Abstract
Maternal influences are an important contributing factor to offspring survival, development, and behavior. Common environmental pathogens can induce maternal immune responses and affect subsequent development of offspring. There are likely sensitive periods during pregnancy when animals are particularly vulnerable to environmental disruption. Here we characterize the effects of maternal immunization across pregnancy and postpartum on offspring physiology and behavior in Siberian hamsters (Phodopus sungorus). Hamsters were injected with the antigen keyhole limpet hemocyanin (KLH) 1) prior to pairing with a male (pre-mating), 2) at separation (post-mating), 3) at mid-pregnancy, or 4) after birth (lactation). Maternal food intake, body mass, and immunity were monitored throughout gestation, and litters were measured weekly for growth until adulthood when social behavior, hormone concentrations, and immune responses were determined. We found that immunizations altered maternal immunity throughout pregnancy and lactation. The effects of maternal treatment differed between male and female offspring. Aggressive behavior was enhanced in offspring of both sexes born to mothers treated post-mating and thus early in pregnancy relative to other stages. In contrast, maternal treatment and maternal stage differentially affected innate immunity in males and females. Offspring cortisol, however, was unaffected by maternal treatment. Collectively, these data demonstrate that maternal immunization affects offspring physiology and behavior in a time-dependent and sex-specific manner. More broadly, these findings contribute to our understanding of the effects of maternal immune activation, whether it be from environmental exposure or immunization, on immunological and behavioral responses of offspring.
Keywords: KLH, cortisol, bacterial killing, innate immunity, resident-intruder, maternal effects
Introduction
Non-genetic, environmental influences contribute to offspring phenotype, including behavior and physiology (Crews, 2010). While the role of environmental factors has been traditionally under-appreciated, recent studies have highlighted the importance of parental effects on an organism’s phenotype (Kappeler and Meaney, 2010; Dolinoy et al., 2007). In particular, maternal influences, such as energy and nutrient availability, oxygen levels, and hormone concentrations, are important contributing factors to offspring survival, development, and behavior (reviewed in: Mousseau and Fox, 1998; Holekamp and Dloniak, 2009).
Pregnancy and lactation represent developmental periods characterized by prolonged physical association between mother and offspring. One current hypothesis posits that during normal reproduction, an activated maternal innate immune response is adaptive and necessary for successful pregnancy (Schminkey and Groer, 2014). A wide variety of environmental factors (e.g., stress, nutrition, toxin exposure) can alter the prenatal environment and consequentially contribute to offspring development. For example, maternally derived antibodies are produced in response to pathogens and can be transferred across the placenta and in the mother’s milk, providing a transgenerational defense. Because neonates have limited ability to synthesize antibodies, maternal antibodies have the capacity to protect the offspring from pathogens, as well as program the developing immune system (Grindstaff et al., 2006). While the role of the maternal immune system in offspring development is not well understood, maternal inflammation can induce altered stress responses, anxiety, and social behaviors such as reduced activity and decreased aggression in mouse offspring (Hava et al., 2006). In humans, maternal immune activation may contribute to several psychiatric conditions including autism and schizophrenia (Bilbo and Schwarz, 2009; Boksa, 2010; Patterson, 2002; Patterson, 2009). For example, influenza infection, particularly in the second trimester, significantly increases the risk of schizophrenia (Boksa, 2010).
Accumulating evidence suggests that neonatal or early-life exposure to elevated cytokines (e.g., in response to lipopolysaccharide (LPS) or polyinosinic:polycytidylic acid (poly I:C)) can result in prolonged neural and behavioral abnormalities (reviewed in: Bilbo and Schwarz, 2009). Further, emerging evidence suggests that elevated cytokine concentrations during pregnancy can cause marked changes in fetal brain development and therefore likely contribute to downstream changes in offspring behavior (Patterson et al., 2009; Patterson, 2002). Therefore, common environmental pathogens such as viral or bacterial organisms that induce maternal inflammatory responses have the potential to alter the embryonic/fetal environment via multiple pathways (Smith et al., 2007; Welberg and Seckl, 2001; Mouihate et al., 2008). It is also apparent that inflammatory responses in pregnancy can have direct implications for behavior and learning (Grindstaff et al., 2012) and regulation of the HPA axis responses to stress (Hava et al., 2006) and immune system (Clark et al., 2004; Patterson et al., 2009; Lemke and Lange, 1999; French et al., 2013a; Onore et al., 2014). It should be noted that maternal immune activation is not necessarily negative for developing offspring or maternal fitness. Evidence suggests immune activation during pregnancy could prime offspring immune system for its external environment allowing for a better phenotype-environment match, i.e., the transgenerational priming of immunity hypothesis (Eggert et al., 2014; Shikano et al., 2015).
Whereas LPS treatment and associated sickness responses (i.e., fever, cytokine release) provide important models with which to examine developmental responses to severe bacterial infection, not all maternal immune insults result in overt sickness, nor is it known whether certain stages of development are more sensitive than others. Less severe infections are probably more common in nature, but may go unnoticed despite immune system activation. Little is known, however, of the effects of these subtler challenges on offspring physiology and behavior. To address this question we used a relatively innocuous antigen, keyhole limpet hemocyanin (KLH), derived from the giant keyhole limpet (Megathura crenulata). In contrast to LPS, KLH generates an antibody response without eliciting changes in the hypothalamo-pituitary-adrenal (HPA) axis, a critical part of a stress response, and without making the animals sick (e.g., inflammation, anorexia, lethargy, or fever) (Dixon et al., 1966). Therefore, KLH can be used to evaluate the effects of immune activation during pregnancy without a potential confound of elevated cortisol or pro-inflammatory cytokine levels or altered nutrient intake and energy expenditure. KLH does, however, affect TH–mediated anti-inflammatory cytokine production as well as B and T cell populations. Specifically, KLH stimulates B cell proliferation and the generation of antibodies through a TH2 response and associated cytokines (e.g., interleukins [IL]-5 and IL-10). These cytokines, as well as associated antibodies, potentially cross through the placenta and affect the brain and immune system development of the offspring (Lemke and Lange, 1999).
Variation in the effects of maternal immune activation could be due to differences in physiology as pregnancy progresses, such as changes in placental buffering capabilities throughout gestation, which temporally vary embryo/fetal susceptibility to maternal influences (Meyer et al., 2006). Characterizing the effects of maternal immune activation across multiple time points is critical to understanding the contributions of the developmental changes that occur throughout pregnancy and lactation. To test this, we immunized animals at different times during pregnancy: pre- and post-mating, when energy investment in current reproductive effort is relatively low, and mid-pregnancy and early lactation, when energy investment is typically very high. We examined the social behavior, hormone concentrations, and immune function of offspring into adulthood, shortly after reaching sexual maturity. Measures of both the innate and acquired arms of the immune system are of particular importance because altered investment in the development of the immune system due to immunization may result in altered functioning of one or both arms later in life. Immune activation in the perinatal period may alter adult immune function and HPA activity, particularly in the mid-gestation period when the nervous and immune systems are developing (Pearce, 2001). Early lactation may be a particularly important time, because rodents undergo substantial brain development similar to the human third trimester during this period (Dobbing and Sands, 1979). Regardless, continued exposure to pathogens is commonplace in the natural environment, and any resulting effects on offspring physiology and behavior could not only impact maternal fitness but have the potential to lead to persistent transgenerational effects as well.
Materials and Methods
Animals and Housing
Ninety-three adult (between 5–6 months of age) nulliparous female Siberian hamsters (Phodopus sungorus) were obtained from the breeding colony maintained at Indiana University. An additional 25 females (from the same in-house breeding colony and were even dispersed throughout cohorts) were later added to the study for a total of 118 females. All animals were initially group housed (2–4 per cage with same-sex siblings upon weaning at 21 days of age). Seven days before the start of the experiment, animals were housed individually in polypropylene cages (28 × 17 × 12 cm) in an experimental room with a 16:8 (light:dark) hour photoperiod. Temperature was maintained at 20 ± 2°C, and relative humidity was 50 ± 10%. All animals were given ad libitum access to food (Purina rodent chow, St. Louis, MO, USA) and water throughout the study. All animals were treated in accordance with the Bloomington Institutional Animal Care and Use Committee (BIACUC).
Experimental Methods
Maternal treatments
All females in the study were initially paired with a male for three days to allow for mating to occur. Of all animals in the study, 76 became pregnant (control = 34, KLH = 42) while 42 did not (control = 15, KLH = 27). In order to examine the effects of maternal immune response at different stages of pregnancy and early lactation on offspring physiology and behavior, female hamsters were randomly assigned to one of four treatment schedules: 1) injection one day prior to mate pairing (control = 8, KLH = 13), 2) early pregnancy - one day following un-pairing mates (control = 7, KLH = 10), 3) mid-pregnancy (control = 9, KLH = 10), and 4) early lactation (control=10, KLH = 9). Hamsters in each treatment schedule received either an injection of keyhole limpet hemocyanin (KLH; 2.86 mg/kg; Calbiochem, San Diego, CA) or a sham injection with the saline vehicle (control). The non-pregnant hamsters were included as time-point controls and thus treated identically to pregnant hamsters in ways.
Maternal food, mass and litter size
Maternal food intake was monitored daily and body mass every three days throughout the study except for the four days while the female was paired with the male to avoid additional stress. Initial litter mass and size was taken on the day of birth and growth of the litters was assessed with weekly litter mass measurements.
Maternal immunity and cortisol
The magnitude of the immune response following KLH injection in the mothers was assessed by measuring antibody production and the ability of blood serum components (e.g., complement proteins) to kill E. coli. Blood samples were collected from the mothers on Day 5 and Day 10 following the KLH injection. These time points reflect the peak levels of serum immunoglobulin M (IgM) and immunoglobulin G (IgG), respectively (Zysling et al., 2009). Additionally, samples were analyzed for bacterial killing ability, which is an assessment of innate immunity, and may be affected by immune activation and stage of pregnancy. Serum aliquots were stored at −80° C until assayed for bacteria killing ability, peak anti-KLH IgM and IgG levels, and cortisol.
Offspring
One female and one male offspring were randomly chosen from each mother’s litter for adult offspring physiology and behavior analyses. After weaning (at 21 days), offspring were housed with same-sex siblings under standard housing protocol until three months old. At 13 weeks of age, a blood sample was collected one week prior to the start of all social interaction trials in order to assess baseline circulating cortisol and bacterial killing ability. Following social interaction, all hamsters received a single subcutaneous injection of KLH. Blood samples were drawn on Days 5 and 10 post-KLH immunization as described above. Serum aliquots were stored at −80° C until assayed for peak anti-KLH IgM and IgG levels and bacterial killing abilities (see above ‘maternal immunity’ section).
Cortisol Enzyme Immunoassay
Serum cortisol was measured in the mothers at Day 5 post-KLH injection and in the offspring baseline sample. Cortisol is the predominant glucocorticoid produced in Siberian hamsters at ~100× that of corticosterone (Reburn and Wynne-Edwards, 1999). Cortisol concentrations were determined with a commercially available enzyme immunoassay (EIA) kit (Correlate-EIA™, Assay Designs, Ann Arbor, MI). This assay was previously validated for use with Siberian hamsters (Demas et al., 2004) and is highly specific for cortisol. The cross-reactivity of corticosterone is 27.7% and other steroid hormones are < 0.1%. The sensitivity of the assay is 56.72 pg/ml. Intra-assay and inter-assay CVs were less than 10.0%.
Immunoglobulin Enzyme-Linked Immunosorbent Assay
To assess humoral immunity, serum anti-KLH antibody concentrations were assayed using an enzyme-linked immunosorbant assay(ELISA) (Demas et al., 2003). Briefly microtiter plates were coated with antigen by incubating overnight at 4°C with 0.5 mg/ml KLH in sodium bicarbonate buffer (pH 9.6). Plates were washed with phosphate buffered saline (PBS) (pH 7.4) containing 0.05% Tween 20 (PBS-T) at pH 7.4, then blocked with 5% non-fat dry milk in PBS overnight at 4°C to reduce non-specific binding, and washed again with PBS-T. Thawed serum samples were diluted 1:20 with PBS-T, and 150 μl of each serum dilution was added in duplicate to the wells of the antigen-coated plates. Positive control samples (pooled sera from hamsters previously determined to have high levels of anti-KLH antibody, similarly diluted with PBS-T) were added in duplicate. Plates were sealed, incubated at 37°C for 3 h, and then washed with PBS-T. Secondary antibody (alkaline phosphatase-conjugated-anti hamster IgG or IgM diluted 1:500 with PBS-T; Rockland, Gilbertsville, PA) was added to the wells, and the plates were sealed and incubated for 1 h at 37°C. Plates were then washed again with PBS-T and 150 μl of the enzyme substrate p-nitro-phenyl phosphate (Sigma, St Louis, MO; 0.1mg/ml in diethanolamine substrate buffer) was added to each well. Plates were protected from light during the enzyme-substrate reaction. The optical density (OD) of each well was determined using a plate reader (Bio-Rad, Benchmark Richmond, CA) equipped with a 405 nm wavelength filter, and the mean OD for each sample was expressed as a ratio of its plate positive control OD for statistical analysis. The intra-assay CVs for IgG and IgM were less than 6.8% and 7.3%, respectively.
Bacterial Killing Assay (BKA)
As a functional assessment of an animal’s ability to clear a bacterial infection, we utilized an ex vivo bacterial killing assay (BKA), based on a published protocol (Matson et al., 2006) that was modified by French et al. (2009). This assay quantifies the relative number of E. coli colony forming units (CFU) that grow after incubation with serum. Differences in CFU presumably represent differences in serum proteins. Briefly, E. coli (ATCC #8739, Microbiologics, St. Cloud, MN) (1 pellet = 107 CFU) was added to 40 ml 1M sterile PBS warmed to 35–37°C and vortexed to create a bacterial stock solution, which was activated by incubation for 30 min at 37°C. Serum samples were stored at −80C following collection and were stored for no longer than 4 weeks before running the assay. Serum samples were diluted 1:40 in glutamine enriched CO2-independent media (Invitrogen Corp., Carlsbad, CA). This dilution was validated for serum with a dose response curve prior to the experiment. The stock bacteria solution (500,000 CFU/ml) was diluted with sterile 1M PBS to create a 50,000 CFU/ml working solution. To obtain estimates of bacterial numbers (i.e., positive control), the working solution was diluted 1:10 with glutamine enriched CO2-independent media. For each sample, the working solution was added at a 1:10 ratio to the diluted serum sample. The bacteria/serum cocktails were incubated for 30 min at 37°C. All samples were vortexed and 50 μl was added to Petri plates in duplicate and spread with a flame-sterilized spreader. All plates were stored upside down overnight at 37°C. Following incubation, bacteria colonies were counted on each plate, and duplicates were averaged. The mean value for each sample was expressed as a percent of bacteria killed relative to the control plates in which no killing occurred. The CV for the bacteria killing assay was 9.1%.
Offspring social behavior
At 14 weeks of age (i.e., young adulthood), male and female offspring (same individuals as above) were observed in a dyadic social interaction with a single same-sex hamster. Individuals were placed in the cages of individually-housed dominant, aggressive resident hamsters for 5 min (modified from Scotti et al., (2007). Sessions occurred within the first 2 h of the dark phase of the light:dark cycle. All interactions were observed in order to determine whether resident aggressors attacked the experimental animals in the defeat group. Furthermore, we determined if the experimental animals displayed any submissive behaviors. All experimental trials were performed under low illumination (25W) red light conditions, which allowed for sufficient light during video recording and observations without disturbing the behavior of the hamsters. To identify the experimental offspring from the residents, small patches of fur were shaved on the dorsal surface. Behavioral interactions were scored using ODlog™ software (Macropod Software) by a trained observer blind to treatment. The numbers of attacks by the resident and defensive behaviors (i.e., escape and on back submissive posture) were quantified via scoring the number of occurrences and the duration of submissive behavior (i.e., only behavior that was sustained for a recordable length of time, < 1–2 seconds). We utilized this design so that we could more directly compare with a similar maternal effects study using a different mitogen LPS. This social defeat protocol allows us to evaluate reactions to an ecologically and behaviorally relevant social stressor, as might occur during competition for food, mates, or territory. Defeat is immunomodulatory, and we are interested in the repercussions of different developmental backgrounds on this effect.
Statistical Analyses
All data were analyzed with JMP.IN (v.8.0.1, SAS Institute Inc., Cary, NC, USA). All data were checked for normality and homogeneity of variance prior to analyses. Cortisol values were log transformed to meet assumptions of normality for both mothers and offspring. Differences among all dependent measures were determined using analyses of variance (ANOVA) analyzing treatment (saline or KLH) X stage (pre, post, mid, lactation) X pregnancy (yes or no). Tukey’s honestly significant difference post-hoc comparisons were conducted when significant interactions were present. Changes in body mass and food intake were compared using repeated measures ANOVA. Because control animals did not receive KLH, anti-KLH IgM (measured 5 days following challenge) was determined using Kruskal-Wallis tests for nonparametric data between different stages and pregnancy, and anti-KLH IgG (measured both 5 and 10 days following KLH challenge) was determined using a repeated measures ANOVA including stage and pregnancy in the model. Within-subject comparisons violated assumptions of sphericity, and were therefore Greenhouse-Geisser (GG)-corrected.
Two offspring from every litter, one male and one female, were included in analyses to avoid issues of non-independence. For offspring analyses, we built a GLM including sex, maternal treatment, and maternal treatment stage, and all interactions on all offspring dependent physiological variables. Tukey’s HSD post-hoc comparisons were conducted when significant interactions were present. Offspring behavior could not be transformed to achieve normality and so a Kruskal-Wallis test was run testing effects of maternal treatment, maternal group, and sex on each behavior with corrections for multiple comparisons. In all cases, differences between group means were considered statistically significant if p ≤ 0.05 unlessotherwise noted.
Results
Maternal Measures
Food Intake and Body Mass
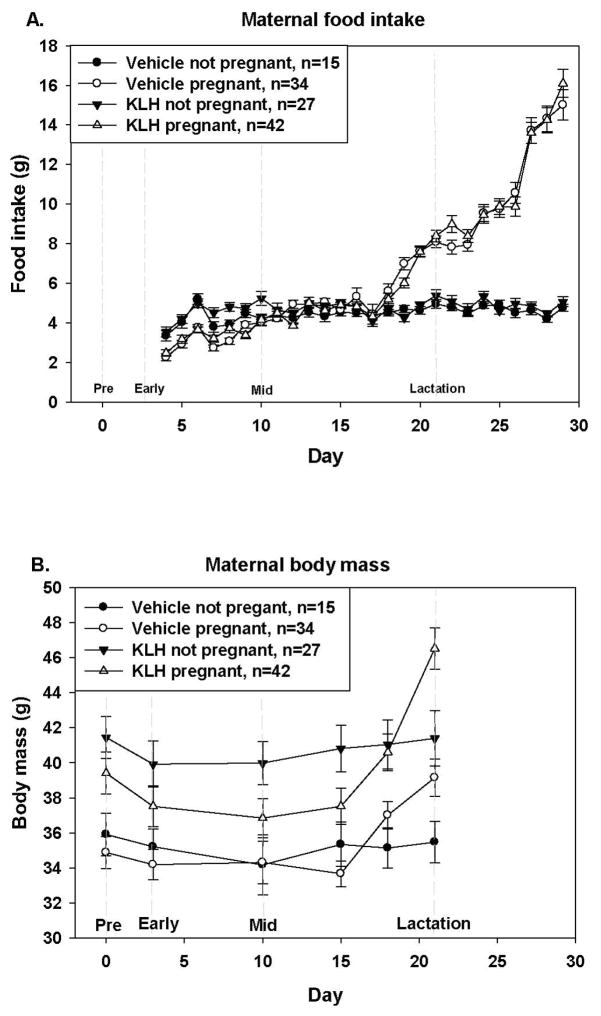
Food intake was analyzed in two periods, 1) from unpairing the female (Day 4) to prior to birth (Day 17) and 2) from birth until weaning (Day 29). Repeated measures ANOVAs for animals from day 4–17 (pregnancy) revealed that during this time there was a significant interaction of time x pregnancy (Ftime x preg = 8.27; d.f. = 6.1, 646.8; p < 0.001; GG-corrected), of time x treatment (Ftime x treat = 3.72; d.f. = 6.1, 646.8; p < 0.001; GG-corrected), and of time x stage (Ftime x stage = 1.65; d.f. = 18.3, 646.8; p = 0.004; GG-corrected), and food intake increased over time (Ftime = 50.77; d.f. = 6.1, 646.8; p < 0.001; GG-corrected). Not surprisingly pregnancy had a significant effect on food intake (Fpreg = 12.75; d.f. = 1, 106; p < 0.001; Fig. 1A), but not treatment (Ftreat = 0.24; d.f. = 1, 106; p = 0.629), or stage at treatment (Fstage = 0.34; d.f. = 3, 106; p = 0.794). Likewise, during lactation (after birth) all interactions were significant (All F > 2.14; p < 0.002; GG-corrected), except for interaction between time X treatment (Ftime x treat = 1.92; d.f. = 6.8, 682.8; p = 0.066). Animals that were rearing pups ate more (Fpreg = 212.6; d.f. = 1, 100; p < 0.001; Fig 1A) and intake increased over time as pups grew (Ftime = 78.17; d.f. = 6.8, 682.8; p < 0.001; GG-corrected). There was no overall effect of treatment (Ftreat = 0.06; d.f. = 1, 100; p = 0.811) or stage at treatment (Fstage = 0.18; d.f. = 3, 100; p = 0.913).
Figure 1. Maternal food intake (A) and body mass (B) starting with day of pairing with a male.
Food intake and body mass of pregnant (KLH n= 42; vehicle n = 34) and not pregnant (KLH n= 27; vehicle n = 15) females either treated with KLH or saline over the course of the experiment (A). There was no overall effect of timing of KLH challenge so groups represent animals challenged at different times lumped together. Pregnancy significant impacted food intake, such that pregnant females ate more than females that did not get pregnant (Fpreg = 12.75; d.f. = 1, 106; p < 0.001) (B). Treatment with KLH did impact body mass where KLH treated females weighed significantly more than vehicle treated control females (Ftreat = 6.55; d.f. = 1, 85; p = 0.012) (B).
There was a significant time X pregnancy effect on body mass and this is likely primarily driven by pregnant animals gaining body mass over time (Ftime X preg = 28.36; d.f = 2.3, 192.8; p < 0.001; GG-corrected). Stage at treatment, and interactions time x treatment and time x stage, were not significant (all F < 1.44, all p > 0.237; GG-corrected). There was also a significant effect of treatment on body mass (Ftreat = 6.55; d.f. = 1, 85; p = 0.012; Fig. 1B) where KLH animals were heavier. There was also a significant effect of time on body mass (Ftime = 26.02; d.f. = 2.3, 192.8; p < 0.001; GG-corrected), such that animals gained body mass over time (likely driven by pregnant females). These results indicate that stage at immune challenge affects food intake and that treatment with KLH affects weight gain during pregnancy.
Serum Cortisol Concentrations
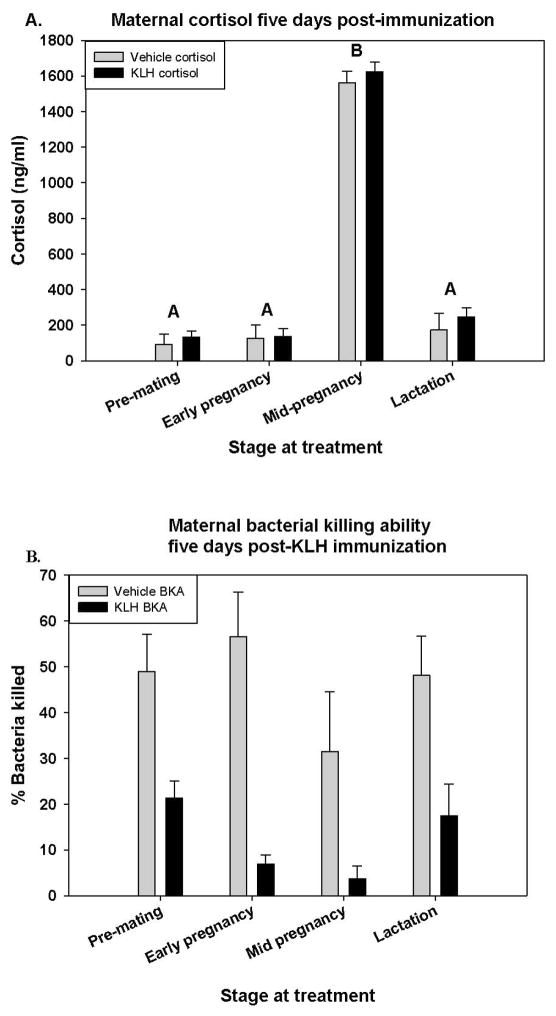
Serum cortisol was measured in samples collected 5 days after KLH injection. Concentrations in mothers differed by stage (Fstage = 105.16; d.f. = 3, 48; p < 0.001; Fig 2A). Animals that were injected at mid-pregnancy were bled just before parturition, when cortisol levels naturally rise, and these animals had the highest cortisol. However, there were no differences according to stage x treatment, treatment, pregnancy, or (Fstage X treat = 0.423; d.f. = 3, 40; p = 0.738, Ftreat = 1.26; d.f. = 1, 40; p = 0.268, and Fpreg = 2.12; d.f. = 1, 40; p = 0.153, respectively).
Figure 2. Maternal cortisol (A) and bacterial killing ability (B).
Cortisol levels in mothers 5 days post-treatment with either KLH or vehicle. Pregnancy stage but not treatment effected cortisol, where females mid-pregnancy had significantly higher cortisol levels (Fstage = 105.16; d.f. = 3, 48; p < 0.001) (A). Percent bacteria killed in mothers 5 days following either KLH or saline injection. Different letters denote significant differences (α=0.05) (B).
Bacteria Killing Ability
Maternal treatment had a significant effect on bacteria killing ability at 5 days after KLH injection (Ftreat = 47.894; d.f. = 1, 99; p < 0.001; Fig 2B). KLH treated animals had significantly reduced bacteria killing ability than saline control females (Fig 2B). The effect of maternal stage was approaching significance (Fstage = 2.61; d.f. = 3, 99; p = 0.055), with bacterial killing ability being lowest during mid-pregnancy when cortisol is highest. There was no significant effect of pregnancy and no significant interaction among stage and treatment (Fpreg = 0.04; d.f. = 1, 99; p = 0.851, and Fstage X treat = 1.18; d.f. = 3, 99; p = 0.321, respectively) on BKA. In some cases, the presence of serum enhanced the survival of bacteria, leading to more colonies than the control plate. These cases were assigned a 0 value for killing ability.
Antibody Production
Anti-KLH IgM was measured in the Day 5 post-KLH samples, when IgM reaches its peak production (Zysling and Demas, 2007). There effect of stage on IgM approached significance (χ2 = 7.37, p = 0.061; Table 1), but there was no effect of pregnancy on IgM (χ2 < 0.01, p = 0.99; Table 1). The lactation stage had lowest IgM and premating the highest, with early and mid- pregnancy falling intermediate. Anti-KLH immunoglobulin G levels increased from Day 5 to Day 10 following KLH challenge (Ftime = 147.00; d.f. = 1, 57; p < 0.001). However, there was no significant effect of stage at immune challenge, pregnancy, or interactions on IgG levels (Fstage = 0.24; d.f. = 3, 57; p = 0.862, and Fpreg = 0.75; d.f. = 1, 57; p = 0.392; all Finteractions < 0.832, all p > 0.365; respectively; Table 1).
Table 1.
Serum anti-keyhole limpet hemocyanin (KLH) antibodies days 5 and 10 post-immunization during non-pregnant, pre-mating, early-pregnancy, mid-pregnancy and lactation in female hamsters (i.e., mothers).
| Day 5 | Day 10 | ||
|---|---|---|---|
| Maternal stage | Anti-KLH IgM | Anti-KLH IgG | anti-KLH IgG* |
| Not pregnant | 0.788 ± 0.065AB | 0.525 ± 0.024 | 0.799 ± 0.051 |
| Pre-mating | 0.911 ± 0.096A | 0.557 ± 0.048 | 0.877 ± 0.036 |
| Early pregnancy | 0.885 ± 0.107AB | 0.544 ± 0.049 | 0.873 ± 0.057 |
| Mid-pregnancy | 0.726 ± 0.084AB | 0.443 ± 0.041 | 0.779 ± 0.060 |
| Lactation | 0.640 ± 0.101B | 0.485 ± 0.074 | 0.889 ± 0.149 |
Different letters denote statistically significant differences across IgM groups (α = 0.05).
IgG levels are significantly higher at day 10 relative to day 5 but do not differ according to reproductive stage within a sampling period (i.e., day 5 versus 10).
Offspring Measures
Growth
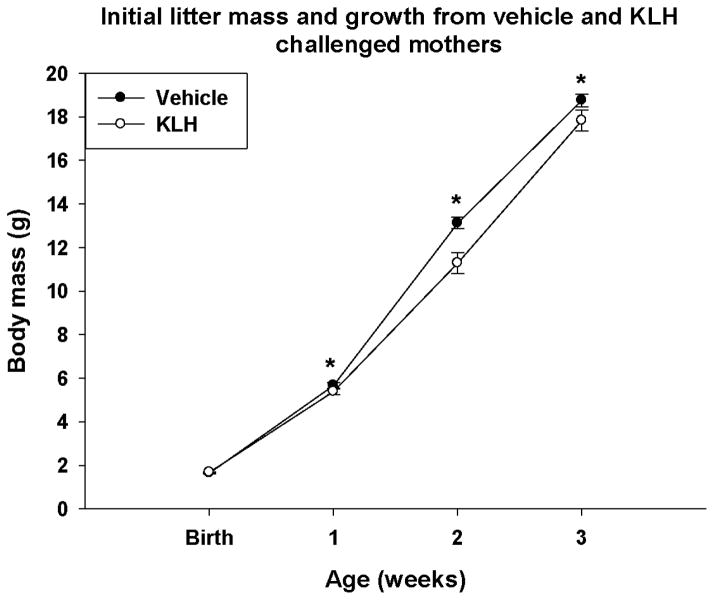
Initial litter mass at birth did not vary according to maternal treatment or stage at treatment, when including maternal mass as a covariate (Table 2; all F < 0.71, all p > 0.549). There was also no difference among groups in the sex ratio of the litters or number of pups eaten by mom (Table 2; all F < 2.11, all p < 0.152). Further analysis reveals a positive relationship between number of pups initially in the litter and the number of pups consumed by KLH-treated moms just post-parturition (F = 16.00, d.f. = 1, 32, P < 0.001, r^2 = 0.33), but this same relationship is not present in vehicle treated moms (F = 2.56, d.f. = 1, 29, P = 0.120., r^2 = 0.08). This relationship is only present in animals treated with KLH during pre-mating and early pregnancy (all F > 6.44, all p < 0.028, all r^2 > 0.37), but not mid-pregnancy or lactation (all F < 0.20, all p > 0.673, all r^2 < 0.04). However, using repeated measures analysis with maternal weight as a covariate, from birth to week 3 (weaning), the mean mass of offspring from KLH-treated mothers was significantly lower than control offspring as they grew (Ftreat = 5.61; d.f. = 1, 61; p = 0.021; Fig 3; Ftimextreat = 7.76; d.f. = 1.1, 68.6; p = 0.005; GG corrected; Fig. 3). There was no effect of mothers’ stage at treatment or any other significant interaction (all F < 2.01, all p < 0.114).
Table 2.
Initial litter size and number, number of each sex upon weaning, and number of pups eaten post-parturition from mothers treated with vehicle and KLH during pre-mating, early-pregnancy, mid-pregnancy and lactation in female hamsters.
| Maternal stage | Initial litter mass (g) | Initial number Pups | Number female pups | Number male pups | Number pups eaten |
|---|---|---|---|---|---|
| Pre-mating | |||||
| Vehicle | 10.09 ± 0.87 | 6.13 ± 0.67 | 3.29 ± 0.42 | 2.71 ± 0.42 | 0.71 ± 0.36 |
| KLH | 11.56 ± 0.72 | 6.85 ± 0.37 | 3.46 ± 0.29 | 2.69 ± 0.31 | 0.69 ± 0.24 |
| Early pregnancy (post-mating) | |||||
| Vehicle | 8.47 ± 0.73 | 5.14 ± 0.51 | 2.57 ± 0.57 | 2.00 ± 0.53 | 0.57 ± 0.43 |
| KLH | 11.29 ± 0.74 | 6.70 ± 0.62 | 3.44 ± 0.41 | 2.44 ± 0.41 | 0.78 ± 0.32 |
| Mid-pregnancy | |||||
| Vehicle | 10.63 ± 0.58 | 6.67 ± 0.37 | 3.00 ± 0.47 | 3.44 ± 0.60 | 0.22 ± 0.15 |
| KLH | 9.99 ± 0.81 | 6.10 ± 0.55 | 3.29 ± 0.42 | 2.71 ± 0.36 | 0.57 ± 0.20 |
| Lactation | |||||
| Vehicle | 10.04 ± 0.52 | 6.30 ± 0.40 | 2.43 ± 0.48 | 2.57 ± 0.61 | 1.13 ± 0.58 |
| KLH | 11.37 ± 0.73 | 6.89 ± 0.42 | 4.20 ± 0.20 | 2.00 ± 0.32 | 0.60 ± 0.24 |
Figure 3. Offspring growth.
Average body mass per individual increased from birth through weaning. Offspring of KLH treated mothers were significantly smaller than control offspring. Statistically significant differences between group means are denoted by an asterisk (*) (Ftreat = 5.61; d.f. = 1, 61; p = 0.021; Ftimextreat = 7.76; d.f. = 1.1, 68.6; p = 0.005; GG corrected).
Following weaning, litters were segregated by sex and body mass was analyzed separately for each sex. After separation there was a significant time x sex interaction (Ftime x sex = 26.77; d.f. = 2.9, 333.4; p < 0.001, GG-corrected) on litter mass such that the sexes grew at different rates, effect of sex (Fsex = 105.22; d.f. = 1, 116; p < 0.001), and of time (Ftime = 1049.03; d.f. = 2.9, 333.4; p < 0.001, GG-corrected). However, there was no effect of maternal treatment, stage at maternal treatment, or any other interactions on offspring mass over time (all F < 2.10, all p > 0.150).
Serum Cortisol Concentrations
There was no significant interaction among sex X maternal stage at treatment (F = 2.30; d.f. = 3, 85; p = 0.083). Baseline serum cortisol differed between the offspring sexes, with females having higher concentrations (Table 3; F = 14.23; d.f. = 1,85; p < 0.001). There was also no effect of maternal treatment, maternal stage at treatment, or any interaction on offspring cortisol levels (all F < 1.68, all p > 0.178).
Table 3.
Serum cortisol, anti-KLH Immunoglobulin G (IgG) and aggressive behavior in male and female offspring from KLH and vehicle treated mothers combined. There were significant sex differences for all of the measures with the exception of intruder attacks. There were no treatment or stage effects for any of the below except intruder attacks.
| Measure | Females | Males |
|---|---|---|
| Cortisol (ng/ml) | 105.121 ± 6.403* | 78.741 ± 2.877 |
| Anti-KLH IgG (% plate positive) | 63.173 ± 2.860* | 52.427 ± 3.072 |
| Resident attacks (n) | 1.349 ± 0.625 | 5.383 ± 1.091* |
| Intruder attacks (n) | 4.186 ± 0.967 | 4.702 ± 1.287 |
| Submissions (n) | 0.326 ± 0.159 | 2.213 ± 0.523* |
| Submission duration (sec) | 0.465 ± 0.298 | 5.255 ± 1.495* |
| Chases (n) | 0.465 ± 0.442 | 0.936 ± 0.283* |
Asterisks denote statistically significant differences among the sexes (α = 0.05), and are positioned on the sex demonstrating the greater value for each measure.
Bacteria Killing Ability
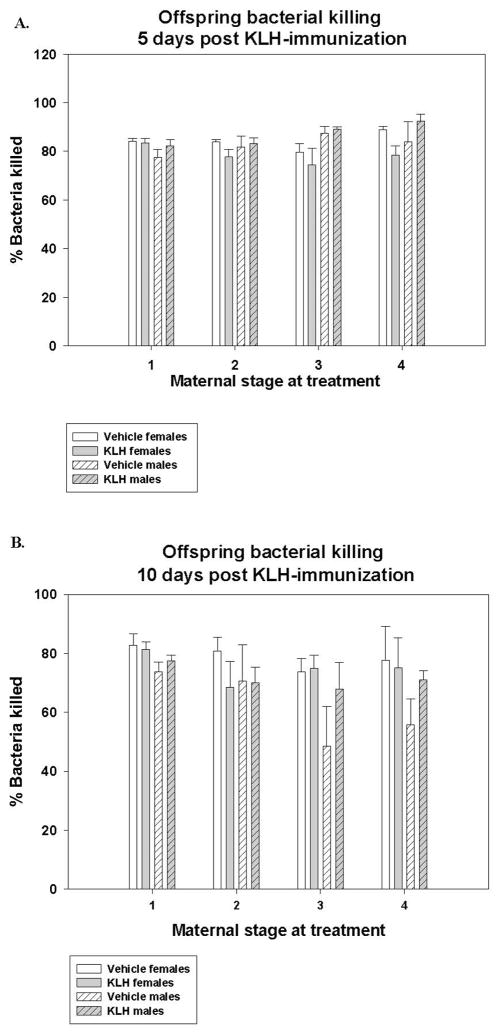
At Day 5 post-KLH injection, there were significant sex x maternal treatment (Fsex x treatment = 5.48; d.f. = 1, 71; p = 0.022; Fig. 4A) and sex x stage at maternal treatment (Fsex x stage = 3.59; d.f. = 3, 71; p = 0.018; Fig. 4A) interactions, suggesting that the sexes respond differently depending on maternal treatment. Specifically, post hoc tests show that males from KLH treated mothers have the highest BKA, females from KLH mothers have the lowest and offspring of both sexes from vehicle treated mothers are intermediate (p < 0.05). We see a similar separation of males having significantly greater BKA than females from mid-pregnancy treated mothers, while all other stages are intermediate (p < 0.05).
Figure 4. Offspring bacterial killing ability on Day 5 (A) and 10 (B) 10 post challenge with KLH.
Percent bacteria killed from male and female offspring of KLH and vehicle treated mothers, 5 days post challenge with KLH there were significant interactions among offspring sex and maternal treatment (Fsex x treatment = 5.48; d.f. = 1, 71; p = 0.022) and offspring sex x stage at maternal treatment (Fsex x stage = 3.59; d.f. = 3, 71; p = 0.018) such that the sexes differed in their BKA depending on maternal treatment and stage at maternal treatment (A). 10 days post-challenge with KLH there was a significant effect of sex (Fsex = 6.92; d.f. = 1, 69; p = 0.011) and maternal stage at treatment (Fstage = 2.936; d.f. = 3, 69; p = 0.039), where females had greater BKA than males and offspring from mothers treated mid pregnancy had the lowest BKA, early pre-pregnancy had the highest, and early pregnancy and lactation were intermediate (B).
At Day 10 post-KLH injection, there was a significant effect of sex (Fsex = 6.92; d.f. = 1, 69; p = 0.011; Fig. 4B), maternal stage at treatment (Fstage = 2.936; d.f. = 3, 69; p = 0.039; Fig 4B). No other effects or interactions where significant (all F < 3.02, all p > 0.087). Finally, there is a greater change for males than females between day 5 to day 10 post KLH-injection (Fsex = 10.56; d.f. = 1, 68; p = 0.002) and that offspring from mothers treated mid-pregnancy have a greater change that those from mother treated pre-mating, with other groups (early pregnancy, lactation) falling intermediate between the two (Fstage = 3.80; d.f. = 3, 68; p = 0.014). Again no other effects or interactions are significant (all F < 2.22, all p > 0.094).
Antibody Production
There was a significant effect of sex on immunoglobulin production of anti-KLH antibodies (Fsex = 6.71; d.f. = 1, 80; p = 0.011; Table 3), where female offspring had greater antibody production than male offspring. However, nothing else was significant (all F < 2.00, all p > 0.121; Table 3).
Agonistic Behavior
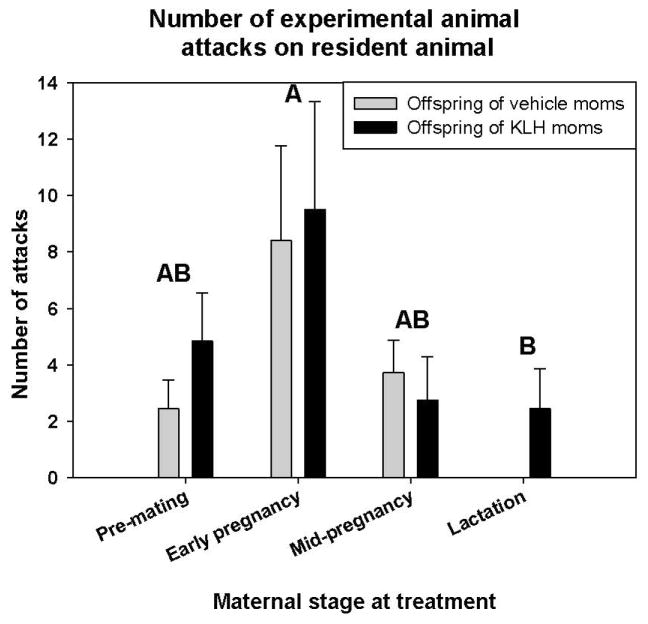
Quantified behaviors included the number of attacks by the resident aggressor, attacks by the experimental animal, and the number and duration of submissive bouts by the experimental animal. There was a significant difference between the sexes for number of resident attacks (χ2 = 11.91, p < 0.001; Table 3), number of submissions (χ2 = 16.90, p < 0.001; Table 3), duration of submissions (χ2 = 19.31, p < 0.001; Table 2), and the number of chases (χ2 = 9.30, p = 0.002; Table 3), but not for the number of experimental animal attacks (χ2 = 0.16, p = 0.693; Table 3). There was also an effect of stage at maternal treatment on attacks by the experimental animal (χ2 = 8.27, p = 0.041; Fig. 5). Post hoc tests showed that offspring from mothers treated early pregnancy, just following un-pairing, engaged in a greater number of attacks than offspring from mothers treated during lactation (p < 0.05; Fig. 5). Offspring from mothers treated pre-mating and mid-pregnancy fell intermediate between the other two groups. Maternal treatment and stage at maternal treatment did not affect any other behaviors (all χ2 < 6.16, all p > 0.104; Fig. 5).
Figure 5. Offspring aggressive behavior.
Aggressive behaviors scored from offspring from vehicle and KLH treated mothers in resident intruder trials. Offspring of both sexes from females treated early in pregnancy engaged in a greater number of attacks than offspring of mothers treated during lactation, while the other groups fell intermediate (χ2 = 8.27, p = 0.041). Different letters denote significant differences (α=0.05).
Discussion
Maternal Effects
The implementation of KLH as an immune challenge, unlike using live pathogens or viruses, allows greater control over the time course and dose of administration of an immune-stimulating agent. Also, KLH creates an immune response without the accompanying sickness; therefore, the effects are specifically due to B cell activation and associated cytokine production. Circulating IgG antibodies specific to KLH levels were consistent across stages of pregnancy and not different from non-pregnant female hamsters. IgM antibodies did not significantly differ in any group relative to the non-pregnant control, but those animals exposed during lactation had a trend toward fewer antibodies than the pre-mating exposure. All animals were tested for antibodies five days following the KLH injection, therefore the samples were taken at different times during pregnancy. Though maternal production of antibodies was not significantly affected by pregnancy stage, the relative quantity that offspring were exposed to could still differ, due to altered maternal transfer of antibodies through the placenta. It is unclear at this time whether the amount of antibody that transferred to the fetuses differed between treatment times.
Maternal bacteria killing ability varied greatly by treatment and the stage of pregnancy or lactation in which the mother was sampled. Overall, bacteria killing was suppressed in KLH-treated females relative to saline controls suggesting a trade-off between acquired and innate immunity in those animals challenged with KLH to mount a specific immune response. During normal human pregnancies, for example, immune responses are shifted to favor humoral responses, as opposed to cell mediated responses (Kelemen et al., 1998). This type of immune trade-off has been theorized on a large scale (Lee, 2006), and has been more specifically documented in African buffalo (Ezenwa et al., 2010) and Darwin’s finches (Lindström et al., 2004).
To further support the idea of trade-offs in reproduction, we found a significant relationship between offspring consumption by mothers post-parturition and litter size, suggesting that mothers could not sustain larger litters and so culled them early on. Interestingly this effect was driven primarily by KLH injected females that were treated just before pregnancy and early in pregnancy and not control females. The immune challenge dependent effect on litter reduction suggests that mounting an immune response during certain stages of pregnancy adds significant costs to reproductive females, causing them to adjust their litter size. Conversely, it was previously demonstrated that providing signals of elevated energy to reproductive females decreases post-parturition litter reduction (French et al., 2009).
We also observed a close to significant effect of stage of pregnancy on maternal bacterial killing ability (P=0.055), where bacterial killing was suppressed during mid-pregnancy when maternal cortisol was at its highest circulating concentrations. Specifically, though the mid-pregnancy group was injected with KLH on gestational day ~11, the blood sample for the bacteria killing assay was taken just before parturition. Serum cortisol was also significantly elevated during mid-pregnancy. Just before parturition, cortisol greatly increases (reviewed in Hillman et al., 2012), which promotes fetal lung development before birth (Smith et al., 1973). Cortisol also promotes gluconeogenesis in the liver, leading to release of sugar into the blood stream (Hillman et al., 2012). At this time point food intake in the females was also elevated. The period immediately prior to parturition may be a time when blood glucose levels in the females are high, to cope with metabolically costly physiological processes such as offspring maturation and milk production.
Offspring Effects
Significant changes occurred in the offspring of KLH treated mothers, some manifesting during development and others during adulthood. Furthermore, these effects varied depending on when the mother was challenged during pregnancy and the sex of the offspring. The present finding is particularly interesting given the mild nature of the KLH treatment, in that it does not induce sickness behaviors or fever. Thus, even very minor immune challenges can alter offspring phenotype. These effects could potentially be mediated by altered resource allocation or availability, cytokine profiles, or glucocorticoid effects (reviewed in: Patterson et al., 2009).
We found significant effects of maternal KLH treatment on offspring growth, such that offspring of KLH treated mothers were smaller. Similar results were found in using the mitogen LPS in this same model system, where litter size and number were reduced in LPS treated mothers (French et al., 2013b). In the present study this effect was present in the first 3 weeks until weaning. After weaning and separating siblings by sex, males remained smaller. Likely, the males were the driving factor of the litter differences prior to weaning as well. This is highly significant, because decreased growth rates have potentially deleterious consequences for survival and fitness (McAdam and Millar, 1999).
Unlike treatment with more severe mitogens such as LPS, there was no effect of maternal KLH treatment on cortisol levels in offspring. For example, in the same species, maternal treatment with LPS during pregnancy resulted in offspring with elevated cortisol in response to stressful stimuli (French et al., 2013b). Similarly, prenatal exposure to LPS resulted in elevations in basal levels of an alternative glucocorticoid, corticosterone, and upstream adrenal corticotropin-releasing hormone in male Wistar rats (Reul et al., 1994). Neonatal rats exposed to LPS also showed elevated corticosterone levels relative to control animals, but only in response to an acute stressor (Walker et al., 2008). LPS exposure causes a significant elevation of cortisol in pregnant females in some studies (Schlafer et al., 1994), but not in others (Mouihate et al., 2005). Whether or not KLH elicits the same effects is not yet clear. Thus, together these results suggest that dysregulation of the HPA axis may be at least partially responsible for the altered endocrine and behavioral responses in offspring prenatally exposed to immune challenges such as LPS, but more mild challenges such as KLH are likely working via different pathways.
Offspring immunocompetence was tested in adulthood at a time when all maternal immune components (i.e., antibodies) have been metabolized, and thus was not directly contributing to immune function (reviewed in Madani and Heiner, 1989). At five days post-KLH challenge we found that the sexes respond differently depending on maternal treatment. Specifically, males from KLH treated mothers had the highest BKA, whereas females from KLH mothers had the lowest. Offspring of both sexes from vehicle treated mothers were intermediate between the KLH treated offspring. At 10 day post KLH-challenge, we found that females had overall greater bacterial killing ability than males, providing evidence for the fact that this immune response is dynamic. This provides some support for the transgenerational priming of immunity hypothesis (Grindstaff et al., 2006; Sadd et al., 2005), in that a prenatal or neonatal innate immune challenge resulted in enhanced innate immunity. Thus the effects of maternal immune activation are not necessarily deleterious and instead may help the offspring’s immune system better match the outside environment (Eggert et al., 2014; Shikano et al., 2015). The sex difference also indicates altered signaling or reception of maternal factors depending on offspring sex following the immune challenge, which needs to be addressed in future studies.
We also see a timing effect of maternal immune challenge on offspring immunity. Specifically, we see a similar separation of males having significantly greater BKA than females from mid-pregnancy treated mothers, while offspring from mothers treated at all other stages fall intermediate. Similarly, at 10 days post-KLH challenge offspring from mothers treated during mid- pregnancy had significantly lower bacterial killing ability relative to offspring from mothers treated pre-mating, whereas other groups (early pregnancy and lactation) were intermediate. These results may indicate that neonatal exposure to maternal antibodies at specific time points can alter innate immunity later in life. While these results do not give a definitive critical window when offspring are particularly susceptible to maternal immune challenge, they shed much needed light on the developing systems that are affected at different time points across gestation and early lactation (Knudsen, 2004). For example, preliminarily, offspring from immune-challenged mothers during mid-pregnancy showed altered immune function, while offspring from mothers challenged with either vehicle or KLH during early pregnancy display more aggressive behavior than controls and offspring of other groups, suggesting that if immune and brain development are being affected, their sensitivity varies depending on gestational stage.
There is evidence that exposure to specific antibodies during the perinatal period can lead to tolerance of the corresponding antigen upon subsequent exposure (Carlier and Truyens, 1995). However, in the current study, we found no evidence of this phenomenon. Instead we observed that immunoglobulin levels in adult male offspring were lower than females regardless of maternal treatment. This is consistent with females having greater immunocompetence in most species (Rolff, 2002).
Collectively, the results of the present study provide evidence that stimulation of the maternal immune response via a mild antigenic challenge during pregnancy has consequences on offspring that manifest throughout growth and into adulthood. Also, similar to challenges with other mitogens (i.e., LPS) that these effects can manifest in a sex-dependent manner (French et al., 2013b). Essentially, a challenge that produces no behavioral symptoms and minimal inflammation in mothers is still capable of affecting the phenotype of offspring. It will be important to identify causative agents mediating these behavioral changes (e.g., specific cytokines, cortisol) to understand the proximate mechanisms underlying the resultant immune and behavioral abnormalities and subsequently employing pharmacological agents that target specific physiological mechanisms. This may allow for the eventual development of effective treatment strategies to attenuate behavioral abnormalities caused by maternal immune activation. Regardless, these findings have significant implications for the effects of natural pathogens on behavioral interactions and neuroendocrine interactions in individuals in the wild.
Acknowledgments
Supporting Grant: This work was supported by National Science Foundation Grant IOB-0543798, National Institutes of Health Training Grant T32HD049336 “Common Themes in Reproductive Diversity”, and Indiana University
References
- Bilbo SD, Schwarz JM. Early-life programming of later-life brain and behavior: A critical role for the immune system. Frontiers in Behavioral Neuroscience. 2009;4:12. doi: 10.3389/neuro.08.014.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksa P. Effects of prenatal infection on brain development and behavior: A review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Carlier Y, Truyens C. Influence of maternal infection on offspring resistance towards parasites. Parasitology Today. 1995;11:94–99. doi: 10.1016/0169-4758(95)80165-0. [DOI] [PubMed] [Google Scholar]
- Clark DA, Manuel J, Lee L, Chaouat G, Gorczynski RM, Levy GA. Ecology of danger-dependent cytokine-boosted spontaneous abortion in the cbaxdba/2 mouse model. I. Synergistic effect of lps and (tnf-α + ifn-γ) on pregnancy loss. American Journal Of Reproductive Immunology. 2004;52:370–378. doi: 10.1111/j.1600-0897.2004.00237.x. [DOI] [PubMed] [Google Scholar]
- Crews D. Epigenetics, brain, behavior, and the environment. Horm. 2010;9:41–50. doi: 10.14310/horm.2002.1251. [DOI] [PubMed] [Google Scholar]
- Demas GE, Drazen DL, Nelson RJ. Reductions in total body fat decrease humoral immunity. Proc R Soc Lond Ser B-Biol Sci. 2003;270:905–911. doi: 10.1098/rspb.2003.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Johnson C, Polacek KM. Social interactions differentially affect reproductive and immune responses of siberian hamsters. Physiol Behav. 2004;83:73–79. doi: 10.1016/j.physbeh.2004.06.025. [DOI] [PubMed] [Google Scholar]
- Dixon FJ, Jacotgui H, JMP Antibody responses of rabbits and rats to hemocyanin. J Immunol. 1966;97:350. [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Dolinoy DC, Weidman JR, Jirtle RL. Epigenetic gene regulation: Linking early developmental environment to adult disease. Reprod Toxicol. 2007;23:297–307. doi: 10.1016/j.reprotox.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Eggert H, Kurtz J, Diddens-de Buhr MF. Different effects of paternal trans-generational immune priming on survival and immunity in step and genetic offspring. Proc R Soc Lond Ser B-Biol Sci. 2014:281. doi: 10.1098/rspb.2014.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezenwa V, xa O, Etienne R, xa S, Luikart G, Beja x, Pereira A, Jolles A, xa E. Hidden consequences of living in a wormy world: Nematode−induced immune suppression facilitates tuberculosis invasion in african buffalo. In: Gregory ED, Mark AM, editors. The American Naturalist. Vol. 176. 2010. pp. 613–624. [DOI] [PubMed] [Google Scholar]
- French SS, Chester EM, Demas GE. Maternal immune activation affects litter success, size and neuroendocrine responses related to behavior in adult offspring. Physiol Behav. 2013a;119:175–184. doi: 10.1016/j.physbeh.2013.06.018. [DOI] [PubMed] [Google Scholar]
- French SS, Chester EM, Demas GE. Maternal immune activation affects litter success, size and neuroendocrine responses related to behavior in adult offspring. Physiol Behav. 2013b;119:175–184. doi: 10.1016/j.physbeh.2013.06.018. [DOI] [PubMed] [Google Scholar]
- French SS, Greives TJ, Zysling DA, Chester EM, Demas GE. Leptin increases maternal investment. Proc R Soc Lond Ser B-Biol Sci. 2009;276:4003–4011. doi: 10.1098/rspb.2009.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff JL, Hasselquist D, Nilsson JA, Sandell M, Smith HG, Stjernman M. Transgenerational priming of immunity: Maternal exposure to a bacterial antigen enhances offspring humoral immunity. Proc R Soc B-Biol Sci. 2006;273:2551–2557. doi: 10.1098/rspb.2006.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindstaff JL, Hunsaker VR, Cox SN. Maternal and developmental immune challenges alter behavior and learning ability of offspring. Horm Behav. 2012;62:337–344. doi: 10.1016/j.yhbeh.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hava G, Vered L, Yael M, Mordechai H, Mahoud H. Alternations in behaviour in adults offspring mice following maternal inflammation during pregnancy. Dev Psychobiol. 2006;48:162–168. doi: 10.1002/dev.20116. [DOI] [PubMed] [Google Scholar]
- Hillman NH, Kallapur SG, Jobe AH. Physiology of transition from intrauterine to extrauterine life. Clin Perinatol. 2012;39:769–783. doi: 10.1016/j.clp.2012.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holekamp KE, Dloniak SM. In: Maternal effects in mammals. Maestripieri D, Mateo JM, editors. Chicago: University of Chicago Press; 2009. [Google Scholar]
- Kappeler L, Meaney MJ. Epigenetics and parental effects. Bioessays. 2010;32:818–827. doi: 10.1002/bies.201000015. [DOI] [PubMed] [Google Scholar]
- Kelemen K, Paldi A, Tinneberg H, Torok A, Szekeres-Bartho J. Early recognition of pregnancy by the maternal immune system. American Journal Of Reproductive Immunology. 1998;39:351–355. doi: 10.1111/j.1600-0897.1998.tb00368.x. [DOI] [PubMed] [Google Scholar]
- Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cognit Neurosci. 2004;16:1412–1425. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- Lee KA. Linking immune defenses and life history at the levels of the individual and the species. Integr Comp Biol. 2006;46:1000–1015. doi: 10.1093/icb/icl049. [DOI] [PubMed] [Google Scholar]
- Lemke H, Lange H. Is there a maternally induced immunological imprinting phase a la konrad lorenz? Scand J Immunol. 1999;50:348–354. doi: 10.1046/j.1365-3083.1999.00620.x. [DOI] [PubMed] [Google Scholar]
- Lindström KM, Foufopoulos J, Pärn H, Wikelski M. Immunological investments reflect parasite abundance in island populations of darwin’s finches. Proc R Soc Lond Ser B-Biol Sci. 2004;271:1513–1519. doi: 10.1098/rspb.2004.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madani G, Heiner DC. Antibody transmission from mother to fetus. Curr Opin Immunol. 1989;1:1157–1164. doi: 10.1016/0952-7915(89)90009-5. [DOI] [PubMed] [Google Scholar]
- McAdam AG, Millar JS. Dietary protein constraint on age at maturity: An experimental test with wild deer mice. Journal of Animal Ecology. 1999;68:733–740. [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouihate A, Ellis S, Harre EM, Pittman QJ. Fever suppression in near-term pregnant rats is dissociated from lps-activated signaling pathways. Am J Physiol- Regul Integr Comp Physiol. 2005;289:R1265–R1272. doi: 10.1152/ajpregu.00342.2005. [DOI] [PubMed] [Google Scholar]
- Mouihate A, Harré E-M, Martin S, Pittman QJ. Suppression of the febrile response in late gestation: Evidence, mechanisms and outcomes. J Neuroendocrinol. 2008;20:508–514. doi: 10.1111/j.1365-2826.2008.01666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousseau TA, Fox CW. The adaptive significance of maternal effects. Trends Ecol Evol. 1998;13:403–407. doi: 10.1016/s0169-5347(98)01472-4. [DOI] [PubMed] [Google Scholar]
- Onore CE, Schwartzer JJ, Careaga M, Berman RF, Ashwood P. Maternal immune activation leads to activated inflammatory macrophages in offspring. Brain Behav Immun. 2014;38:220–226. doi: 10.1016/j.bbi.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection: Window on neuroimmune interactions in fetal brain development and mental illness. Current Opinion in Neurobiology. 2002;12:115–118. doi: 10.1016/s0959-4388(02)00299-4. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behavioural Brain Research. 2009;204:313–321. doi: 10.1016/j.bbr.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Patterson PH, Xu W, Smith SEP, Devarman BE. Maternal immune activation, cytokines and autism. Autism. 2009:289–307. [Google Scholar]
- Pearce BD. Schizophrenia and viral infection during neurodevelopment: A focus on mechanisms. Molecular Psychiatry. 2001;6:634–646. doi: 10.1038/sj.mp.4000956. [DOI] [PubMed] [Google Scholar]
- Reburn CJ, Wynne-Edwards KE. Hormonal changes in males of a naturally biparental and a uniparental mammal. Hormones and Behavior. 1999;35:163–176. doi: 10.1006/hbeh.1998.1509. [DOI] [PubMed] [Google Scholar]
- Reul JM, Stec I, Wiegers GJ, Labeur MS, Linthorst AC, Arzt E, Holsboer F. Prenatal immune challenge alters the hypothalamic-pituitary-adrenocortical axis in adult rats. J Clin Invest. 1994;93:2600–2607. doi: 10.1172/JCI117272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolff J. Bateman’s principle and immunity. Proc R Soc Lond Ser B-Biol Sci. 2002;269:867–872. doi: 10.1098/rspb.2002.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadd BM, Kleinlogel Y, Schmid-Hempel R, Schmid-Hempel P. Trans-generational immune priming in a social insect. Biol Lett. 2005;1:386–388. doi: 10.1098/rsbl.2005.0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlafer DH, Yuh B, Foley GL, Elssaser TH, Sadowsky D, Nathanielsz PW. Effect of salmonella endotoxin administered to the pregnant sheep at 133–142 days gestation on fetal oxygenation, maternal and fetal adrenocorticotropic hormone and cortisol, and maternal plasma tumor necrosis factor alpha concentrations. Biol Reprod. 1994;50:1297–1302. doi: 10.1095/biolreprod50.6.1297. [DOI] [PubMed] [Google Scholar]
- Schminkey DL, Groer M. Imitating a stress response: A new hypothesis about the innate immune system’s role in pregnancy. Medical hypotheses. 2014;82:721–729. doi: 10.1016/j.mehy.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Scotti M-AL, Place NJ, Demas GE. Short-day increases in aggression are independent of circulating gonadal steroids in female siberian hamsters (phodopus sungorus) Horm Behav. 2007;52:183–190. doi: 10.1016/j.yhbeh.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Shikano I, Oak MC, Halpert-Scanderbeg O, Cory JS. Trade-offs between transgenerational transfer of nutritional stress tolerance and immune priming. Funct Ecol. 2015;29:1156–1164. [Google Scholar]
- Smith BT, Torday JS, Giroud CJP. The growth promoting effect of cortisol on human fetal lung cells. Steroids. 1973;22:515–524. doi: 10.1016/0039-128x(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Smith SEP, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker FR, Knott B, Hodgson DM. Neonatal endotoxin exposure modifies the acoustic startle response and circulating levels of corticosterone in the adult rat but only following acute stress. J Psychiatr Res. 2008;42:1094–1103. doi: 10.1016/j.jpsychires.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Welberg LAM, Seckl JR. Prenatal stress, glucocorticoids and the programming of the brain. J Neuroendocrinol. 2001;13:113–128. doi: 10.1046/j.1365-2826.2001.00601.x. [DOI] [PubMed] [Google Scholar]
- Zysling D, Demas G. Metabolic stress suppresses humoral immune function in long-day, but not short-day, siberian hamsters (phodopus sungorus) J Comp Physiol (B) 2007;177:339–347. doi: 10.1007/s00360-006-0133-4. [DOI] [PubMed] [Google Scholar]
- Zysling DA, Garst AD, Demas GE. Photoperiod and food restriction differentially affect reproductive and immune responses in siberian hamsters phodopus sungorus. Funct Ecol. 2009;23:979–988. [Google Scholar]