Abstract
At the time that Paracelsus coined his famous dictum, “What is there that is not poison? All things are poison and nothing is without poison. Solely the dose determines that a thing is not a poison,” embryonic toxicology was a fairly focused discipline that mainly dealt with occupational poisonings and side effects of pharmaceuticals, such as mercury. While Paracelsus paved the way for the modern threshold concept and the no-adverse effect level, modern-day toxicology is now tussling with highly complex issues, such as developmental exposures, genetic predisposition and other sources of hypersusceptibility, multiple causes of underestimated toxicity, and the continuous presence of uncertainty, even in regard to otherwise well-studied mercury compounds. Further, the wealth of industrial chemicals now challenges the “untested-chemical assumption,” that the lack of documentation means that toxic potentials can be ignored. Unfortunately, in its ambition to provide solid evidence, toxicology has been pushed into almost endless replications, as evidenced by the thousands of toxicology publications every year that focus on toxic metals, including mercury, while less well-known hazards are ignored. From a public health viewpoint, toxicology needs to provide better guidance on decision-making under ever-present uncertainty. In this role, we need to learn from the stalwart Paracelsus the insistence on relying on facts rather than authority alone to protect against chemical hazards.
Keywords: Developmental effects, drug toxicity, environmental toxicology, epidemiology, genetic predisposition, individual susceptibility, public health, uncertainty
A standard textbook on pharmacology notes: “Toxicology is often regarded as the science of poisons or poisoning, but developing a strict definition for poison is problematic”[1]. The reason for this paradox is that, in principle, any substance, including any drug or essential nutrient, has the capacity to harm a living organism and thus behave like a poison. The discovery of this conundrum is credited to the famous Renaissance physician Paracelsus (1493–1541), often referred to as the ”Father of Toxicology.” He was mainly writing in German, and a modern translation of his central dictum can be given as: “What is there that is not poison? All things are poison and nothing is without poison. Solely the dose determines that a thing is not a poison.” In this way, he paved the way for modern colleagues to scrutinize the threshold concept and to define the no-adverse effect level (NOAEL) as the highest tested dose or concentration of a substance, at which no adverse effect is found. In fact, by defining that a (lower) dose will prevent adverse effects, Paracelsus laid the groundwork for the modern separation of the terms hazard and risk. I shall further discuss these issues below.
Many biographies on Paracelsus have been published, although much is unknown about him. Even his birth date is not at all certain, but sometime in late 1493, Philippus Theophrastus Aureolus Bombastus von Hohenheim was born. In the brief summary and the quotes that follow, I shall rely on a few excellent and easily accessible sources [2–8] and the most commonly used English translation [9].
Paracelsus was an academic name that he chose for himself, although he soon became known as a verdant critic of old masters like Galen and Hippocrates, and he infuriated his academic colleagues by burning the treasured classical textbooks. Further, he communicated in German, not in Latin, and refused to wear black robes. So his academic name should not be misinterpreted as a sign that he, like his contemporaries, based his thinking and teaching only on classic traditions and beliefs.
In fact, Paracelsus was not a particularly likable person. Though he was intelligent, well-educated and deeply religious, he was also an unpredictable, stubborn, free-thinking and independent iconoclast. He gained a reputation for being arrogant and soon garnered the anger of other physicians when he demanded that they rely on facts and not on authority alone. As he said: “My accusers complain that I have not entered the temple of knowledge through the right door. But which one is the truly legitimate door - Galen and Avicenna, or Nature? I have entered through the door of Nature. Her light, not the lamp of an apothecary’s shop, has illuminated my way.”
He held a position at the University of Basel for less than a year and was forced from the city after a legal dispute over a physician’s fee he sued to collect. Paracelsus’ response highlights controversies that have reappeared many times since then: “The best of our popular physicians are the ones that do the least harm. But, unfortunately, some poison their patients with mercury; others purge them or bleed them to death. There are some who have learned so much that their learning has driven out all their common sense, and there are others who care a great deal more for their own profit than for the health of their patients.”
Paracelsus clearly made an impact, and he was aware that he looked peculiar (fig. 1) and acted peculiar: “I am different, let this not upset you.” But people did get upset by Paracelsus, and this is a main reason that he had difficulty getting his books published. In particular, the Carinthian Trilogy that included the first printed version of ”The Third Defense” – and his famous statement on poisons – was written in 1538 but was not published until 1564, more than 20 years after his death. Thus, it was only during the following decades, indeed, centuries, that the most visionary of his writings became appreciated.
Fig. 1.
Paracelsus, at age 45, three years before he died in 1541 (contemporary copper engraving). Note Paracelsus’ motto on top.
After the skirmishes in Basel, Paracelsus wandered in Europe, Africa and Asia Minor in the pursuit of knowledge. Paracelsus may have reached Denmark, and it has been speculated that he served as a military physician in Christian II’s army for as much as three years [10]. Paracelsus’ own wording does not provide much detail, though: ”Ich hab auch gesehen zu Stockhalma in Denmarck ein wunttrank bei einer edlen frauen” [10]. At the time, Christian II ruled and relied on the help of Mother Sigbrit (mother of the beautiful Dyveke) – was she the ”edle frau”? We can speculate – and I would like to believe that Paracelsus traveled through Odense, now almost 500 years ago.
Paracelsus is also famous for his clear description of the target organ concept and the occupational diseases in miners, among others. But his most famous clinical toxicology work was on mercury, which was applied as an important drug at the time, though limited by frequent side effects. I shall refer to mercury below, as it remains an important public health hazard, and compare it with a group of modern industrial chemicals now universally present in the environment and in ourselves, thereby complicating toxicology and Paracelsus’ dose concept.
The elusive thresholds
Paracelsus’ ingenuity was his emphasis that lower doses – below a threshold – could cause otherwise poisonous substances to become harmless. While he recommended mercury (in the form of calomel and other inorganic mercury compounds) as a therapeutic in proper doses, side effects limited this application [11]. A widened perspective emerged when methylmercury, an organometal compound with a covalent bond between carbon and mercury, turned out to behave entirely differently and appeared to be even more toxic than elemental and inorganic mercury [12]. Further, the neurological signs of methylmercury poisoning in adults were unique [13]. This was particularly apparent during widespread poisoning incidents, which happened when seed grain treated with methylmercury as a fungicide was mistakenly used for bread-making. The most serious of these occurred during a famine in Iraq in 1970–1971 [14].
In the Japanese fishing village, Minamata, release of mercury-laden waste water from a local factory resulted in contamination of the fish, and an unusual clinical picture emerged among the local fishermen’s families in the 1950s. Most remarkably, apparently healthy women gave birth to children with a spastic paresis syndrome and mental retardation [15]. Recognition of the so-called Minamata disease as methylmercury poisoning was delayed, in part because thin-layer chromatography identification of the organometal compound was not possible until 1962 [16]. Soon, elevated methylmercury concentrations were documented in seafood, in tissues of deceased patients, and in umbilical cord samples from poisoned infants [17]. The discovery of the pervasive adverse effects on foetal development at dose levels that spared the mother supported a new paradigm later named Developmental Origins of Health and Disease (DOHaD) [18] – an important extension of Paracelsus’ teaching: “for exposures sustained during early development, another critical, but largely ignored, issue is that ‘the timing makes the poison’” [19].
Developmental vulnerability soon turned out to be only one of the reasons that susceptibility to a toxicant might vary. Genetic predisposition to toxic effects can render an individual highly vulnerable to particular toxicants [20]. When some individuals are much more vulnerable to a toxicant like methylmercury, then the average effect, as documented in epidemiological studies, can be grossly misleading. For example, in a British birth cohort, the mercury concentration in cord tissue served as a marker also of maternal fish intake during pregnancy and showed no correlation with the children’s IQ at age 8 years. In contrast, among children with a genetic predisposition, each doubling of the methylmercury exposure was associated with a loss of as much as 6 IQ points [21]. Thus, we are not all equally sensitive to the risk of toxicity.
As a further complication, the exact dose absorbed by each environmentally exposed individual is often not known. Dietary questionnaires are inherently imprecise, and better data are usually obtained from determining the mercury concentrations in biological samples, such as blood or scalp hair [22]. Although these measurements can be affected by exposures to different forms of mercury, in seafood consumers they mainly reflect the methyl species. Laboratory analysis is normally associated with a relative imprecision better than 5% that can usually be ignored. However, several factors can influence the amount of mercury in the sample, whether external contamination, binding properties or temporal variability. Thus, for hair-mercury analyses, the total imprecision has been estimated to be about 50%, i.e., more than 10-fold greater than the analytical variability [23]. Unfortunately, this issue has not been widely appreciated, and a biomarker result, e.g., the hair-mercury concentration is generally assumed to represent an individual’s methylmercury exposure without error, thereby reflecting the amount that has reached the target organ. High imprecision levels cause misclassification of the exposure and thereby a bias toward the null [23]. Accordingly, risk assessments should take into account the consequences of imprecision on underestimation of risks [24]. For example, the benchmark dose level decreased by about 50% after adjustment for imprecise exposure data [24], and the exposure limits recommended by the U.S. EPA [25] and the World Health Organization (WHO) [26] would now probably need to be halved.
One more complication has been found to be of substantial importance for risk assessment. Methylmercury occurs as a common contaminant of seafood that, at the same time, contains essential nutrients [27]. The total impact of eating seafood therefore depends both on the contamination and the nutrient contents, and more nutritious seafood can appear to compensate for methylmercury toxicity [28]. At the same time, the benefits from otherwise healthy seafood are then diminished by the toxicant and can be exceeded by adverse effects at greater contamination levels. As the two factors operate in different directions, epidemiologists have dubbed this masking effect as negative (or reverse) confounding [29]. Clearly, we want our seafood to be safe and not to contain unwanted substances that diminish the beneficial effects, not to speak about adverse effects. Nonetheless, regulatory agencies usually aim only at securing a net benefit from seafood diets rather than optimizing the benefits by minimizing the risk of mercury toxicity.
Mercury compounds, in particular methylmercury, therefore serve as important reminders of complications (table 1) that can result in adverse effects being easily underestimated and thresholds overestimated. Some conclusions from international risk assessments illustrate this tendency. “The occurrence of prenatal intoxication also calls for caution,” was the scientific consensus in 1972, although this warning had no consequence at all [30]. Later on, WHO experts also recognized that “clinical data from Japan indicate that the foetus is more sensitive than the mother,” but no special protection ensued [31]. Risk assessment for methylmercury continued to rely on average toxicity in adults and remained that way until 2003 [26], when developmental toxicity was finally recognized, but the other considerations mentioned in table 1 remain to be addressed. Thus, even today, exposure limits for methylmercury are far less protective than officially claimed.
Table 1.
Complications in mercury toxicology that Paracelsus did not have to deal with.
|
The dose concept under uncertainty
Paracelsus’ poison dictum heralded the modern application of the NOAEL as a key concept in regulatory pharmacology. However, due to a variety of complexities (table 2), environmental toxicology has benefitted much less. While drugs must be tested for their efficacy and for adverse effects, most industrial chemicals have escaped such regulations. In the United States, the Toxic Substances Control Act, which dates back to the late 1970s, did not require testing of substances already in commerce at the time. It is even possible that the legislation discouraged chemical producers from testing substances that had already received blanket approval [32]. As a result, it is difficult today to judge whether or not a particular chemical is a “poison,” simply because of the lack of information on the dose-response relationship [32].
Table 2.
Modern challenges in toxicology where Paracelsus’ candor and obstinacy might be helpful.
|
An additional problem is that industrial chemicals, especially those that are resistant to breakdown, can disseminate into the global environment and reappear in food chains far from the source. This was the case with the perfluorinated alkyl substances (PFASs) that were discovered in extremely high concentrations in the liver of remote polar bears and pilot whales [33]. The PFASs have been in use for over 60 years [34], the main uses being non-stick kitchenware, raingear, impregnation of furniture textiles and carpets, other water- and stain-repellant uses, and additional uses, such as aqueous film-forming foams used for fire-fighting purposes [34]. By about 2000, their global environmental dissemination became publicly known [35]. Today, virtually all Americans have detectable PFAS concentrations in their serum [36]. However, due to the multiple pathways, e.g., through consumer products, house dust, food chain contamination, and food packaging materials, the exposure sources are difficult to pinpoint, and the only known method to reduce the unwanted body burden is phlebotomy [37].
When foreign chemicals are unexpectedly detected in blood samples, the possible risks deserve to be ascertained, and that can be a protracted process. Thus, the U.S. Environmental Protection Agency issued a draft risk assessment for PFASs in 2005, but a final version has yet to appear, and the limits for drinking water issued in 2009 remain provisional [38].
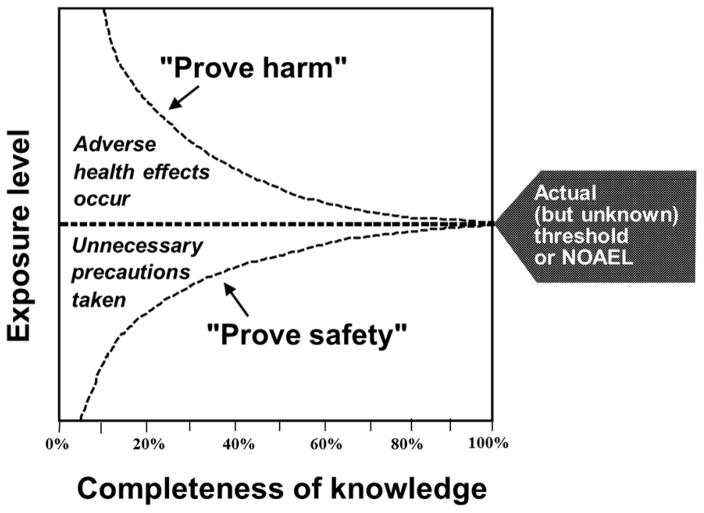
Standardized animal testing may not necessarily reveal the effects that are thought to be critical in human beings, such as developmental neurotoxicity [39]. With the PFASs, early toxicology reports relied on spleen microscopy and general clinical chemistry to conclude that the immune system did not show any significant effects in non-human primates [40]. Later on, more sensitive methods applied in a rodent model showed deficient antibody responses to foreign proteins [41], even at serum concentrations that were fairly close to those seen in exposed human populations. With time, adverse effects were found at lower and lower doses, as illustrated by the top curve in fig. 2.
Fig. 2.
With time, better knowledge allows appreciation that lower exposures must be ensured in order to avoid adverse health effects, and it is also realized that very small doses are tolerated without any risk of adverse effects. However, only with near-perfect knowledge will the two curves meet. It is the purpose of modern toxicology to responsibly interpret science so that adverse effects are avoided, although with due recognition that some low-level exposures must be allowed. Redrawn from (63).
While human data are crucial in order to evaluate the possible health risks, most evidence refers to cross-sectional data that are difficult to interpret, especially if the causative exposure may have happened sometime in the past [42], as suggested by the DOHaD concept [18]. When the hypothesis of immunotoxicity was tested in children’s response to childhood immunizations, the maternal pregnancy serum-PFAS concentration showed a strong negative correlation with the children’s vaccine antibody concentrations at age 5 years, where a doubling in exposure was associated with an antibody loss of 40% or more [43]. A prospective study design involved blood collection from the pregnant mother and from the children 5 years later. These findings have been confirmed in one additional study [44], which, in addition, showed increased frequencies of common cold and gastroenteritis, but replications of such multi-year study designs complicate the desire to obtain confirmatory results within a reasonably short time frame.
The difficulty in obtaining crucial human evidence was recently highlighted by the Agency for Toxic Substances and Disease Registry (ATSDR), which stressed a number of uncertainties and then disregarded the recent reports on developmental PFAS exposures and their associations with adverse effects in children [45]. In regard to human immunotoxicity, we had calculated the so-called benchmark dose level [46], which is used by regulatory agencies as a basis for deriving safe levels of exposures [47, 48]. Our results suggested that current exposure limits [38, 45] may be more than 100-fold too high to protect against immunotoxicity in children. ATSDR disregarded this evidence, as our study did not include a “control group,” despite the fact that no such condition is warranted [47]. In the conclusions, the ATSDR draft instead focused on classical toxicity signs, such as changes in liver weight, in animal models [45].
The PFASs provide a clear and unfortunate example of the “untested-chemical assumption” that the lack of documentation means that no regulatory action is required [49]. In this case, the assumption ignored preliminary evidence on plausible effects and thus failed to inspire further exploration of possible risks. Clearly, the absence of replicated documentation from epidemiological studies should not be considered as a reason to conclude that adverse effects have not and will not occur [50]. I am sure that Paracelsus would have objected against chemicals not being considered poisons simply because no evidence of toxicity had been garnered. Thus, the PFASs reflect a failed scientific and regulatory approach to chemical safety [49]. The question is how toxicology should deal with this challenge in the future.
The need for audacity in toxicology science
Scientific tradition demands solid evidence and replication before drawing conclusions, especially those that can have an impact on decisions in society. According to this conventional approach, about 20 years ago, a group of scientists reviewed the evidence on DOHaD-related toxicity and concluded: ”Differences in sensitivity between children and adults are chemical-specific and must be studied and evaluated on a case-by-case basis” [51]. Certainly, acetaminophen is less toxic to small children with an immature liver metabolism, but does that really mean that all industrial chemicals must be tested for age-dependent toxicity before providing children with additional protection?
Obtaining the necessary documentation can be difficult, especially when a study that fails to reach statistical significance is said to be “negative” or even misinterpreted as evidence that an association is absent [52]. As outlined above, toxicology research is always affected by uncertainties, each of which can easily blur a real association between a chemical hazard and its adverse effects. In particular, risks may be easily underestimated when exposure assessments are imprecise [23]. Uncertainty is often misunderstood to refer only to the risks, so that statistical acceptance of the null hypothesis is interpreted as proof of safety. In reality, uncertainty is not asymmetric and must also be viewed as an equal challenge to alleged safety. The latter aspect is illustrated by the lower curve in fig. 2. As better information becomes available, we are able to endorse the existence of safety at increasing levels of exposure – much in Paracelsus’ spirit. When perfect knowledge is available, we will also know exactly how much of a toxicant an individual can tolerate, although unfortunately, individual vulnerability may make it impossible to extrapolate this knowledge to the population level.
The demands for documentation and replication can impede preventive interventions, but they have also harmed research in a more general sense. When we identified the industrial chemicals covered by the major toxicology and public health journals in 2000–2009, the most frequently studied substances were mainly metals, mercury among them, each of which had resulted in hundreds of new articles every year in peer-reviewed journals [53]. This wealth of recent publications with a focus on well-studied compounds reflects a so-called Matthew Effect [54], where the popularity among researchers and ongoing deliberations generate justification for continued research on the very same substances. Feasibility, funding, publication pace, and institutional agendas, are of course major determinants for academic research, along with the demands for replication, but all of these factors foster inflexibility and inertia, a vicious circle with a perpetual, positive feed-back that acts against the very purpose of toxicology.
As a further sign that science is not serving the changing needs of society in regard to potential chemical hazards, the environmental chemicals identified as a top research priority by the U.S. Environmental Protection Agency in 2006 [55] were barely covered by academic research up to 2009 [53], and not much since then. Accordingly, research on environmental chemicals primarily considers well-known problems, many of which still pose challenges, but little research focuses on the environmental problems of the future.
Part of the responsibility for demanding meticulous and repeated verification is due to industry interests [56] that may view new toxicology evidence as a financial threat. In my own case, when methylmercury from seafood emerged as a public health hazard, the tuna fishing industry set aside $25 million for a campaign to convince consumers that polluted tuna was safe [57]. This way, vested interests inject exaggerated or even manufactured uncertainties into the debate, sometimes through hidden sponsorships of alleged research studies [58].
When public scares nonetheless happen, they are often considered ‘false positive’ events. Likewise, erroneous conclusions may occur in major medical journals [59], but such false positive findings seem to particularly affect certain fields, such as clinical research. On close examination of more than 80 alleged false positive cases relevant to public health, only a handful, such as the swine flu, were truly false negatives that resulted in wasted efforts, because the hazard did not materialize. Thus, costs due to overreactions are rare, while the costs due to false negatives can be truly excessive [60]. In regard to environmental chemicals, the majority has been poorly documented, if at all [61]. Ignoring the potential risks from poorly studied chemical hazards likely involves a very large number of potentially false negative conclusions. Some of these errors may in the end turn out to be extremely costly, as illustrated by asbestos, lead, mercury and many other hazards that were at first ignored [60, 62].
The so-called “untested-chemical assumption” therefore needs to be countered [49]. In fact, like climate change, a potential chemical hazard may still need to be taken seriously, even when a definite proof is not yet at hand. Thus, toxicology conclusions must always be based on a prudent interpretation of our current scientific knowledge, but they also need to take into account what could be realistically known by now, given the research studies completed so far.
Paracelsus’ motto was “Alterius non sit qui suus esse potest” which means “Do not try to be someone different, if you can be yourself.” Toxicology science needs audacity to stand by its values as a public health science and recognize that the modern complexities of chemical exposures require precaution and attention to the doses that can be tolerated without adverse effects (the lower curve in fig. 2) rather than a narrow focus solely on adverse effects (the upper curve).
Conclusions
Developmental exposures, genetic predisposition and other sources of hypersusceptibility, multiple reasons for underestimated toxicity, and the continuous presence of uncertainty, put demands on toxicology that Paracelsus did not have to deal with. However, his example, with his stalwartness and unrelenting insistence on relying on facts rather than authority alone, with the purpose of protecting fellow human beings, should inspire toxicology to become an even more essential tool in public health and in dealing safely with industrial chemicals.
Acknowledgments
This article relies on research supported by public sources, in particular the National Institute of Environmental Health Sciences (NIH), grants ES009797, ES012199, and ES021477. None of this research would have been possible without the thoughtful and determined support by my colleagues in Boston, Odense and Tórshavn.
Footnotes
Conflicts of interest
The author declares no conflicts of interest.
References
- 1.Goodman LS, Brunton LL, Chabner B, Knollmann BrC. Goodman & Gilman’s the pharmacological basis of therapeutics. 12. New York: McGraw-Hill; 2011. [Google Scholar]
- 2.de Vries A. Paracelsus. Sixteenth-century physician-scientist-philosopher. N Y State J Med. 1977;77:790–8. [PubMed] [Google Scholar]
- 3.Deichmann WB, Henschler D, Holmsted B, Keil G. What is there that is not poison? A study of the Third Defense by Paracelsus. Arch Toxicol. 1986;58:207–13. doi: 10.1007/BF00297107. [DOI] [PubMed] [Google Scholar]
- 4.Davis A. Paracelsus: a quincentennial assessment. J R Soc Med. 1993;86:653–6. doi: 10.1177/014107689308601114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernoulli R. Paracelsus--physician, reformer, philosophy, scientist. Experientia. 1994;50:334–8. doi: 10.1007/BF02026633. [DOI] [PubMed] [Google Scholar]
- 6.Borzelleca JF. Paracelsus: herald of modern toxicology. Toxicol Sci. 2000;53:2–4. doi: 10.1093/toxsci/53.1.2. [DOI] [PubMed] [Google Scholar]
- 7.Siddiqui MA, Mehta NJ, Khan IA. Paracelsus: the Hippocrates of the Renaissance. J Med Biogr. 2003;11:78–80. doi: 10.1177/096777200301100207. [DOI] [PubMed] [Google Scholar]
- 8.Stenersen J. Hvem var denne Paracelsus? Toksikologen. 2005;15:27–34. [Google Scholar]
- 9.Hartmann F. The life of Philippus Theophrastus Bombast of Hohenheim, known by the name of Paracelsus: and the substance of his teachings concerning cosmology, anthropology, pneumatology, magic and sorcery, medicine, alchemy and astrology, philosophy and theosophy. 2. London: Kegan Paul, Trench, Trübner, & Co. Ltd; 1896. revised and enlarged. [Google Scholar]
- 10.Fink-Jensen M. Paracelsus og Danmark: Medicin og teologi i 1500- og 1600-tallet. In: Appel C, Henningsen P, Hybel N, editors. Mentalitet & Historie. Ebeltoft: Skippershoved; 2002. pp. 95–118. [Google Scholar]
- 11.Goldwater LJ. Mercury; a history of quicksilver. Baltimore: York Press; 1972. [Google Scholar]
- 12.Hepp P. Über quecksilberäthylverbindungen und über das verhältniss der quecksilberäthyl- zur quecksilbervergiftung. Naunyn Schmiedebergs Arch Pharmakol Exp Pathol. 1887;23:91–128. [Google Scholar]
- 13.Hunter D, Russell DS. Focal cerebellar and cerebellar atrophy in a human subject due to organic mercury compounds. J Neurol Neurosurg Psychiatry. 1954;17:235–41. doi: 10.1136/jnnp.17.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, al-Rawi NY, et al. Methylmercury poisoning in Iraq. Science. 1973;181:230–41. doi: 10.1126/science.181.4096.230. [DOI] [PubMed] [Google Scholar]
- 15.Harada M. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol. 1995;25:1–24. doi: 10.3109/10408449509089885. [DOI] [PubMed] [Google Scholar]
- 16.Irukayama K, Kai F, Fujiki M, Kondo T. Studies on the origin of the causative agent of Minamata disease. III. Industrial wastes containing mercury compounds from Minamata Factory. Kumamoto Med J. 1962;15:57–68. [PubMed] [Google Scholar]
- 17.Nishigaki S, Harada M. Methylmercury and selenium in umbilical cords of inhabitants of the Minamata area. Nature. 1975;258:324–5. doi: 10.1038/258324a0. [DOI] [PubMed] [Google Scholar]
- 18.Heindel JJ, Balbus J, Birnbaum L, Brune-Drisse MN, Grandjean P, Gray K, et al. Developmental Origins of Health and Disease: Integrating Environmental Influences. Endocrinology. 2015;156:3416–21. doi: 10.1210/EN.2015-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grandjean P, Bellinger D, Bergman A, Cordier S, Davey-Smith G, Eskenazi B, et al. The Faroes statement: human health effects of developmental exposure to chemicals in our environment. Basic Clin Pharmacol Toxicol. 2008;102:73–5. doi: 10.1111/j.1742-7843.2007.00114.x. [DOI] [PubMed] [Google Scholar]
- 20.Julvez J, Grandjean P. Genetic susceptibility to methylmercury developmental neurotoxicity matters. Front Genet. 2013:4. doi: 10.3389/fgene.2013.00278. 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Julvez J, Smith GD, Golding J, Ring S, Pourcain BS, Gonzalez JR, et al. Prenatal methylmercury exposure and genetic predisposition to cognitive deficit at age 8 years. Epidemiology. 2013;24:643–50. doi: 10.1097/EDE.0b013e31829d5c93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Budtz-Jorgensen E, Grandjean P, Jorgensen PJ, Weihe P, Keiding N. Association between mercury concentrations in blood and hair in methylmercury-exposed subjects at different ages. Environ Res. 2004;95:385–93. doi: 10.1016/j.envres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Grandjean P, Budtz-Jorgensen E. An ignored risk factor in toxicology: The total imprecision of exposure assessment. Pure Appl Chem. 2010;82:383–91. doi: 10.1351/PAC-CON-09-05-04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Budtz-Jorgensen E, Keiding N, Grandjean P. Effects of exposure imprecision on estimation of the benchmark dose. Risk Anal. 2004;24:1689–96. doi: 10.1111/j.0272-4332.2004.00560.x. [DOI] [PubMed] [Google Scholar]
- 25.National Research Council. Toxicological effects of methylmercury. Washington, DC: National Academy Press; 2000. [Google Scholar]
- 26.JECFA. Summary and conclusions. Sixty-first meeting of the Joint FAO/WHO Expert Committee on Food Additives; Rome. 10–19 June 2003; [accessed, 30 March, 2016]. ftp://ftp.fao.org/es/esn/jecfa/jecfa61sc.pdf. [Google Scholar]
- 27.Mahaffey KR, Sunderland EM, Chan HM, Choi AL, Grandjean P, Marien K, et al. Balancing the benefits of n-3 polyunsaturated fatty acids and the risks of methylmercury exposure from fish consumption. Nutr Rev. 2011;69:493–508. doi: 10.1111/j.1753-4887.2011.00415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Budtz-Jorgensen E, Grandjean P, Weihe P. Separation of risks and benefits of seafood intake. Environ Health Perspect. 2007;115:323–7. doi: 10.1289/ehp.9738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi AL, Cordier S, Weihe P, Grandjean P. Negative confounding in the evaluation of toxicity: the case of methylmercury in fish and seafood. Crit Rev Toxicol. 2008;38:877–93. doi: 10.1080/10408440802273164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.JECFA. [accessed, 30 March, 2016];Evaluation of mercury, lead, cadmium and the food additives amaranth, diethylpyrocarbonate, and octyl gallate. 1972 http://www.inchem.org/documents/jecfa/jecmono/v004je02.htm.
- 31.JECFA. [accessed, 30 March, 2016];Evaluation of certain food additives and contaminants (Twenty-second report of the Joint FAO/WHO Expert Committee on Food Additives) 1978 http://apps.who.int/iris/bitstream/10665/41330/1/WHO_TRS_631.pdf. [PubMed]
- 32.Sass J. The chemical industry delay game. Washington, D.C: Natural Resources Defense Council; 2011. [accessed, 30 March, 2016]. https://www.nrdc.org/sites/default/files/IrisDelayReport.pdf. [Google Scholar]
- 33.Bossi R, Riget FF, Dietz R, Sonne C, Fauser P, Dam M, et al. Preliminary screening of perfluorooctane sulfonate (PFOS) and other fluorochemicals in fish, birds and marine mammals from Greenland and the Faroe Islands. Environ Pollut. 2005;136:323–9. doi: 10.1016/j.envpol.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 34.Lindstrom AB, Strynar MJ, Libelo EL. Polyfluorinated compounds: past, present, and future. Environ Sci Technol. 2011;45:7954–61. doi: 10.1021/es2011622. [DOI] [PubMed] [Google Scholar]
- 35.Olsen GW, Lange CC, Ellefson ME, Mair DC, Church TR, Goldberg CL, et al. Temporal trends of perfluoroalkyl concentrations in American Red Cross adult blood donors, 2000–2010. Environ Sci Technol. 2012;46:6330–8. doi: 10.1021/es300604p. [DOI] [PubMed] [Google Scholar]
- 36.Lewis RC, Johns LE, Meeker JD. Serum Biomarkers of Exposure to Perfluoroalkyl Substances in Relation to Serum Testosterone and Measures of Thyroid Function among Adults and Adolescents from NHANES 2011–2012. Int J Environ Res Public Health. 2015;12:6098–114. doi: 10.3390/ijerph120606098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Genuis SJ, Liu Y, Genuis QI, Martin JW. Phlebotomy treatment for elimination of perfluoroalkyl acids in a highly exposed family: a retrospective case-series. PLoS One. 2014;9:e114295. doi: 10.1371/journal.pone.0114295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.U.S. Environmental Protection Agency. Provisional health advisories for perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) Washington, DC: U.S. Environmental Protection Agency; 2009. Jan 8, [accessed, 30 March, 2016]. https://www.epa.gov/sites/production/files/2015-09/documents/pfoa-pfos-provisional.pdf. [Google Scholar]
- 39.National Research Council; Agents CoTTaAoE, editor. Toxicity Testing in the 21st Century: A Vision and a Strategy. Washington, DC: National Academy Press; 2007. [Google Scholar]
- 40.Butenhoff J, Costa G, Elcombe C, Farrar D, Hansen K, Iwai H, et al. Toxicity of ammonium perfluorooctanoate in male cynomolgus monkeys after oral dosing for 6 months. Toxicol Sci. 2002;69:244–57. doi: 10.1093/toxsci/69.1.244. [DOI] [PubMed] [Google Scholar]
- 41.DeWitt JC, Peden-Adams MM, Keller JM, Germolec DR. Immunotoxicity of perfluorinated compounds: recent developments. Toxicol Pathol. 2012;40:300–11. doi: 10.1177/0192623311428473. [DOI] [PubMed] [Google Scholar]
- 42.Stein CR, McGovern KJ, Pajak AM, Maglione PJ, Wolff MS. Perfluoroalkyl and polyfluoroalkyl substances and indicators of immune function in children aged 12–19 y: National Health and Nutrition Examination Survey. Pediatr Res. 2016;79:348–57. doi: 10.1038/pr.2015.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grandjean P, Andersen EW, Budtz-Jorgensen E, Nielsen F, Molbak K, Weihe P, et al. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307:391–7. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Granum B, Haug LS, Namork E, Stolevik SB, Thomsen C, Aaberge IS, et al. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol. 2013;10:373–9. doi: 10.3109/1547691X.2012.755580. [DOI] [PubMed] [Google Scholar]
- 45.Agency for Toxic Substances and Disease Registry. [accessed, 30 March, 2016];Draft toxicological profile for perfluoroalkyls. 2015 http://www.atsdr.cdc.gov/ToxProfiles/tp200.pdf. [PubMed]
- 46.Grandjean P, Budtz-Jorgensen E. Immunotoxicity of perfluorinated alkylates: calculation of benchmark doses based on serum concentrations in children. Environ Health. 2013;12:35. doi: 10.1186/1476-069X-12-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.EFSA Scientific Committee. Guidance of the Scientific Committee on Use of the benchmark dose approach in risk assessment. The EFSA Journal. 2009;1150:1–72. [Google Scholar]
- 48.U.S. Environmental Protection Agency. Benchmark dose technical guidance. Washington, DC: Risk Assessment Forum, U.S. Environmental Protection Agency; 2012. Jun, Report No.: EPA/100/R-12/001. [Google Scholar]
- 49.National Research Council. Science and decisions: advancing risk assessment. Washington, D.C: National Academy Press; 2009. [PubMed] [Google Scholar]
- 50.Grandjean P. Science for precautionary decision-making. In: Gee D, Grandjean P, Hansen SF, van den Hove S, MacGarvin M, Martin J, Nielsen G, Quist D, Stanners D, editors. Late Lessons from Early Warnings. II. Copenhagen: European Environment Agency; 2013. pp. 517–35. [Google Scholar]
- 51.Guzelian PS, Henry CJ, Olin SS. Similarities and differences between children and adults: Implication for risk assessment. Washington, DC: ILSI Press; 1992. [Google Scholar]
- 52.Grandjean P. Seven deadly sins of environmental epidemiology and the virtues of precaution. Epidemiology. 2008;19:158–62. doi: 10.1097/EDE.0b013e31815be031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Grandjean P, Eriksen ML, Ellegaard O, Wallin JA. The Matthew effect in environmental science publication: a bibliometric analysis of chemical substances in journal articles. Environ Health. 2011;10:96. doi: 10.1186/1476-069X-10-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Merton RK. The Matthew effect in science. The reward and communication systems of science are considered. Science. 1968;159:56–63. [PubMed] [Google Scholar]
- 55.Environmental Protection Agency (EPA) [accessed, 30 March, 2016];Risk-Based Prioritization (RBP) Decisions Summary. 2009 http://www.epa.gov/champ/pubs/hpv/2009-RBP-Decisions.pdf.
- 56.Needleman HL. The removal of lead from gasoline: historical and personal reflections. Environ Res. 2000;84:20–35. doi: 10.1006/enrs.2000.4069. [DOI] [PubMed] [Google Scholar]
- 57.Rodgers T. As canned tuna sales dive, companies plan ad blitz to reel buyers back. The San Diego Union; Tribune: 2005. Jul 27, [Google Scholar]
- 58.Michaels D. Doubt is their product: how industry’s assault on science threatens your health. Oxford; New York: Oxford University Press; 2008. [Google Scholar]
- 59.Ioannidis JP. Why most discovered true associations are inflated. Epidemiology. 2008;19:640–8. doi: 10.1097/EDE.0b013e31818131e7. [DOI] [PubMed] [Google Scholar]
- 60.Late Lessons from Early Warnings: Science, Precaution, Innovation. Copenhagen: European Environment Agency; 2013. [Google Scholar]
- 61.Environmental Protection Agency (EPA) Chemical Hazard Data Availability Study. [acessed, 30 March, 2016];What do we really know about the safety of high production volume chemicals? EPA’s 1998 baseline of hazard information that is readily available to the public. 1998 http://www.epa.gov/hpv/pubs/general/hazchem.pdf.
- 62.Late lessons from early warnings: the precautionary principle 1896–2000. Copenhagen: European Environment Agency; 2002. [Google Scholar]
- 63.Schettler T, Stein J, Reich F, Valenti M, Wallinga D. Harm’s Way: Toxic Threats to Child Development. Cambridge, MA: Greater Boston Physicians for Social Responsibility; 2000. [Google Scholar]