Abstract
Background
Non-surgical bleeding (NSB) is the most common adverse event in patients with continuous-flow left ventricular assist devices (LVADs) and is caused by arteriovenous malformations (AVMs). We hypothesized that deregulation of an angiogenic factor, Angiopoietin-2 (Ang-2), in LVAD patients leads to increased angiogenesis and higher NSB.
Methods
Ang-2 and thrombin levels were measured by ELISA and Western blotting, respectively, in blood samples from 101 patients with heart failure (HF), LVAD, or orthotopic heart transplant (OHT). Ang-2 expression in endothelial biopsy was quantified by immunofluorescence. Angiogenesis was determined by in vitro tube formation using serum from each patient with or without Ang-2-blocking antibody. Ang-2 gene expression was measured by RT-PCR in endothelial cells incubated with plasma from each patient with or without the thrombin receptor blocker Vorapaxar.
Results
Compared with HF or OHT patients, serum levels and endothelial expression of Ang-2 were higher in LVAD patients (p=0.001 and p<0.001, respectively). This corresponded with increased angiogenic potential of serum from patients with LVADs (p<0.001), which was normalized with Ang-2 blockade. Furthermore, plasma from LVAD patients contained higher amounts of thrombin (p=0.003) which was associated with activation of the contact coagulation system. Plasma from LVAD patients induced more Ang-2 gene expression in endothelial cells (p<0.001) which was reduced with thrombin receptor blockade (p=0.013). LVAD patients with Ang-2 levels above the mean (12.32 ng/mL) had more NSB events compared with patients with Ang-2 levels below the mean (p=0.003).
Conclusions
Our findings indicate that thrombin-induced Ang-2 expression in LVAD patients leads to increased angiogenesis in vitro and may be associated with higher NSB events. Ang-2 therefore may contribute to AVM formation and subsequent bleeding in LVAD patients.
Keywords: LVAD, angiogenesis, angiopoietin-2, non-surgical bleeding, thrombin, cardiac transplant
In the last decade, left ventricular assist devices (LVADs) have emerged as an important therapeutic option for supporting patients with advanced heart failure both as a bridge to heart transplantation or as destination therapy. While the use of LVADs has improved survival and quality of life for patients with advanced heart failure, non-surgical bleeding (NSB) defined as bleeding at a non-operative site, commonly complicates the post-LVAD course and frequently leads to increased morbidity and mortality.1 Arteriovenous malformations (AVMs) in the gastrointestinal (GI) tract, nasopharynx, brain, and other tissues, are the most common cause of NSB in patients with continuous-flow LVADs2 although the etiology remains unknown. Interestingly, myocardial capillary density has been shown to be increased after LVAD implantation.3 This finding coupled with the development of AVMs suggests that LVAD implantation may be associated with deregulated angiogenesis at multiple sites.
The angiopoietins (Ang-1 and Ang-2) are a family of molecules that promote angiogenesis. Ang-1 is synthesized by perivascular cells and acts as an agonist of Tie-2, a receptor tyrosine kinase expressed on the surface of endothelial cells. Ang-1 promotes vessel maturity and stability and promotes normal vessel growth in conjunction with vascular endothelial growth factor (VEGF)4. In contrast, Ang-2 is synthesized exclusively by endothelial cells and is stored with von Willebrand Factor (vWF) within Weibel-Palade Bodies.5 Upon exocytosis from endothelial cells, Ang-2 also binds to Tie-2, thereby competitively inhibiting Ang-1, and in concert with VEGF promotes altered vessel growth.4 While both Ang-1 and Ang-2 act in concert with VEGF to promote angiogenesis, Ang-1 promotes normal vessel growth while Ang-2 promotes abnormal growth associated with vascular destabilization and inflammation.4, 6, 7 Ang-2 over-expressing mice develop dilated, redundant, tortuous capillaries and lesions of the alimentary tract.8 Indeed, increased expression of Ang-2 with a corresponding decrease in Ang-1 is associated with vascular malformations9 and gastrointestinal angiodysplasia.10
Activation of the thrombin receptor (Protease-activated Receptor-1, PAR-1) on the endothelial cell surface promotes Ang-2 expression and release from endothelial cells.11–15 Prior studies have suggested that plasma levels of thrombin may be elevated in patients with LVADs.16–18 Given the known relationship between thrombin-dependent PAR-1 activation and Ang-2 expression and release, we hypothesized that thrombin-induced Ang-2 overexpression in patients with LVADs may promote altered blood vessel growth. This study aims to evaluate the expression of Ang-2 in LVAD patients and assess its correlation with neovascularization and NSB.
Methods
Study Subjects
A cross sectional study was performed. The study included 3 groups of patients: (1) Adult patients supported with an LVAD (Thoratec Heartmate II or Heartware HVAD) at least 30 days post-implantation, (2) heart failure patients with reduced ejection fraction (HFrEF) (defined as a left ventricular ejection fraction less than 40%) without an LVAD, (3) patients with history of orthotopic heart transplantation (OHT) at least 30 days post transplantation. Patients were recruited in the outpatient Cardiology clinic or in the cardiac catheterization laboratory and were clinically stable at the time of enrollment. Patients were excluded from the study if they had decompensated heart failure, active cancer within 1 year, untreated hypoxic conditions, acute thrombosis within 6 months, severe renal disease defined as an estimated glomerular filtration rate (eGFR) less than 30 ml/min*1.73m2, or acute illness of any kind. Patients treated with direct thrombin inhibitors or Factor Xa inhibitors at the time of screening were also excluded to avoid confounding effects of these drugs on the measured activity of thrombin and associated biomarkers. Clinical information was obtained from the medical record. LVAD parameters were obtained from the LVAD control module. Blood pressure was measured using an automated blood pressure cuff which has recently been reported as the most accurate method for measuring blood pressure in patients with LVADs.19 All subjects were studied in the fasting state. The study protocol was approved by the University of Chicago Institutional Review Board and all participants provided written informed consent.
Endothelial Cell Culture
Human umbilical vein endothelial cells (HUVECs) were purchased from Lonza (Basel, Switzerland) and grown on T-75 flasks (Falcon) using Endothelial Growth Medium-2 (EGM-2, Lonza) under standard conditions (37 °C, 5% CO2). Cells were grown to 70% confluence, washed in PBS, trypsinized, and passaged. For all experiments, HUVECs were used for experiments prior to passage 7.
Measurement of Circulating Biomarkers
Peripheral venous blood was obtained by venipuncture and collected in vacutainer tubes (BD Bioscience) containing ethylenediaminetetraacetic acid (EDTA), sodium heparin, sodium citrate, or silica clot activator. Samples were immediately centrifuged at 2000 × g for 20 minutes at 4 °C. The plasma and serum fractions were collected, divided, and frozen at −80 °C for future analysis. VEGF and Ang-1 levels were measured in platelet-poor plasma (EDTA) and Ang-2 and soluble Tie-2 (sTie-2) levels were measured in serum by ELISA (R&D Systems, Minneapolis, MN). As certain phlebotomy techniques could induce artefactual thrombin formation, both thrombin and prothrombin were measured in plasma (EDTA) drawn through an 18-gauge butterfly needle from the antecubital vein. Thrombin and prothrombin levels were then determined by Western Blot using rabbit anti-human thrombin/prothrombin primary antibody (1:1000 dilution; Abcam), and horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:2500 dilution; Bio-Rad). Factor XIIa and Factor XIa levels were measured by Western blot using rabbit anti-human Factor XII C-terminal antibody (1:1000 dilution; Abcam) and mouse anti-human Factor XI light chain antibody (1 µg/mL dilution; R&D Systems) respectively and horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse secondary antibody (1:2500 dilution; Bio-Rad). Blot images were analyzed using a ChemiDoc imaging system with Bio-Rad Image Lab 5.1 software.
Harvesting Endothelial Cells from Patients
Vena caval endothelial cells were obtained from guide wires used during right heart catheterization as described.20–24 Briefly, central venous access was obtained using a modified Seldinger technique and a 6F venous sheath was placed into the femoral vein or internal jugular vein over a 0.035-inch J-wire (Arrow International, Reading PA). The J-wire was advanced as far as possible through the vena cava and endothelial cells were collected by incidental abrasion with the vessel wall. Endothelial cells were recovered from the wire by centrifugation in a dissociation buffer and plated on poly-L-lysine coated microscope slides (Sigma, St. Louis, MO). Cells were fixed immediately in 4% paraformaldehyde, washed in PBS, dried, and stored at −80 °C for further processing.
Assessment of Protein Expression by Quantitative Immunofluorescence
Samples were analyzed as described.23 Briefly, fixed endothelial cells were stained with primary antibodies against Ang-2 (1:150 dilution; R&D Systems) and vWF (1:300 dilution; Dako, Carpentaria, CA) followed by fluorescent-labeled secondary antibodies (1:200 dilution; Invitrogen, Carlsbad, CA), and then mounted under glass coverslips with Vectashield containing DAPI for nuclear identification (Vector Laboratories, Burlingame, CA). For each batch of patient-derived cells, a control slide of cultured HUVECs taken from a single passage was stained contemporaneously. Slides were imaged on an Olympus BX41 fluorescent microscope at 20 × magnification and analyzed using Image J software.25 Fluorescent intensity of Ang-2 was quantified in 20 random cells from each patient and the results averaged. Fluorescent intensity for each patient sample was then normalized to the intensity of the HUVEC control slide for the corresponding batch to correct for batch-to-batch variability in staining. Intensity is expressed in arbitrary units (AU) calculated by dividing the average fluorescent intensity from the patient sample by the average fluorescent intensity of the HUVEC control sample and multiplying by 100. Quantifications were performed by technicians, who were blinded to patient identity and cohort status.
Assessment of Angiogenic Potential of Patients’ Serum
24-well cell culture plates (Falcon) were coated with Matrigel (Corning Life Sciences, Corning, NY) and allowed to solidify at 37 °C for 1 hour. Cultured HUVECs were then washed with PBS, trypsinized, centrifuged, and resuspended in a mixture of 50% serum from individual patients with HF, LVAD, or OHT and 50% Endothelial Basal Medium-2 (EBM-2, Lonza) with growth factor additives such that the final concentration of each exogenous growth factor in the serum/EBM-2 mixture was equal to that in EGM-2 (Lonza). This mixture containing 200,000 HUVECs was then gently pipetted into the Matrigel-coated wells and incubated for 18 hours under standard conditions in the presence or absence of an Ang-2 blocking antibody (150 ng/mL, azide-free mouse-anti-human-Ang-2, Adipogen, San Diego, CA), which specifically inhibits binding of Ang-2 to Tie-2 but does not affect binding of Ang-1 to Tie-2. Cultures were then stained with Calcein (Corning) to improve microtube visibility. Microtube formation was assessed by microscopy as described26. Briefly, total tube number in a low power field was quantified (5 fields per well were averaged). All quantifications were performed by technicians who were blinded to patient identity and cohort status.
Assessment of the Effect of Thrombin on Angiopoietin-2 Expression
Plasma was obtained from an antecubital vein through an 18-gauge butterfly needle and anticoagulated with fondaparinux (1 µg/mL). Samples were gently mixed, centrifuged at 2000 × g for 20 minutes at 4 °C, divided, and frozen at −80 °C. HUVECs were grown to 70% confluence on 6-well plates (Falcon) under standard conditions and serum-starved overnight in EBM-2 supplemented with 2% fetal bovine serum (FBS, Life Technologies) but devoid of supplemental growth factors. Cultures were then incubated with the thrombin receptor blocker Vorapaxar (100 µg/mL, Adooq Bioscience, Irvine, CA) or vehicle for 2 hours. During this time, the frozen plasma samples obtained previously were warmed to 37 °C, mixed, and centrifuged at 2000 × g for 10 minutes. The plasma sample from each patient was then divided and mixed with either Vorapaxar (100 µg/mL in PBS) or an equal amount of PBS alone. After 2 hours, the cell culture media was carefully aspirated from each well and the plasma samples with or without Vorapaxar were added to each well. Cultures were then incubated for an additional 4 hours under standard conditions. After incubation, the plasma was aspirated and the cultures were washed with PBS. RNA was isolated using a PureLink RNA Mini Kit (Life Technologies) and Ang-2 gene expression was quantified by RT-PCR.
Statistical Analyses
Statistical analyses were preformed using SPSS version 23.0. Continuous variables such as biomarkers were compared among groups using the Kruskal-Wallis test, followed by pairwise post hoc comparisons using the Mann-Whitney U test with Bonferroni adjustment when the omnibus test indicated a significant difference among the cohorts. Treatment conditions were compared within groups using the Wilcoxon Signed-Rank test. Categorical variables such as bleeding events were compared using Fisher’s exact test. Pearson correlation was used to evaluate the relationship between serum levels of Ang-2 and endothelial tube formation on Matrigel. Clinical characteristics were compared using ANOVA or Student’s t-test for continuous variables or Pearson Chi-Square testing for categorical variables as appropriate. Ordinal variables such as NYHA HF class were compared using the Wilcoxon Rank Sum test. Data are presented as mean ± standard deviation unless otherwise indicated. A 2-sided P value of <0.05 was considered statistically significant.
Results
We enrolled 32 patients with HF, 44 patients with LVADs, and 25 patients with OHT. Clinical characteristics are shown in the Table with subgroups shown in Tables S1–3. All groups were similar in age, sex, race, and renal function. As expected, the NYHA HF Class was higher (worse) for the HF group compared with the LVAD group. Both the LVAD and OHT groups were studied approximately 300 days post-implant. Among the LVAD cohort, 32 patients with a Thoratec Heartmate II and 12 patients with a Heartware HVAD were studied. LVAD flow (HM2 5.4±1.2, HVAD 4.6±1.3 L/min, p=0.077), C-reactive protein (HM2 77.9±44.8, HVAD 39.2±37.5 mg/dL, p=0.192), and lactate dehydrogenase (HM2 386.7±206.0, HVAD 309.3±205.0 U/L, p=0.272) were similar between both groups. Pulse pressure was slightly higher in the HVAD group compared with Heartmate II (35.17±12.50 vs. 27.86±5.64 mmHg, p=0.008). Mean rotor speed was 9115.5±389.3 rpm for patients with a Heartmate II and 2746.7±135.7 rpm for patients with an HVAD. The pulsatility index for Heartmate II patients was 5.6±1.2.
Table.
Clinical Characteristics
| HF | LVAD | OHT | p-value | |
|---|---|---|---|---|
| Number of participants | 32 | 44 | 25 | |
| Age (years) | 62.8±11.9 | 58.8±10.7 | 54.1±10.9 | 0.016 |
| Female (%) | 31 | 27 | 24 | 0.829 |
| Black race (%) | 34 | 41 | 32 | 0.422 |
| Left Ventricular Ejection Fraction (%) | 27.3±9.1 | --- | 59.6±8.4 | <0.001 |
| Days Post Implant (Median) | --- | 295.0±479.2 | 311.0±1257.9 | 0.100 |
| BMI (kg/m2) | 32.7±13.9 | 30.7±7.6 | 28.1±4.7 | 0.217 |
| eGFR (ml/min*1.73m2) | 68.7±20.6 | 59.6±24.5 | 65.8±21.9 | 0.208 |
| Dilated Cardiomyopathy (%) | 53 | 59 | 44 | 0.482 |
| Ischemic Cardiomyopathy (%) | 41 | 43 | 40 | 0.959 |
| Myocarditis (%) | 0 | 2 | 12 | 0.052 |
| Hypertension (%) | 56 | 46 | 52 | 0.640 |
| Diabetes mellitus (%) | 22 | 36 | 48 | 0.115 |
| Dyslipidemia (%) | 50 | 50 | 56 | 0.873 |
| NYHA HF Class (%) | <0.001 | |||
| 1 | 3 | 9 | --- | |
| 2 | 22 | 70 | --- | |
| 3 | 69 | 21 | --- | |
| 4 | 0 | 0 | --- | |
| Heart rate (beats/min) | 78.1±17.5 | 83.0±16.9 | 95.1±11.8 | 0.001 |
| Mean Arterial Pressure (mmHg) | 88.3±14.7 | 84.4±17.6 | 96.2±11.1 | 0.011 |
| Pulse Pressure (mmHg) | 49.6±15.7 | 29.8±8.8 | 47.0±9.7 | <0.001 |
| Hemoglobin (mg/dL) | 12.7±1.7 | 11.5±1.7 | 12.8±1.7 | 0.002 |
| B-type natriuretic peptide (ng/L) | 3025.3±3492.0 | 2345.6±1996.1 | --- | 0.371 |
| Total cholesterol (mg/dL) | 173.0±55.2 | 135.4±47.3 | 166.9±35.8 | 0.010 |
| HDL (mg/dL) | 51.9±24.3 | 34.3±14.1 | 46.2±14.8 | 0.003 |
| LDL (mg/dL) | 95.4±44.0 | 74.9±35.1 | 93.3±29.4 | 0.096 |
| INR | 1.6±0.7 | 1.8±0.5 | 1.1±0.1 | <0.001 |
| Platelet count (#/µL) | 225.3±63.2 | 223.6±71.7 | 193.0±72.2 | 0.150 |
| Statin (%) | 44 | 59 | 96 | <0.001 |
| Warfarin (%) | 44 | 93 | 4 | <0.001 |
| ACE-I/ARB (%) | 84 | 46 | 12 | <0.001 |
| Anti-platelets (%) | 53 | 90 | 52 | <0.001 |
Elevated Circulating Ang-2 and Associated Biomarkers in Patients with LVADs
To evaluate the circulating levels of Ang-2, Ang-1, sTie-2, and VEGF in patients with and without an LVAD, we measured serum levels of Ang-2 and sTie-2 and platelet-poor plasma levels of Ang-1 and VEGF by ELISA (Figure 1a–d). Notably, Ang-2 was higher in patients with LVADs compared with HF or OHT (12.32±9.57, 5.24±2.98, and 4.39±2.00 ng/mL respectively, omnibus p=0.001, HF vs. LVAD p=0.012, LVAD vs. OHT p=0.003). Soluble Tie-2 was similarly elevated in patients with LVADs (LVAD 22.73±6.85, HF 18.97±4.39, and OHT 16.05±4.92 ng/mL, omnibus p=0.004, HF vs. LVAD p=0.213, LVAD vs. OHT p=0.004), possibly due to receptor shedding in response to the action of Ang-2. In contrast, Ang-1 trended lower in patients with LVADs compared with HF (3.73±3.66, 6.94±6.39 ng/mL respectively, p=0.055) and the Ang-1/Ang-2 ratio was lower in patients with LVADs. Interestingly, VEGF was not significantly different in patients with LVADs or HF but trended lower in patients with OHT (147.17±185.57, 181.77±182.46, and 50.48±33.17 pg/mL respectively, omnibus p=0.191). This finding is consistent with prior reports that VEGF is not significantly different in patients with LVADs compared with HF27 but is decreased after OHT28. These findings demonstrate a shift in Tie-2 regulation from Ang-1 in patients with HF to Ang-2 in patients with LVADs in the presence of continued over-expression of VEGF, a constellation that favors abnormal angiogenesis.7, 29–31 In subset analysis, Ang-2 was significantly higher in LVAD patients with HVAD vs. Heartmate II without a significant difference in the other biomarkers measured (Table S4). No significant relationship was noted between length of LVAD support and any biomarker tested (Table S5). The relationships between biomarkers and warfarin or antiplatelets were challenging to interpret due to extremely small patient numbers in the sub-groups (Tables S6a–d). Levels of VEGF appeared to be higher in LVAD patients whose aortic valve opened with every beat while no relationship was seen between aortic valve opening and the other biomarkers (Table S7a).
Figure 1. Altered blood levels of angiogenic proteins in patients with LVADs.
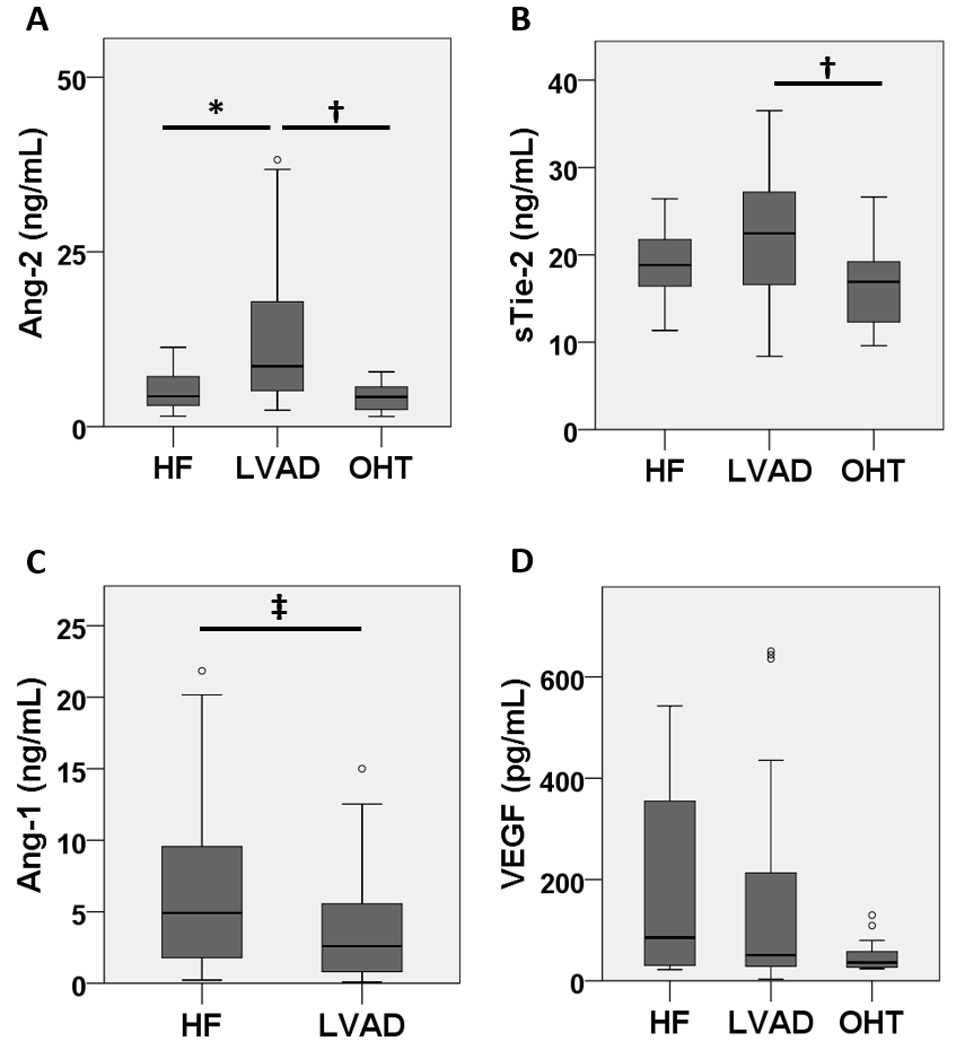
Blood levels of Ang-1, Ang-2, VEGF, and Tie-2 were measured by ELISA and patients with HF, LVAD, or OHT (n=17, 38, and 14 repsectively). (a) Ang-2 was significantly higher in LVAD patients compared with HF and OHT. (b) Soluble Tie-2 (sTie-2) was significantly higher in LVAD patients compared with OHT and increased non-significantly from patients with HF. (c) Ang-1 trended lower in LVAD patients compared with HF. (d) Plasma VEGF remained elevated in patients with LVADs compared with HF but trended lower in patients with OHT. * p<0.05, † p<0.01, ‡ p=0.055
Elevated Ang-2 Expression in Freshly Isolated Endothelial Cells from Patients with LVADs
To investigate the source of the elevated circulating Ang-2 in patients with LVADs, we analyzed freshly-isolated vena caval endothelial cells from patients with HF, LVAD, or OHT using quantitative immunofluorescence. Consistent with our finding of elevated circulating Ang-2 in blood of patients with LVADs, Ang-2 protein expression in freshly isolated endothelial cells was also higher in patients with LVADs compared with HF or OHT (52.4±19.9, 24.2±10.2, 35.0±11.2 AU, omnibus p<0.001, HF vs. LVAD p<0.001, LVAD vs. OHT p=0.029) (Figure 2). These findings suggest that over-expression of Ang-2 in the endothelium may be responsible for the elevated circulating Ang-2 levels in patients with LVADs.
Figure 2. Endothelial Angiopoietin-2 expression is increased in patients with LVADs.
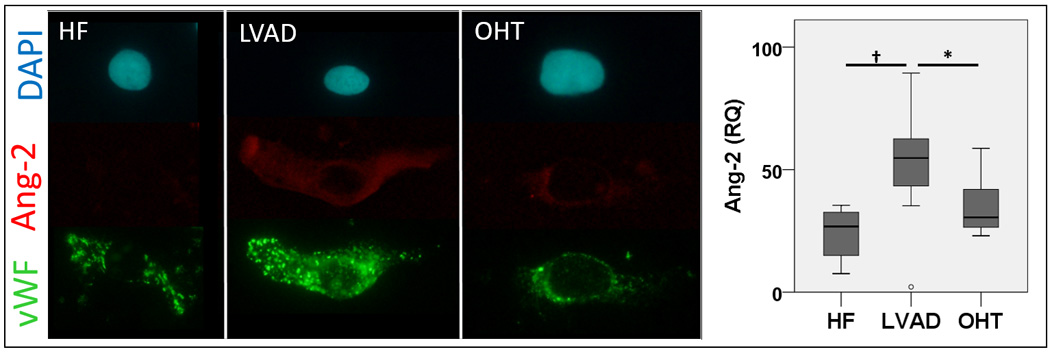
Endothelial biopsy was performed in patients with HF, LVAD, or OHT (n=10, 13, and 12 respectively) and endothelial cells were isolated by centrifugation. Cells were plated onto microscope slides and stained with fluorescent-labeled antibodies. Protein content was measured by quantitative immunofluorescence. Endothelial expression of Angiopoietin-2 (red) was significantly higher in patients with LVADs compared with HF or OHT. * p<0.05, † p<0.01
Elevated Ang-2 in Serum from Patients with LVADs Induces Angiogenesis
Previous studies have shown that Ang-2 increases endothelial tube formation on Matrigel.32 To investigate whether the elevated Ang-2 in serum from patients with LVADs could induce endothelial tube formation, we incubated HUVECs grown on Matrigel with serum from patients with HF, LVAD, or OHT in the presence or absence of an Ang-2 blocking antibody. Serum from patients with LVADs induced more microtube formation than did serum from patients with HF or OHT (38.15±10.42, 27.44±11.34, and 27.50±6.18 tubes per low power field respectively, omnibus p<0.001, HF vs. LVAD p=0.003, LVAD vs. OHT p<0.001) (Figure 3a). This effect was abolished by the Ang-2 blocking antibody (20.71±6.98 tubes per low power field respectively, p<0.001), indicating that elevated Ang-2 levels in the serum from patients with LVADs is the driving factor for the increased microtube formation. No significant difference was observed in the HF or OHT groups in response to the Ang-2 blocking antibody (27.14±9.66 and 24.57±7.33 tubes per low power field respectively, p=NS). Among the LVAD patients, tubule formation correlated strongly with serum Ang-2 level (R2=0.645, p<0.001, Figure 3b).
Figure 3. Elevated Angiopoietin-2 in serum from patients with LVADs induces angiogenesis in human endothelium.
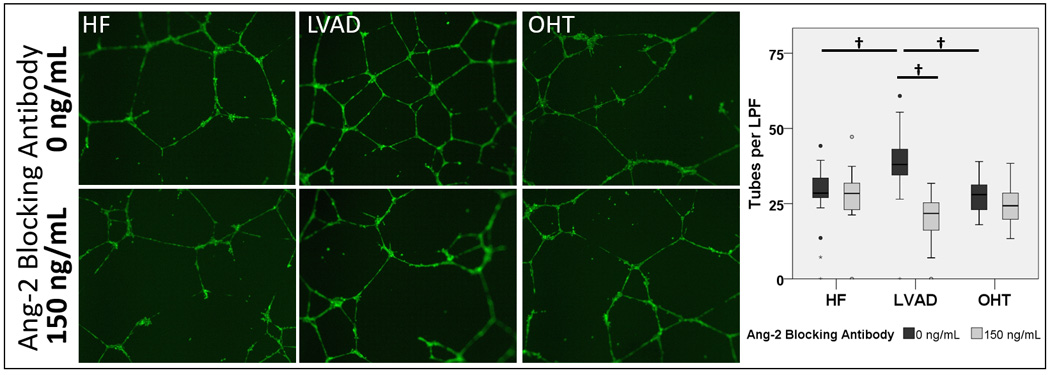
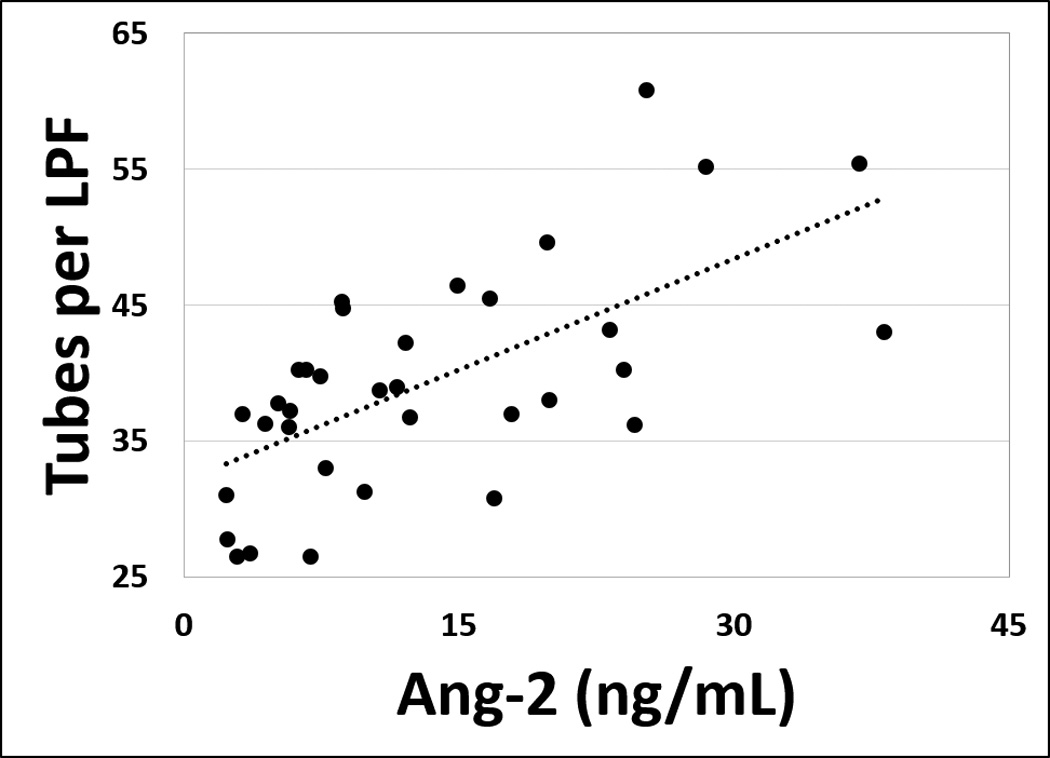
(a) Human umbilical vein endothelial cells were assayed on Matrigel and incubated overnight with serum from patients with HF, LVAD, or OHT (n=17, 35, and 14 respectively) in the presence or absence of an Ang-2 blocking antibody. Tubule formation was then quantified visually by an investigator blinded to sample identity. Tubule formation was significantly greater in cultures treated with serum from patients with LVADs compared with controls. Co-treatment with Ang-2 blocking antibody significantly reduced angiogenic growth in cells treated with serum from patients with LVADs but had no significant effect in cells treated with serum from HF or OHT patients. † p<0.01 (b) Among LVAD patients, tubule formation correlated strongly with serum Ang-2 concentration. R2=0.645, p<0.001
Elevated Thrombin in Plasma from Patients with LVADs Increases Ang-2 Gene Expression in Endothelium
Previous studies have suggested that plasma levels of thrombin may be elevated in patients with LVADs.16–18 To confirm this, we measured thrombin and prothrombin in plasma from patients with HF, LVAD, or OHT by Western Blot. Indeed, thrombin was elevated in plasma from patients with LVADs compared with HF or OHT (5.88±6.70, 0.93±0.79, and 0.57±0.43 AU respectively, omnibus p=0.003, HF vs. LVAD p=0.039, LVAD vs. OHT p=0.003) (Figure 4a–b). Prothrombin, however, was not different among the three groups.
Figure 4. Elevated plasma levels of thrombin in patients with LVADs.

Thrombin was measured by Western Blot in plasma from patients with HF, LVAD, or OHT (n=14, 13, and 15 respectively). (a–b) Thrombin was significantly higher in plasma from patients with LVADs compared with HF or OHT while prothrombin was unchanged. To investigate one possible source of this elevated thrombin, we measured Factor XIIa and XIa by Western Blot in plasma from these patients. (c–f) Both Factor XIIa and XIa were significantly higher in LVAD patients. Taken together, our findings suggest that activation of the contact coagulation system contributes to increased production of thrombin in LVAD patients. * p<0.05, † p<0.01, ‡ p=0.060
To identify the most likely source of the increased thrombin in LVAD patients, we investigated key regulators of the contact coagulation system, specifically Factor XIIa and its downstream effector Factor XIa in plasma from patients with HF, LVAD, or OHT by Western Blot. Both Factor XIIa (HF 0.50±0.26, LVAD 1.01±0.58, and OHT 0.63±0.28 AU, omnibus p=0.037, HF vs. LVAD p=0.034, LVAD vs OHT p=0.377) and Factor XIa (HF 0.94±0.47, LVAD 2.29±1.49, OHT 0.86±0.21 AU, omnibus p=0.014, HF vs. LVAD p=0.060, LVAD vs. OHT p=0.028) were higher in LVAD patients (Figure 4c–f), suggesting that activation of the contact coagulation system is a likely source of the increased thrombin seen in LVAD patients.
To evaluate whether the increased thrombin in plasma from patients with LVADs could be responsible for increased Ang-2 gene expression, we incubated HUVECs for 4 hours with plasma anticoagulated with fondaparinux in the presence or absence of Vorapaxar, a thrombin receptor (PAR-1) antagonist. The plasma from patients with LVADs induced higher Ang-2 gene expression in the cultured endothelial cells compared with plasma from patients with HF or OHT (1.88±0.63, 0.67±0.15, and 0.53±0.14 RQ respectively, omnibus p<0.001, HF vs. LVAD p=0.003, LVAD vs. OHT p<0.001) (Figure 5). This increased Ang-2 expression was significantly reduced with thrombin receptor blockade (0.95±0.22 RQ, p=0.013). In contrast, a small, but non-significant decrease in Ang-2 gene expression in the presence of Vorapaxar was noted in endothelial cells receiving plasma from patients with HF or OHT (0.53±0.21 and 0.43±0.12 RQ respectively, p=NS). Taken together, these data suggest that elevated thrombin in plasma from patients with LVADs induces increased endothelial Ang-2 expression.
Figure 5. Elevated plasma levels of thrombin in patients with LVADs induce endothelial overexpression of Ang-2.

Plasma samples from patients with HF, LVAD, or OHT (n=14, 13, and 15 respectively) were anticoagulated with fondaparinux to prevent artefactual thrombin generation ex vivo. Cultured HUVECs were starved overnight and then incubated with plasma from each patient in the presence or absence of Vorapaxar, a thrombin receptor blocker. Ang-2 gene expression was measured by RT-PCR. Plasma from patients with LVADs induced significantly higher expression of Ang-2 compared with HF or OHT. This effect was normalized with thrombin receptor blockade. * p<0.05, † p<0.01
Elevated Ang-2 in Serum from Patients with LVADs is Associated with an Increased Risk of Non-Surgical Bleeding Events Within 3-months of Sample Collection
To investigate the role of serum Ang-2 in predicting NSB events in patients with LVADs, we reviewed the electronic medical record of all patients with an LVAD enrolled in the present study for instances of GI bleeding, intracranial hemorrhage (ICH), or epistaxis. GI bleeding was defined as a report of blood in the stool or vomitus, a finding of hemoccult positive stool, or a finding of pathologic bleeding on endoscopy. Intracranial hemorrhage was defined as a radiographic finding of bleeding within the cranium. Epistaxis was defined as a report of bleeding from the nose or a finding of pathologic bleeding within the nasopharynx on endoscopy. Among patients with an LVAD and a serum Ang-2 level above the mean of 12.32 ng/mL (n=13), 5 patients had at least one NSB event within 3 months of sample collection (4 GI bleeds, 1 epistaxis, 0 ICH) while 0 patients with serum Ang-2 level below the mean (n=25) experienced bleeding (p=0.003). However, there was no relationship between Ang-2 and NSB at 6 months after sample collection (8 GI bleeds, 1 epistaxis, 0 ICH), suggesting a critical time interval between elevation of Ang-2 and bleeding events. Ang-2 was significantly higher in patients who experienced bleeding events (27.69±9.74 ng/mL) compared with those who did not (9.99±7.18 ng/mL, p<0.001). However, no significant relationship was observed between aortic valve opening and NSB (Table S7B).
Discussion
This is the first study to assess the role of Ang-2 in patients supported with LVADs. We found that patients with LVADs have elevated circulating Ang-2 levels and higher Ang-2 protein expression in the endothelial cells, representing overexpression of Ang-2. The increased Ang-2 levels in patients with LVADs led to increased angiogenesis, which was inhibited by Ang-2-blocking antibody. Furthermore, LVAD patients have elevated thrombin levels, which stimulate Ang-2 overexpression, and LVAD patients with elevated Ang-2 levels have a higher risk for NSB.
Ang-2 disrupts vital inter-cellular connections that are associated with vessel maturation33 and induces endothelial inflammation and abnormal angiogenesis,7, 29–31 resulting in tortuous, fragile vessels that are prone to bleeding. Indeed, Ang-2 over-expressing mice develop dilated, tortuous, redundant vessels8 reminiscent of AVMs in patients with LVADs. Blockade of Ang-2 signaling is currently being investigated in the treatment of various cancers where neovascularization is associated with accelerated tumor growth.4 Numerous Ang-2 blockers are commercially available and many have shown promise in decreasing tumor size4. However, it is not known whether Ang-2 blockade can prevent or is an effective therapy for LVAD-related NSB.
In the current study, we found that Ang-2 levels were markedly higher in the blood of patients with LVADs with even further elevation in patients with HVADs. However, Ang-2 levels were not elevated in patients with OHT suggesting that the LVAD, and not the associated surgery or the increase in cardiac output, is most likely responsible for the conditions leading to Ang-2 overexpression. Notably, the increase in Ang-2 levels in patients with LVADs was mirrored by an increase in soluble Tie-2 (the angiopoietin receptor) and was accompanied by a decrease in Ang-1 levels without a decrease in VEGF levels compared with patients with HF.
Our results provide insight into the potential mechanism and consequences of Ang-2 overexpression in patients with LVADs. Known inducers of Ang-2 secretion include thrombin, catecholamines, and hypoxia. However, catecholamine levels tend to decrease after LVAD implant,34 which makes it unlikely that they are the cause of increased Ang-2 expression in these patients. Hypoxia is the best-characterized stimulator of Ang-2 expression, but patients are typically not hypoxic after LVAD implantation. In addition, patients with untreated hypoxia were excluded from our study. In contrast, thrombin has been shown to upregulate Ang-2 expression and release in vitro14 and prior studies have suggested thrombin activity may be increased in patients with LVADs16, 17 due to interaction of coagulation factors with the materials of the LVAD.18 Specifically, our data suggest that the contact coagulation system (regulated by Factor XIIa and Factor XIa) appears to be activated in LVAD patients, which could be one possible source of thrombin generation in these patients. Titanium, the primary material of the LVAD rotor, is known to strongly activate the contact coagulation system35 suggesting that the use of alternate materials or inhibition of the contact coagulation system might improve LVAD hemocompatibility and/or reduce complications.
In addition to thrombin’s role in converting fibrinogen to fibrin, thrombin is known to activate PAR-1.12, 36 PAR-1 activates the Phospholipase-C-β signaling cascade, inducing Weibel-Palade Body exocytosis and Ang-2 expression.15, 37 In line with this mechanism, our findings implicate thrombin-induced PAR-1 activation as the most likely mechanism for the increased Ang-2 found in patients with LVADs. The further increase in Ang-2 levels in patients with HVADs is of unclear significance and may represent device-specific factors or patient specific factors as all patients in our study with an HVAD received the device as part of a bridge-to-transplant (BTT) strategy while patients with a Heartmate II used as BTT or destination therapy were studied.
Most studies showing thrombin activation in patients with LVADs studied first-generation pulsatile LVADs, not the continuous-flow LVADs used in the present study.16, 18 While one limited study did suggest the presence of increased thrombin activity in modern LVAD recipients (through observation of fibrin split products and thrombin/antithrombin complexes),17 an increase in circulating active thrombin after LVAD has not been shown previously and the net effect of modern LVAD implantation on the coagulation system remains controversial. Study of overall thrombin activity in these patients is challenging because all patients with modern LVADs are treated with warfarin. However, patients treated with warfarin still have residual thrombin activity38 and patients with LVADs retain a significant risk of LVAD thrombosis despite the use of warfarin.39 The use of warfarin and antiplatelets remains an unavoidable confounder in our study.
Our study extends our current understanding of the pathogenesis of LVAD-related AVM formation by demonstrating for the first time in patients with LVADs, the activation of a pathway well known to cause abnormal blood vessel growth. Previously, the transition from pulsatile flow to continuous flow after LVAD implantation has been hypothesized to drive abnormal vessel growth through an unknown mechanism.40 Wever-Pinzon and colleagues reported an association between pulsatility and NSB in LVAD recipients.41 However, in the present study, we found no significant association between aortic valve opening or pulse pressure and NSB events or Ang-2 expression. While we cannot exclude a role for altered flow conditions in the induction of Ang-2 expression, prior studies have offered conflicting data. Some authors suggest Ang-2 expression is increased by continuous flow versus pulsatile flow42 while others suggest the opposite.43 Furthermore, while the term “continuous flow” is commonly used to describe flow in patients with LVADs, blood flow in the aorta of LVAD patients is actually quite turbulent due to high shear forces produced by the LVAD.44, 45 Also, flow in the small arterioles, capillaries, and venous system is normally non-pulsatile and these beds make up an overwhelming majority of the total vascular surface area. In the present study, we show that Ang-2 expression is elevated in the vena caval endothelial cells, where flow is normally non-pulsatile. Therefore, a non-mechanical cause of Ang-2 overexpression is more consistent with known data as the vessels facing altered flow represent a small minority of total vessel area and are distinct from the beds involved in angiogenesis.
Our study has several limitations. Ideally, we would have liked to explore whether pharmacologic inhibition of Ang-2 in LVAD patients reduces AVM formation and NSB. Such agents are currently in Phase III clinical trials for the treatment of various cancers and we hope that they will be available for studies in LVAD patients. Specifically AMG 386, a novel small peptide, which blocks both Ang-1 and Ang-2, appears to reduce blood flow to tumors46 and appears well tolerated by patients.47 Similarly, the monoclonal antibody PF-4856884 reduces circulating levels of Ang-2 and reduces tumor blood flow.48 Numerous other agents are currently in development.4 The freshly isolated endothelial cells obtained from the patients in this study are vena caval in origin while it is the capillary endothelial cells that likely contribute to deregulated angiogenesis in response to Ang-2. However, according to our hypothesis, both the stimulus (thrombin activity) and the effector (Ang-2) are circulating freely and are able to interact with all vascular beds. These systemic changes induce effects on vascular beds distinct from their origin and systemic deregulation of the Ang/Tie-2 axis is a likely driver of angiogenesis. The use of warfarin and antiplatelets are unavoidable confounders as nearly all LVAD patients use these drugs and the INR goal in most non-LVAD patients taking warfarin is typically different from that of LVAD patients. Further, common indications for warfarin in non-LVAD patients include atrial fibrillation which itself is associated with increased Ang-2.49 While it is not possible to account for all confounding variables in human studies, we sought to minimize confounding through the enrollment of well-matched control groups. While patients with OHT are useful to control for effects of increased flow, sternotomy, and general anesthesia, patients with OHT are also treated with immunosuppressants, which are not used in patients with LVADs and may affect angiogenic signaling pathways.50 While this confounder is unavoidable, we have attempted to address this issue by enrolling two control groups, patients with OHT and patients with stable HF. Finally, while this series of experiments was designed to identify the molecular basis for deregulated angiogenesis in patients with LVADs, we acknowledge that blood flow conditions in patients with LVADs are markedly different from patients without LVADs. While evidence suggesting a link between flow conditions and Ang-2 expression is limited and conflicting, we have chosen to focus on the molecular causes of Ang-2-dependent angiogenesis in this study. While elevated Ang-2 was associated with a higher risk of NSB, the relationship between Ang-2 and AVM formation was not directly addressed in this study. Nevertheless, all of the patients who experienced NSB were found to have AVMs on endoscopy. However, patients who did not experience bleeding did not undergo endoscopy, and therefore, the prevalence of asymptomatic AVMs in our study remains unknown. Despite these shortcomings, several studies have shown a strong association between Ang-2 and the development of vascular malformations,9 small bowel angiodysplasia,10 and increased capillary density.51 Lastly, due to limitations in sample availability, we were unable to perform all assays in this study on all samples collected and therefore used subsets when necessary as indicated.
In summary, we have demonstrated that LVAD implantation is associated with increased thrombin-dependent overexpression of Ang-2, which leads to increased angiogenesis. Furthermore, we have shown that elevated Ang-2 is associated with NSB events in patients with LVADs. As the reliance on LVADs for the treatment of advanced heart failure continues to rise, the identification of novel therapeutic targets to treat LVAD-related complications will grow in importance. It remains to be determined whether pharmaceutical agents that antagonize Ang-2, which are currently in development, will hold promise for the treatment or prevention of LVAD-related AVM formation. Further studies are necessary to determine whether modulation of the Ang/Tie-2 pathway could have therapeutic benefits in reducing the complications associated with LVADs.
Supplementary Material
Clinical Perspective.
What is new?
Angiodysplasia leading to non-surgical bleeding (NSB) is a common complication in patients with left ventricular assist devices (LVADs). However, the cause for this remains unknown. We found that Angiopoietin-2 (Ang-2), a potent angiogenic mediator, is elevated in patients with LVADs. Elevated levels of Ang-2 in these patients increased angiogenesis in vitro and were associated with bleeding events.
Furthermore, we found that increased thrombin levels in LVAD patients were associated with elevated Ang-2 levels. Our findings, therefore, suggest that high levels of thrombin induce endothelial Ang-2 expression, which may contribute to angiodysplasia and NSB in LVAD patients.
What are the clinical implications?
Our findings link the activation of the coagulation system in LVAD patients with altered angiogenesis. This may contribute to the development of angiodysplasia and NSB. Because several Ang-2 inhibitors are currently being developed for the treatment of cancer, these inhibitors might also be useful in preventing or treating LVAD-related angiodysplasia and NSB. In addition, inhibition of the contact coagulation system including Factor XII may reduce thrombin generation and Ang-2 expression in LVAD patients.
Our findings suggest that further clinical studies using Ang-2 and Factor XII inhibitors may show therapeutic benefits in preventing NSB in LVAD patients.
Acknowledgments
Sources of Funding
This work was support by grants from the NIH (HL052233) to JKL and the American Heart Association (14POST20380109) and the University of Chicago Institute for Translational Medicine (CTSA UL1 TR000430) to CET.
Footnotes
Disclosures
None.
References
- 1.Morgan JA, Paone G, Nemeh HW, Henry SE, Patel R, Vavra J, Williams CT, Lanfear DE, Tita C, Brewer RJ. Gastrointestinal bleeding with the HeartMate II left ventricular assist device. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2012;31:715–718. doi: 10.1016/j.healun.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 2.Suarez J, Patel CB, Felker GM, Becker R, Hernandez AF, Rogers JG. Mechanisms of bleeding and approach to patients with axial-flow left ventricular assist devices. Circulation Heart failure. 2011;4:779–784. doi: 10.1161/CIRCHEARTFAILURE.111.962613. [DOI] [PubMed] [Google Scholar]
- 3.Yuzefpolskaya M, Godier-Furnemont A, Levin AP, Dionizovik-Dimanovski M, Takayama H, Naka Y, Uriel N, Colombo PC, Vunjak-Novakovic G, Jorde UP. Myocardial Microvascular Density Increases After Chronic Continuous Flow Left Ventricular Assist Device (CF-LVAD) Support. The Journal of Heart and Lung Transplantation. 33:S236–S237. [Google Scholar]
- 4.Cascone T, Heymach JV. Targeting the angiopoietin/Tie2 pathway: cutting tumor vessels with a double-edged sword? Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2012;30:441–444. doi: 10.1200/JCO.2011.38.7621. [DOI] [PubMed] [Google Scholar]
- 5.Metcalf DJ, Nightingale TD, Zenner HL, Lui-Roberts WW, Cutler DF. Formation and function of Weibel-Palade bodies. Journal of cell science. 2008;121:19–27. doi: 10.1242/jcs.03494. [DOI] [PubMed] [Google Scholar]
- 6.Randi AM, Laffan MA, Starke RD. Von Willebrand Factor, Angiodysplasia and Angiogenesis. Mediterranean journal of hematology and infectious diseases. 2013;5:e2013060. doi: 10.4084/MJHID.2013.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liersch Rd, Berdel WE, Kessler T. Angiogenesis inhibition. Heidelberg: Springer; 2010. [Google Scholar]
- 8.Ziegler T, Horstkotte J, Schwab C, Pfetsch V, Weinmann K, Dietzel S, Rohwedder I, Hinkel R, Gross L, Lee S, Hu J, Soehnlein O, Franz WM, Sperandio M, Pohl U, Thomas M, Weber C, Augustin HG, Fassler R, Deutsch U, Kupatt C. Angiopoietin 2 mediates microvascular and hemodynamic alterations in sepsis. The Journal of clinical investigation. 2013;123:3436–3445. doi: 10.1172/JCI66549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Redondo P, Aguado L, Marquina M, Paramo JA, Sierra A, Sanchez-Ibarrola A, Martinez-Cuesta A, Cabrera J. Angiogenic and prothrombotic markers in extensive slow-flow vascular malformations: implications for antiangiogenic/antithrombotic strategies. Br J Dermatol. 2010;162:350–356. doi: 10.1111/j.1365-2133.2009.09513.x. [DOI] [PubMed] [Google Scholar]
- 10.Holleran G, Hall B, O'Regan M, Smith S, McNamara D. Expression of Angiogenic Factors in Patients With Sporadic Small Bowel Angiodysplasia. J Clin Gastroenterol. 2014;49:831–836. doi: 10.1097/MCG.0000000000000260. [DOI] [PubMed] [Google Scholar]
- 11.Smadja DM, Laurendeau I, Avignon C, Vidaud M, Aiach M, Gaussem P. The angiopoietin pathway is modulated by PAR-1 activation on human endothelial progenitor cells. Journal of thrombosis and haemostasis : JTH. 2006;4:2051–2058. doi: 10.1111/j.1538-7836.2006.02101.x. [DOI] [PubMed] [Google Scholar]
- 12.Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- 13.Bae JS, Rezaie AR. Thrombin upregulates the angiopoietin-Tie2 Axis: endothelial protein C receptor occupancy prevents the thrombin mobilization of angiopoietin 2 and P-selectin from Weibel-Palade bodies. Journal of thrombosis and haemostasis : JTH. 2010;8:1107–1115. doi: 10.1111/j.1538-7836.2010.03812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang YQ, Li JJ, Hu L, Lee M, Karpatkin S. Thrombin induces increased expression and secretion of angiopoietin-2 from human umbilical vein endothelial cells. Blood. 2002;99:1646–1650. doi: 10.1182/blood.v99.5.1646. [DOI] [PubMed] [Google Scholar]
- 15.Ju R, Zhuang ZW, Zhang J, Lanahan AA, Kyriakides T, Sessa WC, Simons M. Angiopoietin-2 secretion by endothelial cell exosomes: regulation by the phosphatidylinositol 3-kinase (PI3K)/Akt/endothelial nitric oxide synthase (eNOS) and syndecan-4/syntenin pathways. The Journal of biological chemistry. 2014;289:510–519. doi: 10.1074/jbc.M113.506899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spanier T, Oz M, Levin H, Weinberg A, Stamatis K, Stern D, Rose E, Schmidt AM. Activation of coagulation and fibrinolytic pathways in patients with left ventricular assist devices. The Journal of thoracic and cardiovascular surgery. 1996;112:1090–1097. doi: 10.1016/S0022-5223(96)70111-3. [DOI] [PubMed] [Google Scholar]
- 17.John R, Panch S, Hrabe J, Wei P, Solovey A, Joyce L, Hebbel R. Activation of endothelial and coagulation systems in left ventricular assist device recipients. The Annals of thoracic surgery. 2009;88:1171–1179. doi: 10.1016/j.athoracsur.2009.06.095. [DOI] [PubMed] [Google Scholar]
- 18.Wagner WR, Johnson PC, Heil BV, Thompson KA, Kormos RL, Griffith BP. Thrombin activity resides on LVAD Dacron inflow and outflow grafts. ASAIO journal. 1992;38:M634–M637. doi: 10.1097/00002480-199207000-00114. [DOI] [PubMed] [Google Scholar]
- 19.Colombo PC, Lanier GM, Orlanes K, Yuzefpolskaya M, Demmer RT. Usefulness of a standard automated blood pressure monitor in patients with continuous-flow left ventricular assist devices. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2015;34:1633–1635. doi: 10.1016/j.healun.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Colombo PC, Ashton AW, Celaj S, Talreja A, Banchs JE, Dubois NB, Marinaccio M, Malla S, Lachmann J, Ware JA, Le Jemtel TH. Biopsy coupled to quantitative immunofluorescence: a new method to study the human vascular endothelium. Journal of applied physiology. 2002;92:1331–1338. doi: 10.1152/japplphysiol.00680.2001. [DOI] [PubMed] [Google Scholar]
- 21.Feng L, Stern DM, Pile-Spellman J. Human endothelium: endovascular biopsy and molecular analysis. Radiology. 1999;212:655–664. doi: 10.1148/radiology.212.3.r99au28655. [DOI] [PubMed] [Google Scholar]
- 22.Shenouda SM, Widlansky ME, Chen K, Xu G, Holbrook M, Tabit CE, Hamburg NM, Frame AA, Caiano TL, Kluge MA, Duess MA, Levit A, Kim B, Hartman ML, Joseph L, Shirihai OS, Vita JA. Altered mitochondrial dynamics contributes to endothelial dysfunction in diabetes mellitus. Circulation. 2011;124:444–453. doi: 10.1161/CIRCULATIONAHA.110.014506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabit CE, Shenouda SM, Holbrook M, Fetterman JL, Kiani S, Frame AA, Kluge MA, Held A, Dohadwala MM, Gokce N, Farb MG, Rosenzweig J, Ruderman N, Vita JA, Hamburg NM. Protein kinase C-beta contributes to impaired endothelial insulin signaling in humans with diabetes mellitus. Circulation. 2013;127:86–95. doi: 10.1161/CIRCULATIONAHA.112.127514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombo PC, Onat D, Harxhi A, Demmer RT, Hayashi Y, Jelic S, LeJemtel TH, Bucciarelli L, Kebschull M, Papapanou P, Uriel N, Schmidt AM, Sabbah HN, Jorde UP. Peripheral venous congestion causes inflammation, neurohormonal, and endothelial cell activation. European heart journal. 2014;35:448–454. doi: 10.1093/eurheartj/eht456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rasband WS. ImageJ. Bethesda, Maryland, USA: U.S. National Institutes of Health; 1997–2014. http://imagej.nih.gov/ij/ [Google Scholar]
- 26.Ponce ML. Tube formation: an in vitro matrigel angiogenesis assay. Methods in molecular biology. 2009;467:183–188. doi: 10.1007/978-1-59745-241-0_10. [DOI] [PubMed] [Google Scholar]
- 27.Manginas A, Tsiavou A, Sfyrakis P, Giamouzis G, Tsourelis L, Leontiadis E, Degiannis D, Cokkinos DV, Alivizatos PA. Increased number of circulating progenitor cells after implantation of ventricular assist devices. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2009;28:710–717. doi: 10.1016/j.healun.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Aharinejad S, Schafer R, Hofbauer R, Abraham D, Blumer R, Miksovsky A, Traxler H, Pullirsch D, Alexandrowicz R, Taghavi S, Kocher A, Laufer G. Impact of cardiac transplantation on molecular pathology of ET-1, VEGF-C, and mitochondrial metabolism and morphology in dilated versus ischemic cardiomyopathic patients. Transplantation. 2001;72:1043–1049. doi: 10.1097/00007890-200109270-00011. [DOI] [PubMed] [Google Scholar]
- 29.Tse V, Xu L, Yung YC, Santarelli JG, Juan D, Fabel K, Silverberg G, Harsh Gt. The temporal-spatial expression of VEGF, angiopoietins-1 and 2, and Tie-2 during tumor angiogenesis and their functional correlation with tumor neovascular architecture. Neurol Res. 2003;25:729–738. doi: 10.1179/016164103101202084. [DOI] [PubMed] [Google Scholar]
- 30.Reiss Y, Knedla A, Tal AO, Schmidt MH, Jugold M, Kiessling F, Burger AM, Wolburg H, Deutsch U, Plate KH. Switching of vascular phenotypes within a murine breast cancer model induced by angiopoietin-2. J Pathol. 2009;217:571–580. doi: 10.1002/path.2484. [DOI] [PubMed] [Google Scholar]
- 31.Reiss Y, Machein MR, Plate KH. The role of angiopoietins during angiogenesis in gliomas. Brain Pathol. 2005;15:311–317. doi: 10.1111/j.1750-3639.2005.tb00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu A, Shively JE. Angiopoietins-1 and-2 play opposing roles in endothelial sprouting of embryoid bodies in 3D culture and their receptor Tie-2 associates with the cell-cell adhesion molecule PECAM1. Exp Cell Res. 2011;317:2171–2182. doi: 10.1016/j.yexcr.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hakanpaa L, Sipila T, Leppanen VM, Gautam P, Nurmi H, Jacquemet G, Eklund L, Ivaska J, Alitalo K, Saharinen P. Endothelial destabilization by angiopoietin-2 via integrin beta1 activation. Nat Commun. 2015;6:5962. doi: 10.1038/ncomms6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koerner MM, El-Banayosy A, Eleuteri K, Kline C, Stephenson E, 3rd, Pae W, Ghodsizad A. Neurohormonal regulation and improvement in blood glucose control: reduction of insulin requirement in patients with a nonpulsatile ventricular assist device. The heart surgery forum. 2014;17:E98–E102. doi: 10.1532/HSF98.2013323. [DOI] [PubMed] [Google Scholar]
- 35.Hong J, Andersson J, Ekdahl KN, Elgue G, Axen N, Larsson R, Nilsson B. Titanium is a highly thrombogenic biomaterial: possible implications for osteogenesis. Thrombosis and haemostasis. 1999;82:58–64. [PubMed] [Google Scholar]
- 36.Zhang C, Srinivasan Y, Arlow DH, Fung JJ, Palmer D, Zheng Y, Green HF, Pandey A, Dror RO, Shaw DE, Weis WI, Coughlin SR, Kobilka BK. High-resolution crystal structure of human protease-activated receptor 1. Nature. 2012;492:387–392. doi: 10.1038/nature11701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nightingale T, Cutler D. The secretion of von Willebrand factor from endothelial cells; an increasingly complicated story. Journal of thrombosis and haemostasis : JTH. 2013;11(Suppl 1):192–201. doi: 10.1111/jth.12225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Massicotte P, Leaker M, Marzinotto V, Adams M, Freedom R, Williams W, Vegh P, Berry L, Shah B, Andrew M. Enhanced thrombin regulation during warfarin therapy in children compared to adults. Thrombosis and haemostasis. 1998;80:570–574. [PubMed] [Google Scholar]
- 39.Lindenfeld J, Keebler ME. Left ventricular assist device thrombosis: another piece of the puzzle? JACC Heart failure. 2015;3:154–158. doi: 10.1016/j.jchf.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Kitchens CS, Kessler CM, Konkle BA. Consultative hemostasis and thrombosis. 3rd. Philadelphia, PA: Elsevier/Saunders; 2013. [Google Scholar]
- 41.Wever-Pinzon O, Selzman CH, Drakos SG, Saidi A, Stoddard GJ, Gilbert EM, Labedi M, Reid BB, Davis ES, Kfoury AG, Li DY, Stehlik J, Bader F. Pulsatility and the risk of nonsurgical bleeding in patients supported with the continuous-flow left ventricular assist device HeartMate II. Circulation Heart failure. 2013;6:517–526. doi: 10.1161/CIRCHEARTFAILURE.112.000206. [DOI] [PubMed] [Google Scholar]
- 42.Yee A, Bosworth KA, Conway DE, Eskin SG, McIntire LV. Gene expression of endothelial cells under pulsatile non-reversing vs. steady shear stress; comparison of nitric oxide production. Annals of biomedical engineering. 2008;36:571–579. doi: 10.1007/s10439-008-9452-9. [DOI] [PubMed] [Google Scholar]
- 43.Li R, Beebe T, Jen N, Yu F, Takabe W, Harrison M, Cao H, Lee J, Yang H, Han P, Wang K, Shimizu H, Chen J, Lien CL, Chi NC, Hsiai TK. Shear stress-activated Wnt-angiopoietin-2 signaling recapitulates vascular repair in zebrafish embryos. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:2268–2275. doi: 10.1161/ATVBAHA.114.303345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Karmonik C, Partovi S, Loebe M, Schmack B, Weymann A, Lumsden AB, Karck M, Ruhparwar A. Computational fluid dynamics in patients with continuous-flow left ventricular assist device support show hemodynamic alterations in the ascending aorta. The Journal of thoracic and cardiovascular surgery. 2014;147:1326 e1–1333 e1. doi: 10.1016/j.jtcvs.2013.09.069. [DOI] [PubMed] [Google Scholar]
- 45.Karmonik C, Partovi S, Schmack B, Weymann A, Loebe M, Noon GP, Piontek P, Karck M, Lumsden AB, Ruhparwar A. Comparison of hemodynamics in the ascending aorta between pulsatile and continuous flow left ventricular assist devices using computational fluid dynamics based on computed tomography images. Artificial organs. 2014;38:142–148. doi: 10.1111/aor.12132. [DOI] [PubMed] [Google Scholar]
- 46.Herbst RS, Hong D, Chap L, Kurzrock R, Jackson E, Silverman JM, Rasmussen E, Sun YN, Zhong D, Hwang YC, Evelhoch JL, Oliner JD, Le N, Rosen LS. Safety, pharmacokinetics, and antitumor activity of AMG 386, a selective angiopoietin inhibitor, in adult patients with advanced solid tumors. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:3557–3565. doi: 10.1200/JCO.2008.19.6683. [DOI] [PubMed] [Google Scholar]
- 47.Mita AC, Takimoto CH, Mita M, Tolcher A, Sankhala K, Sarantopoulos J, Valdivieso M, Wood L, Rasmussen E, Sun YN, Zhong ZD, Bass MB, Le N, LoRusso P. Phase 1 study of AMG 386, a selective angiopoietin 1/2-neutralizing peptibody, in combination with chemotherapy in adults with advanced solid tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16:3044–3056. doi: 10.1158/1078-0432.CCR-09-3368. [DOI] [PubMed] [Google Scholar]
- 48.Rosen LS, Mendelson DS, Cohen RB, Gordon MS, Goldman JW, Bear IK, Byrns B, Perea R, Schoenfeld SL, Gollerkeri A. First-in-human dose-escalation safety and PK trial of a novel intravenous humanized monoclonal CovXbody inhibiting angiopoietin 2. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28(suppl 15) abstr 2524. [Google Scholar]
- 49.Freestone B, Chong AY, Lim HS, Blann A, Lip GY. Angiogenic factors in atrial fibrillation: a possible role in thrombogenesis? Ann Med. 2005;37:365–372. doi: 10.1080/07853890510037392. [DOI] [PubMed] [Google Scholar]
- 50.Choe JY, Lee SJ, Park SH, Kim SK. Tacrolimus (FK506) inhibits interleukin-1beta-induced angiopoietin-1, Tie-2 receptor, and vascular endothelial growth factor through down-regulation of JNK and p38 pathway in human rheumatoid fibroblast-like synoviocytes. Joint Bone Spine. 2012;79:137–143. doi: 10.1016/j.jbspin.2011.03.018. [DOI] [PubMed] [Google Scholar]
- 51.Pappa CA, Alexandrakis MG, Boula A, Thanasia A, Konsolas I, Alegakis A, Tsirakis G. Prognostic impact of angiopoietin-2 in multiple myeloma. J Cancer Res Clin Oncol. 2014;140:1801–1805. doi: 10.1007/s00432-014-1731-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


