Abstract
Purpose
The factors related to early-onset tumor recurrence in patients with spontaneously ruptured hepatocellular carcinoma (HCC) after hepatectomy remain unclear. The aims of the present study were to compare characteristics between early and late recurrence groups in spontaneously ruptured HCC patients who received curative hepatectomy and to identify risk factors for mortality.
Methods
We selected 19 patients who had been diagnosed with spontaneously ruptured HCC and who were treated with curative liver resection between 1998 and 2013. The 19 patients were divided into 2 groups: the early recurrence group of patients who experienced tumor recurrence within 12 months after hepatectomy, and the late recurrence group of patients who experienced recurrence after 12 months or who did not experience recurrence.
Results
The median tumor size was 7.4 cm, and there were no cases of postoperative mortality. Patient survival rates at 1, 3, and 5 years were 78.9%, 58.6%, and 58.6%, respectively. The incidence of tumor protrusion in the early recurrence group was higher than that in the late recurrence group (100% vs. 30%, respectively, P = 0.003). There were no statistically significant differences in other factors between the 2 groups. Multivariate analysis showed that greater than 30% protrusion of the tumor was a predictor of patient survival.
Conclusion
The results from the present study suggests that spontaneously ruptured HCC patients with protrusion should be frequently monitored after hepatectomy in order to achieve early detection of tumor recurrence and improve survival.
Keywords: Hepatocellular carcinoma, Rupture, Survival, Recurrence
INTRODUCTION
Spontaneous rupture of hepatocellular carcinoma (HCC) is a life-threatening complication with a high mortality rate in the range of 25% to 75% [1]. Although the incidence of spontaneous rupture is decreasing with the development of screening for early detection and advanced treatment of HCC, it remains as high as 10% to 14.5% in Asian countries compared to less than 5% in Western countries [2]. In Korea, the incidence of spontaneous rupture has been reported to be in the range of 7% to 12.9% [2,3]. If left untreated, median survival is only 1.2 to 4 months, and the prognosis is very poor because tumor cells spread to the peritoneum after HCC rupture, increasing the possibility of peritoneal metastasis [4].
Several studies have reported better prognosis with staged hepatectomy after primary hemostasis, but whether spontaneous rupture of HCC increases peritoneal recurrence rates remains unclear [4,5]. In addition, the factors related to early-onset tumor recurrence in patients with spontaneously ruptured HCC after hepatectomy have not yet been identified. In this study, we compared characteristics between early and late recurrence groups in spontaneously ruptured HCC patients with curative hepatectomy and identified the risk factors for mortality.
METHODS
Patients
This study included patients who underwent surgical resection of HCC between September 1998 and December 2013. This study was approved by the Samsung Medical Center Institutional Review Board and was performed according to the guidelines of the Helsinki Declaration. One hundred thirty-two patients were admitted to our hospital with a diagnosis of spontaneous rupture of HCC. The diagnosis of ruptured HCC was performed based on dynamic contrast-enhanced liver or abdominal CT with typical findings of extravasation of contrast material and hemoperitoneum. HCC was confirmed from the pathologic results after surgical resection. While 107 patients underwent only transarterial chemoembolization (TACE) for management of hemostasis for ruptured HCC, 24 patients underwent surgery for management of ruptured HCC with or without transarterial embolization (TAE). However, we excluded 5 patients from this study aimed to investigate the survival and recurrence rate after curative hepatectomy of ruptured HCC (1 patient with concomitant stomach and colon invasion with ruptured HCC was lost to follow-up, 2 patients underwent only palliative operations with multiple seeding lesions on the peritoneum, 1 patient had iatrogenic HCC rupture during liver mass biopsy and 1 patient underwent only evacuation and bleeding control with drainage). After excluding these 5 patients, 19 patients underwent curative hepatectomy and were included in this study. Demographic, preoperative, and pathologic data of 19 patients were collected from electronic medical records and retrospectively reviewed. Tumor recurrence and survival data were recorded.
We divided the 19 patients into 2 groups. The early recurrence group included intrahepatic or extrahepatic recurrence distinguished by follow-up CT or MRI within 12 months after hepatectomy, while the late recurrence group involved the other patients, including 3 recurrence-free patients. We followed the 19 patients from surgical intervention to the study end point, death, or recurrence. No patients in either group received postoperative adjuvant therapy before recurrence.
Surgery and pathology
The surgical and pathological procedures used after liver resection have been described previously [6,7]. Liver function was evaluated using the Child-Pugh classification system, and standard operative techniques for hepatectomy were used. Adequate mobilization was achieved based on the part of the liver to be resected. Selective clamping of the portal vein and hepatic artery was performed when feasible; if not, the intermittent Pringle maneuver was used. Parenchymal transection was performed using a Cavitron Ultrasonic Surgical Aspirator under low central venous pressure.
Postoperative histological assessment and reporting included maximal tumor size, differentiation, staging, encapsulation, intrahepatic metastasis, microvascular invasion, protrusion, and cirrhosis. The histologic differentiation of HCC was assigned according to the Edmonson-Steiner system as well-differentiated (grade I), moderately differentiated (grade II), or poorly differentiated (grades III, IV). Tumor staging was determined based on the American Joint Committee on Cancer, 7th edition, TNM classification. Tumor protrusion was calculated by dividing the length of the protruded mass from the liver surface by the tumor size. We measured the tumor size and length of the protruded tumor in the preoperative CT. Cirrhosis was defined as the presence of stage 4 fibrosis.
Surveillance after surgical resection
The procedures used for surveillance after liver resection were described previously [8]. All patients were followed postoperatively every 2 to 3 months. Follow-up parameters included physical examination, serum α-FP, liver function tests, and chest X-rays. Abdominal CT was performed every 3 months or when recurrence was suspected. MRI and/or PET scans were performed if CT did not show definitive evidence of recurrence. Patients with intrahepatic recurrences were treated with radiofrequency ablation, TACE, or sorafenib according to their functional liver reserve and the pattern of recurrence. The follow-up time was considered to be the length of time from surgery to last follow-up or death. No patients were lost to follow-up and all 19 patients were included in survival analysis.
Statistical analysis
All statistical analyses were performed using IBM SPSS Statistics ver. 21.0 (IBM Co., Armonk, NY, USA). Continuous variables are described as median with range, and categorical variables are expressed as number and percentage of subjects. Fisher exact test was conducted to evaluate differences in the frequencies of categorical variables between the groups. Mann-Whitney U analysis was conducted to evaluate the differences in the continuous variables between the 2 groups. The Kaplan-Meier survival method was performed to evaluate differences in patient survival between the two groups. To identify prognostic factors of patient survival, Cox regression analysis was performed. All tests were two-sided, and statistical significance was defined as P < 0.05
RESULTS
Baseline characteristics
Table 1 shows the baseline characteristics of patients who underwent curative hepatectomy for spontaneously ruptured HCC. Thirteen patients were male (73.7%), and the median age of 19 patients was 56 years (range, 31–81 years). There were no cases of postoperative mortality, and none of the patients had positive lymph nodes. The tumor stage of ruptured HCC was assessed based on tumor size and number excluding the rupture factor (T4). We observed eight patients with high tumor stage, and 11 patients had stage I or II tumors, all of which were solitary. Of 19 patients, only 2 were Child-Pugh class B and 17 were Child-Pugh class A. The median tumor size was 7.4 cm (range, 4–14 cm). Fourteen patients (73.7%) underwent staged hepatectomy after TAE for hemostasis. Among these 14 patients, two underwent laparoscopic right hemihepatectomy and laparoscopic left hemihepatectomy, respectively. Major hepatectomy was conducted in nine patients (7 right hepatectomy, 2 left hepatectomy). Minor hepatectomy was performed in 10 patients (6 left lateral sectionectomy, 3 right sectionectomy, 1 tumorectomy).
Table 1. Characteristics of spontaneously ruptured hepatocellular carcinoma patients.
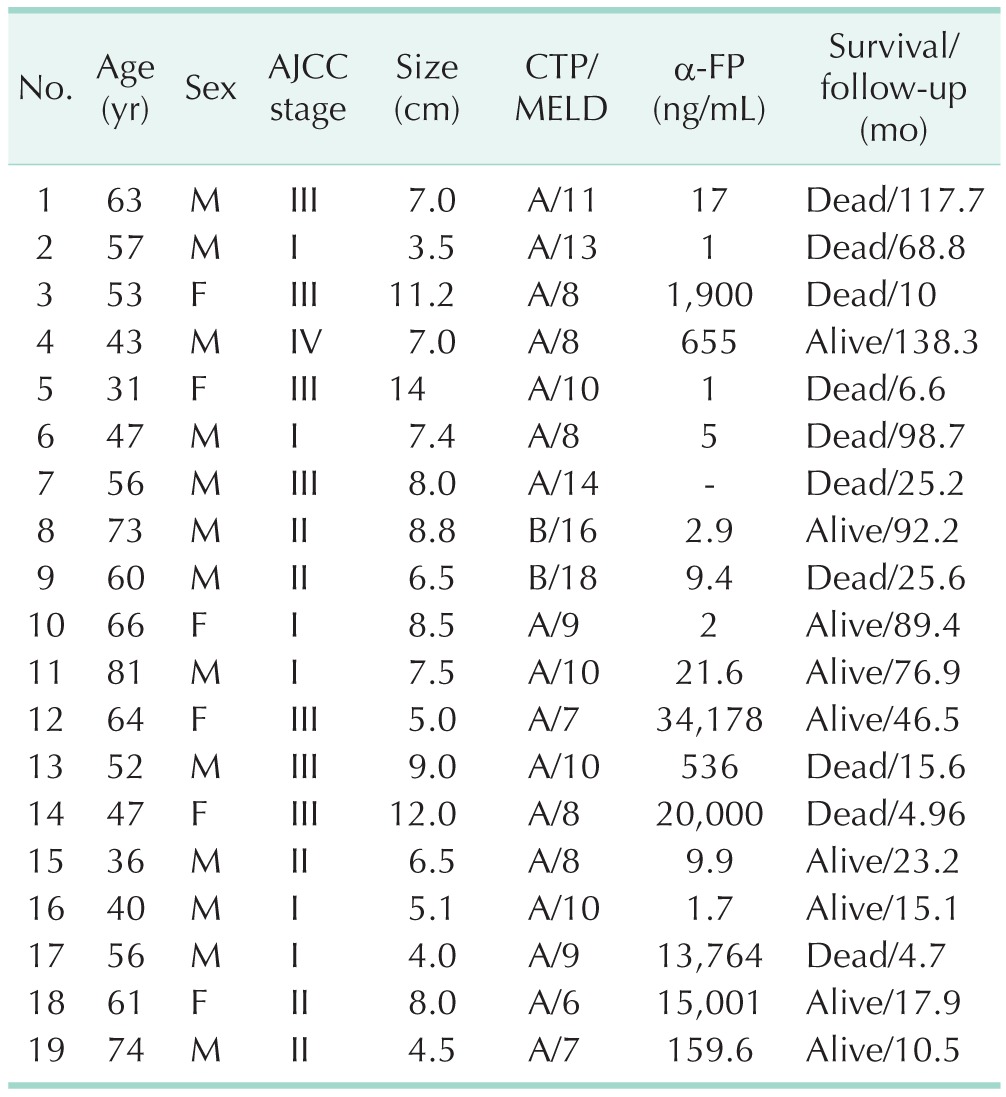
AJCC, American Joint Committee on Cancer; CTP, Child-Turcotte-Pugh; MELD, model for end-stage liver disease; α-FP, α-fetoprotein.
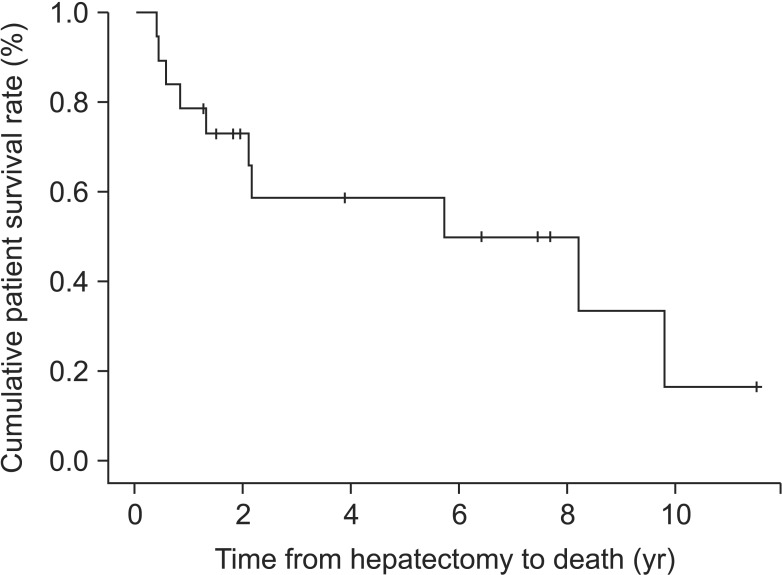
The patient survival rates at 1, 3, and 5 years were 78.9%, 58.6%, and 58.6%, respectively (Fig. 1). Four patients died within 12 months after surgery. Sixteen patients developed HCC recurrence, but tumor recurrence was not detected in 3 patients after surgery. In HCC recurrence, 9 patients had intrahepatic metastasis, five had extrahepatic metastasis, and 2 had synchronous intrahepatic and extrahepatic metastasis. Aside from 2 patients with synchronous intra and extrahepatic metastasis, 5 of 9 patients (55.6%) with intrahepatic metastasis experienced extrahepatic metastasis within 12 months. However, there were no cases of tumor extension from extrahepatic to intrahepatic metastasis. The most common sites of extrahepatic metastasis were lung (n = 9, 81.8%) or peritoneal seeding (n = 5, 45.5%) including 2 patients (18%) with diaphragm invasion and metastatic lymph node and 1 case of inferior vena cava with tumor thrombi. Among three tumor recurrence-free patients, 1 underwent curative right hepatectomy with right adrenalectomy because of direct invasion to the right adrenal gland with stage IV disease.
Fig. 1. Patient survival in spontaneous hepatocellular carcinoma rupture after curative hepatectomy.
Comparison between early and late recurrence groups
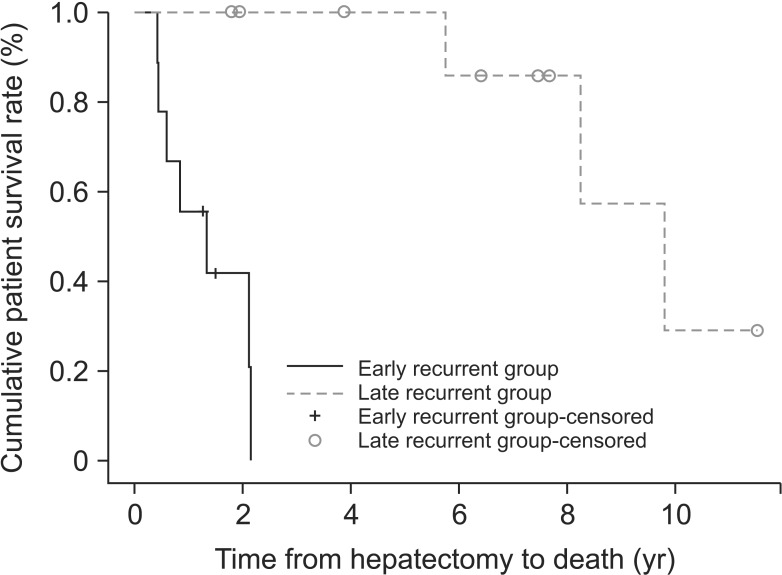
The characteristics of the early and late recurrence groups are summarized in Table 2. The 2 groups showed significant differences in frequency of greater than 30% tumor protrusion (100% vs. 30%; P = 0.003). Regarding age, etiology, Child-Pugh class, model for end-stage liver disease score, α-FP, preoperative TAE, operation time, estimated blood loss, tumor size, tumor number, portal vein tumor thrombosis, differentiation, intrahepatic metastasis, microvascular invasion, encapsulization, cirrhosis, and T stage, there were no statistically significant differences between the 2 groups. We identified the response to preoperative TACE (n = 14) between the 2 groups (Table 3). For seven patients, we could not determine the response to TACE because they did not undergo follow-up CT after preoperative TACE. The 1-, 2-, and 3-year patient survival rates in the early recurrence group were 55.6%, 41.7%, and 0%, respectively, while the 5-year patient survival rate was 100% in the late recurrence group (Fig. 2).
Table 2. Characteristics of spontaneously ruptured hepatocellular carcinoma (HCC) patients according to onset of recurrence.
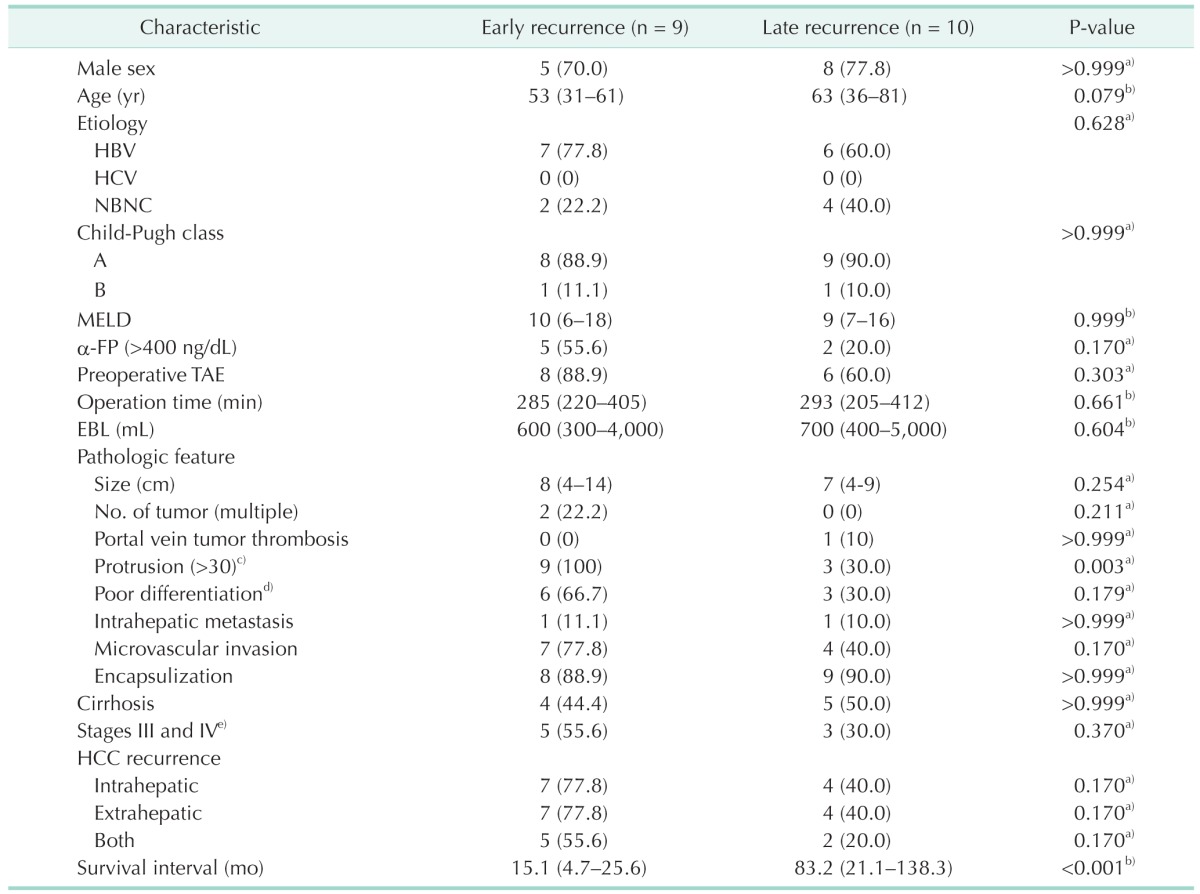
Values are presented as number (%) or median (range).
NBNC, non-B, non-C; MELD, model for end stage liver disease; TAE, transarterial embolization; EBL, expected blood loss.
a)Fisher exact test. b)Mann-Whitney U-test. c)(length of protruded tumor from liver surface/tumor size) × 100. d)Edmondson grade III/IV. e)American Joint Committee on Cancer 7th edition.
Table 3. Response to preoperative transarterial chemoembolization between early and late recurrence groups.
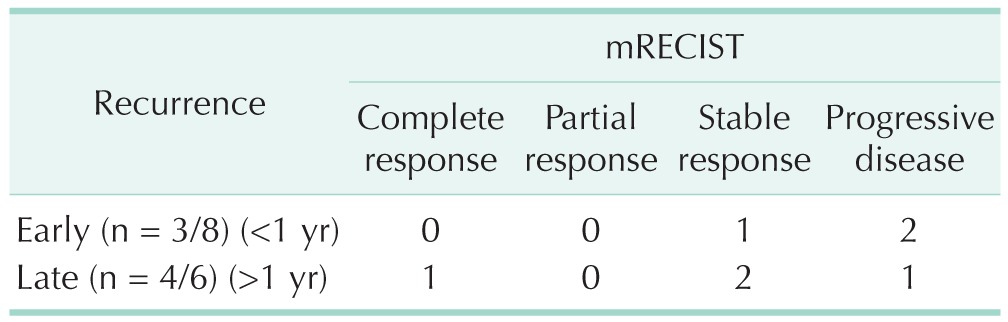
mRECIST, modified response evaluation criteria in solid tumors.
Fig. 2. Patient survival between early and late recurrence groups. P < 0.001.
On univariate and multivariate analyses, greater than 30% protrusion of the tumor was significantly associated with patient survival (Table 4).
Table 4. Cox regression analysis of prognostic factors for overall survival.
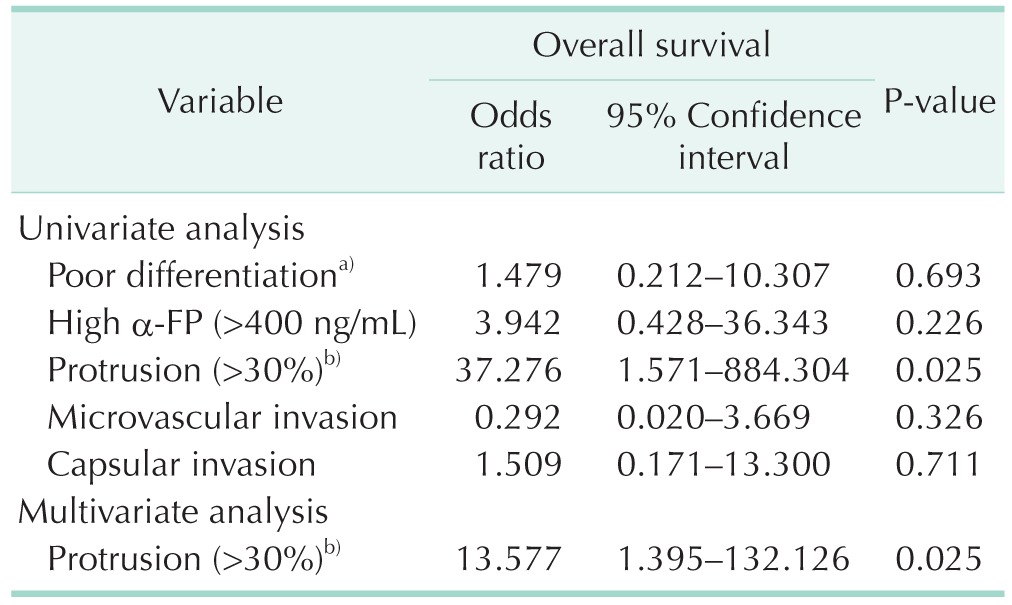
a)Edmondson grade III/IV. b)(length of protruded tumor from liver surface/tumor size) × 100.
DISCUSSION
In the present study, the 5-year survival rate was 58.6% after curative hepatectomy in spontaneously ruptured HCC patients, and three patients were alive without recurrence. We hypothesized that there are important factors contributing to survival, so we divided the tumor recurrence group based on a period of 12 months after surgical resection. In the present study, when comparing the early recurrence group and the late recurrence group, greater than 30% of the tumor size from the liver surface was seen in all 9 patients in the early recurrence group and only in 3 patients in the late recurrence group (100% vs. 30%, respectively; P = 0.003). We also observed a significantly different survival curve in the late recurrence group compared to the early recurrence group (P < 0.001). When we investigated the prognostic factors related to patient survival, our results revealed that protrusion was a significant prognostic factor.
The results of hepatectomy in spontaneously ruptured HCC patients are controversial because several studies have reported similar outcomes after hepatectomy between ruptured and nonruptured HCC groups [4,5,9]. However, recent study reported that patient survival and disease-free survival in ruptured HCC after liver resection were lower than in cases of nonruptured HCC [10]. In a nationwide survey of large ruptured HCC cases from Japan, tumor rupture itself was observed to have a negative impact on survival [11]. However, there were no significant differences between ruptured and nonruptured HCC groups in not only patient survival rate, but also disease-free survival rate after recalibration of patient characteristics [11].
In the present study, we compared ruptured HCC cases only, which could indicate a somewhat different natural course of intra-parenchymal HCC. Our findings could be useful for improving the treatment of ruptured HCC. In a Japanese nationwide survey [11], the overall survival curves showed significantly different patterns. The slope of the survival curve in ruptured HCC showed a steep decline, with more than 70% of ruptured patients dying within 12 months. After 12 months, the slope of the survival curve gradually declined. These patterns were similar to those of our study. Spontaneously ruptured HCC patients selectively underwent liver resection, which may positively affect survival. Our results encourage an aggressively surgical approach in HCC patients with spontaneous rupture.
Although the exact mechanism of HCC rupture is not clear, it appears to be more frequent when the tumor is peripherally located [12], possibly due to rapid growth with necrosis, erosion of vessels, increased intratumor pressure with hepatic veins occlusion due to tumor thrombi, or direct invasion individually or combined and coagulopathy Several studies have reported that vascular dysfunction, which results from degeneration of elastin and type IV collagen, might be associated with rupture of HCC [13]. Our study revealed that protrusion may be associated with rapid growth of tumor cells and poor tumor biology.
The present study had several limitations. First, this was a retrospective study with a small number of patients. Second, this study included a select group of patients who underwent hepatic resection and who had good liver function.
In conclusion, the present study showed a 5-year patient survival rate of 50% in patients with spontaneously ruptured HCC after liver resection, and encouraged surgical treatment in selected patients. HCC protrusion was a predictor of early recurrence after hepatectomy.
Footnotes
CONFLICTS OF INTEREST: No potential conflict of interest relevant to this article was reported.
References
- 1.Han XJ, Su HY, Shao HB, Xu K. Prognostic factors of spontaneously ruptured hepatocellular carcinoma. World J Gastroenterol. 2015;21:7488–7494. doi: 10.3748/wjg.v21.i24.7488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Min HJ, Lee OJ, Kang DY, Lee EJ, Lee JH, Kim HJ, et al. The clinical study on spontaneously ruptured hepatocellular carcinoma. Korean J Gastroenterol. 2004;44:160–167. [PubMed] [Google Scholar]
- 3.Kim YI, Ki HS, Kim MH, Cho DK, Cho SB, Joo YE, et al. Analysis of the clinical characteristics and prognostic factors of ruptured hepatocellular carcinoma. Korean J Hepatol. 2009;15:148–158. doi: 10.3350/kjhep.2009.15.2.148. [DOI] [PubMed] [Google Scholar]
- 4.Lee HS, Choi GH, Kang DR, Han KH, Ahn SH, Kim DY, et al. Impact of spontaneous hepatocellular carcinoma rupture on recurrence pattern and long-term surgical outcomes after partial hepatectomy. World J Surg. 2014;38:2070–2078. doi: 10.1007/s00268-014-2502-6. [DOI] [PubMed] [Google Scholar]
- 5.Mizuno S, Yamagiwa K, Ogawa T, Tabata M, Yokoi H, Isaji S, et al. Are the results of surgical treatment of hepatocellular carcinoma poor if the tumor has spontaneously ruptured? Scand J Gastroenterol. 2004;39:567–570. doi: 10.1080/00365520410005135. [DOI] [PubMed] [Google Scholar]
- 6.Kim JM, Kwon CH, Joh JW, Park JB, Lee JH, Kim SJ, et al. Outcomes after curative hepatectomy in patients with non-B non-C hepatocellular carcinoma and hepatitis B virus hepatocellular carcinoma from non-cirrhotic liver. J Surg Oncol. 2014;110:976–981. doi: 10.1002/jso.23772. [DOI] [PubMed] [Google Scholar]
- 7.Kim JM, Kwon CH, Joh JW, Park JB, Lee JH, Kim SJ, et al. Differences between hepatocellular carcinoma and hepatitis B virus infection in patients with and without cirrhosis. Ann Surg Oncol. 2014;21:458–465. doi: 10.1245/s10434-013-3302-1. [DOI] [PubMed] [Google Scholar]
- 8.Kim JM, Kwon CH, Joh JW, Park JB, Ko JS, Lee JH, et al. The effect of alkaline phosphatase and intrahepatic metastases in large hepatocellular carcinoma. World J Surg Oncol. 2013;11:40. doi: 10.1186/1477-7819-11-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yeh CN, Lee WC, Jeng LB, Chen MF, Yu MC. Spontaneous tumour rupture and prognosis in patients with hepatocellular carcinoma. Br J Surg. 2002;89:1125–1129. doi: 10.1046/j.1365-2168.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- 10.Chan AC, Dai JW, Chok KS, Cheung TT, Lo CM. Prognostic influence of spontaneous tumor rupture on hepatocellular carcinoma after interval hepatectomy. Surgery. 2016;159:409–417. doi: 10.1016/j.surg.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Aoki T, Kokudo N, Matsuyama Y, Izumi N, Ichida T, Kudo M, et al. Prognostic impact of spontaneous tumor rupture in patients with hepatocellular carcinoma: an analysis of 1160 cases from a nationwide survey. Ann Surg. 2014;259:532–542. doi: 10.1097/SLA.0b013e31828846de. [DOI] [PubMed] [Google Scholar]
- 12.Vergara V, Muratore A, Bouzari H, Polastri R, Ferrero A, Galatola G, et al. Spontaneous rupture of hepatocelluar carcinoma: surgical resection and long-term survival. Eur J Surg Oncol. 2000;26:770–772. doi: 10.1053/ejso.2000.1001. [DOI] [PubMed] [Google Scholar]
- 13.Zhu LX, Geng XP, Fan ST. Spontaneous rupture of hepatocellular carcinoma and vascular injury. Arch Surg. 2001;136:682–687. doi: 10.1001/archsurg.136.6.682. [DOI] [PubMed] [Google Scholar]