Abstract
Maternally-derived corticosterone in the egg and corticosterone produced endogenously by altricial nestling birds play essential roles during development. Although persistently high corticosterone levels can be harmful, moderately elevated levels above baseline can lead to reallocation of resources between growth and maintenance to ensure immediate survival or to enhance the development of fitness-related traits. We tested two hypotheses concerning the fitness consequences of elevated corticosterone during pre-natal and post-natal development in altricial house wrens: (1) elevated corticosterone shifts resources away from growth and immune function and (2) elevated corticosterone serves as a signal to allocate resources to fitness-related traits. We also explored the development of the stress response, hypothesizing that early-stage nestlings have little endogenously produced corticosterone, but that their baseline and stress-induced corticosterone levels increase with age. Nestlings hatching from corticosterone-injected eggs were lighter at hatching, but through compensatory growth, ended up heavier than controls near the time of fledgling, an important, fitness-related trait. Nestlings that hatched from corticosterone-injected eggs and those given oral doses of corticosterone did not differ from controls in three other fitness-related traits: immunoresponsiveness, size, or haematocrit. Early- and late-stage nestlings had similar baseline corticosterone levels, and all nestlings increased their plasma corticosterone levels in response to a capture-and-restraint protocol, with older nestlings mounting a stronger stress-induced response than younger nestlings. These results suggest that pre-natal exposure to corticosterone is important in shaping offspring phenotype, and are consistent with the hypothesis that maternally derived corticosterone in the egg can have long-term, fitness-related effects on offspring phenotype.
Keywords: corticosterone, development, house wren, maternal effects, stress, Troglodytes aedon
Glucocorticoid hormones are considered ‘stress hormones’ because they typically increase in response to environmental challenges (Bonier et al., 2009; Constantini et al., 2011; Pankhurst, 2011; Wingfield, 2013). Corticosterone, the primary glucocorticoid in some vertebrates, is released following activation of the hypothalamic-pituitary-adrenal (HPA) axis. One of its functions during acute stress is to reallocate energy toward overcoming environmental challenges by shifting resources away from reproduction and other non-essential processes (Sapolsky et al., 2000; Martin et al., 2005; Brooks and Mateo, 2013; Wingfield, 2013; Hau et al, 2016).
Developing birds are exposed to corticosterone during both the pre-natal (i.e., embryonic) and post-natal (i.e., nestling and fledgling) stages of development, and differences in corticosterone levels during these stages can have profound effects, some of which may be species- or context-specific, on the adult phenotype and, hence, fitness (Schoech et al., 2009; Henriksen et al., 2011; Schoech et al., 2011). Birds provide a particularly suitable model system for studying the developmental effects of elevated corticosterone exposure as bird embryos develop outside their mother. Thus, they develop independently of the conditions that their mothers experience post-oviposition and are amenable to experimental manipulation at the earliest stages of development.
In the pre-natal stage, corticosterone is passed from mother to embryo via the yolk of the egg (Love and Williams, 2008a, b; Moore and Johnston, 2008), where it influences the developing embryo’s phenotype as a maternal effect (Groothuis et al., 2005; Adkins-Regan et al., 2013). The maternal environment, including exposure to predators during egg-production (Saino et al., 2005) and differences in environmental quality (Love et al., 2008), clearly influences the amount of corticosterone transferred to eggs, and females with corticosterone-filled implants produce eggs with higher corticosterone concentrations than control females (Hayward and Wingfield, 2004). These and other studies suggest that the amount of corticosterone in the egg reflects maternal condition, environment, and plasma corticosterone levels at the time of egg synthesis (Hayward and Wingfield, 2004). In the post-natal stage, nestlings can also experience elevated corticosterone levels from endogenous sources when faced with environmental challenges, such as food shortages (Kitaysky et al., 2001a; Quillfeldt et al., 2007; Honarmand et al., 2010), the presence of ectoparasites (Raouf et al., 2006), low parental attentiveness (Rensel et al., 2010a), and sibling competition (Kern et al., 2001).
Although corticosterone is essential for development (Sapolsky et al., 2000), pre-natal exposure to elevated concentrations in the egg can have significant, detrimental post-hatching phenotypic effects, including decreased cutaneous immune response (Rubolini et al., 2005) and slowed growth and small body size (Saino et al., 2005; Hayward et al., 2006). Post-natal elevation of corticosterone can reduce nestling growth (Loiseau et al., 2008; Love and Williams, 2008a; Wada and Breuner, 2008) and decrease immune response (Saino et al., 2003; Loiseau et al., 2008; Stier et al., 2009). Such findings suggest that corticosterone mediates the reallocation of resources in a way that promotes immediate survival by temporarily shifting resources away from costly processes, such as growth and immune responses (Råberg et al., ’98; Saino et al., 2003; Wada and Breuner, 2008).
Positive effects of pre-natally elevated corticosterone levels have also been reported (reviewed in Crino and Breuner, 2015; Crossin et al., 2016). European starlings (Sturnus vulgaris) hatching from eggs with experimentally elevated corticosterone developed heavier, more mature flight muscles and performed better during flight-performance trials than those hatching from control eggs (Chin et al., 2009). Similar results have been reported in non-avian taxa (Dantzer et al., 2013; Sopinka et al., 2015). Positive effects of post-natal increases in corticosterone have also been reported, including increased aggression and begging by precocial chicks that resulted in increased food provisioning by the parents (Kitaysky et al., 2001b; Kitaysky et al., 2003). Adult zebra finches (Taeniopygia guttatta) exposed to elevated corticosterone during post-natal development solved a novel foraging task faster than their control siblings (Crino et al., 2014). Studies with positive results such as these suggest that corticosterone may be acting in a preparative way for predictable changes in the environment. Because poor conditions can lead to an increase in pre- and post-natal corticosterone levels, the increase may serve as a signal to the offspring that prepares them for a low-quality environment (Mathis et al., 2008; Schoech et al., 2011).
The responsiveness of the HPA axis during post-natal development varies among bird species. Hatchlings in precocial species (e.g., domestic chicken, wild turkey, mallard duck) are able to mount a stress-induced corticosterone response shortly after hatching (Wada, 2008), whereas altricial species (e.g., white-crowned sparrow, Florida scrub-jay) produce moderate to no corticosterone in response to a stressful stimulus shortly after hatching, but later, when nearing the time to leave the nest, they can mount a significant response (Wada et al., 2007; Wada et al., 2009; Rensel et al., 2010b). At present, there are few data on the maturation of the HPA axis in altricial birds, although the evidence suggests that younger altricial nestlings have a less robust response than older nestlings (Schwabl, ’99; Sims and Holberton, 2000; Wada et al., 2007; Wada et al., 2009; Pakkala et al., in press).
We tested two hypotheses concerning the role of corticosterone during house wren (Troglodytes aedon) development, focusing on its effects on nestling traits (body mass, body size, cutaneous immune response, and haematocrit) that previous research on the study population has shown to be related to nestling fitness (Bowers et al., 2014, Sakaluk et al., 2014). (1) Elevated corticosterone shifts resources away from costly processes, such as growth and immune function. If true, pre- or post-natal treatment with corticosterone should produce nestlings in poorer condition, as measured by fitness-related traits, than controls. (2) Elevated corticosterone during development serves as a signal to offspring to allocate resources to fitness-related traits. If true, pre- or post-natal treatment with corticosterone should produce nestlings in better condition, as measured by fitness-related traits, than controls. We also explored the development of the individual stress response, hypothesizing that early-stage nestlings have little endogenously produced corticosterone, but that their baseline and stress-induced corticosterone levels increase with age. If true, baseline corticosterone levels should be undetectable or lower in early-stage than late-stage nestlings, and late-stage nestlings should respond to a stressful situation by producing more corticosterone than early-stage nestlings.
METHODS
Study Species and Site
House wrens are small (10–12 g), insectivorous songbirds with a breeding range extending from Canada to extreme southern South American (Johnson, 2014). House wrens in the migratory population that breeds on our study area in central Illinois typically produce two broods each breeding season. First-brood clutches are initiated shortly after the wrens return from their wintering grounds in late April-early May and second-brood clutches in early July (Styrsky et al., ’99; Johnson, 2014). Our study took place at the 130-ha Mackinaw study area in McLean County, Illinois (40.665ºN, 88.89ºW) in 2012–2014. The Mackinaw study area has 700 standardized, uniformly spaced nestboxes (5.4 boxes/ha) that house wrens readily accept as nesting sites (DeMory et al., 2010); see Lambrechts et al., (2010) for details on nestbox dimensions and materials. Female house wrens typically lay 6–8 eggs (modal clutch size=7) in first-brood clutches and 4–7 (modal clutch size=6) in second-brood clutches; the incubation period is 12–13 days, and nestlings usually fledge on brood-days 15–18 (brood-day 0 is the day the first egg hatches) (Bowers et al., 2013). The altricial nestlings are warmed by the female during the first half of the nestling period and provisioned by both parents until about two weeks after leaving the nest (i.e., fledging). During the nestling period, house wrens undergo a period of rapid sigmoidal growth with their mass increasing from ≈1 g at hatching to 9–11 g 11 days later. See Johnson (2014) for additional details on the biology of the house wren.
All experiments were carried out with Institutional Animal Care and Use Committee protocol approvals 05–2010 and 04–2013, and under U.S. Geological Survey Banding Permit 09211, U.S. Fish and Wildlife Service Collecting Permit MB692148-0, and Illinois Department of Natural Resources Scientific Permit NH15.0004a
Pre-Natal Manipulation of Corticosterone
We manipulated levels of pre-natal corticosterone by injecting eggs with varying doses of corticosterone. Nests were monitored for the start of egg-laying in 2013, and randomly assigned to one of three treatments on the day the first egg was laid: control (N=15 nests), low corticosterone increase (N=14 nests), or high corticosterone increase (N=13 nests). Treatments were assigned to whole nests to avoid the difficulty of associating nestlings with differently treated eggs. The corticosterone concentrations of eggs in the low and high corticosterone treatment groups were increased one and two standard deviations, respectively, above the mean corticosterone concentration in yolk of house wren eggs sampled during the 2012 field season (1.80±0.24 ng/g, N = 17 eggs; M. Strange, unpublished data) as determined using a standard radioimmunoassay (details found in Paitz et al., 2011; Haussmann et al., 2012; Bowers et al., 2015). The doses we used were intended to increase in ovo corticosterone to levels comparable to those that stressed females in other species deposit in their eggs (Hayward and Wingfield, 2004; Hayward et al., 2006).
Injections occurred on the day the egg was laid to mimic maternal deposition of corticosterone and to avoid manipulating an egg with a well-developed embryo after incubation had begun. We replaced the egg that we temporarily removed from the nest for injection with a plastic egg so that the number of eggs remained the same. We moved a sufficient distance away from the nest to perform the injection so as not to disturb the parents. Eggs were candled using a LED miniature flashlight to visualize and to avoid puncturing the yolk. We sterilized the acute pole of the egg by applying a small amount of betadine solution, and used a sterile, 27-gauge needle to drill a small hole in the sterilized area. A Hamilton syringe (Hamilton Company, Reno, NV, USA) with a 26-gauge needle was inserted through the hole in the shell, and the solution was injected into the albumen near the yolk. We injected the solution into the albumen to avoid damaging the yolk. Corticosterone is lipophilic, so it migrates to the yolk (Moore and Johnston, 2008). Eggs from nests in the low and high corticosterone treatments were injected with 0.35 ng and 0.7 ng of corticosterone, respectively, dissolved in 5 µL of sesame oil. Control eggs were injected with 5 µL of the sesame oil vehicle only. The hole was sealed using a small amount of cyanoacrylate glue. After the glue dried, we returned the egg to the nest and removed the artificial egg.
Of the 42 nests in which eggs were injected, nine were abandoned before hatching occurred (two control, four low-corticosterone and three high-corticosterone nests). Hatchability was low (~26%; Table 1) compared with uninjected eggs, but it was not affected by treatment (F2, 38 =0.25, P=0.78), nor by the number of eggs incubated (F1, 38 =2.34, P=0.13). We conclude, therefore, that although piercing and sealing of the eggshell decreases egg viability (a common feature of these types of studies; see Bowden et al., 2009), it is not influenced by corticosterone injection per se. A further nine nests (three in each treatment) were depredated or abandoned following hatching, leaving a total of 24 nests (10 control, 7 low, 7 high) with which to compare offspring phenotype across treatments (Table 1).
Table 1.
Samples sizes, hatching success and nestling survival following pre-natal manipulation of corticosterone.
| Treatment | |||
|---|---|---|---|
| Variable | Control | Low CORT | High CORT |
| Number of nests injected |
15 | 14 | 13 |
| Mean clutch size | 5.9 | 5.7 | 6 |
| Total eggs incubated1 | 79 | 69 | 69 |
| Hatchability | 22.8% | 27.5% | 27.5% |
| Mean no. of hatched young |
1.2 | 1.36 | 1.46 |
| Total no. of brood-day 0 hatchlings |
18 | 19 | 19 |
| Total no. of brood-day 11 nestlings (No. Nests) |
15 (10) | 14 (7) | 14 (7) |
The number of eggs incubated is slightly less than what would be expected based on clutch size owing to the loss of a few eggs before incubation commenced.
Post-Natal Manipulation of Corticosterone
We manipulated levels of post-natal corticosterone by orally dosing nestlings with corticosterone in the 2013 breeding season. We employed a randomized block design in which half of the nestlings within each of 56 nests were randomly assigned to a control or experimental treatment on brood-day 4. We used a dose of 0.87 µg of corticosterone/g body mass for the experimental nestlings, which is based on published information on the doses used in a study of nestling song sparrows (Melospiza melodia) that resulted in a transient increase in circulating corticosterone levels in the blood similar to the levels seen during restraint-induced stress (Schmidt et al., 2012). This dosage mimics the anticipated endogenous response to a repeated acute stressor, such as short-term periods of food shortage. Doses were adjusted for differences in the body mass of the rapidly growing nestlings. Corticosterone was dissolved in peanut oil following the procedures described in Breuner et al. (’98). Nestlings in the experimental treatment group were fed 5.1 µg of corticosterone dissolved in 15 µL of peanut oil once each morning on brood-days 5 and 6 and 7.0 µg of corticosterone dissolved in 15 µL of oil on brood-days 7 and 8. Nestlings in the control treatment were fed 15 µL of peanut oil once each morning on brood-days 5–8. We used a Gilson Pipetman (Gilson, Inc., Middleton, WI, USA) inserted briefly into the nestlings’ œsophagus to deliver the liquid.
Confirmation of Post-Natal Elevation in Corticosterone
A different set of nine nests was used in 2013 to document that the oral administration of corticosterone successfully increases corticosterone levels in nestling blood. We used the same methods and dosage described above for administrating the treatment, but used nestlings on brood-day 11 because they were sufficiently large to provide an adequate plasma sample. Nestlings were fed 8.7 µg of corticosterone (representing 0.87 µg /g body mass after accounting for the average brood-day 11 mass) dissolved in 15 µL of peanut oil; control nestlings were fed 15 µL of peanut oil only. A blood sample was taken 10 min after the nestling was fed the supplement, as Schmidt et al. (2012) found that the highest levels of circulating corticosterone occur 10 min after being given an oral supplement. We took a blood sample by puncturing the left brachial vein and used a heparinized micro-capillary tube to collect the blood. Blood samples were stored on ice in a cooler until they were taken back to the laboratory and centrifuged on the same day to separate the plasma and red blood cells.
Plasma corticosterone concentrations of nestlings in the post-natal confirmation experiment were measured using an enzyme-linked immunoassay (EIA) (Corticosterone EIA Kit, Cat. No. ADI-900-097, Enzo Life Sciences, Plymouth Meeting, PA, USA). We followed the manufacturer’s protocol except for the volume of plasma prescribed. Instead, we used a modified procedure (Wada et al., 2007) that accommodates smaller plasma volumes (10 µL), and if 10 µL of plasma were not obtained, we added an appropriate amount of Assay 15 Buffer to the sample to reach a volume of 10 µL. All plasma samples and standards were run in duplicate to obtain an average value for each sample. We read the plates containing the samples with a microplate reader at 405 nm (corrected at 580 nm), and used a 4-parameter logistic curve-fitting program from Gen5 1.11.5 (BioTek Instruments, Inc., Winooski, VT, USA) to generate a standard curve. We applied a conversion factor for each volume of plasma used per sample in each calculation of final corticosterone concentration to account for the difference in actual volumes used in the assay (per well). Two plates were used in this analysis, and the inter-assay coefficient of variation (CV) was low (0.6%), allowing for across-plate comparison of the results. The intra-assay CV was low for both plates at 2.4% and 3.3% (mean ± s.e.m.=2.9±0.4 %).
HPA Axis Reactivity of Nestlings
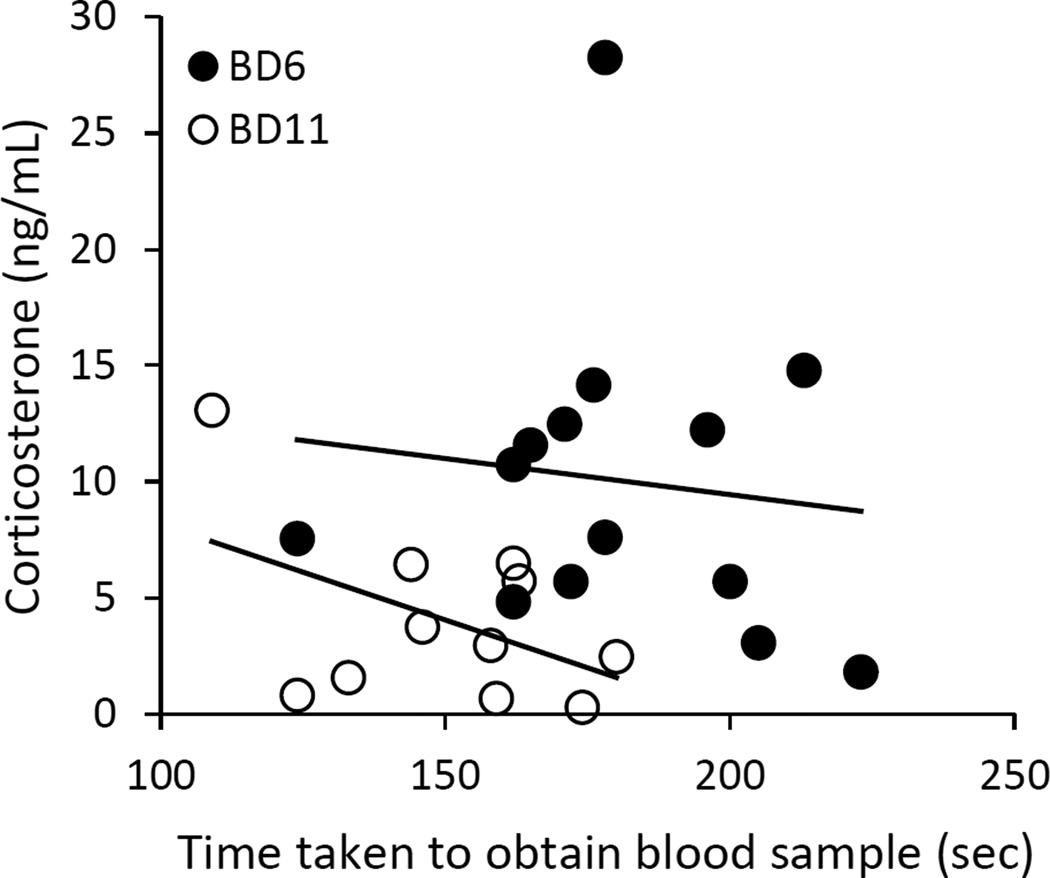
The HPA axis reactivity of nestlings was assessed in 2014 using a standardized capture-and-restraint protocol (Wingfield, ’94). Nests were monitored for hatching and randomly assigned to either brood-day 6 (N=17 nests) or brood-day 11 (N=16 nests) for assessment of the stress response. On the assigned day, three nestlings were removed from the nest and placed in separate open-mesh bags. One blood sample was taken from each nestling during the stress series: within 4 min of removal from the nest, 15 min after removal, or 30 min after removal (hereafter, referred to as baseline, 15 min, or 30 min, respectively). Baseline blood samples from brood-day 6 nestlings were occasionally difficult to obtain in under 3 min as recommended (Romero and Reed, 2005; Lothery et al., 2014), but there was no relationship between time to obtain the blood sample and corticosterone concentration (Fig. 1), which indicates that baseline corticosterone levels rather than elevated stress-induced levels of corticosterone were measured (linear regression brood-day 6: F1=0.17, P=0.69, Adj. R2=− 0.07; brood-day 11: F1=2.58, P=0.14, Adj. R2=0.14). The 15-min and 30-min sampling times were chosen to measure a stress response because corticosterone levels are typically elevated 15 min after a disturbance and peak and plateau at 30 min (Rensel et al., 2010b). Blood samples were collected using the same technique described above.
Figure 1.
The relationship between the time taken to obtain a blood sample and circulating corticosterone concentration in brood-day 6 and brood-day 11 nestlings.
Plasma corticosterone concentrations of nestlings were measured using an EIA kit (Corticosterone EIA Kit, Cat. No. K014-H1; Arbor Assays, Ann Arbor, MI, USA) designed to accommodate small volumes of plasma (5 µL) and to measure low concentrations of corticosterone (limit of detection: 0.0169 ng/mL). Obtaining more than 5 µL of plasma from brood-day 6 nestlings can be difficult, and brood-day 6 nestlings have low plasma corticosterone concentrations, necessitating the use of this particular kit. If 5 µL of plasma were not obtained, an appropriate amount of Assay Buffer was added to increase the sample volume to 5 µL. All plasma samples and standards were run in duplicate to obtain an average value for each sample, and the plates containing the samples were read as described above. Three plates were used in this analysis, and the inter-assay CV was 11.4% allowing for across-plate comparison of the results. The intra-assay CV was low for all plates, ranging from 1.9% to 5.6% (mean ± s.e.m.=3.9±1.1%).
Measurement of Fitness-Related Traits of Nestlings
Fitness-related traits of nestlings were measured in both the pre- and post-natal experimental manipulations of corticosterone. On brood-day 11, all nestlings were banded with a numbered, aluminum U.S. Geological Survey band, and body mass was measured to the nearest 0.1 g using a digital scale (Acculab Pocket Pro PP 201, Edgewood, NY, USA). Tarsus length (a measure of structural body size) was measured to the nearest 0.1 mm with dial calipers. To determine sex and to measure haematocrit, blood samples were collected from each nestling as described above. To measure a component of nestling immunoresponsiveness, we conducted a PHA (phytohemagglutinin) test. The PHA test is commonly used as a measure of cutaneous immune activity, which involves both the adaptive and innate branches of the immune system (Martin et al., 2006). On brood-day 11, the thickness of the left wing-web (prepatagium) was measured three consecutive times to the nearest 0.1 mm with a digital thickness gauge (Mitutoyo no. 547–500, Aurora, IL, USA) to obtain a mean initial wing-web thickness. We then used a 30-gauge needle to inject 50 µL of phosphate-buffered saline (PBS) containing 5 mg/mL of PHA (Sigma-Aldrich cat. # L8754, St. Louis, MO, USA) into the wing-web. Approximately 24 h later, we re-measured the wing-web three consecutive times to obtain a mean post-injection thickness, and then took the difference in the mean pre- and post-injection measurements to obtain a measure of post-injection swelling.
Blood samples were centrifuged at 1930 g for 60 sec to separate red blood cells from plasma (Hematastat II, Separation Technology, Inc., Sanford, FL, USA). We then measured haematocrit in the micro-capillary tubes as the percentage of whole blood constituted by the packed red blood cells, averaging three consecutive measures as recommended by the manufacturer. Afterwards, we used a 100-µL Hamilton syringe to collect and measure the volume of plasma in the micro-capillary tube. Plasma was stored in a micro-centrifuge tube at −20°C and red blood cells were placed in micro-centrifuge tubes with Queen’s lysis buffer for storage at −4°C until further analysis.
We determined the sex of each nestling to investigate whether the effect of the corticosterone treatment depended on sex. Because the sexes are not morphologically distinguishable on brood-day 11, polymerase chain reaction (PCR) was used to amplify the sex-specific sequences of DNA extracted from nestling red blood cells (Kahn et al., ’98; Bowers et al., 2011). After amplification, the DNA was separated by electrophoresis on a 1.8% agarose gel. Samples from male and female adult house wrens were placed in adjacent lanes of each gel as a control. Gels were then stained with ethidium bromide and visualized under ultraviolet light.
Statistical Analysis
All statistical analyses were performed using SAS 9.3 (SAS Institute, Inc., Cary, NC, USA) and were two-tailed with α=0.05. All means reported are least-squares means unless otherwise noted. Nest was included as a random effect to account for the statistical non-independence of nestlings within a brood for all analyses where multiple measurements were taken from one nest. Reduced models were obtained in the final analyses by employing backward elimination of non-significant effects (P>0.15), beginning with non-significant two-way interactions.
We utilized general linear models (PROC GLM) to determine the effect of pre-natal corticosterone treatment on incubation period, hatching success, mean mass at hatching, and nestling survival. Preplanned contrasts were made between the pre-natal control and corticosterone treatments using the CONTRAST statement to assess the effects of corticosterone on mean mass at hatching. These dependent variables all represent properties of the nest, so it was not necessary to include nest as a random factor in these analyses. To determine the effect of treatment on hatching success, the number of eggs that hatched was the dependent variable with the number of eggs incubated included as a covariate. Similarly, to determine the effect of the treatment on nestling survival, brood size on brood-day 11 was the dependent variable, with the number of eggs that hatched included as a covariate.
We used a mixed-model ANOVA (PROC MIXED) to examine the effect of pre-natal corticosterone treatment on brood-day 11 nestling mass, tarsus length, haematocrit, and PHA response. Nestling sex and treatment were fixed factors in the models, and hatching date and brood size were included as covariates. In the analysis of nestling mass on brood-day 11, the mean mass of hatchlings in the nest was included as an additional covariate to assess nestling growth.
We employed a mixed-model ANOVA (PROC MIXED) to confirm that our post-natal treatment elevated circulating corticosterone levels and to examine the effect of post-natal corticosterone treatment on brood-day 11 nestling mass, tarsus length, haematocrit, and PHA response. Nestling sex and treatment were fixed factors in the models, and hatching date and brood size were included as covariates.
To determine the effect of age on the magnitude of the response to the capture-and-restraint protocol, we employed a mixed model ANOVA (PROC MIXED) with sampling time during the stress response and age as fixed factors, and hatching date and brood size as covariates. We predicted that if age influences the magnitude of the stress response, there should be a significant age × time interaction. Post-hoc contrasts were used to determine if brood-day 6 and brood-day 11 nestlings elevated their circulating corticosterone levels in response to the capture-and-restraint protocol.
RESULTS
Pre-Natal Manipulation of Corticosterone
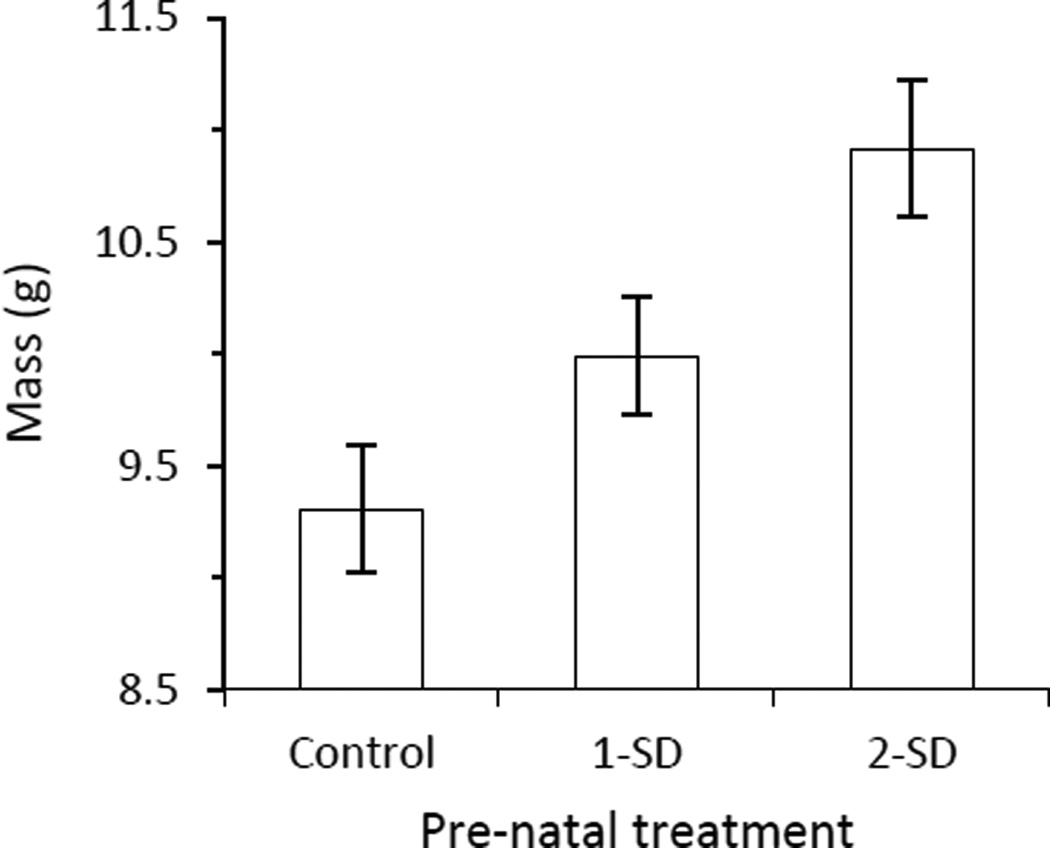
There was no difference across treatments in the length of the incubation period (mean ± s.e.m., control: 12.6±0.3 days; low corticosterone: 13.2±0.3 days; high corticosterone: 13.2±0.3 days; Table 2). Mean mass at hatching was, however, affected by treatment (mean ± s.e.m., control: 1.21±0.05 g; low corticosterone: 0.98±0.06 g; high corticosterone: 1.12±0.07 g; Table 2), with control nestlings weighing more at hatching than nestlings hatching from corticosterone-injected eggs (Preplanned contrasts: low corticosterone and high corticosterone nestlings pooled, F1, 23 =5.35, P=0.03). On brood-day 11, nestlings in the three treatments still differed in mass (Fig. 2), after controlling for initial hatching mass by including mean hatching mass as a covariate in the analysis (Table 2). Post-hoc contrasts revealed that high corticosterone nestlings weighed significantly more on brood-day 11 than both the control (F1, 29=15.95, P=0.0004) and low corticosterone nestlings (F1, 29=4.99, P=0.03); control and low corticosterone nestlings did not differ in body mass on brood-day 11 (F1, 29=2.49, P=0.13). Nestling immune response, tarsus length, and haematocrit did not differ among treatments on brood-day 11(Table 2).
Table 2.
Linear mixed model summary of the effect of the manipulation of pre-natal corticosterone on nestling traits.
| Estimate ± s.e. | df | F-value | P-value | |
|---|---|---|---|---|
| Incubation Period | ||||
| Intercept | 12.61 ± 0.28 | |||
| Treatment | 2,30 | 1.33 | 0.2786 | |
| Control | -- | |||
| Low | 0.58 ± 0.42 | |||
| High | 0.58 ± 0.42 | |||
| Hatching mass | ||||
| Intercept | 1.21 ± 0.05 | |||
| Treatment | 2,23 | 4.56 | 0.0215 | |
| Control | -- | |||
| Low | −0.23 ± 0.08 | |||
| High | −0.08 ± 0.08 | |||
| Nestling mass at BD11 | ||||
| Intercept | 7.32 ±1.36 | |||
| Treatment | 2, 29 | 8.13 | 0.0016 | |
| Control | -- | |||
| Low | 0.68 ± 0.43 | |||
| High | 1.61 ± 0.40 | |||
| Hatching mass | 1.75 ± 1.05 | |||
| PHA response | ||||
| Intercept | 0.56 ± 0.07 | |||
| Treatment | 2, 16.3 | 0.76 | 0.48 | |
| Control | -- | |||
| Low | −0.06 ± 0.11 | |||
| High | 0.08 ± 0.11 | |||
| Tarsus length (mm) | ||||
| Intercept | 17.45 ± 0.39 | |||
| Treatment | 2, 14.2 | 2.16 | 0.15 | |
| Control | -- | |||
| Low | 0.77 ± 0.40 | |||
| High | 0.91 ± 0.38 | |||
| Sex | 1, 17.4 | 0.15 | 0.71 | |
| Male | -- | |||
| Female | 0.57 ± 0.30 | |||
| Sex × Treatment | −0.51 ± 0.43 | 2, 17.5 | 2.70 | 0.10 |
| Brood size | 0.24 ± 0.15 | 1, 13.4 | 2.63 | 0.13 |
| Haematocrit | ||||
| Intercept | 118.17 ± 23.30 | |||
| Treatment | 2, 17.8 | 1.58 | 0.23 | |
| Control | -- | |||
| Low | −3.28 ± 1.99 | |||
| High | −0.32 ± 1.99 | |||
| Hatching Date | −0.37 ± 0.11 | 1, 17.6 | 10.71 | 0.004 |
Figure 2.
Brood-day 11 mass (LS mean ± SE) of late-stage nestlings following pre-natal manipulation of corticosterone. Controls received the vehicle, lows received a dose one standard deviation above mean yolk corticosterone, and highs received a dose two standard deviations above mean yolk corticosterone.
The number of nestlings surviving to brood-day 11 did not differ among treatments (Table 1; F2, 29 = 0.45, P=0.64). Irrespective of treatment, the number of surviving nestlings increased significantly with the number of eggs that hatched in the clutch (F1, 29 =38.85, P<0.0001).
Post-Natal Manipulation of Corticosterone
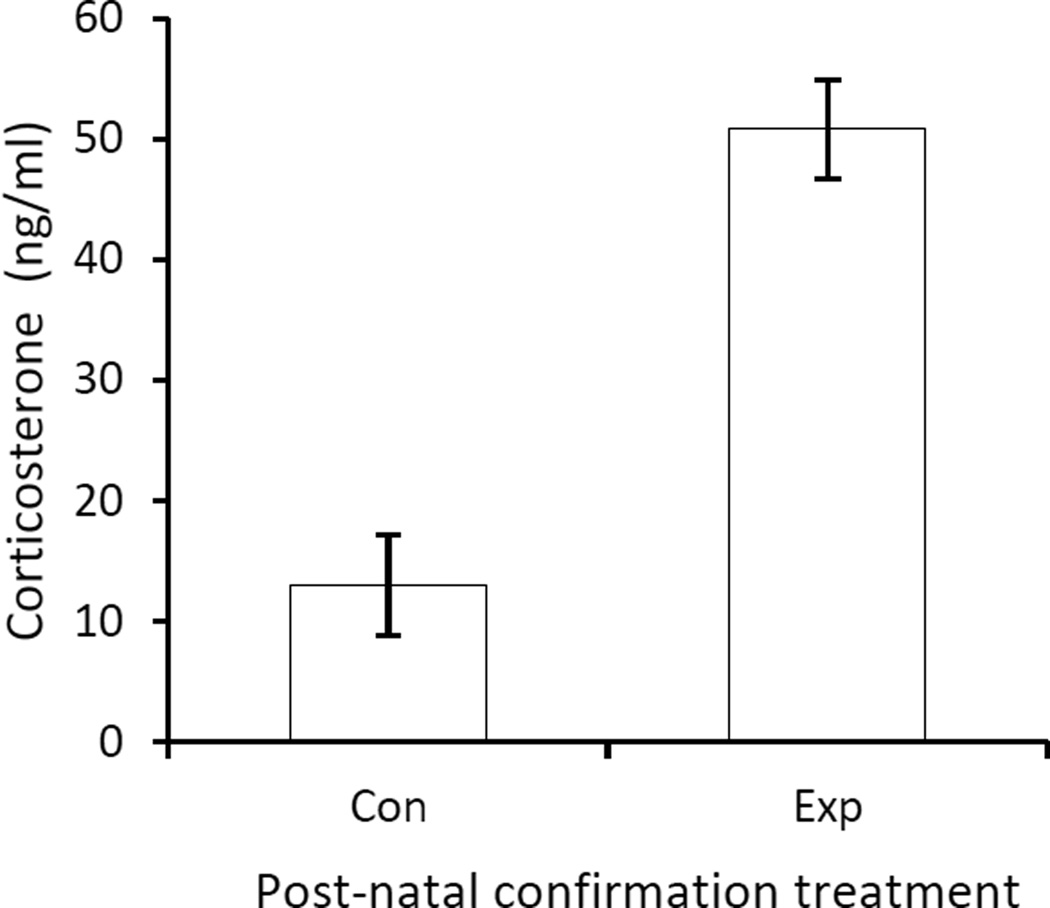
We confirmed that oral administration of corticosterone resulted in an approximately four-fold increase in corticosterone levels in nestling blood on brood-day 11 (F1, 43.1=80.54, P<0.0001; Fig. 3). Oral administration on brood-days 5 to 8 did not affect nestling mass on brood-day 11 (control: 10.33±0.08, experimental: 10.36±0.07; Table 3), although mass did differ between the sexes (male: 10.44±0.09, female: 10.26±0.07). Additionally, nestling mass was affected by brood size, with lighter nestlings occurring in larger broods. Immune response, tarsus length, and haematocrit did not differ between control and experimental nestlings (Table 3).
Figure 3.
Circulating corticosterone concentations after post-natal oral administration of corticosterone. Circulating corticosterone concentration (LS mean ± SE) in brood-day 11 (late-stage) nestlings 10 min after receiving post-natal treatment. Controls received the vehicle and the experimental received corticosterone dissolved in the vehicle.
Table 3.
Linear mixed model summary of the effect of post-natal oral application of corticosterone on nestling traits.
| Estimate ± s.e. | df | F-value | P-value | |
|---|---|---|---|---|
| Nestling mass at BD11 | ||||
| Intercept | 11.24 ± 0.39 | |||
| Treatment | 1, 162 | 0.19 | 0.66 | |
| Control | −0.03 ± 0.07 | |||
| Experimental | -- | |||
| Sex | 1, 182 | 4.67 | 0.0319 | |
| Male | -- | |||
| Female | −0.18 ± 0.08 | |||
| Brood size | −0.12 ± 0.06 | 1, 49.4 | 4.20 | 0.0459 |
| PHA response | ||||
| Intercept | 2.28 ± 0.41 | |||
| Treatment | 1, 273 | 0.00 | 0.96 | |
| Control | −0.001 ± 0.02 | |||
| Experimental | -- | |||
| Hatching Date | −0.009 ± 0.003 | 1, 52.1 | 13.39 | 0.0006 |
| Tarsus length | ||||
| Intercept | 18.94 ± 0.10 | |||
| Treatment | 1, 132 | 2.00 | 0.16 | |
| Control | 0.10 ± 0.07 | |||
| Experimental | -- | |||
| Sex | 1, 140 | 10.95 | 0.001 | |
| Male | -- | |||
| Female | −0.26 ± 0.08 | |||
| Haematocrit | ||||
| Intercept | 43.00 ± 0.61 | |||
| Treatment | 1, 270 | 0.38 | 0.54 | |
| Control | −0.28 ± 0.46 | |||
| Experimental | -- | |||
HPA Axis Reactivity of Nestlings
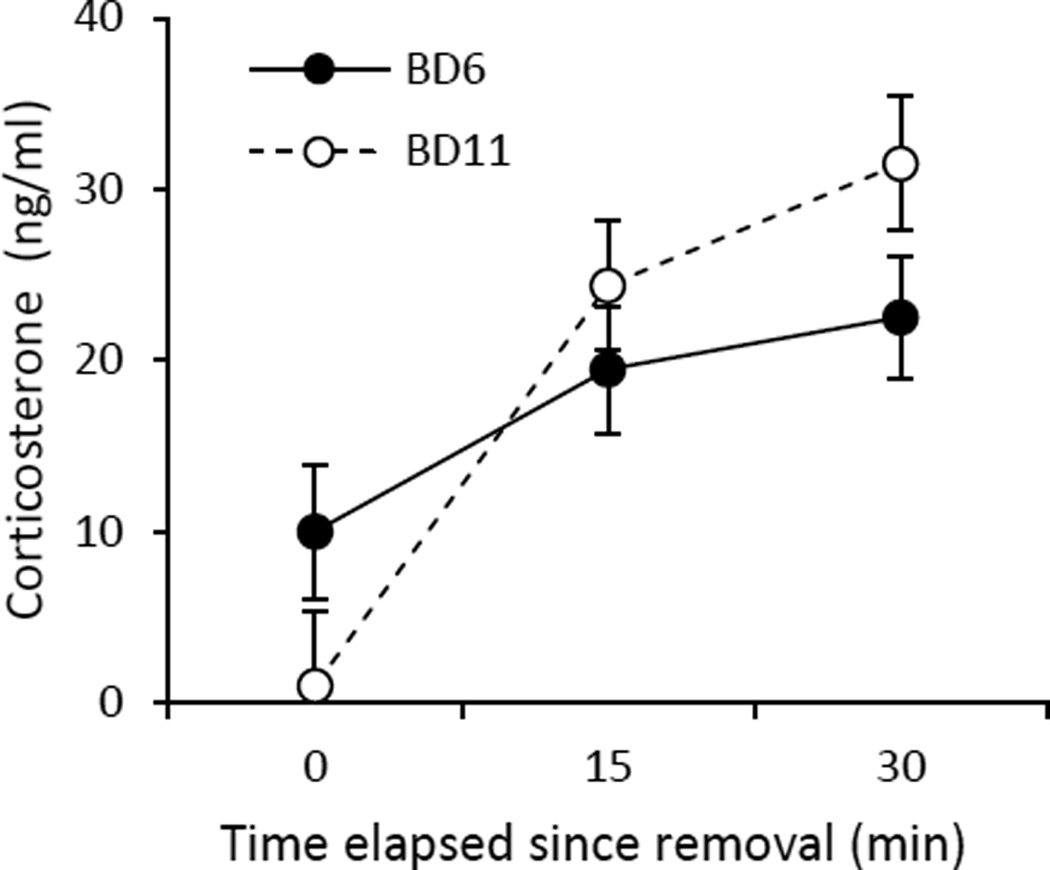
Both brood-day 6 and brood-day 11 nestlings had detectable levels of plasma corticosterone, and although brood-day 6 nestlings had slightly higher baseline corticosterone than brood-day 11 nestlings (Fig. 4), the difference was not statistically significant (F1, 26=2.34, P =0.138). Corticosterone concentration significantly increased in response to the capture-and-restraint protocol in both brood-day 6 (F1, 29=7.71, P=0.01; Fig. 4) and brood-day 11 nestlings (F1, 23=35.52, P<0.0001; Fig. 4), but more rapidly in brood-day 11 than in brood-day 6 nestlings (age × time interaction: F2, 51.4=3.68, P=0.03; Fig. 4).
Figure 4.
Stress-induced responses of brood-day 6 and brood-day 11 nestlings. Circulating corticosterone concentrations (LS mean ± SE) in response to capture-and-restraint protocol for brood-day 6 and brood-day 11 nestlings.
DISCUSSION
Under natural circumstances, it is likely that stress levels of parents and offspring would be correlated as they both experience the same environment. In the current study, we attempted to decouple parental and offspring stress by experimentally manipulating offspring corticosterone independently of the parent during both pre-natal and post-natal periods. Our manipulation of the offspring, but not their parents, allowed us to investigate how offspring alone respond to stress levels of corticosterone without the complicating factor of parents also simultaneously responding to experimentally increased corticosterone.
Pre-Natal Manipulation of Corticosterone
Nestlings from control eggs weighed more at hatching than those from corticosterone-treated eggs, but, by brood-day 11, nestlings from eggs treated with the higher dose of corticosterone were the heaviest of those in the three treatments. Thus, nestlings hatching from eggs receiving the higher dose compensated for their initial lower mass by the time they reached their asymptotic mass. Such compensatory growth by nestlings usually occurs once resources become available, or when the stressor that caused the setback is eliminated (Müller et al., 2009). Notwithstanding the apparent compensatory growth of nestlings hatching from corticosterone-treated eggs, the mechanism leading to their lower hatching mass remains unclear. If elevated corticosterone in the egg affects the catabolism of protein and lipids by the embryo, this could conceivably result in decreased mass at hatching (Hepp et al., 2006). Alternatively, elevated corticosterone may suppress pre-natal growth (as it can post-natal growth; Müller et al., 2009), necessitating, perhaps, a longer period of incubation and increased maintenance costs and energy expenditures for the developing embryo (Olson et al., 2006). This too could result in lower hatching mass. However, we detected no differences across treatments in the length of the incubation period in this study.
The finding that pre-natal elevation in corticosterone leads to lower mass at hatching and then increased mass near the time of fledging was unexpected, with negative effects of experimentally elevated levels of corticosterone on body mass more commonly reported (e.g., Hayward and Wingfield, 2004; Saino et al., 2005). In house wrens, survival shortly after leaving the nest (Young, ’96) and recruitment to future breeding populations (Bowers et al., 2014) is positively correlated with body mass close to nest-leaving. In altricial birds, the mortality rate is high during the fledgling and juvenile periods (Young, ’96; Anders et al., ’97; Rush and Stutchbury, 2008), the commonest causes of which are starvation and predation (Sullivan, ’89; Overskaug et al., ’99; Naef-Daenzer et al., 2001). Heavier fledglings may be better able than lighter nestlings to withstand food shortages that occur after they leave the nest (Magrath, ’91; but see Thompson and Flux, ’88; Thompson et al., ’93). We caution, however, that although compensatory growth of the type we found here does have immediate benefits (e.g., fledging at a higher mass), there may be associated costs, such as a shortened lifespan (Birkhead et al., ’99; Metcalfe and Monaghan, 2001). However, at least in house wrens, it is possible that by increasing levels of corticosterone in eggs, mothers can influence the mass and, therefore, survival and success of their offspring long after they have left the nest.
The mechanism by which corticosterone deposited in the egg elicited its effect on brood-day 11 body mass may have been by its influence on growth hormone production or by increased nestling begging, or both. Corticosterone treatment increases the production of pituitary growth-hormone-secreting cells in chicken (Gallus gallus) embryos (Porter, 2005) and positively affects production of growth hormone mRNAs in cultured embryonic pituitary cells (Jenkins et al., 2013). Although there has been considerable debate about the role, whether positive or negative, of glucocorticoids in regulating growth hormone production, Vakili and Cattini (2012) argue that glucocorticoids clearly play a positive role in the development of growth hormone-producing cells and of growth hormone gene expression in the anterior pituitary. Pre-natal (Love and Williams, 2008b; Bowers et al., in press), as well as post-natal (Loiseau et al., 2008), administration of corticosterone can increase nestling begging, and increased begging typically stimulates increased parental provisioning (Budden and Wright, 2001). However, house wren parents do not always increase provisioning rates in response to increased nestling begging (Barnett et al., 2011). Because we did not measure nestling begging or parental provisioning, we cannot determine the role, if any, they played in producing the heavier nestlings hatching from corticosterone-treated eggs. Although it is clear that elevated pre-natal exposure to corticosterone can influence the developing phenotype, it should be noted that there is evidence of embryonic corticosterone metabolism early in incubation. Radiolabeled corticosterone injected into chicken eggs was found in embryonic tissue by day 6 of incubation. The steroid was in a conjugated, polarized form (von Engelhardt et al., 2009), which suggests that avian embryos may modulate the effects of maternally or experimentally derived corticosterone (Vassallo et al., 2014) and other steroids (Paitz et al., 2011) early during incubation. Although the fate of maternally deposited corticosterone in eggs and the role of its metabolites remain largely unexplored, there is little doubt that elevated pre-natal levels of corticosterone can produce significant fitness-related effects on the phenotype of altricial nestlings, effects that ultimately influence their success as fledglings and adults.
Post-Natal Manipulation of Corticosterone
In contrast to the results of the pre-natal manipulation, increased post-natal exposure to corticosterone had no detectable effect on nestling phenotype. The lack of response is unlikely to be attributable to the use of low dosages because the post-natal experiment raised circulating corticosterone four-fold above the circulating corticosterone concentration of brood-day 11 control nestlings, and this falls within the range of stress-induced levels of corticosterone for nestlings of other altricial species (Kern et al., 2001; Saino et al., 2003; Honarmand et al., 2010). Further, the dose used in the post-natal experiment increased the mean circulating corticosterone levels to about 80% of the maximum level that we detected of endogenously produced corticosterone by brood-day 11 nestlings in response to the standardized capture-and-restraint protocol. Thus, dosing levels successfully simulated nestling response to short-term stress conditions, but the treatment period in our study was short compared with the length of treatment in other studies investigating the effects of post-natal corticosterone elevations. For example, in a study that found that post-natal corticosterone elevation increased foraging skills, oral boluses of corticosterone were given twice a day for 17 days (Crino et al., 2014). Additionally, many studies have used silastic implants, which simulate chronic stress by keeping corticosterone levels constantly elevated (Kitaysky et al., 2001b). In contrast, the daily oral boluses used in our study result in a peak in corticosterone levels that then return to baseline (Schmidt et al., 2012) and are, thus, typical of levels reached during periods of short-term stress. Our shorter treatment period may provide at least a partial explanation for the lack of post-natal treatment effects, but we also think that the response to short-term corticosterone elevation likely depends on the stage of development at which it occurs.
When corticosterone was administered pre-natally to chickens, distress vocalizations and latency to detect a predator increased in the chicks, but there was no effect when administered post-natally (Freire et al., 2006). Similarly, a pre-natal corticosterone treatment in common lizards (Lacerta vivipara) resulted in an increase in time spent moving, whereas a post-natal corticosterone treatment did not (Belliure et al., 2004). Such results suggest that there is greater sensitivity to corticosterone exposure during the pre-natal stage of development than during the post-natal stage. Corticosterone may work during an early, critical stage of development to organize tissues, with long-term effects on the phenotype (Hayward et al., 2006; Love and Williams, 2008a, b; Schoech et al., 2011). This, in turn, may provide an explanation for the outcome of our study because we applied our pre-natal treatment on the day the egg was laid, thereby potentially exposing the developing embryo to an extended period of elevated corticosterone, whereas the post-natal treatment was not applied until brood-day 5, a time when nestlings are growing rapidly and have attained about 50% of their asymptotic mass. At this point, the transient elevation of corticosterone may not have led to any obvious, long-term effects because the nestlings had passed the period in development during which the fitness-related traits that we measured are sensitive to the effects of transient increases in corticosterone.
HPA Axis Reactivity of Nestlings
Baseline plasma corticosterone concentrations were somewhat, but not significantly, higher in early-stage nestlings than in late-stage nestlings. In other species, late-stage nestlings tend to have higher baseline corticosterone concentrations than early-stage nestlings (Schwabl, ’99; Love et al., 2003; Wada et al., 2007; Pakkala et al., in press), but there are reports of both the opposite relationship (Walker et al., 2005) and little or no difference (Sims and Holberton, 2000; Blas et al., 2006; Wada et al., 2009; Taves et al., 2016).
Both early-stage and late-stage nestlings were able to mount a stress-induced response, but the stress response increased more steeply in late-stage nestlings. The late-stage nestlings are well developed, near adult mass and size, and within 4–6 days of fledging (Bowers et al., 2013). Studies have linked increases in corticosterone to increased locomotor activity in other altricial birds (Breuner et al., ’98), which may facilitate their transition from a sedentary life in the nest to the mobile fledgling period (Heath, ’97; Pakkala et al., in press).
Although early-stage nestlings did have a stress-induced response of lower magnitude than late-stage nestlings, they did respond to the capture-and-restraint protocol by significantly elevating their endogenously produced corticosterone levels. A muted stress response in early-stage nestlings is not unexpected as altricial nestlings at this stage in development are unlikely to need to mount a large response as they are entirely dependent on their parents for food and would be unable to escape from a predator. Further, being less responsive to stressors may allow early-stage nestlings to avoid the deleterious effects that have been reported when corticosterone was experimentally elevated (Wada and Breuner, 2008; Loiseau et al., 2008; Stier et al., 2009). Two possible advantages of being able to mount a modest stress response at so young an age are that increased corticosterone leads to increased begging (Loiseau et al., 2008; Love and Williams, 2008b), which may, but not always (Barnett et al., 2011), result in increased provisioning by parents, and may promote successful competition among siblings in the nest (Braasch et al., 2014).
In conclusion, our results suggest that pre-natal exposure to corticosterone is important in shaping offspring phenotype and are consistent with the hypothesis that maternally derived corticosterone in the egg can have long-term effects that alter offspring phenotype in ways that may increase fitness.
Acknowledgments
The ParkLands Foundation (Merwin Preserve) and the Sears and Butler families generously provided access to their properties. We thank Cassie Lothery and Joseph Casto for providing assay training and support, E. Keith Bowers for assistance with data collection and statistical analysis, and C. J. Clark, Erin Dorset, Rachel Gocker, Christine Hodges, Caroline Imhoff, Philip Kohlmeier, Chris Loebach, Ryan Machett, Tony Martini, Colleen McGrath, Jamie Pomahac, Marion Sakaluk, Annie Voigt, Morgan Walder, and Jillian Wormington for assistance in the field and laboratory.
This research was supported by grants from the National Science Foundation [IBN-0316580 to C.F.T and S.K.S.]; National Institutes of Health [R15HD076308-01 to S.K.S. and C.F.T. and R15ES023995 to R.M.B. and R.T. Paitz]; Illinois State University (Faculty Research Award to S.K.S.); the Beta Lambda Chapter of the Phi Sigma Biological Honor Society to M.S.S; and the Health Resources and Services Administration, Department of Health and Human Services [DHP 20062]. Additional support was provided by Research Internships in Science and Engineering (RISE) from the Deutscher Akademischer Austauschdienst to Philip Kohlmeier and Annie Voigt, and the School of Biological Sciences at Illinois State University to M.S.S., C.F.T., and S.K.S.
LITERATURE CITED
- Adkins-Regan E, Banerjee SB, Correa SM, Schweitzer C. Maternal effects in quail and zebra finches: behavior and hormones. Gen Comp Endocrinol. 2013;190:34–41. doi: 10.1016/j.ygcen.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Anders AD, Dearborn DC, Faaborg J, Thompson FR. Juvenile survival in a population of Neotropical migrant birds. Conserv Biol. 1997;11:698–707. [Google Scholar]
- Barnett CA, Clairardin SG, Thompson CF, Sakaluk SK. Turning a deaf ear: a test of the manipulating androgens hypothesis in house wrens. Anim Behav. 2011;81:113–120. [Google Scholar]
- Belliure J, Meylan S, Clobert J. Prenatal and postnatal effects of corticosterone on behavior in juveniles of the common lizard, Lacerta vivipara. J Exp Zool. 2004;301A:401–410. doi: 10.1002/jez.a.20066. [DOI] [PubMed] [Google Scholar]
- Birkhead TR, Fletcher F, Pellatt EJ. Nestling diet, secondary sexual traits and fitness in the zebra finch. Proc R Soc Lond B. 1999;266:385–390. [Google Scholar]
- Blas J, Baos R, Bortolotti GR, Marchant TA, Hiraldo F. Age-related variation in the adrenocortical response to stress in nestling white storks (Ciconia ciconia) supports the developmental hypothesis. Gen Comp Endocrinol. 2006;148:172–180. doi: 10.1016/j.ygcen.2006.02.011. [DOI] [PubMed] [Google Scholar]
- Bonier F, Martin PR, Moore IT, Wingfield JC. Do baseline glucocorticoids predict fitness? Trends Ecol Evol. 2009;24:634–642. doi: 10.1016/j.tree.2009.04.013. [DOI] [PubMed] [Google Scholar]
- Bowden RM, Smithee L, Paitz RT. A modified yolk biopsy technique improves survivorship of turtle eggs. Physiol Biochem Zool. 2009;82:611–615. doi: 10.1086/596579. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Adaptive sex allocation in relation to hatching synchrony and offspring quality in house wrens. Am Nat. 2011;177:617–629. doi: 10.1086/659630. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Sakaluk SK, Thompson CF. Sibling cooperation influences the age of nest leaving in an altricial bird. Am Nat. 2013;181:775–786. doi: 10.1086/670244. [DOI] [PubMed] [Google Scholar]
- Bowers EK, Hodges CJ, Forsman AM, Vogel LA, Masters BS, Johnson BGP, Johnson LS, Thompson CF, Sakaluk SK. Neonatal body condition, immune responsiveness, and hematocrit predict longevity in a wild bird population. Ecology. 2014;95:3027–3034. doi: 10.1890/14-0418.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Bowden RM, Sakaluk SK, Thompson CF. Immune activation generates corticosterone-mediated terminal reproductive investment in a wild bird. Am Nat. 2015;185:769–783. doi: 10.1086/681017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers EK, Bowden RM, Thompson CF, Sakaluk SK. Elevated corticosterone during egg production elicits increased maternal investment and promotes nestling growth in a wild songbird. Horm Behav. doi: 10.1016/j.yhbeh.2016.05.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braasch A, Becker PH, Groothuis TGG. Response of testosterone and corticosterone plasma levels to the challenge of sibling competition: a study in common terns. Gen Comp Endocrinol. 2014;204:95–103. doi: 10.1016/j.ygcen.2014.05.007. [DOI] [PubMed] [Google Scholar]
- Breuner CW, Greenberg AL, Wingfield JC. Noninvasive corticosterone treatment rapidly increases activity in Gambel’s white-crowned sparrows (Zonotrichia leucophrys gambelii) Gen Comp Endocrinol. 1998;111:386–394. doi: 10.1006/gcen.1998.7128. [DOI] [PubMed] [Google Scholar]
- Brooks KC, Mateo JM. Chronically raised glucocorticoids reduce innate immune function in Belding’s ground squirrels (Urocitellus beldingi) after an immune challenge. Gen Comp Endocrinol. 2013;193:149–157. doi: 10.1016/j.ygcen.2013.07.019. [DOI] [PubMed] [Google Scholar]
- Budden AE, Wright J. Begging in nestling birds. Curr Ornithol. 2001;16:83–118. [Google Scholar]
- Chin EH, Love OP, Verspoor JJ, Williams TD, Rowley K, Burness G. Juveniles exposed to embryonic corticosterone have enhanced flight performance. Proc R Soc B. 2009;276:499–505. doi: 10.1098/rspb.2008.1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantini D, Marasco V, Møller AP. A meta-analysis of glucocorticoids as modulators of oxidative stress in vertebrates. J Comp Physiol B. 2011;181:447–456. doi: 10.1007/s00360-011-0566-2. [DOI] [PubMed] [Google Scholar]
- Crino OL, Driscoll SC, Ton R, Breuner CW. Corticosterone exposure during development improves performance on a novel foraging task in zebra finches. Anim Behav. 2014;91:27–32. [Google Scholar]
- Crino OL, Breuner CW. Developmental stress: evidence for positive phenotypic and fitness effects in birds. J Ornithol. 2015;156(Suppl 1):S389–S399. [Google Scholar]
- Crossin GT, Love OP, Cooke SJ, Williams TD. Glucocorticoid manipulations in free-living animals: considerations of dose delivery, life-history context and reproductive state. Funct Ecol. 2016;30:116–125. [Google Scholar]
- Dantzer B, Newman AEM, Boonstra R, Palme R, Boutin S, Humphries MM, McAdam AG. Density triggers maternal hormones that increase adaptive offspring growth in a wild mammal. Science. 2013;340:1215–1217. doi: 10.1126/science.1235765. [DOI] [PubMed] [Google Scholar]
- DeMory ML, Thompson CF, Sakaluk SK. Male quality influences male provisioning in house wrens independent of attractiveness. Behav Ecol. 2010;21:1156–1164. [Google Scholar]
- Dobbs RC, Styrsky JD, Thompson CF. Clutch size and the costs of incubation in the house wren. Behav Ecol. 2006;17:849–856. [Google Scholar]
- Freire R, van Dort S, Rogers LJ. Pre- and post-hatching effects of corticosterone treatment on behavior of the domestic chick. Horm Behav. 2006;49:157–165. doi: 10.1016/j.yhbeh.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Groothuis TGG, Müller W, von Engelhardt N, Carere C, Eising C. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci Biobehav Rev. 2005;29:329–352. doi: 10.1016/j.neubiorev.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Hau M, Casagrande S, Ouyang JQ, Baugh AT. Glucocorticoid-mediated phenotypes in vertebrates: multilevel variation and evolution. Adv Study Behav. 2016;48 [Google Scholar]
- Haussmann MF, Longenecker AS, Marchetto NM, Juliano SA, Bowden RM. Embryonic exposure to corticosterone modifies the juvenile stress response, oxidative stress and telomere length. Proc R Soc B. 2012;279:1447–1456. doi: 10.1098/rspb.2011.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward LS, Wingfield JC. Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen Comp Endocrinol. 2004;135:365–371. doi: 10.1016/j.ygcen.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Hayward LS, Richardson JB, Grogan MN, Wingfield JC. Sex differences in the organizational effects of corticosterone in the egg yolk of quail. Gen Comp Endocrinol. 2006;146:144–148. doi: 10.1016/j.ygcen.2005.10.016. [DOI] [PubMed] [Google Scholar]
- Heath J. Corticosterone levels during nest departure of juvenile American kestrels. Condor. 1997;99:806–811. [Google Scholar]
- Henriksen R, Rettenbacher S, Groothuis TGG. Prenatal stress in birds: pathways, effects, function and perspectives. Neurosci Biobehav Rev. 2011;35:1484–1501. doi: 10.1016/j.neubiorev.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Hepp GR, Kennamer RA, Johnson MH. Maternal effects in wood ducks: incubation temperature influences incubation period and neonate phenotype. Funct Ecol. 2006;20:307–314. [Google Scholar]
- Honarmand M, Goymann W, Naguib M. Stressful dieting: nutritional conditions but not compensatory growth elevate corticosterone levels in zebra finch nestlings and fledglings. PLoS One. 2010;5:e12930. doi: 10.1371/journal.pone.0012930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins SA, Ellestad LE, Mukherjee M, Narayana J, Cogburn LA, Porter TE. Glucocorticoid-induced changes in gene expression in embryonic anterior pituitary cells. Physiol Genomics. 2013;45:422–433. doi: 10.1152/physiolgenomics.00154.2012. [DOI] [PubMed] [Google Scholar]
- Johnson LS. House Wren (Troglodytes aedon) In: Poole A, editor. The Birds of North America Online. Ithaca: Cornell Lab of Ornithology; 2014. Retrieved from the Birds of North America Online: http://bna.birds.cornell.edu/bna/species/380. [Google Scholar]
- Kahn NWSt, John J, Quinn TW. Chromosome-specific intron size differences in the avian CHD gene provide an efficient method for sex identification in birds. Auk. 1998;115:1074–1078. [Google Scholar]
- Kern M, Bacon W, Long D, Cowie RJ. Possible roles for corticosterone and critical size in the fledging of nestling pied flycatchers. Physiol Biochem Zool. 2001;74:651–659. doi: 10.1086/322927. [DOI] [PubMed] [Google Scholar]
- Kitaysky AS, Kitaiskaia EV, Wingfield JC, Piatt JF. Dietary restriction causes chronic elevation of corticosterone and enhances stress response in red-legged kittiwake chicks. J Comp Physiol B. 2001a;171:701–709. doi: 10.1007/s003600100230. [DOI] [PubMed] [Google Scholar]
- Kitaysky AS, Wingfield JC, Piatt JF. Corticosterone facilitates begging and affects resource allocation in the black-legged kittiwake. Behav Ecol. 2001b;12:619–625. [Google Scholar]
- Kitaysky AS, Kitaiskaia EV, Piatt JF, Wingfield JC. Benefits and costs of increased levels of corticosterone in seabird chicks. Horm Behav. 2003;43:140–149. doi: 10.1016/s0018-506x(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Lambrechts MM, Adriaensen F, Ardia DR, Artemyev AV, Atiénzar F, Bańbura J, Barba E, Bouvier J-C, Camprodon J, Cooper CB, et al. The design of artificial nestboxes for the study of secondary hole-nesting birds: a review of methodological inconsistencies and potential biases. Acta Ornithol. 2010;45:1–26. [Google Scholar]
- Loiseau C, Sorci G, Dano S, Chastel O. Effects of experimental increase of corticosterone levels on begging behavior, immunity and parental provisioning rate in house sparrows. Gen Comp Endocrinol. 2008;155:101–108. doi: 10.1016/j.ygcen.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Lothery CJ, Thompson CF, Lawler ML, Sakaluk SK. Food supplementation fails to reveal a trade-off between incubation and self-maintenance in female house wrens. PLoS One. 2014;9:e106260. doi: 10.1371/journal.pone.0106260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love OP, Bird DM, Shutt LJ. Plasma corticosterone in American kestrel siblings: effects of age, hatching order, and hatching asynchrony. Horm Behav. 2003;43:480–488. doi: 10.1016/s0018-506x(03)00033-3. [DOI] [PubMed] [Google Scholar]
- Love OP, Williams TD. Plasticity in the adrenocortical response of a free-living vertebrate: the role of pre- and post-natal developmental stress. Horm Behav. 2008a;54:496–505. doi: 10.1016/j.yhbeh.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Love OP, Williams TD. The adaptive value of stress-induced phenotypes: effects of maternally derived corticosterone on sex-biased investment, cost of reproduction, and maternal fitness. Am Nat. 2008b;172:E135–E149. doi: 10.1086/590959. [DOI] [PubMed] [Google Scholar]
- Love OP, Wynne-Edwards KE, Bond L, Williams TD. Determinants of within- and among-clutch variation in yolk corticosterone in the European starling. Horm Behav. 2008;53:104–111. doi: 10.1016/j.yhbeh.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Magrath RD. Nestling weight and juvenile survival in the blackbird, Turdus merula. J Anim Ecol. 1991;60:335–351. [Google Scholar]
- Martin LB, Gilliam J, Han P, Lee K, Wikelski M. Corticosterone suppresses cutaneous immune function in temperate but not tropical house sparrows, Passer domesticus. Gen. Comp Endocrinol. 2005;140:126–135. doi: 10.1016/j.ygcen.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Martin LB, Han P, Lewittes J, Kuhlman JR, Klasing KC, Wikelski M. Phytohemagglutinin-induced skin swelling in birds: histological support for a classic immunoecological technique. Funct Ecol. 2006;20:290–299. [Google Scholar]
- Mathis A, Ferrari MCO, Windel N, Messier F, Chivers DP. Learning by embryos and the ghost of predation future. Proc R Soc B. 2008;275:2603–2607. doi: 10.1098/rspb.2008.0754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe NB, Monaghan P. Compensation for a bad start: grow now, pay later? Trends Ecol Evol. 2001;16:254–260. doi: 10.1016/s0169-5347(01)02124-3. [DOI] [PubMed] [Google Scholar]
- Moore MC, Johnston GIH. Toward a dynamic model of deposition and utilization of yolk steroids. Integr Comp Biol. 2008;48:411–418. doi: 10.1093/icb/icn079. [DOI] [PubMed] [Google Scholar]
- Müller C, Jenni-Eiermann S, Jenni L. Effects of a short period of elevated circulating corticosterone on postnatal growth in free-living Eurasian kestrels Falco tinnunculus. J Exp Biol. 2009;212:1405–1412. doi: 10.1242/jeb.024455. [DOI] [PubMed] [Google Scholar]
- Naef-Daenzer B, Widmer F, Nuber M. Differential post-fledging survival of great and coal tits in relation to their condition and fledging date. J Anim Ecol. 2001;70:730–738. [Google Scholar]
- Olson CR, Vleck CM, Vleck D. Periodic cooling of bird eggs reduces embryonic growth efficiency. Physiol Biochem Zool. 2006;79:927–936. doi: 10.1086/506003. [DOI] [PubMed] [Google Scholar]
- Overskaug K, Bolstad JP, Sunde P, Øien IJ. Fledgling behavior and survival in northern tawny owls. Condor. 1999;101:169–174. [Google Scholar]
- Paitz RT, Bowden RM. A proposed role of the sulfotransferase/sulfatase pathway in modulating yolk steroid effects. Integr Comp Biol. 2008;48:419–427. doi: 10.1093/icb/icn034. [DOI] [PubMed] [Google Scholar]
- Paitz RT, Bowden RM, Casto JM. Embryonic modulation of maternal steroids in European starlings (Sturnus vulgaris) Proc R Soc B. 2011;278:99–106. doi: 10.1098/rspb.2010.0813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pakkala JJ, Norris DR, Sedinger JS, Newman AEM. Experimental effects of early-life corticosterone on the hypothalamic-pituitary-adrenal axis and pre-migratory behaviour in a wild songbird. Funct Ecol. in press. [Google Scholar]
- Pankhurst NW. The endocrinology of stress in fish: an environmental perspective. Gen Comp Endocrinol. 2011;170:265–275. doi: 10.1016/j.ygcen.2010.07.017. [DOI] [PubMed] [Google Scholar]
- Porter TE. Regulation of pituitary somatotroph differentiation by hormones of peripheral endocrine glands. Domest Anim Endocrinol. 2005;29:52–62. doi: 10.1016/j.domaniend.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Quillfeldt P, Poisbleau M, Chastel O, Masello JF. Corticosterone in thin-billed prion Pachyptila belcheri chicks: diel rhythm, timing of fledging and nutritional stress. Naturwissenschaften. 2007;94:919–925. doi: 10.1007/s00114-007-0275-6. [DOI] [PubMed] [Google Scholar]
- Råberg L, Grahn M, Hasselquist D, Svensson E. On the adaptive significance of stress-induced immunosuppression. Proc R Soc Lond B. 1998;265:1637–1641. doi: 10.1098/rspb.1998.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raouf SA, Smith LC, Brown MB, Wingfield JC, Brown CR. Glucocorticoid hormone levels increase with group size and parasite load in cliff swallows. Anim Behav. 2006;71:39–48. [Google Scholar]
- Rensel MA, Wilcoxen TE, Schoech SJ. The influence of nest attendance and provisioning on nestling stress physiology in the Florida scrub-jay. Horm Behav. 2010a;57:162–168. doi: 10.1016/j.yhbeh.2009.10.009. [DOI] [PubMed] [Google Scholar]
- Rensel MA, Boughton RK, Schoech SJ. Development of the adrenal stress response in the Florida scrub-jay (Aphelocoma coerulescens) Gen Comp Endocrinol. 2010b;165:55–261. doi: 10.1016/j.ygcen.2009.07.002. [DOI] [PubMed] [Google Scholar]
- Romero LM, Reed JM. Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp Biochem Physiol A. 2005;140:73–79. doi: 10.1016/j.cbpb.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Rubolini D, Romano M, Boncoraglio G, Ferrari RP, Martinelli R, Galeotti P, Fasola M, Saino N. Effects of elevated egg corticosterone levels on behavior, growth, and immunity of yellow-legged gull (Larus michahellis) chicks. Horm Behav. 2005;47:592–605. doi: 10.1016/j.yhbeh.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Rush SA, Stutchbury BJM. Survival of fledgling hooded warblers (Wilsonia citrina) in small and large forest fragments. Auk. 2008;125:183–191. [Google Scholar]
- Saino N, Suffritti C, Martinelli R, Rubolini D, Møller AP. Immune response covaries with corticosterone plasma levels under experimentally stressful conditions in nestling barn swallows (Hirundo rustica) Behav Ecol. 2003;14:318–325. [Google Scholar]
- Saino N, Romano M, Ferrari RP, Martinelli R, Møller AP. Stressed mothers lay eggs with high corticosterone levels which produce low-quality offspring. J Exp Zool. 2005;303A:998–1006. doi: 10.1002/jez.a.224. [DOI] [PubMed] [Google Scholar]
- Sakaluk SK, Wilson AJ, Bowers EK, Johnson LS, Masters BS, Johnson BGP, Vogel LA, Forsman AM, Thompson CF. Genetic and environmental variation in condition, cutaneous immunity, and haematocrit in house wrens. BMC Evol Biol. 2014;14:242. doi: 10.1186/s12862-014-0242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Romero LM, Munck AU. How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- Schmidt KL, MacDougall-Shackleton EA, MacDougall-Shackleton SA. Developmental stress has sex-specific effects on nestling growth and adult metabolic rates but no effect on adult body size or body composition in song sparrows. J Exp Biol. 2012;215:3207–3217. doi: 10.1242/jeb.068965. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Rensel MA, Bridge ES, Boughton RK, Wilcoxen TE. Environment, glucocorticoids, and the timing of reproduction. Gen Comp Endocrinol. 2009;163:201–207. doi: 10.1016/j.ygcen.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Schoech SJ, Rensel MA, Heiss RS. Short- and long-term effects of developmental corticosterone exposure on avian physiology, behavioral phenotype, cognition, and fitness: a review. Curr Zool. 2011;57:514–530. [Google Scholar]
- Schwabl H. Developmental changes and among-sibling variation of corticosterone levels in an altricial avian species. Gen Comp Endocrinol. 1999;116:403–408. doi: 10.1006/gcen.1999.7379. [DOI] [PubMed] [Google Scholar]
- Sims CG, Holberton RL. Development of the corticosterone stress response in young northern mockingbirds (Mimus polyglottos) Gen Comp Endocrinol. 2000;119:193–201. doi: 10.1006/gcen.2000.7506. [DOI] [PubMed] [Google Scholar]
- Sopinka NM, Hinch SG, Healy SJ, Harrison PM, Patterson DA. Egg cortisol treatment affects the behavioural response of coho salmon to a conspecific intruder and threat of predation. Anim Behav. 2015;104:115–122. [Google Scholar]
- Stier KS, Almasi B, Gasparini J, Piault R, Roulin A, Jenni L. Effects of corticosterone on innate and humoral immune functions and oxidative stress in barn owl nestlings. J Exp Biol. 2009;212:2085–2091. doi: 10.1242/jeb.024406. [DOI] [PubMed] [Google Scholar]
- Styrsky JD, Eckerle KP, Thompson CF. Fitness-related consequences of egg mass in nestling house wrens. Proc R Soc Lond. 1999;266:1253–1258. [Google Scholar]
- Sullivan KA. Predation and starvation: age-specific mortality in juvenile juncos (Junco phaenotus) J Anim Ecol. 1989;58:275–286. [Google Scholar]
- Taves MD, Losie JA, Rahim T, Schmidt KL, Sandkam BA, Ma C, Silversides FG, Soma KK. Locally elevated cortisol in lymphoid organs of the developing zebra finch but not Japanese quail or chicken. Dev Comp Immunol. 2016;54:116–125. doi: 10.1016/j.dci.2015.09.004. [DOI] [PubMed] [Google Scholar]
- Thompson CF, Flux JEC. Body mass and lipid content at nest-leaving of European starlings in New Zealand. Ornis Scand. 1988;19:1–6. [Google Scholar]
- Thompson CF, Flux JEC, Tetzlaff VT. The heaviest nestlings are not necessarily the fattest nestlings. J Field Ornithol. 1993;64:426–432. [Google Scholar]
- Vakili H, Cattini PA. The hidden but positive role for glucocorticoids in the regulation of growth hormone-producing cells. Mol Cell Endocrinol. 2012;363:1–9. doi: 10.1016/j.mce.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Vassallo BG, Paitz RT, Fasanello VJ, Haussmann MF. Glucocorticoid metabolism in the in ovo environment modulates exposure to maternal corticosterone in Japanese quail embryos (Coturnix japonica) Biol Lett. 2014;10:20140502. doi: 10.1098/rsbl.2014.0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Engelhardt N, Henriksen R, Groothuis TGG. Steroids in chicken egg yolk: metabolism and uptake during early embryonic development. Gen Comp Endocrinol. 2009;163:175–183. doi: 10.1016/j.ygcen.2009.04.004. [DOI] [PubMed] [Google Scholar]
- Wada H. Glucocorticoids: mediators of vertebrate ontogenetic transitions. Gen Comp Endocrinol. 2008;156:441–453. doi: 10.1016/j.ygcen.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Wada H, Hahn TP, Breuner CW. Development of stress reactivity in white-crowned sparrow nestlings: total corticosterone response increases with age, while free corticosterone response remains low. Gen Comp Endocrinol. 2007;150:405–413. doi: 10.1016/j.ygcen.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Wada H, Breuner CW. Transient elevation of corticosterone alters begging behavior and growth of white-crowned sparrow nestlings. J Exp Biol. 2008;211:1696–1703. doi: 10.1242/jeb.009191. [DOI] [PubMed] [Google Scholar]
- Wada H, Cristol DA, McNabb FMA, Hopkins WA. Suppressed adrenocortical responses and thyroid hormone levels in birds near a mercury-contaminated river. Environ Sci Technol. 2009;43:6031–6038. doi: 10.1021/es803707f. [DOI] [PubMed] [Google Scholar]
- Walker BG, Wingfield JC, Boersma PD. Age and food deprivation affects expression of the glucocorticosteroid stress response in Magellanic penguin (Spheniscus magellanicus) chicks. Physiol Biochem Zool. 2005;78:78–89. doi: 10.1086/422769. [DOI] [PubMed] [Google Scholar]
- Wingfield JC. Modulation of the adrenocortical response to stress in birds. In: Davey KG, Peter RE, Tobe SS, editors. Perspectives in comparative endocrinology. Ottawa: National Research Council; 1994. pp. 520–528. [Google Scholar]
- Wingfield JC. Ecological processes and the ecology of stress: the impacts of abiotic environmental factors. Funct Ecol. 2013;27:37–44. [Google Scholar]
- Young BE. An experimental analysis of small clutch size in tropical house wrens. Ecology. 1996;77:472–488. [Google Scholar]