Abstract
Regulatory T cells (Tregs) are functionally suppressive CD4 T cells, critical for establishing peripheral tolerance and controlling inflammatory responses. Previous reports of Tregs during chronic HIV disease have conflicting results with higher or lower levels compared to controls. Identifying true Tregs with suppressive activity proves challenging during HIV infection, as traditional Treg markers, CD25 and FOXP3, may transiently up-regulate expression as a result of immune activation. Helios is an Ikaros family transcription factor that marks natural Tregs with suppressive activity and does not up-regulate expression after activation. Coexpression of FOXP3 and Helios has been suggested as a highly specific marker of “bona fide” Tregs. We evaluated Treg subsets by FOXP3 co-expressed with either CD25 or Helios and their association with HIV disease progression in perinatally-infected HIV positive children. Identifying Tregs by FOXP3 coexpression with Helios rather than CD25 revealed markedly higher Treg frequencies, particularly in HIV+ children. Regardless of ART, HIV infected children had a selective expansion of memory FOXP3+Helios+ Tregs. The rise in memory Tregs correlated with declining HIV clinical status, indicated by falling CD4 percentages and CD4:CD8 ratios and increasing HIV plasma viremia and immune activation. In addition, untreated HIV+ children exhibited an imbalance between the levels of Tregs and activated T cells. Finally, memory Tregs expressed immune activation markers CD38 and Ki67 and exhaustion marker, PD-1, that tightly correlated with a similar phenotype in memory CD4 T cells. Overall, HIV infected children had significant disruptions of memory Tregs that associated with advancing HIV disease.
Keywords: HIV, children, Helios, Regulatory T-Cell, Immune Activation, CD38, Programmed cell death receptor-1
Introduction
Regulatory T cells (Tregs) are functionally suppressive CD4 T cells, critical for establishing peripheral tolerance, and controlling autoimmune and inflammatory responses1,2. An imbalance of Tregs and effector T cells can blunt immune responses to infectious diseases and malignant cells or facilitate inflammation-mediated pathologies1,3. In HIV infection, chronic immune activation is a key causal factor of disease progression, mortality, and much of the pathologic sequelae, such as cardiovascular disease, metabolic disorders and neurocognitive dysfunction4-6. Several studies have probed whether an imbalance of Tregs contributes to this characteristic inflammation in HIV infected individuals, in whom Tregs may play either a beneficial role to alleviate immune activation or a detrimental role by impairing HIV-specific immune responses3. While many previous studies observed a relative increase in Treg proportions in CD4 T cells during HIV infection, these reports have been variable, stemming from the lack of consensus markers to evaluate Tregs3,7. Moreover, recent data suggests that Tregs are not a homogenous population; rather they consist of cytokine producing and non-producing Treg subsets with some degree of plasticity8,9.
Tregs have traditionally been identified by coexpression of markers FOXP3 and CD25. Transcription factor FOXP3 is necessary for Treg development and function, and therefore expressed by all Tregs. However, activation of non-suppressive T cells by TGFβ signaling also transiently upregulates FOXP3, without conferring suppressive capacity1,10-12. Thus FOXP3 expression alone does not always distinguish a regulatory phenotype in human T cells. Likewise, CD25, a canonical Treg marker, may also be transiently upregulated in recently activated conventional CD4 T cells1. With these variable expression patterns, FOXP3 and CD25 coexpression may not represent true Tregs with suppressive function. Other markers differentially expressed on Tregs such as CD127, CTLA-4, CD39, and CD62L, may similarly alter during T cell differentiation or immune activation states, and thus also fail to sufficiently identify Tregs1,11,12. GARP was demonstrated to be a highly specific Treg marker expressed only on activated Tregs, yet its pertinence in ex vivo studies identifying resting Tregs is unclear11,13.
More recently, another transcription factor, Helios, was proposed to represent natural Tregs originating in the thymus11,13-16. Helios is an Ikaros family zinc finger transcription factor that regulates IL-2 production. Together with FOXP3, Helios divides Tregs into two distinct populations: Helios positive Tregs, which do not produce inflammatory cytokines, and Helios negative Tregs that secrete IL-17 and possibly other cytokines15. Both subsets exhibit suppressive functions. More importantly, T cell activation and differentiation states do not modify Helios expression14,15. Thus Helios coexpression with FOXP3 identifies bona fide Tregs with suppressive function and with minimal cytokine production.
In HIV positive adults virally suppressed with antiretroviral therapy (ART), we have previously shown memory FOXP3+Helios+ Tregs selectively increase, while memory FOXP3+Helios- frequency remains similar to healthy adults15. Interestingly, in a study of infants, Rabe et al. revealed neonates express higher levels of Helios in Tregs compared to adults17. Very few reports examine Tregs in HIV infected children, in whom a disruption may have the added consequence of altering responses to essential childhood vaccines. Moreover, persistent inflammation in children may have long-term health and developmental consequences4,5,18. In this study, we examined regulatory T cells in a perinatally infected HIV+ pediatric cohort from Mombasa, Kenya by FOXP3 coexpressed with Helios or CD25 and their correlation with HIV disease progression. We reveal a larger bona fide Treg population using FOXP3 coexpressed with Helios than with CD25. We further demonstrate an expanded memory FOXP3+Helios+ Treg subset in HIV+ children compared to HIV negative-unexposed children that correlates with progressive HIV disease status, measured by CD4 levels, CD4:CD8 ratios, HIV plasma viremia and immune activation. Last, we show a disruption in the correlative balance between Tregs and activated or exhausted T cells in HIV infected children.
Methods
Participants
Ethical approval for this study was obtained from New York University (10-02586) and Kenyatta National Hospital / University of Nairobi (P283/07/2011). Written informed consent and verbal assent when appropriate was obtained from all participants and/or parents. We enrolled a total of 93 perinatally-infected HIV+ and 46 HIV negative-unexposed children ages 5-20 years old from Bomu Hospital in Mombasa, Kenya between 2011-2012. HIV+ children included 48 antiretroviral therapy naïve (ART-) and 45 HIV+ children on antiretroviral treatment for at least six months (ART+). Individuals with a recent acute illness, active Mycobaterium tuberculosis or malaria infection, or pregnancy within one year were ineligible for study entry.
Plasma and peripheral blood mononuclear cells (PBMC) were isolated from peripheral blood by centrifugation and Ficoll-Hypaque (GE Healthcare) density gradient centrifugation then cryopreserved. HIV RNA PCR was performed on diluted plasma samples with Roche, COBAS® AmpliPrep/COBAS® TaqMan®HIV-1 Test, version 2.0 (limit of detection 110 copies/ml).
HIV-, ART-, and ART+ were matched for age and gender (Sup. Table 1). Median CD4% in HIV- children was 38 (IQR 33-42). ART- had median CD4% of 24 (IQR 13-29) and HIV viral load of 4.6 (IQR 3.9-5.2) log copies/ml. ART+ had median CD4% and HIV viral load of 30 (IQR 17-36) and 2 (IQR 2-3.8) log copies/ml respectively (Sup. Table 1).
Flow Cytometric Studies
Cryopreserved PBMCs were thawed then evaluated with 10-color flow cytometry. Cells were stained with fixable viability dye (eBioscence) in PBS for 30 minutes, washed, then stained with fluorescent-conjugated antibodies at 4°C for 30 minutes in PBS buffer containing 2% FCS and 0.1% sodium azide. For intracellular staining, cells were fixed and permeabilized using FOXP3 staining kit (eBioscience) then stained with intracellular antibodies. Stained cells were analyzed using LSRII flow cytometer (BD Bioscience) and FlowJo software (Tree Star). The following anti-human antibodies were used: CD3, CD4, CD8, CD25, CD38, CD45RO, HLA-DR, PD-1, FOXP3 (clone 259D), Helios, and Ki67 (Biolegend). Singlet lymphocytes were gated based on forward and side scatter properties. Dead cells were excluded based on viability dye. All populations are reported as percent of CD4 or CD8 T cell parent populations. Memory populations are defined as CD45RO+, and naïve as CD45RO- in CD4 T cells.
Plasma sCD14
Plasma levels of sCD14 were quantified by ELISA assay using Human CD14 Duoset kit (R&D Systems) per manufacturer's instructions. Plasma was diluted 1500 fold and each test performed in duplicate. Results reported are the average of duplicate results.
Statistics
All statistical analyses were performed using GraphPad Prism software. Comparisons between participant categories were computed with the two-sided Mann-Whitney test. Pairwise comparisons between %FOXP3+CD25+ and %FOXP3+Helios+ memory Tregs were analyzed with Wilcoxon matched-pairs signed rank test. Correlations were calculated with the Spearman's rank test. Threshold of significance for all tests was 0.05.
Results
Conventional Treg marker CD25 underestimates memory Tregs in HIV uninfected and infected children
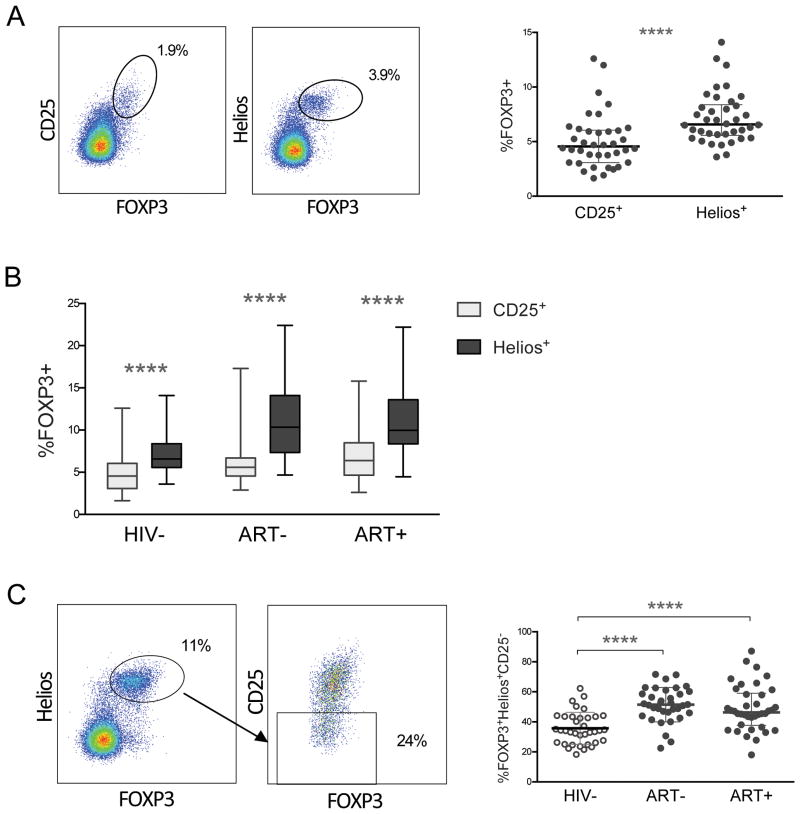
We first identified Tregs in HIV- children by FOXP3 coexpression with either traditional marker CD25 or with Helios (Fig. 1A). In healthy children, paired comparison of the total percent of memory Tregs by each marker consistently showed higher percentages of memory regulatory T cells with FOXP3+Helios+ as opposed to FOXP3+CD25+ coexpression (p<0.0001; Fig. 1A). Next, we asked whether Helios coexpression with FOXP3 identified a larger proportion of memory Tregs than CD25 in HIV+ children. Indeed, in pairwise comparisons, ART- and ART+ children also had higher percentages of FOXP3+Helios+ compared with FOXP3+CD25+ memory Tregs (p<0.0001; Fig. 1B). We subsequently investigated whether the proportion of memory FOXP3+Helios+ Tregs negative for CD25 expression differs in HIV- compared to HIV+ children (Fig. 1C). In aggregate, ART- and ART+ children had relatively higher CD25- cells in FOXP3+Helios+ memory Tregs compared with HIV- children (p<0.0001; Fig. 1C). Helios expression in memory FOXP3+CD25+ cells averaged 77-82% (Sup. Fig. 1). Further, HIV+ children had significantly higher Helios+ cells in the CD25- population compared to HIV- (p<0.0001; Sup. Fig. 1). Indeed, in most HIV+ children nearly half of memory FOXP3+Helios+ Tregs were CD25 negative, regardless of ART (Fig. 1C).
Figure 1. Conventional Treg marker CD25 underestimates memory Tregs in HIV uninfected and infected children.
(A) Singlet live CD4 T cells were first gated on CD45RO+ (memory) cells, then FOXP3+CD25+ or FOXP3+Helios+ cells to identify memory Tregs. Right graph shows paired comparison of %FOXP3+CD25+ with %FOXP3+Helios+ memory CD4 T cells in HIV negative children, with median with interquartile range. P-value reported for Wilcoxon matched-pairs signed rank test. (B) Paired comparison of %FOXP3+CD25+ (grey) and %FOXP3+Helios+ (black) memory Treg populations in HIV-, ART-, and ART+ children. P-values for paired comparisons between %FOXP3+CD25+ and %FOXP3+Helios+ memory Tregs were calculated with Wilcoxon matched-pairs signed rank test. (C) Cells were first gated on the FOXP3+Helios+ population in CD45RO+ CD4 T cells. Within FOXP3+Helios+ memory Tregs, CD25 negative cells were identified. Aggregate %CD25 negative cells within FOXP3+Helios+ memory CD4 T cells in HIV-, ART-, and ART+ children are shown on the right. Graph shows median with interquartile range. P-value reported for two-sided Mann-Whitney test. **** indicates p<0.0001.
Selective expansion of memory FOXP3+Helios+ Tregs in HIV infected children correlates with advancing HIV disease status
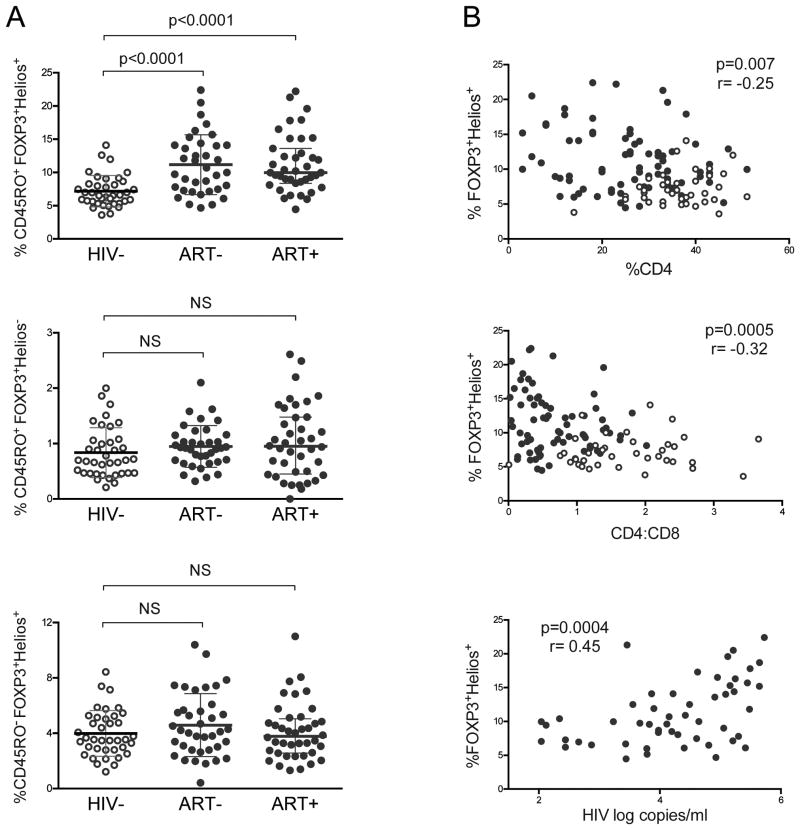
We asked if a particular subset of Tregs altered during HIV disease. First, we evaluated whether CD45RO+ (memory) FOXP3+Helios+ and FOXP3+Helios- Tregs were disrupted in HIV+ children. The percent of memory FOXP3+Helios+ Tregs was expanded in HIV+ children regardless of whether they had been treated with ART (p<0.0001; Fig. 2A). There was no significant difference between frequency of memory FOXP3+Helios- Tregs, in HIV-, ART-, and ART+ (Fig. 2A). HIV- and HIV+ children also had similar percentages of CD45RO- (naive) FOXP3+Helios+ Tregs (Fig. 2A). The frequency of naïve and memory Treg subsets had no correlation with age (Sup. Fig. 2). Total FOXP3 in CD4 T cells and in memory CD4 T cells were higher in ART- and ART+ compared to HIV- (Sup. Fig. 3A-B). Memory FOXP3+CD25+ Tregs were also higher in ART- (p=0.04) and ART+ (p=0.003) compared to HIV- and did not correlate with age (Sup. Fig. 3C-D). Thus there was a selective increase of memory FOXP3+Helios+ Tregs, but not naïve FOXP3+Helios+ or memory FOXP3+Helios- Tregs, in HIV infected children, which remained despite ART.
Figure 2. HIV infected children have selective expansion of memory FOXP3+Helios+ Tregs that correlates with advancing HIV disease status.
(A) Frequencies of FOXP3+Helios+ and FOXP3+Helios- in CD45RO+ (memory) CD4 T cells and FOXP3+Helios+ in CD45RO- (naïve) CD4 T cells in HIV-, ART-, and ART+ children. Graphs show medians with interquartile ranges. P-value reported for two-sided Mann-Whitney test with threshold significance of 0.05. (B) Percent of FOXP3+Helios+ in memory CD4 T cells vs. CD4 percent in total lymphocytes (top) and CD4:CD8 ratio (middle) in HIV-, ART-, and ART+ children. (B, bottom) Percent of FOXP3+Helios+ in memory CD4 T cells vs. plasma HIV RNA log copies/ml in ART- and ART+ children with HIV viral load above the detection limit 110 copies/ml. HIV- shown in open circles (○); HIV+ in filled circles (●). P- and r- values were calculated with Spearman's correlation test.
Next we investigated whether the expansion of memory FOXP3+Helios+ Tregs in HIV+ children correlates with their HIV disease status. We found an inverse correlation between the percent of memory FOXP3+Helios+ Tregs and CD4% in children (p=0.007, Fig. 2B). Additionally, memory FOXP3+Helios+ Tregs had a significant negative correlation with CD4:CD8 ratios, a predictor of morbidity and mortality in HIV infection (p=0.0005; Fig. 2B)19. Finally, we probed whether HIV plasma viremia was associated with the proportion of peripheral memory Tregs. Indeed, in viremic HIV+ children, HIV RNA levels correlated with increasing memory FOXP3+Helios+ Tregs (p=0.0004; Fig. 2B). Taken together, advancing HIV disease state, demonstrated by falling %CD4 and CD4:CD8 ratios and increasing HIV plasma viremia, correlated with the expansion of memory FOXP3+Helios+ Tregs in children.
Disrupted balance between memory FOXP3+Helios+ Tregs and inflammatory markers in untreated HIV infected children
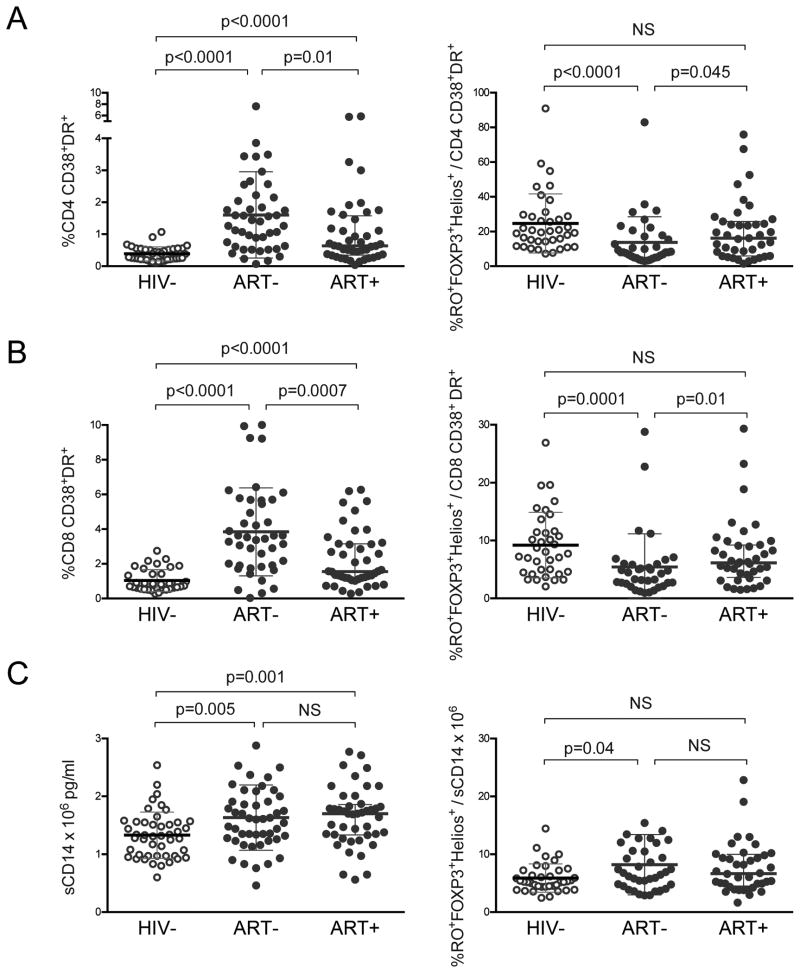
Markers of immune activation (IA), such as CD38 and HLA-DR expression on CD4 and CD8 T cells and monocyte activation marker sCD14, all rise with HIV disease progression20-22. We evaluated whether our HIV+ cohort had elevated IA markers and whether the balance between Tregs and inflammation was disrupted during HIV infection. We first observed ART- children had higher proportions of CD38+HLADR+ CD4 and CD8 T cells compared to HIV- (p<0.0001; Fig. 3A-B; gating strategy shown in Sup. Fig. 4A). ART+ had CD38+HLADR+ CD4 and CD8 T cells significantly higher than HIV- (p<0.0001), and lower than ART- children (CD4 p=0.01, CD8 p=0.0007; Fig 3A-B). Next we evaluated the ratio of memory Tregs to CD38+HLADR+ CD4 and CD8 T cells. Interestingly, ART- children had decreased ratios of Tregs/IA in CD4 and CD8 T cells (p≤0.0001, Fig 3A-B). Last, we evaluated sCD14 and found elevated levels of sCD14 in ART- (p=0.005) and ART+ (p=0.001) compared to HIV- children; additionally ART- had an increased ratio of Tregs/sCD14 (Fig 3C).
Figure 3. Disrupted balance between Tregs and inflammatory markers in untreated HIV infected children.
Comparison of the following immune activation markers in HIV-, ART-, and ART+ children: (A) %CD38+HLA-DR+ in CD4 T cells, (B) %CD38+HLA-DR+ in CD8 T cells, and (C) sCD14 plasma levels. Graphs on the right show the ratio of %FOXP3+Helios+ in CD45RO+ CD4 T cells to each marker. Comparison graphs depict medians with interquartile ranges; p-values reported for two-sided Mann-Whitney test.
Elevated CD38, Ki67, and PD-1 expression in memory FOXP3-Helios- CD4 T cells and FOXP3+Helios+ Tregs of HIV+ children
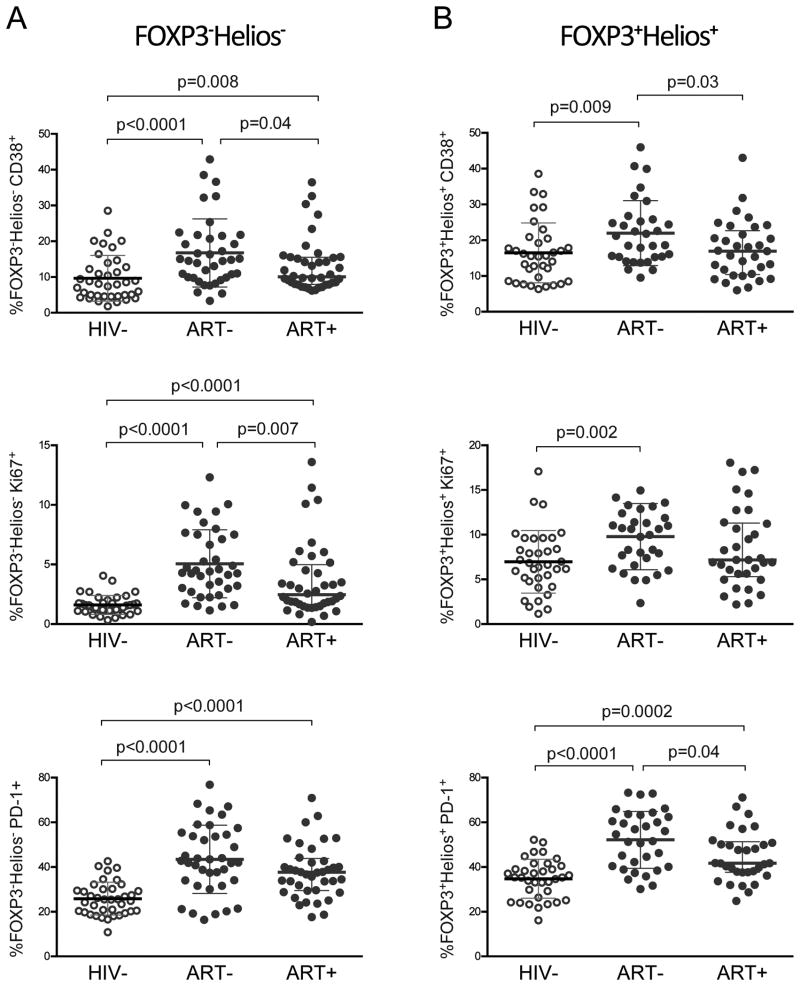
We next assessed markers of IA, CD38 and Ki67, on memory conventional CD4 T cells negative for FOXP3 and Helios and memory FOXP3+Helios+ Tregs (gating strategy shown in Sup. Fig. 4B). HIV+ children had higher memory FOXP3-Helios- CD38+ CD4 T cells compared with HIV- (ART- p<0.0001, ART+ p=0.008; Fig. 4A). Similarly, Ki67 expression in memory FOXP3-Helios- CD4 T cells was raised in ART- and ART+ compared to HIV- children (p<0.0001; Fig. 4A). We further evaluated PD-1 expression, which marks activation in early disease states and immune exhaustion in chronic disease states. PD-1+ memory FOXP3-Helios- CD4 T cells were elevated in ART- and ART+ compared to HIV- (p<0.0001; Fig. 4A)
Figure 4. Elevated CD38, Ki67, and PD-1 expression in memory FOXP3-Helios- CD4 T cells and FOXP3+Helios+ Tregs of HIV+ children.
(A) Comparison of percent CD38 (top), Ki67 (middle), and PD-1 (bottom) expression between HIV-, ART-, and ART+ children in (A) FOXP3-Helios- and (B) FOXP3+Helios+ in CD45RO+ CD4 T cells. Graphs depict median with interquartile range and p-value for two-sided Mann-Whitney test.
Next, we investigated whether CD38, Ki67, and PD-1, which were elevated in memory FOXP3-Helios- CD4 T cells from HIV+ children, were also expressed at higher levels in memory FOXP3+Helios+ Tregs (Sup. Fig 4B). ART- but not ART+ children, had higher CD38 and Ki67 expression in memory FOXP3+Helios+ Tregs than HIV- children (CD38: p=0.009, Ki67: p=0.002; Fig. 4B). The proportion of memory FOXP3+Helios+ Tregs expressing PD-1 was higher in both ART- (p<0.0001) and ART+ (p=0.0002) compared to HIV- children (Fig. 4B). Lastly, we evaluated CD39, a late activation marker of memory CD4 T cells and Tregs. CD39 expression in memory Tregs did not differ between HIV- and HIV+ children, but was higher in memory FOXP3-Helios- CD4 T cells of ART- and ART+ children (Sup. Fig. 5). Thus, HIV infected children had relatively increased proportions of CD38+, Ki67+, and PD-1+ memory FOXP3-Helios- CD4 T cells, with higher expression of these activation/exhaustion markers in memory FOXP3+Helios+ Tregs of ART- children.
CD38+, Ki67+, and PD-1+ memory CD4 T cells correlate with frequency and phenotype of memory FOXP+Helios+ Tregs in HIV infected children
In our final analysis, we investigated whether the activated or exhausted phenotype of memory FOXP3-Helios- CD4 T cells correlated with the frequency of memory FOXP3+Helios+ Tregs. Indeed, the proportion of CD38+, Ki67+, and PD-1+ memory FOXP3-Helios- CD4 T cells each correlated with elevated memory FOXP3+Helios+ Tregs in HIV+ (p<0.0001; Fig. 5A), but not HIV- children (Sup. Fig 6).
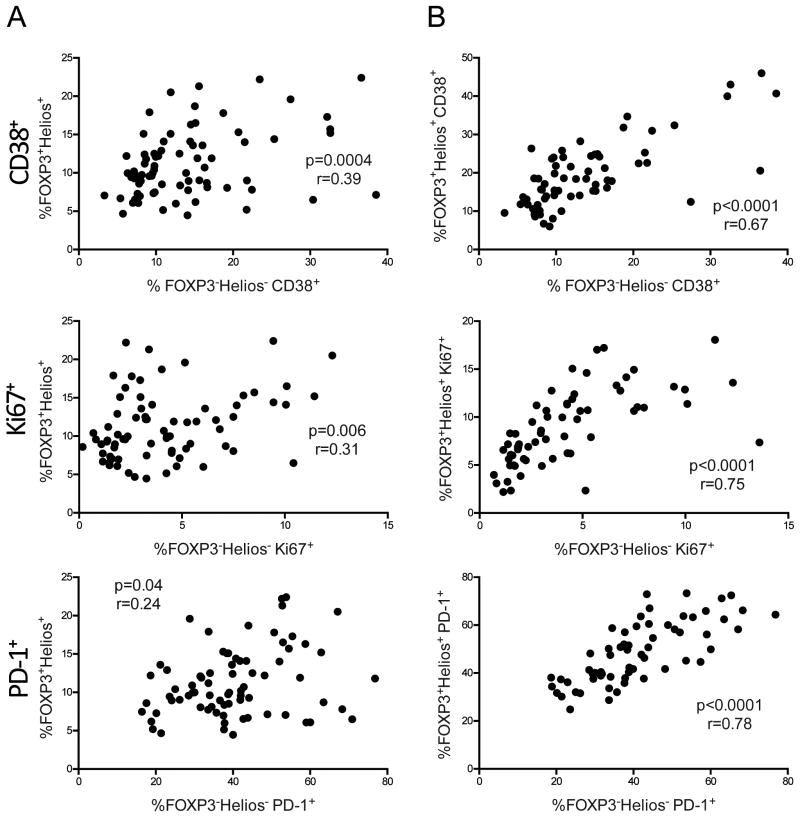
Figure 5. CD38+, Ki67+, and PD-1+ memory CD4 T cells correlate with frequency and phenotype of memory FOXP+Helios+ Tregs in HIV infected children.
(A) Correlation between CD38+, Ki67+, and PD-1+ memory FOXP3-Helios- CD4 T cells and memory FOXP3+Helios+ Tregs in HIV infected children. (B) Correlation between FOXP3-Helios- and FOXP3+Helios+ memory CD4 T cells expressing CD38, Ki67, and PD-1 in HIV infected children. All data shown was gated on CD45RO+ memory CD4 T cells. P- and r- values were calculated with Spearman's correlation test.
We next asked whether there was an association between immune activation/exhaustion phenotypes in memory FOXP3-Helios- CD4 T cells and FOXP3+Helios+ Tregs. Remarkably, there was a strong correlation between the percent of CD38+ and Ki67+ cells in memory FOXP3-Helios- CD4 T cells and FOXP3+Helios+ Tregs in HIV+ children (p<0.0001; Fig. 5B). PD-1 expression in memory FOXP3-Helios- CD4 T cells also tightly correlated with frequency in memory FOXP3+Helios+ Tregs in HIV+ children (p<0.0001; Fig. 5B). Interestingly, these correlations between phenotype in memory Tregs and CD4 T cells were also present in HIV- children (Sup. Fig 6). Thus, CD38, Ki67, and PD-1 expression in memory FOXP3+Helios+ Tregs all closely mirrored levels in memory FOXP3-Helios- CD4 T cells.
Discussion
We demonstrated that defining peripheral human Tregs by FOXP3 expression in conjunction with Helios rather than CD25 reveals markedly higher frequencies of Tregs, particularly in HIV+ children. Second, HIV infected children had a selective expansion of memory FOXP3+Helios+ Tregs regardless of ART. Third, the rise in Tregs correlated with advancing HIV disease status, indicated by falling CD4 percentages and CD4:CD8 ratios and increasing HIV plasma viremia. Fourth, the balance between Tregs and systemic immune activation is disrupted in untreated HIV+ children. Finally, increased expression of activation and exhaustion markers, CD38, Ki67, and PD-1, in memory CD4 T cells was associated with elevated memory Tregs and tightly correlated with a similar phenotype in FOXP3+Helios+ Tregs during HIV infection.
We postulated Helios may be an advantageous marker to identify bona fide Tregs in the context of conditions causing immune activation such as HIV, because unlike CD25, its expression remains stable during T cell activation. In our cohort of HIV negative-unexposed children from Kenya, the FOXP3+Helios+ subset identified markedly more Tregs than FOXP3 and CD25 coexpression. Approximately 75% of FOXP3+CD25+ Tregs were Helios+, a level consistent with our previous study of healthy and HIV+ adults and other reports in healthy adults, neonates, and vertically infected HIV+ children in the United States14,15,17,23. Importantly, the traditional Treg phenotype of FOXP3+CD25+ cells underestimated the proportion of Tregs in HIV+ children considerably more than in HIV- children. Regardless of ART, HIV infected children had significantly more CD25 negative cells in the FOXP3+Helios+ Treg population, amounting to nearly half of Helios+ Tregs. These findings highlight the importance of identifying Tregs by FOXP3 and Helios coexpression rather than CD25, particularly in HIV, and perhaps in other chronic diseases with inflammatory states.
Next, we demonstrated that both treated and untreated HIV+ children have significantly higher memory FOXP3+Helios+ Tregs compared to HIV- children. Interestingly, naïve FOXP3+Helios+ Tregs and memory FOXP3+Helios- Tregs were not disrupted in HIV infected children. These findings are similar to our evaluation of an adult HIV- and ART+ cohort in the United States15. Moreover, frequency of memory FOXP3+Helios+ Tregs was similar in children and adults from Kenya and the United States respectively, suggesting geography and varying cohorts may not account for the variation in previous reports of Tregs. Although this was a cross-sectional study, the cohorts of ART- children, who had low CD4 percentages and the presence of HIV plasma viremia, and ART+ children, who had recovering CD4 levels and HIV viral suppression, provide some insight into whether immunologic disruptions may reverse with antiretroviral drugs. The significant increase in memory FOXP3+Helios+ Treg frequency in both ART- and ART+ children suggests that this specific perturbation fails to normalize despite effective ART. Whether this increase in Treg frequency occurs due to enhanced Treg proliferation or resistance to cell death is unclear. Increased Ki67 expression in memory FOXP3+Helios+ Tregs in ART- children suggests that the higher frequency of memory FOXP3+Helios+ Tregs in untreated HIV+ children may be due to active proliferation of this subset. ART+ children, who had Ki67 expression in memory FOXP3+Helios+ Tregs similar to HIV- children, conceivably may have prolonged survival of memory Tregs.
Overall, the rise of memory FOXP3+Helios+ Tregs was strongly associated with falling CD4 percentages and CD4:CD8 ratios and increasing HIV plasma viremia. One possible explanation for this finding is the expanded memory Tregs may inhibit HIV specific immune responses, leading to plasma viremia and depletion of CD4 T cells. Hunt et al. demonstrated low regulatory T cells were associated with higher HIV specific T cell responses in HIV controllers, which supports this explanation24. In our cohort, both ART- and ART+ had evidence of T cell and monocyte activation with elevated expression of CD38, HLA-DR and Ki-67 and increased levels of sCD14 compared to HIV- children. Moreover, ART+ children had relatively lower expression of these markers compared to ART-, suggesting ART may partially mitigate but not completely eliminate immune activation. Our findings pose the question of why chronic immune activation remains despite substantial increases in memory Tregs, which presumptively function to suppress this activation. One potential explanation may be that such a high degree of chronic inflammation exceeds the suppressive capacity of Tregs, despite an expanded pool. The diminished ratio of Tregs to activated CD4 and CD8 T cells in untreated HIV+ children suggests that, until the virus is suppressed, immune activation dominates over the relative rise of Tregs. Alternatively, a portion of Tregs may be non-functional, or exhausted, similar to effector T cells in chronic HIV infection. This may explain persistent IA in treated HIV+ children, who maintained a balance of Tregs/IA, but had increased PD-1+ Tregs compared to HIV- children.
Remarkably, memory FOXP3+Helios+ Tregs also expressed activation markers CD38 and Ki67, with higher expression in ART- compared with HIV- children. Expression of CD38 and Ki67 in memory FOXP3+Helios+ Tregs directly correlated with their expression in FOXP3-Helios- memory CD4 T cells, suggesting Tregs are also susceptible to the generalized immune activation that occurs in HIV infection. Although ART+ children had elevated CD38 and Ki67 levels in memory CD4 T cells, they did not have a similar increase in memory Tregs, indicating ART may alleviate the activated state in Tregs. Memory Tregs also expressed inhibitory molecule PD-1 with expression levels equivalent or greater than in memory CD4 T cells. Both ART+ and ART- children had higher PD-1+ memory FOXP3-Helios- CD4 T cells and FOXP3+Helios+ Tregs compared with HIV- children. Interestingly, a recent study in the mouse lymphocytic choriomeningitis virus model noted upregulation of PD-1 on Tregs in chronically infected mice. These PD-1+ Tregs had enhanced suppressive capacity mediated by the interaction between PD-1 on Tregs and PD-1 ligand on CD8 T cells25. Whether human PD-1+ Tregs have a similar function remains to be determined in future studies.
In children, an additional consequence of increased memory Tregs may be dampened responses to essential vaccines. Prior studies demonstrate HIV+ infants on ART develop protective immunity following some childhood vaccines such as pneumococcus, tetanus toxoid, and diphtheria toxoid vaccines26-30. However, their responses to haemophilus influenza, hepatitis B and yearly influenza vaccines highly vary26,31-34. Linking vaccine responses to Treg frequency, one study demonstrated weakened responses to H1N1 pandemic influenza vaccine in HIV infected youth with higher proportions of Tregs35. Thus, development of immune therapies targeting Tregs may allow an improved response to these vaccines and perhaps even enhance HIV-specific immune responses. Treg depletion may also be a strategy to boost the efficacy of potential HIV vaccine candidates. Exploring such immunotherapies are particularly important for HIV infected children, as the long-term consequences of persistent inflammation beginning at a young age may include impaired neurocognitive development, early cardiovascular disease, metabolic complications, and premature aging4,5,18.
In conclusion, our findings demonstrate major perturbations in the Treg compartment of HIV infected children. We have shown FOXP3 and Helios coexpression revealed a significant portion of bona fide Tregs undetected by conventional Treg markers. Despite ART, HIV infected children had significantly elevated memory FOXP3+Helios+ Tregs, which correlated with falling CD4 percentages and CD4:CD8 ratios, and increasing HIV plasma viremia and systemic immune activation. Additionally, the balance between Tregs and inflammatory markers was disrupted in untreated HIV+ children. Finally, memory FOXP3+Helios+ Tregs exhibited an activated and exhausted phenotype that tightly correlated with a similar phenotype in memory FOXP3-Helios- CD4 T cells. Questions for future research include evaluating whether this expanded Treg population protects or impairs the host's overall clinical state and investigating the functional significance of activation and inhibitory molecules expressed on Tregs. Understanding the role of Tregs during HIV infection may lead to novel biotherapeutic targets that mitigate HIV disease progression or boost HIV vaccine efficacy.
Supplementary Material
Supplemental Table 1: Demographic and clinical characteristics of participants
Age, sex, %CD4, and HIV viral load in three participant categories of children ages 5-20 years: (1) HIV unexposed and uninfected (HIV-), (2) HIV+, naïve to antiretroviral therapy (ART-), and (3) HIV+ on antiretroviral therapy for at least 6 months (ART+). (*) Median values are shown with upper and lower quartile ranges. P-values were calculated with significance threshold of 0.05 using (a) Kruskal-Wallis test for age and percent CD4, (b) Chi-Square test for sex, and (c) two-sided Mann-Whitney test for plasma HIV RNA log copies/ml.
Supplemental Figure 1: Helios expression in memory FOXP3+CD25+ and FOXP3+CD25- populations
(A) FOXP3+CD25+ and FOXP3+CD25- populations in CD45RO+ CD4 T cells, and the percent of Helios+ cells within each of these gates. (B) Aggregate data of percent of Helios+ cells within FOXP3+CD25+ (left) and FOXP3+CD25- (right) memory CD4 T cells in HIV-, ART-, and ART+ children.
Supplemental Figure 2: Treg subsets do not correlate with age
Age in years vs. percent of (A) CD45RO+ (memory) FOXP3+Helios+, (B) memory FOXP3+Helios-, and (C) CD45RO- (naïve) FOXP3+Helios+ Treg subsets. P-values were calculated with Spearman's correlation test and significance threshold of 0.05.
Supplemental Figure 3: Expanded memory FOXP3+CD25+ Tregs in HIV+ children
Comparison of %FOXP3+ cells within (A) total CD4 T cells and (B) memory CD45RO+ CD4 T cells. (C) FOXP3+CD25+ Tregs gated on CD45RO+ CD4 T cells in HIV-, ART-, and ART+ children. Graph shows median with interquartile range. P-value reported for two-sided Mann-Whitney test. (D) Age in years vs. percent of CD45RO+ FOXP3+CD25+ Tregs. P-values were calculated with Spearman's correlation test and significance threshold of 0.05.
Supplemental Figure 4: FACS plots and gating strategy for CD38, HLA-DR, Ki67, and PD-1
Representative FACS plots and gating strategies are shown for activation and exhaustion markers. (A) Cells were first gated on CD4+ and CD8+ populations within CD3+ T cells. CD38+HLA-DR+ gate is shown within CD4 and CD8 T cells. (B) FOXP3-Helios- and FOXP3+Helios+ populations gates were made on CD4 T cells. CD45RO vs. CD38, Ki67, and PD-1 plots, are shown to identify the CD38+, Ki67+, and PD-1+ populations within memory CD45RO+ CD4 T cells.
Supplemental Figure 5: CD39 in memory FOXP3-Helios- CD4 T cells and FOXP3+Helios+ Tregs
Comparison of percent CD39 expression in (A) memory FOXP3-Helios- CD4 T cells and (B) memory FOXP3+Helios+ CD4 T cells in HIV-, ART-, and ART+ children. Graphs depict medians with interquartile ranges and p-value for two-sided Mann-Whitney test.
Supplemental Figure 6: Correlation between CD38+, Ki67+, and PD-1+ memory CD4 T cells and frequency and phenotype of memory FOXP+Helios+ Tregs in HIV negative children
(A) Correlation between CD38+, Ki67+, and PD-1+ memory FOXP3-Helios- CD4 T cells and memory FOXP3+Helios+ Tregs in HIV negative children. (B) Correlation between FOXP3-Helios- and FOXP3+Helios+ memory CD4 T cells expressing CD38, Ki67, and PD-1 in HIV uninfected children. All data shown was gated on CD45RO+ memory CD4 T cells. P- and r- values were calculated with Spearman's correlation test.
Acknowledgments
We thank all of the children and families who participated in this study. We thank Dr. Mengling Liu for biostatistics consultation. Last, we thank Dave Mellert and Lina Kozhaya for critical reading and valuable suggestions. This study was funded by NIH grants 5K08AI093235-02 to AK and R01AI065303 to DU.
Funding Sources: This study was funded by NIH grants 5K08AI093235-02 to AK and R01AI065303 to DU.
Footnotes
Disclosures: The authors have no financial conflicts of interest.
References
- 1.Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nature reviews Immunology. 2008;8(7):523–532. doi: 10.1038/nri2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakaguchi S, Vignali DA, Rudensky AY, Niec RE, Waldmann H. The plasticity and stability of regulatory T cells. Nature reviews Immunology. 2013;13(6):461–467. doi: 10.1038/nri3464. [DOI] [PubMed] [Google Scholar]
- 3.Chevalier MF, Weiss L. The split personality of regulatory T cells in HIV infection. Blood. 2013;121(1):29–37. doi: 10.1182/blood-2012-07-409755. [DOI] [PubMed] [Google Scholar]
- 4.Hearps AC, Martin GE, Rajasuriar R, Crowe SM. Inflammatory co-morbidities in HIV+ individuals: learning lessons from healthy ageing. Curr HIV/AIDS Rep. 2014;11(1):20–34. doi: 10.1007/s11904-013-0190-8. [DOI] [PubMed] [Google Scholar]
- 5.Narayan KM, Miotti PG, Anand NP, et al. HIV and noncommunicable disease comorbidities in the era of antiretroviral therapy: a vital agenda for research in low- and middle-income country settings. J Acquir Immune Defic Syndr. 2014;67(Suppl 1):S2–7. doi: 10.1097/QAI.0000000000000267. [DOI] [PubMed] [Google Scholar]
- 6.Cerrato E, Calcagno A, D'Ascenzo F, et al. Cardiovascular disease in HIV patients: from bench to bedside and backwards. Open Heart. 2015;2(1):e000174. doi: 10.1136/openhrt-2014-000174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moreno-Fernandez ME, Presicce P, Chougnet CA. Homeostasis and function of regulatory T cells in HIV/SIV infection. J Virol. 2012;86(19):10262–10269. doi: 10.1128/JVI.00993-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hori S. Lineage stability and phenotypic plasticity of Foxp3(+) regulatory T cells. Immunol Rev. 2014;259(1):159–172. doi: 10.1111/imr.12175. [DOI] [PubMed] [Google Scholar]
- 9.Sawant DV, Vignali DA. Once a Treg, always a Treg? Immunol Rev. 2014;259(1):173–191. doi: 10.1111/imr.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou AX, Kozhaya L, Fujii H, Unutmaz D. GARP-TGF-beta complexes negatively regulate regulatory T cell development and maintenance of peripheral CD4+ T cells in vivo. Journal of immunology. 2013;190(10):5057–5064. doi: 10.4049/jimmunol.1300065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang R, Kozhaya L, Mercer F, Khaitan A, Fujii H, Unutmaz D. Expression of GARP selectively identifies activated human FOXP3+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(32):13439–13444. doi: 10.1073/pnas.0901965106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran DQ, Ramsey H, Shevach EM. Induction of FOXP3 expression in naive human CD4+FOXP3 T cells by T-cell receptor stimulation is transforming growth factor-beta dependent but does not confer a regulatory phenotype. Blood. 2007;110(8):2983–2990. doi: 10.1182/blood-2007-06-094656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran DQ, Andersson J, Wang R, Ramsey H, Unutmaz D, Shevach EM. GARP (LRRC32) is essential for the surface expression of latent TGF-beta on platelets and activated FOXP3+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(32):13445–13450. doi: 10.1073/pnas.0901944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thornton AM, Korty PE, Tran DQ, et al. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. Journal of immunology. 2010;184(7):3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mercer F, Khaitan A, Kozhaya L, Aberg JA, Unutmaz D. Differentiation of IL-17-producing effector and regulatory human T cells from lineage-committed naive precursors. Journal of immunology. 2014;193(3):1047–1054. doi: 10.4049/jimmunol.1302936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Himmel ME, MacDonald KG, Garcia RV, Steiner TS, Levings MK. Helios+ and Helios- cells coexist within the natural FOXP3+ T regulatory cell subset in humans. Journal of immunology. 2013;190(5):2001–2008. doi: 10.4049/jimmunol.1201379. [DOI] [PubMed] [Google Scholar]
- 17.Rabe H, Nordstrom I, Andersson K, Lundell AC, Rudin A. Staphylococcus aureus convert neonatal conventional CD4(+) T cells into FOXP3(+) CD25(+) CD127(low) T cells via the PD-1/PD-L1 axis. Immunology. 2014;141(3):467–481. doi: 10.1111/imm.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warriner AH, Burkholder GA, Overton ET. HIV-related metabolic comorbidities in the current ART era. Infect Dis Clin North Am. 2014;28(3):457–476. doi: 10.1016/j.idc.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 19.Serrano-Villar S, Sainz T, Lee SA, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. 2014;10(5):e1004078. doi: 10.1371/journal.ppat.1004078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klatt NR, Funderburg NT, Brenchley JM. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013;21(1):6–13. doi: 10.1016/j.tim.2012.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 22.Shive CL, Jiang W, Anthony DD, Lederman MM. Soluble CD14 is a nonspecific marker of monocyte activation. Aids. 2015;29(10):1263–1265. doi: 10.1097/QAD.0000000000000735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Degaffe G, Zakhour R, Zhang W, et al. Forkhead box protein 3(+) regulatory T cells and Helios(+) subset in perinatally acquired HIV. Clin Exp Immunol. 2015;180(1):108–117. doi: 10.1111/cei.12560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hunt PW, Landay AL, Sinclair E, et al. A low T regulatory cell response may contribute to both viral control and generalized immune activation in HIV controllers. PLoS One. 2011;6(1):e15924. doi: 10.1371/journal.pone.0015924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park HJ, Park JS, Jeong YH, et al. PD-1 Upregulated on Regulatory T Cells during Chronic Virus Infection Enhances the Suppression of CD8+ T Cell Immune Response via the Interaction with PD-L1 Expressed on CD8+ T Cells. Journal of immunology. 2015;194(12):5801–5811. doi: 10.4049/jimmunol.1401936. [DOI] [PubMed] [Google Scholar]
- 26.Sutcliffe CG, Moss WJ. Do children infected with HIV receiving HAART need to be revaccinated? The Lancet Infectious diseases. 2010;10(9):630–642. doi: 10.1016/S1473-3099(10)70116-X. [DOI] [PubMed] [Google Scholar]
- 27.Ching N, Deville JG, Nielsen KA, et al. Cellular and humoral immune responses to a tetanus toxoid booster in perinatally HIV-1-infected children and adolescents receiving highly active antiretroviral therapy (HAART) European journal of pediatrics. 2007;166(1):51–56. doi: 10.1007/s00431-006-0184-2. [DOI] [PubMed] [Google Scholar]
- 28.Abzug MJ, Song LY, Levin MJ, et al. Antibody persistence and immunologic memory after sequential pneumococcal conjugate and polysaccharide vaccination in HIV-infected children on highly active antiretroviral therapy. Vaccine. 2013;31(42):4782–4790. doi: 10.1016/j.vaccine.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bamford A, Kelleher P, Lyall H, et al. Serological response to 13-valent pneumococcal conjugate vaccine in children and adolescents with perinatally acquired HIV infection. Aids. 2014;28(14):2033–2043. doi: 10.1097/QAD.0000000000000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Borkowsky W, Rigaud M, Krasinski K, Moore T, Lawrence R, Pollack H. Cell-mediated and humoral immune responses in children infected with human immunodeficiency virus during the first four years of life. The Journal of pediatrics. 1992;120(3):371–375. doi: 10.1016/s0022-3476(05)80899-6. [DOI] [PubMed] [Google Scholar]
- 31.Mangtani P, Mulholland K, Madhi SA, Edmond K, O'Loughlin R, Hajjeh R. Haemophilus influenzae type b disease in HIV-infected children: a review of the disease epidemiology and effectiveness of Hib conjugate vaccines. Vaccine. 2010;28(7):1677–1683. doi: 10.1016/j.vaccine.2009.12.011. [DOI] [PubMed] [Google Scholar]
- 32.Madhi SA, Kuwanda L, Saarinen L, et al. Immunogenicity and effectiveness of Haemophilus influenzae type b conjugate vaccine in HIV infected and uninfected African children. Vaccine. 2005;23(48-49):5517–5525. doi: 10.1016/j.vaccine.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 33.O'Loughlin RE, Edmond K, Mangtani P, et al. Methodology and measurement of the effectiveness of Haemophilus influenzae type b vaccine: systematic review. Vaccine. 2010;28(38):6128–6136. doi: 10.1016/j.vaccine.2010.06.107. [DOI] [PubMed] [Google Scholar]
- 34.Madhi SA, Petersen K, Khoosal M, et al. Reduced effectiveness of Haemophilus influenzae type b conjugate vaccine in children with a high prevalence of human immunodeficiency virus type 1 infection. The Pediatric infectious disease journal. 2002;21(4):315–321. doi: 10.1097/00006454-200204000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Weinberg A, Muresan P, Fenton T, et al. High proportions of regulatory B and T cells are associated with decreased cellular responses to pH1N1 influenza vaccine in HIV-infected children and youth (IMPAACT P1088) Hum Vaccin Immunother. 2013;9(5):957–968. doi: 10.4161/hv.23774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sandler NG, Wand H, Roque A, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: Demographic and clinical characteristics of participants
Age, sex, %CD4, and HIV viral load in three participant categories of children ages 5-20 years: (1) HIV unexposed and uninfected (HIV-), (2) HIV+, naïve to antiretroviral therapy (ART-), and (3) HIV+ on antiretroviral therapy for at least 6 months (ART+). (*) Median values are shown with upper and lower quartile ranges. P-values were calculated with significance threshold of 0.05 using (a) Kruskal-Wallis test for age and percent CD4, (b) Chi-Square test for sex, and (c) two-sided Mann-Whitney test for plasma HIV RNA log copies/ml.
Supplemental Figure 1: Helios expression in memory FOXP3+CD25+ and FOXP3+CD25- populations
(A) FOXP3+CD25+ and FOXP3+CD25- populations in CD45RO+ CD4 T cells, and the percent of Helios+ cells within each of these gates. (B) Aggregate data of percent of Helios+ cells within FOXP3+CD25+ (left) and FOXP3+CD25- (right) memory CD4 T cells in HIV-, ART-, and ART+ children.
Supplemental Figure 2: Treg subsets do not correlate with age
Age in years vs. percent of (A) CD45RO+ (memory) FOXP3+Helios+, (B) memory FOXP3+Helios-, and (C) CD45RO- (naïve) FOXP3+Helios+ Treg subsets. P-values were calculated with Spearman's correlation test and significance threshold of 0.05.
Supplemental Figure 3: Expanded memory FOXP3+CD25+ Tregs in HIV+ children
Comparison of %FOXP3+ cells within (A) total CD4 T cells and (B) memory CD45RO+ CD4 T cells. (C) FOXP3+CD25+ Tregs gated on CD45RO+ CD4 T cells in HIV-, ART-, and ART+ children. Graph shows median with interquartile range. P-value reported for two-sided Mann-Whitney test. (D) Age in years vs. percent of CD45RO+ FOXP3+CD25+ Tregs. P-values were calculated with Spearman's correlation test and significance threshold of 0.05.
Supplemental Figure 4: FACS plots and gating strategy for CD38, HLA-DR, Ki67, and PD-1
Representative FACS plots and gating strategies are shown for activation and exhaustion markers. (A) Cells were first gated on CD4+ and CD8+ populations within CD3+ T cells. CD38+HLA-DR+ gate is shown within CD4 and CD8 T cells. (B) FOXP3-Helios- and FOXP3+Helios+ populations gates were made on CD4 T cells. CD45RO vs. CD38, Ki67, and PD-1 plots, are shown to identify the CD38+, Ki67+, and PD-1+ populations within memory CD45RO+ CD4 T cells.
Supplemental Figure 5: CD39 in memory FOXP3-Helios- CD4 T cells and FOXP3+Helios+ Tregs
Comparison of percent CD39 expression in (A) memory FOXP3-Helios- CD4 T cells and (B) memory FOXP3+Helios+ CD4 T cells in HIV-, ART-, and ART+ children. Graphs depict medians with interquartile ranges and p-value for two-sided Mann-Whitney test.
Supplemental Figure 6: Correlation between CD38+, Ki67+, and PD-1+ memory CD4 T cells and frequency and phenotype of memory FOXP+Helios+ Tregs in HIV negative children
(A) Correlation between CD38+, Ki67+, and PD-1+ memory FOXP3-Helios- CD4 T cells and memory FOXP3+Helios+ Tregs in HIV negative children. (B) Correlation between FOXP3-Helios- and FOXP3+Helios+ memory CD4 T cells expressing CD38, Ki67, and PD-1 in HIV uninfected children. All data shown was gated on CD45RO+ memory CD4 T cells. P- and r- values were calculated with Spearman's correlation test.