated using TRIzol ® Reagent (Invitrogen Life Technologies, Carlsbad, CA) with homogenization, followed by chloroform separation, isopropanol precipitation and rehydration with nuclease free water. Reverse transcription was carried out with Superscript ® II Reverse Transcriptase (Invitrogen Life Technologies, Carlsbad, CA) using oligo (deoxythymidine) primers to synthesize first strand complementary DNA (cDNA). The following TaqMan Gene Expression Assays (Applied Biosystems, Foster City, CA) were used for qRT-PCR: Hs00194353_m1 (NGAL); Hs00234579_m1 (MMP-9); Hs00265497_m1 (RPL30); and Hs99999907_m1 (B2M). Each 20 μl reaction consisted of 1 μl cDNA (500 ng), 1 μl of TaqMan Gene Expression Assay, 10 μl TaqMan Fast Advanced Master Mix (Applied Biosystems, Foster City, CA), and 8 μl of nuclease free water. Amplifications were performed on the StepOnePlus Real-Time PCR System (Applied Biosystems, Foster City, CA).
2.9. Statistical analysis
Statistical analyses were performed with Sigma Stat, version 2.03 (SPSS Inc., Chicago, IL) and MedCalc (Broekstraat, Belgium) statistical software. Normality testing was performed using the Shapiro-Wilk test. Data were compared with Mann-Whitney Rank Sum test, 1-way ANOVA followed by Holm-Sidak tests (parametric) or Kruskal-Wallis ANOVA on ranks followed by Dunn's tests (non-parametric). Statistical analysis of the immunoassays data was performed after logarithmic transformation of data. Spearman correlations were used to measure co-linearity between the selected independent variables. Comparisons between proportions were done with Chi-square tests. P <0.05 was considered significant throughout the analysis.
3. RESULTS
3.1. Clinical and laboratory characteristics of the study population
Demographic and outcome characteristics of the women at amniocentesis are presented in Table 1. Women who had a genetic amniocentesis had a greater maternal age, and similar to those who had lung maturity testing, more often delivered at >37 weeks. PPROM and clinical chorioamnionitis were encountered only in the group of women who had an amniocentesis to rule-out infection.
Table I.
Demographic clinical and outcome characteristics of the women who provided AF samples for NGAL, MMP-9 and NGAL•MMP-9 complex (n=308).
| Variable | 2nd trimester genetic amniocenteses n = 23 | 3rd trimester lung maturity amniocenteses n = 25 | Rule-out infection amniocenteses n = 260 | P value |
|---|---|---|---|---|
| Characteristics at amniocentesis | ||||
| Maternal age, years, † | 34 [30 – 39] | 30 [26 – 34] | 27 [22 – 33] | <0.001 |
| Parity † | 0 [0 – 1] | 1 [0 – 2] | 1 [0 – 1] | 0.018 |
| Gravidity † | 2 [1 – 3] | 3 [2 – 4] | 2 [1 – 4] | 0.384 |
| GA, weeks † | 19 [17 – 20] | 36 [35 – 37] | 29 [25 – 31] | <0.001 |
| Uterine contractions ‡ | 0 (0) | 0 (0) | 127 (50) | <0.001 |
| Cervical dilatation > 3 cm ‡ | 0 (0) | 0 (0) | 46 (18) | 0.007 |
| PPROM ‡ | 0 (0) | 0 (0) | 121 (47) | <0.001 |
| Clinical chorioamnionitis ‡ | 0 (0) | 0 (0) | 19 (7) | 0.153 |
| Outcome characteristics | ||||
| Term delivery (≥ 37 weeks) ‡ | 23 (100) | 13 (52) | 43 (17) | <0.001 |
| Preterm birth at < 34 weeks ‡ | 0 (0) | 0 (0) | 204 (78) | <0.001 |
| GA at delivery, weeks † | 39 [39 – 41] | 37 [36 – 37] | 31 [26 – 33] | <0.001 |
| Amniocentesis-delivery time, days † | 144 [137 – 156] | 2 [1 – 8] | 2 [0 – 16] | <0.001 |
Data presented as median [interquartile range] and analyzed by Kruskal-Wallis ANOVA on Ranks.
Data presented as n (%) and analyzed by Chi square tests.
Abbreviations: PPROM, preterm premature rupture of membrane; GA, gestational age
The clinical, laboratory and outcome characteristics for the “rule-out infection” group were analysed separately and are presented in Table 2. Women with positive IAI were delivered at earlier GAs, had a higher frequency of clinical chorioamnionitis, shorter amniocentesis-to-delivery interval and more often delivered babies of lower birth weight. The results of the chemical and microbiological studies of the AF showed the group of women with IAI had higher AF levels of LDH, white blood cell (WBC) count, and lower AF glucose levels. They also had a higher frequency of a positive microbial culture or Gram stain. Histological examination of the fetal membranes, chorionic plate and chorio-decidua demonstrated significantly higher grades of histological inflammation in the group of women with IAI.
Table II.
Demographic, clinical, laboratory and outcome characteristics of women with symptoms of preterm birth that provided AF samples for NGAL, MMP-9 and NGAL•MMP-9 complex levels (n=260).
| Variable | Rule-out infection amniocenteses | |||
|---|---|---|---|---|
| no IAI* & TB n = 39 | no IAI* & PTB n = 132 | yes IAI* & PTB n = 89 | P value | |
| Clinical and outcome characteristics at amniocentesis and at delivery | ||||
| Maternal age, years, † | 24 [19 – 31] | 28 [23 – 32] | 27 [23 – 34] | 0.055 |
| Parity † | 0 [0 – 1] | 0 [0 – 1] | 1 [0 – 2] | 0.450 |
| Gravidity † | 2 [1 – 3] | 2 [1 – 4] | 3 [2 – 3] | 0.257 |
| History of preterm birth ‡ | 4 (11) | 36 (27) | 24 (27) | 0.090 |
| GA at amniocentesis, weeks † | 30 [26 – 32] | 30 [27 – 32] | 26 [24 – 29] | <0.001 |
| GA at delivery, weeks † | 39 [38 – 39] | 31 [28 – 33] | 26 [25 – 29] | <0.001 |
| Amniocentesis-delivery interval, days † | 61 [44 – 90] | 4 [1 – 11] | 0 [0 – 1] | <0.001 |
| Amniocentesis-delivery < 7 days | 1 (3%) | 83 (63%) | 83 (93%) | <0.001 |
| Birthweight, grams † | 3,150 [2,899 – 3,505] | 1,708 [1,183 – 2,100] | 930 [753 – 1,373] | <0.001 |
| AF laboratory test results | ||||
| Glucose, mg/dL † | 32 [25 – 46] | 28 [19 – 39] | 4 [2 – 12] | <0.001 |
| LDH activity, U/L † | 156 [118 – 204] | 176 [125 – 263] | 744 [443 – 21,870] | <0.001 |
| WBC count, cells/mm3 † | 4 [1 – 10] | 6 [2 – 21] | 895 [180 – 1,967] | <0.001 |
| Positive Gram stain ‡ | 0 (0%) | 11 (8%) | 53 (60%) | <0.001 |
| Positive culture results ‡ | 0 (0%) | 15 (11%) | 65 (73%) | <0.001 |
| Placental histology results | n=8 | n=122 | n=88 | |
| Chorionic plate inflammation, stage † | 1 [0 – 2] | 1 [0 – 2] | 3 [3 – 3] | <0.001 |
| Amnionitis, grade † | 0 [0 – 0] | 0 [0 – 1] | 3 [2 – 3] | <0.001 |
| Chorio-deciduitis, grade † | 1 [0 – 3] | 2 [0 – 3] | 3 [3 – 4] | <0.001 |
| Funisitis, grade † | 0 [0 – 1] | 0 [0 – 0] | 2 [0 – 4] | <0.001 |
IAI was defined by an MR score 3 or 4 which is indicative of severe intra-amniotic inflammation.
Data presented as median [interquartile range] and analyzed by Kruskal-Wallis ANOVA on Ranks.
Data presented as n (%) and analyzed by Chi square test.
Abbreviations: IAI, intra-amniotic inflammation; PTB, preterm birth <37weeks; TB, term birth ≥37weeks; GA, gestational age; LDH, lactate dehydrogenase
3.2. NGAL, MMP-9 and NGAL•MMP-9 complex levels are increased in amniotic fluid of women with intra-amniotic inflammation and preterm birth
First, we looked at whether the NGAL concentration in AF exhibits GA regulation in the absence of IAI. For this purpose, we limited our analysis to AF from women with normal pregnancy outcome. This included women who had an amniocentesis for either genetic amniocentesis (n=23), rule-out infection and ultimately delivered at term (n=39), or lung maturity (n=25) purposes. We found that AF NGAL concentration varied significantly across human gestation, with lower levels at term (Fig. 1A&D, r= −0.470, P<0.001). There was no statistical significant correlation between GA and MMP-9 (Fig. 1B&D, r= −0.171, P>0.05), GA and NGAL•MMP-9 complex (Fig. 1C&D, r= 0.160, P>0.05) or GA and IL-6 (Fig. 1D, r=−0.098, P>0.05) across gestation. The molar ratio of NGAL/MMP-9 decreased >2-fold in AF from lung maturity compared to 2nd trimester genetic amniocenteses. Despite this, NGAL remained at least two orders of magnitude in excess of MMP-9 (molar ratio genetic: 667, 95%CI [299-991] vs. lung maturity 287, 95%CI [230-407]). Among women with normal pregnancy outcome only a minority (18%, 16/87) had detectable immunoreativity for the NGAL•MMP-9 complex. Levels of the NGAL•MMP-9 complex correlated with MMP-9 (Fig. 1D, r=0.649, P<0.001) but not with NGAL immunoreactivity (Fig. 1D, r=0.086, P>0.05).
Fig 1.
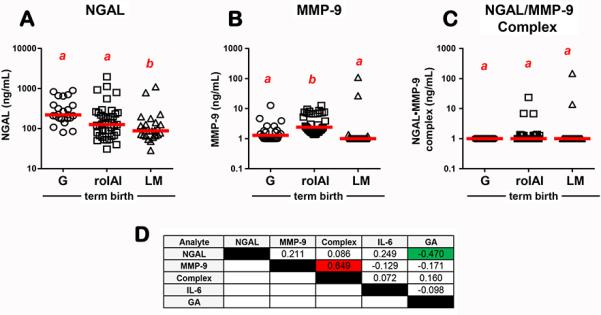
Gestational age (GA) regulation of amniotic fluid (A) NGAL; (B) MMP-9 and (C) NGAL•MMP-9 complex in women with normal pregnancy outcome who had the amniocentesis for either genetic (G), rule-out intraamniotic infection and inflammation (roIAI), or lung maturity (LM) testing. The red lines represent group medians. Data analyzed by Kruskal-Wallis ANOVA followed by post-hoc Dunn's tests. Superscripts with different letters indicate statistically significant differences at P<0.05. (D) Spearman correlation coefficients between AF analytes measured in our study and GA. Significant direct correlations are marked by red highlight and significant inverse correlations are marked green highlight.
Next, we analyzed AF samples retrieved from women with signs or symptoms of PTB who had an amniocentesis to rule-out infection. Cases were grouped by membrane status and IAI severity. We determined that women with severe IAI (MR=3-4) had significantly increased AF NGAL (P<0.001, Fig. 2A) and MMP-9 (P<0.001, Fig. 2B) concentrations compared to all other groups. As the magnitude of MMP-9's increase exceeded that of NGAL this resulted in a >5-fold diminished NGAL/MMP-9 molar ratio in women with severe IAI (MR=3-4: 30, 95%CI [10-84] vs. MR=0-2: 160, 95%CI [83-303]. The majority (92%, 82/89) of women with severe IAI had detectable NGAL•MMP-9 complex. This proportion was significantly lower among the groups with MR=0-2 who either delivered preterm (54%, 71/132, P<0.001) or at term (36%, 14/39, P<0.001). Immunoreactive levels of NGAL•MMP-9 complex were significantly elevated in women with severe IAI (MR=3-4) compared to all other groups (P<0.001, Fig. 2C). The level of significance maintained even when only samples with detectable complex where included in the analysis (MR=0-2: 1.0 mg/mL vs. MR=3-4: 46.4 ng/mL, P<0.001). There were significant direct correlations between all analytes studied which upheld after correction for GA and membrane status (P<0.001 for all, Fig. 2D).
Fig 2.
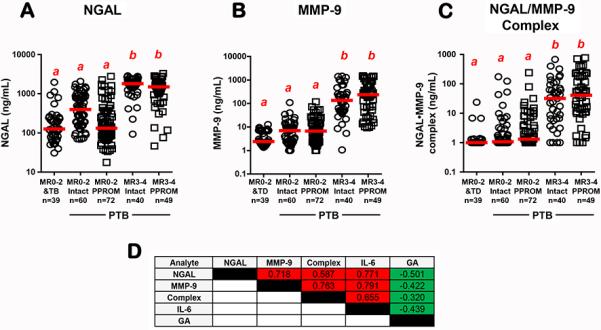
Amniotic fluid levels of (A) NGAL; (B) MMP-9 and (C) NGAL•MMP-9 complex levels in women with categorized by severity of intra-amniotic inflammation (IAI) and membrane status (intact or PPROM). Severity of IAI was assessed by the proteomics MR score and dichotomized as absence (MR=0-2) or presence of severe IAI (MR=3-4). Women with MR=0-2 and intact membranes were grouped based on pregnancy outcome as term (TB) or preterm birth (PTB). The red lines represent group medians. Data analyzed by Kruskal-Wallis ANOVA followed by post-hoc Dunn's tests. Superscripts with different letters indicate statistically significant differences at P<0.05. (D) Spearman correlation coefficients between AF analytes measured in our study and GA. Significant direct correlations are marked by red highlight and significant inverse correlations are marked green highlight.
To further validate the ELISA findings and to investigate the proteoforms underlying NGAL immunoreactivity, we performed western blots for NGAL and MMP-9. We first compared AF of women with infection induced-PTB versus AF of women matched for GA at amniocentesis who had a term delivery (Fig. 3A). As shown, the anti-NGAL antibody also detected a band at 25 kDa which corresponds to the expected size of monomeric NGAL but only in women with IAI. Bands corresponding to the NGAL•MMP-9 complex (125 kDa) were detected by both antibodies. We next examined AF samples from women with varying degrees of AF inflammation and NGAL•MMP-9 complex immunoreactivity both under reducing and non-reducing conditions. In addition to the 125 kDa NGAL•MMP-9 heterodimer and the 25 kDa NGAL monomer visible under reducing conditions, an additional intense band at ~50 kDa was detected under non-reducing conditions (Fig. 3B). Interestingly, the difference in banding pattern at 125 kDa in AF samples with MR=4 was apparent in reducing but not non-reducing conditions. This observation suggests that in vivo in AF of women with severe IAI, the NGAL•MMP-9 complex may be part of a higher molecular weight complex linked through both covalent and non-covalent bonds.
Fig 3.
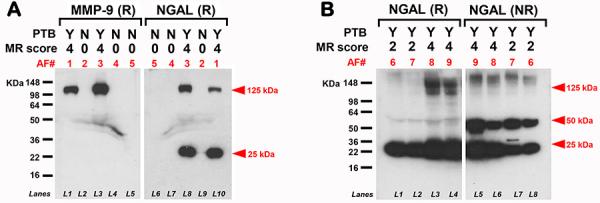
Representative western blots demonstrating immunoreactive proteoforms of (A) human amniotic fluid (AF) MMP-9 and NGAL in reducing (R) conditions. Samples were from women who delivered preterm (PTB=Y) or term (PTB=N) in the setting of either severe (MR=4)] or absent (MR=0) intra-amniotic inflammation (IAI). As shown, AF of women with severe IAI (AF 1&3 in lanes L1, L3, L8, L10) display MMP-9 immunoreactivity at ~125 kDa (higher than the expected mass for pro-MMP-9). Two NGAL proteoforms are detected: ~25 KDa (NGAL monomer) and ~125 kDa (NGAL•MMP-9 hetrodimer). (B) Representative AF western blot gels of women with PTB varying degrees of IAI. NGAL•MMP-9 complex immunoreactivity was identified in both R and non-reducing (NR) conditions at ~125 kDa and ~25 kDa. In addition, a strong band at ~50 kDa was detected under NR conditions likely representing the NGAL hono-dimer. Note the difference in banding pattern at 125 kDa between reducing and non-reducing conditions in AF samples with severe IAI (MR=4: AF 8&9 in lanes L3, L4, L5, L6) versus mild IAI (MR=2: AF 6&7 in lanes L1, L2, L7, L8).
3.3. Expression level of NGAL, MMP-9 in fetal membranes and villous trophoblast of women with IAI
By immunohistochemistry we found NGAL was expressed in both fetal membranes and placenta albeit fetal membranes stained more intensely. Both amnion epithelium and resident cells in the choriodecidua showed strong NGAL immunoreactivity both in the absence (Fig. 4A-B) and presence of (Fig. 4C-D) IAI. The NGAL signal was further enhanced in fetal membranes from pregnancies complicated by IAI primarily through the intense staining of infiltrating inflammatory cells (Fig. 4C&D). Overall, compared to fetal membranes, the placenta had less abundant NGAL staining in resident cells. Villous cytotrophoblasts stained positive for NGAL conferring predominance to the villous stroma over the syncytiotrophoblast (Fig. 4F&G). In women with IAI, inflammatory cells in maternal vascular spaces stained conspicuously for NGAL (Fig. 4H&I). Negative control slides had the primary antibody omitted and was devoid of staining (Fig.4E&J).
Fig 4.
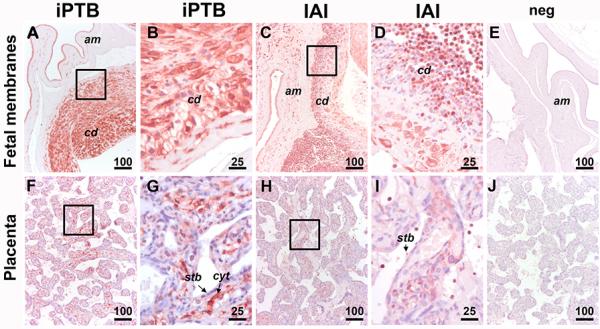
Representative immunohistochemical staining for NGAL in fetal membranes (A-D) and placenta villous tissue (F-J) of a patient with idiopathic spontaneous preterm birth ( iPTB, MR=0) as compared to a patient with histologic chorioamnionitis and severe IAI (MR=4) and histological chorioamnionitis. Amnion epithelium (am) and resident cells in the choriodecidua (cd) showed strong NGAL immunoreactivity both in the absence (A&B) and presence of severe IAI (C&D). The NGAL signal was augmented in fetal membranes from pregnancies complicated by IAI primarily due to the increased staining of the infiltrating inflammatory cells. Placenta had less abundant NGAL staining in resident cells both in the absence (F&G) and presence (H&D) of IAI. Villous cytotrophoblasts (cyt) stained stronger for NGAL compared to syncytiotrophoblast (stb). (E-J)
By qRT-PCR we found that the mRNA expression for both NGAL (Fig.5 A) and MMP-9 (Fig. 5 B) was elevated (P<0.001) in fetal membranes of women with IAI compared to iPTB and term amniochorion. In placenta, the mRNA for NGAL (Fig. 5C) was elevated by IAI while expression of MMP-9 mRNA (Fig. 5D) remained statistically unchanged (P>0.05).
Fig 5.
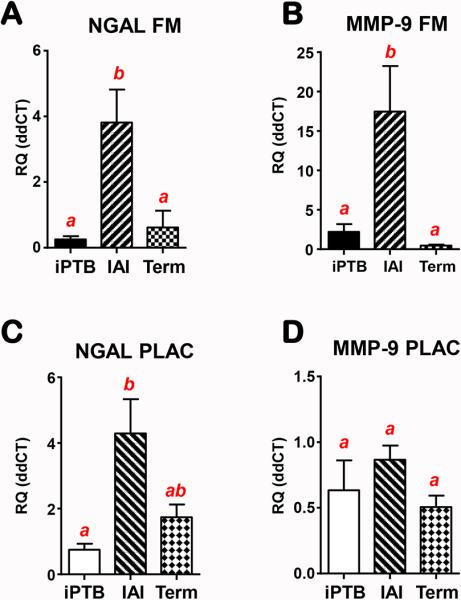
Real-time quantitative PCR results of fetal membranes (FM) (A&B) and placental villous tissue (C&D). NGAL (A&C) and MMP-9 (B&D) mRNA levels were determined in women with preterm birth and severe intraamniotic inflammation (IAI, n=16), idiopathic preterm (iPTB, n=12) or normal term pregnancy undergoing elective C-section (Term, n=7). Error bars show standard error. Bars with at least one common letters indicate groups that are not statistically different at P>0.05.
4. DISCUSSION
In this study, we identified that NGAL, a protein with recognized bacteriostatic function, is abundantly present in normal AF and its levels decrease as GA advances. We further observed that women with severe IAI had significantly AF increased levels of NGAL, MMP-9 and NGAL•MMP-9 complex compared to women with absent and mild inflammation. The presence of NGAL in AF from women with severe IAI displayed a positive correlation with the proteomics MR score and the inflammatory cytokine IL-6. Therefore, our findings support the notion that elevated levels of AF NGAL and NGAL•MMP-9 complex are part of the intra-amniotic response to infection and may represent a marker of severe inflammation.
NGAL is a member of the lipocalin family that was first identified in the granules of neutrophils.7 Since that time it has been recognized in epithelial cells (i.e. lungs, bowel, prostate, kidney), adipocytes and monocytes/macrophages as an up-regulator of growth factors and cytokines.9,10 Lipocalins have a highly conserved molecular conformation comprised of an antiparallel β-barrel that encloses an internal ligand-binding-cavity. The structure of the binding site allows lipocalins to bond and transport small hydrophobic molecules and to engage in complex formation with various soluble macromolecules and metals.20 One such molecule is iron which is essential for almost all life processes (i.e. respiration, DNA synthesis).21 As iron is essential for survivability of almost all pathogenic bacteria, they have evolved a mechanism to trap iron from their environment for their own metabolic use and growth by releasing siderophores (high-affinity iron-chelating compounds).22 NGALs ability to sequester bacterial siderophores, thereby limiting bacterial growth, has provided evidence for its role in innate immunity.23 The observed GA regulation, with higher AF NGAL levels early pregnancy, may reflect a high requisite for anti-microbial defense mechanisms earlier in gestation, when most intra-amniotic infections occur.24
The ability of NGAL to conjugate covalently with pro-MMP-9 protecting it from auto-degradation has recently emerged as a critical determinant of cancer aggressiveness.25 This has led to intensive investigations of the NGAL•MMP-9 heterodimer as a marker for cancer disease status. This body of knowledge, raises the question whether the abundant constitutive expression of NGAL in fetal membranes may play a role in pathogenesis of PPROM through NGAL•MMP-9 complex augmenting collagenolytic effects of MMP-9. Although this is still possible, our data suggests that inflammation was a more important determinant of NGAL and NGAL•MMP-9 levels than membrane status alone. Moreover, as neutrophils infiltrating the fetal membranes were noted to express high levels of NGAL, this suggests that infiltrating neutrophils rather than resident amniochorion cells are more likely the source of the AF NGAL•MMP-9 complex. This assertion is substantiated by our western blot findings which failed to identify NGAL immunoreactive bands in AF of the majority of women with genetic or lung maturity amniocenteses. Weather the level of NGAL in normal AF is below the detection limit of western blotting or AF NGAL immunoreactivity is part of a yet unidentified higher molecular weight complex remains to be further determined.
NGAL exists in multiple molecular forms which are thought to have preferential function. NGALs role in innate immunity, inflammation and its increased production during epithelial damage has led to extensive investigation of NGAL proteoforms in urine as biomarkers of acute kidney injury.26,27The pattern of NGAL immunoreactivity has been proposed as marker to distinguish among different pathologic processes leading to kidney injury. Interestingly, the NGAL band detected at ~50 kDa under non-reducing conditions which most likely represents the dimeric form, has been previously identified in urine of patients with urinary tract infections and is thought to be preferentially secreted by activated neutrophils.28 In contrast kidney epithelial cells reportedly secrete the monomeric proteoform. Thus, in addition to providing a description of proteoforms representing NGAL immunoreactivity in human AF, a novel finding of our study was the association between presence of NGAL dimers in AF and IAI.
Unfortunately the knowledge linking NGAL proteoforms to specific antimicrobial and siderophore-binding activity is severely limited. While dimerization does not appear to affect the structure of the protein, it is not known how this affects the ability to activate pro-MMP-9.29 Moreover, NGAL's ability to form complexes and to impact on innate immunity seems to be less specific than previously thought.30 The recent finding of NGAL rescuing from the inhibitory effects of bacterial siderophores, myeloperoxidase, an important neutrophil bactericidal enzyme,31 suggests that AF NGAL could be an important modulator of host-microbe interactions in intra-amniotic infection.
5. CONCLUSIONS
Our findings reveal that the levels of AF NGAL are GA regulated in pregnancies with normal outcome. In pregnancies complicated by IAI secondary to infection, high levels of AF MMP-9, NGAL and NGAL•MMP-9 complex were detected. The amniochorion expresses NGAL constitutively in decidual, amnion and extravillous trophoblast cells. In pregnancies complicated by IAI and histologic chorioamnionitis, the increased expression of NGAL from infiltrating neutrophils may not only augment the collegenolytic activity of MMP-9 but also modulate host-responses to invading bacteria.
ACKNOWLEDGMENTS
We are indebted to the nurses, fellows and residents at Yale New Haven Hospital, Department of Obstetrics and Gynecology and Reproductive Sciences, and to all patients who participated in the study. CSB and IAB were supported by National Institutes of Health/ Eunice Kennedy Shriver National Institute of Child Health and Human Development (NIH/NICHD) R01HD062007-01A1. The funding source had no involvement in study design, interpretation of data, writing of the report or decision to submit the paper for publication.
Footnotes
CONFLICT OF INTERESTS
The authors have no conflicts to declare.
REFERENCES
- 1.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;5(371):75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buhimschi IA, Nayeri UA, Laky CA, Razeq SA, Dulay AT, Buhimschi CS. Advances in medical diagnosis of intra-amniotic infection. Expert Opin Med Diagn. 2013;7(1):5–16. doi: 10.1517/17530059.2012.709232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez JM, Franzke CW, Yang F, Romero R, Girardi G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am J Pathol. 2011;179(2):838–849. doi: 10.1016/j.ajpath.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernhard EJ, Gruber SB, Muschel RJ. Direct evidence linking expression of matrix metalloproteinase 9 (92-kDa gelatinase/collagenase) to the metastatic phenotype in transformed rat embryo cells. Proc Natl Acad Sci U S A. 1994;91(10):4293–4297. doi: 10.1073/pnas.91.10.4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu P, Alfaidy N, Challis JR. Expression of matrix metalloproteinase (MMP)-2 and MMP-9 in human placenta and fetal membranes in relation to preterm and term labor. J Clin Endocrinol Metab. Mar. 2002;87(3):1353–1361. doi: 10.1210/jcem.87.3.8320. [DOI] [PubMed] [Google Scholar]
- 6.Candido S, Maestro R, Polesel J, Catania A, Maira F, Signorelli SS, McCubrey JA, Massimo L. Roles of neutrophil gelatinase-associated lipocalin (NGAL) in human cancer. Oncotarget. 2014;5(6):1576–1594. doi: 10.18632/oncotarget.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Devarajan P. Neutrophil gelatinase-associated lipocalin: new paths for an old shuttle. Cancer Ther. 2007;5:463–470. [PMC free article] [PubMed] [Google Scholar]
- 8.Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38(3):414–420. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sprenkle P, Russo P. Molecular markers for ischemia, do we have something better then creatinine and glomerular filtration rate? Arch Esp Urol. 2013;66(1):99–114. [PubMed] [Google Scholar]
- 10.Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Molecular Cell. 2002;10(5):1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 11.Chakraborty S, Kaur S, Guha S, Batra SK. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826(1):129–169. doi: 10.1016/j.bbcan.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Candido S, Di Maso M, Serraino D, McCubrey JA, Bortolus R, Zanin M, Battiston M, Salemi R, Libra M, Polesel J. Diagnostic value of neutrophil gelatinase-associated lipocalin/matrix metalloproteinase-9 pathway in transitional cell carcinoma of the bladder. Tumour Biol. 2016 doi: 10.1007/s13277-016-4872-x. PMID: 26810191. [DOI] [PubMed] [Google Scholar]
- 13.Tadesse S, Luo G, Shin Park J, Jae Kim B, Snegovkikh V, Zheng T, Hodgson E, Arcuri F, Toti P, Parikh C, Guller S, Norwitz ER. Intra-amniotic infection upregulates neutrophil gelatinase-associated lipocalin (NGAL) expression at the maternal-fetal interface at term: implications for infection-related preterm birth. Reproductive Sciences. 2011;18(8):713–722. doi: 10.1177/1933719110396722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vajrychova M, Kacerovsky M, Tambor V, Hornychova H, Lenco J. Microbial invasion and histological chorioamnionitis up-regulate neutrophil-gelatinase associated lipocalin in preterm prelabor rupture of membranes. J Matern Fetal Neonatal Med. 2015;27:1–10. doi: 10.3109/14767058.2014.991305. [DOI] [PubMed] [Google Scholar]
- 15.Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary sequence of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268:10425–32. [PubMed] [Google Scholar]
- 16.Naeye RL. Disorders of the placenta and decidua. In: Naeye RL, editor. Disorder of the Placenta, Fetus and Neonate: Diagnosis and Clinical Significance. Mosby; St. Louis: 1992. pp. 118–247. [Google Scholar]
- 17.Salafia CM, Weigl C, Silberman L. The prevalence and distribution of acute placental inflammation in uncomplicated term pregnancies. Obstet Gynecol. 1989;73:383–389. [PubMed] [Google Scholar]
- 18.Buhimschi IA, Christner R, Buhimschi CS. Proteomic biomarker analysis of amniotic fluid for identification of intra-amniotic inflammation. BJOG. 2005;112:173–181. doi: 10.1111/j.1471-0528.2004.00340.x. [DOI] [PubMed] [Google Scholar]
- 19.Buhimschi CS, Bhandari V, Hamar B, Bahtiyar MO, Zhao G, Sfakianaki AK, Pettker CM, Magloire LK, Norwitz ER, Funai E, Paidas M, Weiner CP, Copel J, Lockwood CJ, Buhimschi IA. Proteomic profiling of the amniotic fluid to detect inflammation, infection and neonatal sepsis. PLoS Med. 2007;4:e18. doi: 10.1371/journal.pmed.0040018. PMC1769412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chakraborty S, Kaur S, Tong Z, Batra SK, Guha S. Neutrophil Gelatinase Associated Lipocalin: Structure, Function and Role in Human Pathogenesis, Acute Phase Proteins - Regulation and Functions of Acute Phase Proteins. Prof. Francisco Veas (Ed.) ISBN: 2011; 978-953-307-252-4. [Google Scholar]
- 21.Cassat JE, Skaar EP. Iron in infection and immunity. Cell Host Microbe. 2013;13(5):509–19. doi: 10.1016/j.chom.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saha M, Sarkar S, Sarkar B, Sharma BK, Bhattacharjee S, Tribedi P. Microbial siderophores and their potential applications: a review. Environ Sci Pollut Res Int. 2015 doi: 10.1007/s11356-015-4294-0. PMID: 25758420. [DOI] [PubMed] [Google Scholar]
- 23.Nasioudis D, Witkin SS. Neutrophil gelatinase-associated lipocalin and innate immune responses to bacterial infections. Med Microbiol Immunol. 2015;204(4):471–479. doi: 10.1007/s00430-015-0394-1. [DOI] [PubMed] [Google Scholar]
- 24.Pacora P, Maymon E, Gervasi MT, et al. Lactoferrin in intrauterine infection, human parturition, and rupture of fetal membranes. Am J Obstet Gynecol. 2000;183:904–910. doi: 10.1067/mob.2000.108882. [DOI] [PubMed] [Google Scholar]
- 25.Provatopoulou X, Gounaris A, Kalogera E, Zagouri F, Flessas I, Goussetis E, Nonni A, Papassotiriou I, Zografos G. Circulating levels of matrix metalloproteinase-p (MMP-9), neutrophil gelatinase-associated lipocalin (NGAL) and their complex MMP-9/NGAL in breast cancer disease. BMC Cancer. 2009;9:390. doi: 10.1186/1471-2407-9-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahn JY, Lee MJ, Seo JS, Choi D, Park JB. Plasma neutrophil gelatinase-associated lipocalin as a predictive biomarker for the detection of acute kidney injury in adult poisoning. Clin Toxicol (Phila) 2016;54(2):127–133. doi: 10.3109/15563650.2015.1118487. [DOI] [PubMed] [Google Scholar]
- 27.Martensson J, Xu S, Bell M, Martling CR, Venge P. Immunoassays distinguishing between HNL/NGAL released in urine from kidney epithelial cells and neutrophils. Clinica Chimica Acta. 2012;413:1661–1667. doi: 10.1016/j.cca.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Cai L, Rubin J, Han W, Venge P, Xu S. The origin of multiple molecular forms in urine of HNL/NGAL. Clin J Am Soc Nephrol. 2010;5(12):2229–2235. doi: 10.2215/CJN.00980110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goetz DH, Willie ST, Armen RS, Bratt T, Borregaard N, Strong RK. Ligand preference inferred from the structure of neutrophil gelatinase associated lipocalin. Biochemistry. 2000;39(8):1935–41. doi: 10.1021/bi992215v. [DOI] [PubMed] [Google Scholar]
- 30.Johnstone TC, Nolan EM. Beyond iron: non-classical biological functions of bacterial siderophores. Dalton Trans. 2015;44(14):6320–39. doi: 10.1039/c4dt03559c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh V, Yeoh BS, Xiao X, Kumar M, Bachman M, Borregaard N, Joe B, Vijay-Kumar M. Interplay between enterobactin, myeloperoxidase and lipocalin 2 regulates E. coli survival in the inflamed gut. Nat Commun. 2015;12(6):7113. doi: 10.1038/ncomms8113. [DOI] [PMC free article] [PubMed] [Google Scholar]


