Abstract
It has been recently shown that gut microbes modulate whole host immune and hormonal factors impacting the fate of distant preneoplastic lesions toward malignancy or regression. This raises the possibility that the tumor microenvironment interacts with broader systemic microbial-immune networks. These accumulated findings suggest novel therapeutic opportunities for holobiont engineering in emerging tumor microenvironments.
Keywords: tumor microenvironment, tumor macroenvironment, commensal microbes, carcinogenesis
Introduction
Accumulated cancer research has revealed that the tumor microenvironment contributes to neoplastic disease progression, invasion, and metastasis [1–3]. Recent findings in mice, however, take this notion further by showing that many tumors are less autonomous than previously thought. Intriguing data reveal that immune cells and other factors in the whole host environment determine the fate of dysplastic and preneoplastic lesions toward carcinogenesis or regression [4–11]. This systemic modulation of neoplastic disease may be described as the “tumor-macroenvironment.” [5]. In this context, the host microbiome is emerging as an important modulator of the tumor microenvironment even in extra-intestinal sites.
The Whole Host Shapes the Tumor Microenvironment
Using Paget’s “seed and soil” paradigm, where “seed” is a cancerous cell and “soil” is the tissue environment, the “soil” may either promote or hinder the carcinogenic process. Neoplastic outcomes appear to depend upon coinciding systemic immune-related events [1–4], perhaps explaining why people commonly exhibit pre-neoplastic lesions throughout their body though they don’t develop cancer [12]. Recognizing that human genes could be mutated up to 1010 times during an individual’s lifetime, it follows that ‘seeds’ are inevitable and ‘soil’ is a tractable target to modulate cancer growth.
Among elements of “soil” the immune system is composed of cellular elements and secreted factors that convey powerful signals to preserve homeostasis in epithelia throughout the body [4]. When functioning optimally, a balanced immune system conveys potent immune-stimulatory responses to external challenges with rapid restoration of homeostasis and minimal collateral tissue damage. More specifically, clinically silent gastrointestinal (GI) tract immune networks including neutrophils and regulatory T cells are integrated with commensal microbiota in whole host balance directing cancer development and growth [4, 6–11, 13–15]. Following this reasoning, harnessing the natural power of microbe-immune synergy may serve to suppress carcinogenic processes throughout the body.
At the whole organism level, the interactions of gut bacteria with the immune system is only one aspect of the complex, mechanistically inter-related systemic effects of gut microbiota. Accumulating evidence suggests that gut bacteria and products of their metabolic activities such as short-chain fatty acids, choline and bile salts, influence important metabolic pathways of the host relating to food intake, adiposity and lipid and energy homeostasis [11, 13, 15–23]. Gut bacteria and their products have also been shown to affect the hypothalamic-pituitary-adrenal axis and the production of neuroactive substances and various hormones including oxytocin, testosterone and thyroxin [17, 19, 20, 22, 24]. On this basis, the association of gut bacterial dysbiosis with a wide range of disease syndromes is not surprising. Changes in gut microbiota were originally linked mainly with inflammatory disorders such as autoimmune diseases and allergy. Recent data, however, expand the array of gut bacteria-associated diseases to include metabolic (obesity, type 2 diabetes and fatty liver), psychiatric (autism, depression and chronic fatigue syndrome) and senility-related disorders (hypogonadism, sarcopenia) [17, 18, 20, 22, 24, 25].
Commensal Microbes Help Define the Tumor Environment
It follows that the immune system, the metabolic profile, and the psychological condition of the host are all influenced by the microbiome. These factors, which also affect each other at the whole organism level, are important determinants in carcinogenesis and tumor progression. In accordance with the ‘holobiont’ concept, it was recently introduced that the gut microbiome shapes whole host biology or ‘tumor macroenvironment’ impacting tumor growth [5, 6]. Although there is substantial evidence that carcinogenic effects of gut bacteria are transplantable in mice when using purified immune cells alone [7, 8, 11, 26–29], there may be other direct effects of microbiota. According to recent data gut bacteria metabolites and inflammatory molecules such as bacterial lipopolysaccharide(LPS) may enter blood circulation and affect neoplasia in tissues locating distally from the GI tract [21, 30]. Ohtani’s group has recently described that the obesity-related intestinal microbiota increases the circulating deoxycholic acid, which, by inducing a senescence-associated secretory phenotype in hepatic stellate cells promotes liver carcinogenesis [21, 23].
These modulatory effects of microbiota upon cancer are testable in animal model systems using targeted infections or transplantable immune cell populations to survey interplay between bacteria and systemic processes (Fig. 1) [8, 13]. Clinically silent gastrointestinal (GI) tract immune networks are integrated with commensal microbiota for good health, or alternatively to stimulate distant smoldering carcinogenic processes in distant mammary and prostate glands [4, 8, 10, 13, 14, 28, 31]. In these studies, consuming certain microbes or their sterile products imparts downstream health benefit via systemic homeostasis, a microbe-based strategy that may ultimately overcome therapeutic limitations in preventing or treating cancer [7, 10, 32]. These promising findings put cancer into a new broader context of the “holobiont” comprised of the mammalian host plus resident microbes.
Figure 1.
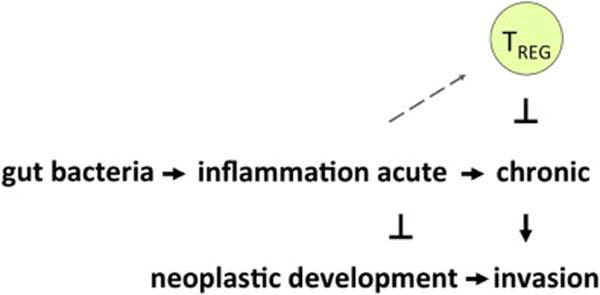
The holobiont regulates neoplastic development, growth and invasion via bacterial interactions that modulate systemic inflammatory tone. Earlier microbe exposures stimulate the immune system culminating in robust yet tightly regulated host immunity to rapidly restore homeostasis with minimal collateral tissue damage. Thus, neoplastic development and growth is framed in the context of the holobiont, including native resident microbes or those we choose to engineer for personalized or public health goals.
The Hygiene Hypothesis and Cancer
The biological significance of bacterial exposures much earlier in mammalian life is now becoming more fully appreciated [15, 33, 34]. Indeed, the “hygiene hypothesis” concept links bacteria with good health [10, 35, 36]. Conversely, pathologies arise later in life after too few perinatal and infant microbe exposures [4, 33–35, 37]. Studies in mouse models show that early-life exposures to bacteria [10, 11] and sterile [lysis-killed] microbes are sufficient for later-life anti-cancer effects [7]. It remains to be determined whether data from mouse models linking early- life microbe exposures with later cancer risks will translate to novel therapies for human patients. While many formative processes occur in utero or during infancy, recent data suggest that commensal bacteria-host crosstalk is also continuous and reciprocal throughout life. In this way, microbe-immune interactions constitute a vast gut-immune-endocrine-brain signaling axis that continuously modulates interferon (IFN)-γ and CD25 expression and host inflammatory tone [8, 13, 33, 34]. For example, dissecting temporal dynamics of wound healing as a proxy for cancer revealed that oral microbe [L reuteri] supplementation serves to enhance host inflammatory response (i.e. microbes were immune-stimulatory), with expedited healing, minimal collateral tissue damage, and return to homeostasis afterwards [20]. In those studies, expedited skin wound repair was attributable to microbe-induced hormone oxytocin, IFN-γ, and CD4+CD25+ immune cells. Likewise, mice supplemented orally with the same bacteria were resistant to both western diet-induced and ErbB2 oncogene-associated mammary carcinogenesis later in life [8].
Optimizing Gut Bacteria and Host Animal Signaling to Prevent Cancer
Earlier evidence for microbe-immune interactions in cancer was provided more than a decade ago by Erdman et al (2003) showing that immune-deficient Rag-knockout (KO) mice colonized with commensal bacteria exist in a chronic, smoldering pro-inflammatory and pro-tumorigenic proximal state [4]. By contrast, their wild-type immune-competent counterparts were resistant to neoplasms due to a competent adaptive immune system with potent anti-neoplastic properties [4]. Subsequent adoptive cell transfer experiments showed CD4+ T cells counteract carcinogenic processes, depending upon prior exposures and the composition of the host microbiome [4, 7, 8, 11, 13, 27, 31, 38]. Earlier infections may serve to reinforce immune system health to protect form cancer in a use-it-or-lose-it sort of way. Amazingly, killed sterile forms of microbes appear to convey similar anti-cancer benefits [7]. Emerging unpublished data from the same lab suggest that commensal bacteria serve to upregulate expression of epithelial Forkhead box protein [Fox]N1 and thus stimulate thymogenesis [39] culminating in CD4+ T cell-mediated immune balance. Interestingly, symbiotic microbiota such as human breast-milk borne L. reuteri appear to have transgenerational impact upon thymogenesis, with interesting implications in mammalian ontogeny [15]. In this way, exposures to bacteria may be used therapeutically for epigenetic control of resident immune cell populations in combating cancer [4]. In sum, these data from animal models raise the possibility of using synthetic microbe cocktails or sterile microbial products to stimulate immunity and lower risk of cancer [4].
Conclusions
Taken together, these findings offer exciting new microbe-based avenues for developing personalized or population-based medicine strategies to decrease the risk of malignancy. As the tumor microenvironment concept first put cancer cells into context within a lesion [2, 3], the notion of tumor macroenvironment puts carcinogenesis into a whole-body context that extends beyond the mammalian host to microbial passengers we may choose to engineer for our therapeutic benefit.
Highlights.
Data reveal that the milieu of immune cells and factors in the whole host environment determine the fate of dysplastic and preneoplastic lesions toward carinogenesis or regression
Clinically silent gastrointestinal tract immune networks and commensal microbiota may impart healthful phenotypes or alternatively stimulate distant smoldering carcinogenic processes in the mammary and prostate glands
Microbes may thus be determining the fate of the distal cancer microenvironment
Taken together, these results suggest that exposures to bacteria may be used therapeutically for epigenetic control of resident immune cell populations combatting cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Coussens LM. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell. 2012;21:309–322. doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 3.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erdman SE, Poutahidis T. Cancer inflammation and regulatory T cells. Int J Cancer. 2010;127:768–779. doi: 10.1002/ijc.25430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erdman SE, Poutahidis T. The microbiome modulates the tumor macroenvironment. Oncoimmunology. 2014;3 doi: 10.4161/onci.28271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erdman SE, Poutahidis T. Gut bacteria and cancer. Biochim Biophys Acta. 2015;1856:86–90. doi: 10.1016/j.bbcan.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erdman SE, Rao VP, Olipitz W, Taylor CL, Jackson EA, Levkovich T, Lee CW, Horwitz BH, Fox JG, Ge Z, Poutahidis T. Unifying roles for regulatory T cells and inflammation in cancer. Int J Cancer. 2010;126:1651–1665. doi: 10.1002/ijc.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakritz JR, Poutahidis T, Levkovich T, Varian BJ, Ibrahim YM, Chatzigiagkos A, Mirabal S, Alm EJ, Erdman SE. Beneficial bacteria stimulate host immune cells to counteract dietary and genetic predisposition to mammary cancer in mice. Int J Cancer. 2014;135:529–540. doi: 10.1002/ijc.28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lakritz JR, Poutahidis T, Mirabal S, Varian BJ, Levkovich T, Ibrahim YM, Ward JM, Teng EC, Fisher B, Parry N, Lesage S, Alberg N, Gourishetti S, Fox JG, Ge Z, Erdman SE. Gut bacteria require neutrophils to promote mammary tumorigenesis. Oncotarget. 2015;6:9387–9396. doi: 10.18632/oncotarget.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rao VP, Poutahidis T, Fox JG, Erdman SE. Breast cancer: should gastrointestinal bacteria be on our radar screen? Cancer Res. 2007;67:847–850. doi: 10.1158/0008-5472.CAN-06-3468. [DOI] [PubMed] [Google Scholar]
- 11.Rao VP, Poutahidis T, Ge Z, Nambiar PR, Boussahmain C, Wang YY, Horwitz BH, Fox JG, Erdman SE. Innate immune inflammatory response against enteric bacteria Helicobacter hepaticus induces mammary adenocarcinoma in mice. Cancer Res. 2006;66:7395–7400. doi: 10.1158/0008-5472.CAN-06-0558. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J, Kalluri R. Cancer without disease. Nature. 2004;427:787. doi: 10.1038/427787a. [DOI] [PubMed] [Google Scholar]
- 13.Poutahidis T, Kleinewietfeld M, Smillie C, Levkovich T, Perrotta A, Bhela S, Varian BJ, Ibrahim YM, Lakritz JR, Kearney SM, Chatzigiagkos A, Hafler DA, Alm EJ, Erdman SE. Microbial reprogramming inhibits Western diet-associated obesity. PLoS One. 2013;8:e68596. doi: 10.1371/journal.pone.0068596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rutkowski MR, Stephen TL, Svoronos N, Allegrezza MJ, Tesone AJ, Perales-Puchalt A, Brencicova E, Escovar-Fadul X, Nguyen JM, Cadungog MG, Zhang R, Salatino M, Tchou J, Rabinovich GA, Conejo-Garcia JR. Microbially driven TLR5-dependent signaling governs distal malignant progression through tumor-promoting inflammation. Cancer Cell. 2015;27:27–40. doi: 10.1016/j.ccell.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poutahidis T, Varian BJ, Levkovich T, Lakritz JR, Mirabal S, Kwok C, Ibrahim YM, Kearney SM, Chatzigiagkos A, Alm EJ, Erdman SE. Dietary microbes modulate transgenerational cancer risk. Cancer Res. 2015;75:1197–1204. doi: 10.1158/0008-5472.CAN-14-2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flint HJ, Scott KP, Louis P, Duncan SH. The role of the gut microbiota in nutrition and health. Nat Rev Gastroenterol Hepatol. 2012;9:577–589. doi: 10.1038/nrgastro.2012.156. [DOI] [PubMed] [Google Scholar]
- 17.Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, Jia W, Pettersson S. Host-gut microbiota metabolic interactions. Science. 2012;336:1262–1267. doi: 10.1126/science.1223813. [DOI] [PubMed] [Google Scholar]
- 18.Clemente JC, Ursell LK, Parfrey LW, Knight R. The impact of the gut microbiota on human health: an integrative view. Cell. 2012;148:1258–1270. doi: 10.1016/j.cell.2012.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varian BJ, Poutahidis T, Levkovich T, Ibrahim YM, Lakritz JR, Chatzigiagkos A, Scherer-Hoock A, Alm EJ, Erdman SE. Beneficial Bacteria Stimulate Youthful Thyroid Gland Activity. J Obes Weight Loss Ther. 2014;4 [Google Scholar]
- 20.Poutahidis T, Kearney SM, Levkovich T, Qi P, Varian BJ, Lakritz JR, Ibrahim YM, Chatzigiagkos A, Alm EJ, Erdman SE. Microbial Symbionts Accelerate Wound Healing via the Neuropeptide Hormone Oxytocin. PLoS One. 2013;8:e78898. doi: 10.1371/journal.pone.0078898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ohtani N. Microbiome and cancer. Seminars in immunopathology. 2015;37:65–72. doi: 10.1007/s00281-014-0457-1. [DOI] [PubMed] [Google Scholar]
- 22.Poutahidis T, Springer A, Levkovich T, Qi P, Varian BJ, Lakritz JR, Ibrahim YM, Chatzigiagkos A, Alm EJ, Erdman SE. Probiotic microbes sustain youthful serum testosterone levels and testicular size in aging mice. PLoS One. 2014;9:e84877. doi: 10.1371/journal.pone.0084877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimoto S, Loo TM, Atarashi K, Kanda H, Sato S, Oyadomari S, Iwakura Y, Oshima K, Morita H, Hattori M, Honda K, Ishikawa Y, Hara E, Ohtani N. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 24.Dinan TG, Stanton C, Cryan JF. Psychobiotics: a novel class of psychotropic. Biol Psychiatry. 2013;74:720–726. doi: 10.1016/j.biopsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Bischoff SC. Microbiota and aging. Curr Opin Clin Nutr Metab Care. 2015 doi: 10.1097/MCO.0000000000000242. [DOI] [PubMed] [Google Scholar]
- 26.Rao VP, Poutahidis T, Ge Z, Nambiar PR, Horwitz BH, Fox JG, Erdman SE. Proinflammatory CD4+ CD45RB(hi) lymphocytes promote mammary and intestinal carcinogenesis in Apc(Min/+) mice. Cancer Res. 2006;66:57–61. doi: 10.1158/0008-5472.CAN-05-3445. [DOI] [PubMed] [Google Scholar]
- 27.Erdman SE, Rao VP, Poutahidis T, Rogers AB, Taylor CL, Jackson EA, Ge Z, Lee CW, Schauer DB, Wogan GN, Tannenbaum SR, Fox JG. Nitric oxide and TNF-{alpha} trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc Natl Acad Sci U S A. 2009 doi: 10.1073/pnas.0812347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poutahidis T, Cappelle K, Levkovich T, Lee CW, Doulberis M, Ge Z, Fox JG, Horwitz BH, Erdman SE. Pathogenic intestinal bacteria enhance prostate cancer development via systemic activation of immune cells in mice. PLoS One. 2013;8:e73933. doi: 10.1371/journal.pone.0073933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levkovich T, Poutahidis T, Cappelle K, Smith MB, Perrotta A, Alm EJ, Erdman SE. ‘Hygienic’ Lymphocytes Convey Increased Cancer Risk. Journal of Analytical Oncology. 2014;3 doi: 10.6000/1927-7229.2014.03.03.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paul B, Barnes S, Demark-Wahnefried W, Morrow C, Salvador C, Skibola C, Tollefsbol TO. Influences of diet and the gut microbiome on epigenetic modulation in cancer and other diseases. Clinical epigenetics. 2015;7:112. doi: 10.1186/s13148-015-0144-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poutahidis T, Rao VP, Olipitz W, Taylor CL, Jackson EA, Levkovich T, Lee CW, Fox JG, Ge Z, Erdman SE. CD4+ lymphocytes modulate prostate cancer progression in mice. Int J Cancer. 2009;125:868–878. doi: 10.1002/ijc.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Malkov SV, Markelov VV, Polozov GY, Sobchuk LI, Zakharova NG, Barabanschikov BI, Kozhevnikov AY, Vaphin RA, Trushin MV. Antitumor features of Bacillus oligonitrophilus KU-1 strain. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2005;38:96–104. [PubMed] [Google Scholar]
- 33.Shanahan F. The gut microbiota-a clinical perspective on lessons learned. Nat Rev Gastroenterol Hepatol. 2012;9:609–614. doi: 10.1038/nrgastro.2012.145. [DOI] [PubMed] [Google Scholar]
- 34.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rook GA. Review series on helminths, immune modulation and the hygiene hypothesis: the broader implications of the hygiene hypothesis. Immunology. 2009;126:3–11. doi: 10.1111/j.1365-2567.2008.03007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belkaid Y, Rouse BT. Natural regulatory T cells in infectious disease. Nat Immunol. 2005;6:353–360. doi: 10.1038/ni1181. [DOI] [PubMed] [Google Scholar]
- 37.Schuijs MJ, Willart MA, Vergote K, Gras D, Deswarte K, Ege MJ, Madeira FB, Beyaert R, van Loo G, Bracher F, von Mutius E, Chanez P, Lambrecht BN, Hammad H. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science. 2015;349:1106–1110. doi: 10.1126/science.aac6623. [DOI] [PubMed] [Google Scholar]
- 38.Erdman SE, Rao VP, Olipitz W, Taylor CL, Jackson EA, Levkovich T, Lee CW, Horwitz BH, Fox JG, Ge Z, Poutahidis T. Unifying roles for regulatory T cells and inflammation in cancer. Int J Cancer. 2009;126:1651–1665. doi: 10.1002/ijc.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bredenkamp N, Ulyanchenko S, O’Neill KE, Manley NR, Vaidya HJ, Blackburn CC. An organized and functional thymus generated from FOXN1-reprogrammed fibroblasts. Nature cell biology. 2014;16:902–908. doi: 10.1038/ncb3023. [DOI] [PMC free article] [PubMed] [Google Scholar]


