Abstract
There is a health disparity for both bacterial vaginosis (BV) and human immunodeficiency virus type 1 (HIV‐1) infection in African American women that may be linked. The evidence that BV predisposes women to higher risk for HIV infection is well documented. The underlying mechanisms to support the epidemiological connections will require further investigations. This review explores the risk factors for BV disease with implications for HIV‐1 acquisition in the context of race as a potential driver of the 20‐fold increase in HIV‐1 acquisition for African American women compared to white women. Specifically, it explores (i) disparities for BV in African American women, (ii) racial disparity for HIV‐1 acquisition in African American women, (iii) common factors associated with BV and HIV acquisition in African American women, and (iv) potential mechanisms of the enhancement of HIV‐1 transmission by BV.
Keywords: BV, inflammation, pathogenesis, race, stress, vaginal epithelium
Introduction
Bacterial vaginosis (BV) is a common vaginal disorder in women first reported by Gardner and Dukes in 1955.1 In women of childbearing age, BV is the most common cause of vaginitis and has also been associated with fetal loss, chorioamnionitis, cervicitis, endometritis, urinary tract infections, cervical intraepithelial neoplasia, pelvic inflammatory disease (PID), preterm labor, and delivery of low birth weight infants.2, 3, 4, 5 BV occurs in nearly 29% of 14‐ to 49‐year‐old women in the United States with an approximate 50% infection rate in African American women and higher infection rates for women in sub‐Saharan Africa.6, 7 African American women are also disproportionately impacted by HIV/AIDS with infection rates more than 20× the rate for white females and account for 66% of all new AIDS cases in women.8 In recent years, BV has been significantly associated with an increased incidence of HIV infection.9, 10, 11, 12, 13 Analyses performed by Atashili et al.14 suggested that BV increases the risk for HIV‐1 acquisition by 60%. This review explores the epidemiologic synergy between the two infections as well as the biologic basis for this synergy.
Racial disparity for BV in African American women
Black and Mexican American women have significantly higher rates of BV with prevalence rates of 51.6% and 32.1%, respectively, than white, non‐Hispanic women (23.2%).5 The prevalence of BV has been associated with several demographic and behavioral factors that include race, age, the number of sexual partners, the use of hormonal contraceptives, menopausal status, smoking, alcohol consumption, and douching.15 Although vaginal douching is more common among African American and Caribbean women than among white women, this practice appears to be independent of other behavioral factors.16
Bacterial vaginosis is characterized by a loss of vaginal lactobacilli usually found in healthy women, followed by an overgrowth of a mixed vaginal flora consisting mainly of anaerobic and facultative aerobic bacteria that includes Gardnerella vaginalis and Mycoplasma hominis, as well as Atopobium, Mobiluncus, Bacteroides, Prevotella, and Peptostreptococcus species.17, 18 The microbiome in BV (BV‐associated bacteria) is very diverse, and the studies suggested that it varies with ethnicity. Deep sequencing analysis of BV‐associated bacteria (BVAB) has been performed. Srinivasan et al. used deep sequencing of the 16S rRNA gene from BV‐associated bacteria from 220 women with and without BV coupled with species‐level taxonomic identification to determine the associations between the presence of individual bacterial species and clinical diagnostic characteristics of BV. They found that several BV‐associated bacteria showed race‐dependent prevalence when analyzed in separate groups by BV status.19 In their final analysis, 28 bacterial taxa were significantly associated with race and included specific Leptotrichia and Atopobium species.19 These bacteria were more prevalent in African American women without BV than in white women without BV.
Additionally, African American women's vaginal microflora was dominated by Lactobacillus iners and white women's by L. crispatus. This is thought to be significant because women with high levels of L. crispatus generally have low vaginal pH, whereas women with high levels of L. iners have both high and low vaginal pH.20 The presence of L. crispatus has been associated with healthy vaginal microenvironments. The authors suggested that this may contribute to the increased incidence of BV in African American women, but it remains inconclusive.20 The predominance of L. crispatus among a minority of women of African descent exhibits low inflammatory states in the cervicovaginal mucosa; however, the majority of these women have diverse cervicovaginal bacterial communities with a low abundance of lactobacilli21. This would argue that women of African descent are more likely to be colonized by diverse communities of genital bacteria that support a high inflammatory state. In the study performed by Anahtar et al.21 in young South African women with BV, only 37% of participants had lactobacillus‐dominant cervicovaginal bacterial communities. By comparison, studies in developed countries showed 90% and 62% with lactobacillus‐dominant cervicovaginal bacterial communities among black and white women, respectively.22, 23
These findings suggested that there are differences in vaginal bacteria profiles in women of African descent from developing countries compared to women of African descent from developed countries. They also found that among the South African women with lactobacillus‐dominant cervicovaginal communities, 77% were colonized with Lactobacillus iners.21 L. iners is unique among lactobacilli in its ability to survive in diverse cervicovaginal bacterial communities.24 Surprisingly, 45% of the 63% women in the cohort with no lactobacillus showed dominance with Gardnerella. Prevotella species were found at a level of 10% in all cohorts studied.21 This would suggest that Gardnerella is a dominant cervicovaginal species in women of African descent with BV.
A report by Royce et al.25 evaluating vaginal flora and vaginal pH of 842 women at 24–29 weeks of gestation, found that vaginal pH and vaginal flora differed significantly by race/ethnicity; African Americans were more likely to have a vaginal pH > or = 4.5, no lactobacilli, small gram‐variable and gram‐negative rods, and Mobiluncus bacterial species compared to white women after controlling for sociodemographics, sexual activity, sexually transmitted diseases, health behavior, and sexual hygiene. In a report by Friscella et al.26 that examined 13,917 women with BV from largely low‐income backgrounds enrolled during routine pre‐natal visits from 23 to 26 weeks of gestation, no significant difference in vaginal pH level between black and white women was found after controlling for differences in Gram stain score, age, and study site. The relationship between vaginal pH, race, and BV prevalence is conflicting and will require further study. However, colonization with specific types of Lactobacillus strains has been shown to influence vaginal pH as well as CO2‐level exposure when measuring the vaginal pH.27
Racial disparity for HIV‐1 acquisition in African American women
In the United States, women represent 25% of all persons living with HIV/AIDS.28 African American women are disproportionately impacted by HIV/AIDS with infection rates more than 20× the rate of their white female counterparts.29 In 2014, the CDC projected that 1 in 32 African American women in the United States will be diagnosed with HIV at some point in their lifetime.30 In 2010, African American teens and young adults represented 57% of all new infections in that age group.31 HIV infection is a leading cause of death among African American women aged 25–44.32 In the United States, older African women represent one of the fastest growing populations newly infected with HIV.33 Older African women infected with HIV are an emerging population that will increase over time due to the use of antiviral therapy.33
The U.S. criminal justice system has the highest incarceration rate in the world.34 African American women are disproportionately impacted by the U.S. criminal justice system.35 African American women are 7 times more likely to be incarcerated in their lifetime than white women.36 Incarcerated women have higher rates of HIV and sexually transmitted infections (STI) than the general population.37 In 2010, 1.9% of incarcerated adult women in the United States were HIV positive, which is 13 times the rate of adult women in the general population (0.15%).38 There are approximately 23,000 HIV‐infected adult women released from correctional institutions annually in the United States with the majority of being African American women.39
Common factors associated with BV and HIV acquisition in African American women
There is a disproportionately high prevalence of herpes simplex virus type 2 (HSV‐2) infection among African American women.40, 41, 42 In a study carried out by Alsworth et al.43 African American women had an HSV‐2 seroprevalence of 48% compared to 16% for white women. It is well documented that HSV‐2 infection is associated with a threefold increased risk for HIV‐1 acquisition in women.44 This increased risk is present even in the absence of HSV‐2 virus replication.45 The vaginal mucosa is known to retain CCR5+ CD4+ T cells, as well as infiltrating plasmacytoid and myeloid dendritic cells (DCs), even after HSV‐2 lesions heal and in the presence of acyclovir therapy.46, 47 The persistence of HIV target cells in the vaginal mucosa could help to explain the increased risk for HIV transmission in HSV‐2‐positive individuals.
Recent studies showed that HSV‐2 infection promotes a reduction in normal vaginal lactobacilli that could contribute to BV acquisition.48, 49 Esber et al.50 performed a systematic review and meta‐analysis of the link between HSV‐2 infection and BV and found that HSV‐2 is an important risk factor for BV acquisition and that pharmacologic HSV‐2 suppression may reduce BV incidence and BV‐associated adverse events.
Recent studies have shown a higher incidence of BV and HIV infection among women with vitamin D deficiency.51, 52, 53 It has been suggested that African American women and women with heavily pigmented skin may be more prone to develop vitamin D deficiencies because of photoprotection by higher levels of melanin in the skin which prevents the skin from absorbing sunlight needed for vitamin D production.54 However, the exact mechanistic role of melanin in vitamin D synthesis remains unclear.54 In a substudy of the Women's Interagency HIV Study (WIHS), vitamin D deficiency was independently associated with BV among HIV‐infected women compared to HIV‐uninfected women.55 The authors concluded that vitamin D deficiency was significantly associated with BV among HIV‐infected women in the cohort and that the mechanistic role of vitamin D deficiency in BV disease and HIV‐1 acquisition requires further examination.55 The authors suggested that the prevalence of vitamin D deficiency in African American women could explain, in part, the racial disparity associated with BV disease. Other studies have found that risk for BV in association with vitamin D deficiency differs by pregnancy status.56 A study performed by Turner et al., examined the ability of vitamin D and metronidazole therapy to reduce BV recurrence. Their findings revealed that short‐term, high‐dose vitamin D supplementation did not reduce BV recurrence.57 However, previous studies examined BV prevalence rather than the recurrence of BV. These authors stated that it is currently unknown whether sufficient vitamin D supplementation can prevent the initial development of BV.57 The effects of vitamin D supplementation on BV recurrence may require more than a 24‐week vitamin D supplementation and metronidazole therapy regimen as performed in the study by Turner et al. Existing data supporting vitamin D and BV prevalence also focused on pregnant women and may suggest that vitamin D effects on BV prevalence in pregnant women are different because of the unique conditions of pregnancy including hormone levels and immune factors.58, 59
One study suggested that the male partner's race is a risk factor for BV disease, supporting the notion that BV is sexually transmitted and could help explain the disparity of BV in African American women.60 Klebanoff et al.60 showed that BV occurred in 12.8% of 906 sexually active intervals compared to white women of 24.8% of sexually active intervals when the woman reported an African American partner and 10.7% when all partners were white. The small size of this study is a limitation and the results are controversial because the association with race of the male partner BV prevalence could be directly linked to having a previous partner with BV. Taken together, these findings will require further investigation.
Poverty that leads to poor educational opportunities, housing insecurity, and the lack of access to care are some of the common problems for African American women at risk for both BV and HIV acquisition.61, 62 Studies that examined BV acquisition among socioeconomically disadvantaged women suggested that women with low socioeconomic status are at an increased risk for BV acquisition.63, 64 These women are more likely to be exposed to sexually transmitted infections and participate in unhealthy behaviors like cigarette smoking which has also been associated with the increased risk for BV.65, 66
Exposure to chronic stress can predispose individuals to disease by the impairment of immune function, leading to the overproduction of glucocorticoid hormones and catecholamines.67 Stress‐related immune dysfunction has been associated with poor responsiveness to vaccines, increased risks for upper respiratory tract infections, impaired wound healing, and progression of HIV infection.68, 69 Studies reported that African American women are exposed to higher levels of psychological and physiological stressors than white women and that increased chronic stress is associated with the increased risk for BV.70, 71, 72 In a study by Culhane et al.73 after adjusting for maternal age, race, education, parity, douching, the number of sexual partners, sexual practices, and the use of illicit drugs, women with high psychological stress were 2.2 times more likely to develop BV. In addition, stressors that include perceived racial discrimination, poverty, poor housing, and exposure to crime over a life course have also been associated with the increased risk for BV.74
Potential mechanisms of enhancement of HIV‐1 transmission by BV
Klebanoff et al.75 reported that the presence and level of lactobacilli in the vaginal fluid could influence heterosexual transmission of HIV via the bacterium's release of hydrogen peroxide that results in the inactivation of HIV. Therefore, women with no or low levels of hydrogen peroxide‐producing vaginal lactobacilli would be at an increased risk for HIV‐1 infection.76 Interactions between interleukin receptor 2 (IL‐1R2) and Toll‐like receptor 4 (TLR4) have been shown to be associated with proinflammatory cervical cytokine concentrations. A report by Rkycman et al.77 examining single nucleotide polymorphisms (SNPs) in cytokine genes associated with cervical cytokine levels in African American and white women found significant gene–gene interactions between IL‐1R2 rs485127 and two SNPs in TLR4 (rs1554973 and rs7856729) and with IL‐1β. Differences were observed in allele frequencies between African Americans and white women. These gene–gene interactions likely impact regulatory mechanisms involving cervical cytokine concentrations that can be influenced under conditions like BV.78 This heightened proinflammatory state of the cervical epithelium could result in the increased recruitment of target cell populations and subsequent increased risks for HIV‐1 acquisition.
Bacterial vaginosis enhances HIV‐1 replication and leads to the increased vaginal shedding of HIV.79 The bacterial flora in BV has been shown to induce HIV‐1 transcriptional activation and replication by stimulating and recruiting HIV‐infected cells.80 BV bacterial lysates have been shown to increase the transcriptional activity of HIV81, and our research suggested that the persistence of BV‐associated bacteria may affect the structural integrity of the vaginal epithelium.81 This in turn would allow HIV to pass between vaginal epithelial cells, which otherwise prevent the virus’ ability to gain access to submucosal CD4+ T cells, the virus’ preferred host cell type. The persistent presence of BV bacteria could heighten the inflammatory state of the vaginal epithelium, resulting in the recruitment of cells permissive for HIV infection.
When squamous vaginal epithelial cells (VK2) are exposed with both macrophage and T‐cell tropic strains of HIV, no significant evidence for direct infection is observed even at high multiplicities of infection. This finding is not surprising because VK2 cells do not have receptors or coreceptors for HIV. In our studies, we observed upregulation of TNF‐α, IL‐6, IL‐8, and IL‐1β just 1 hr post‐exposure to a pure culture of the BV‐associated bacteria G. vaginalis 81 (Marrs and Alcendor, 2012, Fig. 1). G. vaginalis infection also decreased the expression levels of tight junction proteins ZO‐1 and ZO‐2, with no significant change in adherens junction protein expression levels (Alcendor et al., personal communications, Fig. 1). After characterization of the expression profile of vaginal epithelial cells exposed to G. vaginalis, Marrs et al.81 observed that the cytoskeletal protein vimentin was upregulated within 18 hrs post‐exposure (Fig. 1). Vimentin is a class III cytoskeletal intermediate filament protein that supports cell strength and tissue integrity. Vimentin has also been shown to interact with the ibeA gene product of E. coli K1, a known virulence factor associated with the binding and invasion of E. coli K1 into human brain microvascular endothelial cells.82 Marrs et al.81 performed transmission electron microscopy and confocal microscopy experiments, which showed the internalization of G. vaginalis by vaginal epithelial cells (VECs). These tight junction and cytoskeletal protein modifications could represent a potential mechanism for bacterial‐mediated uptake and internalization by vaginal epithelial cells. The ability of G. vaginalis to be taken up and internalized by VECs could contribute to the persistence of the bacteria in the vaginal epithelium. A report by Fichorova et al.83 showed the intracellular presence of Atopobium vaginae and Prevotella bivia in vaginal epithelial cells, which supports the notion that many vaginal bacteria have an intracellular presence in BV.
Figure 1.
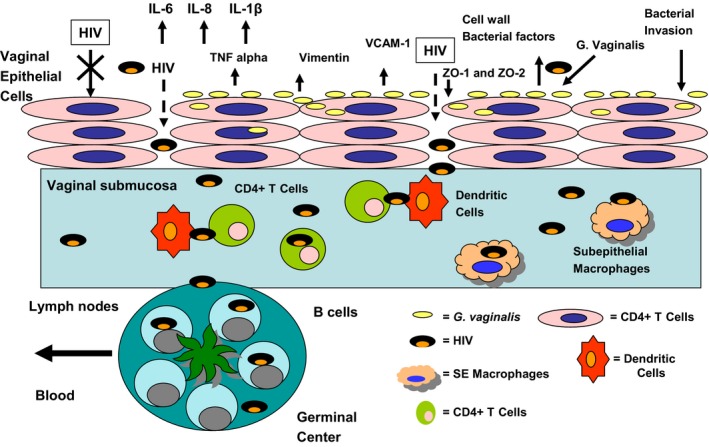
Exposure of vaginal epithelial cells to Gardnerella vaginalis results in upregulation of proinflammatory cytokines, namely IL‐6, IL‐8, TNF‐α, and IL‐1β. Vimentin is upregulated after G. vaginalis exposure. Upregulation of vimentin influences uptake and internalization of bacteria. Tight junction proteins (TJ) ZO1 and ZO2 are downregulated in vaginal epithelial cells exposed to G. vaginalis. Downregulation of TJ proteins could result in HIV passing in between cells (paracellular transport) in route to the vaginal submucosa where subepithelial T cells and macrophages reside and are highly permissive for HIV infection. Trafficking of infected T cells by resident lymph nodes would facilitate HIV dissemination via the blood.
Internalization would allow these bacteria to survive metronidazole and clindamycin therapy, the current standard of care for BV, thus contributing to the high rate of recurrence of BV in some women.84, 85 Moreover, the intracellular presence of G. vaginalis, A. vaginae, and P. bivia in the genital tract could allow these organisms to evade immune responses. A persistent intracellular presence of these bacteria would stimulate cytokine cascades, heightening the inflammatory state of the vaginal epithelium and making it more susceptible to infections like HIV‐1, a virus known to benefit from the inflammatory recruitment of CD4+ T cells. In addition, downregulation of tight junction proteins could facilitate the paracellular transport of HIV into the vaginal submucosa (Fig. 1). This would provide a mechanism to help explain increased HIV‐1 transmission rates in BV‐positive women. Additional studies are needed to provide direct evidence for tight junction disruption of the vaginal epithelium by G. vaginalis or other BV‐related bacteria. Fig. 1 shows vaginal epithelial cells after exposure to G. vaginalis as a prototype BV‐associated bacteria, but similar mechanisms may be applicable to other BV bacteria such as Atopobium vaginae and Prevotella bivia.
G. vaginalis is thought to be necessary for the establishment of BV, but alone is not sufficient to produce BV. Other bacterial species, including Atopobium vaginae and Prevotella bivia, are found in BV.83 In a study by Mitchell et al.86 they detected lower levels of alpha defensins 1–3 in pregnant black and Hispanic women with BV compared to white women with BV. They also determined that increased concentrations of Atophobium vaginae, BVAB1, and BVAB2 were associated with lower levels of human beta defensin‐3 (HBD3), but not human beta defensin 2 (HBD2) or alpha defensins 1–3.86 Human beta defensins have been shown to be protective against HIV‐1 infection.87, 88, 89 Herrera et al.90 showed HIV inactivation and a reduction in HIV transepithelial transmission by human beta defensin‐3 in adult oral epithelial cells.
Conclusions
Going forward, the epidemiological trends for African American women at risk for BV and HIV are discouraging. Therefore, targeted interventions that will reduce the incidence of vaginal infections, treat dietary deficiencies, enhance social support structures, and reduce poverty and its downstream effects on at‐risk communities will likely reduce the incidence of BV and HIV acquisition in African American women. Understanding abnormal vaginal bacteria/host interactions in vaginal epithelium in women of different ethnic backgrounds will allow us to determine the underlying mechanisms involving vaginal bacterial species, their link to BV pathogenesis, and its contribution to increased risk for HIV‐1 sexual transmission. With the well‐documented disparity in HIV incidence between black and white women, there may also be a genetic basis for BV occurrence in black women disproportionate to women of other ethnic backgrounds. This in turn would also increase the risk of HIV‐1 transmission in African American women. BV is a treatable condition. Increased screening for BV and its subsequent treatment could greatly reduce the burden of HIV and AIDS among black women. Long‐term results from these studies may also provide direction for therapeutic BV/HIV strategies resulting in changes in health policy to reduce the burden of HIV‐related health disparities.
Acknowledgements
I would like to acknowledge Jane Schwebke for reviewing the manuscript and Jared Elzey of the Meharry Research Concierge Services (supported by NIH grants U54MD007593 and UL1TR000445) for editing and comments. D.J.A. was supported by the Meharry Translational Research Center (NIH U54 MD0007593‐07); Tennessee Center for AIDS Research (NIH P30 AI110527).
Alcendor Donald J.. Evaluation of health disparity in bacterial vaginosis and the implications for HIV‐1 acquisition in African American women. Am J Reprod Immunol 2016; 76: 99–107. doi: 10.1111/aji.12497
References
- 1. Gardner HL, Dukes CD: Haemophilus vaginalis vaginitis: a newly defined specific infection previously classified nonspecific vaginitis. Am J Obstet Gynecol 1955; 5:962–976. [PubMed] [Google Scholar]
- 2. Gibbs RS, Weiner MH, Walmer K, St Clair PJ. Microbiologic and serologic studies of Gardnerella vaginalis in intra‐amniotic infection. Obstet Gynecol 1987; 2:187–190. [PubMed] [Google Scholar]
- 3. Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, Cotch MF, Edelman R, Pastorek JG 2nd, Rao AV: Association between bacterial vaginosis and preterm delivery of a low‐birth‐weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med 1995; 26:1737–1742. [DOI] [PubMed] [Google Scholar]
- 4. Holst E, Goffeng AR, Andersch B: Bacterial vaginosis and vaginal microorganisms in idiopathic premature labor and association with pregnancy outcome. J Clin Microbiol 1994; 1:176–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Meis PJ, Goldenberg RL, Mercer B, Moawad A, Das A, McNellis D, Johnson F, Iams JD, Thom E, Andrews WW: The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal‐Fetal Medicine Units Network. Am J Obstet Gynecol 1995; 4:1231–1235. [DOI] [PubMed] [Google Scholar]
- 6. Ness RB, Hillier S, Richter HE, Soper DE, Stamm C, Bass DC, Sweet RL, Rice P: Can known risk factors explain racial differences in the occurrence of bacterial vaginosis? J Natl Med Assoc 2003; 3:201–212. [PMC free article] [PubMed] [Google Scholar]
- 7. Allsworth JE, Peipert JF: Prevalence of bacterial vaginosis: 2001‐2004 National Health and Nutrition Examination Survey data. Obstet Gynecol 2007; 109:114–120. [DOI] [PubMed] [Google Scholar]
- 8. Centers for Disease Control (CDC) . HIV/AIDS Surveillance Report, 2005. Vol. 17 Rev ed. Atlanta: US Department of Health and Human Services, CDC; 2007: 1–46. [Google Scholar]
- 9. Martin HL, Richardson BA, Nyange PM, Lavreys L, Hillier SL, Chohan B, Mandaliya K, Ndinya‐Achola JO, Bwayo J, Kreiss J: Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. J Infect Dis 1999; 6:1863–1868. [DOI] [PubMed] [Google Scholar]
- 10. Sewankambo N, Gray RH, Wawer MJ, Paxton L, McNaim D, Wabwire‐Mangen F, Serwadda D, Li C, Kiwanuka N, Hillier SL, Rabe L, Gaydos CA, Quinn TC, Konde‐Lule J: HIV‐1 infection associated with abnormal vaginal flora morphology and bacterial vaginosis. Lancet 1997; 9077:546–550. [DOI] [PubMed] [Google Scholar]
- 11. Taha TE, Gray RH, Kumwenda NI, Hoover DR, Mtimavalye LA, Liomba GN, Chiphangwi JD, Dallabetta GA, Miotti PG: HIV infection and disturbances of vaginal flora during pregnancy. J Acquir Immune Defic Syndr Hum Retrovirol 1999; 1:52–59. [DOI] [PubMed] [Google Scholar]
- 12. Taha TE, Hoover DR, Dallabetta GA, Kumwenda NI, Mtimavalye LA, Yang LP, Liomba GN, Broadhead RL, Chiphangwi JD, Miotti PG: Bacterial vaginosis and disturbances of vaginal flora: association with increased acquisition of HIV. AIDS 1998; 13:1699–1706. [DOI] [PubMed] [Google Scholar]
- 13. Cohen CR, Duerr A, Pruithithada N, Rugpao S, Hillier S, Garcia P, Nelson K: Bacterial vaginosis and HIV seroprevalence among female commercial sex workers in Chiang Mai, Thailand. AIDS 1995; 9:1093–1097. [DOI] [PubMed] [Google Scholar]
- 14. Atashili J, Poole C, Ndumbe PM, Adimora AA, Smith JS: Bacterial vaginosis and HIV acquisition: a meta‐analysis of published studies. AIDS 2008; 22:1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Koumans EH, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, Markowitz LE: The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis 2007; 11:864–869. [DOI] [PubMed] [Google Scholar]
- 16. Cottrell BH: An updated review of evidence to discourage douching. MCN Am J Matern Child Nurs 2010; 2:102–107. [DOI] [PubMed] [Google Scholar]
- 17. Hillier SL: Diagnostic microbiology of bacterial vaginosis. Am J Obstet Gynecol 1993; 169:455–459. [DOI] [PubMed] [Google Scholar]
- 18. Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK: Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 1983; 1983:14–22. [DOI] [PubMed] [Google Scholar]
- 19. Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, Ross FJ, McCoy CO, Bumgarner R, Marrazzo JM, Fredricks DN: Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS ONE 2012; 6:e37818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hummelen R, Fernandes AD, Macklaim JM, Dickson RJ, Changalucha J, Gloor GB, Reid G: Deep sequencing of the vaginal microbiota of women with HIV. PLoS ONE 2010; 8:e12078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anahtar MN, Byrne EH, Doherty KE, Bowman BA, Yamamoto HS, Soumillon M, Padavattan N, Ismail N, Moodley A, Sabatini ME, Ghebremichael MS, Nusbaum C, Huttenhower C, Virgin HW, Ndung'u T, Dong KL, Walker BD, Fichorova RN, Kwon DS. Cervicovaginal bacteria are a major modulator of host inflammatory responses in the female genital tract. Immunity. 2015; 5:965–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ: Vaginal microbiome of reproductive‐age women. Proc Natl Acad Sci USA 2011; 1:4680–4687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhou X, Brown CJ, Abdo Z, Davis CC, Hansmann MA, Joyce P, Foster JA, Forney LJ: Differences in the composition of vaginal microbial communities found in healthy Caucasian and black women. ISME J 2007; 1:121–133. [DOI] [PubMed] [Google Scholar]
- 24. Macklaim JM, Fernandes AD, Di Bella JM, Hammond JA, Reid G, Gloor GB: Comparative meta‐RNA‐seq of the vaginal microbiota and differential expression by Lactobacillus iners in health and dysbiosis. Microbiome 2013; 1:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Royce RA, Thorp J, Granados JL, Savitz DA. Bacterial vaginosis associated with HIV infection in pregnant women from North Carolina. J Acquir Immun Defic Syndr Hum Retrovirol. 1999; 20:382–386. [DOI] [PubMed] [Google Scholar]
- 26. Fiscella K, Klebanoff MA: Are racial difference in vaginal pH explained by vaginal flora? Am J Obstet Gynecol 2004; 3:747–750. [DOI] [PubMed] [Google Scholar]
- 27. Mirmonsef P, Spear GT: The barrier to HIV transmission provided by genital tract lactobacillus colonization. Am J Reprod Immunol 2014; 16:531–536. [DOI] [PubMed] [Google Scholar]
- 28. Centers for Disease Control and Prevention . (2013a). HIV among women. Atlanta, GA: Author. Retrieved from http://www.cdc.gov/hiv/risk/gender/women/facts/index.html [Google Scholar]
- 29. Centers for Disease Control and Prevention . (2013b). HIV surveillance report, 2011. Atlanta, GA: Author. Retrieved from http://www.cdc.gov/hiv/topics/surveillance/resources/reports/ [Google Scholar]
- 30. Centers for Disease Control and Prevention . (2014). Fact sheet: HIV among African Americans. Atlanta, GA: Author. Retrieved from http://www.cdc.gov/hiv/risk/racialethnic/aa/facts/ [Google Scholar]
- 31. CDC . HIV Surveillance Supplemental Report, Vol. 17, No. 4; December 2012. [Google Scholar]
- 32. Center for Disease Control and Prevention: National Center for Health Statistics (2011b). Deaths: final data for 2009. Natl Vital Stat Rep. 2011; 60: 1–117 [PubMed] [Google Scholar]
- 33. McCord LR: Attention HIV: older African American women define sexual risk. Cult Health Sex 2014; 1:90–100. [DOI] [PubMed] [Google Scholar]
- 34. Glaze LE, Kaeble D. Correctional Populations in the United States, 2013. Washington DC: Office of Justice Programs, Bureau of Justice Statistics; 2014. Available at: http://www.antoniocasella.eu/nume/Glaze_Kaeble_dec14.pdf. Accessed July 3, 2015. [Google Scholar]
- 35. Hispanic prisoners in the United States. Washington, DC: The Sentencing Project; 2003. [Google Scholar]
- 36. Kouyoumdjian FG, Leto D, John S, Henein H, Bondy S: A systematic review and meta‐analysis of the prevalence of chlamydia, gonorrhoea and syphilis in incarcerated persons. Int J STD AIDS 2012; 4:248–254. [DOI] [PubMed] [Google Scholar]
- 37. Fogel CI, Crandell JL, Neevel AM, Parker SD, Carry M, White BL, Fasula AM, Herbst JH, Gelaude DJ: Efficacy of an adapted HIV and sexually transmitted infection prevention intervention for incarcerated women: a randomized controlled trial. Am J Public Health 2015; 4:802–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Centers for Disease Control and Prevention . HIV Surveillance Report, 2010. Vol. 22 Atlanta, GA: US Dept of Health and Human Services; 2012. Available at: http://www.cdc.gov/hiv/surveillance/resources/reports/2010 report/pdf/2010_HIV_Surveillance_Report_vol_22. pdf. Accessed May 21, 2014. [Google Scholar]
- 39. Spaulding AC, Seals RM, Page MJ, Brzozowski AK, Rhodes W, Hammett TM: HIV/AIDS among inmates of and releasees from US correctional facilities, 2006: declining share of epidemic but persistent public health opportunity. PLoS ONE 2009; 11:e7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Douglas JM Jr, Berman SM: Screening for HSV‐2 infection in STD clinics and beyond: a few answers but more questions. Sex Transm Dis 2009; 11:729–731. [DOI] [PubMed] [Google Scholar]
- 41. Pouget ER, Kershaw TS, Blankenship KM, Ickovics JR, Niccolai LM: Racial/ethnic disparities in undiagnosed infection with herpes simplex virus type 2. Sex Transm Dis 2010; 9:538–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xu F, Sternberg MR, Kottiri BJ, McQuillan GM, Lee FK, Nahmias AJ, Berman SM, Markowitz LE: Trends in herpes simplex virus type 1 and type 2 seroprevalence in the United States. JAMA 2006; 8:964–973. [DOI] [PubMed] [Google Scholar]
- 43. Allsworth J, Peipert J: Prevalence of bacterial vaginosis: 2001–2004 national health and nutrition examination survey data. Obstet Gynecol 2007; 1:114–120. [DOI] [PubMed] [Google Scholar]
- 44. Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ: Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta‐analysis of longitudinal studies. AIDS 2006; 20:73–83. [DOI] [PubMed] [Google Scholar]
- 45. Barnabas RV, Wasserheit JN, Huang Y, Janes H, Morrow R, Fuchs J, Mark KE, Casapia M, Mehrotra DV, Buchbinder SP, Corey L, NIAID HIV Vaccine Trials Network . Impact of herpes simplex virus type 2 on HIV‐1 acquisition and progression in an HIV vaccine trial (the Step study). J Acquir Immune Defic Syndr 2011; 57:238–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhu J, Hladik F, Woodward A, Klock A, Peng T, Johnston C, Remington M, Magaret A, Koelle DM, Wald A, Corey L: Persistence of HIV‐1 receptor‐positive cells after HSV‐2 reactivation is a potential mechanism for increased HIV‐1 acquisition. Nat Med 2009; 15:886–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Celum C, Wald A, Lingappa JR, Magaret AS, Wang RS, Mugo N, Mujugira A, Baeten JM, Mullins JI, Hughes JP, Bukusi EA, Cohen CR, Katabira E, Ronald A, Kiarie J, Farquhar C, Stewart GJ, Makhema J, Essex M, Were E, Fife KH, deBruyn G , Gray GE, McIntyre JA, Manongi R, Kapiga S, Coetzee D, Allen S, Inambao M, Kayitenkore K, Karita E, Kanweka W, Delany S, Rees H, Vwalika B, Stevens W, Campbell MS, Thomas KK, Coombs RW, Morrow R, Whittington WL, McElrath MJ, Barnes L, Ridzon R, Corey L, Partners in Prevention HSV/HIV Transmission Study Team . Acyclovir and transmission of HIV‐1 from persons infected with HIV‐1 and HSV‐2. N Engl J Med. 2010; 362: 427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cherpes TL, Hillier SL, Meyn LA, Busch JL, Krohn MA: A delicate balance: risk factors for acquisition of bacterial vaginosis include sexual activity, absence of hydrogen peroxide‐producing lactobacilli, black race, and positive herpes simplex virus type 2 serology. Sex Transm Dis 2008; 35:78–83. [DOI] [PubMed] [Google Scholar]
- 49. Kaul R, Nagelkerke NJ, Kimani J, Ngugi E, Bwayo JJ, Macdonald KS, Rebbaprgada A, Fonck K, Temmerman M, Ronald AR, Moses S, Kibera HIV: Study Group. Prevalent herpes simplex virus type 2 infection is associated with altered vaginal flora and an increased susceptibility to multiple sexually transmitted infections. J Infect Dis 2007; 196:1692–1697. [DOI] [PubMed] [Google Scholar]
- 50. Esber A, Vicetti Miguel RD, Cherpes TL, Klebanoff MA, Gallo MF, Turner AN. Risk of bacterial vaginosis among women with herpes simplex virus type 2 infection: a systematic review and meta‐analysis. J Infect Dis. 2015; 212:8–17. [DOI] [PubMed] [Google Scholar]
- 51. French AL, Adeyemi OM, Agniel DM, Evans CT, Yin MT, Anastos K, Cohen MH: The association of HIV status with bacterial vaginosis and vitamin D in the United States. J Womens Health 2011; 10:1497–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hensel KJ, Tandis TM, Gelber SE, Ratner AJ. Pregnancy‐specific association of vitamin D deficiency and bacterial vaginosis. Am J Obstet Gynecol. 2011; 204:41.e1–e9. [DOI] [PubMed] [Google Scholar]
- 53. Bodnar LM, Krohn MA, Simhan HN: Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr Epidemiol 2009; 139:1157–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Springbett P, Buglass S, Young AR: Photoprotection and vitamin D status. J Photochem Photbiol B 2010; 2:160–168. [DOI] [PubMed] [Google Scholar]
- 55. French AL, Adeyemi OM, Agniel DM, Evans CT, Yin MT, Anastos K, Cohen MH: The association of HIV status with bacterial vaginosis and vitamin D in the United States. J Womens Health 2011; 2011:1497–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hensel KJ, Tandis TM, Gelber SE, Ratner AJ. Pregnancy‐ specific association of vitamin D deficiency and bacterial vaginosis. Am J Obstet Gynecol. 2011; 204:41.e1–e9. [DOI] [PubMed] [Google Scholar]
- 57. Turner AN, Carr Reese P, Fields KS, Anderson J, Ervin M, Davis JA, Fichorova RN, Roberts MW, Klebanoff MA, Jackson RD. A blinded, randomized controlled trial of high‐dose vitamin D supplementation to reduce recurrence of bacterial vaginosis. Am J Obstet Gynecol. 2014; 5:479.e1–479.e479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dunlop AL1, Taylor RN, Tangpricha V, Fortunato S, Menon R. Maternal vitamin D, folate, and polyunsaturated fatty acid status and bacterial vaginosis during pregnancy. Infect Dis Obstet Gynecol. 2011; 2011:216–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hensel KJ1, Randis TM, Gelber SE, Ratner AJ. Pregnancy‐specific association of vitamin D deficiency and bacterial vaginosis. Am J Obstet Gynecol. 2011; 204: 41.e1–41.e9. [DOI] [PubMed] [Google Scholar]
- 60. Klebanoff MA, Andrews WW, Zhang J, Brotman RM, Nansel TR, Yu KF, Schwebke JR: Race of male sex partner and occurrence of bacterial vaginosis. Sex Transm Dis 2010; 3:184–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Koumans EH1, Sternberg M, Bruce C, McQuillan G, Kendrick J, Sutton M, Markowitz LE. The prevalence of bacterial vaginosis in the United States, 2001–2004; associations with symptoms, sexual behaviors, and reproductive health. Sex Transm Dis. 2007; 11:864–869. [DOI] [PubMed] [Google Scholar]
- 62. Hrostowski S1, Camp A. The Unchecked HIV/AIDS Crisis in Mississippi. Soc Work Health Care 2015; 5: 474–483. [DOI] [PubMed] [Google Scholar]
- 63. Paul K, Boutain D, Manhart L, Hitti J: Racial disparity in bacterial vaginosis: the role of socioeconomic status, psychosocial stress, and neighborhood characteristics, and possible implications for preterm birth. Soc Sci Med 2008; 67:824–833. [DOI] [PubMed] [Google Scholar]
- 64. Aral SO, Fenton KA, Holmes KK: Sexually transmitted diseases in the USA: temporal trends. Sexually Transmitted Infections 2007; 83:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ness RB, Hillier S, Richter HE, Soper DE, Stamm C, Bass DC, Sweet RL, Rice P: Can known risk factors explain racial differences in the occurrence of bacterial vaginosis? J Natl Med Assoc 2003; 95:201–212. [PMC free article] [PubMed] [Google Scholar]
- 66. Graham H, Inskip HM, Francis B, Harman J: Pathways of disadvantage and smoking careers: evidence and policy implications. J Epidemiol Community Health 2006; 60:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Elenkov IJ, Webster EL, Torpy DJ, Chrousos GP: Stress, corticotropin‐releasing hormone, glucocorticoids, and the immune/inflammatory response: acute and chronic effects. Ann NY Acad Sci 1999; 876:1–11. [DOI] [PubMed] [Google Scholar]
- 68. McIntosh RC, Rosselli M: Stress and coping in women living with HIV: a meta‐analytic review. AIDS Behav 2012; 8:2144–2159. [DOI] [PubMed] [Google Scholar]
- 69. Woo KY: Exploring the effects of pain and stress on wound healing. Adv Skin Wound Care 2012; 1:38–44. [DOI] [PubMed] [Google Scholar]
- 70. Culhane JF, Rauh V, McCollum KF, Elo IT, Hogan V: Exposure to chronic stress and ethnic differences in rates of bacterial vaginosis among pregnant women. Am J Obstet Gynecol 2002; 187:1272–1276. [DOI] [PubMed] [Google Scholar]
- 71. Culhane JF, Rauh V, McCollum KF, Hogan VK, Agnew K, Wadhwa PD: Maternal stress is associated with bacterial vaginosis in human pregnancy. Matern Child Health J 2001; 5:127–134. [DOI] [PubMed] [Google Scholar]
- 72. Paul K, Boutain D, Manhart L, Hitti J: Racial disparity in bacterial vaginosis: the role of socioeconomic status, psychosocial stress, and neighborhood characteristics, and possible implications for preterm birth. Soc Sci Med 2008; 67:824–833. [DOI] [PubMed] [Google Scholar]
- 73. Culhane JF1, Rauh VA, Goldenberg RL. Stress, bacterial vaginosis, and the role of immune processes. Curr Infect Dis Rep. 2006; 6:459–464. [DOI] [PubMed] [Google Scholar]
- 74. McIntosh RC, Rosselli M: Stress and coping in women living with HIV: a meta‐analytic review. AIDS Behav 2012; 8:2144–2159. [DOI] [PubMed] [Google Scholar]
- 75. Klebanoff SJ, Coombs RW: Viricidal effect of Lactobacillus acidophilus on human immunodeficiency virus type 1: possible role in heterosexual transmission. J Exp Med 1991; 1741:289–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Klebanoff SJ1, Kazazi F. Inactivation of human immunodeficiency virus type 1 by the amine oxidase‐peroxidase system. J Clin Microbiol. 1995; 8:2054–2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ryckman KK, Williams SM, Krohn MA, Simhan HN: Racial difference in cervical cytokine concentrations between pregnant women with and without bacterial vaginosis. J Reprod Immunol 2008; 78:166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ryckman KK, Simhan HN, Krohn MA, Williams SM: Cervical cytokine network patterns during pregnancy: The role of bacterial vaginosis and geographic ancestry. J Reprod Immunol 2009; 79:174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Cu‐Uvin S, Hogan JW, Caliendo AM, Harwell J, Mayer KH, Carpenter CC: Association between bacterial vaginosis and expression of human immunodeficiency virus type 1 RNA in the female genital tract. HIV Epidemiol Res Study Clin Infect Dis 2001; 33:894–896. [DOI] [PubMed] [Google Scholar]
- 80. Hashemi FB, Ghassemi M, Faro S, Aroucheva A, Spear GT. Induction of human immunodeficiency virus type 1 expression by anaerobes associated with bacterial vaginosis. J Infect Dis 2000; 181:1574–1580. [DOI] [PubMed] [Google Scholar]
- 81. Marrs C. N., Zhu W. Q, Sweet S., Chaudhry A., Alcendor DJ. Evidence for Gardnerella vaginalis uptake and internalization by squamous vaginal epithelial cells: Implications for the pathogenesis of bacterial vaginosis. Microbes Infect. 2012; 14: 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Chi F, Jong TD, Wang L, Ouyang Y, Wu C, Li W, Huang SH: Vimentin‐mediated signaling is required for IbeA+ E. coli K1 invasion of human brain microvascular endothelial cells. Biochem J 2010; 1:79–90. [DOI] [PubMed] [Google Scholar]
- 83. Fichorova RN, Buck OR, Yamamoto HS, Fashemi T, Dawood HY, Fashemi B, Hayes GR, Beach DH, Takagi Y, Delaney ML, Nibert ML, Singh BN, Onderdonk AB: The villain team‐up or how Trichomonas vaginalis and bacterial vaginosis alter innate immunity in concert. Sex Transm Infect 2013; 89:460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Francis SC, Looker C, Vandepitte J, Bukenya J, Mayanja Y, Nakubulwa S, Hughes P, Hayes RJ, Weiss HA, Grosskurth H. Bacterial vaginosis among women at high risk for HIV in Uganda: high rate of recurrent diagnosis despite treatment. Sex Transm Infect. 2015; 1–7, sextrans‐2015‐052160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hay P: Recurrent bacterial vaginosis. Curr Infect Dis 2002; 2002:506–512. [DOI] [PubMed] [Google Scholar]
- 86. Mitchell C, Gottsch ML, Liu C, Fredricks DN, Nelson DB. Associations between vaginal bacteria and levels of vaginal defensins in pregnant women. Am J Obstet Gynecol 2013; 208:132.e1–132.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zapata W, Aguilar‐Jiménez W, Feng Z, Weinberg A, Russo A, Potenza N, Estrada H, Rugeles MT. Identification of innate immune antiretroviral factors during in vivo and in vitro exposure to HIV‐1. Microbes Infect. 2015; S1286–S4579, doi:/10.1016/j.micinf.2015.10.009 [DOI] [PubMed] [Google Scholar]
- 88. Weinberg A1, Quiñones‐Mateu ME, Lederman MM. Role of human beta‐defensins in HIV infection. Adv Dent Res. 2006; 19:42–48. [DOI] [PubMed] [Google Scholar]
- 89. Sun L1, Finnegan CM, Kish‐Catalone T, Blumenthal R, Garzino‐Demo P, La Terra Maggiore GM, Berrone S, Kleinman C, Wu Z, Abdelwahab S, Lu W, Garzino‐Demo A. Human beta‐defensins suppress human immunodeficiency virus infection: potential role in mucosal protection. J Virol. 2005; 79:14318–14329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Herrera R, Morris M, Rosbe K, Feng Z, Weinberg A, Tugizov S: Human beta‐defensins 2 and ‐3 cointernalize with human immunodeficiency virus via heparan sulfate proteoglycans and reduce infectivity of intracellular virions in tonsil epithelial cells. Virology 2015; 487:172–187. [DOI] [PMC free article] [PubMed] [Google Scholar]


