Abstract
Epidemiological studies have linked increased incidence of inflammatory diseases and intestinal cancers in the developed parts of the world to the consumption of diets poor in dietary fibers and rich in refined carbohydrates. Gut bacteria residing in the intestinal lumen exclusively metabolize dietary fibers. Butyrate, propionate and acetate, which are collectively called short-chain fatty acids (SCFAs), are generated by fermentation of dietary fibers by gut microbiota. Evidences indicate that SCFAs are key players in regulating beneficial effect of dietary fibers and gut microbiota on our health. SCFAs interact with metabolite-sensing G protein-coupled receptors GPR41, GPR43 and GPR109A expressed in gut epithelium and immune cells. These interactions induce mechanisms that play a key role in maintaining homeostasis in gut and other organs. This review summarizes the protective roles of GPR41, GPR43 and GPR109A in dietary fibers-, gut microbiota- and SCFAs- mediated suppression of inflammation and carcinogenesis in gut and other organs.
Keywords: Dietary fibers, short-chain fatty acids, short chain fatty acid receptors, gut microbiota, inflammation and cancer
1. Introduction
Diet has a profound and long-lasting effect on our health. The effects of specific dietary components such as dietary fibers goes beyond their nutritional value and positively influence multiple aspects of human health. Industrial development in modern era has been associated with a change in life style leading to consumption of refined foods and decreased intake of whole grains, fruits and vegetables, which are major source of dietary fibers. The change in life style in the recent years has coincided with increase in inflammatory diseases such as ulcerative colitis, Crohn’s disease (together called as inflammatory bowel diseases or IBDs), allergies, intestinal cancers and others (Hansen, et al., 2012; Hou, Abraham, & El-Serag, 2011; Schatzkin, et al., 2007; Thorburn, Macia, & Mackay, 2014). Ever since these observations have been made, role of dietary fibers and underlying mechanisms in the prevention of inflammatory diseases and cancers have been extensively investigated. Bacteria residing in the gut metabolize dietary fibers into SCFAs (Hamer, et al., 2008). SCFAs are generated at ~ 100mM concentration in the colonic lumen at an approximate ratio of 60:20:20 for acetate, propionate and butyrate, respectively (Ganapathy, Thangaraju, Prasad, Martin, & Singh, 2013). Significance of SCFAs in promotion of health is strengthened by two lines of epidemiological findings: 1) reduction in specific constituents of gut microbiota that play a key role in fermentation of dietary fibers into butyrate in feces of individuals with colorectal cancer and ulcerative colitis (Frank, et al., 2007; Wang, et al., 2012), 2) lower intake of dietary fibers is associated with enhanced risk for development of ulcerative colitis, Crohn disease and colorectal cancers (Hansen, et al., 2012; Hou, et al., 2011; Schatzkin, et al., 2007). Concentrations of SCFAs are highest in colonic lumen and almost negligible in peripheral blood (Bergman, 1990) suggesting that they act locally on epithelium and immune cells present in the colon to induce health-promoting effects. The objective of this review is to provide current evidences, which demonstrate that GPR41, GPR43 and GPR109A act as molecular links between gut microbiota, dietary fibers, SCFAs, and promotion of health.
2. Dietary Fibers
Dietary fibers are carbohydrates that are indigestible in small intestine of mammals due to lack of enzymes. Dietary fibers are complex mixture of branched and unbranched polysaccharides composed of short to long chains of monosaccharides. Different dietary fibers differ in their ability to undergo fermentation in colonic lumen (Eswaran, Muir, & Chey, 2013). Soluble dietary fibers such as oligofructose, inulin, psyllium, and cornstarch have higher fermentability, and thus generate higher amounts of SCFAs. In contrast, insoluble dietary fibers such as cellulose and hemicellulose have low fermentability and, therefore, contribute minimally to SCFA production in colon. In general, dietary fibers with smaller and unbranched chains tend to be more soluble. Dietary fibers with long-chain carbohydrates have either high solubility (e.g. inulin), intermediate solubility (e.g. psyllium) or no solubility (cellulose). Due to their high fermentability, soluble dietary fibers act as energy source for selected group of gut bacteria, possess ability to promote growth of beneficial microorganisms in intestine and thus are used as “prebiotics” (Gibson, et al., 2010).
3. Gut Microbiota
Human intestinal lumen is inhabited by trillions of microorganisms collectively termed as gut microbiota (O'Hara & Shanahan, 2006; Tsai & Coyle, 2009). Metabolic activity of the gut microbiota is qualitatively and quantitatively similar to an organ. In addition, various molecular, cellular, and metabolic component of gut microbiota constantly interact with our organs and impact our health. Therefore, gut microbiota has also been called as forgotten organ (O'Hara & Shanahan, 2006). Gut microbiota consists of ~100-1000 different bacterial species. Collective genome of gut microbiota contain ~ 150 times more genes than number of genes in our body and therefore have also been refereed to as our second genome (Grice & Segre, 2012). Colonization of gut begins immediately after birth and is a continuously ongoing process through out the life of an individual. Members belonging to phyla Bacteroidetes and Firmicutes dominate the composition of gut mcirobiota, where as members from Actinobacteria, Proteobacteria, Fusobacteria, Cyanobacteria and Verrucomicrobia are minor constituents of gut microbiota (Eckburg, et al., 2005; Sekirov, Russell, Antunes, & Finlay, 2010). Relationship between host and most of the gut microbiota has evolved as mutualistic or symbiotic (Backhed, Ley, Sonnenburg, Peterson, & Gordon, 2005; Mazmanian, Round, & Kasper, 2008). Multiple health benefits of symbiotic bacteria or symbionts on human health have been recognized and well appreciated. Positive effects of gut microbiota on human health includes providing vitamins and energy source to host, helping in development of intestinal tissue and immune system, limiting inflammatory responses at local and distal organs, decreasing carcinogenesis, and inhibiting colonization of gut with pathogenic microorganisms (Y. K. Lee & Mazmanian, 2010; Ley, 2010; Nicholson, et al., 2012; Rakoff-Nahoum, Paglino, Eslami-Varzaneh, Edberg, & Medzhitov, 2004; Vijay-Kumar, et al., 2010). Notwithstanding with evolution of symbiotic bacteria in gut, certain bacterial inhabitants of gut which are called pathobionts exert disease-promoting effects on hosts, such as inducing inflammation, carcinogenesis and obesity (Chow, Tang, & Mazmanian, 2011; Elinav, et al., 2011; Garrett, et al., 2010; Palmer, 2011). Thus, gut microbiota possess properties that both positively and negatively affect health of the host.
Owing to a complex and intricate relationship, a dynamic equilibrium exists between host and gut microbiota, which plays a critical role in maintaining intestinal homeostasis. Within gut microbiota, several distinct bacterial communities live at a certain ratio under steady state condition (Faith, et al., 2013). A change in environmental factors, life style, disease and infections lead to alteration in composition of bacterial communities, and this process is termed as dysbiosis (Carding, Verbeke, Vipond, Corfe, & Owen, 2015). Dysbiosis is present in many inflammatory diseases such as IBD, metabolic syndrome and colorectal cancers. Epidemiological studies have shown a decrease in butyrate-producing gut bacteria, such as those belonging to genus Roseburia and family Lachnospiraceae, in feces of individuals with colon cancer compared to healthy donors. Similarly, feces from individuals with ulcerative colitis, a risk factor for development of colorectal cancers, also contain significantly reduced numbers of butyrate-producing gut bacteria belonging to family Lachnospiraceae (Frank, et al., 2007; Wang, et al., 2012). There are evidences suggesting that dysbiosis may be involved in the development of certain diseases. Transfer of gut microbiota from diseased animals into germ-free or susceptible mice causes pathologies in recipients similar to those present in donors (Garrett, et al., 2007; Kostic, et al., 2013; Shanahan & Quigley, 2014; Turnbaugh, Backhed, Fulton, & Gordon, 2008; Vijay-Kumar, et al., 2010). Similarly, correction of dysbiosis is associated with alleviation of the diseases (Everard, et al., 2013). Collectively, these findings suggest that manipulation of gut microbiota may serve as an attractive target for designing therapeutic modalities for prevention and or treatment of certain diseases.
4. Short-chain fatty acids (SCFAs)
Among SCFAs, butyrate has been extensively investigated for its role in suppression of colonic inflammation and carcinogenesis (Hamer, et al., 2008). Fermentation of dietary fibers into butyrate is a stepwise process, which is facilitated by distinct constituents of gut microbiota. In colon, majority of butyrate-producing bacteria are anaerobes and belong to Clostridium clusters IV and XIVa (Nagano, Itoh, & Honda, 2012). Studies performed in vitro using butyrate-producing colonic bacteria such as Roseburia intestinalis DSM14610 and Anaerostipes caccae DSM14662 (both are member of Clostridium cluster XIVa) show that they are poor fermenters of dietary fibers (Falony, Vlachou, Verbrugghe, & De Vuyst, 2006). Butyrate production was minimum in these cultures even in the presence of dietary fibers. On the other hand Bifidobacterium ferment dietary fibers vigorously to produce acetate, fructose, and lactate but no butyrate. In mixed cultures where both Bifidobacterium and Roseburia intestinalis or Anaerostipes caccae are present, addition of dietary fibers lead to butyrate production (Belenguer, et al., 2006). Mechanistic studies show that when acetate, and fructose, which are released following fermentation of dietary fibers by Bifidobacterium are added to the cultures of R. intestinalis or A. caccae respectively, butyrate production is observed (Falony, et al., 2006). Thus, metabolites generated by Bifidobacterium are used by R. intestinalis or A. caccae for their growth and this process is called cross-feeding. The magnitude at which this cross-feeding exist in vivo remains poorly defined. In several human and animal studies, dietary fibers regularly increase number of Bifidobacterium in gut (Gibson, et al., 2010). Efforts have made to analyze the effect of dietary fibers on numbers of butyrate-producing gut bacteria. The protein product of Butyryl-CoA:acetate CoA transferase (BcoA) gene catalyzes the critical final step in butyrate production among gut microbiota. A recent human study found that dietary fibers enhanced the co-occurrence between potential butyrate-producers such as Eubacterirum rectale etc and bacteria associated with complex carbohydrate utilization such as Oscillospria guillermondii (O'Keefe, et al., 2015). This study showed a significant increase in copy number of BcoA. However, the same study failed to find a meaningful change in number of any single butyrate producing bacterial species following dietary fiber intake. Therefore, a significant increase in BcoA gene is most likely caused by sum of all increases in individual butyrate producers.
After being produced in colonic lumen, SCFAs are transported across the epithelium by diffusion, low-affinity transport mechanism such as HCO3−/SCFA exchange [KM 25 mM], medium-affinity transport mechanism involving monocarboxylate transporter 1 [MCT1, KM 2.6 mM] or via high-affinity transport mediated by sodium-coupled monocarboxylate transporter 1 (SMCT1 or SLC5A8, KM ~50 μM) into colon (Charney, Micic, & Egnor, 1998; Hadjiagapiou, Schmidt, Dudeja, Layden, & Ramaswamy, 2000; Mascolo, Rajendran, & Binder, 1991; Thangaraju, et al., 2008). Once transported into colonic tissue, most of SCFAs are metabolized into lipids or ketone bodies such as β-hydroxybutyrate or acetoacetate by colonic epithelium (Bergman, 1990; Zambell, Fitch, & Fleming, 2003), resulting in significant drop in SCFAs exiting the colonic tissue into portal circulation. SCFAs reaching the liver are metabolized into lipids. Therefore, negligible amount of butyrate (4-10 μM), propionate (~4-10 μM) and acetate (~100-μM) are present in peripheral blood (Bergman, 1990; Cummings, Pomare, Branch, Naylor, & Macfarlane, 1987). EC50 of acetate to induce Ca++ flux through GPR43 is ~ 52 μM, and thus only acetate is present in concentration high enough to induce GPR43-dependent signaling in peripheral tissue (Brown, et al., 2003).
Understanding the complex molecular mechanisms underlying protective effects of SCFAs on colonic health has been difficult because they interact with multiple signaling molecules expressed by colonic epithelium and immune cells (Ganapathy, et al., 2013). Most widely studied targets of butyrate and propionate are histone deacetylases or HDACs. Classical HDACs is a family of proteins that consist of 2 groups. Group I HDACs contain mammalian HDAC-1, -2 -3 and -8, whereas group II HDACs comprises of mammalian HDAC4, -5, -6, -7, -9 and -10. Butyrate and propionate specifically inhibit activity of HDAC-1 and -3 (Thangaraju, Carswell, Prasad, & Ganapathy, 2009). Inhibition of HDACs’ activity leads to increase in acetylation at specific lysine residues in histones and thus decreases positive charge on histones. The loss of positive charge inhibits binding of histones to the negatively charged DNA, resulting in open structure of DNA/chromatin, which becomes accessible to transcriptional machinery to initiate transcription of genes. Thus, in principle, inhibition of histone acetylation should result in increase in transcription of genes, however, treatment of cells with butyrate results in both induction and repression of specific genes. Nonetheless, many studies have demonstrated a positive correlation between the ability of butyrate to inhibit HDACs and to induce apoptosis and/or cell cycle arrest in colon cancer cells (Thangaraju, Carswell, et al., 2009). Changes in histone acetylation leads to global change in chromatin structure, which could potentially affect expression pattern of the several genes. How global change in chromatin structure induced by butyrate/propionate-mediated inhibition of HDACs results in expression or repression of specific genes needs to be explored in greater detail.
5. SCFA receptors
Free fatty acid receptor 3 (FFAR3 or GPR41), FFAR2 (GPR43) and GPR109A (also known as hydroxycarboxylic acid receptor 2 or HCA2) are expressed on cell surface and have been explored as mediators of biological effects of dietary fibers and SCFAs (Table 1). While mouse genome has only one gene for Gpr109 (called Gpr109a), human genome contains two, GPR109A and GPR109B. GPR109B originated from duplication of GPR109A and thus these genes are highly similar (Ahmed, Tunaru, & Offermanns, 2009). The translatable parts of the genes encoding the SCFA receptors are intron-less sequences. Using rapid amplification of cDNA ends (RACE), a 5′ untranslated exon has been identified in human GPR43 (Ang, Er, & Ding, 2015). GPR43 and GPR109A polypeptide are 330 and 363 amino acids long and thus their expected molecular weight is approximately 37 and 40 kDa, respectively. Detection of FLAG signal with specific antibody on total cell lysates of 293 cells expressing FLAG-tagged GPR43 and GPR109A and resolved on SDS-PAGE revealed the spread of FLAG-specific signal from top of resolving gel (MW > 250 kDa) to the middle of the gel (~50 kDa). Higher intensity was present in upper most part of the gel (our unpublished data). These findings suggest that GPR43 and GPR109A undergo extensive posttranslational modifications at least in some cells. Therefore, it is very critical to identify these modifications and their effect on agonist binding to fully appreciate the physiological importance and function of SCFA receptors.
Table 1.
Short chain fatty acid receptors, agonists, signaling expression and physiological functions
| Receptor (alias) | Ligands | Signaling | Expression | Biological Effects | References |
|---|---|---|---|---|---|
| GPR41 (G protein- coupled receptor 41; free fatty acid receptor 3; GPCR41; FFAR3; FFA3R; FFA3) |
acetate, propionate, butyrate, |
Gαi/o, β- gustducin |
Pancreas, enteroendocrine cells and enteric neurons |
DC maturation, inhibits gut motility and insulin secretion |
Brown et al., 2003
Le Poul et al., 2003 Samuel et al., 2008 Tang et al., 2015 Tolhurst et al., 2012 Trompette et al., 2014 |
| GPR43 (G ptotein- coupled receptor 43; free fatty acid receptor 2, GPCR43; FFAR2; FFA2R; FFA2) |
acetate, propionate, butyrate, |
Gαi/o, Gαq, β- arrestin-2 |
Adipocytes, enteroendocrine cells, innate immune cells, gut epithelium |
Gut homeostasis, Treg proliferation, inhibits insulin secretion, tumor suppressor, neutrophil chemotaxis, GLP- 1 secretion |
Brown et al., 2003
Hong et al., 2005 Le Poul et al., 2003 Li et al., 2013 Maslowski et al., 2009 Sina et al 2009 Smith et al., 2013 Tang et al., 2015 Tolhurst et al., 2012 |
| GPR109A (G protein-coupled receptor 109A; hydroxycarboxylic acid receptor; nicotinic acid receptor; GPCR019A; HCAR2; HCA2; NIACR1; HM74A) |
niacin, β- hydroxybutyrate, butyrate |
Gαi/o, β- arrestin-1 |
Adipocytes, innate immune cells, intestinal epithelium |
homeostasis of colonic Treg cells, inhibits lipolysis, atherosclerosis, and inflammation in brain |
Chen et al., 2014
Lukasova et al., 2011 Rahman et al., 2014 Singh et al., 2014 Taggart et al., 2005 Tunaru et al., 2005 |
5.1 Tissue distribution of SCFA receptors
Enteric neurons and intestinal leukocytes express GPR41 and GPR43 respectively, while intestinal endocrine cells express both GPR43 and GPR41 (Kaji, Karaki, & Kuwahara, 2014; Karaki, et al., 2006; Karaki, et al., 2008; Tazoe, et al., 2009). White adipose tissue express GPR43, whereas expression of GPR41 on adipose tissue is questionable (Table 1) (Hong, et al., 2005; Le Poul, et al., 2003). GPR109A is expressed in adipose tissue, innate immune cells, intestinal epithelium, keratinocytes and retinal-pigmented epithelium (Table 1) (Ganapathy, et al., 2013; Singh, et al., 2014). Gpr109a expression is silenced in colons of germ-free (GF) and antibiotic-treated mice. Tributyrin, a butyrate prodrug, induces Gpr109a expression in antibiotic-treated animals, which suggests that Gpr109a expression is regulated by gut microbiota (G. Cresci, Nagy, & Ganapathy, 2013; G. A. Cresci, Thangaraju, Mellinger, Liu, & Ganapathy, 2010).
5.2 Ligand specificity of SCFA receptors
All SCFA receptors are specific to deprotonated fatty acids. Esters of the fatty acids are unable to bind to the receptors, suggesting that positively charged amino acids within transmembrane region of SCFA receptors mediate binding of SCFA to these receptors (Le Poul, et al., 2003; Tunaru, Lattig, Kero, Krause, & Offermanns, 2005). GPR41 and GPR43 share 43% amino acid identity between them (Stoddart, Smith, Jenkins, Brown, & Milligan, 2008) and bind to all three SCFAs, acetate, propionate and butyrate as well as pentanoate, a medium-chain fatty acid (Table 1). While GPR43 has preference for shorter chain fatty acids such as acetate and propionate, the reverse is true for GPR41 (Le Poul, et al., 2003). Thus, GPR41 shows better binding to pentanoate, butyrate and propionate than acetate. GPR109B and GPR109B shows 96% identity at protein level, however GPR109B does not bind to any short chain fatty acids (Taggart, et al., 2005). In contrast to GPR41 and GPR43, among SCFAs, only butyrate activates GPR109A (Taggart, et al., 2005) (Thangaraju, Cresci, et al., 2009). Beta-hydroxybutyrate, produced during starvation as a result of ketogenesis is also a ligand of GPR109A (Table 1) (Taggart, et al., 2005). Colonic epithelium has been shown to synthesize β-hydroxybutyrate from butyrate (Roediger, 1982). Thus, in colon GPR109A can be activated by either butyrate or β-hydroxybutyrate. It will be attractive to investigate whether some of the beneficial effects of butyrate on colon are mediated via β-hydroxybutyrate. Originally, GPR109A was identified as a receptor for vitamin B3 or niacin (Tunaru, et al., 2003). Under normal conditions, steady state level of niacin in plasma is too low to bind and activate GPR109A. However, after consumption of pharmacological dose of niacin, its concentration in plasma is high enough to activate GPR109A (Tunaru, et al., 2003). SCFAs are detected in ileum too at a concentration of ~ 10 mM and at present it is unclear whether they are generated locally in ileum or it is due to reflux from colon/caecum. Notably, concentration of SCFAs present in ileum is more than sufficient to activate signaling through GPR41 and GPR43 (Le Bourgot, et al., 2014).
5.3 Signaling mechanisms of SCFA receptors
GPR41, GPR43 and GPR109A are coupled with Gi/o and therefore, following ligand binding, they diminish activity of adenylate cyclase, leading to inhibition of cyclic adenosine monophosphate (cAMP) production via pertussis toxin (PTX)-sensitive mechanism (Table 1). GPR43 also exhibits Gq/11-dependent activity, such as inducing influx of [Ca++] into cytoplasm. Additionally, there is sufficient evidence to indicate the involvement of other signaling pathways too by these receptors, such as engagement of β-arrestins by GPR109A and GPR43, and gustducin by GPR41 (S. U. Lee, et al., 2013; Li, Kokrashvili, Mosinger, & Margolskee, 2013; Walters, et al., 2009). Signaling mechanisms used by these receptors may be cell-type and ligand specific. For example, in adipocytes, GPR109A signaling decreases cAMP, whereas in Langerhans cells and macrophages, its activation leads to production of prostaglandin D2 (PGD2). GPR109A -agonist MK-0354 inhibits adenylate cyclase in adipocytes, while it does not transduce any signal in Langerhans cells or macrophages (Gaidarov, et al., 2013). Gpr43 couples with Gα12, Gα13 and Gα14 in S. cerevisiae (Brown, et al., 2003). Taken together, these observations suggest that, depending on coupling of different subunits of G proteins or β-arrestins, signaling through SCFA receptors could result in different outcomes in different cell types.
6. SCFA receptors in inflammation and cancer
6.1 SCFA receptor and intestinal inflammation
Opposing roles of Gpr43 in suppression of colonic inflammation have been reported. Mice lacking Gpr43 (Gpr43−/−) are hypersusceptible to colonic inflammation induced by either dextran sulfate sodium (DSS) or trinitrobenzene sulfonic acid (TNBS) (Maslowski, et al., 2009). Treatment of neutrophils with acetate treatment reduces surface expression of chemotactic receptors C5aR and CXCR2 and thus inhibits their chemotaxis in a Gpr43-dependent mechanism (Figure 1). Consistent with this, colon of Gpr43−/− animals exhibited higher neutrophil infiltration following DSS treatment than WT mice. Moreover, acetate suppresses DSS-induced colonic inflammation and wasting diseases in germ-free (GF) animals. The ability of acetate to suppress colonic inflammation is significantly attenuated in Gpr43−/− animals. This indicates that Gpr43 signaling plays a critical role in SCFAs-induced suppression of colonic inflammation (Maslowski, et al., 2009). NACHT, LRR and PYD domains-containing protein 3 (NLRP3) plays a key role in the activation of inflammasome, resulting in production of mature IL-18 and IL-1β from corresponding pro-peptides (Zaki, Boyd, et al., 2010). Binding of agonist to Gpr43 induces K+ efflux, leading to activation of Nlrp3 inflammasome (Figure 1) (Macia, et al., 2015). Mice defective in Nlrp3 inflammasome activation are at risk of developing colonic inflammation and colon cancers (Dupaul-Chicoine, et al., 2010; Zaki, Boyd, et al., 2010; Zaki, Vogel, Body-Malapel, Lamkanfi, & Kanneganti, 2010). Notably, dietary fibers-mediated activation of Nlrp3 is also Gpr43 dependent (Figure 1) (Macia, et al., 2015). In contrast to these findings, a different study showed that in mouse model of chronic colitis induced by DSS, Gpr43−/− mice are more resistant to develop colonic inflammation than their WT counterparts (Sina, et al., 2009). In TNBS-, ethanol- and Citrobacter rodenticum- induced colonic inflammation models, Gpr43−/− and Gpr41−/− animals exhibited significantly reduced colonic inflammation than WT mice, suggesting a pro-inflammatory role of Gpr41 and Gpr43 in colon (Kim, Kang, Park, Yanagisawa, & Kim, 2013). What are the reasons for these conflicting findings? Presence of different gut microbiota in different facilities may be one of the reasons. Following Citrobacter rodenticum infection, WT mice showed significantly enhanced impairment of gut epithelial barrier function and higher bacterial load in the tissues than Gpr41−/− or Gpr43−/− mice at earlier time points (4-8 days post infection, whereas reverse was true at later time points (20-24 days post infection) (Kim, et al., 2013). This finding argues that Gpr41 and Gpr43 may play a critical role in colonization and/or clearance of some if not all of the gut bacteria, which may either promote or suppress colonic inflammation. Later it was found that Gpr43−/− mice co-housed with WT mice were protected from DSS-induced colonic inflammation than singly housed counterparts. This study suggested that during cohousing some unknown good gut bacteria got transferred from WT mice to Gpr43−/− mice and offered protection against DSS-induced colonic inflammation. Gpr109a−/− mice exhibit more severe colonic inflammation, and diarrhea in DSS-induced model of colitis than WT mice (Singh, et al., 2014). Gpr109a ligands butyrate and niacin also induced transcription of IL-18, a wound healing cytokine, in colonic epithelium (Singh, et al., 2014). Similar to other mouse strains with reduced levels of IL-18 in their colon, feces of Gpr109a−/− mice harbor drastically elevated number of Prevotellaceae family of bacteria than WT mice (Singh, et al., 2014). Gpr109a agonists butyrate and niacin reduced LPS-induced NF-κB activation in colons of WT mice (Figure 1). Inhibitory effect of butyrate and niacin was dependent on GPR109A, because these agonists inhibited LPS-induced NF-κB activation in colon cancer cell lines KM12L4 and HCT116 transfected with GPR109A whereas they failed to do so in parental cells, which do not express GPR109A (Thangaraju, Cresci, et al., 2009). Taken together, these findings suggest that GPR41, GPR43 and GPR109A induce non-overlapping pathways to regulate gut microbial ecology and/or intestinal inflammation.
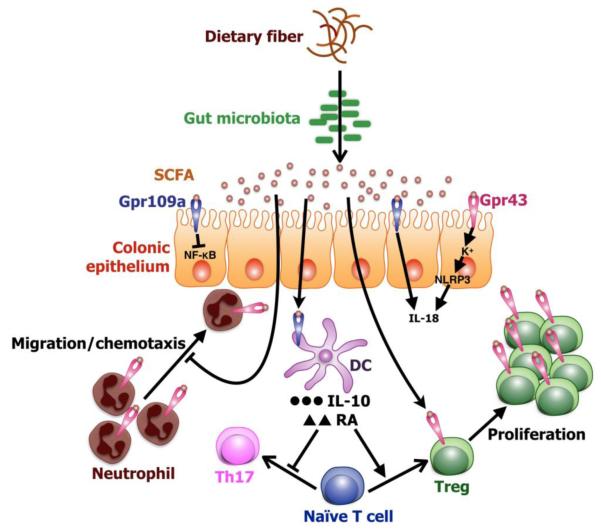
Figure 1.
SCFA receptors link dietary fibers and gut microbiota to intestinal homeostasis. A simplified model showing regulation of gut homeostasis by SCFA receptors. Beneficial gut bacteria (symbionts) ferment dietary fibers into SCFAs. Butyrate induces production of IL-10 and retinoic acid (RA) by dendritic cells. These DCs stimulate conversion of naïve T cells into Treg cells and suppress generation of Th17 cells. Activation of Gpr43 on Treg cells by SCFAs induces Treg cells proliferation. Treg cells are known to suppres colonic inflammation and carcinogenesis, where as Th17 cells promote inflammation and carcinogenesis in colon. Additionally, Gpr109a signaling induces transcription of IL-18, whereas Gpr43 signaling induces K+ flux, which activates Nlrp3 inflasmmasome resulting in maturation of IL-18 from its pro-peptide. Activation of Gpr43 downregulates expression chemotactic receptor CXCR2 in neutrophils and thus inhbits their chemotaxis. Gpr109a signaling inhbitis activation of NF-κB in colonic epithelium.
6.2 SCFA receptors in homeostasis of colonic Treg cells
T regulatory (Treg) cells represent the most dominant arm of immunosuppressive mechanisms. A qualitative or quantitative defect in Treg cells leads to development of intestinal inflammation in multiple animal models (Huber, et al., 2011; Izcue, Coombes, & Powrie, 2009; Rubtsov, et al., 2008). At the molecular level, Treg cells produce large amount of IL-10 which plays an indispensible role in suppression of intestinal inflammation (Rubtsov, et al., 2008). IL-10 is also required for induction and maintenance of Treg cells. Oral administration of SCFAs increases the number of colonic Treg cells by stimulating their proliferation (Figure 1) (Smith, et al., 2013). Moreover, propionate increased Treg cell numbers in WT mice, whereas it failed to do so in Gpr43−/− animals. However, colons of Gpr43−/− animal contain similar number and frequency of Tregs under normal steady state condition (Smith, et al., 2013). Following recognition of cognate antigen bound to MHC class II protein on the surface of antigen-presenting cells (APCs), depending on signals and cues from microenvironment, naïve T cells differentiate into immunogenic subsets Th1, Th2, Th17 or immunosuppressive Treg cells. When mice were fed with acetate, propionate or butyrate fortified diets, only butyrate-supplemented diet increased Treg cell numbers in colon (Arpaia, et al., 2013; Furusawa, et al., 2013). This increase in Treg cell numbers was confined to neuropillin 1-negative (Nrp1−) subset, suggesting differentiation (induction) of naïve T cells into Treg by butyrate in colon. Production of IL-10 and aldehyde dehydrogenase 1a (Aldh1a), which catalyzes rate limiting step in production of retinoic acid (RA) by intestinal DCs and macrophages empowers them with ability to induce differentiation of naïve T cells into Treg cells (Coombes, et al., 2007; Liu, Tonkonogy, & Sartor, 2011; Sun, et al., 2007). Gpr109a agonists niacin and butyrate induced IL-10 and Aldh1a in DCs and macrophages, which facilitated conversion of naïve T cells into Treg cells (Figure 1) (Singh, et al., 2014). Consistent with this, number and frequency of Treg cells is decreased, whereas those of IL-17a+CD4+ T cells are increased in colons of Gpr109a−/− mice compared to WT mice. These findings suggest that SCFA receptors regulate homeostasis of colonic Treg cells via multiple pathways.
6.3 SCFA receptors as regulators of inflammation in extraintestinal organs
Limited studies have been carried out investigating the role of dietary fibers in regulating inflammation outside the gut. Mice fed with a high-fiber diet developed significantly attenuated allergic inflammation in lung when exposed to house dust mite (HDM) (Trompette, et al., 2014). Propionate treatment also reduced HDM-induced allergic inflammation in lungs of WT and Gpr43−/− mice, but had no effect in Gpr41−/− mice, suggesting a critical role for Gpr41 in dietary fibers/SCFAs-mediated suppression of lung inflammation. SCFAs are present at undetectable levels in lung. Mechanistic experiment showed that SCFAs affected developing macrophages and DCs in bone marrow that, upon homing to lung, were defective in inducing allergic inflammation. Surprisingly, in this model Gpr41−/− and Gpr43−/− mice were less susceptible than their WT counter parts in developing lung inflammation. On the other hand, in systemic priming and ovalbumin (OVA) airway challenge model, Gpr43−/− mice exhibit significantly more lung inflammation than WT mice (Maslowski, et al., 2009). Consistent with a role for Gpr43 in activation of inflammasome, in a mouse model of gout, Gpr43−/− mice were protected against monosodium urate monohydrate (MSU)-induced joint inflammation (Vieira, et al., 2015). Tecfidera (dimethyl fumerate or DMF) is a FDA-approved drug for treatment of individuals with relapsing multiple sclerosis (MS). DMF gets metabolized into monomethyl fumerate (MMF), which is an agonist for GPR109A (Chen, et al., 2014). In experimental autoimmune encephalomyelitis (EAE), which is a mouse model of MS, DMF-mediated protection from immune cell infiltration, and demyelination of the spinal cord was dependent on Gpr109a (Chen, et al., 2014). At mechanistic level, in this model, DMF suppressed neutrophil infiltration to brain via Gpr109a. The ketone body β-hydroxybutyrate (BHB) protects brain from stroke and other neurodegenerative diseases. In a mouse model of stroke, BHB treatment reduced the disease severity in WT mice, whereas same treatment failed to change the outcome of disease in Gpr109a−/− mice (Rahman, et al., 2014). Niacin significantly decreased the atherosclerotic lesions in Ldlr−/− mice fed with high-fat diet and this process was obligatorily dependent on Gpr109a (Lukasova, Malaval, Gille, Kero, & Offermanns, 2011). Bone marrow chimera experiments revealed that Gpr109a expression in hematopoietic cells plays an essential role in the ability of niacin to decrease atherosclerotic lesions in this model. Conflicting data were obtained about role of Gpr43 in regulation of metabolic disorders. Gpr43−/− mice fed with high fat diet (HFD) gained less weight and showed improved glucose tolerance (Bjursell, et al., 2011). Gpr41 induces expression of PYY, an enteroendocrine cell-derived hormone that normally decreases intestinal motility and thus slows down the movement of intestinal contents leading to better extraction of energy from fecal content in colon. Accordingly, Gpr41−/− animals raised in germ free or conventional facilities are leaner than WT mice (Samuel, et al., 2008). In contrast, other studies showed reduced glucose tolerance and higher weight gain in Gpr43−/− mice (Kimura, et al., 2013; Tolhurst, et al., 2012). The protective effects of Gpr43 were mediated by either induction of glucagon-like peptide-1 secretion or suppression of insulin signaling in adipocytes. These opposing findings (about the role of Gpr41 and Gpr43 in regulation of metabolic syndrome) may be related to dysregulated expression of other receptor in mice lacking only one of these receptors, which are co-expressed in many cell types and share overlapping endogenous agonists (Bjursell, et al., 2011). Gpr41 and Gpr43 inhibit insulin secretion by pancreatic beta cells. Mice lacking both Gpr41 and Gpr43 (Gpr41−/−Gpr43−/− mice) exhibit higher insulin levels and better glucose tolerance when fed with high-fat diet (C. Tang, et al., 2015). Collectively, these data suggest that short-chain fatty acids play a key role in regulation of inflammation in organs located outside of gut under normal and pharmacological settings.
6.4 SCFA receptors and intestinal cancers
Expression of GPR109A mRNA is suppressed in primary colon cancer tissues and colon cancer cell lines, and ectopic expression of GPR109A in colon cancer cells induces apoptosis in the presence of GPR109A agonists niacin and butyrate (Thangaraju, Cresci, et al., 2009). In an inflammation-associated colon carcinogenesis model, azoxymethane (AOM, a colon carcinogen) and colonic irritant DSS induced significantly larger and more number of colonic polyps in Gpr109a−/− mice than in WT mice (Singh, et al., 2014). Gpr109a-deficiency also enhanced colon carcinogenesis in ApcMin/+ mice, a genetic model of intestinal carcinogenesis (Singh, et al., 2014). Depletion of microbiota by antibiotics increases susceptibility of mice to DSS-induced colonic inflammation, which may explain enhanced AOM+DSS-induced colon carcinogenesis in antibiotic-treated animals. Niacin administration inhibits both colonic inflammation and carcinogenesis induced by AOM+DSS and this effect is dependent on Gpr109a (Singh, et al., 2014). Consistent with a role for dietary fibers in suppressing colon carcinogenesis, ApcMin/+ mice fed with diet depleted of dietary fibers developed enhanced intestinal cancers. Gpr109a−/− ApcMin/+ mice developed higher number of colonic polyps than ApcMin/+ mice. In addition, depletion of dietary fibers did not change the polyp development in colons of Gpr109a−/− ApcMin/+ mice. Niacin, a Gpr109a agonist, suppressed enhanced intestinal tumorigenesis in ApcMin/+ mice fed with diet depleted of dietary fibers and this effect was obligatorily dependent on Gpr109a. GPR43 expression is lost in human colon cancer cell lines and drastically downregulated in colorectal adenocarcinomas (Y. Tang, Chen, Jiang, Robbins, & Nie, 2011). Expression of GPR43 in colon cancer cell lines resulted in cell cycle arrest and apoptosis following treatment with butyrate and propionate (Y. Tang, et al., 2011). In inflammation-associated colon cancer model, Gpr43−/− animals develop significantly higher number of colonic polyps than WT counter parts (Sivaparasam et al., submitted). Role of Gpr43 in suppression of colon cancer was not limited to chemical-induced carcinogenesis, because it was also observed in Apcmin/+ mice, which is a genetic model of colon carcinogenesis (Sivaparasam et al., submitted for publication). Feeding of animals with inulin-type fructans (ITF), a soluble dietary fiber, reduces accumulation of BaF3 (a leukemia cell line) in liver. Propionate treatment or Gpr43 activation reduced proliferation of BaF3 cells, suggesting that ITF inhibits proliferation of BaF3 via Gpr43 signaling. Both Gpr109a and Gpr43 signal via Gi, and mice lacking Giα2 undergo spontaneous development of colitis and colon cancer (Rudolph, et al., 1995). Therefore, it is important to investigate whether Gpr109a and Gpr43 signaling depends on Giα2. Collectively, these finding demonstrate that Gpr109a and Gpr43 link dietary fibers to the suppression of colon carcinogenesis.
6.5 SCFA receptors as regulators of carcinogenesis in extraintestinal organs
Epidemiological studies indicate that consumption of dietary fibers is associated with reduced risk of carcinogenesis in breast, prostrate and other organs as well. Mammary epithelium cells express GPR109A, whereas primary human breast tumor tissue does not. Forced expression of GPR109A induces apoptosis and cell cycle arrest in breast cancer cell lines. Moreover, Gpr109a deficiency leads to earlier development and enhanced metastasis in MMTV-neu model of breast cancer (Elangovan, et al., 2014). So far there is no report of the presence metabolites of dietary fibers, including SCFAs, in these organs at concentrations high enough to activate their targets. Then, how do dietary fibers inhibit cancer development in these organs? One way to explain these findings is that absence of dietary fibers/SCFAs results in development, differentiation or accumulation of inflammatory immune cells in gut and local lymph nodes. These inflammatory cells then can either travel to target organs or secrete inflammatory molecules that reach target organs where they induce inflammation leading to enhanced carcinogenesis. Infection of Apcmin/+ mice with Helicobacter hepaticus (strain 3B1, ATCC #51449), which only colonizes gut, promotes neoplasia in prostate (Elangovan, et al., 2014). Treatment of these mice with anti-TNF-α antibody suppresses H. hepaticus-mediated neoplasia. Moreover, adoptive transfer of mesenteric lymph node cells from H. hepaticus-infected Apcmin/+ mice promote neoplasia in host Apcmin/+ mice. Dietary fibers are known to increase the number of symbionts at the cost of pathobionts and Gpr43 and Gpr109a are known to influence colonization of gut pathogens and gut microbiota (Gibson, et al., 2010; Kim, et al., 2013; Vieira, et al., 2015). Therefore, it is critical to test whether dietary fibers and SCFA receptor-signaling suppresses carcinogenesis in organs located distant to gut via inhibiting growth of pathobionts and/or inflammatory cells in gut.
7. Therapeutic potentials of SCFA receptors
Recent progress in understanding the role of GPR41, GPR43 and GPR109A in health and disease demonstrate that these receptors do not just regulate inflammation and cancer in intestine, but also influence other gastrointestinal functions, allergies, adipogenesis, central nervous system and cardiovascular health. Therefore, they have been actively explored as a very attractive pharmaceutical target with broad range of therapeutic applications (Bolognini, Tobin, Milligan, & Moss, 2016; Offermanns & Schwaninger, 2015). GPR109A ligand niacin has been used for several decades for improving cardiovascular health. Niacin may improve cardiovascular health via GPR109A-dependent and-independent mechanisms. Deficiency of niacin, a GPR109A agonist leads to spontaneous development of diarrhea, dermatitis and dementia (Hegyi, Schwartz, & Hegyi, 2004). Our finding that Gpr109a−/− mice are at risk of developing colonic inflammation and carcinogenesis, and niacin induces anti-inflammatory and anti-carcinogenic environment in intestine via Gpr109 demonstrates the therapeutic potential for this receptor. GLPG0974, a GPR43-specific antagonist, did not change clinical outcomes over a short period of time in individuals with mild to moderate ulcerative colitis in Phase 2 clinical trial (Bolognini, et al., 2016). Studies have uncovered conflicting data regarding role of these receptors in either promotion or suppression of disease. Therefore, it is important to understand whether other genetic or environmental factors influence outcome of SCFA receptors-signaling on our health. These findings will also help us to decide the choice between agonists and antagonists of these receptors for therapeutic manipulation of a given health condition (Bolognini, et al., 2016; Cornall, Mathai, Hryciw, & McAinch, 2013).
8. Conclusions and Future Directions
SCFAs are the key metabolites that connect dietary fibers and gut microbiota to the intestinal health. Butyrate exerts plethora of biological effects in colon, which play a critical role in establishment and maintenance of intestinal homeostasis. To exert their effect, SCFAs interact with specific cell surface receptors such as GPR41, GPR43, and GPR109A, as well as intracellular molecules such as HDACs. At least some of the beneficial effects of dietary fibers and gut microbiota depend on defined signaling triggered by these molecules following their interaction with SCFAs. Depending on cell type and ligands, these receptors appear to transmit signaling via Gα, Gi/0, Gq or β- arrestins or a combination of them. It is very important to identify the signaling partners associated with beneficial effect of GPR41, GPR43, and GPR109A. This information will be valuable in designing small molecules targeting these receptors or their signaling pathways for therapeutic application. Although beneficial effects of butyrate are more noticeable than other SCFAs, butyrate delivery into colon is still challenging because of low compliance rate of rectal administration and very short half-life of systemically administered butyrate (Van Immerseel, et al., 2010). Identification and delivery of butyrate-producing gut bacteria that promote health is one aspect of solving this problem. Alternatively, new agonists or antagonists specific for GPR41, GPR43, and GPR09B or signaling pathways triggered by these receptors could potentially be targeted to achieve optimum maintenance of intestinal homeostasis and health.
Acknowledgements
This research was supported in part by National Institutes of Health grants R21AI085440 and R01DK103576 to N.S.
Abbreviations
- GPR41
G protein-coupled receptor 41
- GPR43
G protein-coupled receptor 43
- GPR109A
G protein-coupled receptor 109A
- HDAC
Histone Deacetylases
- DSS
dextran sulfate sodium
- AOM
azoxymethane
- APC
adenomatous polyposis
- DC
dendritic cell
- Treg
T Regulatory
- LPS
lipopolysaccride
- GF
Germ free
- SLC5A8
Solute Carrier Family 5 Member 8
- MCT1
Mono Carboxylate Transporter-1
- SCFAs
Short-Chain Fatty Acids
Footnotes
The authors disclose no potential conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed K, Tunaru S, Offermanns S. GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol Sci. 2009;30:557–562. doi: 10.1016/j.tips.2009.09.001. [DOI] [PubMed] [Google Scholar]
- Ang Z, Er JZ, Ding JL. The short-chain fatty acid receptor GPR43 is transcriptionally regulated by XBP1 in human monocytes. Sci Rep. 2015;5:8134. doi: 10.1038/srep08134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- Belenguer A, Duncan SH, Calder AG, Holtrop G, Louis P, Lobley GE, Flint HJ. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl Environ Microbiol. 2006;72:3593–3599. doi: 10.1128/AEM.72.5.3593-3599.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman EN. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiological reviews. 1990;70:567–590. doi: 10.1152/physrev.1990.70.2.567. [DOI] [PubMed] [Google Scholar]
- Bjursell M, Admyre T, Goransson M, Marley AE, Smith DM, Oscarsson J, Bohlooly YM. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. American journal of physiology. Endocrinology and metabolism. 2011;300:E211–220. doi: 10.1152/ajpendo.00229.2010. [DOI] [PubMed] [Google Scholar]
- Bolognini D, Tobin AB, Milligan G, Moss CE. The Pharmacology and Function of Receptors for Short-Chain Fatty Acids. Mol Pharmacol. 2016;89:388–398. doi: 10.1124/mol.115.102301. [DOI] [PubMed] [Google Scholar]
- Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem. 2003;278:11312–11319. doi: 10.1074/jbc.M211609200. [DOI] [PubMed] [Google Scholar]
- Carding S, Verbeke K, Vipond DT, Corfe BM, Owen LJ. Dysbiosis of the gut microbiota in disease. Microb Ecol Health Dis. 2015;26:26191. doi: 10.3402/mehd.v26.26191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charney AN, Micic L, Egnor RW. Nonionic diffusion of short-chain fatty acids across rat colon. The American journal of physiology. 1998;274:G518–524. doi: 10.1152/ajpgi.1998.274.3.G518. [DOI] [PubMed] [Google Scholar]
- Chen H, Assmann JC, Krenz A, Rahman M, Grimm M, Karsten CM, Kohl J, Offermanns S, Wettschureck N, Schwaninger M. Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate's protective effect in EAE. The Journal of clinical investigation. 2014;124:2188–2192. doi: 10.1172/JCI72151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow J, Tang H, Mazmanian SK. Pathobionts of the gastrointestinal microbiota and inflammatory disease. Current opinion in immunology. 2011;23:473–480. doi: 10.1016/j.coi.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombes JL, Siddiqui KR, Arancibia-Carcamo CV, Hall J, Sun CM, Belkaid Y, Powrie F. A functionally specialized population of mucosal CD103+ DCs induces Foxp3+ regulatory T cells via a TGF-beta and retinoic acid-dependent mechanism. The Journal of experimental medicine. 2007;204:1757–1764. doi: 10.1084/jem.20070590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornall LM, Mathai ML, Hryciw DH, McAinch AJ. The therapeutic potential of GPR43: a novel role in modulating metabolic health. Cell Mol Life Sci. 2013;70:4759–4770. doi: 10.1007/s00018-013-1419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresci G, Nagy LE, Ganapathy V. Lactobacillus GG and tributyrin supplementation reduce antibiotic-induced intestinal injury. JPEN. Journal of parenteral and enteral nutrition. 2013;37:763–774. doi: 10.1177/0148607113486809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cresci GA, Thangaraju M, Mellinger JD, Liu K, Ganapathy V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J Gastrointest Surg. 2010;14:449–461. doi: 10.1007/s11605-009-1045-x. [DOI] [PubMed] [Google Scholar]
- Cummings JH, Pomare EW, Branch WJ, Naylor CP, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N, Vallance BA, Saleh M. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elangovan S, Pathania R, Ramachandran S, Ananth S, Padia RN, Lan L, Singh N, Martin PM, Hawthorn L, Prasad PD, Ganapathy V, Thangaraju M. The niacin/butyrate receptor GPR109A suppresses mammary tumorigenesis by inhibiting cell survival. Cancer Res. 2014;74:1166–1178. doi: 10.1158/0008-5472.CAN-13-1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, Flavell RA. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswaran S, Muir J, Chey WD. Fiber and functional gastrointestinal disorders. The American journal of gastroenterology. 2013;108:718–727. doi: 10.1038/ajg.2013.63. [DOI] [PubMed] [Google Scholar]
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB, Guiot Y, Derrien M, Muccioli GG, Delzenne NM, de Vos WM, Cani PD. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faith JJ, Guruge JL, Charbonneau M, Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath AC, Leibel RL, Rosenbaum M, Gordon JI. The long-term stability of the human gut microbiota. Science. 2013;341:1237439. doi: 10.1126/science.1237439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falony G, Vlachou A, Verbrugghe K, De Vuyst L. Cross-feeding between Bifidobacterium longum BB536 and acetate-converting, butyrate-producing colon bacteria during growth on oligofructose. Appl Environ Microbiol. 2006;72:7835–7841. doi: 10.1128/AEM.01296-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:13780–13785. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, Takahashi M, Fukuda NN, Murakami S, Miyauchi E, Hino S, Atarashi K, Onawa S, Fujimura Y, Lockett T, Clarke JM, Topping DL, Tomita M, Hori S, Ohara O, Morita T, Koseki H, Kikuchi J, Honda K, Hase K, Ohno H. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- Gaidarov I, Chen X, Anthony T, Maciejewski-Lenoir D, Liaw C, Unett DJ. Differential tissue and ligand-dependent signaling of GPR109A receptor: implications for anti-atherosclerotic therapeutic potential. Cellular signalling. 2013;25:2003–2016. doi: 10.1016/j.cellsig.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Ganapathy V, Thangaraju M, Prasad PD, Martin PM, Singh N. Transporters and receptors for short-chain fatty acids as the molecular link between colonic bacteria and the host. Current opinion in pharmacology. 2013 doi: 10.1016/j.coph.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Garrett WS, Gallini CA, Yatsunenko T, Michaud M, DuBois A, Delaney ML, Punit S, Karlsson M, Bry L, Glickman JN, Gordon JI, Onderdonk AB, Glimcher LH. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell host & microbe. 2010;8:292–300. doi: 10.1016/j.chom.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett WS, Lord GM, Punit S, Lugo-Villarino G, Mazmanian SK, Ito S, Glickman JN, Glimcher LH. Communicable ulcerative colitis induced by T-bet deficiency in the innate immune system. Cell. 2007;131:33–45. doi: 10.1016/j.cell.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GR, Scott KP, Rastall RA, Tuohy KM, Hotchkiss A, Dubert-Ferrandon A, Gareau M, Murphy EF, Saulnier D, Loh G, Macfarlane S, Delzenne N, Ringel Y, Kozianowski G, Dickmann R, Lenoir-Wijnkoop I, Walker C, Buddington R. Dietary prebiotics: current status and new definition. Food Science & Technology Bulletin: Functional Foods. 2010;7:1–19. [Google Scholar]
- Grice EA, Segre JA. The human microbiome: our second genome. Annu Rev Genomics Hum Genet. 2012;13:151–170. doi: 10.1146/annurev-genom-090711-163814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjiagapiou C, Schmidt L, Dudeja PK, Layden TJ, Ramaswamy K. Mechanism(s) of butyrate transport in Caco-2 cells: role of monocarboxylate transporter 1. American journal of physiology. Gastrointestinal and liver physiology. 2000;279:G775–780. doi: 10.1152/ajpgi.2000.279.4.G775. [DOI] [PubMed] [Google Scholar]
- Hamer HM, Jonkers D, Venema K, Vanhoutvin S, Troost FJ, Brummer RJ. Review article: the role of butyrate on colonic function. Aliment Pharmacol Ther. 2008;27:104–119. doi: 10.1111/j.1365-2036.2007.03562.x. [DOI] [PubMed] [Google Scholar]
- Hansen L, Skeie G, Landberg R, Lund E, Palmqvist R, Johansson I, Dragsted LO, Egeberg R, Johnsen NF, Christensen J, Overvad K, Tjonneland A, Olsen A. Intake of dietary fiber, especially from cereal foods, is associated with lower incidence of colon cancer in the HELGA cohort. International journal of cancer. Journal international du cancer. 2012;131:469–478. doi: 10.1002/ijc.26381. [DOI] [PubMed] [Google Scholar]
- Hegyi J, Schwartz RA, Hegyi V. Pellagra: dermatitis, dementia, and diarrhea. International journal of dermatology. 2004;43:1–5. doi: 10.1111/j.1365-4632.2004.01959.x. [DOI] [PubMed] [Google Scholar]
- Hong YH, Nishimura Y, Hishikawa D, Tsuzuki H, Miyahara H, Gotoh C, Choi KC, Feng DD, Chen C, Lee HG, Katoh K, Roh SG, Sasaki S. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology. 2005;146:5092–5099. doi: 10.1210/en.2005-0545. [DOI] [PubMed] [Google Scholar]
- Hou JK, Abraham B, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. The American journal of gastroenterology. 2011;106:563–573. doi: 10.1038/ajg.2011.44. [DOI] [PubMed] [Google Scholar]
- Huber S, Gagliani N, Esplugues E, O'Connor W, Jr., Huber FJ, Chaudhry A, Kamanaka M, Kobayashi Y, Booth CJ, Rudensky AY, Roncarolo MG, Battaglia M, Flavell RA. Th17 cells express interleukin-10 receptor and are controlled by Foxp3(−) and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izcue A, Coombes JL, Powrie F. Regulatory lymphocytes and intestinal inflammation. Annual review of immunology. 2009;27:313–338. doi: 10.1146/annurev.immunol.021908.132657. [DOI] [PubMed] [Google Scholar]
- Kaji I, Karaki S, Kuwahara A. Short-chain fatty acid receptor and its contribution to glucagon-like peptide-1 release. Digestion. 2014;89:31–36. doi: 10.1159/000356211. [DOI] [PubMed] [Google Scholar]
- Karaki S, Mitsui R, Hayashi H, Kato I, Sugiya H, Iwanaga T, Furness JB, Kuwahara A. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 2006;324:353–360. doi: 10.1007/s00441-005-0140-x. [DOI] [PubMed] [Google Scholar]
- Karaki S, Tazoe H, Hayashi H, Kashiwabara H, Tooyama K, Suzuki Y, Kuwahara A. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J Mol Histol. 2008;39:135–142. doi: 10.1007/s10735-007-9145-y. [DOI] [PubMed] [Google Scholar]
- Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406. e391–310. doi: 10.1053/j.gastro.2013.04.056. [DOI] [PubMed] [Google Scholar]
- Kimura I, Ozawa K, Inoue D, Imamura T, Kimura K, Maeda T, Terasawa K, Kashihara D, Hirano K, Tani T, Takahashi T, Miyauchi S, Shioi G, Inoue H, Tsujimoto G. The gut microbiota suppresses insulin-mediated fat accumulation via the short-chain fatty acid receptor GPR43. Nature communications. 2013;4:1829. doi: 10.1038/ncomms2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic AD, Chun E, Robertson L, Glickman JN, Gallini CA, Michaud M, Clancy TE, Chung DC, Lochhead P, Hold GL, El-Omar EM, Brenner D, Fuchs CS, Meyerson M, Garrett WS. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell host & microbe. 2013;14:207–215. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourgot C, Ferret-Bernard S, Le Normand L, Savary G, Menendez-Aparicio E, Blat S, Appert-Bossard E, Respondek F, Le Huerou-Luron I. Maternal short-chain fructooligosaccharide supplementation influences intestinal immune system maturation in piglets. PloS one. 2014;9:e107508. doi: 10.1371/journal.pone.0107508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Poul E, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van Damme J, Parmentier M, Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem. 2003;278:25481–25489. doi: 10.1074/jbc.M301403200. [DOI] [PubMed] [Google Scholar]
- Lee SU, In HJ, Kwon MS, Park BO, Jo M, Kim MO, Cho S, Lee S, Lee HJ, Kwak YS, Kim S. beta-Arrestin 2 mediates G protein-coupled receptor 43 signals to nuclear factor-kappaB. Biol Pharm Bull. 2013;36:1754–1759. doi: 10.1248/bpb.b13-00312. [DOI] [PubMed] [Google Scholar]
- Lee YK, Mazmanian SK. Has the microbiota played a critical role in the evolution of the adaptive immune system? Science. 2010;330:1768–1773. doi: 10.1126/science.1195568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE. Obesity and the human microbiome. Curr Opin Gastroenterol. 2010;26:5–11. doi: 10.1097/MOG.0b013e328333d751. [DOI] [PubMed] [Google Scholar]
- Li Y, Kokrashvili Z, Mosinger B, Margolskee RF. Gustducin couples fatty acid receptors to GLP-1 release in colon. American journal of physiology. Endocrinology and metabolism. 2013;304:E651–660. doi: 10.1152/ajpendo.00471.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Tonkonogy SL, Sartor RB. Antigen-presenting cell production of IL-10 inhibits T-helper 1 and 17 cell responses and suppresses colitis in mice. Gastroenterology. 2011;141:653–662. 662, e651–654. doi: 10.1053/j.gastro.2011.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukasova M, Malaval C, Gille A, Kero J, Offermanns S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. The Journal of clinical investigation. 2011;121:1163–1173. doi: 10.1172/JCI41651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia L, Tan J, Vieira AT, Leach K, Stanley D, Luong S, Maruya M, Ian McKenzie C, Hijikata A, Wong C, Binge L, Thorburn AN, Chevalier N, Ang C, Marino E, Robert R, Offermanns S, Teixeira MM, Moore RJ, Flavell RA, Fagarasan S, Mackay CR. Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nature communications. 2015;6:6734. doi: 10.1038/ncomms7734. [DOI] [PubMed] [Google Scholar]
- Mascolo N, Rajendran VM, Binder HJ. Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology. 1991;101:331–338. doi: 10.1016/0016-5085(91)90008-9. [DOI] [PubMed] [Google Scholar]
- Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461:1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453:620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- Nagano Y, Itoh K, Honda K. The induction of Treg cells by gut-indigenous Clostridium. Current opinion in immunology. 2012;24:392–397. doi: 10.1016/j.coi.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- O'Hara AM, Shanahan F. The gut flora as a forgotten organ. EMBO Rep. 2006;7:688–693. doi: 10.1038/sj.embor.7400731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe SJ, Li JV, Lahti L, Ou J, Carbonero F, Mohammed K, Posma JM, Kinross J, Wahl E, Ruder E, Vipperla K, Naidoo V, Mtshali L, Tims S, Puylaert PG, DeLany J, Krasinskas A, Benefiel AC, Kaseb HO, Newton K, Nicholson JK, de Vos WM, Gaskins HR, Zoetendal EG. Fat, fibre and cancer risk in African Americans and rural Africans. Nature communications. 2015;6:6342. doi: 10.1038/ncomms7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S, Schwaninger M. Nutritional or pharmacological activation of HCA(2) ameliorates neuroinflammation. Trends Mol Med. 2015;21:245–255. doi: 10.1016/j.molmed.2015.02.002. [DOI] [PubMed] [Google Scholar]
- Palmer R. Fecal matters. Nat Med. 2011;17:150–152. doi: 10.1038/nm0211-150. [DOI] [PubMed] [Google Scholar]
- Rahman M, Muhammad S, Khan MA, Chen H, Ridder DA, Muller-Fielitz H, Pokorna B, Vollbrandt T, Stolting I, Nadrowitz R, Okun JG, Offermanns S, Schwaninger M. The beta-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nature communications. 2014;5:3944. doi: 10.1038/ncomms4944. [DOI] [PubMed] [Google Scholar]
- Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Roediger WE. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982;83:424–429. [PubMed] [Google Scholar]
- Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR, Jr., Muller W, Rudensky AY. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–150. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:16767–16772. doi: 10.1073/pnas.0808567105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzkin A, Mouw T, Park Y, Subar AF, Kipnis V, Hollenbeck A, Leitzmann MF, Thompson FE. Dietary fiber and whole-grain consumption in relation to colorectal cancer in the NIH-AARP Diet and Health Study. Am J Clin Nutr. 2007;85:1353–1360. doi: 10.1093/ajcn/85.5.1353. [DOI] [PubMed] [Google Scholar]
- Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiological reviews. 2010;90:859–904. doi: 10.1152/physrev.00045.2009. [DOI] [PubMed] [Google Scholar]
- Shanahan F, Quigley EM. Manipulation of the microbiota for treatment of IBS and IBD-challenges and controversies. Gastroenterology. 2014;146:1554–1563. doi: 10.1053/j.gastro.2014.01.050. [DOI] [PubMed] [Google Scholar]
- Sina C, Gavrilova O, Forster M, Till A, Derer S, Hildebrand F, Raabe B, Chalaris A, Scheller J, Rehmann A, Franke A, Ott S, Hasler R, Nikolaus S, Folsch UR, Rose-John S, Jiang HP, Li J, Schreiber S, Rosenstiel P. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. Journal of immunology. 2009;183:7514–7522. doi: 10.4049/jimmunol.0900063. [DOI] [PubMed] [Google Scholar]
- Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi H, Thangaraju M, Prasad PD, Manicassamy S, Munn DH, Lee JR, Offermanns S, Ganapathy V. Activation of Gpr109a, receptor for niacin and the commensal metabolite butyrate, suppresses colonic inflammation and carcinogenesis. Immunity. 2014;40:128–139. doi: 10.1016/j.immuni.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM, Glickman JN, Garrett WS. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–573. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoddart LA, Smith NJ, Jenkins L, Brown AJ, Milligan G. Conserved polar residues in transmembrane domains V, VI, and VII of free fatty acid receptor 2 and free fatty acid receptor 3 are required for the binding and function of short chain fatty acids. J Biol Chem. 2008;283:32913–32924. doi: 10.1074/jbc.M805601200. [DOI] [PubMed] [Google Scholar]
- Sun CM, Hall JA, Blank RB, Bouladoux N, Oukka M, Mora JR, Belkaid Y. Small intestine lamina propria dendritic cells promote de novo generation of Foxp3 T reg cells via retinoic acid. The Journal of experimental medicine. 2007;204:1775–1785. doi: 10.1084/jem.20070602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taggart AK, Kero J, Gan X, Cai TQ, Cheng K, Ippolito M, Ren N, Kaplan R, Wu K, Wu TJ, Jin L, Liaw C, Chen R, Richman J, Connolly D, Offermanns S, Wright SD, Waters MG. (D)-beta-Hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J Biol Chem. 2005;280:26649–26652. doi: 10.1074/jbc.C500213200. [DOI] [PubMed] [Google Scholar]
- Tang C, Ahmed K, Gille A, Lu S, Grone HJ, Tunaru S, Offermanns S. Loss of FFA2 and FFA3 increases insulin secretion and improves glucose tolerance in type 2 diabetes. Nature medicine. 2015;21:173–177. doi: 10.1038/nm.3779. [DOI] [PubMed] [Google Scholar]
- Tang Y, Chen Y, Jiang H, Robbins GT, Nie D. G-protein-coupled receptor for short-chain fatty acids suppresses colon cancer. International journal of cancer. Journal international du cancer. 2011;128:847–856. doi: 10.1002/ijc.25638. [DOI] [PubMed] [Google Scholar]
- Tazoe H, Otomo Y, Karaki S, Kato I, Fukami Y, Terasaki M, Kuwahara A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed Res. 2009;30:149–156. doi: 10.2220/biomedres.30.149. [DOI] [PubMed] [Google Scholar]
- Thangaraju M, Carswell KN, Prasad PD, Ganapathy V. Colon cancer cells maintain low levels of pyruvate to avoid cell death caused by inhibition of HDAC1/HDAC3. The Biochemical journal. 2009;417:379–389. doi: 10.1042/BJ20081132. [DOI] [PubMed] [Google Scholar]
- Thangaraju M, Cresci G, Itagaki S, Mellinger J, Browning DD, Berger FG, Prasad PD, Ganapathy V. Sodium-coupled transport of the short chain fatty acid butyrate by SLC5A8 and its relevance to colon cancer. J Gastrointest Surg. 2008;12:1773–1781. doi: 10.1007/s11605-008-0573-0. discussion 1781-1772. [DOI] [PubMed] [Google Scholar]
- Thangaraju M, Cresci GA, Liu K, Ananth S, Gnanaprakasam JP, Browning DD, Mellinger JD, Smith SB, Digby GJ, Lambert NA, Prasad PD, Ganapathy V. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 2009;69:2826–2832. doi: 10.1158/0008-5472.CAN-08-4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorburn AN, Macia L, Mackay CR. Diet, metabolites, and “western-lifestyle” inflammatory diseases. Immunity. 2014;40:833–842. doi: 10.1016/j.immuni.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Tolhurst G, Heffron H, Lam YS, Parker HE, Habib AM, Diakogiannaki E, Cameron J, Grosse J, Reimann F, Gribble FM. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes. 2012;61:364–371. doi: 10.2337/db11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom-Bru C, Blanchard C, Junt T, Nicod LP, Harris NL, Marsland BJ. Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nature medicine. 2014;20:159–166. doi: 10.1038/nm.3444. [DOI] [PubMed] [Google Scholar]
- Tsai F, Coyle WJ. The microbiome and obesity: is obesity linked to our gut flora? Curr Gastroenterol Rep. 2009;11:307–313. doi: 10.1007/s11894-009-0045-z. [DOI] [PubMed] [Google Scholar]
- Tunaru S, Kero J, Schaub A, Wufka C, Blaukat A, Pfeffer K, Offermanns S. PUMA-G and HM74 are receptors for nicotinic acid and mediate its antilipolytic effect. Nature medicine. 2003;9:352–355. doi: 10.1038/nm824. [DOI] [PubMed] [Google Scholar]
- Tunaru S, Lattig J, Kero J, Krause G, Offermanns S. Characterization of determinants of ligand binding to the nicotinic acid receptor GPR109A (HM74A/PUMA-G) Mol Pharmacol. 2005;68:1271–1280. doi: 10.1124/mol.105.015750. [DOI] [PubMed] [Google Scholar]
- Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell host & microbe. 2008;3:213–223. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Immerseel F, Ducatelle R, De Vos M, Boon N, Van De Wiele T, Verbeke K, Rutgeerts P, Sas B, Louis P, Flint HJ. Butyric acid-producing anaerobic bacteria as a novel probiotic treatment approach for inflammatory bowel disease. Journal of medical microbiology. 2010;59:141–143. doi: 10.1099/jmm.0.017541-0. [DOI] [PubMed] [Google Scholar]
- Vieira AT, Macia L, Galvao I, Martins FS, Canesso MC, Amaral FA, Garcia CC, Maslowski KM, De Leon E, Shim D, Nicoli JR, Harper JL, Teixeira MM, Mackay CR. A Role for Gut Microbiota and the Metabolite-Sensing Receptor GPR43 in a Murine Model of Gout. Arthritis Rheumatol. 2015;67:1646–1656. doi: 10.1002/art.39107. [DOI] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S, Sitaraman SV, Knight R, Ley RE, Gewirtz AT. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science. 2010;328:228–231. doi: 10.1126/science.1179721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters RW, Shukla AK, Kovacs JJ, Violin JD, DeWire SM, Lam CM, Chen JR, Muehlbauer MJ, Whalen EJ, Lefkowitz RJ. beta-Arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. The Journal of clinical investigation. 2009;119:1312–1321. doi: 10.1172/JCI36806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Cai G, Qiu Y, Fei N, Zhang M, Pang X, Jia W, Cai S, Zhao L. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 2012;6:320–329. doi: 10.1038/ismej.2011.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki MH, Vogel P, Body-Malapel M, Lamkanfi M, Kanneganti TD. IL-18 production downstream of the Nlrp3 inflammasome confers protection against colorectal tumor formation. Journal of immunology. 2010;185:4912–4920. doi: 10.4049/jimmunol.1002046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambell KL, Fitch MD, Fleming SE. Acetate and butyrate are the major substrates for de novo lipogenesis in rat colonic epithelial cells. The Journal of nutrition. 2003;133:3509–3515. doi: 10.1093/jn/133.11.3509. [DOI] [PubMed] [Google Scholar]