Abstract
Background
Takotsubo cardiomyopathy (TTC) is characterized by reversible left ventricular dysfunction, frequently precipitated by a stressful event. Despite the favorable course and good long-term prognosis, a variety of complications may occur in the acute phase of the disease. The aim of this study was to evaluate the in-hospital and long-term outcomes of a cohort of TTC patients.
Methods
Fifty-five patients (mean age 68.1±12 years) were prospectively followed for a mean of 69.6±32.2 months (64,635 days). In-hospital (death, heart failure, arrhythmias) and long-term events (death and recurrences) were recorded.
Results
Patients were predominantly women (87.3%) who experienced a recent stressful event (emotional or physical) and were admitted to hospital for chest pain. Eleven patients (20%) had a diagnosis of depressive disorder, and arterial hypertension was the most frequent cardiovascular risk factor. The ECG revealed ST-segment elevation in 43.6% of patients. At angiography, seven cases (12.7%) had at least one significant (≥50%) coronary artery stenosis and four patients (7.3%) had myocardial bridging of the left anterior descending artery. During hospitalization, three patients died (one from cardiac causes) and cardiovascular complications occurred in 12 patients. During follow-up, five patients died (none from cardiac causes), six patients had recurrences within the first year. Two patients had two recurrences: one after 114 days, triggered by an asthma attack as the first event, and the other after 1,850 days.
Conclusions
In TTC patients, in-hospital and long-term mortality is primarily due to non-cardiovascular causes. Recurrences are not infrequent and coronary artery disease is not an uncommon finding.
Keywords: coronary artery disease, follow-up study, recurrence, Takotsubo cardiomyopathy
Takotsubo cardiomyopathy (TTC), also known as stress cardiomyopathy, is an acute cardiac syndrome mimicking acute myocardial infarction. It is characterized by chest pain, electrocardiographic changes and transient akinesia/dyskinesia of the left ventricle (apical and mid-ventricular segments) with wall motion abnormalities extending beyond a single vascular territory (1–5). TTC is typically triggered by an acute emotional or physical stress event and occurs mostly in postmenopausal women. Although TTC has a favorable long-term prognosis and usually resolves spontaneously within a few days to several weeks (6), there are conflicting data on in-hospital complications (ranging from 22 to 50%) (6, 7) and early mortality rates (8). Currently, TTC is being diagnosed more frequently, likely because of heightened awareness of this syndrome with the need of a more specific approach.
The aim of this study was to evaluate the clinical and diagnostic features, the short and long-term outcomes of a cohort of TTC patients followed at a single center for 12.9 years.
Methods
Fifty-five TTC patients admitted to the San Daniele Community Hospital (Italy) or followed as outpatients in the cardiology service from May 2003 to August 2014 were included in the study. On admission, demographics, triggering stressful events, clinical history, cardiovascular risk factors, and presenting symptoms were collected in all patients using a uniform definition, as well as clinical data previously described (6). Cardiac catheterization was performed within 48–72 h of admission to the cath lab of the referral hospital (9).
TTC diagnosis and inclusion and exclusion criteria were based on the modified Mayo Clinic criteria (10) and the Takotsubo Italian Network (TIN) (11).
Echocardiography
Two-dimensional echocardiography was performed in all patients on admission, and LV ejection fraction (EF) was calculated using the biplane method of disks (modified Simpson's rule). The wall motion score index (WMSI) was visually assessed on multiple views as in routine clinical practice. The left ventricle was divided into 17 segments as recommended by the American Heart Association (12). Right ventricular (RV) involvement was assessed visually (akinesia or dyskinesia of the mid or apical RV free wall segments). Mitral regurgitation was graded based on color-Doppler flow imaging in the parasternal and apical views (3, 4).
Cardiac catheterization
Cardiac catheterization was performed within 48–72 h of hospital admission using the femoral or radial approach. Significant coronary artery disease was defined as ≥50% luminal diameter narrowing, by visual assessment, of at least one major epicardial artery (13). Myocardial bridging was diagnosed if the typical angiographic ‘milking effect’ (i.e., systolic compression and complete or partial decompression during diastole) was observed in the coronary artery (14–17). Follow-up was performed by outpatient clinic visits or telephone interview, and relapses and all-cause mortality were recorded. All patients gave written informed consent.
Statistical analysis
Descriptive statistics for clinical characteristics are presented as means and SDs for continuous data and as counts and percentages for dichotomous and categorical data. Paired Student's t-test or non-parametric test was used for statistical analysis, as appropriate. A survival analysis using the Kaplan–Meier estimator was performed to determine the proportion of TTC patients who died. SYSTAT 12.0 (Systat Software Inc., San Jose, CA) was used for data analysis.
Results
A total of 55 patients were diagnosed as having TTC (Table 1) and were followed up for 12.9 years. They were predominantly women (n=48, 87.3%; age range: 41–87 years), more frequently of postmenopausal age; 89.5% were older than 65 years, and 10.4% were younger than 50 years. Mean follow-up was 69.6±32.2 months. Eleven patients (20%) were admitted with a diagnosis of anxiety or depressive disorder. According to medical history, the majority of patients had experienced a recent stressful event, either emotional or physical, and the most common clinical symptom at presentation was chest pain. Systemic arterial hypertension was the most frequent cardiovascular risk factor (Table 1). Clinical and laboratory findings are reported in Table 2. The ECG revealed ST-segment elevation in a high proportion of patients (43.6%).
Table 1.
Baseline characteristics of the study population
| No. of patients | 55 |
| Age (years) | 68.2±11.4 |
| Female gender | 48 (87.3%) |
| Cardiovascular risk factors | |
| Hypertension | 36 (65.4%) |
| Diabetes mellitus | 10 (18.1%) |
| Dyslipidemia | 22 (40.0%) |
| Smoking | 10 (18.1%) |
| History of coronary artery disease | 4 (7.2%) |
| Menopause | 45 (90%) |
| Presenting symptom | |
| Chest pain | 36 (65.4%) |
| Dyspnea | 6 (10.0%) |
| Chest pain+dyspnea | 13 (23.6%) |
| Triggering factor | |
| Emotional stress | 25 (45.4%) |
| Physical stress | 13 (23.6) |
| None | 17 (30%) |
Table 2.
Clinical and laboratory findings on admission and in-hospital complications and procedures
| Hemodynamic data | |
| Systolic BP (mmHg) | 142.4±35 |
| Diastolic BP (mmHg) | 84.7±16.8 |
| Heart rate (bpm) | 87.1±21.8 |
| Laboratory findings | |
| Peak troponin I (µg/ml) | 3.16±4.5 |
| White blood count (×103/µl) | 9.61±4.82 |
| Creatinine (mg/dl) | 1.21±1,26 |
| Hemoglobin (g/dl) | 12.9±1.7 |
| ECG | |
| ST-segment elevation | 24 (43.6%) |
| QT interval (ms) | 376.5±52.4 |
| Echocardiography | |
| Mitral regurgitation Moderate to severe |
5 (9%) |
| Mild | 26 (47.3%) |
| LVOT gradient | 1 (1.8%) |
| Right ventricular involvement | 2 (3.6%) |
| LVEF (%) | 44.8±11.6 |
| Mid-apical involvement | 27 (56%) |
| Apical involvement | 20 (41.7%) |
| WMSI | 1.66±2.29 |
| PASP (mmHg) | 36.3±16.5 |
| In-hospital complications | 12 (21.8%) |
| Cardiogenic shock | 4 (7.2%) |
| Congestive heart failure | 3 (5.4%) |
| Intraventricular thrombus | 1 (1.8%) |
| Cerebral Ischemic lesion | 1 (1.8%) |
| Pulmonary artery systolic hypertension | 1 (1.8%) |
| Paroxysmal atrial fibrillation | 2 (3.6%) |
| In-hospital procedures | 3 (5.4%) |
| Stent implantation | 2 (3.6%) |
| CABG | 1 (1.8%) |
BP=blood pressure; LVOT=left ventricular outflow tract; LVEF=left ventricular ejection fraction; WMSI=wall motion score index; PASP=pulmonary artery systolic pressure; CABG=coronary artery bypass grafting.
Echocardiography
In 48 patients, left ventricular ejection fraction (LVEF) was low on admission but improved at discharge (43.7±11.6% vs. 55.5±9.0%, p<0.0001). Two patients had RV involvement. Five patients (9%) had moderate to severe mitral regurgitation, and 26 (47.3%) had mild mitral regurgitation (Table 2). In 38 patients, pre-discharge echocardiography showed complete recovery of LV and RV regional wall motion abnormalities.
Coronary angiography
Among the 55 TTC patients, coronary angiography revealed significant coronary stenosis (≥50%) in seven cases (12.7%) and a myocardial bridge within the intramural course of the left anterior descending coronary artery in four cases (7.3%). All of the seven patients with critical coronary lesions had one-vessel disease (60% occlusion of the right coronary artery in 1, complete occlusion of the posterior descending artery in 1, and left anterior descending coronary artery with hemodynamically significant lesions in 5). Five lesions showed moderate coronary stenosis (50–75%), and four lesions showed severe coronary stenosis (>75%), including one chronic total occlusion of the posterior descending branch.
In-hospital management, complications, and mortality
In the acute phase, patients received aspirin (100%), clopidogrel (67%), heparin (100%), beta-blockers (73%), statins (43.7%), nitrates (18.7%), proton pump inhibitors (100%), diuretics (12%), intravenous inotropic agents (8.3%), and antiarrhythmic drugs (5%). Complications occurred in 12 patients (21.8%), with heart failure being the most frequent one (Table 2). One patient had two stents and one patient had one stent implanted in the left anterior descending coronary artery because of a critical stenosis on coronary angiography, and another patient underwent coronary artery bypass surgery.
Three patients died during hospitalization (Table 3), two from non-cardiac causes and one from a massive cerebral hemorrhagic stroke within 12 h of hospital admission. Heart rate and cardiac troponin I levels were slightly lower and LVEF higher in survivors than in non-survivors, though without reaching statistical significance.
Table 3.
In-hospital and long-term mortality and relapse
| In-hospital mortality | 3 (5.4%) |
| Age (years) | 65.7±10.7 |
| Time to death (days) | 20 (6–45) |
| LVEF (%) | 34.3±0.6 |
| Troponin I (ng/ml) | 4.2±4.0 |
| Cause of death | |
| Acute bone marrow aplasia | 1 |
| Multiorgan failure | 1 |
| Cerebral hemorrhagic stroke | 1 |
| Long-term mortality | 5 (9%) |
| Age (years) | 74.6±6.2 |
| Time to death (days) | 953 (477–1,415) |
| LVEF (%) | 51±8.9 |
| Troponin I (ng/ml) | 7.6±12.0 |
| Cause of death | |
| Femoral fracture and worsening of pre-existing disease | 2 |
| Intestinal occlusion | 2 |
| Complications of respiratory failure | 1 |
| Relapse | 6 (10.9%) |
| Age (years) | 71.2±7.3 |
| Time to relapse (days) | 89.2 (8–243) |
| LVEF (%) | 53,0±15.1 |
| Troponin I (ng/ml) | 0.8±2.1 |
| Trigger event | |
| Asthma attack | 2 |
| No trigger event | 4 |
LVEF=left ventricular ejection fraction.
Follow-up and long-term mortality
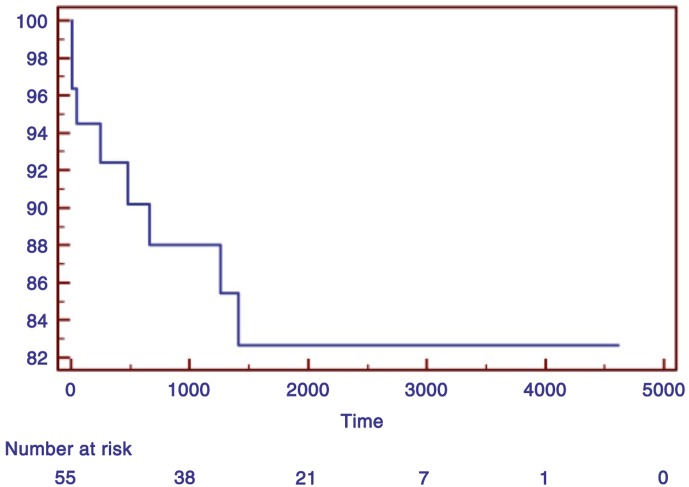
The 52 patients who survived were followed up, and data were 100% complete. Five additional patients died during the follow-up but none from cardiovascular causes (Table 3). Non-survivors were older than survivors, but they did not differ in cardiovascular risk factors (p=ns for systemic arterial hypertension, diabetes, serum cholesterol, and smoking). TTC did not contribute to death in those who died in hospital, and all deaths after discharge were unrelated to cardiac illness. Fatal events occurred within the first 4 years of follow-up (Fig. 1).
Fig. 1.
Kaplan–Meier survival curve.
Relapse
TTC recurrence was experienced by six patients (Table 3). Time to relapse was within the first year (mean 3 months). Consistent with a previous study from our group (7), LVEF and WMSI were less compromised, troponin I levels were lower than those observed during the first episode, and there was complete recovery of LV function with WMSI improvement. Two patients had two recurrences: after 114 days in one patient, triggered by an asthma attack as the first event, and after 1,850 days in the other, in the absence of a trigger event. In the latter, angiography was repeated but no significant coronary lesions were detected.
Discussion
TTC is not rare and is an increasingly recognized clinical syndrome, though probably still underestimated. In the United States, in 2008, TTC was diagnosed in about 0.02% of all hospitalizations (18) and was the final diagnosis in 2% of patients with acute coronary syndrome (19). Recurrence rates were reported to range from 0 to 15% (1, 7). Results from a previous study by our group are consistent with all these data (9). Although TTC is generally considered as a benign cardiac condition with favorable long-term prognosis, complications during the acute phase of the disease have been variably reported (6, 9, 20–24). There is growing and considerable evidence suggesting that enhanced sympathetic activity plays a central role in the pathogenesis of Takotsubo cardiomyopathy: marked elevation in plasma catecholamines (compared to other types of ACS), increased heart rate variability, association with high catecholamine states (e.g., pheochromocytoma), and nuclear imaging showing increased sympathetic activity in the akinetic segments (25) have all been demonstrated in TTC.
The present series of TTC patients is among the few studies with very long-term follow-up. Clinical characteristics of the enrolled population were similar to those previously described by our group and others: most patients were postmenopausal women, the main cardiovascular risk factor was arterial hypertension, and 20% of the patients were admitted with a diagnosis of anxiety or depression. On admission, TTC patients showed depressed LVEF that improved before discharge, wall motion abnormalities extended beyond a single epicardial vascular distribution, and 12.7% had at least one significant coronary lesion. In-hospital complications, including three deaths (5.4%) with only one death due to cardiovascular causes, occurred in 21.8% of the patients. Six patients (11.5%) experienced recurrences within the first 3 months and two patients had two episodes of TTC. Long-term mortality (9%) was due to worsening of preexisting disease.
Anxiety-depressive disorders
Compared to other reports (26, 27), we found less TTC patients affected by anxiety-depressive disorders (21.8%) probably because, aside the trigger event, patients were not specifically questioned about their lifestyle or chronic psychological stress (26, 28, 29). High-anxiety trait in TTC patients is not significantly more frequent than in patients with ST-elevation myocardial infarction and seems not to be associated with a worse clinical outcome in both pathologies (28). However, in a series of 1,750 TTC patients, Templin et al. (30) reported a history of neurologic or psychiatric disorders in more than 50%, with a high incidence of psychiatric illness identified as affective disorder.
Myocardial bridging
In our population, myocardial bridging was observed in 7.3% of cases. This proportion is consistent with previous findings in a large cohort (average 5%, range: 0.5–16%) (15) but is much less than the 62.5 and 76% prevalence reported in a small series by Lemaitre et al. (31) and by Migliore et al. (32), respectively, likely due to the use of cardiac computed tomography, which is far more sensitive than angiography in identifying this congenital coronary anomaly. Notwithstanding this, Stiermaier et al. (33) found no differences in the prevalence of myocardial bridging between TTC patients and controls. Several reports have also suggested that myocardial bridging may be an underlying mechanism of apical ballooning (31, 33–35).
Coronary atherosclerosis
In our series, 12.5% of patients had significant coronary artery stenosis which is consistent with previous findings in large series by Parodi et al. (5) and Templin et al. (30). The TIN investigators showed that the extension of myocardial dysfunction clearly exceeded the area supplied by a single epicardial vessel (5). Given that TTC affects predominantly postmenopausal women with cardiovascular risk factors, the presence of significant coronary artery disease should not be considered an unexpected finding (18, 36).
In-hospital complications
Although TTC is considered a benign reversible condition, it poses a non-trivial risk for adverse events. In our series, complications occurred in 21.8% of the study population, with heart failure being the most frequent one. Early mortality was not negligible (5.4%) compared to the previous reports (from 1.7 to 2.8%) (6, 7, 37), and two deaths were not related to cardiovascular events. In a large TTC group evaluated by Templin et al. (30), complications occurred in 21.8% of patients, and Citro et al. (6) reported in-hospital complications up to 50% of those older than 74 years. Age, multiorgan impairment with irreversible non-cardiac conditions associated with low LVEF, and high troponin adversely affected short-term survival.
Recurrences
Recurrences were recorded in six patients (10.9%) within the first 3 months and two patients had two episodes of TTC. Consistent with our previous experience (9), TTC recurrences were experienced by patients on β-blockers, but cardiac involvement was less severe than at the time of the first event. It may be hypothesized that, although β-blockers are not effective in preventing TTC, they might mitigate its clinical manifestations. In the literature, recurrences are reported to range from 0 to 11.4% up to 4–5 years after the first episode (1, 5, 7, 20–23, 38–48), and relapses are unpredictable. Moreover, patients with recurrences may show different LV patterns, with apical ballooning during the first episode and mid-ventricular ballooning during the second one.
Long-term mortality
In the present study, long-term mortality was 9% and death occurred within the first 2.6 years. No death was due to cardiac causes, suggesting that mortality was independent of TTC. In reviewing available literature on long-term follow-up of TTC, mortality rates differed considerably across studies probably because of either different follow-up lengths and sample groups or the older age of our population (Table 4) (1, 5, 7, 8, 20–23, 30, 38–50). Although in the study of Parodi et al. (5), the results of the standardized mortality ratio analysis showed that TTC patients are three times more likely to die than the general population, TTC seems to be associated with a long-term survival rate that is intermediate between the one of the general population and the one of patients with ST-elevation myocardial infarction. Comorbidities are the main predictors of mortality in TTC patients.
Table 4.
In-hospital and long-term outcome of Takotsubo cardiomyopathy in previous studies
| In-hospital mortality n (%) |
Long-term mortality (n,%) |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Author, year | Patients F/M n (F%) |
Age (years) | Country | CV death | Other causes | Follow-up (months) | Recurrences n (%) | CV death | Other causes |
| Tsuchihashi et al. (1), 2001 |
88 76/12 (86) |
67±13 | Japan | 1 (1) |
0 | 13±14 | 2 (2.7) |
1 (1.3) |
0 |
| Elesber et al. (38), 2007 |
100 95/5 (95) |
66±13 | USA | 2 (1) |
0 | 26±12 | 10 (11.4) |
7 (7) |
10 (10) |
| Burgdorf et al. (39), 2008 |
50 47/3 (94) |
70±10 | Europe | 0 | 3 (6) |
35±19 | – | 3 (6) |
3 (6) |
| Eshtehardi et al. (40), 2009 |
41 35/6 (85) |
65±11 | Europe | 0 | 0 | 23±10 | 2 (5) |
0 | 1 (2.4) |
| Regnante et al. (41), 2009 |
70 67/3 (95) |
67±11 | USA | 1 (1.4) |
0 | 12 | 2 (2.9) |
0 | 2 (2.9) |
| Sharkey et al. (7), 2010 |
136 130/6 (96) |
68±13 | USA | 2 (1.5) |
1 (0.7) |
27.6±24 | 7 (5) |
0 | 17 (12.5) |
| Previtali et al. (42), 2011 |
132 125/7 (98) |
67±11 | Europe | 1 (0.8) |
0 | 13 | 2 (1.5) |
0 | 1 (0.8) |
| Parodi et al. (5), 2011 |
116 106/10 (91) |
73±10 | Italy | 1 | 1 | 24±15 | 2 | 7 (6) | 4 (3) |
| Eitel et al. (43), 2011 |
256 227/29 (89) |
69±12 | Europe/ North America |
3 (1.1) |
1 (0.4) |
1 to 6 | – | 2 (0.8) |
2 (0.8) |
| Samardhi et al. (20), 2012 |
52 51/1 (98) |
64 (43–89) |
Australia | 0 | 0 | 32.66 | 0 | 0 | 0 |
| Looi et al. (44), 2012 |
100 95/5 (95) |
65±11 | New Zealand | 1 (1) |
0 | 36±20 | 7 (7) |
0 | 4 (4) |
| Cacciotti et al. (45), 2012 |
75 73/2 (97.3) |
71.9±9.6 | Europe | 0 |
0 | 26.4±24 | 1 (1.3) |
2 (2.6) |
0 |
| Song et al. (46), 2012 |
137 101/36 (74) |
59 | Korea | 0 | 0 | 68.4 | 0 | 0 | 9 (6.5) |
| Núñez-Gil et al. (23), 2012 |
100 89/11 (89) |
68±13 | Spain | 0 | 0 | 46 | 4 (4) |
3 (3) |
3 (3) |
| Buja et al. (47), 2012 |
54 47/7 (87) |
72.1 | Italy | 1 (1.85) |
1 (1.85) |
18.5 | 2 (3.9) |
2 (3.9) |
2 (3.9) |
| Weihs et al. (21), 2013 |
179 168/11 (94) |
69±11 | Austria | 1 (0.6) |
– | 36.5±18.9 |
4 (2) |
3 (1.6) |
10 (5.5) |
| Ribeiro et al. (22), 2014 |
37 35/2 (94) |
63±13 | Portugal | 0 | 0 | 16±17 |
0 | 1 (2.8) |
0 |
| Vizzardi et al. (48), 2015 |
42 100 (100) |
67±11 | Italy | 0 | 0 | 12 | 0 | 0 | 0 |
| Redfors et al. (8) 2015 |
302 255/47 (84%) |
66±12 | Sweden |
(4) |
36 | 0 | (12) | ||
| Templin et al. (30) 2015 |
1,750 1,571/179 (89.9%) |
66.4±13.1 | Switzerland | 77 (4.1%) |
12 | 1.8% per year | 5.6% per year | ||
| Gopalakrishnan et al (49) 2015 |
56 45/11 (80.4%) |
65.8±14.12 | USA | 5 Not specified |
27.6±21,6 | not reported | 10 Not specified |
||
| Present study |
55 43/5 (89%) |
68.2±11.4 | Italy | 1 | 2 | 69.6±32.2 | 6 | 0 | 5 |
CV=cardiovascular; F=female; M=male.
Study limitations
Several limitations should be acknowledged. First, this is a single-center study, and results cannot be generalized to other ethnic groups. Second, the sample size is relatively small, even though the follow-up period is very long. Third, this is a clinical study and the diagnostic procedures applied were those used in everyday clinical practice and not addressed for answering a specific comorbidity or complication.
Conclusions
The present study is one with the longest follow-up reported to date in TTC patients of a community hospital and takes into account a wide range of clinical and diagnostic features. Anxiety-depressive disorders were not particularly frequent in our TTC patients, and the most frequent acute complication was heart failure. Recurrences mostly occurred within the first 3 months with less severe cardiac involvement and were experienced by patients on β-blockers. Significant coronary artery disease was not an uncommon finding, and angiographic prevalence of myocardial bridging did not differ from that reported in other non-TTC series. Both in-hospital and long-term mortality were confined to patients with malignancies or non-cardiovascular diseases, suggesting an important role of the underlying pathology in patient outcome. TTC did not contribute to death in those who died in hospital, and all deaths after discharge were unrelated to cardiac illness.
Acknowledgements
This study was funded by the Associazione ‘18 Maggio 1370’, San Daniele del Friuli, Italy.
Conflict of interest and funding
The authors have not received any funding or benefits from industry or elsewhere to conduct this study.
References
- 1.Tsuchihashi K, Ueshima K, Uchida T, Oh-Mura N, Kimura K, Owa M, et al. Transient left ventricular apical ballooning without coronary artery stenosis: A novel heart syndrome mimicking acute myocardial infarction. J Am Coll Cardiol. 2001;38(1):11–18. doi: 10.1016/s0735-1097(01)01316-x. [DOI] [PubMed] [Google Scholar]
- 2.Kurisu S, Sato H, Kawagoe T, Ishihara M, Shimatani Y, Nishioka K, et al. Takotsubo like left ventricular dysfunction with ST-segment elevation: A novel cardiac syndrome mimicking acute myocardial infarction. Am Heart J. 2002;143(3):448–55. doi: 10.1067/mhj.2002.120403. [DOI] [PubMed] [Google Scholar]
- 3.Parodi G, Del Pace S, Salvadori C, Carrabba N, Olivotto I, Gensini GF, et al. Left ventricular apical ballooning syndrome as a novel cause of acute mitral regurgitation. J Am Coll Cardiol. 2007;50(7):647–9. doi: 10.1016/j.jacc.2007.04.057. [DOI] [PubMed] [Google Scholar]
- 4.Citro R, Previtali M, Bovelli D, Vriz O, Astarita C, Patella MM, et al. Chronobiological patterns of onset of Takotsubo cardiomyopathy: A multicenter Italian study. J Am Coll Cardiol. 2009;54(2):180–1. doi: 10.1016/j.jacc.2009.03.048. [DOI] [PubMed] [Google Scholar]
- 5.Parodi G, Bellandi B, Del Pace S, Barchielli A, Zampini L, Velluzzi S, et al. Tuscany registry of Takotsubo cardiomyopathy. Natural history of Takotsubo cardiomyopathy. Chest. 2011;139(4):887–92. doi: 10.1378/chest.10-1041. [DOI] [PubMed] [Google Scholar]
- 6.Citro R, Rigo F, Previtali M, Ciampi Q, Canterin FA, Provenza G, et al. Differences in clinical features and in-hospital outcomes of older adults with Takotsubo cardiomyopathy. J Am Geriatr Soc. 2012;60(1):93–8. doi: 10.1111/j.1532-5415.2011.03730.x. [DOI] [PubMed] [Google Scholar]
- 7.Sharkey S, Windenburg D, Lesser J, Maron M, Hauser R, Lesser JN, et al. Natural history and expansive clinical profile of stress (Takotsubo) cardiomyopathy. J Am Coll Cardiol. 2010;55(4):333–41. doi: 10.1016/j.jacc.2009.08.057. [DOI] [PubMed] [Google Scholar]
- 8.Redfors B, Vedad R, Angeras O, Ramunddal T, Petursson P, Haraldsson I, et al. Mortality in Takotsubo syndrome is similar to mortality in myocardial infarction – A report from the SWEDEHEART registry. Int J Cardiol. 2015;185(15):282–9. doi: 10.1016/j.ijcard.2015.03.162. [DOI] [PubMed] [Google Scholar]
- 9.Vriz O, Driussi C, Fazio MG, Arteni F, Mos L, Pertoldi F, et al. Takotsubo cardiomyopathy: Insights from a community hospital. J Cardiovasc Med. 2013;14(8):576–81. doi: 10.2459/JCM.0b013e3283595ab8. [DOI] [PubMed] [Google Scholar]
- 10.Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Takotsubo or stress cardiomyopathy): A mimic of acute myocardial infarction. Am Heart J. 2008;155(3):408–17. doi: 10.1016/j.ahj.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 11.Parodi G, Citro R, Bellandi B, Del Pace S, Rigo F, Marrani M, et al. Takotsubo Italian Network (TIN). Tako-tsubo cardiomyopathy and coronary artery disease: A possible association. Coron Artery Dis. 2013;24(6):527–33. doi: 10.1097/MCA.0b013e3283645c4e. [DOI] [PubMed] [Google Scholar]
- 12.Cerqueira MD, Weissman NJ, Dilsizian V, Jacobs AK, Kaul S, Laskey WK, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RD, Pepine CJ. Coronary angiography: Is it time to reassess? Circulation. 2013;127(17):1760–2. doi: 10.1161/CIRCULATIONAHA.113.002566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Möhlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation. 2002;106(29):2616–22. doi: 10.1161/01.cir.0000038420.14867.7a. [DOI] [PubMed] [Google Scholar]
- 15.Kim PJ, Hur G, Kim SY, Namgung J, Hong SW, Kim YH, et al. Frequency of myocardial bridges and dynamic compression of epicardial coronary arteries: A comparison between computed tomography and invasive coronary angiography. Circulation. 2009;119(10):1408–16. doi: 10.1161/CIRCULATIONAHA.108.788901. [DOI] [PubMed] [Google Scholar]
- 16.Erbel R, Ge J, Möhlenkamp S. Myocardial bridging: A congenital variant as an anatomic risk factor for myocardial infarction? Circulation. 2009;120(5):357–9. doi: 10.1161/CIRCULATIONAHA.109.881367. [DOI] [PubMed] [Google Scholar]
- 17.Ferreira AG, Jr, Trotter SE, König B, Jr, Décourt LV, Fox K, Olsen EG. Myocardial bridges: Morphological and functional aspects. Br Heart J. 1991;66(5):364–7. doi: 10.1136/hrt.66.5.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deshmukh A, Kumar G, Pant S, Rihal C, Murugiah K, Mehta JL. Prevalence of Takotsubo cardiomyopathy in the United States. Am Heart J. 2012;164(1):66–71.e1. doi: 10.1016/j.ahj.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 19.Prasad A, Dangas G, Srinivasan M, Yu J, Gersh BJ, Mehran R, et al. Incidence and angiographic characteristics of patients with apical ballooning syndrome (Takotsubo/stress cardiomyopathy) in the HORIZONS-AMI trial: An analysis from a multicenter, international study of ST-elevation myocardial infarction. Catheter Cardiovasc Interv. 2014;83(3):343–8. doi: 10.1002/ccd.23441. [DOI] [PubMed] [Google Scholar]
- 20.Samardhi H, Raffel OC, Savage M, Sirisena T, Bett N, Pincus M, et al. Takotsubo cardiomyopathy: An Australian single centre experience with medium term follow up. Intern Med J. 2012;42(1):35–42. doi: 10.1111/j.1445-5994.2011.02474.x. [DOI] [PubMed] [Google Scholar]
- 21.Weihs V, Szücs D, Fellner B, Eber B, Weihs W, Lambert T, et al. Stress-induced cardiomyopathy (Takotsubo syndrome) in Austria. Eur Heart J Acute Cardiovasc Care. 2013;2(2):137–46. doi: 10.1177/2048872613483592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro VF, Vasconcelos M, Melão F, Ferreira E, Malangatana G, Maciel MJ. Short and long-term outcome of stress-induced cardiomyopathy: What can we expect? Arq Bras Cardiol. 2014;102(1):80–5. doi: 10.5935/abc.20130228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Núñez-Gil IJ, Molina M, Bernardo E, Ibañez B, Ruiz-Mateos B, García-Rubira JC, et al. Takotsubo syndrome and heart failure: Long-term follow-up. Rev Esp Cardiol. 2012;65(11):996–1002. doi: 10.1016/j.recesp.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Chan C, Troughton R, Elliott J, Zarifeh J, Bridgman P. One-year follow-up of the 2011 Christchurch Earthquake stress cardiomyopathy cases. N Z Med J. 2014;127(1396):15–22. [PubMed] [Google Scholar]
- 25.Wittstein B. Stress cardiomyopathy: A syndrome of catecholamine-mediated myocardial stunning? Cell Mol Neurobiol. 2012;32:847–57. doi: 10.1007/s10571-012-9804-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Delmas C, Lairez O, Mulin E, Delmas T, Boudou N, Dumonteil N, et al. Anxiodepressive disorders and chronic psychological stress are associated with Takotsubo cardiomyopathy – new physiopathological hypothesis. Circ J. 2013;77(1):175–80. doi: 10.1253/circj.cj-12-0759. [DOI] [PubMed] [Google Scholar]
- 27.El-Sayed AM, Brinjikji W, Salka S. Demographic and co-morbid predictors of stress (Takotsubo) cardiomyopathy. Am J Cardiol. 2012;110(9):1368–72. doi: 10.1016/j.amjcard.2012.06.041. [DOI] [PubMed] [Google Scholar]
- 28.Summers MR, Lennon RJ, Prasad A. Pre-morbid psychiatric and cardiovascular diseases in apical ballooning syndrome (Takotsubo/stress-induced cardiomyopathy): Potential pre-disposing factors? J Am Coll Cardiol. 2010;55(7):700–1. doi: 10.1016/j.jacc.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 29.Del Pace S, Parodi G, Bellandi B, Zampini L, Venditti F, Ardito M, et al. Anxiety trait in patients with stress-induced cardiomyopathy: A case-control study. Clin Res Cardiol. 2011;100(6):523–9. doi: 10.1007/s00392-010-0276-x. [DOI] [PubMed] [Google Scholar]
- 30.Templin C, Ghadri JR, Diekmann J, Napp LP, Bataiosu DR, Jaguszewski M, et al. Clinical features and outcomes of Takotsubo (stress) cardiomyopathy. N Engl J Med. 2015;373(73):929–38. doi: 10.1056/NEJMoa1406761. [DOI] [PubMed] [Google Scholar]
- 31.Lemaitre F, Close L, Yarol N, Kemdem A, Silance PG, De Marneffe M, et al. Role of myocardial bridging in the apical localization of stress cardiomyopathy. Acta Cardiol. 2006;61(5):545–50. doi: 10.2143/AC.61.5.2017770. [DOI] [PubMed] [Google Scholar]
- 32.Migliore F, Maffei E, Perazzolo Marra M, Bilato C, Napodano M, Corbetti F, et al. LAD coronary artery myocardial bridging and apical ballooning syndrome. JACC Cardiovasc Imaging. 2013;6(1):32–41. doi: 10.1016/j.jcmg.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 33.Stiermaier T, Desch S, Blazek S, Schuler G, Thiele H, Eitel I. Frequency and significance of myocardial bridging and recurrent segment of the left anterior descending coronary artery in patients with takotsubo cardiomyopathy. Am J Cardiol. 2014;114(8):1204–9. doi: 10.1016/j.amjcard.2014.07.040. [DOI] [PubMed] [Google Scholar]
- 34.Boktor M, Mansi IA, Troxclair S, Modi K. Association of myocardial bridge and Takotsubo cardiomyopathy: A case report and literature review. South Med J. 2009;102(8):957–60. doi: 10.1097/SMJ.0b013e3181b08a30. [DOI] [PubMed] [Google Scholar]
- 35. Andò G, Trio O, de Gregorio C. Eur Heart J Acute Cardiovasc Care 2013. Coronary spasm and myocardial bridging: An elusive pathophysiological mechanism leading to apical ballooning syndrome? [DOI] [PubMed] [Google Scholar]
- 36.Vriz O, Minisini R, Ruscio M, Calabro P, Bossone E. ST2 marker might help to stratify in-hospital high risk patients with Takotsubo cardiomyopathy. Eur J Intern Med. 2015;26(2):144–5. doi: 10.1016/j.ejim.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Parodi G, Del Pace S, Carrabba N, Salvadori C, Memisha G, Simonetti I, et al. Incidence, clinical findings, and outcome of women with left ventricular apical ballooning syndrome. Am J Cardiol. 2007;99(2):182–5. doi: 10.1016/j.amjcard.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 38.Elesber AA, Prasad A, Lennon RJ, Wright RS, Lerman A, Rihal CS. Four-year recurrence rate and prognosis of the apical ballooning syndrome. J Am Coll Cardiol. 2007;50(5):448–52. doi: 10.1016/j.jacc.2007.03.050. [DOI] [PubMed] [Google Scholar]
- 39.Burgdorf C, Kurowski V, Bonnemeier H, Schunkert H, Radke PW. Long-term prognosis of the transient left ventricular dysfunction syndrome (Takotsubo cardiomyopathy): Focus on malignancies. Eur J Heart Fail. 2008;10(10):1015–19. doi: 10.1016/j.ejheart.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Eshtehardi P, Koestner SC, Adorjan P, Windecker S, Meier B, Hess OM, et al. Transient apical ballooning syndrome – clinical characteristics, ballooning pattern, and long-term follow-up in a Swiss population. Int J Cardiol. 2009;135(3):370–5. doi: 10.1016/j.ijcard.2008.03.088. [DOI] [PubMed] [Google Scholar]
- 41.Regnante RA, Zuzek RW, Weinsier SB, Latif SR, Linsky RA, Ahmed HN, et al. Clinical characteristics and four-year outcomes of patients in the Rhode Island Takotsubo Cardiomyopathy Registry. Am J Cardiol. 2009;103(7):1015–19. doi: 10.1016/j.amjcard.2008.12.020. [DOI] [PubMed] [Google Scholar]
- 42.Previtali M, Repetto A, Camporotondo R, Citro R, Faggiano P, Bovelli D, et al. Clinical characteristics and outcome of left ventricular ballooning syndrome in a European population. Am J Cardiol. 2011;107(1):120–5. doi: 10.1016/j.amjcard.2010.08.055. [DOI] [PubMed] [Google Scholar]
- 43.Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, et al. Clinical characteristics and cardiovascular magnetic resonance findings in stress (Takotsubo) cardiomyopathy. JAMA. 2011;306(3):277–86. doi: 10.1001/jama.2011.992. [DOI] [PubMed] [Google Scholar]
- 44.Looi JL, Wong CW, Khan A, Webster M, Kerr AJ. Clinical characteristics and outcome of apical ballooning syndrome in Auckland, New Zealand. Heart Lung Circ. 2012;21(3):143–9. doi: 10.1016/j.hlc.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 45.Cacciotti L, Passaseo I, Marazzi G, Camastra G, Campolongo G, Beni S, et al. Observational study on Takotsubo-like cardiomyopathy: Clinical features, diagnosis, prognosis and follow-up. BMJ Open. 2012;2(5):e001165. doi: 10.1136/bmjopen-2012-001165. doi: http://dx.doi.org/10.1136/bmjopen-2012-001165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Song BG, Yang HS, Hwang HK, Kang GH, Park YH, Chun WJ, et al. The impact of stressor patterns on clinical features in patients with Takotsubo cardiomyopathy: Experiences of two tertiary cardiovascular centers. Clin Cardiol. 2012;35(11):E6–13. doi: 10.1002/clc.22053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Buja P, Zuin G, Cutolo A, Grassi G, Madalosso M, Millosevich P, et al. Left ventricular apical ballooning syndrome in men: A case series. J Cardiovasc Med. 2012;13(12):790–4. doi: 10.2459/JCM.0b013e328346a722. [DOI] [PubMed] [Google Scholar]
- 48.Vizzardi E, Bonadei I, Rovetta R, Sciatti E, D'Aloia A, Pezzali N, et al. Characteristics and mid-term follow-up of a single-center population affected by Takotsubo cardiomyopathy. J Cardiovasc Med. 2015;16(5):326–30. doi: 10.2459/JCM.0b013e328364e710. [DOI] [PubMed] [Google Scholar]
- 49.Gopalakrishnan M, Hassan A, Villines D, Nasr S, Chandrasekaran M, Klein LW. Predictors of short- and long-term outcomes of Takotsubo cardiomyopathy. Am J Cardiol. 2015;116(10):1586–90. doi: 10.1016/j.amjcard.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 50.Sharkey SW, Maron BJ. Epidemiology and clinical profile of Takotsubo cardiomyopathy. Circ J. 2014;78(9):2119–28. doi: 10.1253/circj.cj-14-0770. [DOI] [PubMed] [Google Scholar]