Abstract
Objective of the present study is to investigate the effect of coated edible films with chitosan solutions enriched with essential oil (EO) on the chemical, microbial and sensory properties of Kashar cheese during ripening time. Generally, no differences were found in total aerobic mesophilic bacteria, streptococci and lactoccocci counts among cheeses but these microorganism counts increased during 60 and 90 d storage especially in C1 (uncoated sample) as compared with coated samples. Antimicrobial effectiveness of the films against moulds was measured on 30, 60, and 90 d of storage. In addition of fish EO into chitosan edible films samples were showed to affect significantly decreased the moulds (p<0.05) as 1.15 Log CFU/g in C4 (with fish oil (1% w/v) fortified chitosan film) on the 90th d, while in C1 as 3.89 Log CFU/g on the 90th d of ripening. Compared to other cheese samples, C2 (coated with chitosan film) and C4 coated cheese samples revealed higher levels of water-soluble nitrogen and ripening index at the end of storage. C2 coated cheese samples were preferred more by the panellists while C4 coated cheese samples received the lowest scores.
Keywords: fish oil, essential oil, chitosan, edible coating, shelf-life
Introduction
Traditional dairy products including cheese have an important place in rural food culture thus contributing to the formation of whole country’s culture. Today, most of cheese types have registered trademarks and have their protected geographic indications (Dost et al., 2004; Ercan, 2009). As a type of cheese, Kashar is manufactured and consumed in an extended geography including Turkey, where the production types and techniques of Kashar may vary depending largely on regions, producers and the methods applied and it has gained great diversity. Among the Kashar cheese types manufactured in certain regions and offering originality is the Göbek Kashar of Ardahan. This type of cheese is usually produced from cow milk using traditional methods and consumed after a long ripening period from 3 mon to 1 year. Göbek Kashar appealing to the taste of local people and containing rich nutrition elements (Yangilar, 2015).
Food coatings or edible films with the same functions, which are often composed of edible materials covering or buffering on/between food surfaces in a thin layer (Bravin et al., 2006), may ensure quality positively by affecting the preservation, distribution and marketing of food products (Falguera et al., 2011; Ruiz-Navajas et al., 2015). Chitosan coatings were tested on different types of cheese aiming at prolonging their shelf life, such as Mozzarella (Altieri et al., 2005), Emmental (Coma et al., 2002), Regional Saloio (Cerqueira et al., 2009), Apulia spreadable cheese (Gammariello et al., 2008); chitosan was used by itself, or as carrier of other natural antimicrobials, e.g., lysozyme (Duan et al., 2007), lysozyme and EDTA (Del Nobile et al., 2009). Food related pathogens are susceptible to essential oils (EOs) due to their influential antibacterial properties resulting their high phenolic compound content e.g., carvacrol and eugenol existent majorly in oregano and clove oils, respectively (Burt, 2004; Torlak and Nizamoglu, 2011). EOs are aromatic, oily liquids that are obtained from plant materials (Burt, 2004; Lambert et al., 2001) and humans have used essential oils together with their contents as flavourers in their food throughout the history maybe without knowing their large range of antimicrobial functions (Alzoreky and Nakahara, 2002; Holley and Patel, 2005; Kim et al., 1995; Packiyasothy and Kyle, 2002). The application of essential oils (EOs) has determined to be an effective preservation method that extends the shelf life of foods (Emir et al., 2012; Erkan et al., 2007; Giatrakou et al., 2008; Harpaz et al., 2003; Parry, 1993; Quitral et al., 2009). In addition to their antimicrobial actions, most plant extracts are known to exhibit antioxidant properties (Madsen and Bertelsen, 1995; Schwarz et al., 1996). The liquid fish oil because of the high content of long-chain polyunsaturated fatty acids (LCPUFAs) protect them against oxidation. Microencapsulation of LCPUFAs offer the possibility for the protection and release controlling of lipophilic food ingredients and can be used for supplementation of foods almost without oxidation (Drusch et al., 2007; Farbod et al., 2015). Such a situation is largely useful for both the reduction of food deterioration resulting from free radical-mediate and human health for the protective role of antioxidants in cancer and coronary heart disease (Farbod et al., 2015).
There are several studies conducted on the application of various antimicrobial films in Kashar cheese in literature, however; only few of them are related to the use of the essential oil combined coating material. From this point of view, the objective of the present study is to determine the effectiveness of chitosan edible film fortified with fish EO coatings in the prevention of mould growth and to investigate their effects on microbiological and chemical properties, proteolysis levels and sensory evaluation of traditional Göbek Kashar cheese during ripening.
Materials and Methods
Materials
Cow’s milk obtained from a livestock facility in Göle, Ardahan province was used to produce the cheese together with microbial rennet (1:15000; Mayasan, Turkey) and analytical grade reagents (from Merck, Darmstadt, Germany and Sigma Chemical Co., USA). Fish oil (Ropufa 30 n-3 EPA oil) was obtained by DSM Co, Switzerland.
Production of Göbek Kashar cheese
Since no literature could be found about Göbek Kashar cheese, the term “Göbek” was accepted to be used for this local type and it was prepared following the method composed of the experiences of elderly villagers and cheese producers in the province. Experimental cheese samples were produced from raw cow’s milk in a facility named Alibey in Göle district, Ardahan province using traditional methods without starter culture. Cheese was produced by filtrating and heating raw cow’s milk (400 kg) up to 33℃ and adding rennet diluted in water (1/10) in it for the formation of coagulation. Furthermore the coagulum was cut into cubes and allowed to rest for 15 min. When pH reached 5.5, whey was removed from curd in the tank and the curd was taken to a vat. Boiling process was conducted at 83℃ and 5.5% brine. Boiled cheese paste was cut into pieces in moulding machine at 75 to 83℃, desired weight and put into moulds. After resting in mould for 10 min, mould was removed and past was expanded. After resting for an additional 10 min out of mould, Göbek Kashar cheese was taken on a wooden bench. Cheese samples were stored on benches for 10 h at room temperature and then additional 10 h at 10 to 15℃. Göbek Kashar cheese, a traditional cheese type, gets its name from its shape (göbek means belly in Turkish).
Cheese samples were then divided into four groups C1 [control; without edible films and essential oil], C2 [coated with chitosan film 0.8% w/v], C3 [with fish oil (0.8% w/v) fortified chitosan film 0.8% w/v] and C4 [with fish oil (1% w/v) fortified chitosan film 0.8% w/v]. Cheese samples were first analysed at the end of pre-ripening process and before coating. Other analyses were carried out in 30 d intervals (i.e. on 3rd, 30th, 60th and 90th d) by duplicating all the analyses.
Preparation of fish essential oils enriched chitosan edible film forming solution
The chitosan edible film solution, containing 0.8% w/v chitosan was prepared by mixing of chitosan dissolved in 0.1 N HCl and in distilled water under continuous stirring. The pH of the chitosan solution was adjusted to pH 5.0 with 0.1 N HCl, and the solution was filter-sterilized. Film was prepared by casting 32.5 mL of chitosan (0.8% w/v) solution into polystyrene petri dishes (60×15 mm) in the preparation of coating film according to Di Pierro et al. (2010) and fish essential oil was added to chitosan solution. This solution was applied to cheese sample groups by dipping the cheese wheels for 60 s only twice and then left to dry for 2 h. The residual chitosan allowed to drip off and then, the cheeses were dried during 1 h at 22℃ until coating would be cake.
Microbiological analysis
Edible films with the dried essential oils were removed from each cheese wheel by means of sterile gloves and microbiological analysis was conducted over the sample groups. An 11 g cheese part was taken from each sample and diluted in 99 mL of 0.85% (w/v) sterile saline solution. Homogenisation of the samples was achieved using a Stomacher (Lab. Stomacher Blander 400 BA 7021, Swardmedical) in a sterile polyethylene bag for 1.5 min and a sterile 9 mL and 0.85% (w/v) NaCl solution was used for the dilution. The number of total aerobic mesophilic bacteria (TAMB; Merck, at 30±1℃ for 72 h; Ozdemir and Sert, 1996); lactobacilli (in MRS; Merck, at 30℃ for 48 h in anaerobic conditions; Diliello, 1982); streptococci (in M17; Merck, at 30℃ for 48 h; Sert et al., 2007); coagulase-positive Staphylococci on Baird-Parker agar with egg yolk tellurite enrichment (Merck, at 37℃ for 24 h; Ozdemir and Sert, 1996); coliforms (Violet Red Bile Agar, Oxoid, at 35±2℃ for 48 h; Diliello, 1982) and moulds (Potato Dextrose Agar, Oxoid, at 25℃ for 5 to 7 d; Koburger and Marth, 1984) were determined.
Chemical analysis
After removing edible films with dried essential oil, each cheese sample was shredded completely. Rates of moisture, fat and salt were determined conveniently with the method of Kurt et al. (2007) while titratable acidity was calculated in AOAC Official Method 920.124 (AOAC, 2000). Kjeldahl method (IDF, 1993) was used to measure total nitrogen (TN) while pH was detected using a pH-metre (WTW 340-1) as in the study of Savello et al. (1989).
Nitrogen fractions
The rates of nitrogen soluble in water (WSN), 12% trichloroacetic acid (TCA-SN) and pH 4.6 were detected in percentage in the aliquots of the same cheese extract prepared as in Kuchroo and Fox (1982). A 20-g grated cheese sample was homogenised in 40 mL H2O for 2 min through an Ultra turrax blender (IKA, USA); stored at 40℃ for 1 h; centrifuged at 3000 g for 30 min at 4℃; its fatty layer was removed and the supernatant was filtered with filter paper (Whatman 113). Twenty-five milliliter extract prepared for WSN was taken at an equal volume of 24% (wv) and TCA was added for further fractionation of the nitrogenous compounds. The mixtures were incubated for 2 h at room temperature. Precipitates were filtered through filter paper (Polychroniadou et al., 1999). The contents of WSN, TCA-SN and pH 4.6-SN were determined using Kjeldahl method. The ripening index (RI) was determined using the formula WSN/TN×100.
Sensory evaluation
Cheese samples were evaluated by 8 panellists who had performed sensorial evaluation of Kashar cheese, 3, 30, 60 and 90 d after the samples were stored for ripening based on Bodyfelt et al. (1988) by adjusting sensory criteria according to the characteristics of Kashar cheese. Panellists gave points (1 for poor and 9 for excellent) to the samples based on six characteristics e.g., colour, texture, taste and aroma, odour, saltiness and general acceptability.
Statistical analysis
Experiments were performed in completely randomised design with factorial arrangement i.e., four treatment groups (C1, C2, C3 and C4), four ripening times (3, 30, 60 and 90 d) and two replicates. SAS Statistical Software (SPSS 17.0, USA; SAS, 1998) was used for the analysis. Significance level (p<0.05) of statistical differences was determined using Duncan’s multiple range tests and variance analysis.
Results and Discussion
The gross chemical and physical properties of raw milk
Dry matter, fat and protein contents and acidity and pH rates of the cow milk used in the manufacturing process of Göbek Kashar cheese samples were 12.71±0.28%, 3.5 ±1.24%, 3.21±0.11%, 5.8±0.04 and 6.52±0.07, respectively while the counts of total aerobic mesophilic bacteria, lactobacilli, streptococci, coliform bacteria and moulds of the milk used in the production of Göbek Kashar cheeses were 8.15±0.03, 7.31±0.27, 6.5±0.51, 4.73±0.80 and 4.6±0.25 Log CFU/g, respectively.
Microbiological evaluation
Figs. 1-5 represent the results obtained from microbiological analysis of the cheese samples. Differences between microbiological counts of cheese samples were determined to be significant (p<0.05) during the ripening period. Total aerobic mesophilic bacteria counts of C1 sample was found to be greater than the others on 60th d of ripening. As can be seen in Fig. 1, total number of mesophilic aerobic bacteria in cheese samples was determined to range from 6.64 to 8.16 Log CFU/g. In the study, total aerobic mesophilic bacteria counts often revealed an increase for all samples during ripening time. Similar results were obtained by several researchers (Sarioglu and Oner, 2006; Yilmaz and Dagdemir, 2012). Fajardo et al. (2010) found mesophilic bacteria counts to be in the range between 6.29 and 7.51 Log CFU/g in natamycin coated Saloio cheese. Moatsou et al. (2015) reported that total mesophilic counts were consistently high during ripening in natamycin coating of the hard-Gruyère-type cheese, which is convenient with the findings in the present study. Lucera et al. (2014) stated the total bacterial counts showed an increasing trend in fresh mozzarella cheese. The initial microbial count was about 4.5 Log CFU/g, and in the control samples slightly faster than in the other cheese coated with potassium sorbate. Such results found in previous studies aren’t in convenience with Lucera’s result.
Fig. 1. The changes of total aerobic mesophilic bacteria counts (Log CFU/g) of Göbek Kashar cheese samples. C1 (control, without edible films and essential oil); C2 (coated with chitosan film 0.8% w/v); C3 (with fish oil (0.8% w/v) fortified chitosan film 0.8% w/v); C4 (with fish oil (1% w/v) fortified chitosan film 0.8% w/v).
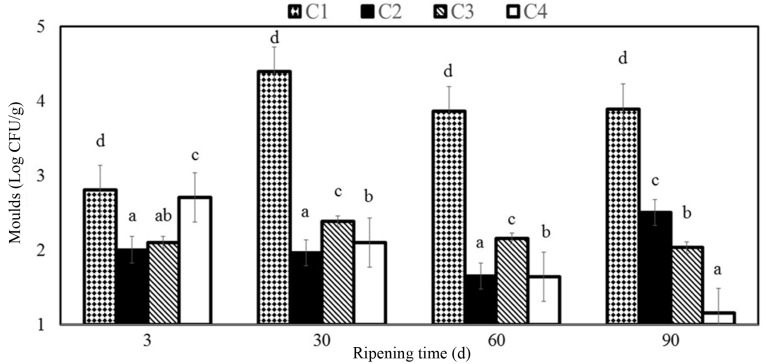
Fig. 5. The changes of moulds counts (Log CFU/g) of Göbek Kashar cheese samples. C1 (control, without edible films and essential oil); C2 (coated with chitosan film 0.8% w/v); C3 (with fish oil (0.8% w/v) fortified chitosan film 0.8% w/v); C4 (with fish oil (1% w/v) fortified chitosan film 0.8% w/v).
Fig. 2. The changes of lactobacilli counts (Log CFU/g) of Göbek Kashar samples. C1 (control, without edible films and essential oil); C2 (coated with chitosan film 0.8% w/v); C3 (with fish oil (0.8% w/v) fortified chitosan film 0.8% w/v); C4 (with fish oil (1% w/v) fortified chitosan film 0.8% w/v).
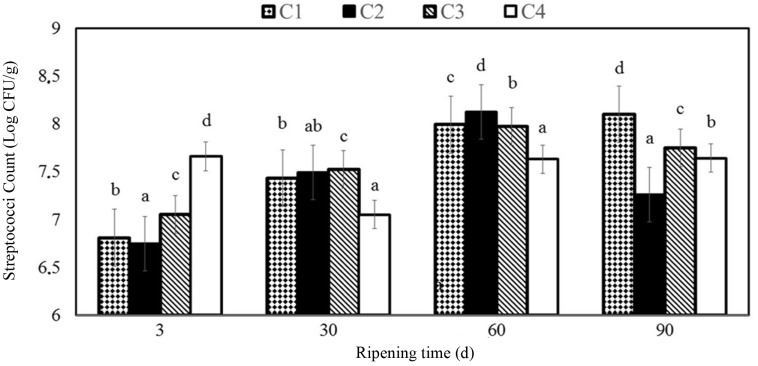
Fig. 3. The changes of streptpcocci counts (Log CFU/g) of Göbek Kashar cheese samples. C1 (control, without edible films and essential oil); C2 (coated with chitosan film 0.8% w/v); C3 (with fish oil (0.8% w/v) fortified chitosan film 0.8% w/v); C4 (with fish oil (1% w/v) fortified chitosan film 0.8% w/v).
Fig. 4. The changes of coliforms counts (Log CFU/g) of Göbek Kashar cheese samples. C1 (control, without edible films and essential oil); C2 (coated with chitosan film 0.8% w/v); C3 (with fish oil (0.8% w/v) fortified chitosan film 0.8% w/v); C4 (with fish oil (1% w/v) fortified chitosan film 0.8% w/v).
Lactobacilli counts were found to be between 6.43 and 8.08 Log CFU/g which were significantly lesser (p<0.05) in C1 sample on 30th d of ripening. It was reported by Di Pierro et al. (2011) that lactobacilli were between 5 and 6 Log CFU/g in the control on the 14th d and the chitosan/whey protein film-coated cheese samples on the 30th d, which are lesser than those in the present study.
Streptococci counts were between 6.75 and 8.12 Log CFU/g in the cheese samples during ripening period and was determined as 7.64 on 90th ripening day in C4 samples. Yangilar (2015) found results to be between 5.84 and 7.25 Log CFU/g for coated Göbek Kashar cheese samples. Yilmaz and Dagdemir (2012) reported similar results changing from 6.73 to 8.13 Log CFU/g for coated Kashar cheese samples with beeswax on 120th d. A consistent increase in total bacteria counts was reported by Lucera et al. (2014) in fresh mozzarella cheese, where baseline microbial count was about 4.5 Log CFU/g slightly greater in control samples than the others coated with potassium sorbate. These results are not convenient with those in the present study, which showed that coating with essential oil enriched chitosan films does not have any negative impact on the growth of cheese maturing microorganisms.
The treatment and ripening processes were found to affect significantly the formation of moulds (p<0.05) in coated samples in the present study changing between 1.15 and 2.7 Log CFU/g. Microbiological analyses showed that samples coated with chitosan fortified essential oil exhibited a decrease in moulds compared to control after 90 d of storage while C sample represented greater mould counts than C3 and C4 samples at the end of the ripening period. Yangýlar (2015) found that samples coated with chitosan and chitosan/whey protein (CWP) exhibited a decrease on moulds counts. Sarioglu and Oner (2006) stated that mould and yeast could not be counted in Na-caseinate film coated Kashar cheese samples from the 60th d while from 90th d in uncoated samples. Fajardo et al. (2010) stated for semi-hard cheese types coated with chitosan containing 0.5 mg/mL natamycin after a 27-d storage that the yeasts and moulds were 1.1 Log CFU/g lesser than with the control. Ramos et al. (2012) showed in their study on the efficacy of edible films containing whey protein isolate, glycerol and natamycin that the natamycin content of the film exhibited cidal effect against Y. lipolytica. Yangilar and Yildiz (2016) reported that samples coated with casein, casein/natamycin and natamycin solutions exhibited a decrease in moulds compared to control after 90 d of storage. Balaguer et al. (2013) reported no fungi in active film coated cheese samples on the 26th d of storage at 4℃ while fungi growth was seen in control samples on 16th d of storage. Ollé Resa et al. (2014) stated in their study where they investigated the effect of natamycin on yeast propagation in Port Salut cheese that it exhibited fungicidal effect on S. cerevisiae. It was stated by Ramos et al. (2012) that natamycin involved in edible films in the rate of 0.05 g/L exhibited a strong inhibitory effect against yeasts. Kallinteri et al. (2011) found that it was also reported in another study that 0.02% natamycin inhibited yeasts significantly in whole storage period reducing their counts to a range from 3.0 to 4.1 Log CFU/g. Treatment of natamycin containing coating material was reported to affect significantly (p<0.05) on the counts of yeast and moulds in hard-Gruyère-type cheese (Moatsou et al., 2015). Dos Santos Pires et al. (2008) reported a 2 - Log CFU/g decrease in yeasts and moulds in sliced Mozzarella covered with natamycin containing film after a 9-d storage period.
Counts of coliform bacteria found in cheese samples were between 0.90 and 1.34 Log CFU/g in the present study while it was <1 Log CFU/g in Yilmaz and Dagdemir (2012). Yangilar (2015) found coliform counts to be between <1 and 3.77 Log CFU/g for coated Göbek Kashar cheese samples. Sarioglu and Oner (2006) stated that counts of coliform microorganisms could not be detected in Na-caseinate film coated Kashar cheese on the 90th d while it was possible to count them in control samples on the 60th d. Gammariello et al. (2008) stated that the Thymus essential oils decreased slightly coliforms in Fior di Latte cheese.
High level of anthropogenic or animal based Staphylococcus in food may show poor sanitation and heating conditions. Antimicrobial films may inhibit S. aureus during ripening. In the present study, S. aureus count was under detectable level (2 Log CFU/g) in all samples which can be attributed to the scalding process applied in the production of Göbek Kashar cheese. A similar result was also reported by Yilmaz and Dagdemir (2012). Pranoto et al. (2005) also mentioned the inhibitor effect of antimicrobial alginate film containing 0.4% garlic oil on S. aureus. Torlak and Nizamoglu (2011) reported in Kashar cheese samples coated with renewable films that the counts of S. aureus decreased on 14th d compared to control group between 0.90 and 2.66 Log CFU/g and all the film types exhibited anti-microbial effect at significant level compared to control group (p<0.05). Mei et al. (2013) reported that the application of starch chitosan film in the storage of Mongolian cheese for controlling microbial populations was effective.
Chemical conclusion
Results of the chemical composition analysis of cheese samples in the present study is given in Table 1 and 2. Chemical changes in the samples were found to be statistically significant (p<0.05) until the end of 90 d ripening period. Dry matter rates of all cheese samples significantly increased during ripening. This increase was higher in the uncoated samples. The results also showed that the coating process with chitosan film enriched with fish oil might have delayed moisture losses compared to control. The packaging material has a significant influence on outage formed by the loss of moisture in cheese. Generally, the crust layer is enclosed on the surface of Kashar cheese during ripening depending on storage conditions. This layer is commonly removed before eating, and for this reason, being a very thick crust is not desirable as it will cause economic loses. Because of therefore, we think that outage formation because of moisture loss may be reduced by coating. Similar results were reported in beeswax coating of Kashar cheese by Yilmaz and Dagdemir (2012) and in casein/natamycin coating of Kashar cheese by Yangilar and Yildiz (2016). Zantar et al. (2014) found that essential oils increased dry matter at the end of the storage period of goat cheese with Thymus vulgaris and Origanum compactum. Yildirim et al. (2006) found that the dry matter content of Kashar cheese samples A (control), C (coating with casein solution), D (coating with casein solution containing natamycin) and E (dipping in natamycin solution) increased until the 60th d of storage (p<0.05) after which it did not change significantly (p>0.05) and these results are in agreement with the results of the present study.
Table 1. Effect of essential oil enriched edible films and storage on chemical characteristics of Göbek Kashar cheeses.
| Cheese samples | Ripening (d) | Dry matter (%) | Fat (%) | Salt (%) | pH | Acidity SH | Salt in dry matter (%) | Fat in dry matter (%) |
|---|---|---|---|---|---|---|---|---|
| C1 | 3 | 58.14±0.00a | 24.50±0.70a | 3.04±0.00b | 5.33±0.00ab | 19.18±0.00a | 5.22±0.00a | 42.13±1.21a |
| 30 | 61.68±0.28c | 25.24±0.35a | 4.21±0.33ab | 5.36±0.03ab | 31.96±4.51ab | 6.82±0.55ab | 40.93±0.38a | |
| 60 | 62.31±0.41c | 26.75±0.35a | 4.68±0.00b | 5.31±0.02b | 35.16±0.00a | 7.51±0.05ab | 42.93±0.85a | |
| 90 | 63.46±0.02c | 27.25±0.35a | 4.91±0.00a | 5.30±0.02b | 35.96±1.10a | 7.67±0.08a | 42.59±0.10a | |
| C2 | 3 | 57.84±0.00a | 26.00±0.00b | 3.60±0.29c | 5.30±0.00a | 21.58±0.00b | 6.23±0.50b | 44.95±0.00b |
| 30 | 57.77±0.06ab | 26.00±0.00b | 4.30±0.79ab | 5.39±0.00b | 28.77±0.00ab | 7.43±1.37ab | 44.71±0.04c | |
| 60 | 58.50±0.30a | 27.10±0.14a | 4.44±0.35ab | 5.27±0.00ab | 38.36±0.00b | 7.59±0.53ab | 46.32±0.48b | |
| 90 | 59.78±0.12a | 29.50±2.12a | 5.14±0.00ab | 5.35±0.03c | 34.37±0.00a | 8.50±0.01b | 49.33±0.45a | |
| C3 | 3 | 57.89±0.02a | 24.25±0.35a | 2.98±0.07a | 5.35±0.01b | 19.98±1.13ab | 5.16±0.13a | 41.88±0.02a |
| 30 | 58.10±0.09b | 25.23±0.00a | 4.50±0.77b | 5.32±0.02a | 33.16±2.81b | 7.75±0.14b | 43.45±0.53b | |
| 60 | 58.49±0.34a | 28.00±0.00a | 4.86±0.02b | 5.24±0.01a | 37.5±0.00b | 8.29±0.02b | 47.85±0.23b | |
| 90 | 61.88±0.07b | 29.00±0.00a | 5.50±0.32b | 5.24±0.02a | 42.36±1.13b | 8.88±0.07b | 46.86±0.05a | |
| C4 | 3 | 57.30±0.01a | 25.00±0.00ab | 2.93±0.31a | 5.32±0.02ab | 18.37±1.40a | 5.10±0.09a | 43.62±0.77ab |
| 30 | 57.02±0.04a | 26.17±0.44b | 3.09±0.22a | 5.34±0.03ab | 24.75±1.12a | 5.45±0.38a | 45.56±0.41c | |
| 60 | 59.75±0.16b | 27.60±0.84a | 3.96±0.34a | 5.30±0.02ab | 34.36±1.12a | 6.62±0.70a | 46.15±1.54b | |
| 90 | 61.79±0.13b | 29.50±2.10a | 4.68±0.34a | 5.22±0.01a | 41.56±0.00b | 7.56±0.50a | 47.63±3.37a |
*Mean values followed by different superscripts in the same column are significantly different (p<0.05).
Abbreviations are: C1 (control, without edible films and essential oil); C2 (coated with chitosan edible film 0.8% w/v); C3 (with fish oil (0.8% w/v) fortified chitosan film (0.8% w/v)); C4 (with fish oil (1% w/v) fortified chitosan film (0.8% w/v)).
Table 2. Effect of essential oil enriched edible films and storage on proteolysis fractions of Göbek Kashar cheeses.
| Cheese samples | Ripening (d) | Protein (%) | WSN (%) | Ripening index (%) | TCA-SN (%) | pH 4.6-SN (%) |
|---|---|---|---|---|---|---|
| C1 | 3 | 27.17±0.00b | 1.66±0.00a | 6.11±0.00a | 4.14±0.03c | 4.22±0.11a |
| 30 | 26.00±0.12a | 2.25±0.00b | 8.70±0.04a | 5.25±0.00c | 4.84±0.02a | |
| 60 | 26.64±0.36b | 2.79±0.01a | 10.48±0.08a | 6.41±0.03c | 5.31±0.04a | |
| 90 | 26.95±0.12a | 3.42±0.17bc | 12.69±0.59ab | 7.15±0.06c | 7.98±0.19c | |
| C2 | 3 | 28.75±0.00c | 2.59±0.00b | 9.00±0.01b | 3.80±0.00b | 4.07±0.00a |
| 30 | 25.60±0.07a | 2.10±0.06a | 8.24±0.58a | 4.17±0.03b | 4.49±0.24a | |
| 60 | 25.20±0.00a | 2.97±0.04b | 11.79±0.17b | 4.30±0.00a | 5.17±0.03a | |
| 90 | 25.81±0.47a | 3.04±0.05a | 11.78±0.02a | 4.62±0.00a | 6.25±0.11a | |
| C3 | 3 | 26.04±0.72a | 2.57±0.03b | 9.85±0.41b | 3.19±0.12a | 4.48±0.36a |
| 30 | 25.09±0.18a | 2.32±0.01b | 9.23±0.28b | 3.89±0.06a | 5.20±0.27b | |
| 60 | 26.19±0.21b | 3.24±0.16c | 12.35±0.19c | 4.79±0.02b | 6.65±0.04c | |
| 90 | 26.37±0.62a | 3.52±0.02c | 13.36±0.24b | 6.15±0.00b | 8.53±0.11d | |
| C4 | 3 | 26.29±0.21ab | 3.31±0.09c | 12.61±0.45c | 4.47±0.00d | 7.55±0.03b |
| 30 | 25.65±0.07a | 3.18±0.00c | 12.40±0.45c | 4.19±0.09b | 7.86±0.36c | |
| 60 | 26.53±0.06b | 2.81±0.04a | 10.58±0.84a | 4.76±0.19b | 6.19±0.14b | |
| 90 | 25.76±1.05a | 3.24±0.01ab | 12.56±0.57ab | 6.33±0.02b | 6.80±0.27b |
*Mean values followed by different superscripts in the same column are significantly different (p<0.05).
Abbreviations are: C1 (control, without edible films and essential oil); C2 (coated with chitosan edible film 0.8% w/v); C3 (with fish oil (0.8% w/v) fortified chitosan film (0.8% w/v)); C4 (with fish oil (1% w/v) fortified chitosan film (0.8% w/v)).
As can also be seen in Table 1, treatment and storage processes affected pH significantly (p<0.05). The pH level of cheese samples during ripening are shown in Table 1 and decreased linearly during ripening. Farbod et al. (2015) reported a significant decrease in pH level of Iranian UF-Feta cheese fortified with fish oil or fish oil powder from 4.7 to 4.5 during 60-d storage at 5℃. Fluctuations in pH during ripening were also reported by Yildirim et al. (2006) in Kashar cheese coated with casein solution. It was found by Di Pierro et al. (2011) that pH values of chitosan/whey protein film coated Ricotta cheese decreased, after 7 d of storage. Gulec et al. (2004) stated that pH ranged from 5.14 to 5.25 in casein coated and uncoated Kashar cheese samples on 90th d of storage, which is consistent with the present study. Lucera et al. (2014) determined pH of mozzarella cheese, monitored during the entire observation period, ranged between 6.50 and 6.30.
Acidity rates of the samples in the present study varied between 18.37 and 42.36 SH. Di Pierro et al. (2011) reported that titratable acidity of Ricotta cheese, coated with a chitosan/whey protein film, reached the same level as measured in the control sample at the end of storage period. Similar results (between 21.01 and 35.46 SH) related to acidity rates were also found by Yangilar (2015) in coated Göbek Kashar samples.
Results of the present study showed that saltiness rates of the samples changed between 2.93 and 5.14%. Gulec et al. (2004) also found the lowest and the highest saltiness rates of casein coated Kashar cheese samples and control to range from 1.54% to 2.54% and 1.54% to 2.43%, respectively.
Both largest and smallest fat contents in the present study were found in the control sample to be 24.25% and 29.50%, respectively while for the same study material (coated Kashar cheese), Sarioglu and Oner (2006) also determined the lowest and highest fat contents to be 31.37% and 43.25%, respectively while in control group, these rates were found to be 30.5% and 42.25%, respectively. Similar results were reported also by Yilmaz and Dagdemir (2012) and Yildirim et al. (2006). Farbod et al. (2015) stated that fat content of Iranian UF-Feta cheese fortified with fish oil or fish oil powder changed insignificantly during the storage time.
Proteolysis fractions of Göbek Kashar cheeses
Results of the proteolysis fractions carried out over cheese samples are given in Table 2. It was observed in the present study that protein rates of the samples ranged between 25.09 and 28.75%. Gulec et al. (2004) reported the lowest and highest rates of protein in cheese samples and control Kashar samples to change from 24.5 to 31.36% and 24.5 to 31.28% while Sarioglu and Oner (2006) found this rate in Kashar samples and control group to range from 27.70 to 36.64% and 27.25 to 34.40%, respectively. Farbod et al. (2015) stated that protein content of Iranian UF-Feta cheese fortified with fish oil or fish oil powder changed insignificantly during the storage time.
The rate of WSN increased during ripening period in all cheese samples and found to be 1.66% on 3rd (C1 sample) and 3.52% on 90th d (C2 sample) of ripening in the present study. Yilmaz and Dagdemir (2012) found WSN to be significantly higher in control cheese until day 90, followed by beeswax (single-layer coating).
In the evaluation of the ripening index, the values ranged from 6.11 to 12.69% for C1 sample, 9.00 to 11.79% for C2 sample, 9.23 to 13.36% for C3 sample, and 10.58 to 12.61% for C4 sample. The lowest percentage of ripening index was determined in C1 sample (6.11%) while C2 sample (13.36%) showed the highest percentages. In addition, statistically significant differences were determined between the ripening indexes values obtained on 90th d. Such results are similar to those found in Yilmaz and Dagdemir (2012). Yildirim et al. (2006) reported that the ripening index values in Kashar cheese samples increased steadily until the 60th d of ripening while Gulec et al. (2004) stated that ripening index values of all samples were similar until 60th d and ripening index values of control increased more than coated samples on 90th d. In order to ensure a good texture, taste and aroma in ripened cheese proteins should well be degraded into peptides and amino acids. Proteolysis occurrence in cheese is generally understandable by quantifying WSN fractions containing whey proteins, medium- and small-sized peptides from the degradation of caseins and free amino acids (Christensen et al., 1991; Yilmaz and Dagdemir, 2012). Yangilar (2015) reported that the ripening index value ranged from 14.71 to 21.11% for control sample and 12.45 to 19.95% for chitosan with coated cheese samples and 14.10 to 18.66% for chitosan/whey protein with coated cheese samples.
Table 2 shows that 12% TCA-SN values increased with increasing ripening time significantly (p<0.05) in cheese samples. A similar result was also found by Yýlmaz and Dagdemir (2012) to be a significant increase (p<0.05) during ripening process. Aydemir (2010) reported that the increase in TCA-SN values were low when the Kashar cheese samples were ripened at 4±1℃ following the preripening.
The pH 4.6-SN value ranged from 5.94 to 10.59% for control sample, 3.19 to 8.32% for N1 sample, 5.30 to 8.34% for N2 sample, 6.39 to 10.19% for N3 sample, 5.76 to 6.69% for N4 sample and 5.34 to 9.17% for N5 sample. The pH 4.6-SN was significant (p<0.05) during the ripening time and these results were affected by the coating materials and their interactions. Yangilar (2015) stated that the pH 4.6-SN ranged from 6.52 to 8.69% for the control samples, 7.22 to 9.21% for chitosan with coated cheese samples and 4.25 to 6.46% for chitosan/whey protein with coated cheese samples during ripening time. The values of pH 4.6-SN were found to be 10.72-23.76% of Kashar cheese samples in Hayaloglu (2009). Moatsou et al. (2015) reported that the features such as overall composition and proteolytic pattern of hard-Gruyère-type cheese coated with natamycin during ripening did not change significantly.
Sensory evaluation
Results of the sensory evaluation of 90 d cheese samples on a scale from 1 (poor) to 9 (excellent) are shown in Table 3. A significant difference (p<0.05) was found to be between the samples in colour, texture, taste and aroma, odour, saltiness and general acceptability. Control cheese (C1) received the highest texture scores (8.45) while fish oil fortified chitosan film coated cheese (C4) had the lowest texture score (6.30) and C2 sample was mostly preferred by the panellists in overall scores may be because of its high protein and fat content affecting the panellists’ scores. C4 sample was given the lowest overall acceptability score by the panellists on 60th d. It is possible that the use of edible films may increase sensory properties of cheese during ripening. Smith-Palmer et al. (2001) reported that plant essential oils in effective concentrations may change organoleptic properties of food. Farbod et al. (2015) stated that sensory scores of FO sample were significantly higher than FOP sample (p<0.05) by reaching an overall acceptability up to 70% on 30th and 60th d of storage maybe due to its better hardness, texture and flavour. It was reported by Zantar et al. (2014) that cheese sample aromatized using T. vulgaris got the highest overall acceptability score and it was followed by the control and the sample aromatized using O. compactum. Di Pierro et al. (2011) stated that compared to control, Ricotta cheese exhibited better texture conditions when it was coated with chitosan/whey protein film while no difference was found in visual appearance, texture, flavour and odour between uncoated and chitosan/whey protein film coated Ricotta cheese samples. Cetinkaya and Soyutemiz (2004) reported significant differences between Kashar samples coated with beeswax in terms of aroma, flavour, colour, appearance and texture.
Table 3. Effect of essential oils enriched edible films and storage on sensory characteristics of Göbek Kashar cheese.
| Cheese samples | Ripening (d) | Colour | Texture | Taste and Aroma | Odour | Saltiness | General acceptability |
|---|---|---|---|---|---|---|---|
| C1 | 3 | 7.90±0.14b | 8.05±0.07ab | 8.15±0.07b | 8.40±0.14bc | 7.49±0.23ab | 8.40±0.07a |
| 30 | 6.97±0.03b | 7.05±0.07a | 7.21±0.07b | 6.35±0.21a | 7.36±0.21a | 6.60±0.14a | |
| 60 | 7.98±0.02b | 8.05±0.04b | 8.13±0.18c | 6.45±0.07a | 7.35±0.21ab | 7.38±0.14b | |
| 90 | 8.45±0.07d | 8.32±0.04c | 7.95±0.07b | 7.40±0.14b | 7.46±0.19b | 8.05±0.07c | |
| C2 | 3 | 8.10±0.10b | 8.20±0.98b | 9.00±0.02c | 8.80±0.28c | 7.90±0.14c | 8.45±0.14a |
| 30 | 7.10±0.14b | 7.95±0.05c | 7.75±0.05c | 6.75±0.35a | 7.73±0.09a | 8.00±0.00b | |
| 60 | 8.25±0.07b | 7.55±0.63a | 8.03±0.07c | 6.90±0.14a | 7.90±0.42b | 7.30±0.28b | |
| 90 | 7.99±0.01c | 8.21±0.04c | 7.84±0.03b | 7.90±0.08c | 7.80±0.00b | 8.00±0.07c | |
| C3 | 3 | 8.55±0.07c | 8.35±0.77b | 7.75±0.00a | 7.85±0.21ab | 7.60±0.00ab | 8.35±0.23a |
| 30 | 7.98±0.02c | 7.05±0.07a | 8.05±0.08d | 8.40±0.14c | 7.58±0.12a | 8.15±0.21b | |
| 60 | 7.47±0.66ab | 7.41±0.21a | 7.45±0.14b | 6.72±0.35a | 6.92±0.35a | 6.90±0.03ab | |
| 90 | 6.45±0.07b | 7.47±0.14b | 6.91±0.07a | 6.93±0.14a | 6.82±0.23a | 7.32±0.01a | |
| C4 | 3 | 7.45±0.08a | 7.25±0.63a | 7.62±0.66a | 7.35±0.20a | 7.25±0.11a | 8.15±0.07a |
| 30 | 6.30±0.28a | 7.33±0.21b | 7.03±0.04a | 7.61±0.21b | 7.81±0.24a | 6.70±0.42a | |
| 60 | 6.71±0.07a | 7.02±0.63a | 6.75±0.09a | 6.70±0.30a | 7.80±0.47b | 6.05±0.28a | |
| 90 | 6.15±0.03a | 6.30±0.09a | 6.86±0.03a | 6.58±0.05a | 7.73±0.08b | 6.35±0.21a |
*Mean values followed by different superscripts in the same column are significantly different (p<0.05).
Abbreviations are: C1 (control, without edible films and essential oil); C2 (coated with chitosan edible film 0.8% w/v); C3 (with fish oil (0.8% w/v) fortified chitosan film (0.8% w/v)); C4 (with fish oil (1% w/v) fortified chitosan film (0.8% w/v)).
It can be stated that coating process may significantly reduce mould growth during the ripening and thus extending shelf-life when compared to control. Results of the study indicate also that the use of fish fortified coating film with chitosan can inhibit mould growth during ripening without any adverse effects on cheese quality. In addition, effect of the coating with the substances mentioned above on the rate and extent of proteolysis was estimated quantitatively by considering the level of soluble nitrogen components in cheese during ripening. In terms of sensorial evaluation, panellists gave high scores especially to C1 and C2 group. This study is essentially relevant since it indicates successfully through the coated samples with chitosan enriched fish oil which could be used as good coating materials in the manufacture of traditional Göbek Kashar cheese.
References
- 1.Altieri C., Scrocco C., Sinigaglia M., Del Nobile M. A. Use of chitosan to prolong mozzarella cheese shelf life. J. Dairy Sci. 2005;88:2683–2688. doi: 10.3168/jds.S0022-0302(05)72946-5. [DOI] [PubMed] [Google Scholar]
- 2.Alzoreky N. S., Nakahara K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food Microbiol. 2003;80:223–230. doi: 10.1016/S0168-1605(02)00169-1. [DOI] [PubMed] [Google Scholar]
- 3.Horwitz W., editor. AOAC. Acidity of cheese. Official Method 920.124. Official Methods of Analysis. (17th Ed.) Association of Official Analytical Chemists; Gaithersburg, Maryland, USA: 2000. [Google Scholar]
- 4.Aydemir O. The Characterization of Kars Kashar Cheese. Ph.D Thesis. Ondokuz Mayýs University, Graduate School of Natural and Applied Sciences Samsun; Turkey: 2010. [Google Scholar]
- 5.Balaguer M. P., Lopez-Carballo G., Catala R., Gavara R., Hernandez-Munoz P. Antifungal properties of gliadin films incorporating cinnamaldehyde and application in active food packaging of bread and cheese spread food-stuffs. Int. J. Food Microbiol. 2013;166:369–377. doi: 10.1016/j.ijfoodmicro.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Bodyfelt F. W., Tobias J., Trout G. M. Sensory evaluation of ice cream and related products. The Sensory Evaluation of Dairy Products. AVI; Westport, Conn.: 1988. [Google Scholar]
- 7.Bravin B., Peressini D., Sensidoni A. Development and application of polysaccharide-lipid edible coating to extend shelf-life of dry bakery products. J. Food Eng. 2006;76:280–290. doi: 10.1016/j.jfoodeng.2005.05.021. [DOI] [Google Scholar]
- 8.Burt S. Essential oils: their antibacterial properties and potential applications in foods: a revienw. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 9.Cerqueira M. A., Lima A. M., Souza B. W. S., Teixeira J. A., Moreira R. A., Vicente A. A. Functional polysaccharides as edible coatings for cheese. J. Agric. Food Chem. 2009;57:1456–1462. doi: 10.1021/jf802726d. [DOI] [PubMed] [Google Scholar]
- 10.Cetinkaya F., Soyutemiz G. E. A study on survival of Listeria monocytogenes during manufacture and ripening of Kashar cheese. Turk. J. Vet. Anim. Sci. 2004;28:927–932. [Google Scholar]
- 11.Christensen T. M. I. E., Bech A. M., Werner H. Methods for crude fractionation (extraction and precipitation) of nitrogen components in cheese. International Dairy Federation; Brussels: 1991. pp. 4–9. IDF Bulletin 261. [Google Scholar]
- 12.Coma V., Martial-Gros A., Garreau S., Copinet A., Salin F., Deschamps A. Edible antimicrobial films based on chitosan matrix. J. Food Sci. 2002;67:1162–1169. doi: 10.1111/j.1365-2621.2002.tb09470.x. [DOI] [Google Scholar]
- 13.Del Nobile M. T., Gammariello D., Conte A., Attanasio M. A combination of chitosan, coating and modified atmosphere packaging for prolonging Fior di latte cheese shelf life. Carbohydr. Polym. 2009;78:151–156. doi: 10.1016/j.carbpol.2009.03.017. [DOI] [Google Scholar]
- 14.Di Pierro P., Mariniello L., Sorrentino A., Villalonga R., Chico B., Porta R. Putrescine-polysaccharide conjugates as transglutaminase substrates and their possible use in producing crosslinked films. Amino Acids. 2010;38:669–675. doi: 10.1007/s00726-009-0427-4. [DOI] [PubMed] [Google Scholar]
- 15.Di-Pierro P. D., Sorrentino A., Mariniello L., Giosafatto C. V. L., Porta R. Chitosan/whey protein film as active coating to extend ricotta cheese shelf-life. LWT-Food Sci. Technol. 2011;44:2324–2327. doi: 10.1016/j.lwt.2010.11.031. [DOI] [Google Scholar]
- 16.Diliello L. R. Methods in food and dairy microbiology. AVI Publishing Co. Inc.; 1982. [Google Scholar]
- 17.Dos Santos Pires A. C., De Fátima Ferreira Soares N., De Andrade N. J., Da Silva L. H. M., Camilloto G. P., Bernardes P. C. Development and evaluation of active packaging for sliced mozzarella preservation. Packag. Technol. Sci. 2008;21:375–383. doi: 10.1002/pts.815. [DOI] [Google Scholar]
- 18.Dost A., Yenikan H., Okumus F., Isikli N. D. Bazi Geleneksel Peynirlerin Üretim Yöntemleri; Geleneksel Gidalar Sempozyumu; Van. 2004. [Google Scholar]
- 19.Drusch S., Serfert Y., Scampicchio M., Schmidt-Hansberg B., Schwaz K. Impact of physicochemical characteristics on the oxidative stability of fish oil microencapsulated by spray-drying. J. Agric. Food Chem. 2007;55:11044–11051. doi: 10.1021/jf072536a. [DOI] [PubMed] [Google Scholar]
- 20.Duan J., Park S. I., Daeschel M. A., Zhao Y. Antimicrobial chitosan Lysozyme (CL) films and coatings for enhancing microbial safety of mozzarella cheese. Food Microbiol. Safe. 2007;72:355–361. doi: 10.1111/j.1750-3841.2007.00556.x. [DOI] [PubMed] [Google Scholar]
- 21.Ercan D. Quality Characteristics of Traditional Sepet Cheese. A Thesis. Izmir: 2009. Submitted to the Graduate School of Engineering and Sciences of Izmir Institute of Technology in Partial Fulfilment of the Requirements for the Degree of Master of Science in Food Engineering, July. [Google Scholar]
- 22.Erkan N., Ozden O., Inugur M. The effects of modied atmosphere and vacuum packaging on quality of chub mackerel. Int. J. Food Sci. Technol. 2007;42:1297–1304. doi: 10.1111/j.1365-2621.2006.01325.x. [DOI] [Google Scholar]
- 23.Fajardo P., Martins J. T., Fuciños C., Pastrana L., Teixeira J. A., Vicente A. A. Evaluation of a chitosan-based edible film as carrier of natamycin to improve the storability of Saloio cheese. J. Food Eng. 2010;101:349–356. doi: 10.1016/j.jfoodeng.2010.06.029. [DOI] [Google Scholar]
- 24.Falguera V., Quintero J. P., Jiménez A., Muñoz J. A., Ibarz A. Edible films and coatings: Structures, active functions and trends in their use. Trends Food Sci. Technol. 2011;22:292–303. doi: 10.1016/j.tifs.2011.02.004. [DOI] [Google Scholar]
- 25.Farbod F., Kalbasi A., Moini S., Emam-Djomeh Z., Razavi H., Mortazavi A. Effects of storage time on compositional, micro-structural, rheological and sensory properties of low fat Iranian UF-Feta cheese fortified with fish oil or fish oil powder. J. Food Sci. Technol. 2015;52:1372–1382. doi: 10.1007/s13197-013-1163-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gammariello D., Chillo S., Mastromatteo M., Di Giulio S., Attanasio M., Del Nobile M. A. Effect of chitosan on the rheological and sensorial characteristics of apulia spreadable cheese. J. Dairy Sci. 2008;91:4155–4163. doi: 10.3168/jds.2008-1280. [DOI] [PubMed] [Google Scholar]
- 27.Giatrakou V., Kykkidou S., Papavergou A., Kontominas M. G., Savvaidis I. N. Potential of oregano essential oil and MAP to extend the shelf life of fresh swordsh: A comparative study with ice storage. J. Food Sci. 2008;73:M167–M173. doi: 10.1111/j.1750-3841.2008.00729.x. [DOI] [PubMed] [Google Scholar]
- 28.Gulec F., Bayram M., Yildirim M., Yildirim Z. Some properties of Kashar cheese coated with casein, Süleyman Demirel University, Recent Developments In Dairy Science and Technology; International Dairy Symposium; May 24-28; 2004. pp. 58–66. [Google Scholar]
- 29.Harpaz S., Glatman L., Drabkin V., Gelman A. Effects of herbal essential oils used to extend the shelf-life of freshwater reared Asian sea bass sh (Lates calcarifer) J. Food Protect. 2003;66:410–417. doi: 10.4315/0362-028x-66.3.410. [DOI] [PubMed] [Google Scholar]
- 30.Hayaloglu A. A. Volatile composition and proteolysis in traditionally produced mature Kashar cheese. Int. J. Food Sci. Technol. 2009;44:1388–1394. doi: 10.1111/j.1365-2621.2009.01968.x. [DOI] [Google Scholar]
- 31.Holley R. A., Patel D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005;22:273–292. doi: 10.1016/j.fm.2004.08.006. [DOI] [Google Scholar]
- 32.International Dairy Federation (IDF) Milk: Determination of the Nitrogen (Kjeldahl Method) and Calculation of the Crude Protein Content. IDF Standard 20B. International Dairy Federation; Brussels: 1993. [Google Scholar]
- 33.Kallinteri L. D., Kostoula O. K., Savvaidis I. N. Efficacy of nisin and/or natamycin to improve the shelf-life of Galotyri cheese. Food Microbiol. 2013;36:176–181. doi: 10.1016/j.fm.2013.05.006. [DOI] [PubMed] [Google Scholar]
- 34.Kim J., Marshall M. R., Wei C. I. Antibacterial activity of some essential oil components against five food-borne pathogens. J. Agric. Food Chem. 1995;43:2839–2845. doi: 10.1021/jf00059a013. [DOI] [Google Scholar]
- 35.Koburger J. A., Marth E. H. Yeasts and moulds In: Marvin L. Speck., editor. Compendium of Methods for the Examination of Foods. A. P. H. A.; Washington D. C.: 1984. pp. 197–202. [Google Scholar]
- 36.Kuchroo C. N., Fox P. F. Soluble nitrogen in cheese: comparison of extraction procedures. Milchwissenschaft. 1982;37:331–335. [Google Scholar]
- 37.Kurt A., Cakmakci S., Caglar A. A Guide Book of Analysis Methods of Milk and Milk Products. Ataturk University, Agriculture Faculty; Erzurum, Turkey: 2007. [Google Scholar]
- 38.Lambert R. J., Skandamis P. N., Coote P., Nychas G. J. E. A study of the minimum inhibitory concentration and model of action of oregano essential oil, thymol and carvacrol. J. Appl. Microbiol. 2001;91:453–462. doi: 10.1046/j.1365-2672.2001.01428.x. [DOI] [PubMed] [Google Scholar]
- 39.Lucera M., Mastromatteo M., Conte A., Zambrini A. V., Faccia M., Del Nobile M. A. Effect of active coating on microbiological and sensory properties of fresh mozzarella cheese. Food Pack. Shelf Life. 2014;1:25–29. doi: 10.1016/j.fpsl.2013.10.002. [DOI] [Google Scholar]
- 40.Madsen H. L., Bertelsen G. Spices as antioxidants. Trends Food Sci. Technol. 1995;6:271–277. doi: 10.1016/S0924-2244(00)89112-8. [DOI] [Google Scholar]
- 41.Mei J., Yuan Y., Wu Y., Li Y. Characterization of edible starch-chitosan film and its application in the storage of Mongolian cheese. Int. J. Biol. Macromol. 2013;57:17–21. doi: 10.1016/j.ijbiomac.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Moatsou G., Moschopoulou E., Beka A., Tsermoula P., Pratsis D. Effect of natamycin-containing coating on the evolution of biochemical and microbiological parameters during the ripening and storage of ovine hard-Gruyère-type cheese. Int. Dairy J. 2015;50:1–8. doi: 10.1016/j.idairyj.2015.05.010. [DOI] [Google Scholar]
- 43.Ollé Resa C. P., Jagus R. J., Gerschenson L. N. Natamycin efficiency for controlling yeast growth in models systems and on cheese surfaces. Food Cont. 2014;35:101–108. doi: 10.1016/j.foodcont.2013.06.049. [DOI] [Google Scholar]
- 44.Ozdemir S., Sert S. Gida Mikrobiyolojisi Tatbikat Notlari. Atatürk Üniversitesi Ziraat Fakültesi Yayinlari, Erzurum, No.128. 1996. p. 111. (in Turkish) [Google Scholar]
- 45.Packiyasothy E. V., Kyle S. Antimicrobial properties of some herb essential oils. Food Aust. 2002;54:384–387. [Google Scholar]
- 46.Parry R. T. Introduction In: Parry R. T., editor. Principles and Application of Modied Atmosphere Packaging Food. Blackie Academic and Professional; Glasgow: 1993. pp. 1–17. [Google Scholar]
- 47.Polychroniadou A., Michaelidou A., Paschaloudis N. Effect of time, temperature and extraction method on the trichloroaceticacid-soluble nitrogen of cheese. Int. Dairy J. 1999;9:559–568. doi: 10.1016/S0958-6946(99)00122-3. [DOI] [Google Scholar]
- 48.Pranoto Y., Salokhe V. M., Rakshit S. K. Physical and antibacterial properties of alginate-based edible film incorporated with garlic oil. Food Res. Int. 2005;38:267–272. doi: 10.1016/j.foodres.2004.04.009. [DOI] [Google Scholar]
- 49.Quitral V., Donoso M. L., Ortiz J., Herrera M. V., Araya H., Aubourg S. P. Chemical changes during the chilled storage of Chilean jack mackerel (Trachurus murphyi): Effect of a plant-extract icing system. LWT-Food Sci. Technol. 2009;42:1450–1454. doi: 10.1016/j.lwt.2009.03.005. [DOI] [Google Scholar]
- 50.Ramos Ó. L., Silva S. I., Soares J. C., Fernandes J. C., Poças M. F., Pintado M. E., Malcata F. X. Features and performance of edible films, obtained from whey protein isolate formulated with antimicrobial compounds. Food Res. Int. 2012;45:351–361. doi: 10.1016/j.foodres.2011.09.016. [DOI] [Google Scholar]
- 51.Ruiz-Navajas Y., Viuda-Martos M., Barber X., Sendra E., Perez-Alvarez J. A., Fernández-López J. Effect of chitosan edible films added with Thymus moroderi and Thymus piperella essential oil on shelf-life of cooked cured ham. J. Food Sci. Technol. 2015:1–9. doi: 10.1007/s13197-015-1733-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sarioglu T., Oner Z. Usage possibilities of an edible film for coating Kashar cheese and its effects on cheese quality. Food. 2006;31:3–10. [Google Scholar]
- 53.SAS. Changes and Enhancements through Release 6.12. SAS Institute Inc.; Cary, NC, USA: 1998. [Google Scholar]
- 54.Savello P. A., Ernstrom C. A., Kalab M. Microstructure and meltability of model process cheese made with rennet and acid casein. J. Dairy Sci. 1989;72:1–11. doi: 10.3168/jds.S0022-0302(89)79073-1. [DOI] [Google Scholar]
- 55.Schwartz J. L. The dual roles of nutrients as antioxidants and pro-oxidants: Their effects on tumor cell growth. J. Nutr. 1996;126:1221S. doi: 10.1093/jn/126.suppl_4.1221S. [DOI] [PubMed] [Google Scholar]
- 56.Sert D., Ayar A., Akin N. The effects of starter culture on chemical composition microbiological and sensory characteristics of Turkish Kashar cheese during ripening. Int. J. Dairy Technol. 2007;60:245–252. doi: 10.1111/j.1471-0307.2007.00339.x. [DOI] [Google Scholar]
- 57.Smith-Palmer A., Stewart J., Fyfe L. The potential application of plant essential oils as natural food preservatives in soft cheese. Food Microbiol. 2001;18:463–470. doi: 10.1006/fmic.2001.0415. [DOI] [Google Scholar]
- 58.Torlak E., Nizamoglu M. Effectiveness of edible chitosan films containing essential oils on Staphylococcus aureus and Escherichia coli O157:H7. J. Kafkas Univ. Vet. Fac. 2011;17:S125–S129. [Google Scholar]
- 59.Yangilar F. Evaluation of the effects of chitosan/whey protein (CWP) coating on quality of Göbek Kashar cheese during ripening. Korean J. Food Sci. An. 2015;35:216–224. doi: 10.5851/kosfa.2015.35.2.216. [DOI] [Google Scholar]
- 60.Yangilar F., Yildiz P. O. Casein/natamycin edible films efficiency for controlling mould growth and on microbiological, chemical and sensory properties during the ripening of Kashar cheese. J. Sci. Food Agric. 2016;96:2328–2336. doi: 10.1002/jsfa.7348. [DOI] [PubMed] [Google Scholar]
- 61.Yildirim M., Gulec F., Bayram M., Yildirim Z. Properties of Kashar cheese coated with casein as a carrier of natamycin. Ital. J. Food Sci. 2006;18:127–138. [Google Scholar]
- 62.Yilmaz F., Dagdemir E. The effects of beeswax coating on quality of Kashar cheese during ripening. Int. J. Food Sci. Technol. 2012;47:2582–2589. doi: 10.1111/j.1365-2621.2012.03137.x. [DOI] [Google Scholar]
- 63.Zantar S., Yedri F., Mrabet R., Laglaoui A., Bakkali M., Zerrouk M. H. Effect of Thymus vulgaris and Origanum compactum essential oils on the shelf life of fresh goat cheese. J. Essent. Oil Res. 2014;26:76–84. doi: 10.1080/10412905.2013.871673. [DOI] [Google Scholar]