Abstract
Cobalamin and other corrinoids are essential cofactors for many organisms. The majority of microbes with corrinoid-dependent enzymes do not produce corrinoids de novo, and instead must acquire corrinoids produced by other organisms in their environment. However, the profile of corrinoids produced in corrinoid-dependent microbial communities, as well as the exchange and modification of corrinoids among community members have not been well studied. In this study, we applied a newly developed LC/MS/MS–based corrinoid detection method to examine relationships between corrinoids, their lower ligand bases, and specific microbial groups in microbial communities containing Dehalococcoides mccartyi that has an obligate requirement for benzimidazole-containing corrinoids for trichloroethene-respiration. We found that p-cresolylcobamide ([p-Cre]Cba) and cobalamin were the most abundant corrinoids in the communities. It suggests that members of the family Veillonellaceae are associated with the production of [p-Cre]Cba. The decrease of supernatant-associated [p-Cre]Cba and the increase of biomass-associated cobalamin were correlated with the growth of D. mccartyi by dechlorination. This supports the hypothesis that D. mccartyi is capable of fulfilling its corrinoid requirements in community through corrinoid remodeling, in this case, by importing extracellular [p-Cre]Cba and 5,6-dimethylbenzimidazole (DMB) (the lower ligand of cobalamin), to produce cobalamin as a cofactor for dechlorination. This study also highlights the role of DMB, the lower ligand produced in all of the studied communities, in corrinoid remodeling. These findings provide novel insights on roles played by different phylogenetic groups in corrinoid production and corrinoid exchange within microbial communities. This study may also have implications for optimizing chlorinated solvent bioremediation.
Keywords: corrinoid, Dehalococcoides, microbial community, reductive dechlorination, trichloroethene
Introduction
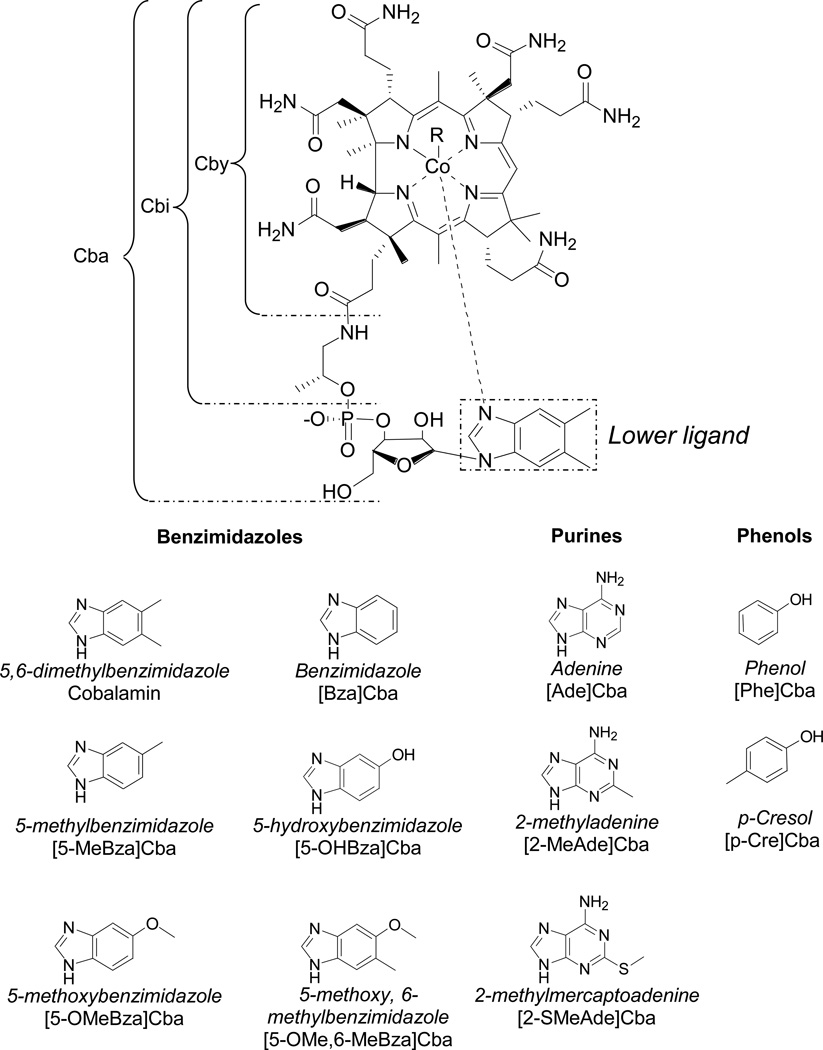
Corrinoids, which include cobalamin (vitamin B12) and other structurally related compounds (Fig. 1), are a family of cofactors that function in three classes of enzymes: isomerases, methyltransferases, and reductive dehalogenases (RDases) (Banerjee and Ragsdale, 2003; Brown, 2005). Although corrinoids are synthesized solely by a subset of Bacteria and Archaea, they function as cofactors for a variety of organisms including many Eukaryotes (Martens et al., 2002; Ryzhkova, 2003). At least sixteen corrinoids with structural variability in the lower axial ligand have been identified, and can be classified into three groups defined by the structure of the lower ligand: benzimidazole, purine, or phenolic cobamides (Fig. 1) (Guimaraes et al., 1994; Renz, 1999; Kräutler et al., 2003; Allen and Stabler, 2008). Previous studies have shown that even corrinoids within one lower ligand class may not necessarily be functionally equivalent as cofactors (Yi et al., 2012). Therefore, organisms that rely on corrinoids produced by other members of their community must have mechanisms to obtain corrinoids with the appropriate lower ligand. Corrinoid remodeling, in which an organism removes the lower ligand of an imported corrinoid and replaces it with its functional lower ligand, has been demonstrated in a number of microorganisms including Dehalococcoides mccartyi (Escalante-Semerena, 2007; Gray and Escalante-Semerena, 2009a; Gray and Escalante-Semerena, 2009b; Yan et al., 2012; Yi et al., 2012; Keller et al., 2013).
Figure 1.
Structures of corrinoids and lower ligand bases together with abbreviated designation. The names of the lower ligands are italicized and the abbreviation of each cobamide is given below. Cby: cobyric acid; Cbi: cobinamide; Cba: cobamide.
D. mccartyi strains are the only known Bacteria capable of complete dechlorination of the common groundwater contaminants tetrachloroethene (PCE), and trichloroethene (TCE) to the innocuous end product ethene (Maymó-Gatell et al., 1997; He et al., 2003; Cupples et al., 2004). Corrinoids are essential cofactors for reductive dehalogenases (RDases), the enzymes that catalyze organohalide respiration in many different microorganisms, including D. mccartyi strains (Maymó-Gatell et al., 1997; Siebert et al., 2002; Kräutler et al., 2003; Maillard et al., 2003; Müller et al., 2004; Seshadri et al., 2005; Reinhold et al., 2012; Schipp et al., 2013). The corrinoid cofactors bind to the reductive dehalogenases, serving as the enzyme active site, where dehalogenation occurs either through halide elimination forming an organocobalt adduct or by using Co(I) as the electron donor (Banerjee and Ragsdale, 2003). Genomic analyses of the sequenced D. mccartyi strains indicate that they are unable to produce corrinoids de novo, and therefore exogenous cobalamin is regularly added to D. mccartyi isolates and D. mccartyi-containing communities to enhance dechlorination performance (Lesage et al., 1996; Richardson et al., 2002; He et al., 2003; Cupples et al., 2004; Duhamel et al., 2004; Schipp et al., 2013). We recently showed that [5-MeBza]Cba and [5-OMeBza]Cba also support growth and dechlorination in D. mccartyi strain 195 (strain 195) (Yi et al., 2012). However, other corrinoids do not function as cofactors for strain 195, indicating a strict requirement for specific benzimidazole cobamides.
D. mccartyi coexists with corrinoid-producing organisms such as acetogens, methanogens, and sulfate-reducing bacteria in dechlorinating communities (Kräutler et al., 1988; Stupperich et al., 1988; Guimaraes et al., 1994; Renz, 1999), and is likely to encounter a variety of different corrinoids in its environment. Though none of the sequenced D. mccartyi strains possesses the complete corrinoid biosynthesis pathway, genes encoding corrinoid salvaging and remodeling pathways have been identified in each strain (Yi et al., 2012; Schipp et al., 2013). The remodeling of seven nonfunctional corrinoids (i.e [Ade]Cba, [2-SMeAde]Cba, [5-OHBza]Cba, [Bza]Cba, [5-MeBza]Cba, [p-Cre]Cba and Cbi) has been observed in a number of D. mccartyi strains in isolation or defined consortia. When provided with the lower ligand of cobalamin, 5,6-dimethylbenzimidazole (DMB), strain 195 was able to grow and dechlorinate TCE by remodeling nonfunctional corrinoids to cobalamin (Yi et al., 2012). D. mccartyi strains incubated with corrinoid-producing microorganisms Geobacter sulfurreducens, Methanosarcina barkeri or Sporomusa sp. also exhibited dechlorination activity when DMB was added to the cobalamin-free medium (Yan et al., 2012; Yan et al., 2013). Similar dechlorination activities have been observed in enrichments grown without exogenous cobalamin (Men et al., 2013), leading to the hypothesis that other bacteria provide corrinoids to D. mccartyi that can be used directly or after remodeling. However, comprehensive profiles of corrinoids produced by other bacteria and corrinoid remodeling in dechlorinating microbial communities have not been reported. Additionally, little is known about the generation and availability of DMB in anaerobic communities, although the availability of DMB is crucial for corrinoid remodeling by D. mccartyi according to previous studies with pure cultures and defined consortia (Yan et al., 2012; Yi et al., 2012; Yan et al., 2013).
Limitations of analytical techniques for differentiating and quantifying corrinoids impede our understanding of corrinoid content and functions associated with specific corrinoids in microbial communities. Previous studies have largely relied on bioassays to analyze corrinoid content, although these do not allow identification of specific corrinoids or the detection of corrinoid forms that cannot be utilized by chosen bioassay strains (Chanarin and Muir, 1982; Guggisberg et al., 2012; Yan et al., 2013). Indeed, the range of corrinoids that bioassays can detect is unknown. Moreover, no methods have been reported for detecting free benzimidazole lower ligands present in complex mixtures. In this study, we established a liquid chromatography tandem mass spectrometry (LC/MS/MS) method that is able to not only differentiate various corrinoids and benzimidazole lower ligands, but also to quantify them at low levels.
We successfully applied the LC/MS/MS method to examine thirteen corrinoids and three benzimidazoles in two sets of dechlorinating enrichments derived from geographically different inocula (Supporting Information Table S1).One set of enrichments was inoculated with ANAS, a long-term TCE dechlorinating enrichment initially derived from contaminated soil in California (Freeborn et al., 2005), grown with and without exogenous cobalamin (SANASB12 and SANAS). The other set of enrichments was inoculated with microbial cells collected from contaminated groundwater from a field site in New Jersey (NJ enrichments), and grown under four different conditions exploring two parameters: low and high TCE amendment (resulting in uninhibited and inhibited methanogenic activity, respectively) and with and without cobalamin amendment (LoTCEB12 and HiTCEB12, LoTCE and HiTCE) (Men et al., 2013).
In this study, we examine the contributions of specific microbial groups to corrinoid production and modification by analyzing corrinoid profiles in the two sets of enrichments as well as in cultures exposed to growth perturbations. This represents the first comprehensive analysis of corrinoid and lower ligand profiles in dechlorinating microbial communities, and allows us to better understand the roles played by different microbial groups on corrinoid production of the community, corrinoid modification by corrinoid scavengers, and the ecological interactions associated with corrinoid producers and corrinoid auxotrophs.
Results
Development and validation of an analytical method for the detection of corrinoids and free benzimidazoles
In order to measure the composition of corrinoids in microbial communities, we developed a new LC/MS/MS-based analytical method for quantification of thirteen different corrinoids. Combined with the 1000× concentration by solid phase extraction prior to LC/MS/MS, the overall limit of detection was 200 pM for phenolic corrinoids, and 1–2 pM for all other corrinoids. The overall limit of quantification was 1 nM for phenolic corrinoids, and 2–5 pM for all other corrinoids (Table S1). This method was validated by a direct comparison with a previously described LC/MS analytical method (Allen and Stabler, 2008). We found concentrations of detectable corrinoids to be comparable using the two methods, with the exception of Cbi, for which the LC/MS/MS method yielded a lower limit of detection (Table S2). The LC/MS/MS method was also able to measure three benzimidazoles: DMB, 5-MeBza, and 5-OMeBza with an overall limit of detection of 1 pM and an overall limit of quantification of 2 pM (Table S1). Subsequent experiments in this study relied solely on the newly established LC/MS/MS method.
Corrinoid and benzimidazole profiles in TCE-dechlorinating enrichments
We applied the LC/MS/MS method to the two sets of enrichments that reductively dechlorinate TCE. The enrichment conditions, a description of subculture feeding regimes, as well as D. mccartyi growth are listed in Table S3. The ANAS subcultures contained Bacterial species related most closely to D. mccartyi, Clostridium sp., Eubacterium sp., Bacteroides sp., Citrobacter sp., Spirochaeta sp., and δ-proteobacteria (Freeborn et al., 2005). In the NJ enrichments, besides D. mccartyi, 7 other Bacterial operational taxonomic units (OTUs) have been identified, which, according to the closest genus, were designated in Genbank as Pelosinus_GW, Dendrosporobacter_GW, Sporotalea_GW, Desulfovibrio_GW, Clostridium_GW, Spirochaetes_GW, and Bacteroides_GW (Men et al., 2013), “GW” (short for groundwater) is used in order to differentiate the OTU name from the genus name.
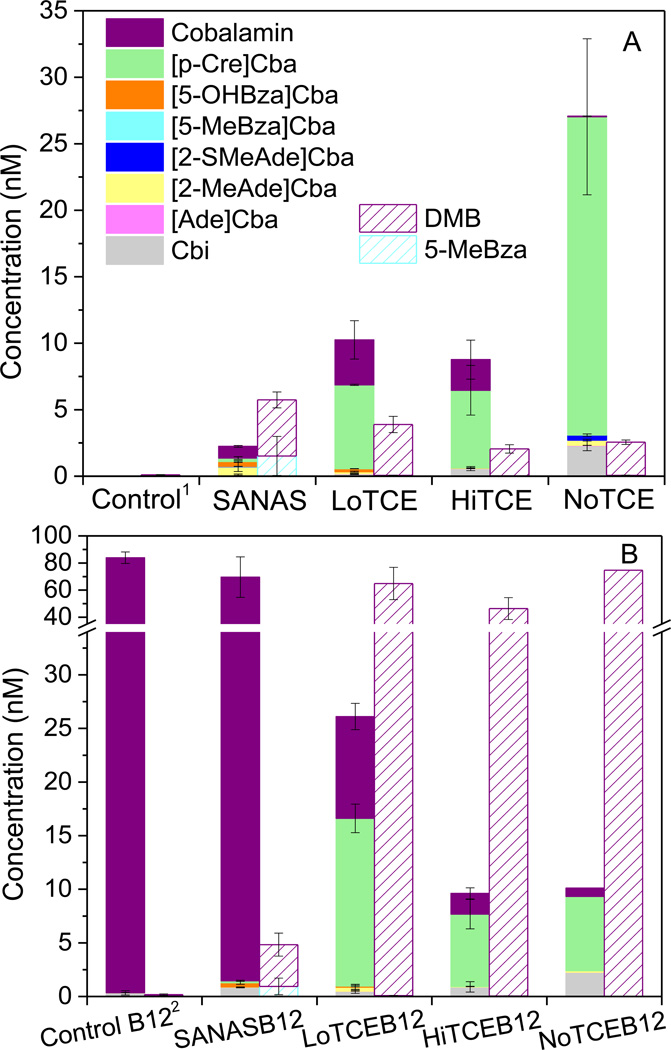
Corrinoids were quantified in each of the enrichments after the added TCE was degraded (13–14 days for NJ enrichments and 18 days for SANAS and SANASB12), or in the case of the NoTCE enrichment, after 13 days. The major corrinoids detected in SANAS were cobalamin, [2-MeAde]Cba, [5-OHBza]Cba, and [p-Cre]Cba, though smaller amounts of [5-MeBza]Cba, [Ade]Cba, [2-SMeAde]Cba, and Cbi were also present (Fig. 2A & Table S4). Of the corrinoids in SANAS, only cobalamin was present at concentrations above 0.74 nM (c.a. 1 µg/L), the reported minimum requirement for growth of strain 195 (He et al., 2007). In SANASB12, 80% of the added cobalamin was detected. With the exception of [2-SMeAde]Cba, corrinoids detected in SANAS were also present in SANASB12 (Fig. 2B). The level of [2-MeAde]Cba decreased, and Cbi increased in SANASB12 compared to SANAS. Similar levels of DMB and 5-MeBza lower ligand bases were detected in SANAS and SANASB12, consistent with the detection of both cobalamin and [5-MeBza]Cba (Fig. 2 & Table S5).
Figure 2.
Corrinoid and benzimidazole lower ligand concentrations in B12-unamended (A) and B12-amended (B) enrichments at the end of 13–18 days’ subculturing cycle, error bars represents standard deviation, n=3 (1Abiotic control without exogenous vitamin B12; 2Abiotic control with 74 nM (c.a.100 µg/L) vitamin B12; Note: (A) and (B) share the same legend, but different y axis scales. Most of the cobalamin in SANASB12 was detected in the supernatant, while most corrinoids were detected in the cell pellets of the other enrichments. Lower ligands were mostly detected in the supernatant).
The corrinoid profiles of the NJ enrichments differed from SANAS and SANASB12. In the NJ enrichments without exogenous cobalamin (LoTCE and HiTCE), [p-Cre]Cba was the most abundant corrinoid, followed by cobalamin (Fig. 2A). Cobalamin was detected at levels above 0.74 nM in LoTCE and HiTCE (3.2 nM and 2.2 nM, respectively), similar to SANAS (Fig. 2A), indicating that the amount of cobalamin present was sufficient for the growth of D. mccartyi. Free DMB was detected at levels similar to those detected in SANAS (Fig. 2A). Interestingly, greater [p-Cre]Cba concentrations were detected in LoTCEB12 and HiTCEB12 (enrichments with exogenous cobalamin) than in LoTCE and HiTCE. [2-MeAde]Cba, [Ade]Cba, and Cbi were also detected in the four NJ enrichments, but at levels lower than 0.74 nM (Table S4). Neither [5-MeBza]Cba nor its associated lower ligand 5-MeBza was detected in the NJ enrichments (Fig. 2). Notably, [5-OHBza]Cba was only present in methanogenic NJ enrichments, LoTCE and LoTCEB12 (Fig. 2).
The fate of exogenously added cobalamin was substantially different in SANASB12 versus the NJ enrichments (LoTCEB12 and HiTCEB12). In the SANASB12, 68 nM out of 83 nM added cobalamin was detected (Fig. 2B), and the majority (80%) was in the culture supernatant; whereas 90% of the other corrinoids were detected in the SANASB12 cell pellet. In contrast, in LoTCEB12 and HiTCEB12, only 9.45 nM and 1.95 nM out of 83 nM added cobalamin was detected, respectively, most of which was in the cell pellet (Table S4). One possible reason for the disappearance of cobalamin in the two NJ enrichments is the remodeling of cobalamin, however, the generation of other corrinoids by corrinoid remodeling was not sufficient to account for the decrease of cobalamin in LoTCEB12 and HiTCEB12. [p-Cre]Cba increased by 9.3 nM and 1.3 nM in LoTCEB12 and HiTCEB12, compared with LoTCE and HiTCE, respectively, but no increase was observed for the other corrinoids targeted in this study. The decrease in cobalamin was accompanied by the apparent liberation of DMB, which is reflected by the profiles of free DMB. In contrast to SANASB12, where only 3.7 nM DMB was detected in the culture supernatant, 45–64 nM DMB was found in the supernatant of the two B12-amended NJ enrichments (Fig. 2B & Table S5), suggesting that DMB was cleaved from the amended cobalamin by bacteria present in the NJ enrichment that are different from those in SANASB12.
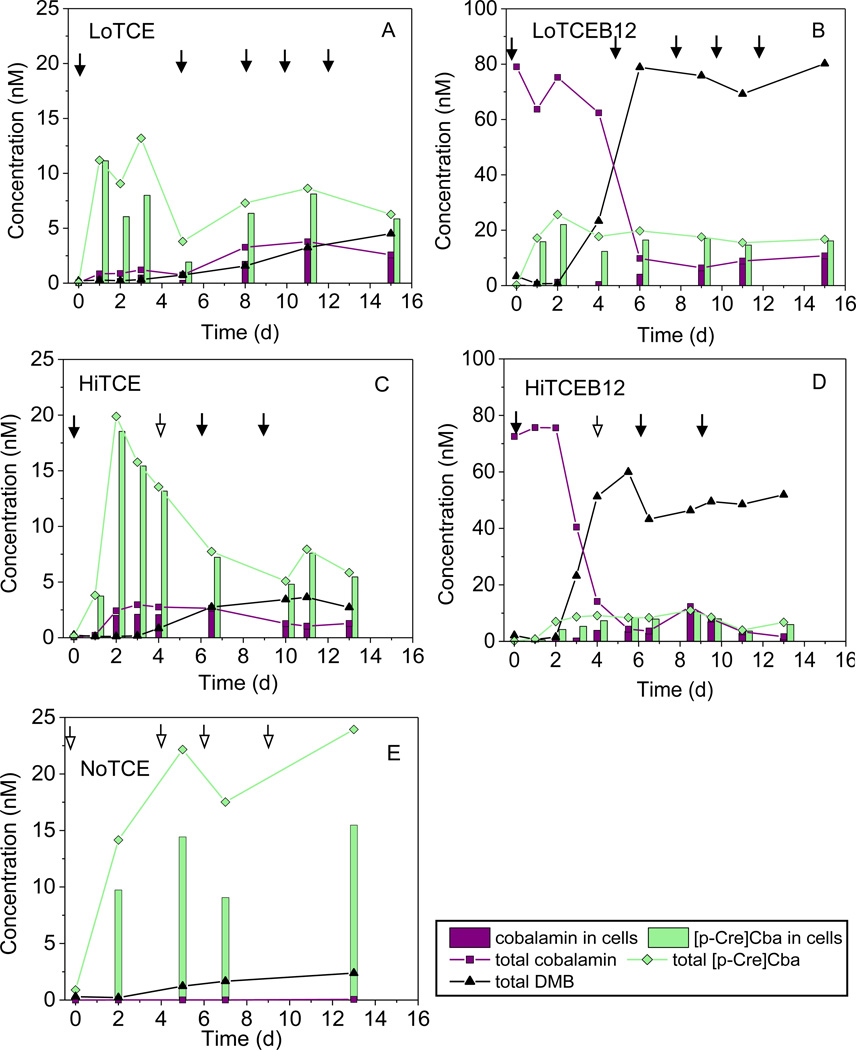
In order to examine the dynamics of corrinoid production and modification, temporal changes of the corrinoids and lower ligand bases in the four NJ enrichments were monitored over a 13–15 day feeding cycle. Concentrations of [p-Cre]Cba and cobalamin, the most abundant corrinoids in these cultures, as well as DMB are shown in Fig. 3. In all cultures, [p-Cre]Cba accumulated to near maximum levels during the first 2–3 days of incubation. Subsequently, [p-Cre]Cba levels declined substantially in cultures without cobalamin added (LoTCE and HiTCE) (Fig. 3A & C), while those in the B12-amended cultures (LoTCEB12 and HiTCEB12) exhibited little change (Fig. 3B & D). Cobalamin in LoTCE and HiTCE cultures increased from 0 to 1–2 nM during the first two days, and reached the highest levels at day 11 and day 3, respectively. Free DMB slowly accumulated in the supernatant, and reached maximum concentrations by day 15 for LoTCE and by day 11 for HiTCE (Fig. 3A & C). In LoTCEB12 and HiTCEB12, added cobalamin was primarily detected in the supernatant during the first 2–4 days of incubation, but subsequently decreased dramatically accompanied by an increase in free DMB in the supernatant (Fig. 3B & D).
Figure 3.
Temporal changes of [p-Cre]Cba, Cobalamin, and DMB in groundwater enrichments (A: LoTCE, B: LoTCEB12, C: HiTCE, D: HiTCEB12, E: NoTCE. ↓ indicates amendments of lactate and TCE, ↓ indicates amendment of lactate only, added amounts are according to Table 1. Note: Y-axis Scales in A, C, E are different from those in B & D).
The effect of dechlorination metabolism and growth of D. mccartyi on the corrinoid profiles of NJ enrichments
Since free DMB (a prerequisite for corrinoid remodeling by strain 195) was detected in the supernatant of the cultures without exogenous cobalamin, we hypothesized that D. mccartyi strains in the NJ enrichments generate cobalamin by remodeling other corrinoids with DMB. To test this hypothesis, we examined whether active dechlorination and D. mccartyi cell growth affected the corrinoid profile of the community. To limit the growth of D. mccartyi, we constructed enrichments NoTCE and NoTCEB12 by subculturing HiTCE and HiTCEB12 cultures without TCE (Table S3).
We analyzed the corrinoid profiles of NoTCE and NoTCEB12 after 6 subculturing events when the growth of D. mccartyi in these two cultures was significantly inhibited (<104 cells/mL compared to 109 total Bacteria). Interestingly, only trace amounts of cobalamin were detected in NoTCE throughout the entire incubation (< 0.1 nM versus 2.3 nM in HiTCE) (Fig. 2A and Fig. 3E), while [p-Cre]Cba was produced at concentrations as high as 24 nM. These results, together with the observed decrease in [p-Cre]Cba in the HiTCE culture after day 2 (Fig. 3C) suggest that D. mccartyi may be responsible for remodeling [p-Cre]Cba to form cobalamin in these cultures. Moreover, about 20–50% of total [p-Cre]Cba in NoTCE was detected in the supernatant (Fig. 3E), while in HiTCE, almost all of the [p-Cre]Cba was detected in the cell pellets (Fig. 3C). Despite the trace production of cobalamin, the concentration of free DMB in NoTCE was similar to that found in HiTCE (Fig. 2A), confirming anaerobic production of DMB in these communities, a result corroborated by alternative DMB detection methods (Sinorhizobium meliloti bluB bioassays, Fig. S1). Similar to other B12-amended NJ enrichments, a low concentration of cobalamin was detected in NoTCEB12, while a high concentration of DMB was generated (Fig. 2B). This result, in the absence of actively growing D. mccartyi, indicates that microorganisms other than D. mccartyi were active in modification of the added cobalamin.
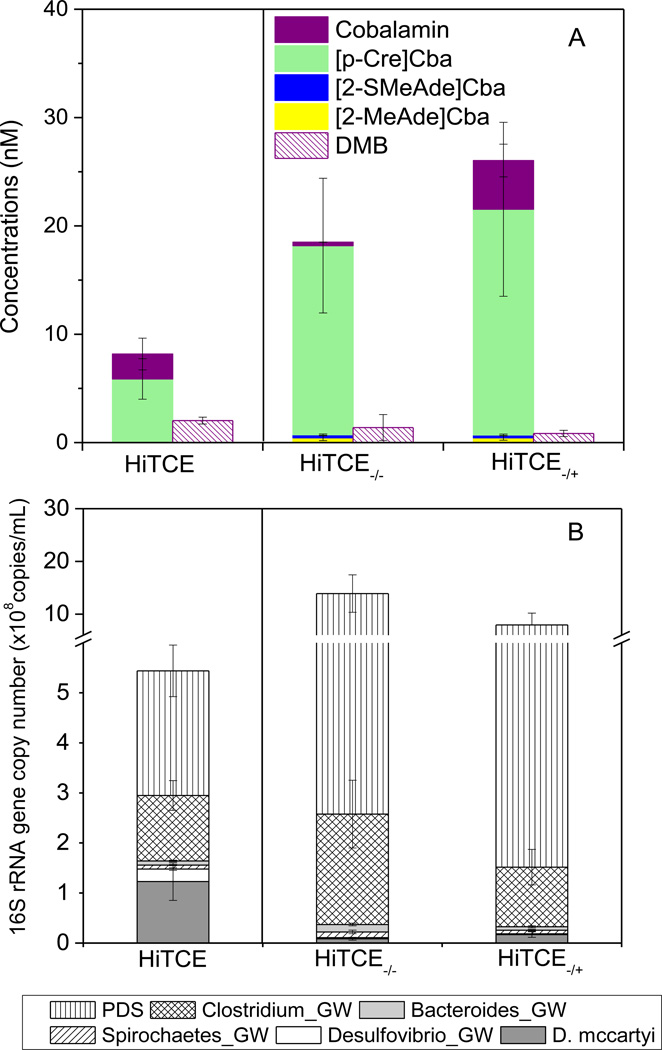
Corrinoid profiles were also determined when D. mccartyi activity was perturbed over the course of two feeding cycles. HiTCE−/+ was constructed by subculturing HiTCE into TCE-free medium, incubating for one feeding cycle (13 days) and then re-amending 77 µmol of TCE on day 14 for another feeding cycle (Table S6). HiTCE−/− was HiTCE subcultured without TCE for two feeding cycles (Table S6). As expected, D. mccartyi numbers were about 10 times lower in HiTCE−/− than in HiTCE (Fig. 4B), and in HiTCE−/+, they were about double the amount in HiTCE−/−, indicating a rebound in D. mccartyi caused by the TCE re-amended during the second feeding cycle. The concentration of cobalamin was 86% lower in HiTCE−/− than in HiTCE (Fig. 4A), while in HiTCE−/+, it rebounded to two times the concentration in HiTCE. No substantial difference in other corrinoids or in free DMB was observed between the two perturbed cultures. D. mccartyi and the other seven OTUs previously identified in the NJ enrichments (Men et al., 2013) have been quantified by qPCR (Fig. 4B). Interestingly, numbers of the three dominant OTUs, Pelosinus_GW, Dendrosporobacter_GW and Sporotalea_GW (collectively designated PDS”) exhibited a 5-fold increase in the two perturbed cultures compared to HiTCE (Fig. 4B), accompanied by an increase in [p-Cre]Cba concentrations (Fig. 4A).
Figure 4.
Comparison of corrinoid and lower ligand production (A) and 16S rRNA gene copy numbers of the OTUs (B) between HiTCE−/− and HiTCE−/+, HiTCE is shown as reference. Error bars represents standard deviation, n=3. (PDS represents the summation of Pelosinus_GW, Dendrosporobacter_GW and Sporotalea_GW).
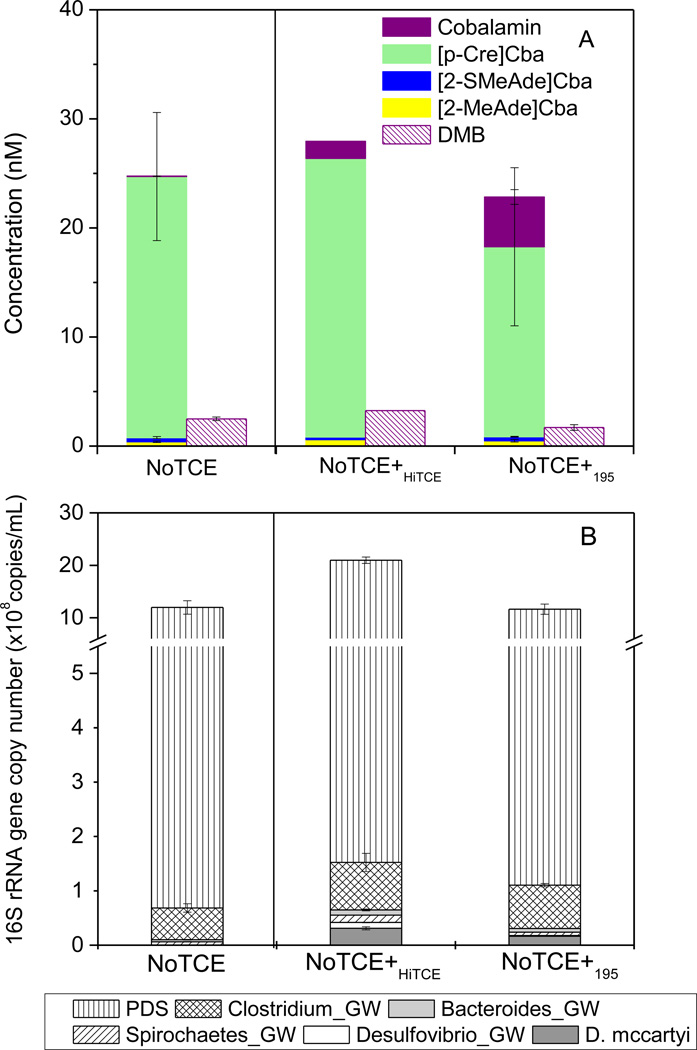
When we attempted to evaluate whether the production of cobalamin would be recovered when TCE was re-amended to the NoTCE culture, no dechlorination occurred even after a prolonged incubation (60 days), likely due to the extremely low D. mccartyi numbers resulting from multiple sub-culturing events. Therefore, NoTCE was subsequently bioaugmented with HiTCE, the original inoculation culture for NoTCE, to construct NoTCE+HiTCE (Table S6). NoTCE+HiTCE dechlorinated TCE to VC and ethene after 14 days, together with a 32-fold increase in the cobalamin concentration and a 103-time increase in the D. mccartyi cell number. To determine whether these effects could be specifically attributed to D. mccartyi, NoTCE was also bioaugmented with strain 195 isolate (1%, v/v) to construct NoTCE+195 (Table S6), in which substantial increases were observed in the cobalamin concentration (92-fold) and the D. mccartyi cell number (103-time), similar to NoTCE+HiTCE (Fig. 5). Interestingly, increases in Desulfovibrio were also observed in both bioaugmented cultures (20-fold in NoTCE+HiTCE and 3-fold in NoTCE+195) compared with NoTCE (Fig. 5B).
Figure 5.
Comparison of corrinoid and lower ligand production (A) and 16S rRNA gene copy numbers of the OTUs (B) between NoTCE+HiTCE, and NoTCE+195, NoTCE is shown as reference. Error bars represent standard deviation, n=3. (PDS represents the summation of Pelosinus_GW, Dendrosporobacter_GW and Sporotalea_GW).
Discussion
The exchange of metabolites among members of microbial communities enables the catabolism of complex substrates and supports the growth of auxotrophic microbes. Better understanding of the roles played by nutrient providers and scavengers in communities will help to elucidate ecological relationships among community members. Corrinoids are produced by less than half of the microbes that have corrinoid-dependent enzymes, and as such, the exchange of corrinoid cofactors is crucial for functionally integrated microbial communities (Martens et al., 2002). Since different corrinoids do not function equivalently as cofactors, some microbes require specific corrinoids for survival. Microorganisms in a community that require specific corrinoids have three options: 1) de novo biosynthesis; 2) import of the specific corrinoid generated by other community members; or 3) salvaging and remodeling corrinoids with an appropriate free lower ligand. Previous studies have examined the prevalence of genes involved in corrinoid biosynthesis, import, and modification among sequenced microbes, but there are few known biomarkers indicative of the specific corrinoid an organism produces in communities. The traditional corrinoid bioassays are not capable of distinguishing between different corrinoids (Guggisberg et al., 2012). Moreover, the total corrinoids detected by bioassays are limited to the corrinoid utilization capabilities of the applied bacterium, potentially resulting in underestimation. For example, Yan et al. (2013) reported that [p-Cre]Cba and [Phe]Cba were not detectable using the Lactobacillus delbrueckii bioassay. This might be due to the absence of the co-ordination between the Co ion and the lower ligand (base-off conformation) in the two cobamides, making them nonfunctional in L. delbrueckii. By applying a crude extraction procedure combined with LC/MS/MS, the new corrinoid detection method developed in this study enables the identification of the specific corrinoids present in a microbial community, which may have important implications for community function. The analysis of corrinoid profiles in microbial communities, such as the corrinoid-dependent dechlorinating enrichments studied here, will contribute to the understanding of the roles played by specific microbial groups in corrinoidproduction and modification in the environment.
The dechlorinating enrichments evaluated in this study were able to sustain robust continuous growth of D. mccartyi in the absence of exogenous cobalamin amendment. However, corrinoid profiles and community structures differed in the enrichments inoculated from different locations (CA or NJ). In CA derived SANAS, cobalamin was the dominant corrinoid detected at concentrations above the minimal requirement of D. mccartyi, while in the NJ enrichments, [p-Cre]Cba was the most abundant corrinoid. The dominance of different corrinoids is likely due to the difference in the microbial compositions of these two sets of enrichments. The dominance of cobalamin in SANAS is likely attributed to the de novo anaerobic cobalamin biosynthesis, which has been shown in Acetobacterium woodii, Eubacterium limosum, and Clostridium barkeri (Stupperich et al., 1988; Munder et al., 1992), and members of these genera have been identified in ANAS, the original inoculum of SANAS (Richardson et al., 2002; Freeborn et al., 2005). In addition, recent metagenomic analysis of ANAS found that one D. mccartyi strain (ANAS2) in this community possesses a nearly complete corrinoid biosynthesis pathway (Brisson et al., 2012), however the function has not been confirmed in this strain. In contrast, the abundance of [p-Cre]Cba in NJ enrichments is likely related to the dominance of Pelosinus-like bacteria in Veillonellaceae family (Men et al., 2013), which were not detected in ANAS subcultures. According to our recent study, Pelosinus fermentans strain R7 is another bacterium to generate [p-Cre]Cba, following previously reported Sporomusa ovata and Veillonella parvula (Stupperich et al., 1988; Stupperich et al., 1989; Chan and Escalante-Semerena, 2011; Crofts et al., 2013; Men et al., 2014).
The effect of community structure on corrinoid profiles was also reflected by the detection of [5-OHBza]Cba in only the methanogenic enrichments. This is consistent with previous studies which have shown that [5-OHBza]Cba is produced by a number of methanogens (Pol et al., 1982; Whitman and Wolfe, 1984; Kräutler et al., 1987; Stupperich et al., 1987; Stupperich and Krautler, 1988). Methanogens have been shown to be able to produce various corrinoids, and the role of methanogens in communities containing D. mccartyi has long been of interest. However, given that the amounts of [5-OHBza]Cba in all enrichments were very low, and that NJ enrichments with inhibited methanogenic activity and no detectable [5-OHBza]Cba also successfully supported D. mccartyi growth and dechlorination, the contribution of methanogens to the corrinoid supply of D. mccartyi is relatively small in the communities examined in this study.
D. mccartyi is the only dechlorinating bacterial species found in the NJ enrichments (Men et al., 2013). The correlation between cobalamin production and D. mccartyi growth in B12-unamended NJ enrichments suggests corrinoid salvaging and remodeling carried out by D. mccartyi. Genes encoding enzymes involved in the corrinoid remodeling pathway, including the amidohydrolase CbiZ and the adenosylcobinamide-phosphate guanylyltransferase CobU (Escalante-Semerena, 2007), are present in all sequenced D. mccartyi strains (Kube et al., 2005;Seshadri et al., 2005; McMurdie et al., 2009; Yi et al., 2012), as well as the D. mccartyi strains in the NJ enrichments (Men et al., 2013). A previous study with strain 195 revealed that this strain is capable of remodeling added nonfunctional corrinoids including [p-Cre]Cba into cobalamin in the presence of DMB (Yi et al., 2012). Results of this study suggest that the D. mccartyi strains in the NJ enrichments also employ the remodeling mechanism to obtain cobalamin: when the growth of D. mccartyi was inhibited, little cobalamin was produced, and the level of [p-Cre]Cba increased; when the growth of D. mccartyi was restored in perturbed cultures, cobalamin concentrations rebounded. This correlation between cobalamin concentration and D. mccartyi growth supports the hypothesis that D. mccartyi acquires corrinoids and free DMB from the other microbes and remodels them into cobalamin. The dominance of [p-Cre]Cba in the NJ enrichments indicates that [p-Cre]Cba, likely produced by Pelosinus-like strains, serves as a major substrate for corrinoid remodeling by D. mccartyi. The low level of [p-Cre]Cba in the supernatant of HiTCE and the increase of [p-Cre]Cba in the supernatant of NoTCE (Fig. 3C&E) strongly favor the hypothesis that [p-Cre]Cba was exchanged between its producers and D. mccartyi by being released into culture supernatant, and was salvaged by D. mccartyi for corrinoid remodeling. This hypothesis has recently been tested with experiments in which P. fermentans strain R7 successfully supported the growth of strain 195 in B12-unamended defined consortia by generating [p-Cre]Cba in the presence of DMB. These results highlight the potential role of Pelosinus-like species on corrinoid exchange in the NJ enrichments (Men et al., 2014).
Besides reductive dehalogenases, isomerases involved in fermentation pathways are also corrinoid-dependent (Banerjee and Ragsdale, 2003). Recently, Pelosinus sp. strain HCF1 has been reported to ferment lactate to propionate and acetate through methylmalonyl-CoA pathway (Beller et al., 2013), in which methylmalonyl-CoA mutase is a corrinoid-dependent isomerase (Banerjee and Ragsdale, 2003). Given that a total of 5.3 mmol lactate was provided to the NJ enrichments per feeding cycle, most of which was fermented to propionate and acetate (Men et al., 2013), it is possible that the [p-Cre]Cba detected in the NJ enrichments is generated for this fermentation process.
A notable finding of this study is the detection of free DMB in community supernatants of all cultures examined. Studies of D. mccartyi isolates (Yi et al., 2012) and constructed co-cultures (Yan et al., 2012; Yan et al., 2013) indicate the importance of DMB in corrinoid remodeling. This study is the first to detect and quantify DMB in microbial communities and provides evidence for endogenous DMB production (at nM levels) in anaerobic microbial communities enriched from contaminated soil and groundwater. Although the only known physiological role of DMB is as the lower ligand of cobalamin (Munder et al., 1992; Taga et al., 2007), the generation of DMB in the absence of cobalamin in the NoTCE enrichment suggests that DMB is produced independently of cobalamin biosynthesis. Under aerobic conditions, DMB is biosynthesized from flavin mononucleotide (FMN) catalyzed by the enzyme BluB (Campbell et al., 2006; Gray and Escalante-Semerena, 2007; Taga et al., 2007). However, information on anaerobic DMB production is still very limited. Previous labeling studies showed that the anaerobe Eubacterium limosum synthesizes DMB from substrates such as glutamine, glycine and formate, which are also used in purine-nucleotide biosynthesis (Munder et al., 1992). However, the enzymes involved in anaerobic DMB biosynthesis have not yet been identified. Due to the importance of DMB in the corrinoid remodeling processes of D. mccartyi and other cobalamin-salvaging anaerobes, further investigations are needed to understand DMB synthesis in anaerobic communities.
In summary, this study sheds light on the correlation between corrinoid production and community structure, the corrinoid salvaging and modification in D. mccartyi-containing communities, as well as ecological relationships between D. mccartyi and other community members. Greater insights into corrinoid production, modification, and utilization in microbial communities will help us better understand how nutrient exchange shapes these communities, and what ecological roles are played by individual community members.
Experimental Procedures
Cultures and growth conditions
SANASB12 and SANAS are the subcultures of ANAS, a well-maintained TCE-dechlorinating enrichment characterized in previous studies (Richardson et al., 2002; Freeborn et al., 2005; Lee et al., 2006). They were constructed by inoculating 5 mL ANAS culture (5%, v/v) into 95 mL basal medium with N2/CO2 headspace (90:10, v/v) and 74 nM (c.a. 100 µg/L) vitamin B12 (SANASB12) and without the addition of B12 (SANAS). The composition of the basal medium was the same as described elsewhere (He et al., 2007), except that a modified Wolin vitamin stock excluding B12 was used (Wolin et al., 1963). It contained (per liter) 1 g of NaCl, 0.5 g of MgCl2∙6H2O, 0.2 g of KH2PO4, 0.3 g of NH4Cl, 0.3 g of KCl, 0.015 g of CaCl2 2H2O, 0.2 g of MgSO4∙7H2O, 1 ml of a trace element solution (Pfennig, 1974), 1 ml of a Na2SeO3-Na2WO4 solution (Brysch et al., 1987), and 10 mg of resazurin. Cultures were amended with 48 mM lactate as both carbon source and electron donor, and 2 µL TCE (ca. 0.2 mM, final concentration) was supplied as the terminal electron acceptor. Both SANASB12 and SANAS completely dechlorinated TCE to ethene. Experiments were carried out after five 5% (v/v) subculturing events for SANASB12 and three subculturing events for SANAS.
Another four dechlorinating enrichments (LoTCEB12, LoTCE, HiTCEB12 and HiTCE) used in this study were originally inoculated with contaminated groundwater from New Jersey and maintained under conditions listed in Table S3. The high initial TCE concentration in HiTCEB12 and HiTCE cultures resulted in the inhibition of methanogenesis due to the toxicity of TCE to methanogens. These enrichments are capable of dechlorinating TCE to VC and ethene as described elsewhere (Men et al., 2013). In order to investigate the effects of the presence of D. mccartyi on corrinoid profiles, two enrichments were subsequently constructed from HiTCE and HiTCEB12 using the same growth condition, but with no TCE added (denoted “NoTCE”, “NoTCEB12”, respectively) (Table S3). Experiments were carried out after 40 subculturing events for LoTCE, LoTCEB12, HiTCE and HiTCEB12, and 6 subculturing events for NoTCE and NoTCEB12.
Corrinoid biosynthesis and purification
[Ade]Cba and [2-MeAde]Cba were extracted from Salmonella enterica serovar Typhimurium strain LT2, and [5-OHBza]Cba was extracted from Methanosarcina barkeri strain Fusaro using cyanidation and solid phase extraction as previously described (Gray and Escalante-Semerena, 2009a; Yi et al., 2012). Briefly, cells were collected, resuspended in 20 mL methanol with 20 mg KCN per gram of cells, and incubated at 60 °C for 1.5 hr with periodic mixing. Samples were then dried and resuspended in 20 mL deionized (DI) water, desalted using a C18 Sep-Pak cartridge (Waters Associates, Milford, MA) and eluted in 2 mL methanol. The eluates were dried and redissolved in DI water. [Bza]Cba, [5-MeBza]Cba, [5-OMeBza]Cba, [5-OMe, 6-MeBza]Cba, [2-SMeAdeCba], [Phe]Cba and [p-Cre]Cba were extracted from bacterial cultures and purified as described previously (Allen and Stabler, 2008). A molar extinction coefficient of 30, 800 M−1 cm−1 at 367.5 nm was used for quantification (Allen and Stabler, 2008). The identity of each corrinoid was confirmed by mass spectrometry.
Monocyanocobyric acid standard was prepared as described (Butler et al., 2006), with the following changes: after evaporation to dryness on a rotary evaporator, the reaction mixture residue was dissolved in 0.1 mM KCN in DI water. 5 mL aliquots were desalted with a C18 Sep-Pak cartridge, and eluted in 3 mL methanol. The eluates were dried, resuspended in deionized water, and stored at −80 °C. Monocyanocobyric acid was purified using the solvent gradient as described (Butler et al., 2006).
Extraction of corrinoid and lower ligand bases
Cell pellets were collected from 200–300 mL cultures by centrifugation at 15, 000 × g for 10 min at 4 °C and stored at −80 °C. Supernatants were passed through a 0.2 µm filter and loaded onto a Sep-Pak C18 cartridge. The cartridge was then washed with 50 mL DI water, and eluted with 3 mL 100% methanol. The eluate was stored at −80 °C. Methanol extraction, cyanidation and desalting were carried out as described above. The dried extracts were dissolved in 200–300 µL milliQ water. All samples were stored at −20 °C prior to LC/MS/MS analysis.
Analytical methods
Chlorinated ethenes and ethene were measured by an Agilent 7890A gas chromatograph (GC) equipped with a flame ionization detector (Agilent, Santa Clara, CA), as described elsewhere (Richardson et al., 2002; Men et al., 2013).
Liquid chromatography coupled with tandem mass spectrometry (LC/MS/MS) was performed using an Agilent 6410 liquid chromatograph-triple quadrupole mass spectrometer (Agilent Technologies, Santa Clara, CA). Samples were loaded onto an Agilent Eclipse Plus C18 column, 1.8 µm, 3.0 × 50 mm (Agilent Technologies, Santa Clara, CA) with temperature maintained at 40 °C. LC was performed at 0.5 mL/min with initial mobile phase conditions of 82% miliQ water with 0.1% formic acid (A) and 18% methanol with 0.1% formic acid (B) held for 3 min, increased to 21% B immediately and held for 2 min, increased to 100% B over 0.1min and held for 1 min, decreased back to 18% B over 0.1 min and held for 3.8 min. The injection volume was 10 µL. The fragmentor voltage was set at 135 V for MS2 scan, and the collision energy was 45 V for product ion scan. Multiple reaction monitoring (MRM) was used to capture the signature transition of each corrinoid and lower ligand for quantitative analysis. Corrinoids lacking a lower ligand base (Cby and Cbi) and the phenolic corrinoids ([Phe]Cba and [p-Cre]Cba) were identified by their unique product ions. All other corrinoids are qualified and quantified by tracking the transition of the doubly charged molecular ion to two dominant product ions corresponding to the singly charged lower ligand base, and an ion of unknown structure with an m/z of 912 (Allen and Stabler, 2008). Benzimidazoles were quantified by monitoring the transition from the singly charged molecular ion to unique product ions (Table S1).
Two parallel sets of samples were prepared for the LC/MS/MS method validation. In each set cell pellets and supernatant were separated by centrifugation at 15,000 × g for 15 min at 4 °C. Two fresh media controls with different cyanocobalamin concentrations were prepared in parallel. One set of samples was measured using extraction and detection methods described by Allen and Stabler (Allen and Stabler, 2008), while the other set was extracted and analyzed by the method described in this study.
Biological triplicates were performed for GC measurements. For end-point corrinoid and lower ligand detection, measurements were carried out on one subculture, and measurements on three subsequential subcultures were reported as biological triplicates. For temporal analyses, measurements were carried out on one subculture.
Bioassays for DMB detection
Calcofluor analysis of DMB using a Sinorhizobium meliloti bluB mutant strain was performed as previously described (Campbell et al., 2006; Taga et al., 2007). A modified quantitative bioassay was further performed as described by Croft and Taga (personal communication). Briefly, S. meliloti bluB was grown in M9 minimal media supplemented with 1 mg/mL L-methionine, and was inoculated with serially diluted DMB standards or enrichment samples to a total of 200 µL in a 96-well plate. Calcofluor was added for the final 5 hr, and the fluorescent phenotype was measured by excitation at 360 nm and emission at 460 nm.
DNA isolation and quantification by quantitative PCR (qPCR)
Genomic DNA was extracted from 1.5 mL culture using a DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA) according to manufacturer’s instructions. qPCR was applied using SYBR Green reagent Applied Biosystems, Foster City, CA) and primer sets targeting 16S rRNA gene sequences of the OTUs of interest as described elsewhere (Men et al., 2013).
Supplementary Material
Acknowledgements
This research was supported by the Strategic Environmental Research and Development Program (SERDP) through grant ER-1587, NIEHS Superfund P42ES004705, and NSF CBET1336709 to L.A.C. and NSF grant MCB1122046 to M.E.T.
List of Acronyms
- PCE
tetrachloroethene
- TCE
trichloroethene
- Cby
cobyric acid
- Cbi
cobinamide
- Cba
cobamide
- DMB
5,6-dimethylbenzimidazole
- OTU
operational taxonomic unit
- PDS
summation of OTUs related to Pelosinus, Dendrosporobacter, and Sporotalea
Footnotes
There is no conflict of interest to declare.
References
- Allen RH, Stabler SP. Identification and quantitation of cobalamin and cobalamin analogues in human feces. Am J Clin Nutr. 2008;87:1324–1335. doi: 10.1093/ajcn/87.5.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R, Ragsdale SW. The many faces of vitamin B12: Catalysis by cobalamin-dependent enzymes. Annu Rev Biochem. 2003;72:209–247. doi: 10.1146/annurev.biochem.72.121801.161828. [DOI] [PubMed] [Google Scholar]
- Beller HR, Han RY, Karaoz U, Lim H, Brodie EL. Genomic and physiological characterization of the chromate-reducing, aquifer-derived Firmicute Pelosinus sp strain HCF1. Appl Environ Microbiol. 2013;79:63–73. doi: 10.1128/AEM.02496-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson VL, West KA, Lee PKH, Tringe SG, Brodie EL, Alvarez-Cohen L. Metagenomic analysis of a stable trichloroethene-degrading microbial community. ISME J. 2012;6:1702–1714. doi: 10.1038/ismej.2012.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KL. Chemistry and enzymology of vitamin B12. Chem Rev. 2005;105:2075–2149. doi: 10.1021/cr030720z. [DOI] [PubMed] [Google Scholar]
- Brysch K, Schneider C, Fuchs G, Widdel F. Lithoautotrophic growth of sulfate-reducing bacteria, and description of Desulfobacterium autotrophicum gen. nov., sp. nov. Arch Microbiol. 1987;148:264–274. [Google Scholar]
- Butler PA, Murtaza S, Krautler B. Partial synthesis of Co alpha Co beta-dicyano-176-norcobinamide. Monatshefte Fur Chemie. 2006;137:1579–1589. [Google Scholar]
- Campbell GRO, Taga ME, Mistry K, Lloret J, Anderson PJ, Roth JR, Walker GC. Sinorhizobium meliloti bluB is necessary for production of 5,6-dimethylbenzimidazole, the lower ligand of B12. Proc Natl Acad Sci U S A. 2006;103:4634–4639. doi: 10.1073/pnas.0509384103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HC, Escalante-Semerena JC. ArsAB, a novel enzyme from Sporomusa ovata activates phenolic bases for adenosylcobamide biosynthesis. Mol Microbiol. 2011;81:952–967. doi: 10.1111/j.1365-2958.2011.07741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanarin I, Muir M. Demonstration of vitamin B12 analogs in human-sera not detected by microbiological assay. Br J Haematol. 1982;51:171–173. doi: 10.1111/j.1365-2141.1982.tb07301.x. [DOI] [PubMed] [Google Scholar]
- Crofts TS, Seth EC, Hazra AB, Taga ME. Cobamide structure depends on both lower ligand availability and CobT substrate specificity. Chem Biol. 2013;20:1265–1274. doi: 10.1016/j.chembiol.2013.08.006. [DOI] [PubMed] [Google Scholar]
- Cupples AM, Spormann AM, McCarty PL. Comparative evaluation of chloroethene dechlorination to ethene by Dehalococcoides-like microorganisms. Environ Sci Technol. 2004;38:4768–4774. doi: 10.1021/es049965z. [DOI] [PubMed] [Google Scholar]
- Duhamel M, Mo K, Edwards EA. Characterization of a highly enriched Dehalococcoides-containing culture that grows on vinyl chloride and trichloroethene. Appl Environ Microbiol. 2004;70:5538–5545. doi: 10.1128/AEM.70.9.5538-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escalante-Semerena JC. Conversion of cobinamide into adenosylcobamide in Bacteria and Archaea. J Bacteriol. 2007;189:4555–4560. doi: 10.1128/JB.00503-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeborn RA, West KA, Bhupathiraju VK, Chauhan S, Rahm BG, Richardson RE, Alvarez-Cohen L. Phylogenetic analysis of TCE-dechlorinating consortia enriched on a variety of electron donors. Environ Sci Technol. 2005;39:8358–8368. doi: 10.1021/es048003p. [DOI] [PubMed] [Google Scholar]
- Gray MJ, Escalante-Semerena JC. Single-enzyme conversion of FMNH2 to 5,6-dimethylbenzimidazole, the lower ligand of B12. Proc Natl Acad Sci U S A. 2007;104:2921–2926. doi: 10.1073/pnas.0609270104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, Escalante-Semerena JC. The cobinamide amidohydrolase (cobyric acid-forming) CbiZ enzyme: a critical activity of the cobamide remodelling system of Rhodobacter sphaeroides. Mol Microbiol. 2009a;74:1198–1210. doi: 10.1111/j.1365-2958.2009.06928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray MJ, Escalante-Semerena JC. In vivo analysis of cobinamide salvaging in Rhodobacter sphaeroides strain 2.4.1. J Bacteriol. 2009b;191:3842–3851. doi: 10.1128/JB.00230-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggisberg D, Risse MC, Hadorn R. Determination of vitamin B12 in meat products by RP-HPLC after enrichment and purification on an immunoaffinity column. Meat Sci. 2012;90:279–283. doi: 10.1016/j.meatsci.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Guimaraes DH, Weber A, Klaiber I, Vogler B, Renz P. Guanylcobamide and hypoxanthylcobamide - corrinoids formed by Desulfovibrio vulgaris. Arch Microbiol. 1994;162:272–276. [Google Scholar]
- He JZ, Holmes VF, Lee PKH, Alvarez-Cohen L. Influence of vitamin B12 and cocultures on the growth of Dehalococcoides isolates in defined medium. Appl Environ Microbiol. 2007;73:2847–2853. doi: 10.1128/AEM.02574-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JZ, Ritalahti KM, Yang KL, Koenigsberg SS, Löffler FE. Detoxification of vinyl chloride to ethene coupled to growth of an anaerobic bacterium. Nature. 2003;424:62–65. doi: 10.1038/nature01717. [DOI] [PubMed] [Google Scholar]
- Keller S, Ruetz M, Kunze C, Krautler B, Diekert G, Schubert T. Exogenous 5,6-dimethylbenzimidazole caused production of a non-functional tetrachloroethene reductive dehalogenase in Sulfurospirillum multivorans. Environ Microbiol. 2013 doi: 10.1111/1462-2920.12268. [DOI] [PubMed] [Google Scholar]
- Kräutler B, Fieber W, Ostermann S, Fasching M, Ongania KH, Gruber K, et al. The cofactor of tetrachloroethene reductive dehalogenase of Dehalospirillum multivorans is norpseudo-B12, a new type of a natural corrinoid. Helv Chim Acta. 2003;86:3698–3716. [Google Scholar]
- Kräutler B, Kohler HPE, Stupperich E. 5'-Methylbenzimidazolyl-cobamides are the corrinoids from some sulfate-reducing and sulfur-metabolizing bacteria. Eur J Biochem. 1988;176:461–469. doi: 10.1111/j.1432-1033.1988.tb14303.x. [DOI] [PubMed] [Google Scholar]
- Kräutler B, Moll J, Thauer RK. The corrinoid from Methanobacterium thermoautotrophicum (Marburg strain) spectroscopic structure analysis and identification as Coβ-cyano-5'-hydroxybenzimidazolyl-cobamide (Factor-III) Eur J Biochem. 1987;162:275–278. doi: 10.1111/j.1432-1033.1987.tb10596.x. [DOI] [PubMed] [Google Scholar]
- Kube M, Beck A, Zinder SH, Kuhl H, Reinhardt R, Adrian L. Genome sequence of the chlorinated compound respiring bacterium Dehalococcoides species strain CBDB1. Nat Biotechnol. 2005;23:1269–1273. doi: 10.1038/nbt1131. [DOI] [PubMed] [Google Scholar]
- Lee PKH, Johnson DR, Holmes VF, He JZ, Alvarez-Cohen L. Reductive dehalogenase gene expression as a biomarker for physiological activity of Dehalococcoides spp. Appl Environ Microbiol. 2006;72:6161–6168. doi: 10.1128/AEM.01070-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesage S, Brown S, Millar K. Vitamin B12 catalyzed dechlorination of perchloroethylene present as residual DNAPL. Ground Water Monit Rem. 1996;16:76–85. [Google Scholar]
- Maillard J, Schumacher W, Vazquez F, Regeard C, Hagen WR, Holliger C. Characterization of the corrinoid iron-sulfur protein tetrachloroethene reductive dehalogenase of Dehalobacter restrictus. Appl Environ Microbiol. 2003;69:4628–4638. doi: 10.1128/AEM.69.8.4628-4638.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens JH, Barg H, Warren MJ, Jahn D. Microbial production of vitamin B12. Appl Microbiol Biotechnol. 2002;58:275–285. doi: 10.1007/s00253-001-0902-7. [DOI] [PubMed] [Google Scholar]
- Maymó-Gatell X, Chien YT, Gossett JM, Zinder SH. Isolation of a bacterium that reductively dechlorinates tetrachloroethene to ethene. Science. 1997;276:1568–1571. doi: 10.1126/science.276.5318.1568. [DOI] [PubMed] [Google Scholar]
- McMurdie PJ, Behrens SF, Müller JA, Goke J, Ritalahti KM, Wagner R, et al. Localized plasticity in the streamlined genomes of vinyl chloride respiring Dehalococcoides. PLoS Genet. 2009;5:e1000714. doi: 10.1371/journal.pgen.1000714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men Y, Seth EC, Yi S, Allen RH, Taga ME, Alvarez-Cohen L. Sustainable growth of Dehalococcoides mccartyi 195 by corrinoid salvaging and remodeling in defined lactate-fermenting consortia. Appl Environ Microbiol. 2014;80:2133–2141. doi: 10.1128/AEM.03477-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Men YJ, Lee PKH, Harding KC, Alvarez-Cohen L. Characterization of four TCE-dechlorinating microbial enrichments grown with different cobalamin stress and methanogenic conditions. Appl Microbiol Biotechnol. 2013;97:6439–6450. doi: 10.1007/s00253-013-4896-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller JA, Rosner BM, von Abendroth G, Meshulam-Simon G, McCarty PL, Spormann AM. Molecular identification of the catabolic vinyl chloride reductase from Dehalococcoides sp strain VS and its environmental distribution. Appl Environ Microbiol. 2004;70:4880–4888. doi: 10.1128/AEM.70.8.4880-4888.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munder M, Vogt JRA, Vogler B, Renz P. Biosynthesis of vitamin B12 in anaerobic bacteria: experiments with Eubacterium limosum on the incorporation of D-[1-C13]erythrose and [C13]formate into the 5,6-dimethylbenzimidazole moiety. Eur J Biochem. 1992;204:679–683. doi: 10.1111/j.1432-1033.1992.tb16681.x. [DOI] [PubMed] [Google Scholar]
- Pfennig N. Rhodopseudomonas globiformis, sp. n., a new species of Rhodospirillaceae. Arch Microbiol. 1974;100:197–206. [Google Scholar]
- Pol A, Vanderdrift C, Vogels GD. Corrinoids from Methanosarcina barkeri : structure of the α-Ligand. Biochem Biophys Res Commun. 1982;108:731–737. doi: 10.1016/0006-291x(82)90890-7. [DOI] [PubMed] [Google Scholar]
- Reinhold A, Westermann M, Seifert J, von Bergen M, Schubert T, Diekert G. Impact of vitamin B12 on formation of the tetrachloroethene reductive dehalogenase in Desulfitobacterium hafniense strain Y51. Appl Environ Microbiol. 2012;78:8025–8032. doi: 10.1128/AEM.02173-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renz P. Biosynthesis of the 5,6-dimethylbenzimidazole moiety of cobalamin and of the other bases found in natural corrinoids. In: Banerjee R, editor. Chemistry and biochemistry of B12. New York: John Wiley & Sons, Inc.; 1999. pp. 557–575. [Google Scholar]
- Richardson RE, Bhupathiraju VK, Song DL, Goulet TA, Alvarez-Cohen L. Phylogenetic characterization of microbial communities that reductively dechlorinate TCE based upon a combination of molecular techniques. Environ Sci Technol. 2002;36:2652–2662. doi: 10.1021/es0157797. [DOI] [PubMed] [Google Scholar]
- Ryzhkova EP. Multiple functions of corrinoids in prokaryote biology. Appl Biochem Microbiol. 2003;39:115–139. [PubMed] [Google Scholar]
- Schipp CJ, Marco-Urrea E, Kublik A, Seifert J, Adrian L. Organic cofactors in the metabolism of Dehalococcoides mccartyi strains. Philos Trans R Soc, B. 2013;368:20120321. doi: 10.1098/rstb.2012.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri R, Adrian L, Fouts DE, Eisen JA, Phillippy AM, Methe BA, et al. Genome sequence of the PCE-dechlorinating bacterium Dehalococcoides ethenogenes. Science. 2005;307:105–108. doi: 10.1126/science.1102226. [DOI] [PubMed] [Google Scholar]
- Siebert A, Neumann A, Schubert T, Diekert G. A non-dechlorinating strain of Dehalospirillum multivorans: evidence for a key role of the corrinoid cofactor in the synthesis of an active tetrachloroethene dehalogenase. Arch Microbiol. 2002;178:443–449. doi: 10.1007/s00203-002-0473-8. [DOI] [PubMed] [Google Scholar]
- Stupperich E, Eisinger HJ, Krautler B. Diversity of corrinoids in acetogenic bacteria p-cresolylcobamide from Sporomusa ovata-5-methoxy-6-methylbenzimidazolylcobamide from Clostridium formicoaceticum and vitamin B12 from Acetobacterium woodii. Eur J Biochem. 1988;172:459–464. doi: 10.1111/j.1432-1033.1988.tb13910.x. [DOI] [PubMed] [Google Scholar]
- Stupperich E, Eisinger HJ, Krautler B. Identification of phenolyl cobamide from the homoacetogenic bacterium Sporomusa ovata. Eur J Biochem. 1989;186:657–661. doi: 10.1111/j.1432-1033.1989.tb15256.x. [DOI] [PubMed] [Google Scholar]
- Stupperich E, Krautler B. Pseudo vitamin B12 or 5-hydroxybenzimidazolyl cobamide are the corrinoids found in methanogenic bacteria. Arch Microbiol. 1988;149:268–271. [Google Scholar]
- Stupperich E, Steiner I, Eisinger HJ. Substitution of Coα-(5-hydroxybenzimidazolyl)cobamide (factor-III) by vitamin B12 in Methanobacterium thermoautotrophicum. J Bacteriol. 1987;169:3076–3081. doi: 10.1128/jb.169.7.3076-3081.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga ME, Larsen NA, Howard-Jones AR, Walsh CT, Walker GC. BluB cannibalizes flavin to form the lower ligand of vitamin B12. Nature. 2007;446:449–453. doi: 10.1038/nature05611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman WB, Wolfe RS. Purification and analysis of cobamides of Methanobacterium bryantii by high-performance liquid chromatography. Anal Biochem. 1984;137:261–265. doi: 10.1016/0003-2697(84)90380-4. [DOI] [PubMed] [Google Scholar]
- Wolin EA, Wolin MJ, Wolfe RS. Formation of methane by bacterial extracts. J Biol Chem. 1963;238:2882–2886. [PubMed] [Google Scholar]
- Yan J, Im J, Yang Y, Loffler FE. Guided cobalamin biosynthesis supports Dehalococcoides mccartyi reductive dechlorination activity. Philos Trans R Soc, B. 2013;368:20120320. doi: 10.1098/rstb.2012.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Ritalahti KM, Wagner DD, Loffler FE. Unexpected specificity of interspecies cobamide transfer from Geobacter spp. to organohalide-respiring Dehalococcoides mccartyi strains. Appl Environ Microbiol. 2012;78:6630–6636. doi: 10.1128/AEM.01535-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi S, Seth EC, Men YJ, Allen RH, Alvarez-Cohen L, Taga ME. Versatility in corrinoid salvaging and remodeling pathways supports the corrinoid-dependent metabolism of Dehalococcoides mccartyi. Appl Environ Microbiol. 2012;78:7745–7752. doi: 10.1128/AEM.02150-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.