Abstract
In nature Siberian hamsters utilize the decrement in day length following the summer solstice to implement physiological adaptations in anticipation of the forthcoming winter, but also exploit an intrinsic interval timer to initiate physiological recrudescence following the winter solstice. However, information is lacking on the temporal dynamics in natural photoperiod of photoperiodically regulated genes and their relationship to physiological adaptations. To address this, male Siberian hamsters born and maintained outdoors were sampled every month over the course of one year. As key elements of the response to photoperiod, thyroid hormone signalling components were assessed in the hypothalamus. From maximum around the summer solstice (late-June), Dio2 expression rapidly declined in advance of physiological adaptations. This was followed by a rapid increase in Mct8 expression (T3/T4 transport), peaking early-September before gradually declining to minimum expression by the following June. Dio3 showed a transient peak of expression beginning late-August. A recrudescence of testes and body mass occurred from mid-February, but Dio2 expression remained low until late-April of the following year, converging with the time of year when responsiveness to short-day length is re-established. Other photoperiodically regulated genes show temporal regulation, but of note is a transient peak in Gpr50 around late-July.
The Siberian hamster (Phodopus sungorus) is a seasonal mammal and an exemplar for the pre-emptive physiological adaptations made by seasonal mammals to ensure survival and maximize reproductive success1,2,3. Physiological adaptations including body weight and regression of the reproductive axis, in preparation for winter are thought to be triggered by the predictable decrease in day length following the summer solstice, but recrudescence to the summer phenotype is orchestrated independently of day length (known as the photorefractory response) by an endogenous interval timer4,5,6. In continuous short days (SD), once recrudescence has taken place, the summer physiological state remains indefinitely if hamsters are not re-exposed to long-day (LD) photoperiod. Recrudescence requires 10–15 weeks of exposure to LD photoperiod or 4–6 weeks after the vernal equinox in simulated natural photoperiod, to initiate a SD physiological response or respond to melatonin infusions7,8. However, the mechanism involved in the development of refractoriness under SD conditions is unknown, as is the mechanism which terminates refractoriness and allows responsiveness to a SD melatonin signal.
From studies performed in both seasonal mammals and birds the current view of the mechanism governing seasonal phenotype centres on photoperiod regulated availability of thyroid hormone (T3) to the hypothalamus9. In LD, hypothalamic T3 availability is facilitated by TSH produced by the pars tuberalis (PT) acting on hypothalamic tanycytes to increase the expression of type 2 deiodinase (Dio2), enabling a higher rate of conversion of T4 to T3 to drive LD physiology. To enable SD adaptations, TSH expression is abolished which coupled with catabolism of T4 and T3 to inactive metabolites by SD induced type 3 deiodinase (Dio3) expression removes the thyroid hormone drive and initiates SD physiology10,11,12,13. In addition to deiodinase genes, other genes change expression in response to a change in photoperiod including the thyroid hormone transporter monocarboxylate transporter 8 (Mct8), intermediate filament proteins Nestin and Vimentin, components of retinol transport and metabolism, an orphan G-protein coupled receptor, Gpr50 in tanycytes14,15, Vgf (non-acronymic) in the dorsal posterior ARC (dmpARC) and Srif (somatostatin) in the arcuate nucleus (ARC)11,14,15,16. However, the role of each of these genes in adapting physiology to the different seasons is still unknown.
Most studies investigating mechanistic basis of seasonal physiology are performed in a laboratory setting using static photoperiods representing long days (LD) or short days (SD), with hamsters abruptly transferred between static photoperiods to initiate physiological adaptations. While this approach has been instrumental in identifying the role of T3 and genes likely to contribute to the adaption of physiological states, static photoperiods do not reflect the natural environment where the hamster will experience a daily change in day length over the course of the year. The decremental changes following the summer solstice are relevant to registering photoperiodic history and have a strong synchronising effect on the recrudescence of reproductive physiology and body weight5,8,17,18. Therefore the aim of this study was to place gene expression changes into a temporal context in relation to body weight and reproductive physiology. The results reveal an orchestration of gene expression that may not necessarily be predicted from experiments performed with static photoperiods representing LD and SD and indicate that temporal organization will be important to seasonal physiological responses.
Results
Components of thyroid hormone metabolism and transport
The date of cull and the corresponding day length hours for hamsters born during March and April 2009 and culled between June and December 2009 are given in Table 1 and those born between March and July 2008 and culled between January and June 2009 are given in Table 2.
Table 1. Summer to winter transition.
| Date of cull (2009) | Day length (h:min) |
|---|---|
| 12th June | 16:44 |
| 24th July | 15:55 |
| 4th September | 13:24 |
| 16th October | 10:36 |
| 27th November | 8:12 |
| 21st December | 7:40 |
Date of cull and corresponding day length experienced by Siberian hamsters born between March and April 2009 and housed outdoors in natural photoperiod. Note the summer solstice day length duration on the 21st June was 16 h:48 min.
Table 2. Winter to summer transition.
| Date of Cull (2009) | Day length (h:min) |
|---|---|
| 20th January | 8:27 |
| 3rd February | 9:12 |
| 17th February | 10:05 |
| 3rd March | 11:01 |
| 17th March | 11:58 |
| 31st March | 12:55 |
| 21st April | 14:19 |
| 19th May | 15:56 |
| 16th June | 16:47 |
Date of cull and corresponding day length experienced by Siberian hamsters born between March and April 2008 housed outdoors in natural photoperiod.
The body weight of a cohort of hamsters born during March and April ’09 representing the summer to winter transition and sacrificed on 21st December as the final cohort, peaked at 10th August (37.9 ± 1.6 g vs 26.9 ± 1.1 g at 18th May) and declined thereafter, achieving significance at 12th October (31.7 ± 1.6 g, P = 0.002; see Supplementary Fig. S1). Hamsters born between the months of March and July ‘08 entered the experiment at the beginning of January ’09 and represent the winter to summer transition. The representative cohort killed 16th June ’09, started at 27.3 ± 0.3 g on the 6th January and gradually increased, becoming significantly different by 10th February (30.2 ± 1.2 g, P = 0.002, see Supplementary Fig. S1). Parallel changes in body weight, lean and fat tissue masses were evident in hamster cohorts used for analysis of hypothalamic gene expression (see Supplementary Fig. S1).
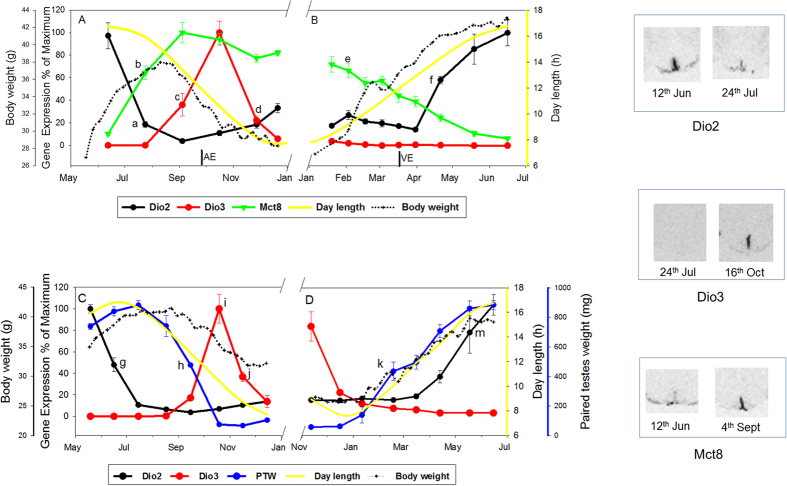
In early summer expression of Dio2 in tanycytes was close to maximal value at 12th June, declining sharply and were close to minimum by the 24th July (18%, P < 0.001), remaining low for the remainder of the year (Fig. 1A). However, body mass continued to increase after July before declining from late August (Fig. 1A and see Supplementary Fig. S1). Dio2 expression remained low until the 21st April when expression increased (58%, P < 0.001) achieving 100% by 16th June (Fig. 1B). Similar temporal kinetics were found in a second experiment (Fig. 1C,D). In the summer to winter transition Dio2 was maximal at 19th May, declining to 45% of maximum at the 16th June (P < 0.001) and continued to decline thereafter. Similarly body and testicular mass continued to increase for several weeks after the 16th June (Fig. 1C). In the winter to summer transition, Dio2 expression remained suppressed until 13th April when the first rise was observed (Fig. 1D).
Figure 1. Temporal profile of gene expression for thyroid hormone signalling components, DIO2, DIO3 and MCT8 in the hypothalamus of Siberian hamsters in natural photoperiod over one year.
Panel A: summer to winter transition and panel B winter to summer transition - experiment 1. In these panels, Dio2 is represented by a solid black line; Dio3 - red line; Mct8 - green line; dotted black line - body weight change of the final cohort of hamsters born between March and April 2009 and killed 21st December 2009 in panel A, and the final cohort of hamsters born March and July 2008 and killed 16th June 2009 in panel B. ‘a’ P < 0.001 Dio2 significantly decreased relative to 12th June. ‘f’ P < 0.001 Dio2 relative to 31st March; ‘b’ P < 0.001 Mct8 relative to June; ‘e’ P < 0.01 Mct8 relative to a peak in August of the previous year; ‘c’ P < 0.001 Dio3 relative to July; ‘d’ P < 0.001 Dio3 relative to October. Panel C: summer to winter transition and panel D winter to summer transition - experiment 2. In these panels, Dio2 is represented by a solid black line; Dio3 - red line; paired testes weight – blue line; dotted black line - body weight change of the final cohort of hamsters born between 6th February and 21st March and killed 14th December 2010 in panel C, and the final cohort of hamsters born between 1st June and 3rd July 2010 and killed 16th June 2011 in panel D. ‘g’ P < 0.001 Dio2 significantly decreased from 16th June relative to 12th May; ‘m’ P < 0.001 Dio2 significantly increased 18th May relative to 13th March. ‘i’ P < 0.001 Dio3 relative to preceding time points; ‘j’ P < 0.01 Dio3 significantly decreased relative to 19th October. ‘h’ paired testes weight significantly decreased relative to 15th July; ‘k’ P < 0.001 paired testes weight significantly increased relative to 11th January. Superimposed on all plots (yellow line) is the actual day length over the months of the year for this transitional period (http://www.timeanddate.com/sun/germany/hannover). AE = autumnal equinox, VE = vernal equinox. N = 6–7 per group. Also Shown are representative autoradiograph images for Dio2, Dio3 and Mct8 at the dates indicated.
Dio3 expression in hypothalamic tanycytes was first detected at the 4th September (36%, P = 0.002) with peak expression at 16th October (P < 0.001), declining sharply by the 27th November (22%, P < 0.001), undetectable by 21st December (Fig. 1A) and during the following months up to 16th June (Fig. 1B). Very similar temporal kinetics were found in a second experiment. In the summer to winter transition Dio3 was undetectable until 15th September, peaking at 19th October (P < 0.001, Fig. 1C) and then declined sharply by 16th November (P < 0.01). In the winter to summer transition Dio3 was undetectable from December through to June (Fig. 1D).
Mct8 expressed in hypothalamic tanycytes rose from a low expression (10%) at the 12th June to 64% by July 24th (P < 0.001) with the highest level at 4th September (Fig. 1A). From September, Mct8 gradually declined to almost complete absence by 16th June, with the decline becoming significant at the 20th January (P = 0.007, Fig. 1B).
Non-thyroid hormone signalling genes
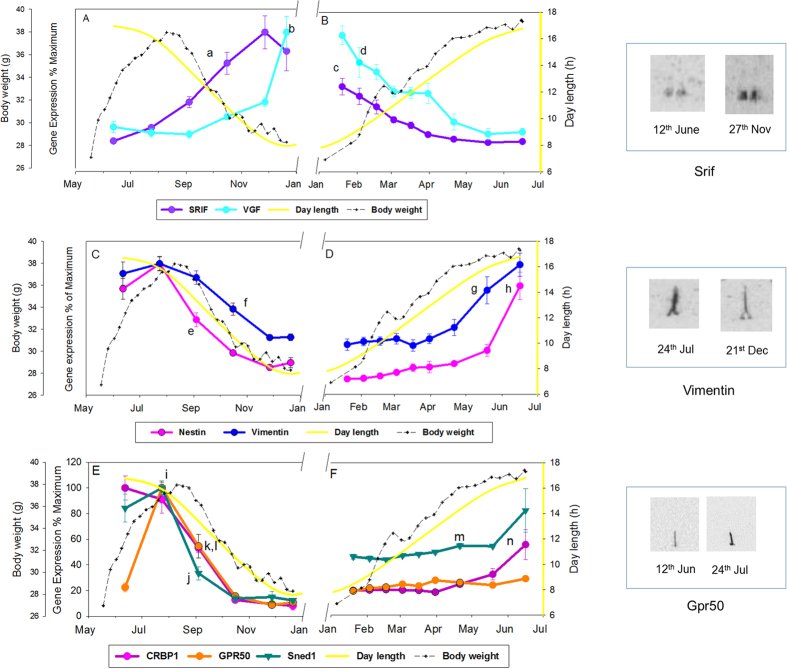
In experiment 1, in situ hybridization was used to investigate the expression of additional neuronal and ependymal expressed genes not involved in thyroid hormone signalling. In the first summer, Srif in the ARC gradually increased from 5% at 12th June to 100% expression by 27th November, with values achieving significance relative to June by 16th October (P < 0.001, Fig. 2A). Srif subsequently declined and was significantly reduced compared to peak value by the 20th January (P = 0.007, Fig. 2B) with expression continuing to decline gradually to less than 10% by 16th June. A similar response was observed for Vgf expression in the dmpARC which gradually increased from 16th October, becoming significant at the 21st December (100% P < 0.001, Fig. 2A), then declining to less than 10%, achieving significance at 56% of maximum (P < 0.001) at the 3rd February (Fig. 2B).
Figure 2. Temporal profile of neuronal or ependymal expressed genes in the hypothalamus of the Siberian hamster in natural photoperiod over 1 year.
Panels A,C and E represent the summer to winter transition while panels B,D and F represent the winter to summer transition in experiment 1. Panels A and B: - Srif is represented by the purple line; Vgf – light blue. ‘a’ P < 0.001 Srif increased relative to 12th June; ‘c’ P < 0.001 Srif decreased relative 27th November of the previous year. ‘b’ P < 0.001 Vgf increased relative to 12th June; ‘d’ P < 0.01 Vgf decreased relative to 21st December of the previous year. Panels C and D: Nestin is represented by the pink line and vimentin - dark blue line. ‘e’ P < 0.001 Nestin decreased relative to 24th July; ‘h’ Nestin increased relative 19th May. ‘f’ P < 0.001 Vimentin decreased relative to 24th July; ‘g’ P < 0.001 Vimentin relative to 21st April. Panels E and F: Gpr50 is represented by the orange line, Sned 1 – dark green, Crpb1 – dark pink. ‘i’ P < 0.001 Gpr50 increased relative to 12th June; ‘k’ P < 0.001 Gpr50 decreased relative to 24th July. ‘j’ Sned 1 decreased relative 24th July; ‘m’ P < 0.05 Sned 1 relative to the minimal value achieved in December of the previous year. ‘l’ P < 0.001 Crpb1 decreased relative to 24th July; ‘n’ P < 0.001 Crpb1 relative to the minimal value at the 31st March. Superimposed on all plots (yellow line) is the actual day length over the months of the year for this transitional period (http://www.timeanddate.com/sun/germany/hannover). Dashed black line on plots represents the body weight change of the final cohort of hamsters born between March and April 2009 and killed 21st December 2009 in panels A,C and E, and the final cohort of hamsters born March and July 2008 and killed 16th June 2009 in panels B,D and F. N = 6–7 per group except April and May for Vgf where n = 5. Also shown are representative autoradiographs for Srif, Vimentin and Gpr50.
Nestin expression in hypothalamic tanycytes increased between 12th June (77%) and 24th July (100%, P = 0.033, Fig. 2C). By the 4th September, nestin expression was reduced (49%, P < 0.001) and continued to decline thereafter (Fig. 2C). Vimentin in tanycytes showed a similar pattern, with maximal expression at the 24th July declining to 34%, by the 16th October (P < 0.001, Fig. 2C). Following the winter solstice, Nestin gradually increased but was not significant until 16th June (71%, P < 0.001, Fig. 2D). Vimentin showed a similar pattern, starting at 32% at the 20th January, gradually increasing, becoming significant relative to the 16th March at 19th May (67%, P < 0.001) and further increased by 16th June (85%, Fig. 2D).
The highest level of cellular retinol binding protein (Crbp1) expression occurred at 12th June, declined sharply by 16th October (12%), reaching significance at the 4th September (P < 0.001, Fig. 2E) and remained low between January and April. Expression significantly increased at the 16th June achieving 46% of maximum expression levels (P = 0.015 relative to 15th May and earlier, Fig. 2F). Expression of the insulin responsive transcription factor, Sned1 was maximal at the 24th July and had declined sharply by 4th September (33%, P < 0.001) and continued to decline to 12% of maximum (Fig. 2E). Following the winter solstice Sned1 had a higher level of expression from the 20th January becoming significant at 21st April (P = 0.045), increasing to 77% expression by the 16th June (Fig. 2F). Gpr50 expression in tanycytes increased sharply to maximum expression levels between the 12th June and 24th July (100%, P < 0.001) and sharply declined by the 4th September (54%, P < 0.001, Fig. 2E). Gpr50 was not detected after the winter solstice at all time points up to 16th June (Fig. 2F).
Discussion
To date, the majority of studies of hypothalamic gene expression in response to photoperiod in any seasonal mammalian species have been performed by transferring animals abruptly between static long photoperiod (usually 16 h light: 8 h dark) and short photoperiod (usually 8 h light:16 h dark). In the Siberian hamster, this approach has successfully identified a substantial number of gene expression changes in several areas of the hypothalamus including the ARC, dmpARC and tanycytes14. Furthermore, only a few studies have investigated temporal changes in gene expression following a change in photoperiod, but again these studies have been performed by abruptly switching hamsters from LD to SD or acclimated hamsters in SD to LD11,19,20. Nevertheless, these studies revealed an intriguing temporal dependent expression of the mRNA for Dio311. Together with experiments using hypothalamic microimplants releasing T3 into the hypothalamus these data provided evidence for a role of T3 in driving the LD physiological state, placing gene expression changes in thyroid hormone metabolism and transport at the centre of the seasonal regulatory mechanism11,21.
However, in their natural ecological domain, the hamster will experience an incremental increase or decrease in day length over the course of the year. These incremental changes in day length are relevant to registering photoperiodic history and have a strong synchronising effect on the recrudescence of reproductive physiology and body weight the following spring in juvenile hamsters born either side of the summer solstice5,8,17,18. Therefore aim of this study was to investigate the temporal kinetics of photoperiod regulated genes in a natural photoperiod over the period of one year in order to place hypothalamic gene expression, particularly genes involved in thyroid hormone signalling, into a temporal context in relation to body weight and reproductive physiology.
Although the current dogma would suggest elevated DIO2 is required to drive long day physiology, surprisingly the present data reveal a decline in Dio2 expression can be initiated before day length shortens. Nevertheless, while Dio2 declines there is a continued increase in body and testes mass indicating a programmed trajectory of growth, a notion supported by continued growth in juvenile hamsters following the large decrease in post-weaning Dio2 expression22,23. One explanation for the temporal kinetics of Dio2 expression in late spring/early summer after birth, is a decline programmed in utero or early post-natal development in these relatively young animals and would be consistent with evidence for a maternal influence of perceived photoperiod either in static or simulated natural photoperiod17,24. Although we were unable to quantify TSHβ expression in our hamsters due fragmentation and loss of PT tissue on removal of the brain or during sectioning of brain tissue, a second explanation for the decline in Dio2 observed in hamsters during the first June after birth could be a LD refractory mechanism similar to that which occurs in sheep, which show a decline in PT derived TSHβ upon prolonged exposure to LD with a concurrent decrease in Dio2 expression in the ependymal layer25,26.
Whether programmed in utero or part of a LD refractory mechanism, the decline in Dio2 expression in LD hamsters offers an explanation for differences in responsiveness of Dio2 to SD photoperiod seen in some studies which may simply reflect the extent to which Dio2 expression has declined prior to SD exposure11,14.
Dio2 expression remained suppressed following the winter solstice until mid-April while body mass and testicular recrudescence was evident in early February. This would suggest that a large increase in hypothalamic T3 is unlikely to be required to initiate the LD phenotype following the winter solstice.
However, the date of the first observed significant increase in Dio2 expression may be highly relevant for the mechanism of breaking photorefractoriness. In studies using simulated natural photoperiod, hamsters have been shown to be refractory to SD until around 4–6 weeks after the vernal equinox at a durational day length of 13 h 49 min or greater8. At the 21st April when Dio2 mRNA was first measureable at 58% of maximum, the day length duration was 14 h 19 min, close to the duration of day length required to break photorefractoriness. Therefore we propose that a substantial rise in T3 would occur at this time and signals to break the photorefractory state. This would be consistent with studies in sheep where thyroidectomy prevents emergence from the photorefractory state27.
This finding has implications for the current dogma on the role of the PT from which LD induced TSH through a clock dependent mechanism of secretion is viewed as the driver of LD physiology28,29. While it is undisputed that TSH can induce Dio2 expression in sheep and hamsters12,13, TSH is unlikely to be involved in the refractory response to SD since Tshβ is not produced in SD photorefractory hamsters or sheep25,30. A recent study in pinealectomized European hamsters held in LD over the course of one year did show a correlation between Tshβ expression in the PT and hypothalamic Dio2 expression31 leading to the suggestion that a circannual rhythm of PT TSH secretion underpins the circannual rhythm in seasonal physiology. However, hamsters in that study were sampled when physiological adaptations had been established with a possibility that important temporal information prior to the changes had been missed.
The temporal kinetics of Dio3 expression in natural photoperiod reflect the kinetics we have previously seen in hamsters transferred from static LD to SD11. Together, current and previous studies suggest a decline in photoperiod is the trigger for Dio3 expression, but the transient expression profile of Dio3 implicates an endogenous mechanism for the offset of expression. A candidate for this mechanism may involve DNA methylation on the Dio3 promoter by photoperiod regulated DNA methyltransferase (DNMT3b) activity32. It is notable that the duration of day length after the summer solstice (16 h 48 min) at which Dio3 increases (approximately 13 h 24 min) is convergent with the critical day length (13 h) determined to be required in static photoperiod experiments to initiate testicular regression33, suggesting the onset of Dio3 expression is a principal event in the initiation of physiological adaptations.
During the decline of Dio2 and before the peak of Dio3 expression, Mct8 expression increases to peak early September. This is coincident with the first increase in Dio3 expression and the time at which body mass and testes start to decline. As DIO2 is a protein with a short half-life34, reduced levels of Dio2 expression would lead to a relatively hypothyroid state in the hypothalamus. Therefore one explanation for the increase in Mct8 expression would be to facilitate synthesis of more thyroid hormone transporter in an attempt to compensate for a reduction in DIO2 enzyme. However, limited evidence would suggest ependymal Mct8 expression is unchanged or decreases in the hypothyroid state35,36. Given that a decline of T3 availability to the hypothalamus is a requirement for the induction of SD physiology, this inverse relationship of Mct8 and Dio2 is not consistent with this transporter maintaining the availability of T3 from the circulation or from the conversion of T4 to T3. Human MCT8 can both transport T3 and T4 in and out of a cell with the direction of transport is likely to depend on a concentration gradient between the extracellular and intracellular environment37. Human MCT8 has an approximately 3-fold higher affinity for T3 over T438. If hamster MCT8 shows similar characteristics, it is likely that due to a 40–50 fold higher concentration of circulating T4 over T3 in the SD Siberian hamster39, T4 will most likely be the principal form of thyroid hormone transported by MCT8. This would be consistent with our hypothesis that this increase in Mct8 contributes to a regulatory mechanism of thyroid hormone availability to the hypothalamus. This mechanism may involve the provision of T4 to enable substrate induced decrease in DIO2 protein half-life, or together with activation of Dio3 expression, T4 conversion to rT3 which is inhibitory for DIO2 activity. Both actions would aid efficient and further depletion of hypothalamic concentrations of T3 by possible DIO2 protein synthesised from low residual Dio2 mRNA40,41.
Based on a hypothesis that increased T4 transport via MCT8 augments a reduction in DIO2 activity and T3 generating capacity, the corollary of this is a decrease in MCT8 would lengthen the half-life of DIO2 deiodinase. Although we cannot rule out small increases in T3 produced by a small increase in Dio2 expression which are below the limit of detection by autoradiography, we would propose the hypothesis that the remarkable gradual decline in Mct8 expression seen from late autumn, coupled with a decrease in circulating T439 permits a gradual increase T3 production. This would allow a gradual increase in body mass and reproductive recrudescence that is perfectly timed with the emergent environmental conditions of spring. Thus the date at which Mct8 was detectably reduced below peak level may hold significance as it falls just ahead of the period when testicular recrudescence and body weight increases occur. This leads us to propose that gradual decline of Mct8 may be instrumental in permitting the refractory response. Consistent with this is the previous observation of reduced Mct8 expression in refractory hamsters in a continuous static SD experiment14. Mct8 continues a decline in winter and spring and by the time of the first significant rise in Dio2, Mct8 is almost at the lowest value, as might be expected to permit maximal T3 production.
Srif (somatostatin) has recently been shown to be up-regulated in the ARC in static SD and reduced by icv TSH indicating negative regulation by T313,22,42. ARC derived somatostatin is proposed as a potential direct or indirect inhibitor of growth hormone secretion from pituitary somatotrophs14,42,43 and the kinetics of Srif expression observed in this study would be consistent with such a role, with maximal expression near the winter solstice when body weight is at the nadir, then through winter and spring of the following year showing a gradual decline. The similarity in the rate of Srif decline, as a gene negatively regulated by T3, parallels the decline of Mct8 and would support the notion that this is reflective of a gradual increase in T3.
The role of Crbp1, vimentin and nestin in the context of the seasonal mammal is not yet clarified. However, a resurgence in Dio2 expression occurred before significant increases in LD regulated genes such as Crbp1, nestin, vimentin and Gpr50 and it would seem likely the role of these genes is downstream of T3 action.
Sned1 is an insulin responsive transcription factor44, but regulated by T3 and photoperiod in tanycytes of rats and hamsters respectively35. LD hamsters have higher levels of circulating insulin in LD42; therefore the increase from January of Sned1 may reflect increasing levels of circulating insulin as the LD phenotype develops after the winter solstice rather than an increase in T3.
In static photoperiods expression of Gpr50 is higher in LD than SD photoperiods15 and might therefore be considered to be a LD expressed gene, possibly regulated by a LD induced TSH drive of PT origin or resultant T3 production. However, the temporal kinetics in natural photoperiod would suggest that Gpr50 may not be regulated directly by TSH or T3 as Gpr50 expression is ascendant as Dio2 and photoperiod decreases, suggesting that this gene may be under the regulation of an intrinsic timing mechanism. A definitive function for Gpr50 is lacking, but Gpr50 knock-out mice are susceptible to food deprivation induced torpor45. The timing of this transient peak of Gpr50 expression may hold significance for the timing of the onset of torpor in the hamster which occurs after 12 weeks of exposure in SD or around mid-December46. By June following the winter solstice, Gpr50 expression only showed a small increase but this was not significant relative to the lowest value in January.
Conclusions
Overall, the data illustrate the orchestration of gene expression in the hypothalamus of the Siberian hamster over the course of changing seasons. Strikingly there is a temporal restriction of components involved in thyroid hormone signalling including Dio2, Dio3 and Mct8. While T3 can undoubtedly drive gene expression and downstream pathways resulting in LD physiology, the data suggest that once established, a high level expression of Dio2 is not necessary for the maintenance of LD phenotype. Timing of maximal Mct8 expression occurs as body mass and testes regress and is coincidental with the emergence of Dio3 expression indicating that the temporal relationship of these three components may be necessary to achieve timely physiological adaptations. A resurgence of Dio2 expression in late spring convergent with the time when photorefractoriness is broken, suggests that a large increase in hypothalamic T3 is not required to drive LD physiology but may be a signal to reset the sensitivity to melatonin.
Materials and Methods
Animals and tissue collection
Animal husbandry and all experiments were in accordance with the German Animal Welfare Act and approved by the Lower Saxony State Office for Consumer Protection and Food Safety. Djungarian hamsters (Phodopus sungorus) were reared under natural photoperiod and natural Ta (Hannover, Germany; ~52°N latitude). They were kept singly in polycarbonate cages (20.7 × 14 × 26.5 cm) and supplied with breeding diet (Altromin 7014) and tap water ad libitum, supplemented by a slice of apple once a week. Cages were provided with wood shavings and tissue for nest building. All animals were weighed weekly throughout the experiment.
Experiment 1 was carried out in two phases (timeline see Supplementary Fig. S1). For the winter-summer transition, 62 male hamsters were born under natural photoperiod and Ta from 30th March to 16th July 2008 and were killed every 2–4 weeks (N = 6–7) from 6th January to 16th June 2009. Correspondingly, these animals had experienced one winter and were at the age of 9–13 months at the respective time point of killing. For the summer-winter transition, 42 male hamsters were born under natural photoperiod, between 16th February and 22nd April 2009. A cohort of seven hamsters were killed every 4–6 weeks between the 12th June and 21st December. They were 3–9 months of age when they were culled. All brains were stored at −80 °C until processed for coronal sections.
In the second experiment (timeline see Supplementary Fig. S2), for the autumn-spring transition group, 48 hamsters were born in natural photoperiod from 1st June to 3rd July 2010 reared under natural photoperiod. From 12th November 2010 a cohort of 6 hamsters were sacrificed at 4–5 week intervals resulting a one cohort per month from 12th November 2010 to 16th June 2011. For the summer-winter transition group 48 hamsters were born between 6th February and 21st March 2011. From the 19th May one cohort of 6 hamsters were sacrificed every 4–5 weeks providing one cohort per month between 19th May and 14th December.
Hamsters were culled 3–4 hours after sunrise (www.timeanddatee.com/sun/germany/hannover). Hamsters were killed with carbon dioxide. Brains were immediately dissected, frozen on dry ice and then stored at −80 °C for later procedure of in situ hybridisation. Whole animal carcasses (minus the head) were sealed in plastic bags and also stored at −80 °C. Later, hamster carcasses sealed in their plastic bags were thawed and heated to 37 °C before being individually scanned by magnetic resonance imaging (MRI) (Echo MRI ™, Whole Body Composition Analyser, Echo Medical Systems, Houston, Texas).
Riboprobes
Riboprobes complementary to fragments of the required DNA sequences were generated from Djungarian hamster, mouse or rat brain cDNAs by RT-PCR as described previously11,15,16,39,47,48,49. Templates for riboprobe synthesis were generated by PCR amplification of the insert from plasmid DNA using M13 forward and reverse primers which spans both insert and polymerase transcription binding and initiation sites in the host vectors. One hundred nanograms of PCR product was used in an in vitro transcription reaction with T7, T3 or SP6 polymerases as appropriate in the presence of 35S-uridine 5-triphosphate (Perkin-Elmer, Buckinghamshire, UK) for radioactive in situ hybridization.
In situ hybridization
Coronal sections of the hypothalamic ARC region (14 μm thick) for each experiment were cut and analysed together. Sections were collected onto glass slides. Adjacent sections were mounted on consecutively numbered slides, permitting a number of mRNAs to be localised and quantified in each brain.
Briefly, frozen slides were fixed in 4% PFA in 0.1 m PBS, acetylated in 0.25% acetic anhydride in 0.1 m TEA, pH 8. Radioactive probes (approximately 106 cpm) were applied to the slides in 70 μl hybridization buffer containing 0.3 M NaCl, 10 mM Tris-HCl (pH 8), 1 mM EDTA, 0.05% tRNA, 10 mM DTT, 0.02% Ficoll, 0.02% polyvinylpyrrolidone, 0.02% BSA, and 10% dextran sulfate. Hybridization was performed overnight at 58 °C. Following hybridisation, slides were washed in 4x SSC (1x SSC is 0.15 M NaCl, 15 mM sodium citrate), then treated with ribonuclease A (20 μg/μl) at 37 °C and finally washed in 0.1x SSC at 60 °C. Slides were dried and apposed to autoradiographic Biomax MR film (Kodak, Rochester, New York) for several hours to days.
Image analysis
Films were scanned at 600 dpi on an Umax scanner and quantification was carried out using Image J 1.37v software (Wayne Rasband, National Institutes of Health, USA) or Image-Pro PLUS analysis software (v4.1.0.0, Media Cybernetics, Wokingham, USA). For each probe, three sections spanning a selected region of the hypothalamus were chosen for image analysis. Integrated optical density for each selected region was obtained by reference to a standard curve generated from the autoradiographic 14C microscale (Amersham). An average (with SEM) for the integrated optical densities for all sections of one animal and for all animals in one group was calculated. The highest value of one group in an assay was set to 100% expression value, and other treatment values were calculated accordingly.
Statistics
The first detectable increase or decrease in body weight was determined using a two-way ANOVA, with main effect terms for time and animal ID followed by a Tukey post-hoc test.
All other statistical analysis was by one-way ANOVA followed by post-hoc Holm-Sidak test for multiple comparisons. In all cases the threshold for significance was P < 0.05.
Additional Information
How to cite this article: Petri, I. et al. Orchestration of gene expression across the seasons: Hypothalamic gene expression in natural photoperiod throughout the year in the Siberian hamster. Sci. Rep. 6, 29689; doi: 10.1038/srep29689 (2016).
Supplementary Material
Acknowledgments
Funding for work in the laboratory of PB was supported by Scottish Government Rural and Environment Science and Analytical Services Division, BBSRC (grant BB/M001504/1), British Society for Neuroendocrinology (research visit grant to IP). Work in the laboratory of SS was supported by a grant from the DFG (Ste 331/8-1). We thank Siegried Hilken, Marianne Brüning, Dr. Esther Lipokatic-Takacs and Dr. Frank Scherbarth at UVMH for technical assistance. We thank Graham Horgan of Bioinformatics, Statistics Scotland for assistance with some of statistical tests.
Footnotes
Author Contributions P.B., A.H. and S.S. conceived the project. I.P., D.W. and J.F.-C. performed in situ hybridizations and image analysis. I.P. and V.D. performed experiments. I.P. and P.B. analysed the data. P.B. and S.S. wrote the paper.
References
- Figala J., Hoffmann K. & Goldau G. Zur Jahresperiodik beim Dsungarischen Zwerghamster Phodopus sungorus Pallas. Oecologia 12, 89–118 (1973). [DOI] [PubMed] [Google Scholar]
- Hoffmann K. The influence of photoperiod and melatonin on testis size, body weight, and pelage colour in the Djungarian hamster (Phodopus sungorus). J. Comp. Physiol. 85, 267–282 (1973). [Google Scholar]
- Schlatt S., Niklowitz P., Hoffmann K. & Nieschlag E. Influence of short photoperiods on reproductive organs and estrous cycles of normal and pinealectomized female Djungarian hamsters, Phodopus sungorus. Biol. Reprod. 49, 243–250 (1993). [DOI] [PubMed] [Google Scholar]
- Hoffmann K. Effects of short photoperiods on puberty, growth and moult in the Djungarian hamster (Phodopus sungorus). J. Reprod fertility 54, 29–35 (1978). [DOI] [PubMed] [Google Scholar]
- Gorman M. R. A plastic interval timer synchronizes pubertal development of summer- and fall-born hamsters Am. J. Physiol. Reg. Integ. Comp. Physiol. 281, 50–55 (2001). [DOI] [PubMed] [Google Scholar]
- Gorman M. R. & Zucker I. Pattern of change in melatonin duration determines testicular response in Siberian hamsters, Phodopus sungorus. Biol Reprod. 56, 668–673 (1997). [DOI] [PubMed] [Google Scholar]
- Kauffman A. S., Freeman D. A. & Zucker I. Termination of neuroendocrine refractoriness to melatonin in Siberian hamsters (Phodopus sungorus). J. Neuroendocrinol. 15, 191–196 (2003). [DOI] [PubMed] [Google Scholar]
- Butler M. P., Turner K. W., Park J. H., Schoomer E. E., Zucker I. & Gorman M. R. Seasonal regulation of reproduction: altered role of melatonin under naturalistic conditions in hamsters. Proc R Soc Lond B 277, 2867–2874 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane Y. & Yoshimura T. Universality and diversity in the signal transduction pathway that regulates seasonal reproduction in vertebrates. Frontiers in Neurosci. 8, 115 10.3389/fnins.2014.00115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao N. et al. Thyrotrophin in the pars tuberalis triggers photoperiodic response. Nature 452, 317–322 (2008). [DOI] [PubMed] [Google Scholar]
- Barrett P. et al. Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinol. 148, 3608–3617 (2007). [DOI] [PubMed] [Google Scholar]
- Hanon E. A. et al. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr. Biol. 18, 1147–1152 (2008). [DOI] [PubMed] [Google Scholar]
- Klosen P., Sébert M., Rasri K., Laran-Chich M. & Simonneaux V. TSH restores a summer phenotype in photoinhibited mammals via the RF-amides RFRP3 and kisspeptin. FASEB J. 27, 2677–2686 (2013). [DOI] [PubMed] [Google Scholar]
- Herwig A. et al. Hypothalamic Ventricular Ependymal Thyroid Hormone Deiodinases Are an Important Element of Circannual Timing in the Siberian Hamster (Phodopus sungorus). PLoS ONE 8, e62003 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett P. et al. Photoperiodic regulation of cellular retinol binding protein, GPR50 and nestin in tanycytes of the third ventricle ependymal layer of the Siberian hamster. J. Endocrinol. 191, 687–698 (2006). [DOI] [PubMed] [Google Scholar]
- Barrett P. et al. Photoperiodic regulation of histamine H3 receptor and VGF messenger ribonucleic acid in the arcuate nucleus of the Siberian hamster. Endocrinol. 146, 1930–1939 (2005). [DOI] [PubMed] [Google Scholar]
- Butler M. P. et al. Simulated natural day lengths synchronze seasonal rhythms of asychronously born mal Siberian hamsters. Am. J. Physiol. Reg. Integ. Comp. Physiol. 293, R402–R412 (2007). [DOI] [PubMed] [Google Scholar]
- Butler M. P., Trumbull J. J., Turner K. W. & Zucker I. Timing of puberty and synchronization of seasonal rhythms by simulated natural photoperiods in female Siberian hamsters. Am. J. Physiol. Reg. Integ. Comp. Physiol. 293, R413–R420 (2007). [DOI] [PubMed] [Google Scholar]
- Ross A. W. et al. Temporal changes in gene expression in the arcuate nucleus precede seasonal responses in adiposity and reproduction. Endocrinol. 146, 1940–1947 (2005). [DOI] [PubMed] [Google Scholar]
- Barrett P. et al. Short photoperiod-induced decrease of histamine H3 receptors facilitates activation of hypothalamic neurons in the Siberian hamster. Endocrinol. 150, 3655–3663 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. et al. Effects of manipulating hypothalamic triiodothyronine concentrations on seasonal body weight and torpor cycles in Siberian hamsters. Endocrinol. 153, 101–112 (2012). [DOI] [PubMed] [Google Scholar]
- Herwig A., Petri I. & Barrett P. Hypothalamic Gene Expression Rapidly Changes in Response to Photoperiod in Juvenile Siberian Hamsters (Phodopus sungorus). J Neuroendocrinol. 24, 991–998 (2012). [DOI] [PubMed] [Google Scholar]
- Prendergast B. J., Pyter L. M., Kampf-Lassin A., Patel P. N. & Stevenson T. J. Rapid induction of hypothalamic iodotyronine deiodinase expression by photoperiod and melatonin in juvenile Siberian hamsters (Phodopus sungorus). Endocrinol 154, 831–841 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson M. H., Elliot J. A. & Goldman B. D. Maternal transfer of photoperiodic information influences the photoperiodic response of prepubertal Djungarian hamsters (Phodopus sungorus sungorus). Biol Repord 34, 664–669 (1986). [DOI] [PubMed] [Google Scholar]
- Saenz de Miera C. et al. Circannual variation in thyroid hormone deiodinases in a short-day breeder. J Neuroendocrinol 2, 412–421 (2013). [DOI] [PubMed] [Google Scholar]
- Wood S. H. et al. Binary switching of calendar cells in the pituitary defines the phase of the circannual cycle in mammals. Curr Biol 25, 1–12 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrun L. S., Dahl G. E., Evans N. P. & Karsch F. J. A critical period for thyroid hormone action on seasonal changes in reproductive neuroendocrine function in the ewe. Endocrinol. 138, 3402–3409 (1997). [DOI] [PubMed] [Google Scholar]
- Wood S. & Loudon A. Clocks for all seasons: Unwinding the roles and mechanisms of circadian and interval timers in the hypothalamus and pituitary J. Endocrinol. 222, R39–R59 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardente H., Hazelrigg D. G. & Ebling F. J. P. Thyroid hormone and seasonal rhythmicity. Frontiers in Endocrinol. 5, article 19 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockers T. M. et al. Initial expression of the common α-chain in hypophyseal pars tuberalis-specific cells in spontaneous recrudescent hamsters. Endocrinol. 138, 4101–4108 (1997). [DOI] [PubMed] [Google Scholar]
- Saenz de Miera C. et al. A circannual clock drives expression of genes central for seasonal reproduction. Curr. Biol. 24, 1500–1506 (2014). [DOI] [PubMed] [Google Scholar]
- Stevenson T. & Prendergast B. J. Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proc.Nat. Acad. Sci. 110, 16651–16656 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman K. The critical photoperiod in the Djungarian hamster Phodopus sungorus. Vertebrate Circadian Systems. In (eds Aschoff J., Daan S., Gross G.) 297–304 (Berlin:Springer Verlag. 1982). [Google Scholar]
- Kim B. W. et al. Endoplasmic reticulum-associated degradation of the human type 2 iodothyronine deiodinase (D2) is mediated via an association between mammalian UBC7 and the carboxyl region of D2. Mol. Endocrinol. 17, 2603–2612 (2003). [DOI] [PubMed] [Google Scholar]
- Herwig A. et al. A thyroid hormone challenge in hypothyroid rats identifies T3 regulated genes in the hypothalamus and in models with altered energy balance and glucose homeostasis. Thyroid 24, 1575–1593 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuer H. et al. The monocarboxylate transporter 8 linked to human physchomotor retardation is highly express in thyroid hormone-sensitive neuron populations. Endocrinol. 141, 1701–1706 (2005). [DOI] [PubMed] [Google Scholar]
- Visser W. E., Friesema E. C. H., Jansen J. & Visser T. J. Thyroid hormone transport in and out of cells Trends Endocrinol Metab. 19, 50–56 (2008). [DOI] [PubMed] [Google Scholar]
- Friesema E. C. H., Kulper G. G. J. M., Jansen J., Visser T. J. & Kester M. H. A. Thyroid hormone transport by the human monocarboxylate transporter 8 and it rate-limiting role in intracellular metabolism. Mol. Endocrinol 20, 2761–2272 (2006). [DOI] [PubMed] [Google Scholar]
- Herwig A. et al. Photoperiod and acute energy deficits interact on components of the thyroid hormone system in hypothalamic tanycytes of the Siberian hamster. Am. J. Physiol. Reg. Integ. Comp. Physiol. 296, R1307–R1315 (2009). [DOI] [PubMed] [Google Scholar]
- Kaiser C. A., Goumaz M. O. & Burger A. G. In vivo inhibition of the 5_- deiodinase type II in brain cortex and pituitary by reverse triiodothyronine. Endocrinol. 119, 762–770 (1986). [DOI] [PubMed] [Google Scholar]
- Silva J. E. & Leonard J. L. Regulation of rat cerebrocortical and adenohypophyseal type II 5′-deiodinase by thoxine, triiodothryoine, and reverse triiodothryoine. Endocrinol. 116, 1627–1635 (1985). [DOI] [PubMed] [Google Scholar]
- Petri I., Dumbell R., Scherbarth F., Steinlechner S. & Barrett P. Effect of exercise on photoperiod-regulated hypothalamic gene expression and peripheral hormones in the seasonal dwarf hamster Phodopus sungorus. PLoS ONE 9, e902531 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumbell R. et al. Somatostatin agonist parsireotide promotes a physiological state resembling short-day acclimation in the photoperiodic male Siberian hamster (Phodopus sungorus). J. Neuroendocrinol. 27, 588–599 (2015). [DOI] [PubMed] [Google Scholar]
- Villafuerte B. C., Phillips L. S., Rane M. J. & Weidong Z. Insulin-response element binding protein 1 – A novel Akt substrate involved in transcriptional action of insulin. J. Biol. Chem. 279, 36650–36659 (2004). [DOI] [PubMed] [Google Scholar]
- Hand L. E. et al. Induction of the metabolic regulator Txnip in fasting-induced torpor and natural torpor. Endocrinol. 154, 2081–2091 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherbarth F., Rozman J., Klingenspor M., Brabant G. & Steinlechner S. Wheel running affects seasonal acclimatization of physiological and morphological traits in the Djungarian hamster (Phodopus sungorus). Am. J. Physiol. Reg. Integ. Comp. Physiol. 293, R1368–R1375 (2007). [DOI] [PubMed] [Google Scholar]
- Drew J. E. et al. Localization of the melatonin-related receptor in the rodent brain and peripheral tissues. J. Neuroendocrinol. 13, 453–458 (2001). [DOI] [PubMed] [Google Scholar]
- Ross A. W. et al. Photoperiodic Regulation of Hypothalamic Retinoid Signaling: Association of Retinoid X Receptor γ with Body Weight. Endocrinol. 145, 13–20 (2004). [DOI] [PubMed] [Google Scholar]
- Ross A. W. et al. Divergent Regulation of Hypothalamic Neuropeptide Y and Agouti-Related Protein by Photoperiod in F344 rats With Differential Food Intake and Growth. J. Neuroendocrinol. 21, 610–619 (2009). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.