Abstract
The maternal brain is remarkably plastic and exhibits multifaceted neural modifications. Neurogenesis has emerged as one of the mechanisms by which the maternal brain exhibits plasticity. This review highlights what is currently known about peripartum-associated changes in adult neurogenesis and the underlying hormonal mechanisms. We also consider the functional consequences of neurogenesis in the peripartum brain and extent to which this process may play a role in maternal care, cognitive function and postpartum mood. Finally, while most work investigating the effects of parenting on adult neurogenesis has focused on mothers, a few studies have examined fathers and these results are also discussed.
Keywords: anxiety, depression, hippocampus, neurogenesis, olfactory bulb, paternal, pregnancy, postpartum, subventricular zone
1. Introduction
Becoming a mother is one of the most monumental events in a female’s life. Across mammalian species, major hormonal shifts during pregnancy, parturition and the postpartum period coupled with experiential factors modify the brain and, as a result, the behavior of the female producing a high level of maternal responsiveness along with changes in maternal mood, cognition and stress regulation (Fleming et al., 1999; Numan and Insel, 2003; Lonstein, 2007; Slattery and Neumann, 2008; Macbeth and Luine, 2010; Numan and Woodside, 2010; Workman et al., 2012; Dulac et al., 2014; Galea et al., 2014; Hillerer et al., 2014; Perani and Slattery, 2014; Rilling and Young, 2014; Pereira and Ferreira, 2015). Together, these changes represent adaptive responses that are necessary to ensure the survival and well-being of the offspring.
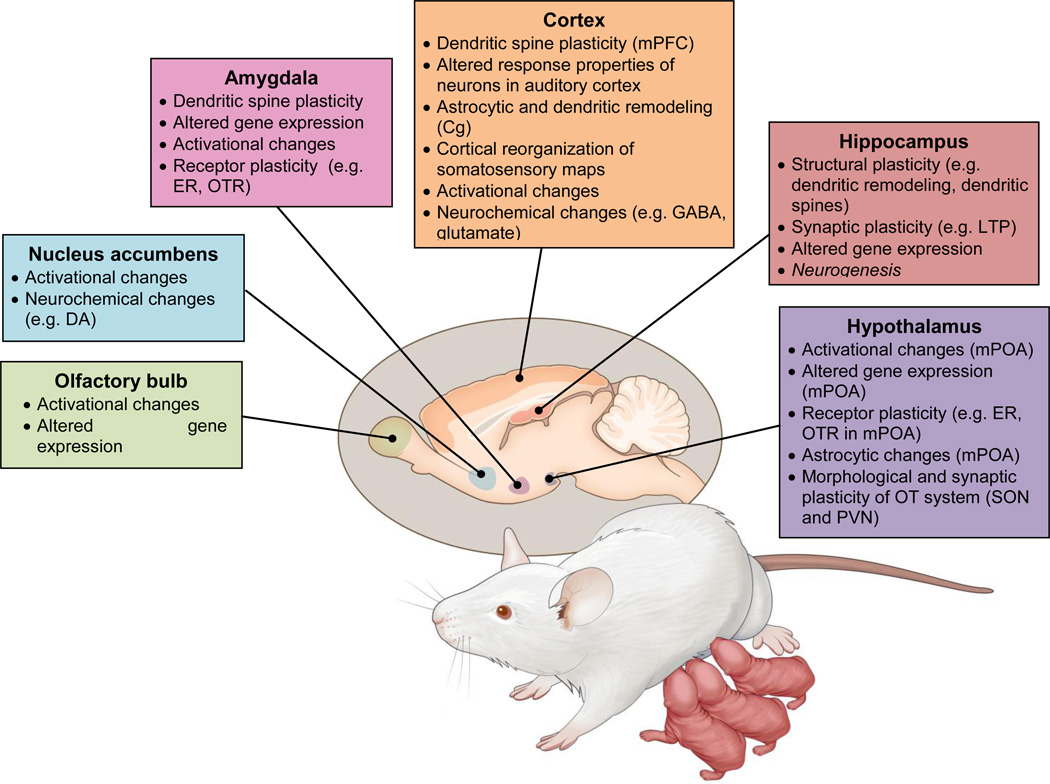
The neural modifications found in the maternal brain are numerous and widespread (Figure 1) consisting of neurochemical, neuroendocrine, activational, morphological, gene expression and functional changes within a distributed but interconnected circuitry (Theodosis et al., 1986; Xerri et al., 1994; Featherstone et al., 2000; Keyser-Marcus et al., 2001; Tomizawa et al., 2003; Rasia-Filho et al., 2004; Febo et al., 2005; Miranda and Liu, 2009; Sanna et al., 2009; Leuner et al., 2010; Canavan et al., 2011; Salmaso et al., 2011a; Salmaso et al., 2011b; Lonstein et al., 2014; Bridges, 2015; Cohen and Mizrahi, 2015; Corona and Levy, 2015; Elyada and Mizrahi, 2015) that includes the hypothalamus, amygdala, nucleus accumbens, olfactory bulb, hippocampus and various cortical areas (i.e. parietal, auditory, somatosensory, prefrontal). Within distinct areas of this circuitry, adult neurogenesis has emerged as another important mechanism that contributes to maternal neuroplasticity (Leuner et al., 2010; Levy et al., 2011; Galea et al., 2014; Pawluski et al., 2015b; Slattery and Hillerer, 2016) and will be the focus of this review.
Figure 1.
Schematic diagram highlighting various modifications in the maternal brain. DA = dopamine, ER = estrogen receptor, OTR = oxytocin receptor, mPFC = medial prefrontal cortex, Cg= cingulate cortex, LTP= long term potentiation, mPOA = medial preoptic area, PVN = paraventriular nucleus, SON = supraoptic nucleus.
2. Neurogenesis in the adult brain
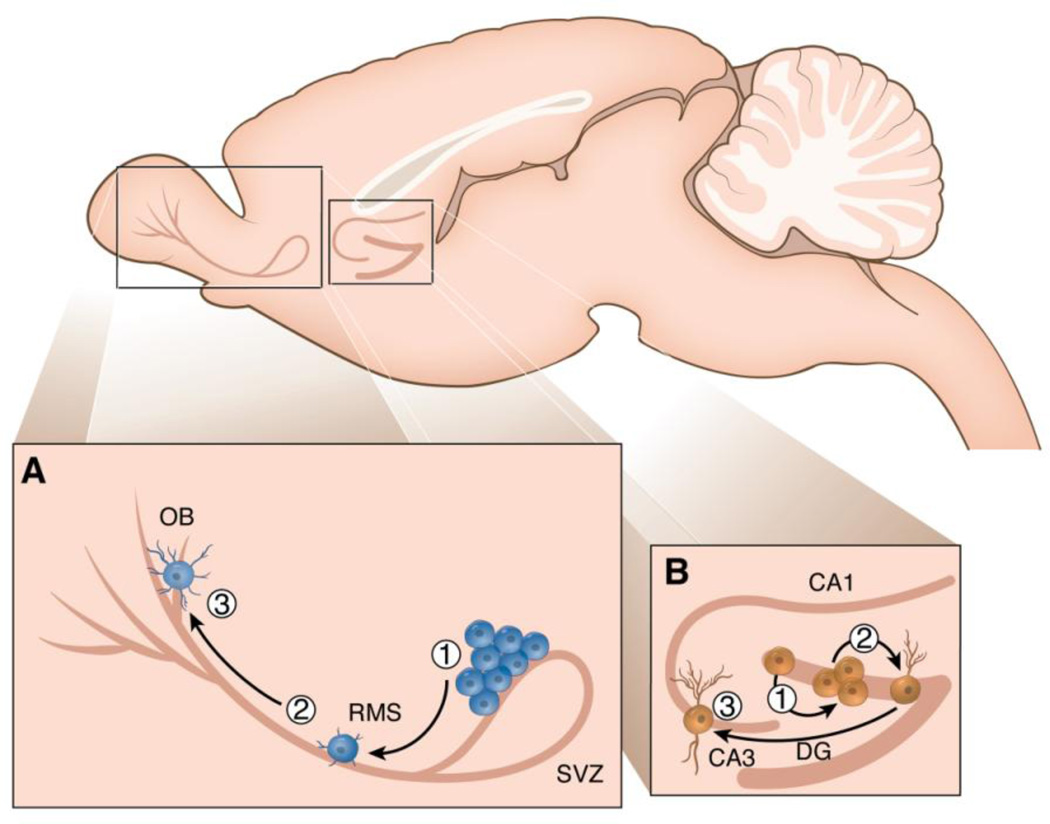
Neurogenesis is the process by which new neurons are generated from neural stem cells or progenitor cells. In most adult mammals, neurogenesis primarily occurs in two main regions, the dentate gyrus (DG) of the hippocampus and the olfactory bulb (OB) (Ming and Song, 2011; Aimone et al., 2014; Lepousez et al., 2015). Within the DG and OB, neurogenesis is a multi-step process that includes proliferation, migration, survival, differentiation and integration of newborn neurons into the existing circuitry (Leuner and Gould, 2010; Ming and Song, 2011). In the OB (Figure 2a), newly-born cells originate from the sub-ventricular zone (SVZ) which lies adjacent to the wall of the lateral ventricle. Neural progenitor cells residing in the SVZ give rise to transient amplifying cells which differentiate into neuroblasts. These neuroblasts then migrate a great distance along the rostral migratory stream (RMS) to reach the OB where they become mostly local inhibitory interneurons which join the neural networks of the olfactory system to participate in a range of odor-guided behaviors (Gheusi et al., 2013; Lepousez et al., 2015). In the DG (Figure 2b), neural progenitor cells proliferate in the sub-granular zone (SGZ) and give rise to neuroblasts that migrate a short distance into the granule cell layer (GCL) where a percentage will survive and mature, becoming glutamatergic granule neurons that synaptically and functionally integrate into the neuronal network of the hippocampus to play a role in cognition, mood and stress regulation (Leuner and Gould, 2010; Snyder et al., 2011; Aimone et al., 2014; Cameron and Glover, 2015).
Figure 2.
Sagittal section of the rodent brain showing the two main regions where neurogenesis occurs in the adult brain, the dentate gyrus (DG) of the hippocampus and the olfactory bulb (OB). In the OB (Figure 2a), neural stem cells residing in the SVZ proliferate (1) then migrate a great distance along the RMS (2) to reach the OB where they become mostly local inhibitory interneurons (3). In the DG (Figure 2b), progenitor cells proliferate in the SGZ (1) then migrate a short distance into the GCL (2) where a percentage will survive and mature, becoming glutamatergic granule neurons that extend axons to the CA3 region of the hippocampus (3). Adapted from Borsini et al., 2015.
In both neurogenic brain regions, adult born neurons serve as excellent candidates for plasticity during pregnancy, parturition and the postpartum period because they are not only sensitive to many hormonal and experiential changes that occur during these times but also because the functions which they have been suggested to contribute to are affected by motherhood. Here we highlight similarities and differences in the effects of motherhood on adult neurogenesis in the DG and SVZ across various species as well as the putative underlying mechanisms. We also consider the extent to which neurogenesis in the peripartum SVZ and/or DG may play a role in maternal care, cognitive function and postpartum mood. Finally, while most work investigating the effects of parenting on adult neurogenesis has focused on mothers, a few studies have examined fathers and these results are also discussed (Leuner et al., 2010; Levy et al., 2011; Lieberwirth and Wang, 2012).
3. Important considerations
Studying a dynamic process like adult neurogenesis during dynamic periods such as pregnancy and the postpartum period is inherently complex. New neurons are born, develop and mature while mothers are carrying or caring for offspring who are doing the same all the while undergoing numerous physiological changes. An additional level of complexity arises when attempting to compare species which can differ with respect to variables such as reproductive strategy, degree of parental investment, developmental maturity of offspring at birth as well as certain temporal features of adult neurogenesis (Snyder et al., 2009; Bonfanti and Peretto, 2011; Lieberwirth and Wang, 2012; Brus et al., 2013). Even between laboratory rats and mice there are differences in the olfactory regulation of maternal care (Corona and Levy, 2015) as well as in the capacity to spontaneously display maternal care (Stolzenberg and Rissman, 2011). Because of these differences, broad generalizations about neurogenesis in the parental brain may be difficult. Like most studies of adult neurogenesis, investigating this mode of plasticity in the parental brain is further complicated by various methodological issues. Many of these are related to bromodeoxyuridine (BrdU), the exogenous marker mostly widely used to label newly born neurons (Taupin, 2007; Leuner and Gould, 2010). After administration, usually by intraperitoneal injection, BrdU becomes incorporated into the DNA of cells during the synthesis stage of the cell cycle, and depending on the survival time employed, different stages of adult neurogenesis can be investigated. For example, short survival times of 2–24 hr are often used to assess cell proliferation while longer survival times are employed in studies of cell survival. BrdU is detected using immunohistochemical methods which can be combined with multiple markers to identify phenotypes of new cells, their maturational stage and/or to assess the expression of various receptors and immediate early genes. Administration and detection of BrdU is relatively straightforward. However, there is considerable variation in how BrdU is used including dose, number of injections and timing of administration which, along with differences in immunohistochemical detection methods, have the potential to influence experimental outcome (Christie and Cameron, 2006; Leuner et al., 2009). Some of these concerns can be addressed by using endogenous markers, such as Ki67 or doublecortin (DCX), although this approach is not without drawbacks which include potential uncertainty about specificity and timeline of expression (Eisch and Mandyam, 2007; Balthazart and Ball, 2014). Collectively, these issues must be kept in mind and special care taken in the interpretation of seemingly contradictory data.
4. Hippocampal neurogenesis during pregnancy and the postpartum period
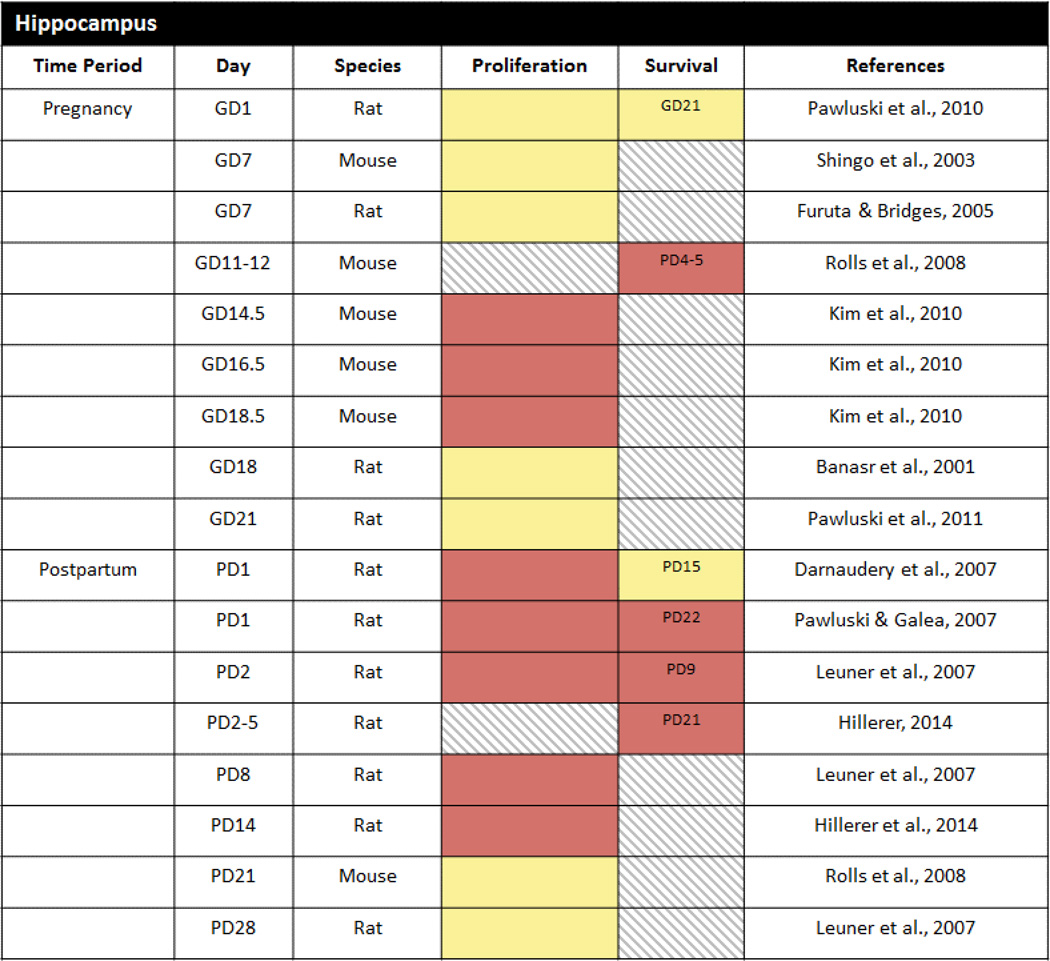
The first demonstration that reproductive status can alter adult neurogenesis found that cell proliferation in the DG was suppressed in pregnant wild female meadow voles trapped during the breeding season relative to non-pregnant females trapped during the non-breeding season (Galea and McEwen, 1999). Subsequent research has focused primarily on laboratory rodents and has investigated both hippocampal cell proliferation and survival (Table 1). In contrast to wild meadow voles, rats do not exhibit alterations in cell proliferation on gestational days (GD) 7, 18 or 21 (Banasr et al., 2001; Furuta and Bridges, 2005; Pawluski et al., 2010; Pawluski et al., 2011; Pawluski et al., 2015a) as compared to nulliparous (virgin) females. There are also no effects of pregnancy on hippocampal cell proliferation when BrdU is injected earlier on GD1 even when the number of pregnancies is taken into account (Pawluski et al., 2010). Likewise, cell survival in the DG is unaltered in the pregnant rat – similar numbers of cells survive across pregnancy from GD0–1 to GD21–22 as survive in virgin females across the same time frame (Pawluski et al., 2010; Pawluski et al., 2011). In fact, the only change detected to date in pregnant rats is a potential increase in differentiation/migration on GD18 as assessed with the marker PSA-NCAM (Banasr et al., 2001).
Table 1.
Cell proliferation and cell survival in the DG during pregnancy and the postpartum period of laboratory rats and mice. Red = decrease, Blue = increase, Yellow = no change, Gray hatched = no data. Day in survival column indicates the timepoint at which cell survival was assessed.
In accordance with the rat studies, Shingo et al. (2003) also showed no change in hippocampal cell proliferation when mice were injected with BrdU during early pregnancy (GD7). However, other work (Kim et al., 2010) using the cell proliferation marker Ki67 did uncover fewer proliferating cells in the mouse DG at later gestational time points (GD14.5, GD16.5 and GD18.5) suggesting that alterations in cell proliferation during pregnancy occur in a species- and time-dependent manner. Effects of pregnancy on cell survival and differentiation have also been demonstrated. Pregnant mice given BrdU on GD 11–12 and sacrificed 2 weeks later during the early postpartum period had fewer BrdU labeled cells and a lower percentage of BrdU labeled cells co-labeled with the immature neuronal marker DCX as compared to age-matched virgin mice (Rolls et al., 2008). Whether this represents a species-specific effect of pregnancy has yet to be determined because of the different experimental timelines used in the rat and mouse studies.
Although available evidence indicates that hippocampal cell proliferation is not altered during pregnancy in female rats, cell proliferation has been consistently shown to be reduced during the early-mid postpartum period on postpartum day 1 (PD1), PD2, PD8 and PD14 (Darnaudery et al., 2007; Leuner et al., 2007; Pawluski and Galea, 2007; Hillerer et al., 2014; Workman et al., 2015) as compared to nulliparous females. Furthermore, the postpartum reduction in cell proliferation occurs in both first time (primiparous) and experienced (multiparous) mothers and thus is not modulated by reproductive experience (Pawluski and Galea, 2007). It may seem counterintuitive that hippocampal neurogenesis would be decreased postpartum, a time of significant plasticity in many brain regions including the hippocampus (Figure 1; Galea et al., 2014; Slattery and Hillerer, 2016). However, considering the high energy demands required for lactation, it may be adaptive to downregulate metabolically costly processes such as cell proliferation. In support of this, reduced cell proliferation during the postpartum period is temporary and is dependent upon lactation and the presence of nursing offspring – it subsides around the time of weaning (PD28), when offspring no longer nurse, and can be prevented by the removal of the offspring shortly after birth (Leuner et al., 2007).
Some studies suggest that along with diminished cell proliferation, cell survival is also suppressed in rats across the postpartum period (Leuner et al., 2007; Pawluski and Galea, 2007; Hillerer et al., 2014 but see Darnaudery et al., 2007). For example, Pawluski and Galea (2007) found that fewer cells labeled with BrdU on PD1 survived to PD22. However, unlike the postpartum reduction in cell proliferation, cell survival was decreased only in primiparous female rats and this reduction occurred independent of pup exposure (Pawluski and Galea, 2007).
Expanding upon the postpartum cell proliferation and survival findings, Workman et al. (2015) recently found that at the end of the postpartum period (PD22), the density of DCX-expressing cells is also significantly lower in the hippocampus of primparious rats. Further analyses revealed that postpartum rats had a lower proportion of more mature postmitotic DCX-expressing cells and a higher proportion of less mature proliferative cells compared with nulliparous rats. Thus, although hippocampal neurogenesis is suppressed during the postpartum period, it begins to recover just prior to weaning.
A small number of studies have also examined whether hippocampal neurogenesis is altered during the postpartum period in species other than rats. To the best of our knowledge, only one has examined cell proliferation in the DG of laboratory mice during the postpartum period on PD21 (Rolls et al., 2008) and it found no difference from virgin females consistent with the restoration of cell proliferation during the late postpartum period of the rat reported by Leuner et al. (2007). Whether there are differences in the number of proliferating cells at earlier postpartum time points in mice as seen in rats requires further investigation. In other work using the biparental California mouse, cell survival was shown to be reduced in the DG of mothers as compared to virgin controls such that there were fewer cells born one-week postpartum that survived to weaning 3 weeks later (Glasper et al., 2011). Cell proliferation and cell survival were also found to be down-regulated in the DG of primiparous postpartum sheep two days after the birth of their offspring (Brus et al., 2010; Brus et al., 2014). However, unlike postpartum rats in which only cell survival remained reduced after pups removal, both effects in ewes were sustained after removal of the lamb at parturition indicating species differences with regard to the role of offspring. Rodent offspring are altricial while sheep offspring are precocial and thus differences in developmental maturity at the time of birth may underlie these species variations in offspring regulation of neurogenesis in the postpartum DG.
Importantly, interactions with young can have opposing effects on hippocampal neurogenesis depending on the reproductive status of the female. In rats, cell proliferation increases in virgins acutely exposed to pups for 2 days while cell survival increases in ‘sensitized’ virgins exposed to pups for 22 days and induced to behave maternally (Pawluski and Galea, 2007). Likewise, offspring seem to have a stimulatory effect in virgin females of other rodent species including prairie voles which are biparental and typically display spontaneous parental care when exposed to foster pups. Specifically, Ruscio et al. (2008) found that virgin female voles briefly exposed to pups for just 20 min exhibit increased cell proliferation in the DG regardless of whether they behaved parentally. It is important to note however that cell proliferation also increased in response to a control stimulus (i.e. exposure to a tootsie roll) raising questions about the extent to which increased cell proliferation is a response to novelty.
5. Neurogenesis in the SVZ during pregnancy and the postpartum period
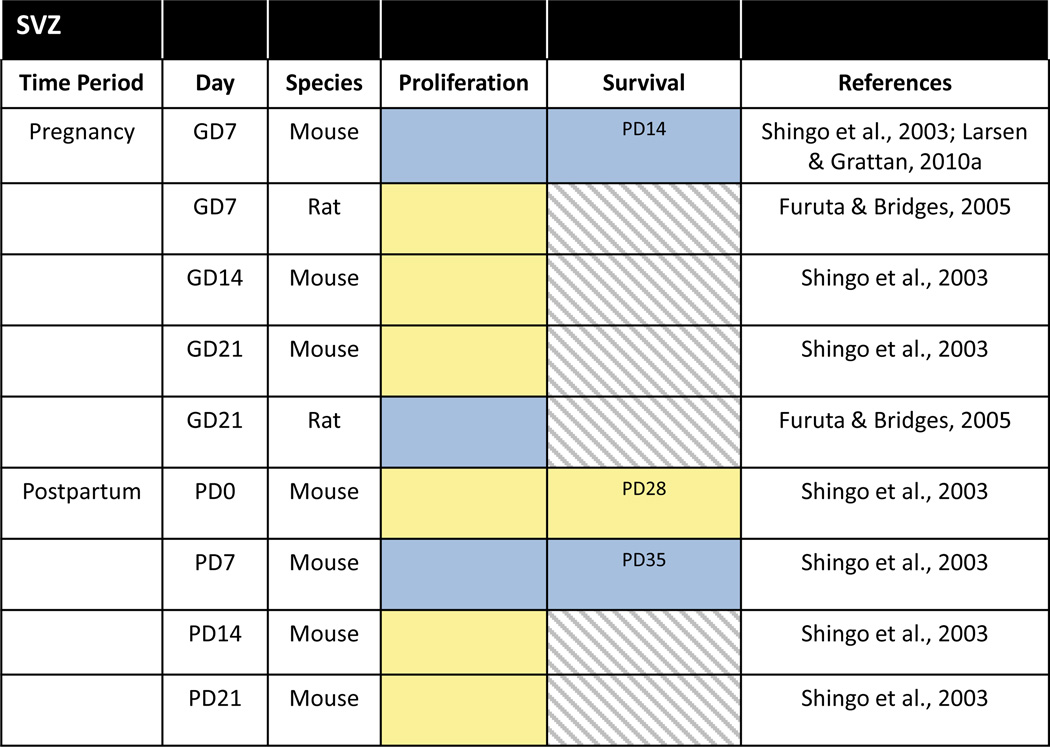
Gestation has also been reported to affect adult neurogenesis within the SVZ of laboratory rats and mice (Table 2). Cell proliferation in rats was shown to be increased in the SVZ during late pregnancy on GD21 but not earlier on GD7 (Furuta and Bridges, 2005). Pregnancy in mice was also associated with increased cell proliferation in the SVZ although the timing was opposite from rats with the increase evident on GD7 but not later on GD14 or GD21 (Shingo et al., 2003; Larsen and Grattan, 2010a). Despite numerous methodological differences in these two studies related to BrdU labeling (i.e. route of administration, number and dose of injections, survival time after BrdU), these are unlikely to account completely for the contrasting effects observed in rats and mice because the increase in cell proliferation on GD7 in mice was also found with the endogenous marker of cell proliferation, Ki67.
Table 2.
Cell proliferation and cell survival in the SVZ/OB during pregnancy and the postpartum period of laboratory rats and mice. Red = decrease, Blue = increase, Yellow = no change, Gray hatched = no data. Day in survival column indicates the timepoint at which cell survival was assessed.
In addition showing an increase in cell proliferation during pregnancy, Shingo et al. (2003) also found that a substantial percentage (> 50%) of the proliferating cells in the SVZ survive for at least 4 weeks and migrate to become olfactory interneurons. Besides the OB, there is also some evidence that newly generated neurons derived from the adult SVZ may migrate to other olfactory, limbic, striatal and cortical regions (Shapiro et al., 2009). This raises the intriguing possibility that there may be other destinations for these new cells born in the SVZ during pregnancy.
During the postpartum period of the mouse, another peak in cell proliferation occurs in the SVZ on PD7, but not PD14 or PD21 (Shingo et al., 2003). As in pregnancy, the postpartum increase in SVZ proliferation is followed by a doubling of the numbers of new OB interneurons (Shingo et al., 2003). However, this enhancement of neurogenesis in the postpartum SVZ may not be a universal phenomenon shared across mammalian species as it was not observed in sheep. Specifically, Brus et al. (2010, 2014) reported reduced cell proliferation in the SVZ, as well as reduced survival of newborn neurons in the OB, during the early postpartum period in ewes that was due to interactions with the lamb as these effects were not observed in ewes separated from their offspring at birth. Here too, various methodological variations in BrdU labeling could contribute to the discrepancies in mice versus sheep but differences in the duration of contact with pups – 7 days in Shingo et al. (2003) versus 2 days in Brus et al. (2010) – could be involved as well. These species differences may further be related to the olfactory regulation of mothering. While odors from the young stimulate maternal care in both mice and sheep, only sheep form selective bonds based on odor recognition of their offspring (Brus et al., 2010; Levy et al., 2011; Corona and Levy, 2015). The results also raise the question of what might be the response in primates, especially humans, where parenting is governed less by olfactory cues than in mice or sheep, although the existence of OB neurogenesis in humans is controversial (Macklis, 2012).
Along with increased cell proliferation and cell survival, adult born neurons in the OB of postpartum mice also exhibit structural features (i.e. fewer, more stable dendritic spines) that are indicative of enhanced synaptic integration into the bulbar circuitry (Kopel et al., 2012). Interestingly, enhanced dendritic maturation of olfactory neuroblasts has been reported in sheep mothers interacting with their lambs despite reduced cell proliferation and survival (Brus et al., 2014). Accelerated maturation and incorporation of newborn neurons into the maternal OB may thus be part of the normal repertoire of adult-born neurons following parturition regardless of species variations in the total number of new OB neurons.
There is no evidence indicating that cell proliferation in the SVZ, at least in the caudal portion, is altered in the rat during the postpartum period on PD2 or PD8 (Leuner et al., 2007). However, ‘sensitized’ virgin rats exposed to pups and induced to display maternal behavior have a greater number of newly born neurons in the SVZ as compared to non-maternal, pup-exposed virgins and virgins not exposed to young (Furuta and Bridges, 2009). These data suggest that maternal behavior itself, independent of pregnancy and lactation, is associated with the stimulation of adult neurogenesis in the rat SVZ.
6. Mechanisms underlying changes in neurogenesis during pregnancy and the postpartum period
The balance of hormones changes dramatically in females during pregnancy, parturition and the postpartum period (Numan and Insel, 2003; Pawluski et al., 2009a; Workman et al., 2012) with many mammals, including rodents and sheep, exhibiting similar profiles of various key hormones during these times. For example, estradiol levels rise during late pregnancy, peak at parturition then drastically drop and remain low during lactation (Chamley et al., 1973; Rosenblatt et al., 1979; Pawluski et al., 2009a). In contrast, progesterone levels, which are high throughout pregnancy, decrease prior to parturition, but in rats again become elevated during mid-lactation (Chamley et al., 1973; Pawluski et al., 2009a). The hypothalamic-pituitary-adrenal (HPA) system is also altered during pregnancy and the postpartum period such that corticosterone (CORT) levels increase during late gestation and remain elevated during lactation (Chamley et al., 1973; Stern and Voogt, 1973; Fischer et al., 1995). Along with these ovarian and adrenal steroid hormones, there are alterations in peptide hormones including prolactin, oxytocin and vasopressin. Levels of the pituitary hormone prolactin are elevated in early pregnancy and rise rapidly again around the time of parturition after which high levels are maintained by suckling stimulation (Chamley et al., 1973; Grattan, 2001; Torner and Neumann, 2002). Similarly, both parturition and lactation are associated with upregulation of the hypothalamic oxytocin and vasopressin systems (Slattery and Neumann, 2008; Bosch and Neumann, 2012).
Hormones are well-known regulators of adult neurogenesis, although it is important to note that their effects often vary according to species, sex, brain region and stage of neurogenesis in addition to numerous other experimental variables (i.e. endogenous vs exogenous; dose, timing and duration of hormone administration), a detailed discussion of which is beyond the scope of this review (see Pawluski et al., 2009a; Schoenfeld and Gould, 2012; Galea et al., 2013). Briefly, acute estradiol treatment increases cell proliferation in the DG of female but not male rats (Tanapat et al., 1999; Barha et al., 2009) while chronic estradiol treatment decreases cell survival in female rats (Barker and Galea, 2008; Chan et al., 2014). In the female mouse SVZ, estradiol seems to influence cell proliferation and survival in a dose- and time-dependent manner (Shingo et al., 2003; Brock et al., 2010; Veyrac and Bakker, 2011). Progesterone’s effects on neurogenesis have been less studied. However, available evidence indicates stimulatory effects on cell proliferation in the female DG when given acutely (Liu et al., 2009) but no significant effects on cell proliferation or cell survival when given chronically (Chan et al., 2014). Furthermore, progesterone interacts with estradiol to modulate hippocampal cell proliferation in females (Tanapat et al., 2005; Galea et al., 2006) and can promote neurogenesis in the DG of males as well (Barha et al., 2009; Zhang et al., 2010). In general, corticosterone has a suppressive effect on neurogenesis in the DG of male and female rats (Brummelte and Galea, 2010b; Schoenfeld and Gould, 2012) independent of reproductive status (Brummelte and Galea, 2010a) and has also been shown to diminish cell proliferation in the SVZ of male rats (Lau et al., 2007). On the other hand, prolactin has been linked to the promotion of adult neurogenesis in both regions of male and female mice (Shingo et al., 2003; Mak and Weiss, 2010; Larsen and Grattan, 2012; Walker et al., 2012). Exogenous oxytocin, but not vasopressin, also stimulates new cell production in the DG of male rats (Leuner et al., 2012) but their effects on neurogenesis in the female DG, or in the SVZ of either sex, have not been investigated to date. Further work is also needed to determine how adult neurogenesis is affected by other peptides associated with pregnancy and motherhood (such as CRF, placental lactogens) as well as the possible interactive effects of these various hormones, which for the most part, have been studied in isolation. These deficiencies notwithstanding, given what is known about the hormonal regulation of neurogenesis, it should not be surprising that at least some of the peripartum-associated modifications in neurogenesis in the DG and SVZ are under the influence of endocrine events.
6.1 Mechanisms: DG
The down-regulation of cell proliferation during the early postpartum period in primiparous rats has been directly linked to hormonal changes associated with nursing pups and lactation, specifically elevated basal glucocorticoid levels. Accordingly, lowering CORT levels by adrenalectomy or preventing the postpartum increase by pup removal blocks the decrease in hippocampal cell proliferation (Leuner et al., 2007). It is notable that both prolactin and oxytocin have the capacity to protect hippocampal neurogenesis from stress or elevated glucocorticoids (Torner et al., 2009; Leuner et al., 2012) and yet the maternal hippocampus remains sensitive to the influence of high glucocorticoids. Because the postpartum period is accompanied by reduced binding capacity of glucocorticoid receptors (GRs) in the hippocampus (Meaney et al., 1989) and decreased corticosteroid-binding globulin (CBG), which normally serves to buffer the amount of biologically active glucocorticoids (Gala and Westphal, 1965; Walker et al., 1992; Pawluski et al., 2009b), these may exacerbate corticosterone action on hippocampal neurogenesis. It is possible that during late pregnancy, increased GR levels (Pawluski et al., 2015a) may be sufficient to buffer against reductions in hippocampal cell proliferation due to elevated glucocorticoids, despite low CBG levels which also occurs at this time (Johnstone et al., 2000; Pawluski et al., 2009b).
The mechanisms underlying the glucocorticoid-mediated reduction in cell proliferation may involve direct effects through GRs on progenitor cells (Cameron et al., 1993; Garcia et al., 2004). Alternatively, glucocorticoids may engage an intermediary factor, for example by acting on granule cell afferents that contain GRs or have other indirect effects on neurogenesis by affecting vascular tone and/or neighboring glial and neuronal cells that express appropriate receptors (Schoenfeld and Gould, 2012; Egeland et al., 2015). In addition, it remains possible that during the postpartum period, glucocorticoids interact with some other biochemical change associated with lactation or maternal care to suppress cell proliferation. As discussed above, the postpartum period is also accompanied by a decrease in estradiol levels (Rosenblatt et al., 1979) and it has been shown that manipulating estradiol using a hormone-simulated pregnancy regimen produces a similar decrease in cell proliferation to that found during the early postpartum period in rats (Green and Galea, 2008). When combined with work showing an interaction between glucocorticoids and estradiol on neurogenesis in the DG (Ormerod and Galea, 2001), it is conceivable that there may be a complex interplay between adrenal and ovarian steroids that contributes to the reduction in hippocampal cell production during the postpartum period. These and potentially other interactive hormonal effects on hippocampal neurogenesis during the peripartum period require further investigation.
It remains to be determined whether similar hormonal mechanisms are responsible for the reduction in cell proliferation also observed in multiparous mothers as well as the hormonal mediators contributing to the effect of parity on cell survival. In this regard, it is worth noting that there are various hormonal and neurochemical differences between primiparous and multiparous rats (Numan and Insel, 2003; Bridges, 2015). For example, multiparty is associated with higher estradiol and lower prolactin during gestation as compared to primiparity (Bridges et al., 1993; Paris and Frye, 2008). Furthermore, reproductive experience differentially affects levels of CORT and CBG such that primiparous females have higher CORT and lower CBG as compared to multiparous females. In addition to hormones, several other factors have been shown to be differentially altered during pregnancy and the postpartum period in multiparous and primiparous rats including dopamine (Felicio et al., 1996) and opioids (Mann and Bridges, 1992) and these could play a role as well.
Hormonal mechanisms are unlikely to underlie the increase in hippocampal neurogenesis seen in pup-exposed and ‘sensitized’ virgin rats. Rather, the presence of offspring and the various types of stimulation (i.e. olfactory, tactile, auditory) they provide may be a form of environmental enrichment which is known to stimulate new neuron production in the DG (van Praag et al., 2000; Brown et al., 2003). These results further suggest that the contrasting effects of pup exposure on hippocampal neurogenesis in virgins and postpartum mothers may be caused by one or more factors that differ in these conditions. These factors may include differences in physiological demands accompanying lactation or resulting from parturition and pregnancy as well as hormonal fluctuations. Thus, increased neurogenesis in virgin female rats following pup exposure may reflect environmental enrichment in the absence of these other changes associated with pregnancy and lactation. In lactating females however, increased glucocorticoid levels seem to override the possible pup-enhancing effects on hippocampal neurogenesis.
Other work investigating the mechanisms underlying the downregulation in hippocampal neurogenesis during pregnancy in mice has raised the possibility that factors besides hormones may be playing a role. In addition to changes in the hormonal milieu, pregnancy is a time of altered immune and inflammatory responses to support and foster the growth of the developing fetus (Luppi, 2003). Among the gestational immunological changes that occur are alterations in the activity of T-cells, immune cells which have been shown to support adult hippocampal neurogenesis (Ziv et al., 2006). To investigate whether the pregnancy-induced decrease in neurogenesis is immune-dependent, Rolls et al. (2008) examined 2 week cell survival in virgin and pregnant (GD11–12) nude immune deficient mice that lack the entire T-cell population and observed that pregnant immune-deficient mice did not show any reduction in the formation of new neurons relative to virgin immune-deficient mice. Thus, both groups presented with low levels of new neuron formation, as both groups lacked immune cells that could promote the generation of new neurons. However, when immune deficient mice were reconstituted with T-cells prior to mating, the pregnancy induced reduction in neurogenesis became evident. Thus, while virgin mice could benefit from the restoration of immune cells, pregnant mice could not because of the immune suppression in pregnancy. The early postpartum period is also associated with immuno-inflammatory changes (Anderson and Maes, 2013) which raises the unexplored possibility that immune factors may be contributing to reduced neurogenesis in the maternal brain as well. It is also worth considering that microglia, resident immune cells in the brain that regulate hippocampal neurogenesis (Aimone et al., 2014), may themselves be impacted during gestation or motherhood (Haim et al., 2015) and directly contribute to the suppression of neurogenesis in pregnant mice and/or postpartum rats. More research is needed to uncover potential neural-immune interactions during the peripartum period.
6.2 Mechanisms: SVZ/OB
In contrast to the regulation of neurogenesis in the DG of postpartum rats by glucocorticoids, various lines of evidence indicate that high prolactin levels are both necessary and sufficient to increase neurogenesis in the SVZ of pregnant mice (Larsen and Grattan, 2012). For example, systemic or central administration of prolactin induces cell proliferation in the SVZ of ovariectomized female mice (Shingo et al., 2003). Conversely, a reduction in prolactin levels pharmacologically with bromocriptine (Larsen and Grattan, 2010b) or physiologically as a consequence of exposure to unfamiliar female pheromones (Larsen and Grattan, 2010a) prevents the early pregnancy increase in SVZ cell proliferation. Enhanced neurogenesis in the SVZ on GD7 was also shown to be attenuated in mice with a targeted disruption of the gene encoding the prolactin receptor (Shingo et al., 2003).
There are numerous open questions pertaining to prolactin regulation of neurogenesis in the SVZ. First, how does prolactin act to stimulate neurogenesis in the SVZ? Some data suggest that prolactin receptors are expressed in the SVZ and specifically on neural progenitors (Mak and Weiss, 2010) and therefore might directly mediate prolactin’s actions on neurogenesis. However, other work could not find evidence for prolactin receptors in the SVZ (Brown et al., 2010) suggesting that prolactin action on neurogenesis in the SVZ may instead be mediated indirectly, potentially through prolactin-sensitive afferent neurons (Larsen and Grattan, 2012). Second, why do female mice show increased SVZ cell proliferation on GD7 but not GD21 while rats show the opposite even though prolactin levels are known to be elevated at both time points in both species? It is possible that there are differences between rats and mice in the regulation of neurogenesis by prolactin or that lactogenic hormones other than prolactin are playing a role. For example, placental lactogens rise during the second half of gestation in rats only and may account for the discrepancy during late pregnancy (Markoff and Talamantes, 1981; Furuta and Bridges, 2005; Levy et al., 2011). Third, is SVZ neurogenesis also increased in multiparous mothers? Given that maternal experience is associated with lowered prolactin during gestation (Bridges et al., 1993), increased neurogenesis in the SVZ may be specific to primigravidy. Lastly, is prolactin also responsible for the enhanced maturation and synaptic integration of adult born neurons in the OB of lactating mice and/or sheep (Kopel et al., 2012; Brus et al., 2014)? Other hormones or changes in the mother’s sensory environment could also be playing a role but further studies are needed to identify the underlying mechanisms and whether they are similar or different across species.
7. Functional consequences of altered neurogenesis during pregnancy and the postpartum period
The constant integration of newborn neurons into the functional circuitry of the DG and OB along with their unique properties that differ from more mature neurons (Ming and Song, 2011; Aimone et al., 2014; Lepousez et al., 2015), suggests that these neurons may serve a very specific function. This function has not been fully elucidated and continues to be a topic of intense investigation and discussion (Gheusi et al., 2009; Aimone et al., 2011; Lazic, 2012; Gheusi et al., 2013; Cameron and Glover, 2015; Miller and Hen, 2015; Opendak and Gould, 2015). Nonetheless, numerous studies have linked adult neurogenesis in the hippocampus to hippocampal-dependent functions including certain cognitive abilities as well as emotion and stress regulation (Leuner and Gould, 2010; Snyder et al., 2011; Cameron and Glover, 2015; Miller and Hen, 2015) while olfactory neurogenesis has been implicated in a range of olfactory related and social behaviors (Gheusi et al., 2009; Lieberwirth and Wang, 2012; Gheusi et al., 2013). All of these functions are known to be altered during pregnancy and/or the postpartum period suggesting that newly-born neurons may be playing a role but the number of studies addressing this possibility are scarce.
7.1 Functional consequences: DG
The function of altered hippocampal neurogenesis during pregnancy and the postpartum period has yet to be determined. Given evidence linking neurogenesis in the DG to various learning and memory functions (Leuner et al., 2006; Cameron and Glover, 2015), it is possible that hippocampal neurogenesis mediates cognitive changes that have been reported to occur during the peripartum period (Macbeth and Luine, 2010; Workman et al., 2012; Duarte-Guterman et al., 2015). One way to explore this possibility would be to look for parallel changes in hippocampal neurogenesis and cognitive abilities that would suggest a possible link between the two effects. Support for such a link is found during the early postpartum period when both hippocampal cell proliferation and spatial cognition are reduced in primiparous females (Darnaudery et al., 2007; Leuner et al., 2007; Pawluski and Galea, 2007). However, neurogenesis may only play a minor role in cognitive changes during other peripartum times. For example, variations in spatial abilities are evident across pregnancy in rats when neither cell proliferation nor survival are altered (Galea et al., 2000; Bodensteiner et al., 2006; Paris and Frye, 2008). Likewise, primiparous female rats during the late postpartum period outperform nulliparous females on spatial tasks despite comparable levels of cell proliferation and reduced cell survival (Kinsley et al., 1999; Darnaudery et al., 2007; Leuner et al., 2007; Pawluski and Galea, 2007; Hillerer et al., 2014). Studies examining spatial cognition in the post-weaning period have found that both primiparous and multiparous mothers exhibit enhanced spatial learning and memory compared to nulliparous rats even well into aging (Kinsley et al., 1999; Gatewood et al., 2005; Lemaire et al., 2006; Pawluski et al., 2006a; Pawluski et al., 2006b; Macbeth et al., 2008; Paris and Frye, 2008; Kinsley et al., 2012; Cost et al., 2014). But as in the late postpartum period, cell proliferation levels in primiparous rats are not significantly different than nulliparous rats (Leuner et al., 2007). During these times, mechanisms other than neurogenesis must be responsible for variations in spatial abilities. For example, alterations in synaptic plasticity (Tomizawa et al., 2003; Lemaire et al., 2006) and additional types of structural plasticity, such as dendritic complexity and spine density, have also been reported during pregnancy and motherhood and in some cases are associated with spatial cognitive performance (Kinsley et al., 2006; Pawluski and Galea, 2006; Brusco et al., 2008; Leuner et al., 2010; Galea et al., 2014; Slattery and Hillerer, 2016). It is also important to keep in mind that the amount of neurogenesis does not always correlate with spatial abilities (Leuner et al., 2006; Duarte-Guterman et al., 2015) and thus it may be that the number of cells is not as important as the function of each individual new cell within the hippocampal network. Consequently, determining the contribution of new neurons to cognitive functions during this time may require alternative methodological approaches. For example, looking at the expression of immediate early genes in new neurons in response to spatial learning may reveal differential activation in mothers.
In addition to changes in cognition across the peripartum period, there are also alterations in stress responsiveness. Thus, while basal glucocorticoid levels are elevated, the response of the hypothalamic-pituitary-adrenal (HPA) axis is reduced postpartum (Slattery and Neumann, 2008; Perani and Slattery, 2014). New neurons have been implicated in the stress regulatory functions of the hippocampus by dampening the HPA response to stress (Snyder et al., 2011) and therefore may play a role in lowered stress responsiveness during the postpartum period. However, reduced numbers of newly born neurons as seen during the postpartum period would be expected to be associated with greater stress responsiveness (Snyder et al., 2011). Likewise, anxiety regulation has been linked to hippocampal neurogenesis such that fewer numbers of new neurons are associated with elevated anxiety (Revest et al., 2009; Opendak and Gould, 2015). But, this relationship doesn’t hold true for the postpartum period when both neurogenesis and anxiety are reduced (Leuner et al., 2007; Lonstein, 2007). Taken together, these data suggest that mechanisms other than neurogenesis may contribute to alterations in these functions during motherhood. However, as with the relationship between postpartum hippocampal neurogenesis and cognition, a role for new neurons in these other hippocampal functions may be revealed if approaches are used to examine whether new neurons in the postpartum brain function differently in response to activation of the HPA axis or anxiety-provoking situations.
The peripartum period, while generally a time of increased calmness and reduced stress responsiveness, also represents a period when women are at their most vulnerable to develop depression. Indeed, postpartum depression represents one of the most common complications following childbirth and has been estimated to affect up to 20% of new mothers (O’Hara and McCabe, 2013; Wisner et al., 2013; Agrati and Lonstein, 2015; Brummelte and Galea, 2015). A number of factors have been identified that may increase the risk of postpartum depression (Robertson et al., 2004; Stone et al., 2015), including stress during pregnancy and/or after birth which can be easily modeled in rodents (Perani and Slattery, 2014). For example, a growing body of research has revealed that repeated exposure to stress while pregnant can induce behavioral despair and impaired maternal care during the postpartum period (Smith et al., 2004; O’Mahony et al., 2006; Haim et al., 2014; Leuner et al., 2014), thus acting as a model of postpartum depression. Interestingly, repeated stress has limited effects on depressive-like behavior in the pregnant female (Baker et al., 2008; Pawluski et al., 2011), suggesting differential effects of stress during pregnancy and lactation.
Stress also affects hippocampal neurogenesis, and stress-induced alterations in hippocampal neurogenesis have been suggested to play a role in depressive-like behaviors (Schoenfeld and Gould, 2012; Miller and Hen, 2015; Schoenfeld and Cameron, 2015). The effects of stress on hippocampal neurogenesis are sex-dependent and are influenced by the type and duration of the stress as well as the stage of neurogenesis assessed (Schoenfeld and Gould, 2012; Gobinath et al., 2014). For example, in virgin females, cell proliferation is unaffected by acute stress (Falconer and Galea, 2003; Shors et al., 2007) but cell survival increases in response to chronic stress (Westenbroek et al., 2004). There are only a limited number of studies which have investigated the effects of stress on hippocampal neurogenesis in the pregnant and postpartum female. These have shown that one week of repeated restraint stress (3 times/day for 45 min) during early (GD5–11) or mid-late gestation (GD11–17) increases the number of proliferating cells in the hippocampus on GD21 without affecting cell survival (Pawluski et al., 2011; Pawluski et al., 2015a). This same stress protocol also increased hippocampal cell proliferation in virgin females indicating that it is not specific to pregnancy (Pawluski et al., 2011). However, there appears to be little effect of gestational stress on hippocampal neurogenesis at the time of weaning (Pawluski et al., 2012). Other work using restraint stress (2 hr/day) for 12 days during the early-mid postpartum period (PD2–13) found increased cell proliferation on PD14 thus preventing the reduction in hippocampal cell proliferation typically observed at this time (Hillerer et al., 2014). In this case, the same stressor was without effect in virgins and, as such, provides the first direct evidence that neurogenesis in the DG is differentially affected by stress during lactation.
In addition to stress, prolonged treatment with high levels of CORT during the postpartum period (PD2–24) has also been used to model postpartum depression (Brummelte and Galea, 2010c; Workman et al., 2016). In this model, depressive-like behavior and impaired maternal care are associated with reduced cell proliferation (Brummelte and Galea, 2010a) and reduced neurogenesis as assessed with DCX (Workman et al., 2016). In virgin females, chronic CORT also increased depressive-like behavior and reduced DG neurogenesis in addition to impacting immature neuron development (Workman et al., 2016). Along with the demonstration that despite reduced neurogenesis, postpartum females exhibit less depressive-like behavior and greater recovery of CORT following stress than virgins (Workman et al., 2016), these data suggest that the postpartum period confers some degree of resilience at least when it comes to chronic glucocorticoids and acute stressors. Together, these studies represent important steps in understanding how parity modifies the impact of stress and glucocorticoids on hippocampal neurogenesis but whether these changes play a causal role in postpartum mood disorders remains to be determined.
Maternal stress and depression are not only detrimental for maternal well-being, they can also adversely affect child development (Lovejoy et al., 2000; Brummelte and Galea, 2015). As such, effective treatment of maternal mood disorders is needed. One of the most common treatments for maternal depression during pregnancy and postpartum are selective serotonin reuptake inhibitor (SSRI) antidepressant medications (Epperson et al., 2003). However, several systematic reviews indicate that such pharmacological antidepressants are not as efficacious to the mother during the postpartum period (Sharma and Sommerdyk, 2013; De Crescenzo et al., 2014; Molyneaux et al., 2014). SSRI medications are thought to alleviate depressive symptoms in part through their effects on neurogenesis – SSRIs promote hippocampal neurogenesis and neurogenesis in turn is required for the behavioral effects of antidepressants (Malberg et al., 2000; Santarelli et al., 2003; Eisch et al., 2008; Miller and Hen, 2015). Together, these data suggest SSRIs may have a different effect on neurogenesis during the peripartum period. Only two studies to date have addressed this possibility. In one, chronic administration of the SSRI fluoxetine (5mg/kg) from PD1–28 was shown to increase hippocampal neurogenesis as assessed with DCX on PD28 but only if mothers had been exposed to gestational stress which itself was without effect (Pawluski et al., 2012). In the other, a larger dose of fluoxetine (10 mg/kg) administered from PD2–23 was effective in increasing neurogenesis in the DG of virgin, but not postpartum, females (Workman et al., 2016). Much more work investigating different dosages and types of antidepressant medications, varying durations of treatment during pregnancy and postpartum, the neurobiological effects of antidepressant treatment in different models of postpartum depression and the behavioral outcomes of these treatments are needed to understand and improve the efficacy of these medications for the treatment of peripartum mood disorders.
7.2 Functional consequences: SVZ/OB
The OB is an important region involved in behavioral responses during motherhood and in several mammalian species, odors from the young are essential to elicit maternal care and recognition of the young (Numan and Insel, 2003; Corona and Levy, 2015). Thus, alterations in SVZ/OB neurogenesis may support some functional aspects of maternal behavior. Using a variety of technical approaches to delete new neurons, several studies have addressed this issue and while some have implicated new neurons in maternal care, others have not.
In mice, the prolactin-induced increase in SVZ neurogenesis during pregnancy would result in new mature granule cells being present in the OB at the time of parturition which could be important for fine tuning the olfactory response to pups and thus influence maternal behavior. As an initial test of this idea, Larsen and Grattan (2010b) suppressed prolactin secretion with the drug bromocriptine during early pregnancy in mice from GD1–4 and this prevented the increase in new cell production specifically in the SVZ on GD7. Another group of bromocriptine-treated mice were then allowed to continue their pregnancy to allow for maternal behavior assessment postpartum. Their results show that low prolactin during early pregnancy and the consequent suppression in SVZ neurogenesis was associated with impaired maternal care. Interestingly, these effects may have been a consequence of increased anxiety, which was also observed in the bromocriptine-treated mothers (Larsen and Grattan, 2010b). These data support the assumption that attenuated anxiety is required for the adequate display of maternal care (Lonstein, 2007) and suggests that increased generation of neural progenitors in the SVZ of the maternal brain during early pregnancy may play a role. How SVZ/OB neurogenesis could mediate mood is unknown but could involve afferent input from other brain regions critical for the display of maternal care and/or anxiety such as the amygdala (Walsh et al., 1996; Larsen and Grattan, 2012). Alternatively, newly generated cells in the SVZ may also migrate to other brain areas and this might contribute to plastic changes in additional circuits involved in postpartum anxiety (Shapiro et al., 2009; Larsen and Grattan, 2012).
It is important to note that although these data were consistent with the hypothesis that changes in SVZ neurogenesis mediated the behavioral effects of low prolactin, they were correlative only and could not rule out the possibility that another distinct action of prolactin in the brain was involved. To specifically test the role of neurogenesis during pregnancy on postpartum anxiety without manipulating prolactin levels, mice were injected with the mitotic inhibitor, methylazoxymethanol (MAM) from days 4 to 7 of pregnancy. As seen in low-prolactin animals, the MAM-treated mice displayed increased anxiety, and apparently normal maternal behavior in their home cage, but when placed into an anxiogenic situation, these mice also exhibited severely impaired maternal behavior (Larsen and Grattan, 2010b). These results strongly implicate increased neurogenesis during early pregnancy in the display of postpartum maternal behaviors and further suggest that failure of these adaptive changes might result in postpartum anxiety or an inability to cope with stressful situation in lactation. However, neurogenesis was significantly reduced not only in the SVZ about also in the DG as a result of MAM treatment. Thus, it cannot be determined from this work how much SVZ neurogenesis during pregnancy alone contributed to the observed effects or whether hippocampal neurogenesis also played a role. A similar criticism applies to another study by Sakamoto et al. (2011) which employed a transgenic mouse approach to continuously ablate newly born neurons. Although this approach produced profound deficits in retrieval and nursing behaviors, resulting in the death of the offspring within 24 hours (Sakamoto et al., 2011), it too blocked neurogenesis in both the SVZ and hippocampus. As noted above, adult hippocampal neurogenesis has been implicated in anxiety regulation (Revest et al., 2009; Opendak and Gould, 2015) and although it is not considered to be a major component of the maternal behavior circuitry, early work suggests that lesions of the hippocampus can impair some aspects of maternal care (Kimble et al., 1967; Slotnick and Nigrosh, 1975). Thus, the presence of new neurons within the hippocampus during gestation may also be contributing to the display of maternal care and reduced maternal anxiety during the postpartum period. In this regard, it is worth noting that maternal care deficits were not found when focal irradiation was used to selectively disrupt neurogenesis the SVZ/OB circuit (Feierstein et al., 2010). Given the varying effects observed, and the possibility that compensatory mechanisms occur when levels of OB neurogenesis are decreased (Saghatelyan et al., 2005), further studies using more specific approaches are needed before definitive conclusions can be reached regarding the contribution of SVZ/OB neurogenesis to maternal care and its possible relationship with maternal anxiety.
8. Effects of fatherhood on adult neurogenesis
For most mammalian species, raising the young is accomplished exclusively by the mother but for a small percentage (~6%), fathers also play a role (Lonstein and De Vries, 2000; Fernandez-Duque et al., 2009; Bales and Saltzman, 2015). Fathers of biparental species, such as the California mouse and prairie vole, engage in all aspects of parental behaviors displayed by the mother, except nursing (Lonstein and DeVries, 2000). Evidence to date suggests that the neural circuitry underlying paternal behavior is similar to that for maternal behavior (Bales and Saltzman, 2015; Perea-Rodriguez et al., 2015). Moreover, the endocrine changes experienced by fathers typically involve testosterone but also some of the same hormones and neuropeptides that regulate maternal care and lactation including estrogen, progesterone, prolactin, oxytocin and vasopressin, although often with notable species variations (Saltzman and Ziegler, 2014; Bales and Saltzman, 2015; Perea-Rodriguez et al., 2015). This raises the question of whether neurogenesis in the adult brain responds similarly in fathers as in mothers.
In the California mouse and the prairie vole, fatherhood has been shown to reduce neurogenesis in the DG without affecting neurogenesis in the SVZ or OB (Glasper et al., 2011; Lieberwirth et al., 2013). The reduction in fathers was shown to be specific to cell survival (Lieberwirth et al., 2013; Hyer et al., 2016) and thus suggests that parenthood in general impairs hippocampal neurogenesis by reducing cell proliferation and/or survival. No studies to date have looked at the potential hormonal mediators reducing neurogenesis during fatherhood. However, unlike postpartum rats, glucocorticoids are probably not involved given that basal glucocorticoid concentrations in biparental rodents typically do not differ between fathers and non-breeding males (e.g.. prairie vole: Campbell et al., 2009; and California mouse: Chauke et al., 2011; Harris and Saltzman, 2013). The extent to which pups play a role in reducing neurogenesis during fatherhood has not been directly tested either. However, there are two studies which have examined how pup exposure, in the absence of fatherhood, affects neurogenesis in the DG of male prairie voles. While one study found no effect of acute (20 min) or chronic (20 min/day for 10 consecutive days) pup exposure on cell proliferation or survival in the DG (Lieberwirth and Wang, 2012), the other found that like females, a single 20 min exposure to pups increased cell proliferation regardless of whether the animal responded parentally (Ruscio et al., 2008). The discrepancies between these studies may be related to the animal’s prior experience (mating and cohabitation with female versus sexually inexperienced, respectively) but neither eliminates a potential role for pups in driving reduced neurogenesis in fathers themselves. Indeed, as previously discussed, contrasting effects of pup exposure on hippocampal neurogenesis have been reported in virgins and postpartum mother rats (Leuner et al., 2007; Pawluski and Galea, 2007; Hillerer et al., 2014).
The functional consequences of having fewer newly born neurons in the hippocampus during fatherhood remains unknown. While the suppression in hippocampal neurogenesis was not accompanied by alterations in hippocampal dependent memory or anxiety-like behavior in California mouse fathers (Glasper et al., 2011), there was a concomitant increase in anxiety- and depression-like behaviors as well as altered aggressive and social recognition memory in prairie vole fathers (Lieberwirth and Wang, 2013). These data suggest that although neurogenesis in the DG responds similarly in two different biparental species, the behavioral outcome may vary but other factors such as differences in the behavioral tests used and timing of testing relative to pup birth may have also contributed to these discrepancies. Either way, these results warrant further investigation of a possible causal link between reduced hippocampal neurogenesis and behavioral changes due to fatherhood.
The effects of paternal experience on neurogenesis have also been examined in laboratory mice (Mak and Weiss, 2010). Most laboratory mice are not naturally paternal but will exhibit caregiving behavior if they mate with a female and are then co-housed with her. In male mice that were induced to display paternal care in this way, there was an increase in the number of proliferating cells in both the SVZ and DG as compared to fathers separated from their pups and their partner immediately after birth and this effect persisted for 10 days after parturition. Moreover, increased neurogenesis in the paternal SVZ and DG resulted from physical interaction with their own, but not alien, pups and ultimately led to a greater number of newly born neurons in the DG and OB several weeks later. Additional mechanistic experiments revealed that infusion of a prolactin neutralizing antibody prevented the enhanced cell proliferation in males interacting with their pups. Similar results were obtained in transgenic mice lacking the prolactin receptor and thus like pregnant females (Shingo et al., 2003), prolactin mediates enhanced neurogenesis the paternal brain. Functionally, it was found that adult born neurons in the OB that were generated as a result of father-pup interactions were preferentially activated in response to the odors of their adult offspring compared to non-offspring. Furthermore, paternal mice with a targeted disruption of the prolactin receptor gene did not exhibit the enhanced neurogenesis normally observed after pup interactions and they were unable to recognize their own offspring as adults. However rescuing this olfactory neurogenesis also restored their recognition. Thus, at least in laboratory mice, newly generated neurons in the paternal brain seem to be important for adult offspring recognition behavior. The mechanism by which related pups induce neurogenesis and unrelated pups do not is an important gap in the story that remains to be filled. Even so, recognition of adult kin is presumably advantageous and may serve to increase reproductive success particularly in mice where paternal care is limited to a relatively brief period postnatally. For example, recognition of male offspring may reduce the possibility that a father would kill his own adult son should a conflict present itself while recognition of adult female offspring could limit the deleterious consequences of consanguineous breeding (McCarthy, 2010; Glasper et al., 2011).
Overall, these studies show that, like motherhood, the effects of fatherhood on neurogenesis in the adult brain are complex and species specific. Given that both prairie voles and California mice are biparental whereas laboratory mice are not naturally biparental, the opposing effects of fatherhood on neurogenesis in California mice and prairie voles vs. laboratory mice may be attributable to species-specific reproductive strategies. It will be important to determine if olfactory neurogenesis plays a similar role in biparental species as well as in mothers, particularly species like sheep for whom it is crucial to recognize their offspring.
9. Effects of pregnancy, parenting and pup exposure on neurogenesis in other regions of the adult brain
In addition to the canonical neurogenic regions, adult neurogenesis has also been documented in other brain structures such as the neocortex (Gould et al., 1999; Dayer et al., 2005), piriform cortex (Bernier et al., 2002; Yuan et al., 2014), striatum (Bedard et al., 2002; Dayer et al., 2005; Bedard et al., 2006; Luzzati et al., 2006; Inta et al., 2008; Ernst et al., 2014), amygdala (Bernier et al., 2002; Fowler et al., 2002; Akbari et al., 2007; Okuda et al., 2009; Lieberwirth and Wang, 2012) and hypothalamus (Huang et al., 1998; Fowler et al., 2002; Kokoeva et al., 2005; Akbari et al., 2007) of mammals. As compared to the DG and SVZ, far fewer numbers of new neurons are generated in these regions during adulthood although better detection methods are likely needed (Gould, 2007; Arisi et al., 2012; Inta et al., 2015; Nacher and Bonfanti, 2015; Schoenfeld and Cameron, 2015). Furthermore, many questions remain about newborn neurons in these areas including their origin (local or SVZ) and functions (Shapiro et al., 2009; Lee and Blackshaw, 2012; Feliciano et al., 2015; Inta et al., 2015). Nonetheless, a growing number of studies have revealed that neurogenesis in at least some of these areas is subject to modulation by a variety of physiological and environmental factors including pregnancy, pup exposure and parenting. For example, in the female prairie vole, Fowler et al. (2002) reported that exposure to a male vole, which resulted in pregnancy, enhanced the number of newborn cells in the amygdala and hypothalamus. Other work in male prairie voles also found a trend for increased cell proliferation in the amygdala in those that were exposed to pups and were spontaneously paternal (Ruscio et al., 2008) although like the DG, there are paradoxical data showing no effect of pup exposure on cell proliferation or survival in the amygdala or hypothalamus even though cell survival in both regions was reduced during fatherhood itself (Lieberwirth et al., 2013). In the rat, postpartum maternal experience was found to increase the survival of new cells in the nucleus accumbens and bed nucleus of the stria terminalis, but not amygdala, following pup interactions (Akbari et al., 2007). Neurogenesis in the hypothalamus of lactating pigs has also been examined but no changes were observed (Raymond et al., 2006). Importantly, these areas are known to be critical for the expression of maternal care as well as maternal aggression and other behavioral processes that are altered during parenthood such as motivation and anxiety (Numan and Insel, 2003). The development of new tools to investigate the functional effects of specifically ablating or increasing these new neuron populations are needed to determine whether or not neurogenesis contributes to these or other behavioral functions during parenthood.
10. Concluding remarks
In the past decade, adult neurogenesis has emerged as another important mechanism that contributes to maternal, as well as paternal, neuroplasticity (Leuner et al., 2010; Levy et al., 2011; Galea et al., 2014; Pawluski et al., 2015b; Slattery and Hillerer, 2016). While it is clear that parenthood can influence adult neurogenesis, the specific effects and their underlying mechanisms are complex and dependent on various factors such as species, brain region and time point investigated. Identifying the reasons for this complexity, and whether they are meaningful, represents a challenge as the field moves forward. Moreover, the vast majority of studies examining the functional importance of neurogenesis in the parental brain are correlative. There a few that have directly manipulated neurogenesis (Mak and Weiss, 2010; Larsen and Grattan, 2010b; Sakamoto et al., 2011), but in most cases it is not known whether new neurons in the SVZ or DG are responsible for the resulting behavioral changes. Thus, more work is needed to determine whether new neurons in these regions have distinct functional roles and whether these are the same or different across various species and sex. It is also important to consider that neurogenesis is just one of the multiple mechanisms of plasticity (Figure 1) that occur in mothers and fathers (Leuner et al., 2010; Galea et al., 2014; Slattery and Hillerer, 2016). As such, delineating what determines which plasticity mechanism is functional and under what conditions will greatly contribute to our understanding of the parental brain (Levy et al., 2011).
Highlights.
Neurogenesis is altered in the DG and SVZ across the peripartum period
The specific effects vary by brain region, species, and peripartum time point
The underlying mechanisms involve hormones, experience and immune factors
Fatherhood also affects adult neurogenesis in the DG and SVZ
Neurogenesis in the parental brain may play a role in caregiving, cognition and mood
Acknowledgments
This work supported by NIH grant NICHDR21-083791.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrati D, Lonstein JS. Affective changes during the postpartum period: Influences of genetic and experiential factors. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH. Regulation and function of adult neurogenesis: from genes to cognition. Physiol Rev. 2014;94:991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari EM, Chatterjee D, Levy F, Fleming AS. Experience-dependent cell survival in the maternal rat brain. Behav Neurosci. 2007;121:1001–1011. doi: 10.1037/0735-7044.121.5.1001. [DOI] [PubMed] [Google Scholar]
- Anderson G, Maes M. Postpartum depression: psychoneuroimmunological underpinnings and treatment. Neuropsychiatr Dis Treat. 2013;9:277–287. doi: 10.2147/NDT.S25320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arisi GM, Foresti ML, Mukherjee S, Shapiro LA. The role of olfactory stimulus in adult mammalian neurogenesis. Behav Brain Res. 2012;227:356–362. doi: 10.1016/j.bbr.2011.03.050. [DOI] [PubMed] [Google Scholar]
- Baker S, Chebli M, Rees S, Lemarec N, Godbout R, Bielajew C. Effects of gestational stress: 1. Evaluation of maternal and juvenile offspring behavior. Brain Res. 2008;1213:98–110. doi: 10.1016/j.brainres.2008.03.035. [DOI] [PubMed] [Google Scholar]
- Bales KL, Saltzman W. Fathering in rodents: Neurobiological substrates and consequences for offspring. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Endogenous versus exogenous markers of adult neurogenesis in canaries and other birds: advantages and disadvantages. J Comp Neurol. 2014;522:4100–4120. doi: 10.1002/cne.23661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. doi: 10.1046/j.0953-816x.2001.01763.x. [DOI] [PubMed] [Google Scholar]
- Barha CK, Lieblich SE, Galea LA. Different forms of oestrogen rapidly upregulate cell proliferation in the dentate gyrus of adult female rats. J Neuroendocrinol. 2009;21:155–166. doi: 10.1111/j.1365-2826.2008.01809.x. [DOI] [PubMed] [Google Scholar]
- Barker JM, Galea LA. Repeated estradiol administration alters different aspects of neurogenesis and cell death in the hippocampus of female, but not male, rats. Neuroscience. 2008;152:888–902. doi: 10.1016/j.neuroscience.2007.10.071. [DOI] [PubMed] [Google Scholar]
- Bedard A, Cossette M, Levesque M, Parent A. Proliferating cells can differentiate into neurons in the striatum of normal adult monkey. Neurosci Lett. 2002;328:213–216. doi: 10.1016/s0304-3940(02)00530-x. [DOI] [PubMed] [Google Scholar]
- Bedard A, Gravel C, Parent A. Chemical characterization of newly generated neurons in the striatum of adult primates. Exp Brain Res. 2006;170:501–512. doi: 10.1007/s00221-005-0233-5. [DOI] [PubMed] [Google Scholar]
- Bernier PJ, Bedard A, Vinet J, Levesque M, Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc Natl Acad Sci U S A. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodensteiner KJ, Cain P, Ray AS, Hamula LA. Effects of pregnancy on spatial cognition in female Hooded Long-Evans rats. Horm Behav. 2006;49:303–314. doi: 10.1016/j.yhbeh.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Bonfanti L, Peretto P. Adult neurogenesis in mammals--a theme with many variations. Eur J Neurosci. 2011;34:930–950. doi: 10.1111/j.1460-9568.2011.07832.x. [DOI] [PubMed] [Google Scholar]
- Borsini A, Zunszain PA, Thuret S, Pariante CM. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 2015;38:145–157. doi: 10.1016/j.tins.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID. Both oxytocin and vasopressin are mediators of maternal care and aggression in rodents: from central release to sites of action. Horm Behav. 2012;61:293–303. doi: 10.1016/j.yhbeh.2011.11.002. [DOI] [PubMed] [Google Scholar]
- Bridges RS. Long-term alterations in neural and endocrine processes induced by motherhood. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS, Felicio LF, Pellerin LJ, Stuer AM, Mann PE. Prior parity reduces post-coital diurnal and nocturnal prolactin surges in rats. Life Sci. 1993;53:439–445. doi: 10.1016/0024-3205(93)90648-m. [DOI] [PubMed] [Google Scholar]
- Brock O, Keller M, Veyrac A, Douhard Q, Bakker J. Short term treatment with estradiol decreases the rate of newly generated cells in the subventricular zone and main olfactory bulb of adult female mice. Neuroscience. 2010;166:368–376. doi: 10.1016/j.neuroscience.2009.12.050. [DOI] [PubMed] [Google Scholar]
- Brown J, Cooper-Kuhn CM, Kempermann G, Van Praag H, Winkler J, Gage FH, Kuhn HG. Enriched environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–2046. doi: 10.1046/j.1460-9568.2003.02647.x. [DOI] [PubMed] [Google Scholar]
- Brown RS, Kokay IC, Herbison AE, Grattan DR. Distribution of prolactin-responsive neurons in the mouse forebrain. J Comp Neurol. 2010;518:92–102. doi: 10.1002/cne.22208. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LA. Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Horm Behav. 2010a;58:769–779. doi: 10.1016/j.yhbeh.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LA. Chronic high corticosterone reduces neurogenesis in the dentate gyrus of adult male and female rats. Neuroscience. 2010b;168:680–690. doi: 10.1016/j.neuroscience.2010.04.023. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LA. Depression during pregnancy and postpartum: contribution of stress and ovarian hormones. Prog Neuropsychopharmacol Biol Psychiatry. 2010c;34:766–776. doi: 10.1016/j.pnpbp.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LA. Postpartum depression: Etiology, treatment and consequences for maternal care. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Brus M, Meurisse M, Franceschini I, Keller M, Levy F. Evidence for cell proliferation in the sheep brain and its down-regulation by parturition and interactions with the young. Horm Behav. 2010;58:737–746. doi: 10.1016/j.yhbeh.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Brus M, Meurisse M, Gheusi G, Keller M, Lledo PM, Levy F. Dynamics of olfactory and hippocampal neurogenesis in adult sheep. J Comp Neurol. 2013;521:169–188. doi: 10.1002/cne.23169. [DOI] [PubMed] [Google Scholar]
- Brus M, Meurisse M, Keller M, Levy F. Interactions with the young down-regulate adult olfactory neurogenesis and enhance the maturation of olfactory neuroblasts in sheep mothers. Front Behav Neurosci. 2014;8:53. doi: 10.3389/fnbeh.2014.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusco J, Wittmann R, De Azevedo MS, Lucion AB, Franci CR, Giovenardi M, Rasia-Filho AA. Plasma hormonal profiles and dendritic spine density and morphology in the hippocampal CA1 stratum radiatum, evidenced by light microscopy, of virgin and postpartum female rats. Neurosci Lett. 2008;438:346–350. doi: 10.1016/j.neulet.2008.04.063. [DOI] [PubMed] [Google Scholar]
- Cameron HA, Glover LR. Adult neurogenesis: beyond learning and memory. Annu Rev Psychol. 2015;66:53–81. doi: 10.1146/annurev-psych-010814-015006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron HA, Woolley CS, Gould E. Adrenal steroid receptor immunoreactivity in cells born in the adult rat dentate gyrus. Brain Res. 1993;611:342–346. doi: 10.1016/0006-8993(93)90524-q. [DOI] [PubMed] [Google Scholar]
- Campbell JC, Laugero KD, Van Westerhuyzen JA, Hostetler CM, Cohen JD, Bales KL. Costs of pair-bonding and paternal care in male prairie voles (Microtus ochrogaster) Physiol Behav. 2009;98:367–373. doi: 10.1016/j.physbeh.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canavan SV, Mayes LC, Treloar HB. Changes in maternal gene expression in olfactory circuits in the immediate postpartum period. Front Psychiatry. 2011;2:40. doi: 10.3389/fpsyt.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamley WA, Buckmaster JM, Cerini ME, Cumming IA, Goding JR, Obst JM, Williams A, Winfield C. Changes in the levels of progesterone, corticosteroids, estrone, estradiol-17 beta, luteinizing hormone, and prolactin in the peripheral plasma of the ewe during late pregnancy and at parturition. Biol Reprod. 1973;9:30–35. doi: 10.1093/biolreprod/9.1.30. [DOI] [PubMed] [Google Scholar]
- Chan M, Chow C, Hamson DK, Lieblich SE, Galea LA. Effects of chronic oestradiol, progesterone and medroxyprogesterone acetate on hippocampal neurogenesis and adrenal mass in adult female rats. J Neuroendocrinol. 2014;26:386–399. doi: 10.1111/jne.12159. [DOI] [PubMed] [Google Scholar]
- Chauke M, Malisch JL, Robinson C, De Jong TR, Saltzman W. Effects of reproductive status on behavioral and endocrine responses to acute stress in a biparental rodent, the California mouse (Peromyscus californicus) Horm Behav. 2011;60:128–138. doi: 10.1016/j.yhbeh.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie BR, Cameron HA. Neurogenesis in the adult hippocampus. Hippocampus. 2006;16:199–207. doi: 10.1002/hipo.20151. [DOI] [PubMed] [Google Scholar]
- Cohen L, Mizrahi A. Plasticity during motherhood: changes in excitatory and inhibitory layer 2/3 neurons in auditory cortex. J Neurosci. 2015;35:1806–1815. doi: 10.1523/JNEUROSCI.1786-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona R, Levy F. Chemical olfactory signals and parenthood in mammals. Horm Behav. 2015;68:77–90. doi: 10.1016/j.yhbeh.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Cost KT, Lobell TD, Williams-Yee ZN, Henderson S, Dohanich G. The effects of pregnancy, lactation, and primiparity on object-in-place memory of female rats. Horm Behav. 2014;65:32–39. doi: 10.1016/j.yhbeh.2013.10.012. [DOI] [PubMed] [Google Scholar]
- Darnaudery M, Perez-Martin M, Del Favero F, Gomez-Roldan C, Garcia-Segura LM, Maccari S. Early motherhood in rats is associated with a modification of hippocampal function. Psychoneuroendocrinology. 2007;32:803–812. doi: 10.1016/j.psyneuen.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Dayer AG, Cleaver KM, Abouantoun T, Cameron HA. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Crescenzo F, Perelli F, Armando M, Vicari S. Selective serotonin reuptake inhibitors (SSRIs) for post-partum depression (PPD): a systematic review of randomized clinical trials. J Affect Disord. 2014;152–154:39–44. doi: 10.1016/j.jad.2013.09.019. [DOI] [PubMed] [Google Scholar]
- Duarte-Guterman P, Yagi S, Chow C, Galea LA. Hippocampal learning, memory, and neurogenesis: Effects of sex and estrogens across the lifespan in adults. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.05.024. [DOI] [PubMed] [Google Scholar]
- Dulac C, O’connell LA, Wu Z. Neural control of maternal and paternal behaviors. Science. 2014;345:765–770. doi: 10.1126/science.1253291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeland M, Zunszain PA, Pariante CM. Molecular mechanisms in the regulation of adult neurogenesis during stress. Nat Rev Neurosci. 2015;16:189–200. doi: 10.1038/nrn3855. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Cameron HA, Encinas JM, Meltzer LA, Ming GL, Overstreet-Wadiche LS. Adult neurogenesis, mental health, and mental illness: hope or hype? J Neurosci. 2008;28:11785–11791. doi: 10.1523/JNEUROSCI.3798-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisch AJ, Mandyam CD. Adult neurogenesis: can analysis of cell cycle proteins move us “Beyond BrdU”? Curr Pharm Biotechnol. 2007;8:147–165. doi: 10.2174/138920107780906540. [DOI] [PubMed] [Google Scholar]
- Elyada YM, Mizrahi A. Becoming a mother-circuit plasticity underlying maternal behavior. Curr Opin Neurobiol. 2015;35:49–56. doi: 10.1016/j.conb.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Epperson CN, Jatlow PI, Czarkowski K, Anderson GM. Maternal fluoxetine treatment in the postpartum period: effects on platelet serotonin and plasma drug levels in breastfeeding mother-infant pairs. Pediatrics. 2003;112:e425. doi: 10.1542/peds.112.5.e425. [DOI] [PubMed] [Google Scholar]
- Ernst A, Alkass K, Bernard S, Salehpour M, Perl S, Tisdale J, Possnert G, Druid H, Frisen J. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- Falconer EM, Galea LA. Sex differences in cell proliferation, cell death and defensive behavior following acute predator odor stress in adult rats. Brain Res. 2003;975:22–36. doi: 10.1016/s0006-8993(03)02542-3. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Fleming AS, Ivy GO. Plasticity in the maternal circuit: effects of experience and partum condition on brain astrocyte number in female rats. Behav Neurosci. 2000;114:158–172. doi: 10.1037//0735-7044.114.1.158. [DOI] [PubMed] [Google Scholar]
- Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci. 2005;25:11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierstein CE, Lazarini F, Wagner S, Gabellec MM, De Chaumont F, Olivo-Marin JC, Boussin FD, Lledo PM, Gheusi G. Disruption of Adult Neurogenesis in the Olfactory Bulb Affects Social Interaction but not Maternal Behavior. Front Behav Neurosci. 2010;4:176. doi: 10.3389/fnbeh.2010.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feliciano DM, Bordey A, Bonfanti L. Noncanonical Sites of Adult Neurogenesis in the Mammalian Brain. Cold Spring Harb Perspect Biol. 2015;7:a018846. doi: 10.1101/cshperspect.a018846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felicio LF, Florio JC, Sider LH, Cruz-Casallas PE, Bridges RS. Reproductive experience increases striatal and hypothalamic dopamine levels in pregnant rats. Brain Res Bull. 1996;40:253–256. doi: 10.1016/0361-9230(96)00008-1. [DOI] [PubMed] [Google Scholar]
- Fernandez-Duque E, Valeggia CR, Mendoza SP. The biology of paternal care in human and nonhuman primates. Annu Rev Anthro. 2009:38. [Google Scholar]
- Fischer D, Patchev VK, Hellbach S, Hassan AH, Almeida OF. Lactation as a model for naturally reversible hypercorticalism plasticity in the mechanisms governing hypothalamo-pituitary- adrenocortical activity in rats. J Clin Invest. 1995;96:1208–1215. doi: 10.1172/JCI118153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, O’day DH, Kraemer GW. Neurobiology of mother-infant interactions: experience and central nervous system plasticity across development and generations. Neurosci Biobehav Rev. 1999;23:673–685. doi: 10.1016/s0149-7634(99)00011-1. [DOI] [PubMed] [Google Scholar]
- Fowler CD, Liu Y, Ouimet C, Wang Z. The effects of social environment on adult neurogenesis in the female prairie vole. J Neurobiol. 2002;51:115–128. doi: 10.1002/neu.10042. [DOI] [PubMed] [Google Scholar]
- Furuta M, Bridges RS. Gestation-induced cell proliferation in the rat brain. Brain Res Dev Brain Res. 2005;156:61–66. doi: 10.1016/j.devbrainres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Furuta M, Bridges RS. Effects of maternal behavior induction and pup exposure on neurogenesis in adult, virgin female rats. Brain Res Bull. 2009;80:408–413. doi: 10.1016/j.brainresbull.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gala RR, Westphal U. Corticosteroid-Binding Globulin in the Rat: Possible Role in the Initiation of Lactation. Endocrinology. 1965;76:1079–1088. doi: 10.1210/endo-76-6-1079. [DOI] [PubMed] [Google Scholar]
- Galea LA, Leuner B, Slattery DA. Hippocampal plasticity during the peripartum period: influence of sex steroids, stress and ageing. J Neuroendocrinol. 2014;26:641–648. doi: 10.1111/jne.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Mcewen BS. Sex and seasonal differences in the rate of cell proliferation in the dentate gyrus of adult wild meadow voles. Neuroscience. 1999;89:955–964. doi: 10.1016/s0306-4522(98)00345-5. [DOI] [PubMed] [Google Scholar]
- Galea LA, Ormerod BK, Sampath S, Kostaras X, Wilkie DM, Phelps MT. Spatial working memory and hippocampal size across pregnancy in rats. Horm Behav. 2000;37:86–95. doi: 10.1006/hbeh.1999.1560. [DOI] [PubMed] [Google Scholar]
- Galea LA, Spritzer MD, Barker JM, Pawluski JL. Gonadal hormone modulation of hippocampal neurogenesis in the adult. Hippocampus. 2006;16:225–232. doi: 10.1002/hipo.20154. [DOI] [PubMed] [Google Scholar]
- Galea LA, Wainwright SR, Roes MM, Duarte-Guterman P, Chow C, Hamson DK. Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. J Neuroendocrinol. 2013;25:1039–1061. doi: 10.1111/jne.12070. [DOI] [PubMed] [Google Scholar]
- Garcia A, Steiner B, Kronenberg G, Bick-Sander A, Kempermann G. Age-dependent expression of glucocorticoid- and mineralocorticoid receptors on neural precursor cell populations in the adult murine hippocampus. Aging Cell. 2004;3:363–371. doi: 10.1111/j.1474-9728.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- Gatewood JD, Morgan MD, Eaton M, Mcnamara IM, Stevens LF, Macbeth AH, Meyer EA, Lomas LM, Kozub FJ, Lambert KG, Kinsley CH. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res Bull. 2005;66:91–98. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Lepousez G, Lledo PM. Adult-born neurons in the olfactory bulb: integration and functional consequences. Curr Top Behav Neurosci. 2013;15:49–72. doi: 10.1007/7854_2012_228. [DOI] [PubMed] [Google Scholar]
- Gheusi G, Ortega-Perez I, Murray K, Lledo PM. A niche for adult neurogenesis in social behavior. Behav Brain Res. 2009;200:315–322. doi: 10.1016/j.bbr.2009.02.006. [DOI] [PubMed] [Google Scholar]
- Glasper ER, Kozorovitskiy Y, Pavlic A, Gould E. Paternal experience suppresses adult neurogenesis without altering hippocampal function in Peromyscus californicus. J Comp Neurol. 2011;519:2271–2281. doi: 10.1002/cne.22628. [DOI] [PubMed] [Google Scholar]
- Gobinath AR, Mahmoud R, Galea LA. Influence of sex and stress exposure across the lifespan on endophenotypes of depression: focus on behavior, glucocorticoids, and hippocampus. Front Neurosci. 2014;8:420. doi: 10.3389/fnins.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gould E, Reeves AJ, Graziano MS, Gross CG. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- Grattan DR. The actions of prolactin in the brain during pregnancy and lactation. Prog Brain Res. 2001;133:153–171. doi: 10.1016/s0079-6123(01)33012-1. [DOI] [PubMed] [Google Scholar]
- Green AD, Galea LA. Adult hippocampal cell proliferation is suppressed with estrogen withdrawal after a hormone-simulated pregnancy. Horm Behav. 2008;54:203–211. doi: 10.1016/j.yhbeh.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Haim A, Lenz K, Leuner B. Society for Neuroscience. Chicago, IL: 2015. Microglial alteration within the postpartum brain. [Google Scholar]
- Haim A, Sherer M, Leuner B. Gestational stress induces persistent depressive-like behavior and structural modifications within the postpartum nucleus accumbens. Eur J Neurosci. 2014;40:3766–3773. doi: 10.1111/ejn.12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris BN, Saltzman W. Effect of reproductive status on hypothalamic-pituitary-adrenal (HPA) activity and reactivity in male California mice (Peromyscus californicus) Physiol Behav. 2013;112–113:70–76. doi: 10.1016/j.physbeh.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillerer KM, Neumann ID, Couillard-Despres S, Aigner L, Slattery DA. Lactation-induced reduction in hippocampal neurogenesis is reversed by repeated stress exposure. Hippocampus. 2014;24:673–683. doi: 10.1002/hipo.22258. [DOI] [PubMed] [Google Scholar]
- Huang L, Devries GJ, Bittman EL. Photoperiod regulates neuronal bromodeoxyuridine labeling in the brain of a seasonally breeding mammal. J Neurobiol. 1998;36:410–420. doi: 10.1002/(sici)1097-4695(19980905)36:3<410::aid-neu8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Hyer MM, Hunter TJ, Katakam J, Wolz T, Glasper ER. Neurogenesis and anxiety-like behavior in male California mice during the mate’s postpartum period. Eur J Neurosci. 2016 doi: 10.1111/ejn.13168. [DOI] [PubMed] [Google Scholar]
- Inta D, Alfonso J, Von Engelhardt J, Kreuzberg MM, Meyer AH, Van Hooft JA, Monyer H. Neurogenesis and widespread forebrain migration of distinct GABAergic neurons from the postnatal subventricular zone. Proc Natl Acad Sci U S A. 2008;105:20994–20999. doi: 10.1073/pnas.0807059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inta D, Cameron HA, Gass P. New neurons in the adult striatum: from rodents to humans. Trends Neurosci. 2015 doi: 10.1016/j.tins.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone HA, Wigger A, Douglas AJ, Neumann ID, Landgraf R, Seckl JR, Russell JA. Attenuation of hypothalamic-pituitary-adrenal axis stress responses in late pregnancy: changes in feedforward and feedback mechanisms. J Neuroendocrinol. 2000;12:811–822. doi: 10.1046/j.1365-2826.2000.00525.x. [DOI] [PubMed] [Google Scholar]
- Keyser-Marcus L, Stafisso-Sandoz G, Gerecke K, Jasnow A, Nightingale L, Lambert KG, Gatewood J, Kinsley CH. Alterations of medial preoptic area neurons following pregnancy and pregnancy-like steroidal treatment in the rat. Brain Res Bull. 2001;55:737–745. doi: 10.1016/s0361-9230(01)00554-8. [DOI] [PubMed] [Google Scholar]
- Kim SK, Hwang IK, Yoo KY, Yoo DY, Bae E, Lee CH, Choi JH, Choi JW, Seong JK, Yoon YS, Won MH. Pregnancy inhibits cell proliferation and neuroblast differentiation without neuronal damage in the hippocampal dentate gyrus in C57BL/6N mice. Brain Res. 2010;1315:25–32. doi: 10.1016/j.brainres.2009.12.029. [DOI] [PubMed] [Google Scholar]
- Kimble DP, Rogers L, Hendrickson CW. Hippocampal lesions disrupt maternal, not sexual, behavior in the albino rat. J Comp Physiol Psychol. 1967;63:401–407. doi: 10.1037/h0024605. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Franssen RA, Meyer EA. Reproductive experience may positively adjust the trajectory of senescence. Curr Top Behav Neurosci. 2012;10:317–345. doi: 10.1007/7854_2011_123. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Madonia L, Gifford GW, Tureski K, Griffin GR, Lowry C, Williams J, Collins J, Mclearie H, Lambert KG. Motherhood improves learning and memory. Nature. 1999;402:137–138. doi: 10.1038/45957. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Trainer R, Stafisso-Sandoz G, Quadros P, Marcus LK, Hearon C, Meyer EA, Hester N, Morgan M, Kozub FJ, Lambert KG. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49:131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- Kokoeva MV, Yin H, Flier JS. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- Kopel H, Schechtman E, Groysman M, Mizrahi A. Enhanced synaptic integration of adult-born neurons in the olfactory bulb of lactating mothers. J Neurosci. 2012;32:7519–7527. doi: 10.1523/JNEUROSCI.6354-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen CM, Grattan DR. Exposure to female pheromones during pregnancy causes postpartum anxiety in mice. Vitam Horm. 2010a;83:137–149. doi: 10.1016/S0083-6729(10)83005-5. [DOI] [PubMed] [Google Scholar]
- Larsen CM, Grattan DR. Prolactin-induced mitogenesis in the subventricular zone of the maternal brain during early pregnancy is essential for normal postpartum behavioral responses in the mother. Endocrinology. 2010b;151:3805–3814. doi: 10.1210/en.2009-1385. [DOI] [PubMed] [Google Scholar]
- Larsen CM, Grattan DR. Prolactin, neurogenesis, and maternal behaviors. Brain Behav Immun. 2012;26:201–209. doi: 10.1016/j.bbi.2011.07.233. [DOI] [PubMed] [Google Scholar]
- Lau WM, Qiu G, Helmeste DM, Lee TM, Tang SW, So KF, Tang SW. Corticosteroid decreases subventricular zone cell proliferation, which could be reversed by paroxetine. Restor Neurol Neurosci. 2007;25:17–23. [PubMed] [Google Scholar]
- Lazic SE. Using causal models to distinguish between neurogenesis-dependent and -independent effects on behaviour. J R Soc Interface. 2012;9:907–917. doi: 10.1098/rsif.2011.0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DA, Blackshaw S. Functional implications of hypothalamic neurogenesis in the adult mammalian brain. Int J Dev Neurosci. 2012;30:615–621. doi: 10.1016/j.ijdevneu.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire V, Billard JM, Dutar P, George O, Piazza PV, Epelbaum J, Le Moal , Mayo W. Motherhood-induced memory improvement persists across lifespan in rats but is abolished by a gestational stress. Eur J Neurosci. 2006;23:3368–3374. doi: 10.1111/j.1460-9568.2006.04870.x. [DOI] [PubMed] [Google Scholar]
- Lepousez G, Nissant A, Lledo PM. Adult neurogenesis and the future of the rejuvenating brain circuits. Neuron. 2015;86:387–401. doi: 10.1016/j.neuron.2015.01.002. [DOI] [PubMed] [Google Scholar]
- Leuner B, Caponiti JM, Gould E. Oxytocin stimulates adult neurogenesis even under conditions of stress and elevated glucocorticoids. Hippocampus. 2012;22:861–868. doi: 10.1002/hipo.20947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Fredericks PJ, Nealer C, Albin-Brooks C. Chronic gestational stress leads to depressive-like behavior and compromises medial prefrontal cortex structure and function during the postpartum period. PLoS One. 2014;9:e89912. doi: 10.1371/journal.pone.0089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Thymidine analog methods for studies of adult neurogenesis are not equally sensitive. J Comp Neurol. 2009;517:123–133. doi: 10.1002/cne.22107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E. Parenting and plasticity. Trends Neurosci. 2010;33:465–473. doi: 10.1016/j.tins.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E. Structural plasticity and hippocampal function. Annu Rev Psychol. 2010;61:111–140. doi: 10.1146/annurev.psych.093008.100359. C111–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, Shors TJ. Is there a link between adult neurogenesis and learning? Hippocampus. 2006;16:216–224. doi: 10.1002/hipo.20153. [DOI] [PubMed] [Google Scholar]
- Leuner B, Mirescu C, Noiman L, Gould E. Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus. 2007;17:434–442. doi: 10.1002/hipo.20278. [DOI] [PubMed] [Google Scholar]
- Levy F, Gheusi G, Keller M. Plasticity of the parental brain: a case for neurogenesis. J Neuroendocrinol. 2011;23:984–993. doi: 10.1111/j.1365-2826.2011.02203.x. [DOI] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Y, Jia X, Liu Y, Wang Z. Fatherhood reduces the survival of adult-generated cells and affects various types of behavior in the prairie vole (Microtus ochrogaster) Eur J Neurosci. 2013;38:3345–3355. doi: 10.1111/ejn.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberwirth C, Wang Z. The social environment and neurogenesis in the adult Mammalian brain. Front Hum Neurosci. 2012;6:118. doi: 10.3389/fnhum.2012.00118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wang J, Zhao L, Nilsen J, Mcclure K, Wong K, Brinton RD. Progesterone increases rat neural progenitor cell cycle gene expression and proliferation via extracellularly regulated kinase and progesterone receptor membrane components 1 and 2. Endocrinology. 2009;150:3186–3196. doi: 10.1210/en.2008-1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS. Regulation of anxiety during the postpartum period. Front Neuroendocrinol. 2007;28:115–141. doi: 10.1016/j.yfrne.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, De Vries GJ. Influence of gonadal hormones on the development of parental behavior in adult virgin prairie voles (Microtus ochrogaster) Behav Brain Res. 2000;114:79–87. doi: 10.1016/s0166-4328(00)00192-3. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Maguire J, Meinlschmidt G, Neumann ID. Emotion and mood adaptations in the peripartum female:complementary contributions of GABA and oxytocin. J Neuroendocrinol. 2014;26:649–664. doi: 10.1111/jne.12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’hare E, Neuman G. Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev. 2000;20:561–592. doi: 10.1016/s0272-7358(98)00100-7. [DOI] [PubMed] [Google Scholar]
- Luppi P. How immune mechanisms are affected by pregnancy. Vaccine. 2003;21:3352–3357. doi: 10.1016/s0264-410x(03)00331-1. [DOI] [PubMed] [Google Scholar]
- Luzzati F, De Marchis S, Fasolo A, Peretto P. Neurogenesis in the caudate nucleus of the adult rabbit. J Neurosci. 2006;26:609–621. doi: 10.1523/JNEUROSCI.4371-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macbeth AH, Luine VN. Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev. 2010;34:452–467. doi: 10.1016/j.neubiorev.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Macbeth AH, Scharfman HE, Maclusky NJ, Gautreaux C, Luine VN. Effects of multiparity on recognition memory, monoaminergic neurotransmitters, and brain-derived neurotrophic factor (BDNF) Horm Behav. 2008;54:7–17. doi: 10.1016/j.yhbeh.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macklis JD. Human adult olfactory bulb neurogenesis? Novelty is the best policy. Neuron. 2012;74:595–596. doi: 10.1016/j.neuron.2012.05.005. [DOI] [PubMed] [Google Scholar]
- Mak GK, Weiss S. Paternal recognition of adult offspring mediated by newly generated CNS neurons. Nat Neurosci. 2010;13:753–758. doi: 10.1038/nn.2550. [DOI] [PubMed] [Google Scholar]
- Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann PE, Bridges RS. Neural and endocrine sensitivities to opioids decline as a function of multiparity in the rat. Brain Res. 1992;580:241–248. doi: 10.1016/0006-8993(92)90950-e. [DOI] [PubMed] [Google Scholar]
- Markoff E, Talamantes F. Serum placental lactogen in mice in relation to day of gestation and number of conceptuses. Biol Reprod. 1981;24:846–851. doi: 10.1095/biolreprod24.4.846. [DOI] [PubMed] [Google Scholar]
- Mccarthy MM. Father and child reunion. Nat Neurosci. 2010;13:652–653. doi: 10.1038/nn0610-652. [DOI] [PubMed] [Google Scholar]
- Meaney MJ, Viau V, Aitken DH, Bhatnagar S. Glucocorticoid receptors in brain and pituitary of the lactating rat. Physiol Behav. 1989;45:209–212. doi: 10.1016/0031-9384(89)90187-x. [DOI] [PubMed] [Google Scholar]
- Miller BR, Hen R. The current state of the neurogenic theory of depression and anxiety. Curr Opin Neurobiol. 2015;30:51–58. doi: 10.1016/j.conb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JA, Liu RC. Dissecting natural sensory plasticity: hormones and experience in a maternal context. Hear Res. 2009;252:21–28. doi: 10.1016/j.heares.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneaux E, Poston L, Ashurst-Williams S, Howard LM. Pre-pregnancy obesity and mental disorders during pregnancy and postpartum: A systematic review and meta-analysis. Pregnancy Hypertens. 2014;4:236. doi: 10.1016/j.preghy.2014.03.022. [DOI] [PubMed] [Google Scholar]
- Nacher J, Bonfanti L. New neurons from old beliefs in the adult piriform cortex? A Commentary on: “Occurrence of new neurons in the piriform cortex”. Front Neuroanat. 2015;9:62. doi: 10.3389/fnana.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numan M, Insel TR. The neurobiology of parental behavior. New York: Springer; 2003. [Google Scholar]
- Numan M, Woodside B. Maternity: neural mechanisms, motivational processes, and physiological adaptations. Behav Neurosci. 2010;124:715–741. doi: 10.1037/a0021548. [DOI] [PubMed] [Google Scholar]
- O’hara MW, Mccabe JE. Postpartum depression: current status and future directions. Annu Rev Clin Psychol. 2013;9:379–407. doi: 10.1146/annurev-clinpsy-050212-185612. [DOI] [PubMed] [Google Scholar]
- O’mahony SM, Myint AM, Van Den Hove D, Desbonnet L, Steinbusch H, Leonard BE. Gestational stress leads to depressive-like behavioural and immunological changes in the rat. Neuroimmunomodulation. 2006;13:82–88. doi: 10.1159/000096090. [DOI] [PubMed] [Google Scholar]
- Okuda H, Tatsumi K, Makinodan M, Yamauchi T, Kishimoto T, Wanaka A. Environmental enrichment stimulates progenitor cell proliferation in the amygdala. J Neurosci Res. 2009;87:3546–3553. doi: 10.1002/jnr.22160. [DOI] [PubMed] [Google Scholar]
- Opendak M, Gould E. Adult neurogenesis: a substrate for experience-dependent change. Trends Cogn Sci. 2015;19:151–161. doi: 10.1016/j.tics.2015.01.001. [DOI] [PubMed] [Google Scholar]
- Ormerod BK, Galea LA. Reproductive status influences cell proliferation and cell survival in the dentate gyrus of adult female meadow voles: a possible regulatory role for estradiol. Neuroscience. 2001;102:369–379. doi: 10.1016/s0306-4522(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Paris JJ, Frye CA. Estrous cycle, pregnancy, and parity enhance performance of rats in object recognition or object placement tasks. Reproduction. 2008;136:105–115. doi: 10.1530/REP-07-0512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluski JL, Barakauskas VE, Galea LA. Pregnancy decreases oestrogen receptor alpha expression and pyknosis, but not cell proliferation or survival, in the hippocampus. J Neuroendocrinol. 2010;22:248–257. doi: 10.1111/j.1365-2826.2010.01960.x. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Brummelte S, Barha CK, Crozier TM, Galea LA. Effects of steroid hormones on neurogenesis in the hippocampus of the adult female rodent during the estrous cycle, pregnancy, lactation and aging. Front Neuroendocrinol. 2009a;30:343–357. doi: 10.1016/j.yfrne.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Charlier TD, Fillet M, Houbart V, Crispin HT, Steinbusch HW, Van Den Hove DL. Chronic fluoxetine treatment and maternal adversity differentially alter neurobehavioral outcomes in the rat dam. Behav Brain Res. 2012;228:159–168. doi: 10.1016/j.bbr.2011.11.043. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Charlier TD, Lieblich SE, Hammond GL, Galea LA. Reproductive experience alters corticosterone and CBG levels in the rat dam. Physiol Behav. 2009b;96:108–114. doi: 10.1016/j.physbeh.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Csaszar E, Savage E, Martinez-Claros M, Steinbusch HW, Van Den Hove D. Effects of stress early in gestation on hippocampal neurogenesis and glucocorticoid receptor density in pregnant rats. Neuroscience. 2015a;290:379–388. doi: 10.1016/j.neuroscience.2015.01.048. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Galea LA. Hippocampal morphology is differentially affected by reproductive experience in the mother. J Neurobiol. 2006;66:71–81. doi: 10.1002/neu.20194. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Galea LA. Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience. 2007;149:53–67. doi: 10.1016/j.neuroscience.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Lambert KG, Kinsley CH. Neuroplasticity in the maternal hippocampus: Relation to cognition and effects of repeated stress. Horm Behav. 2015b doi: 10.1016/j.yhbeh.2015.06.004. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Van Den Hove DL, Rayen I, Prickaerts J, Steinbusch HW. Stress and the pregnant female: Impact on hippocampal cell proliferation, but not affective-like behaviors. Horm Behav. 2011;59:572–580. doi: 10.1016/j.yhbeh.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Vanderbyl BL, Ragan K, Galea LA. First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or ‘mothering’ alone. Behav Brain Res. 2006a;175:157–165. doi: 10.1016/j.bbr.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Pawluski JL, Walker SK, Galea LA. Reproductive experience differentially affects spatial reference and working memory performance in the mother. Horm Behav. 2006b;49:143–149. doi: 10.1016/j.yhbeh.2005.05.016. [DOI] [PubMed] [Google Scholar]
- Perani CV, Slattery DA. Using animal models to study post-partum psychiatric disorders. Br J Pharmacol. 2014;171:4539–4555. doi: 10.1111/bph.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea-Rodriguez JP, Takahashi EY, Amador TM, Hao RC, Saltzman W, Trainor BC. Effects of reproductive experience on central expression of progesterone, oestrogen alpha, oxytocin and vasopressin receptor mRNA in male California mice (Peromyscus californicus) J Neuroendocrinol. 2015;27:245–252. doi: 10.1111/jne.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M, Ferreira A. Neuroanatomical and neurochemical basis of parenting: Dynamic coordination of motivational, affective and cognitive processes. Horm Behav. 2015 doi: 10.1016/j.yhbeh.2015.08.005. [DOI] [PubMed] [Google Scholar]
- Rasia-Filho AA, Fabian C, Rigoti KM, Achaval M. Influence of sex, estrous cycle and motherhood on dendritic spine density in the rat medial amygdala revealed by the Golgi method. Neuroscience. 2004;126:839–847. doi: 10.1016/j.neuroscience.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Raymond AD, Kucherepa NN, Fisher KR, Halina WG, Partlow GD. Neurogenesis of oxytocin-containing neurons in the paraventricular nucleus (PVN) of the female pig in 3 reproductive states: puberty gilts, adult gilts and lactating sows. Brain Res. 2006;1102:44–51. doi: 10.1016/j.brainres.2006.04.113. [DOI] [PubMed] [Google Scholar]
- Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV, Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Young LJ. The biology of mammalian parenting and its effect on offspring social development. Science. 2014;345:771–776. doi: 10.1126/science.1252723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E, Grace S, Wallington T, Stewart DE. Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry. 2004;26:289–295. doi: 10.1016/j.genhosppsych.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Rolls A, Schori H, London A, Schwartz M. Decrease in hippocampal neurogenesis during pregnancy: a link to immunity. Mol Psychiatry. 2008;13:468–469. doi: 10.1038/sj.mp.4002126. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, Hinde RA, Beer CG, Busnel MC. Advances in the study of behavior. New York: Academic Press; 1979. [Google Scholar]
- Ruscio MG, Sweeny TD, Hazelton JL, Suppatkul P, Boothe E, Carter CS. Pup exposure elicits hippocampal cell proliferation in the prairie vole. Behav Brain Res. 2008;187:9–16. doi: 10.1016/j.bbr.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saghatelyan A, Roux P, Migliore M, Rochefort C, Desmaisons D, Charneau P, Shepherd GM, Lledo PM. Activity-dependent adjustments of the inhibitory network in the olfactory bulb following early postnatal deprivation. Neuron. 2005;46:103–116. doi: 10.1016/j.neuron.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Sakamoto M, Imayoshi I, Ohtsuka T, Yamaguchi M, Mori K, Kageyama R. Continuous neurogenesis in the adult forebrain is required for innate olfactory responses. Proc Natl Acad Sci U S A. 2011;108:8479–8484. doi: 10.1073/pnas.1018782108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmaso N, Cossette MP, Woodside B. Pregnancy and maternal behavior induce changes in glia, glutamate and its metabolism within the cingulate cortex. PLoS One. 2011a;6:e23529. doi: 10.1371/journal.pone.0023529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmaso N, Quinlan MG, Brake WG, Woodside B. Changes in dendritic spine density on layer 2/3 pyramidal cells within the cingulate cortex of late pregnant and postpartum rats. Horm Behav. 2011b;60:65–71. doi: 10.1016/j.yhbeh.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Saltzman W, Ziegler TE. Functional significance of hormonal changes in mammalian fathers. J Neuroendocrinol. 2014;26:685–696. doi: 10.1111/jne.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanna E, Mostallino MC, Murru L, Carta M, Talani G, Zucca S, Mura ML, Maciocco E, Biggio G. Changes in expression and function of extrasynaptic GABAA receptors in the rat hippocampus during pregnancy and after delivery. J Neurosci. 2009;29:1755–1765. doi: 10.1523/JNEUROSCI.3684-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Schoenfeld TJ, Cameron HA. Adult neurogenesis and mental illness. Neuropsychopharmacology. 2015;40:113–128. doi: 10.1038/npp.2014.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenfeld TJ, Gould E. Stress, stress hormones, and adult neurogenesis. Exp Neurol. 2012;233:12–21. doi: 10.1016/j.expneurol.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LA, Ng K, Zhou QY, Ribak CE. Subventricular zone-derived, newly generated neurons populate several olfactory and limbic forebrain regions. Epilepsy Behav. 2009;14(Suppl 1):74–80. doi: 10.1016/j.yebeh.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma V, Sommerdyk C. Are antidepressants effective in the treatment of postpartum depression? A systematic review. Prim Care Companion CNS Disord. 2013:15. doi: 10.4088/PCC.13r01529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Mathew J, Sisti HM, Edgecomb C, Beckoff S, Dalla C. Neurogenesis and helplessness are mediated by controllability in males but not in females. Biol Psychiatry. 2007;62:487–495. doi: 10.1016/j.biopsych.2006.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery DA, Hillerer KM. The maternal brain under stress: Consequences for adaptive peripartum plasticity and its potential functional implications. Front Neuroendocrinol. 2016 doi: 10.1016/j.yfrne.2016.01.004. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID. No stress please! Mechanisms of stress hyporesponsiveness of the maternal brain. J Physiol. 2008;586:377–385. doi: 10.1113/jphysiol.2007.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotnick BM, Nigrosh BJ. Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. J Comp Physiol Psychol. 1975;88:118–127. doi: 10.1037/h0076200. [DOI] [PubMed] [Google Scholar]
- Smith JW, Seckl JR, Evans AT, Costall B, Smythe JW. Gestational stress induces post-partum depression-like behaviour and alters maternal care in rats. PsychoneuroEndocrinology. 2004;29:227–244. doi: 10.1016/s0306-4530(03)00025-8. [DOI] [PubMed] [Google Scholar]
- Snyder JS, Choe JS, Clifford MA, Jeurling SI, Hurley P, Brown A, Kamhi JF, Cameron HA. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J Neurosci. 2009;29:14484–14495. doi: 10.1523/JNEUROSCI.1768-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder JS, Soumier A, Brewer M, Pickel J, Cameron HA. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476:458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JM, Voogt JL. Comparison of plasma corticosterone and prolactin levels in cycling and lactating rats. NeuroEndocrinology. 1973;13:173–181. doi: 10.1159/000122235. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Rissman EF. Oestrogen-independent, experience-induced maternal behaviour in female mice. J Neuroendocrinol. 2011;23:345–354. doi: 10.1111/j.1365-2826.2011.02112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Diop H, Declercq E, Cabral HJ, Fox MP, Wise LA. Stressful events during pregnancy and postpartum depressive symptoms. J Womens Health (Larchmt) 2015;24:384–393. doi: 10.1089/jwh.2014.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Gould E. Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J Comp Neurol. 2005;481:252–265. doi: 10.1002/cne.20385. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Chapman DB, Montagnese C, Poulain DA, Morris JF. Structural plasticity in the hypothalamic supraoptic nucleus at lactation affects oxytocin-, but not vasopressin-secreting neurones. Neuroscience. 1986;17:661–678. doi: 10.1016/0306-4522(86)90038-2. [DOI] [PubMed] [Google Scholar]
- Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T, Matsui H. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci. 2003;6:384–390. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- Torner L, Karg S, Blume A, Kandasamy M, Kuhn HG, Winkler J, Aigner L, Neumann ID. Prolactin prevents chronic stress-induced decrease of adult hippocampal neurogenesis and promotes neuronal fate. J Neurosci. 2009;29:1826–1833. doi: 10.1523/JNEUROSCI.3178-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torner L, Neumann ID. The brain prolactin system: involvement in stress response adaptations in lactation. Stress. 2002;5:249–257. doi: 10.1080/1025389021000048638. [DOI] [PubMed] [Google Scholar]
- Van Praag H, Kempermann G, Gage FH. Neural consequences of environmental enrichment. Nat Rev Neurosci. 2000;1:191–198. doi: 10.1038/35044558. [DOI] [PubMed] [Google Scholar]
- Veyrac A, Bakker J. Postnatal and adult exposure to estradiol differentially influences adult neurogenesis in the main and accessory olfactory bulb of female mice. FASEB J. 2011;25:1048–1057. doi: 10.1096/fj.10-172635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CD, Lightman SL, Steele MK, Dallman MF. Suckling is a persistent stimulus to the adrenocortical system of the rat. Endocrinology. 1992;130:115–125. doi: 10.1210/endo.130.1.1309321. [DOI] [PubMed] [Google Scholar]
- Walker TL, Vukovic J, Koudijs MM, Blackmore DG, Mackay EW, Sykes AM, Overall RW, Hamlin AS, Bartlett PF. Prolactin stimulates precursor cells in the adult mouse hippocampus. PLoS One. 2012;7:e44371. doi: 10.1371/journal.pone.0044371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CJ, Fleming AS, Lee A, Magnusson JE. The effects of olfactory and somatosensory desensitization on Fos-like immunoreactivity in the brains of pup-exposed postpartum rats. Behav Neurosci. 1996;110:134–153. doi: 10.1037//0735-7044.110.1.134. [DOI] [PubMed] [Google Scholar]
- Westenbroek C, Den Boer JA, Veenhuis M, Ter Horst GJ. Chronic stress and social housing differentially affect neurogenesis in male and female rats. Brain Res Bull. 2004;64:303–308. doi: 10.1016/j.brainresbull.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Sit DK, Mcshea MC, Rizzo DM, Zoretich RA, Hughes CL, Eng HF, Luther JF, Wisniewski SR, Costantino ML, Confer AL, Moses-Kolko EL, Famy CS, Hanusa BH. Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry. 2013;70:490–498. doi: 10.1001/jamapsychiatry.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman JL, Barha CK, Galea LA. Endocrine substrates of cognitive and affective changes during pregnancy and postpartum. Behav Neurosci. 2012;126:54–72. doi: 10.1037/a0025538. [DOI] [PubMed] [Google Scholar]
- Workman JL, Gobinath AR, Kitay NF, Chow C, Brummelte S, Galea LA. Parity modifies the effects of fluoxetine and corticosterone on behavior, stress response and hippocampal neurogenesis. Neuropharmacology. 2016 doi: 10.1016/j.neuropharm.2015.11.027. [DOI] [PubMed] [Google Scholar]
- Workman JL, Raineki C, Weinberg J, Galea LA. Alcohol and pregnancy: Effects on maternal care, HPA axis function, and hippocampal neurogenesis in adult females. Psychoneuroendocrinology. 2015;57:37–50. doi: 10.1016/j.psyneuen.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xerri C, Stern JM, Merzenich MM. Alterations of the cortical representation of the rat ventrum induced by nursing behavior. J Neurosci. 1994;14:1710–1721. doi: 10.1523/JNEUROSCI.14-03-01710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan TF, Liang YX, So KF. Occurrence of new neurons in the piriform cortex. Front Neuroanat. 2014;8:167. doi: 10.3389/fnana.2014.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Yang R, Zhou R, Li L, Sokabe M, Chen L. Progesterone promotes the survival of newborn neurons in the dentate gyrus of adult male mice. Hippocampus. 2010;20:402–412. doi: 10.1002/hipo.20642. [DOI] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]