Abstract
Background & Aims
A major challenge in the management of nonalcoholic fatty liver disease (NAFLD) is to identify patients with nonalcoholic steatohepatitis (NASH) and early liver fibrosis. The progression of NAFLD is accompanied by distinctive changes in very low density lipoprotein (VLDL), a lipoprotein particle produced exclusively in the liver. Herein, we sought to determine the characteristics of VLDL profiles associated with NASH and liver fibrosis.
Methods
We evaluated VLDL profiles of 128 patients from a single centre NAFLD registry, and examined VLDL size, total and subclass VLDL concentrations in relation to NAFLD activity score (NAS), steatohepatitis and liver fibrosis as determined by liver biopsy.
Results
A near linear relationship was observed between mean VLDL particle size and NAFLD activity score (NAS). In multivariate models, VLDL particle size was significantly associated with both NAS and NASH, after adjustment for BMI and diabetes. A decrease in small VLDL particle concentration was associated with more advanced liver fibrosis. In receiver operative characteristic analyses, mean VLDL size performed similarly to cytokeratin 18 in predicting NASH, whereas small VLDL particle concentration had similar performance to NAFLD fibrosis score in predicting stage 2 or above liver fibrosis.
Conclusions
The increase in mean VLDL size in NASH and decrease in small VLDL particle concentration in liver fibrosis likely reflect changes in the number and state of hepatocytes associated with NASH and fibrosis. In addition to its value in risk stratification of cardiovascular diseases, circulating VLDL profile may provide information for the staging of NAFLD disease severity.
Keywords: lipoprotein metabolism, nonalcoholic fatty liver disease, nonalcoholic steatohepatitis, very low density lipoprotein
Non-alcoholic fatty liver disease (NAFLD) constitutes a spectrum of liver diseases ranging from simple steatosis to steatohepatitis (NASH), progressive liver fibrosis, cirrhosis and hepatocellular carcinoma (1). NAFLD is an increasingly relevant cause of liver disease-related morbidity, mortality and liver transplantation in the United States (2).
Despite its high prevalence, only a small proportion of NAFLD patients eventually develop liver failure and its related complications (3). There is a critical need for effective noninvasive tools to identify individuals with progressive disease. The current standard of care involves percutaneous liver biopsy, which is costly and invasive (4). Fibrosis indices derived from serologic markers and imaging tests such as magnetic resonance elastography and vibration-controlled transient elastography are powerful noninvasive tools to determine the risk of fibrosis in NAFLD (5–7). But they are imperfect, and importantly unable to discern NASH from simple steatosis. Cytokeratin-18 (CK18), a biomarker for liver injury, once was reported to differentiate NASH from simple steatosis (8). But its clinical value has been questioned by more recent studies (9, 10).
It is well established that NAFLD is associated with characteristic lipid profiles, such as elevated fasting triglyceride, increased non-high density lipoprotein (HDL) cholesterol and decreased HDL cholesterol (11–13). Recently, using lipoprotein subfraction analysis methods such as nuclear magnetic resonance (NMR) spectroscopy or ion mobility analysis, several groups have reported associations between NASH and an atherogenic lipoprotein profile (14–16).
Among circulating lipoproteins, very low density lipoprotein (VLDL) has the most intimate relationship with NAFLD, as this represents the essential route of hepatocyte lipid excretion (17). Defects in VLDL processing are directly linked to hepatic steatosis. For example, genetic defects that impair VLDL production seen in familial hypobetalipoproteinemia and abetalipoproteinemia are well-established causes for hepatic steatosis (18). Recently, two genetic polymorphisms, an I148M variant in the patatin-like phospholipase domain-containing 3 (PNPLA3) gene and an E167K variant in the Transmembrane 6 Superfamily Member 2 (TM6SM2) gene, have been shown to confer significant risk of NAFLD and its disease progression (19–22). Both genetic variants appear to impair hepatic VLDL secretion (21, 23).
Beyond inborn risk factors, the production of VLDL is dynamically influenced by the natural history of NAFLD. On the one hand, VLDL particle concentration and size are positively associated with insulin resistance and diabetes, diseases that often co-exist with NAFLD (24, 25). On the other, the production of VLDL, apolipoprotein B and subsequently low-density lipoprotein-cholesterol (LDL-C) decreases as steatohepatitis and liver fibrosis develop (26, 27). These changes likely also result in a decrease in circulating VLDL particle concentration.
Taken together, growing evidence suggests that VLDL plays an important role in the pathogenesis and progression of NAFLD. Herein, we test the association of VLDL concentration and particle size with the histological staging of NASH and liver fibrosis.
Patients and methods
Patient population
Patients for this study came from a prospective NAFLD registry at Beth Israel Deaconess Medical Center (BIDMC). Patients enrolled since 2009 with clinical diagnosis of NAFLD that was confirmed by liver biopsy. Patients with other forms of chronic liver diseases, alternative causes for fatty liver, such as medication, hereditary haemochromatosis or consumption of greater than 20 g alcohol daily were excluded from the registry. Data on patient demographics, medical history and from their physical exam were obtained at the enrolment of this study. Laboratory tests and collection of serum were performed at enrolment. Individuals with non-fasting samples were excluded from this study, as the VLDL fraction may include intestine-derived chylomicrons. Liver biopsy was performed on each individual within three months of the index visit. Baseline serum and liver biopsy tissue were stored at −80°C. At the time of this study, 170 patients had undergone tests required, among whom 128 had fasting blood samples and were included in this study. This study has been approved by the BIDMC institutional review board, and was conducted in accordance with the Helsinki declaration of 1975, as revised in 1983. All participants consented to this study at the enrolment.
Clinical biochemistry and measurements of VLDL profiles
Routine blood tests including complete blood count, chemistry, liver function tests, albumin and lipid panel were processed at the BIDMC clinical laboratory. Lipids were assessed using conventional lipid panels. Total and subclass VLDL concentrations and VLDL size were measured by NMR spectroscopy at LabCorp (previously known as LipoScience, Raleigh, NC, USA). Frozen serum samples were used for VLDL analysis. Samples were thawed, aliquoted (500 µl), and courier delivered to LabCorp, who were blinded to the clinical information. Lipoprotein particle concentration and size were calculated from the measured amplitudes of the spectroscopically distinct signals from different-size lipoprotein subclasses using the LipoProfile-3 algorithm (28). Diameter range estimates for the reported subclasses are as follows: large VLDL, >60 nm; medium VLDL, 42–60 nm; small VLDL, 29–42 nm. Weighted-average lipoprotein particle sizes were derived from the sum of the diameter of each subclass multiplied by its relative mass percentage and reported in nanometer (nm) units.
Liver biopsy
Ultrasound guided liver biopsy was performed at the enrolment of this study. Biopsy results were interpreted by staff pathologists who specialized in hepatopathology, and were blinded to the lipoprotein measurements. All liver biopsies were assessed and reported in a standardized fashion, including fibrosis stages (1–4) and NAFLD activity score (NAS, 0–8) calculated based on the degrees of hepatic steatosis, lobular inflammation and ballooning degeneration (29). The diagnosis of NASH was made based on the presence of macrovesicular steatosis of at least 5% of liver parenchyma, ballooned hepatocytes and inflammation predominantly in a zone 3 distribution. Significant liver fibrosis was defined as fibrosis stages 2 or above.
Serum CK18 measurement
CK18 levels were measured using the PEVIVA in vitro immunoassay M30-Apoptosense ELISA kit (DiaPharma, West Chester, OH, USA) as described previously (30). The test was performed following the manufacturer’s instructions using antibodies and standards provided in the kit. Serum CK18 concentration was expressed in U/L (1 U/L = 1.24 pM recombinant protein standard).
DNA extraction and SNP genotyping
Genomic DNA was extracted from frozen human whole blood samples, using phenol chloroform. Ethanol-precipitated DNA was resuspended in double distilled water. Single nucleotide polymorphisms (SNP), rs738409 in the PNPLA3 gene and rs58542926 in the TM6SF2 gene, were genotyped by TaqMan Allelic Discrimination, using predesigned TaqMan SNP genotyping assays (Applied Biosystems, Grand Island, NY, USA).
Non-invasive fibrosis scores
The NAFLD fibrosis score (NFS) was calculated using the following formula: −1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio − 0.013 × platelet count (×109/L) − 0.66 × albumin (g/dl) as described previously (31). FIB4 was calculated using the following formula: (Age × AST)/[Platelet count (×109/ L) × ALT1/2] (32).
Statistical analysis
We first compared baseline patient characteristics, conventional lipid profiles and NMR lipoprotein profiles among individuals with simple steatosis vs. NASH, minimal fibrosis (stage 0–1) vs. significant liver fibrosis (stage 2–4), using Student’s t-tests and Chi-square tests. For both NASH and fibrosis, a comprehensive VLDL profile was evaluated first, including total and subclass concentrations, VLDL size and the ratios between subclass particles to total VLDL concentration. We focused our analysis on the associations between VLDL size and NASH, and between small VLDL particle concentration and liver fibrosis, because of their stronger associations among other parameters.
To further determine the relationship between mean VLDL size and NASH, we calculated unadjusted beta coefficient for NAS and coefficient adjusted for age, gender, BMI, history of diabetes, the use of statin (n = 41), PNPLA3 I148M and TM6SF2 E167K status. Covariates were selected based on their biological relevance to NASH and VLDL profile. We then calculated the relative risk for NASH, lobular inflammation, ballooning degeneration, as well as beta coefficient for hepatic steatosis score. Given that our data are significantly skewed toward positive outcomes, i.e. NASH, the conventional odds ratio would likely be inflated. We instead calculated relative risk ratio using generalized linear regression. The regression model was calculated using maximum likelihood method with gamma error distributions. The receiver operative characteristics (ROC) of VLDL size in predicting NASH was compared to that of CK18 in our cohort.
For fibrosis, we calculated unadjusted and adjusted relative risk ratio for VLDL characteristics in relationship with stage 2–4 fibrosis on liver biopsy. Generalized linear regression was performed for multivariate analysis using variables, including VLDL subclass concentrations, age, gender, BMI, history of diabetes, the use of statin and PNPLA3 I148M genotype. To compare the strength of association between small VLDL particle concentration, NFS and FIB4, two established non-invasive liver fibrosis indices in predicting fibrosis, we performed regression using both measurements as independent variables in a competition test and examined the Z-statistics and P value. Similarly, we compared the ROC curve of inverse concentration of small VLDL particles to that of NFS.
All statistical analyses were performed in Stata13.0 (Stata corp., College Station, TX, USA). Two-tailed Student’s t-test was calculated for pair-wise comparison of continuous variable. Chi-square test was used for binary variables.
Results
Patient characteristics
The clinical characteristics of the 128 patients included in this study are summarized in Table S1. The average age of the cohort was 50.4, and about 60% of the patients were male. Most of the subjects (n = 103, 80%) had liver biopsies consistent with NASH, with an average NAS of 4.5; 61 (47%) had significant liver fibrosis (stage ≥ 2) and 21 (16%) had advanced liver fibrosis (stage 3–4).
NASH is associated with increased VLDL particle size
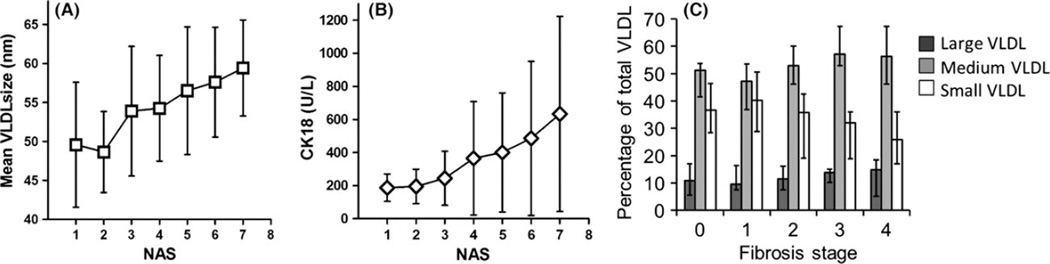
There were no significant differences in age, gender and BMI between individuals with simple steatosis and NASH. Individuals with NASH had higher prevalence of diabetes and PNPLA3 I148M (rs738409) polymorphism (Table S2). Although there were no differences in lipid or lipoprotein concentrations among individuals with NASH vs. simple steatosis, the mean VLDL size in the NASH group was significantly larger (56.1 vs. 52.2 nm, P = 0.02). Indeed, a near linear relationship between mean VLDL size and NAS was noted, with every point increase in NAS score associated with 1.9 nm increase in mean VLDL size (95% CI 1.0–2.8, P < 0.0001) (Fig. 1A). Mean VLDL size, PNPLA3 I148M and TM6SF2 E167K genotypes were positively associated with NAS in univariate and multivariate models, whereas diabetes was only significant in the univariate model (Table 1).
Fig. 1.
Characteristics of VLDL changes associated with NASH and liver fibrosis. (A) Mean VLDL size measured in nm plotted over NAS score. (B) CK18 concentration over NAS score. (C) The changes of VLDL subclass ratios to total VLDL at different stages of liver fibrosis. Dark gray, medium gray and white bars represent the percentage of large, medium and small VLDL particles respectively. P values were calculated by Student’s t-test. Error bars represent standard deviation in A, B and 25–75% IQR in C.
Table 1.
Multivariate analysis of VLDL particle size and NAS
| Unadjusted β | 95% CI | P value | Adjusted β* | 95% CI | P value | |
|---|---|---|---|---|---|---|
| Age | −0.01 | −0.04 to 0.01 | 0.2 | −0.02 | −0.04 to 0.01 | 0.1 |
| Gender (%female) | 0.24 | −0.28 to 0.77 | 0.4 | 0.20 | −0.27 to 0.67 | 0.4 |
| BMI | 0.03 | −0.004 to 0.07 | 0.08 | 0.03 | −0.01 to 0.06 | 0.2 |
| Diabetes | 0.76 | 0.22–1.32 | 0.007 | 0.42 | −0.15 to 0.99 | 0.1 |
| Statin use | 0.36 | −0.20 to 0.91 | 0.2 | 0.31 | −0.25 to 0.86 | 0.3 |
| PNPLA3 I148M | 0.83 | 0.31–1.35 | 0.002 | 0.98 | 0.51–1.45 | <0.001 |
| TM6SF2 E167K | 0.62 | −0.01 to 1.24 | 0.05 | 0.60 | 0.03–1.18 | 0.04 |
| Mean VLDL diameter (nm) | 0.07 | 0.03–0.10 | <0.001 | 0.06 | 0.03–0.09 | <0.001 |
Adjusted risk ratio calculated from multivariate models with all variables in this table.
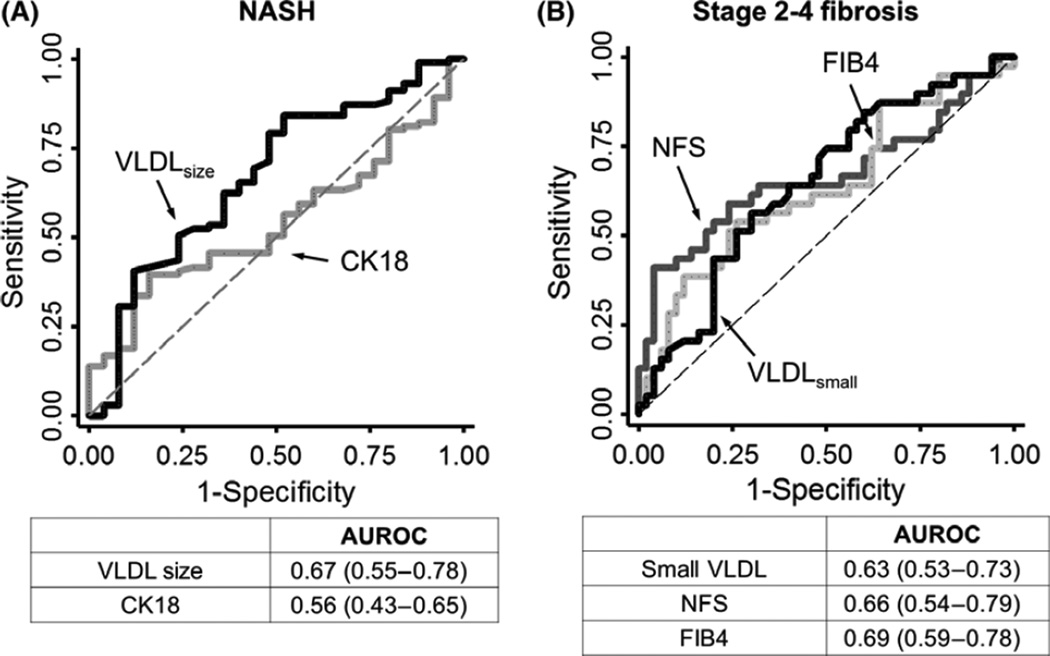
CK18, a proposed NASH biomarker, was also positively associated with NAS in our cohort, with β coefficient of 0.12 (95% CI 0.05–0.18, P = 0.001) in univariate model. Compared to VLDL size, we noted a significant variability in its levels among those with high NAS (Fig. 1B). When we examined their relationships to NASH and its histological features, both mean VLDL particle size and CK18 were positively associated with steatosis score, but only mean VLDL particle size had significant association with ballooning degeneration (Table S3). Each nm increase in mean VLDL size was associated with 1.2% increase (95% CI 0.003–0.02, P = 0.01) in the relative risk of NASH in multivariate model. Mean VLDL particle size had an area under the curve (AUROC) of 0.67 (95% CI 0.55–0.78) whereas CK18 had a slightly lower AUROC of 0.56 (95% CI 0.43–0.65) in our study, although this difference was not significant (P = 0.08) (Fig. 2A).
Fig. 2.
ROC curves for NASH and significant liver fibrosis. (A) Comparison of mean VLDL size (black) and CK18 (gray) in predicting NASH. (B) Comparison of small VLDL concentration (black) and NFS (gray dash) in predicting significant liver fibrosis (stage 2 or above). The predicted AUROC with its 95% CI was shown below each panel.
Significant liver fibrosis is associated with low concentration of small VLDL particles
We then investigated the relationship of VLDL profiles between individuals without significant liver fibrosis (stage 0–1) and those with significant liver fibrosis on biopsy (stage 2 or higher) (Table S4). Individuals with significant liver fibrosis had higher BMI (36.1 ± 7.4 vs. 33.1 ± 5.6, P = 0.01) and were more likely to have diabetes (49.2 vs. 13.2% P < 0.001). CK18 was also higher among those with significant fibrosis (493 ± 465 vs. 300 ± 272 U/l, P = 0.005). Their conventional lipid panel was characterized by lower total cholesterol (181 ± 51 mg/dl vs. 202 ± 40 mg/dl, P = 0.01) and lower LDL-C (102 ± 41 mg/dl vs. 118 ± 37 mg/dl, P = 0.02), but no significant differences in fasting triglyceride. Although there was a lack of significant difference in total VLDL particle concentration or VLDL particle size, the small VLDL particle concentration was significantly lower among those with significant liver fibrosis (23.4 ± 17.4 nmol/l vs. 34.6 ± 30.9 nmol/l, P = 0.02). Upon further examination of the VLDL subclasses in association with liver fibrosis, there was a prominent reciprocal change in the percentage of small and medium VLDL particles with increasing liver fibrosis (Fig. 1C). For every one stage increase in liver fibrosis, there was a 1.6% decrease in the percentage of small VLDL particles (95% CI 0.5–2.8, P = 0.007), and 2.5% increase in the percentage of medium VLDL particles (95% CI 1.0–3.9%, P = 0.001).
We constructed a multivariate regression model to examine the association of significant liver fibrosis with the concentration of three subclasses of VLDL particles, and covariates including age, gender, BMI diabetes, statin use and PNPLA3 I148M genotype (Table 2). In this model, small VLDL particle concentration, PNPLA3 genotype and diabetes were associated with significant liver fibrosis. For every nmol/l decrease in the concentration of small VLDL particles, there was an associated 2.1% increase in the relative risk of significant fibrosis (95% CI 1.003–1.04, P = 0.03). In addition, diabetes and PNPLA3 I148M genotype were associated with 3.5- and 2.1-fold increase in the relative risk of significant fibrosis (95% CI 1.4–8.8, P = 0.008 and 95% CI 1.01– 4.03, P = 0.03) respectively.
Table 2.
Multivariate analysis of VLDL subclass concentration and significant liver fibrosis (stage 2 or above)
| Unadjusted Risk Ratio* |
95% CI | P value | Adjusted Risk Ratio† |
95% CI | P value | |
|---|---|---|---|---|---|---|
| Age | 1.01 | 1.00–1.03 | 0.1 | 1.00 | 0.98–1.03 | 0.9 |
| Gender (%female) | 0.90 | 0.61–1.32 | 0.6 | 0.67 | 0.36–1.26 | 0.2 |
| BMI | 1.03 | 1.00–1.07 | 0.03 | 1.02 | 0.98–1.06 | 0.4 |
| Diabetes | 2.29 | 1.43–3.66 | 0.001 | 3.49 | 1.39–8.79 | 0.008 |
| Statin use | 1.67 | 1.09–2.56 | 0.02 | 1.41 | 0.66–3.03 | 0.4 |
| PNPLA3 I148M | 1.37 | 0.92–2.04 | 0.1 | 2.07 | 1.01–4.03 | 0.03 |
| TM6SF2 E167K | 1.16 | 0.73–1.85 | 0.5 | 0.58 | 0.24–1.43 | 0.2 |
| Large VLDL (nmol/l) | 0.99 | 0.97–1.01 | 0.5 | 1.00 | 0.96–1.03 | 0.8 |
| Medium VLDL (nmol/l) | 1.00 | 0.99–1.01 | 0.8 | 1.00 | 0.98–1.02 | 0.9 |
| Small VLDL (nmol/l) | 0.98 | 0.97–0.99 | 0.002 | 0.98 | 0.96–1.00 | 0.03 |
Risk ratio calculated significant liver fibrosis (stage 2 or above) compared to minimal fibrosis (stage 0–1).
Adjusted risk ratio calculated from multivariate models with all variables in this table.
We compared the predictive value of small VLDL particle concentration to NFS and FIB4, two validated indices for NASH fibrosis (Fig. 2B). When FIB4 was included in the same model as a competition test, small VLDL particle concentration remained significantly associated with stage 2 or above liver fibrosis, with relative risk of 0.98 (95% 0.96–1.00, P = 0.02). In our cohort, small VLDL particle concentration had an AUROC of 0.63 (95% CI 0.53–0.76) in predicting stage 2–4 fibrosis, slightly lower than the AUROC for NFS and FIB4 (0.66, 95% CI 0.54–0.79 and 0.69 95% CI 0.59–0.78 respectively).
Discussion
Lipoprotein metabolism is deeply implicated in the pathogenesis of NAFLD. We undertook this study to determine changes in VLDL characteristics associated with pathological features of NAFLD disease progression in order to define this physiology and explore its clinical use.
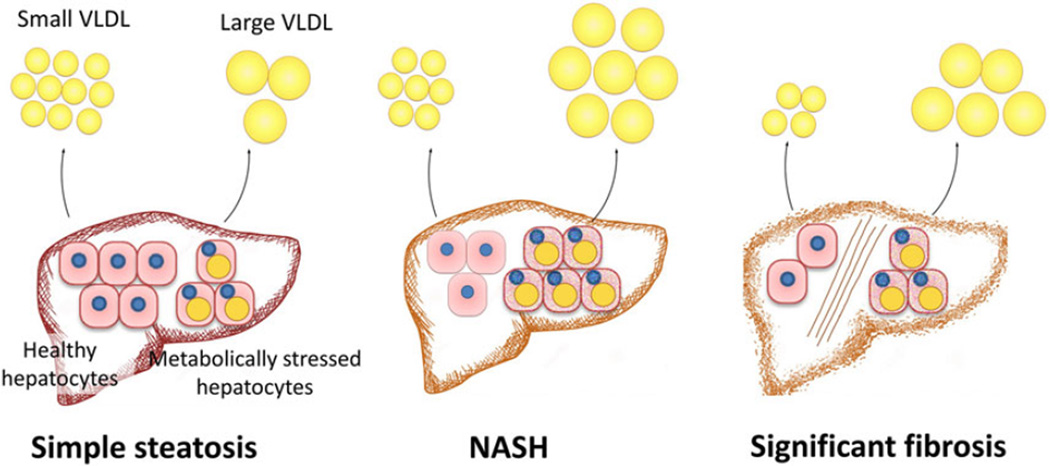
Our study demonstrated that NASH was associated with an increase in VLDL particle size, whereas liver fibrosis was associated with a decrease in the concentration of small VLDL particles. A hypothesis that may explain these observations is a direct link between circulating VLDL profile and the number and state of hepatocytes in the liver parenchyma (Fig. 3). VLDL is the only means by which hepatocytes excrete triglyceride to the systemic circulation (17). Hepatocytes with increased intracellular lipid pool are likely the source for large VLDL particles. Thereby, NASH driven by insulin resistance and an increase in the intrahepatic lipid pool may increase the ratio of large VLDL particles and mean VLDL size (24, 25, 33). On the other hand, liver fibrosis leads to hepatocyte dropout, further decreases the ratio of healthy hepatocytes among remaining hepatocytes, leading to decreased concentration of small VLDL particles.
Fig. 3.
Proposed VLDL profile changes in association with NAFLD disease progression. This hypothesis highlights the relationship between circulating VLDL profile and the state of hepatocytes in the liver parenchyma. An increase in the ratio of metabolically stressed hepatocytes in the setting of NASH produces more large VLDL particles. The development of significant liver fibrosis leads to the loss of hepatocytes, especially healthy ones, leading to decreased concentration of small VLDL particles.
Studies from large cohorts of metabolic syndrome suggested that insulin resistance is associated with both increased VLDL particle concentration and size (24, 34). Interestingly, our data suggest that steatohepatitis is associated much strongly with an increase in VLDL size rather than VLDL particle concentration. This change occurs in a near linear fashion in association with NAS, a validated histological measurement for NASH disease activity. Among NASH patients in our cohort, the mean VLDL size measured at 56.1 ± 7.2 nm. This is larger than previously reported values in association with insulin resistance. For example, the Insulin Resistance Atherosclerosis Study (IRAS) found mean VLDL sizes of 48.1 nm among those with normal glucose tolerance, 51.3 nm among those with impaired glucose tolerance and 55.2 nm among those with diabetes (24). Associations between VLDL profiles and NAFLD have been described in a few recent studies (14, 15). Notably, a recent study by Siddiqui et al. (15) did not observe a statistically significant difference in VLDL size between NASH and simple steatosis. This difference could be methodological as the VLDL concentration observed in their study was ~3 nmol/l, whereas it was 80.2 ± 50.2 nmol/l in our study. It should be noted that VLDL particle concentration was typically in the order of 60–70 nmol/l as observed in multiple large cohorts (24, 34, 35).
Liver fibrosis may be accompanied by subclinical impairment in liver synthetic function. This may result in a decrease in circulating triglyceride and apoB-containing particles, including VLDL and subsequently LDL, its downstream product (36). We have previously shown that fasting triglyceride level, a proxy for VLDL, is inversely associated with liver fibrosis indices (37). Here, we demonstrate a more selective decrease in the concentration of small VLDL particles in association with liver fibrosis. This suggests a specific pattern in which the circulating VLDL profile characteristic for NASH fibrosis can be distinguished from a decrease in fasting triglyceride that can be seen with healthy lifestyle interventions. However, whether this change in the concentration of small VLDL particle is specific to fibrosis from NAFLD or liver fibrosis in general deserves further investigation.
Clearly, the predictive value seen in this analysis is modest, and not enough to replace current noninvasive diagnosis of NASH or NAFLD fibrosis as is. However, it should be noted that they performed comparably to existing non-invasive biomarkers such as CK18 for NASH and NFS or FIB4 for fibrosis. The AUROC for CK18 in our cohort was lower than what have been seen elsewhere (9, 30). This may in part because our cohort skewed heavily toward NASH. A detailed analysis of CK18 in this cohort has been described elsewhere (38). Recently, Hyysalo and coworkers reported an NASH score using fasting insulin, PNPLA3 genotype and AST (39). It will be interesting to compare VLDL size to NASH score in cohorts with fasting insulin measurements. Future work should focus on evaluating the predictive value of VLDL parameters in cohorts with a broader spectrum of disease severities and in the setting of serial measurement for disease follow-up.
In summary, we have shown that an increase in VLDL size is a characteristic feature of NASH, whereas a decrease in small VLDL particle concentration is suggestive of the development of significant liver fibrosis in NAFLD patients. NMR-measured VLDL profile may be of value in non-invasive staging and risk stratification of NAFLD.
Supplementary Material
Key points.
NASH and liver fibrosis are associated with characteristic changes in the circulating VLDL profile
NASH is associated an increase in VLDL particle size
The presence of stage 2 or above liver fibrosis is associated with a decrease in the concentration of small VLDL particles
The serum VLDL profile is a potential biomarker that can be incorporated in NAFLD disease staging
Acknowledgments
Grant support: This work is in part supported by NIH grants to ML (K23DK083439) and MH (R01DK100425).
Disclosure: MAJ and JDO are employees at LabCorp. LabCorp employees were responsible for sample testing, but blinded to any patient level information. Only researches at BIDMC were responsible for data analysis.
Abbreviations
- AUROC
area under the ROC
- HDL
high density lipoprotein
- IDL
intermediate density lipoprotein
- LDL
low density lipoprotein
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NAS
NAFLD activity score
- NFS
NAFLD fibrosis score
- nm
nanometer
- NMR
nuclear magnetic resonance
- PNPLA3
patatin-like phospholipase domain-containing 3
- ROC
receiver operative characteristics
- SNP
single nucleotide polymorphism
- TM6SF2
Transmembrane 6 Superfamily Member 2
- VLDL
very low density lipoprotein
Footnotes
Additional Supporting Information may be found at onlinelibrary.wiley.com/doi/10.1111/liv.13076/suppinfo
Conflict of interest: The authors do not have any disclosures to report.
References
- 1.Afdhal NH. Management of nonalcoholic fatty liver disease: a 60-year-old man with probable nonalcoholic fatty liver disease: weight reduction, liver biopsy, or both? JAMA. 2012;308:608–616. doi: 10.1001/jama.2012.8402. [DOI] [PubMed] [Google Scholar]
- 2.Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol. 2011;9:524–530. e1. doi: 10.1016/j.cgh.2011.03.020. quiz e60. [DOI] [PubMed] [Google Scholar]
- 3.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD American Association for the Study of Liver D. Liver biopsy. Hepatology. 2009;49:1017–1044. doi: 10.1002/hep.22742. [DOI] [PubMed] [Google Scholar]
- 5.Castera L, Vilgrain V, Angulo P. Noninvasive evaluation of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:666–675. doi: 10.1038/nrgastro.2013.175. [DOI] [PubMed] [Google Scholar]
- 6.Tapper EB, Afdhal NH. Vibration-controlled transient elastography: a practical approach to the noninvasive assessment of liver fibrosis. Curr Opin Gastroenterol. 2015;31:192–198. doi: 10.1097/MOG.0000000000000169. [DOI] [PubMed] [Google Scholar]
- 7.Murphy P, Hooker J, Ang B, et al. Associations between histologic features of nonalcoholic fatty liver disease (NAFLD) and quantitative diffusion-weighted MRI measurements in adults. J Magn Reson Imaging. 2015;41:1629–1638. doi: 10.1002/jmri.24755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwok R, Tse YK, Wong GH, et al. Systematic review with meta-analysis: non-invasive assessment of non-alcoholic fatty liver disease – the role of transient elastography and plasma cytokeratin-18 fragments. Aliment Pharmacol Ther. 2014;39:254–269. doi: 10.1111/apt.12569. [DOI] [PubMed] [Google Scholar]
- 9.Cusi K, Chang Z, Harrison S, et al. Limited value of plasma cytokeratin-18 as a biomarker for NASH and fibrosis in patients with non-alcoholic fatty liver disease. J Hepatol. 2014;60:167–174. doi: 10.1016/j.jhep.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 10.Chan WK, Sthaneshwar P, Nik Mustapha NR, Mahadeva S. Limited utility of plasma M30 in discriminating non-alcoholic steatohepatitis from steatosis–a comparison with routine biochemical markers. PLoS ONE. 2014;9:e105903. doi: 10.1371/journal.pone.0105903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang ZG, Mukamal K, Tapper E, Robson SC, Tsugawa Y. Low LDL-C and high HDL-C levels are associated with elevated serum transaminases amongst adults in the United States: a cross-sectional study. PLoS ONE. 2014;9:e85366. doi: 10.1371/journal.pone.0085366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corey KE, Lai M, Gelrud LG, et al. Non-high-density lipoprotein cholesterol as a biomarker for nonalcoholic steatohepatitis. Clin Gastroenterol Hepatol. 2012;10:651–656. doi: 10.1016/j.cgh.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser A, Longnecker MP, Lawlor DA. Prevalence of elevated alanine aminotransferase among US adolescents and associated factors: NHANES 1999–2004. Gastroenterology. 2007;133:1814–1820. doi: 10.1053/j.gastro.2007.08.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Defilippis AP, Blaha MJ, Martin SS, et al. Nonalcoholic fatty liver disease and serum lipoproteins: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2013;227:429–436. doi: 10.1016/j.atherosclerosis.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siddiqui MS, Fuchs M, Idowu MO, et al. Severity of nonalcoholic Fatty liver disease and progression to cirrhosis are associated with atherogenic lipoprotein profile. Clin Gastroenterol Hepatol. 2015;13:1000–1008. e3. doi: 10.1016/j.cgh.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannisto VT, Simonen M, Soininen P, et al. Lipoprotein subclass metabolism in nonalcoholic steatohepatitis. J Lipid Res. 2014;55:2676–2684. doi: 10.1194/jlr.P054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang ZG, Robson SC, Yao Z. Lipoprotein metabolism in nonalcoholic fatty liver disease. J Biomed Res. 2013;27:1–13. doi: 10.7555/JBR.27.20120077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarugi P, Averna M. Hypobetalipoproteinemia: genetics, biochemistry, and clinical spectrum. Adv Clin Chem. 2011;54:81–107. [PubMed] [Google Scholar]
- 19.Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rotman Y, Koh C, Zmuda JM, Kleiner DE, Liang TJ. The association of genetic variability in patatin-like phospholipase domain-containing protein 3 (PNPLA3) with histological severity of nonalcoholic fatty liver disease. Hepatology. 2010;52:894–903. doi: 10.1002/hep.23759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dongiovanni P, Petta S, Maglio C, et al. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61:506–514. doi: 10.1002/hep.27490. [DOI] [PubMed] [Google Scholar]
- 22.Liu YL, Reeves HL, Burt AD, et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with non-alcoholic fatty liver disease. Nat Commun. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pirazzi C, Adiels M, Burza MA, et al. Patatin-like phospholipase domain-containing 3 (PNPLA3) I148M (rs738409) affects hepatic VLDL secretion in humans and in vitro. J Hepatol. 2012;57:1276–1282. doi: 10.1016/j.jhep.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 24.Festa A, Williams K, Hanley AJ, et al. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study. Circulation. 2005;111:3465–3472. doi: 10.1161/CIRCULATIONAHA.104.512079. [DOI] [PubMed] [Google Scholar]
- 25.Mora S, Otvos JD, Rosenson RS, et al. Lipoprotein particle size and concentration by nuclear magnetic resonance and incident type 2 diabetes in women. Diabetes. 2010;59:1153–1160. doi: 10.2337/db09-1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Charlton M, Sreekumar R, Rasmussen D, Lindor K, Nair KS. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology. 2002;35:898–904. doi: 10.1053/jhep.2002.32527. [DOI] [PubMed] [Google Scholar]
- 27.Fujita K, Nozaki Y, Wada K, et al. Dysfunctional very-low-density lipoprotein synthesis and release is a key factor in nonalcoholic steatohepatitis pathogenesis. Hepatology. 2009;50:772–780. doi: 10.1002/hep.23094. [DOI] [PubMed] [Google Scholar]
- 28.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–870. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 29.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 30.Feldstein AE, Wieckowska A, Lopez AR, et al. Cytokeratin-18 fragment levels as noninvasive biomarkers for nonalcoholic steatohepatitis: a multicenter validation study. Hepatology. 2009;50:1072–1078. doi: 10.1002/hep.23050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angulo P, Hui JM, Marchesini G, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 32.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 33.Chan DC, Watts GF, Gan S, et al. Nonalcoholic fatty liver disease as the transducer of hepatic oversecretion of very-low-density lipoprotein-apolipoprotein B-100 in obesity. Arterioscler Thromb Vasc Biol. 2010;30:1043–1050. doi: 10.1161/ATVBAHA.109.202275. [DOI] [PubMed] [Google Scholar]
- 34.Rivellese AA, Patti L, Kaufman D, et al. Lipoprotein particle distribution and size, insulin resistance, and metabolic syndrome in Alaska Eskimos: the GOCADAN study. Atherosclerosis. 2008;200:350–358. doi: 10.1016/j.atherosclerosis.2007.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mackey RH, Mora S, Bertoni AG, et al. Lipoprotein particles and incident Type 2 diabetes in the multi-ethnic study of atherosclerosis. Diabetes Care. 2015;38:628–636. doi: 10.2337/dc14-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cicognani C, Malavolti M, Morselli-Labate AM, et al. Serum lipid and lipoprotein patterns in patients with liver cirrhosis and chronic active hepatitis. Arch Intern Med. 1997;157:792–796. [PubMed] [Google Scholar]
- 37.Jiang ZG, Tsugawa Y, Tapper EB, et al. Low-fasting triglyceride levels are associated with non-invasive markers of advanced liver fibrosis among adults in the United States. Aliment Pharmacol Ther. 2015;42:106–116. doi: 10.1111/apt.13216. [DOI] [PubMed] [Google Scholar]
- 38.Pimentel CF, Jiang ZG, Otsubo T, et al. Poor inter-test reliability between CK18 kits as a biomarker of NASH. Dig Dis Sci. 2015 Oct 13; doi: 10.1007/s10620-015-3916-1. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hyysalo J, Mannisto VT, Zhou Y, et al. A population-based study on the prevalence of NASH using scores validated against liver histology. J Hepatol. 2014;60:839–846. doi: 10.1016/j.jhep.2013.12.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.