Abstract
Chloroplast retrograde signals play important roles in coordinating the plastid and nuclear gene expression and are critical for proper chloroplast biogenesis and for maintaining optimal chloroplast functions in response to environmental changes in plants. Until now, the signals and the mechanisms for retrograde signalling remain poorly understood. Here we identify factors that allow the nucleus to perceive stress conditions in the chloroplast and to respond accordingly by inducing or repressing specific nuclear genes encoding plastid proteins. We show that ABI4, which is known to repress the LHCB genes during retrograde signalling, is activated through phosphorylation by the MAP kinases MPK3/MPK6 and the activity of these kinases is regulated through 14-3-3ω-mediated Ca2+-dependent scaffolding depending on the chloroplast calcium sensor protein CAS. These findings uncover an additional mechanism in which chloroplast-modulated Ca2+ signalling controls the MAPK pathway for the activation of critical components of the retrograde signalling chain.
 Retrograde signalling co-ordinates nuclear gene expression in response to stress perceived in the chloroplast. Here Guo et al. show that a MAPK cascade acting downstream of a plastid derived Ca2+ signal contributes to the regulation of nuclear gene expression by phosphorylation of ABI4 during retrograde signalling.
Retrograde signalling co-ordinates nuclear gene expression in response to stress perceived in the chloroplast. Here Guo et al. show that a MAPK cascade acting downstream of a plastid derived Ca2+ signal contributes to the regulation of nuclear gene expression by phosphorylation of ABI4 during retrograde signalling.
Chloroplasts are vital for the viability of plants. They are not only the site of photosynthesis, but also function as central hubs in plant metabolism1,2. Chloroplasts are considered semi-autonomous organelles in that they have their own genome although most chloroplast proteins are encoded by nuclear genes3. The functional properties of chloroplasts are tightly regulated by the nuclear genome. Intracellular chloroplast retrograde signalling regulates the nuclear gene expression and is essential for the biogenesis of chloroplasts and for maintaining optimal chloroplast function in response to fluxes of metabolites and changes in environmental conditions4,5. Therefore, in addition to their roles as sites of energy conversions and as metabolic hubs, chloroplasts also serve as environmental sensors and evoke a broad range of stress responses of plants6.
During the past two decades, genetic screening of Arabidopsis has identified six mutants with defects in retrograde signalling termed genome uncoupled (gun) mutants, in which the expression of photosynthesis-associated nuclear genes is partially derepressed on treatment with either norflurazon (NF), an inhibitor of carotenoid biosynthesis, or lincomycin (Lin), an inhibitor of plastid protein synthesis4,5. Studies of these mutants revealed the existence of multiple retrograde pathways. Perturbations of the tetrapyrrole pathway, changes in the reduction/oxidation state of the photosynthetic electron transfer chain (PET), inhibition of chloroplast gene expression (PGE) and production of reactive oxygen species (ROS) would trigger retrograde signalling7,8,9,10 and these signals were integrated by GUN1 within the chloroplast. In response to the GUN1-derived signal, the nuclear-localized AP2/ERF class transcription factor ABI4 represses photosynthesis-associated nuclear genes by competing with G-box binding factors (GBFs) for binding to the G-box11. In addition, metabolites such as the recently discovered haem, 3′-phosphoadenosine 5′-phosphate (PAP), β-cyclocitral and methylerythritolcyclodiphosphate (MEcPP) produced in stressed chloroplasts act as novel types of retrograde signals to regulate stress-responsive genes in the nucleus12,13,14,15,16. It is likely that multiple pathways and metabolites form a complex signalling network and are involved in transmitting the chloroplast signals to the nucleus to coordinate gene expression in response to environmental and developmental cues. However, how chloroplasts initiate the retrograde response and the nature of the retrograde signal moving out from the plastid to influence nuclear gene expression are not fully understood.
Perception and transduction of environmental and developmental cues within plant cells involve a set of interconnected intracellular signalling networks. Evolutionarily conserved three-tiered mitogen-activated protein kinase (MAPK) cascades are among the most thoroughly studied signalling cascades17. Arabidopsis MAPK cascades are activated by various endogenous and exogenous stimuli and participate in diverse cellular functions ranging from developmental processes such as stomata development, cell division and petal abscission to biotic and abiotic stress responses18,19,20. A key question in studies of this cascade is how the specificity of different cellular signals can be efficiently achieved through a common set of components. Scaffold proteins that bind several MAPK components contribute to the efficiency and specificity of signal transmission21. The role of scaffold proteins in the spatial-temporal organization and regulation of MAPK pathway is well established in higher eukaryotes. On the other hand, MAPK scaffold proteins could also mediate the crosstalk between signalling pathways. For example, the MAPK scaffold protein Ste5 in yeast links the MAPK cascade to G-protein signalling in the mating pathway through binding to G-protein βγ-subunits22. In mammals, the scaffold protein IQGAP1 couples Ca2+ signalling and MAPK signalling through Ca2+-mediated promotion of binding of IQGAP1 to B-Raf23.
Our previous studies showed that dysfunctional chloroplasts initiate the retrograde response by triggering the processing of PTM (PHD type transcription factor with transmembrane domains), a chloroplast envelope-bound plant homeodomain (PHD) transcription factor. The N-terminal PTM fragment is released and mediates activation of ABI4 at the transcriptional level which in turn leads to the repression of LHCB24. Transgenic plants overexpressing the ABI4 gene did not induce LHCB repression under normal growth conditions (Supplementary Fig. 1), implying that the presence of ABI4 is necessary, but not sufficient for LHCB repression. In this study, we reveal another regulatory module in retrograde signalling that involves phosphorylation-dependent activation of ABI4 through the MPK3/MPK6 cascade. Activation of MAPK involves generation of a cytosolic Ca2+ transient mediated by the chloroplast Ca2+-binding protein CAS. In addition, we show that calcium-binding protein 14-3-3ω links chloroplast-modulated Ca2+signalling to MAPK signalling during retrograde response through its Ca2+-dependent scaffolding function.
Results
MPK3/MPK6 are ABI4-interacting proteins
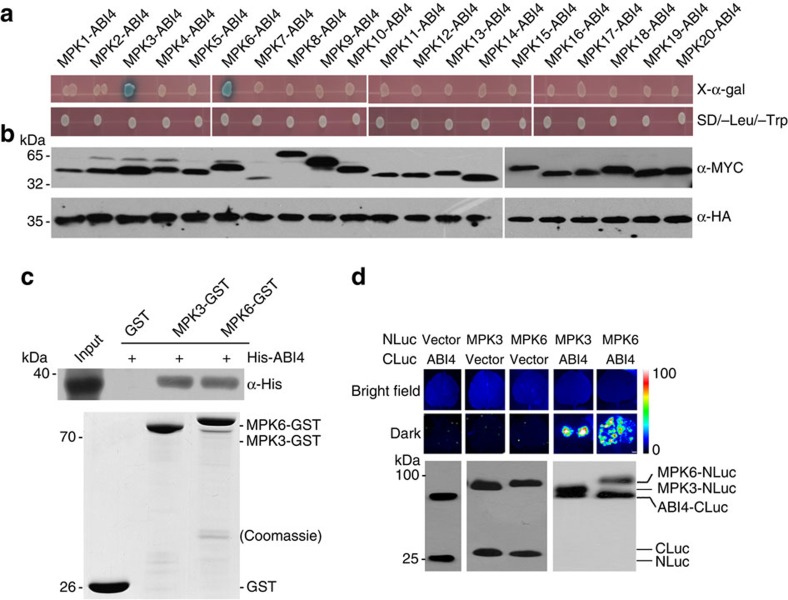
The observations that LHCB gene expression was not suppressed in ABI4 overexpressing transgenic plants under normal growth conditions in the absence of norflurazon (NF) despite the accumulation of ABI4 protein (Supplementary Fig. 1) suggests that a further activation step, possibly at the post-transcriptional level, is required for the ABI4-mediated retrograde response. Thus, the activity of ABI4 may be regulated either by post-translational modifications or through direct protein interactions. To address this possibility, we performed yeast two-hybrid screens using ABI4 lacking its activation domain to isolate interaction partners. Among these we isolated three clones encoding MPK6 (Supplementary Table 1). Because the Arabidopsis genome contains 20 MPK genes which belong to four subfamilies25, we subsequently tested whether other members of the MPK family are also able to directly interact with ABI4 in the yeast two-hybrid system. As shown in Fig. 1a, in addition to MPK6, MPK3, which is often functionally redundant to MPK6, interacted with ABI4 in the yeast two-hybrid system. The levels of the proteins expressed in yeast were checked by immunoblot analysis (Fig. 1b).
Figure 1. ABI4 interacts with MPK3 and MPK6.
(a) Yeast two-hybrid assay for the interaction between ABI4 and MPK3/MPK6. Fusion constructs of the ABI4 (amino acids 1–160) fused with the GAL4 DNA-binding domain (BD) and 20 MAPKs fused with the GAL4 activation domain (AD) were co-transformed into Y2H Gold yeast cells. These strains were grown on SD-Leu-Trp medium (bottom) and subjected to α-galactosidase assay (top). (b) Immunoblot analysis of target fusion proteins in the yeast two-hybrid assays indicated in (a) using HA tag and Myc tag antibodies, respectively. (c) Pull-down assay for the interaction of ABI4 with MPK3/MPK6. His-tagged ABI4 was incubated with immobilized GST or GST-tagged MPK3/MPK6. After washing, bound proteins were eluted and subjected to immunoblot analysis using an antibody against His. Coomassie blue (CBB) staining indicates equal amounts of bait proteins. (d) Luciferase complementation imaging assay for the interaction of ABI4 with MPK3/MPK6. N. benthamiana leaves were co-infiltrated with the agrobacterial strains harbouring the construct pairs of ABI4-CLuc and MPK3/MPK6-NLuc. ABI4-CLuc and NLuc as well MPK3/MPK6-NLuc and CLuc were used as controls. The images were taken after 48 h of infiltration. Bright luminescence indicates direct protein–protein interactions and the pseudocolour bar shows the range of luminescence intensity in the images. The western blot below shows the expression levels of CLuc- and NLuc-fusion proteins using anti-full-length firefly LUC antibody. Scale bar, 1 cm.
To provide further evidence for such interactions, we performed a pull-down assay. On immobilization of recombinant GST-MPK3/MPK6 fusion proteins on glutathione Sepharose beads, we found that both GST-MPK3 and GST-MPK6, but not GST alone, were able to pull down ABI4 in vitro (Fig. 1c). Furthermore, co-expression of ABI4 and MPK3 (or MPK6) in tobacco leaves resulted in strong LUC (firefly luciferase) activity, as demonstrated by luciferase complementation imaging (Fig. 1d). In control experiments when each of the three proteins alone was expressed LUC activity reached only background levels. Taken together, these results confirmed that ABI4 could interact with both MPK3 and MPK6 in vitro and in vivo.
Phosphorylation of ABI4 by MPK3 and MPK6
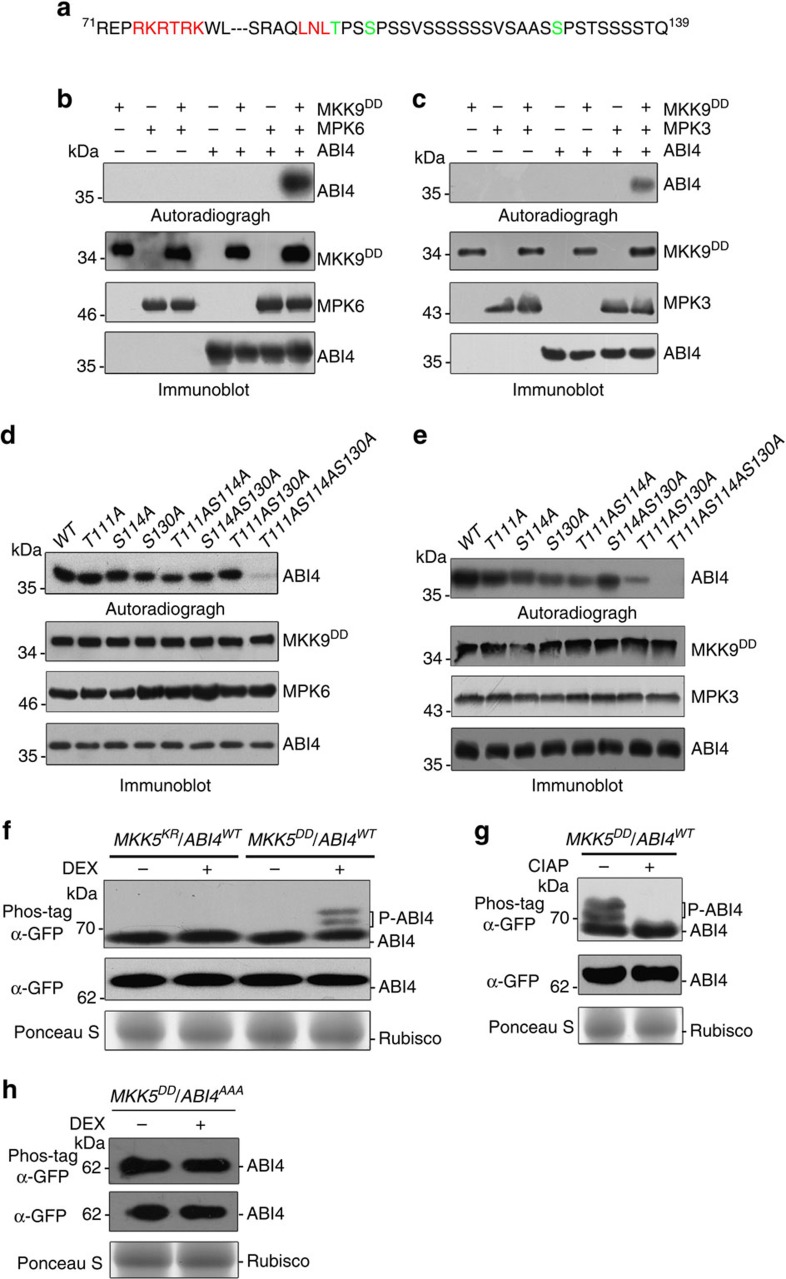
The direct interaction of ABI4 with MPK3 and MPK6 led us to examine whether ABI4 might also be phosphorylated by MPK3 and MPK6. The ability of MAPKs to phosphorylate their substrates is mediated by docking sequences and phosphorylation sites. The docking sequence is a common determinant of both the specificity and efficiency by which substrate proteins are phosphorylated by MAP kinases26. Its characteristic features include a cluster of basic residues located upstream of the LxL motif and sometimes a triplet of hydrophobic residues. The phosphorylation sites of substrates by MPKs are serine or threonine followed by proline (S/T-P motif)27. Sequence analysis indicated that the N terminus of ABI4 contains a typical MAPK docking motif and three potential phosphorylation sites (Thr-111, Ser-114 and Ser-130, designated as T111, S114 and S130, respectively) (Fig. 2a). In vitro phosphorylation assays showed that ABI4 was strongly phosphorylated in the presence of activated recombinant MPK3 and MPK6 (Fig. 2b,c). To identify which amino-acid residues are responsible for phosphorylation, recombinant ABI4 proteins were mutated by replacing Ser (S) or Thr (T) residues with Ala (A) and used as substrates for in vitro MAPK phosphorylation assays. Changes of all the three corresponding residues to Ala (ABI4AAA) in ABI4 led to a loss of phosphorylation whereas MPK3/MPK6 was still able to phosphorylate various single and double mutants (Fig. 2d,e). These results indicate that ABI4 is a substrate of MPK3/MPK6 and that the T111, S114 and S130 are independent MPK3/MPK6 phosphorylation sites.
Figure 2. Phosphorylation of ABI4 by MPK3 and MPK6.
(a) Putative MAPK docking motif and three potential phosphorylation sites in the N terminus of ABI4. The MAPK docking motif is highlighted in red, and the potential phosphorylation sites are highlighted in green. (b,c) Phosphorylation of recombinant ABI4 by the activated MPK3 and MPK6 in vitro. Recombinant MPK3/MPK6 were first activated by MKK9DD and subsequently incubated with ABI4 in the presence of [γ-32P]ATP. Autoradiography was used to visualize phosphorylated proteins (upper panel) and equal input of MKK9DD, MPK3/MPK6 and was confirmed by immunoblot analysis (lower panel). Reactions with various components omitted (−) were used as controls. (d,e) Identification of the MPK3/MPK6 phosphorylation sites in ABI4 based on in vitro phosphorylation assays. Recombinant wild type and mutant ABI4 proteins with single, double and triple mutations were subjected to phosphorylation assays with preactivated recombinant MPK3/MPK6 in the presence of [γ-32P]ATP. The phosphorylation level was visualized by autoradiography (upper panel) and the loading of MKK9DD, MPK3/MPK6 and ABI4 proteins were determined by immunoblot analysis. (f) Phosphorylation of ABI4 is induced in MKK5DD/ABI4WT but not in MKK5KR/ABI4WT transgenic plants after DEX treatment. Total protein was prepared at 3 h after DEX treatment, separated in a Phos-tag SDS–PAGE and immunodetected by an anti-GFP antibody (top). A second immunoblot was performed to detect ABI4 (middle) and Ponceau S staining was used to demonstrate equal loading (bottom). (g) Characterization of up-shifted band of ABI4 by dephosphorylation. The protein extracts from DEX-treated MKK5DD/ABI4WT seedlings were incubated with or without calf intestinal alkaline phosphatase (CIAP) and then subjected to Phos-tag SDS–PAGE (top) and SDS–PAGE analyses (middle). The ABI4WT-GFP protein was detected by immunoblot analysis using anti-GFP antibody. Ponceau S staining was used to demonstrate equal loading (bottom). (h) Effect of mutation of MAPK-phosphorylation sites on ABI4 phosphorylation status in the MKK5DD background after DEX treatment. Protein extracts from MKK5DD/ABI4AAA were prepared at 3 h after DEX treatment, separated in a Phos-tag SDS–PAGE and immunodetected by an anti-GFP antibody (top). Another immunoblot analysis was done at the same time to detect the ABI4 protein (middle). Ponceau S staining was used to demonstrate equal loading (bottom).
To further test whether phosphorylation of ABI4 is dependent on MPK3 and MPK6 activities in vivo, we expressed a ABI4–GFP fusion protein in the conditional gain-of-function MKK5DDand loss-of-function MKK5KR transgenic plants28, in which activation of MPK3/MPK6 is constitutively induced and abolished following DEX treatment, respectively (Supplementary Fig. 2). Phosphorylation of ABI4 was subsequently investigated by Phos-tag gel blot analysis with an anti-GFP antibody. In the gel containing Phos-tag, the phosphorylated proteins specifically binds to the Phos-tag reagent and cause a mobility shift compared with that of the non-phosphorylated counterparts29. As shown in Fig. 2f, phosphorylation of ABI4 was induced in the MKK5DD background, but not in MKK5KR plants, after DEX treatment, as indicated by the upshift of the protein bands. The band shifts of ABI4 were abolished after treatment with calf intestine alkaline phosphatase (CIAP), demonstrating that the additional bands with reduced mobility indeed result from phosphorylation (Fig. 2g). Such upshift was also absent when changes of all the three corresponding residues to Ala (ABI4AAA) in ABI4 under the MKK5DD background after DEX treatment (Fig. 2h), again indicating that the identified MAPK phosphorylation sites were subject to phosphorylation. Together, these results indicate that ABI4 could indeed be phosphorylated on MPK3/MPK6 activation both in vitro and in vivo.
Role of ABI4 phosphorylation during the retrograde response
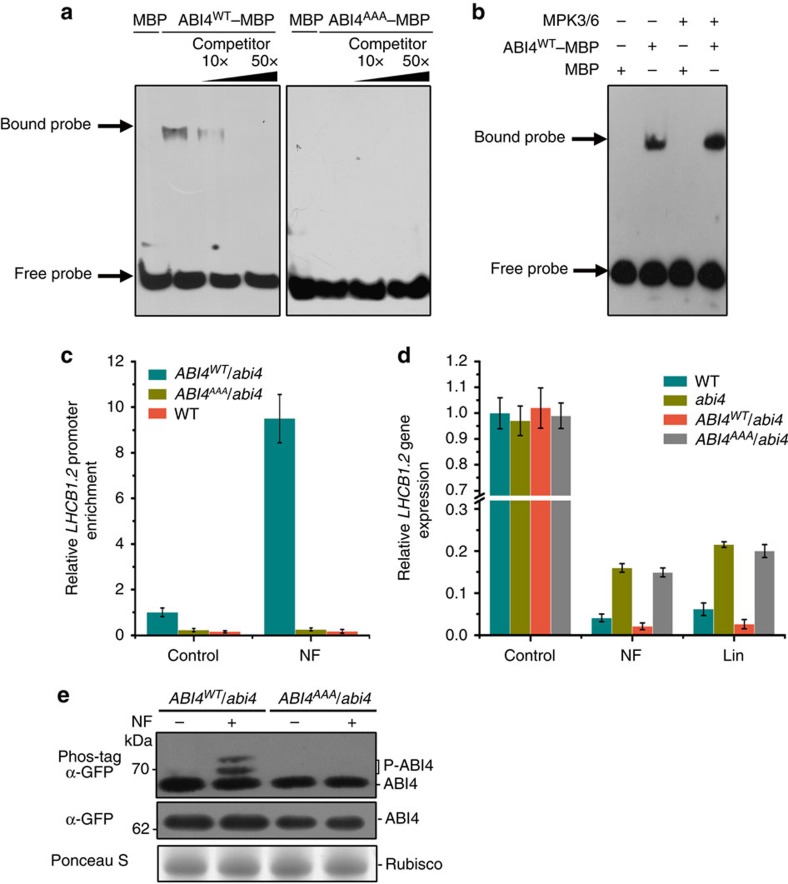
The three phosphorylation sites of ABI4 lie in its DNA-binding domain, which raised the possibility that phosphorylation affects its DNA-binding activity. Therefore, we examined the effect of mutations of the phosphorylation sites of ABI4 on its DNA-binding activity using an electrophoresis mobility shift assay (EMSA). As shown in Fig. 3a, a protein–DNA complex was detected when recombinant ABI4 was incubated with the LHCB promoter. Competition experiments using an unlabelled probe of the LHCB promoter confirmed binding specificity. However, compared with wild type, mutant ABI4 lacking the phosphorylation sites lost DNA-binding activity, suggesting that MPK3/MPK6 phosphorylation sites are required for the DNA-binding activity of ABI4. In addition, the DNA-binding activity of ABI4 was further increased when ABI4 was phosphorylated by activated MPK3/MPK6 (Fig. 3b), demonstrating that the DNA-binding activity of ABI4 is promoted by MAPK-mediated phosphorylation. The result also implied that phosphorylated ABI4 may increase its binding capacity to its target genes in vivo. To test this, we performed a chromatin immunoprecipitation (ChIP) assay with an anti-GFP antibody on the LHCB promoter in the GFP-tagged ABI4WT and ABI4AAA transgenic plants and revealed that the changes of the phosphorylation sites to Ala residues significantly decrease the DNA-binding activity of ABI4 in vivo (Fig. 3c). Moreover, the enrichment of the LHCB promoter precipitated from the ABI4WT/abi4 lines, but not from the ABI4AAA/abi4 lines, was considerably increased after NF treatment. Together, these results demonstrated that MAPK phosphorylation sites are important for the DNA-binding activity of ABI4 and that MPK3/MPK6-mediated phosphorylation could enhance its DNA-binding capacity.
Figure 3. Phosphorylation of ABI4 is required for its function during the retrograde response.
(a) The effect of mutations of phosphorylation sites on ABI4 DNA-binding activity by EMSA analysis. Recombinant ABI4WT and ABI4AAA proteins were incubated with a biotin-labelled LHCB promoter probe and detected by chemiluminescence. Increasing amounts of unlabelled competitors were used for competition and maltose-binding protein (MBP) served as a negative control. (b) ABI4 binding activity to the LHCB promoter upon MAPK-mediated phosphorylation. After incubation with activated MPK3 and MPK6 (equal mixture), phosphorylated ABI4 was subject to EMSA. MBP served as a negative control. (c) ChIP-qPCR analysis of ABI4 binding to the LHCB promoter in ABI4WT/abi4 and ABI4AAA/abi4 transgenic plants. Chromatin was isolated from 7-day-old seedlings in the presence or absence NF treatment. The GFP-tagged ABI4–chromatin complex was immunoprecipated using anti-GFP antibody. The relative DNA enrichment was determined by real-time PCR using primers specific to the promoters of LHCB1.2. A control reaction was processed using wild-type Arabidopsis samples. Values shown are means±s.d. of three biological replicates. (d) Impact of lincomycin (Lin) and NF treatments on LHCB1.2 transcript levels in WT, abi4, ABI4WT/abi4 and ABI4AAA/abi4. Seven-day-old seedlings of WT, abi4, ABI4WT/abi4 and ABI4AAA/abi4 grown on 1/2 MS media containing 500 μM Lin or 5 μM NF were harvested and RNA was isolated. The LHCB mRNA levels were determined by qRT–PCR. The relative transcript level of LHCB in seedlings grown on Lin or NF medium were normalized to values of control seedlings grown on 1/2 MS medium. Values shown are means±s.d. of three biological replicates. (e) Phosphorylation of ABI4 in seedlings after NF treatment. Total protein was extracted from ABI4WT/abi4 and ABI4AAA/abi4 seedlings at 0.5 h after NF treatment and separated in a Phos-tag SDS–PAGE followed by immunoblot analysis with anti-GFP antibody (top). Total protein extracts were separated by SDS–PAGE and the ABI4 protein was immunodetected with anti-GFP antibody at the same time (middle). Ponceau S staining was used to demonstrate equal loading (bottom).
We next investigated the significance of ABI4 phosphorylation in chloroplast retrograde signalling. Analysis of LHCB mRNA level in ABI4WT/abi4 revealed that the gun phenotype of abi4 can be rescued by constitutive expression of ABI4WT but not by ABI4AAA protein lacking the MPK phosphorylation sites, suggesting that the phosphorylation of ABI4 is required for its action in retrograde signalling (Fig. 3d). Moreover, an in vivo phosphorylation assay showed that NF treatment induces the accumulation of phosphorylated ABI4 in ABI4WT/abi4 but not in ABI4AAA/abi4 plants (Fig. 3e). In agreement with the phosphorylation of ABI4, endogenous MPK6 and MPK3 activation could also be observed under NF or Lin treatment (Supplementary Fig. 3a,b). These results suggest that NF or Lin treatment induces the activation of MPK3/MPK6, which subsequently promotes ABI4 phosphorylation, and that the phosphorylation sites are required for the repression of LHCB expression during retrograde signalling.
ABI4 genetically interacts with MPK3 and MPK6
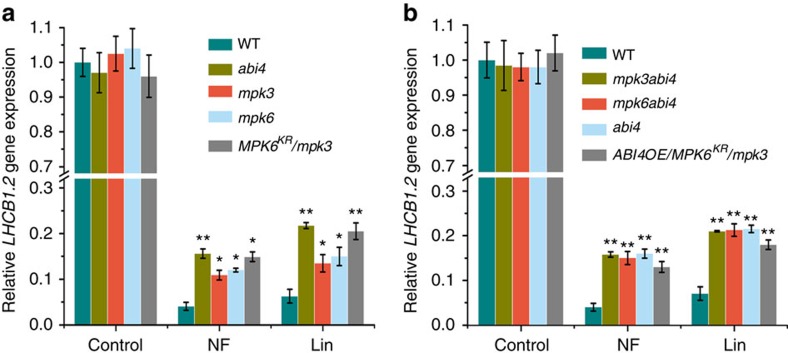
Direct phosphorylation of ABI4 by MPK3/MPK6 suggests that MPK3/MPK6 may be involved in mediating a retrograde response. We next examined whether retrograde response is altered in the mpk3 and mpk6 mutants. As shown in Fig. 4a, after treatments with Lin and NF for 7 days, the transcript levels of LHCB decreased to almost 6 and 4% in wild-type seedlings, respectively. By contrast, ∼21 and 15% of LHCB transcript were retained in abi4 after these treatments. As expected, Lin and NF treatments resulted in a reduction of LHCB to about 15% and 12% of the control in mpk6 mutant, respectively. Similarly, the repression of LHCB transcripts by these inhibitors was less marked in mpk3 mutants. Both mpk3 and mpk6 exhibit gun phenotypes under Lin and NF treatments, suggesting that MPK3 and MPK6 are indeed involved in the retrograde signal transduction pathway. Nevertheless, the gun phenotypes of mpk3 and mpk6 are weaker than that of abi4, which might be due to their functional redundancy. Because plants without both MPK3 and MPK6 display embryo lethality30, we employed mpk3 lines expressing a dominant-negative MPK6 variant (MPK6KR) to assess the gun phenotype31. As expected, MPK6KRmpk3 plants displayed a more severe gun phenotype that was similar to that of abi4 (Fig. 4a).
Figure 4. ABI4 genetically interacts with MPK3 and MPK6 in the retrograde response.
(a) Impact of Lin and NF treatments on LHCB1.2 transcript levels in wild type(WT), abi4, mpk3, mpk6 and MPK6KRmpk3. Arabidopsis seedlings were grown on 1/2 MS medium containing 500 μM Lin or 5 μM NF and harvested 7 days later. RNA was extracted and transcript levels of LHCB were analysed by qRT–PCR. (b) Impact of Lin and NF treatments on LHCB1.2 transcript levels in WT, abi4mpk3, abi4mpk6, abi4, ABI4OE and ABI4OE/MPK6KR/mpk3. Arabidopsis seedlings were grown on 1/2 MS medium containing 500 μM Lin or 5 μM NF and harvested 7 days later. RNA was extracted and the levels of LHCB mRNA were analysed by qRT–PCR. The relative transcript level of LHCB in seedlings grown on Lin or NF medium were normalized to values of control seedlings grown on 1/2 MS medium. The mean ±s.d. of three biological replicates is shown. Asterisks indicate significant differences from WT (*P<0.05 or **P<0.01, Student's t-test).
To investigate the genetic interactions of ABI4 with MPK3 and MPK6 in the retrograde response, we generated double mutants and analysis of double mutants revealed that mpk3abi4 or mpk6abi4 displayed a similar gun phenotype as the abi4 single mutant (Fig. 4b), suggesting that ABI4 acts downstream of MPK3/MPK6. To further test whether MPK3 and MPK6 are required for LHCB transcript regulation, we overexpressed ABI4 in the MPK6KRmpk3 mutant background. As shown in Fig. 4b, overexpression of ABI4 did not suppress the gun phenotype of MPK6KRmpk3 plants, suggesting that MPK3/MPK6 regulates the activity of ABI4 at the post-transcriptional level rather than regulating its accumulation, which is in agreement with the role of MPK3/MPK6 in ABI4 phosphorylation. Collectively, these data suggest that ABI4 functions downstream of MPK3 and MPK6 in the retrograde pathway and ABI4-mediated suppression of LHCB transcription is largely dependent on full activation of MPK3/MPK6.
MAPK activation involves CAS-mediated Ca2+ transients
The above results showed that MAP kinases mediate retrograde signalling by activating the downstream transcription factor ABI4. However, how the chloroplasts signal is emitted to regulate the activation of MAP kinases is unknown. Positive modulation of the Raf-MEK-ERK/MAPK cascade by an increase in intracellular Ca2+ is well established in animal cells32,33. Recent studies have demonstrated that chloroplasts serve as important intracellular calcium stores in plant cells, and they may also influence the entire cellular Ca2+ network by generating cytoplasmic Ca2+ transients34. To investigate a potential connection or overlap between MAPK and the Ca2+ pathway during the retrograde response, we first measured changes of calcium levels in the cytosol in response to retrograde signals using transgenic plants expressing the aequorin bioluminescence reporter35. As shown in Fig. 5a, challenging Arabidopsis seedlings with NF induced a rapid Ca2+ transient in the cytosol within several minutes, after which the signal gradually declined, implying that cytosolic calcium signals might play a role in meditating the chloroplast retrograde response. Because recent studies implied that the chloroplast calcium sensor protein CAS might be associated with cytosolic Ca2+ dynamics35,36, we measured NF-induced cytosol Ca2+elevation in aequorin-expressing plants in cas-1 background. As shown in Fig. 5a, the amplitude of the signal was significantly attenuated in the cas-1 mutant. The integrated amount of NF-induced cytosolic Ca2+ elevation over 30 min in cas-1 mutant was reduced to about half of the wild-type amounts. Similar cytosolic Ca2+ transient patterns were observed after Lin treatment (Fig. 5b). Meanwhile, no obvious changes in cytosolic-free calcium were induced in control experiments in which water was employed instead of NF or Lin (Supplementary Fig. 4). Together, these results indicate that perturbation of plastid functions by NF and Lin causes a transient increase in cytosolic Ca2+ level in a CAS-dependent manner.
Figure 5. Activation of MAPK during retrograde response involves CAS-mediated transient increase in cytosolic Ca2+level.
(a,b) Effect of NF and Lin on cytosolic calcium kinetics in transgenic Arabidopsis expressing apoaequorin in wild type (black lines) and cas-1 mutant (red lines) plants. Six-day-old seedlings were treated with 5 μM NF (a) or 500 μM lincomycin (b) and luminescence was recorded at intervals of 0.2 s. Ca2+ concentrations were calculated from relative luminescence using a mathematical algorithm equation. The vertical dashed line indicates the time at which treatment was initiated. Experiments were repeated at least five times and representative data are shown. The time-integrated cytosolic Ca2+ concentration in wild-type and cas-1 mutants in the first 30 min after treatment with 5 μM NF (a) or 500 μM lincomycin (b) is shown. Data are the means±s.d. of relative values of five replicates. At least five seedlings per genotype were measured in each replicate and wild-type values were set as 100%. (c) MPK3 and MPK6 activation profiles in WT and cas-1 seedlings in response to NF treatment. Total protein was extracted after treatment with 5 μM NF for the indicated times and the activated MPK6 and MPK3 were detected by immunoblot analysis using phosphor-p44/42 MAPK (Erk1/2) antibody. Arrowheads indicate phosphorylated MPK6 and MPK3. Equal loading was indicated by immunoblot analysis with anti-MPK6 antibody.
To investigate the possible role of chloroplast-modulated Ca2+ signalling in the regulation of MAPK signal pathway during retrograde signalling, we subsequently examined the effect of Ca2+ blockers on MPK3/MPK6 activity. We found that pretreatment with EGTA (a calcium-chelator) markedly suppressed Lin- and NF-triggered activation of MPK3 and MPK6. A similar but less pronounced effect was observed when applying lanthanum chloride, a calcium channel blocker (Supplementary Fig. 5a,b), suggesting that Ca2+ is positively involved in the modulation of MPK3/MPK6 activity during the retrograde response. Moreover, MAPK activation in response to retrograde signal was diminished in the cas-1 mutant as compared with wild type (Fig. 5c), indicating that CAS significantly contributes not only to the cytosolic Ca2+ transient, but also to the downstream Ca2+-modulated cytosolic signalling response. NF is known to generate harmful ROS and cause photo-oxidative stress during illumination, which may lead to the activation of MPK3/MPK6 in Arabidopsis. Pretreatment with Tiron and dimethylthiourea (DMTU)37, scavengers for O2− and H2O2, respectively, in dark-grown seedlings barely affected the activation of MPK3/MPK6 induced by NF treatment (Supplementary Fig. 5c), suggesting that ROS production does not play a major role in the activation of MPK3/MPK6.
Calcium-binding protein 14-3-3ω facilitates MAPK activation
The observations that activation of MAPK in response to retrograde signals is regulated by chloroplast-modulated Ca2+ signalling prompted us to search potential signalling components that could link Ca2+ signals to the downstream MAPK cascades during the retrograde response. Scaffold proteins exert substantial control over MAPK signalling, ranging from signal intensity and specificity to crosstalk with other signalling cascades, and increasing evidence supports the existence of scaffold proteins in shaping a MAPK signalling module in Arabidopsis38,39. 14-3-3 proteins, a family of conserved scaffolding or anchoring proteins in eukaryotes for cellular signalling circuits, which can bind multiple signalling components, represent good candidates, as the association of 14-3-3s with some components of the MAPK cascade is well documented40,41,42.
We first investigated the expression profile of all 14-3-3 gene family members and found that 14-3-3ω exhibits a more rapid induction among the isoforms in response to retrograde signal (Supplementary Fig. 6), suggesting a possible role in the process. To test whether14-3-3ω exerts a regulatory role for MAPK pathway by acting as a scaffold during the retrograde signalling, we used the yeast two-hybrid method to test potential physical interactions between 14-3-3ω and components of the MAPK pathway. In Arabidopsis, MPK3 and MPK6 are activated probably through the well-studied MEKK1-MKK4/MKK5-MPK3/MPK6 kinase cascade19. As shown in Fig. 6a, 14-3-3ω specifically interacted with MKK4/MKK5 and MPK3/MPK6, but not with MEKK1. Also, MPK4, another well-characterized member of the MAPK family, as well as MKK9, another upstream MAPKK activator of MPK3 and MPK6, did not interact with 14-3-3ω in the yeast two-hybrid assays, demonstrating the specific interaction of 14-3-3ω with these MAPK pathway components. By contrast, the 14-3-3Φ isoform, the family member most closely related to 14-3-3ω (ref. 43), interacted with neither MPK3/MPK6 nor MKK4/MKK5 (Supplementary Fig. 7). Thus, we focused on 14-3-3ω in the subsequent studies. To confirm the association of 14-3-3ω with these MAPK components, we carried out transient bimolecular fluorescence complementation (BiFC) assays and found that coexpression of 14-3-3ω-nYFP (fusion of 14-3-3ω with the N-terminal fragment of yellow fluorescent protein (YFP)) with MPK3-cYFP (fusion of MPK3 with the C-terminal fragment of YFP), MPK6-cYFP, MKK4-cYFP or MKK5-cYFP produced strong YFP signals in both the nucleus and cytoplasm (Fig. 6b), consistent with the subcellular localization of these proteins44,45, while no fluorescence was detectable in the controls (Supplementary Fig. 8), indicating that MPK3, MPK6, MKK4 and MKK5 can interact with 14-3-3ω in vivo. Since it has been reported that 14-3-3ω undergoes conformational changes on binding of Ca2+at the C-terminal domain and since such binding of divalent cations is often required for the interaction of 14-3-3 with clients46,47, we next examined whether calcium could modulate the interaction between 14-3-3ω and MAPK cascade components in vivo using protoplast transient co-immunoprecipitated assays. Consistent with the yeast two-hybrid and BiFC assays, GFP-tagged MPK3, MPK6, MKK4 and MKK5, all co-immunoprecipitated with FLAG-tagged 14-3-3ω (Fig. 6c). The amounts of 14-3-3ω that were pulled down by MAPKK and MAPKs in the co-immunoprecipitation experiments significantly increased in the presence of Ca2+ (Fig. 6c). However, no 14-3-3ω could be immunoprecipitated when Ca2+ was chelated, suggesting that the 14-3-3ω and MPK3/MPK6–MKK4/MKK5 interaction was likely Ca2+ dependent (Fig. 6c). Likewise, treatment with R18 peptide, a strong competitive inhibitor of 14-3-3 client protein interactions48, also abolished such association (Fig. 6c). Moreover, a significant enhancement of the interaction between 14-3-3ω and the MKK4/MKK5–MPK3/MPK6 module could be observed following Lin and NF treatment in the wild type but such Lin- or NF-mediated promotion of interaction was dramatically reduced in cas-1 mutants (Supplementary Fig. 9), which is consistent with the diminished MAPK activation in cas-1 mutants. Together, these results indicate 14-3-3ω can interact with MKK4/MKK5–MPK3/MPK6 in a calcium-dependent manner and chloroplast-localized CAS enhances their association, possibly through modulation of cytoplasmic Ca2+ transients.
Figure 6. 14-3-3ω facilitates MAPK activation through its Ca2+-dependent scaffolding role that is essential for the retrograde response.
(a) Yeast two-hybrid assay for the interaction of 14-3-3ω with MPK3/MPK6 and MKK4/MKK5. These strains were grown on synthetic dropout medium lacking Leu, Trp and His (SD-Leu-Trp–His) containing 40 μg ml−1 X-α-gal (5-bromo-4-chloro-3-indolyl-α-D-galactopyranoside). (b) BiFC analyses for the interaction of 14-3-3ω with MPK3/MPK6 and MKK4/MKK5 in Arabidopsis protoplasts. YFP fluorescence was detected and the positions of nuclei were shown by 4, 6-diamidino-2-phenylindole (DAPI) staining. Scale bar, 10 μm. (c) Co-immunoprecipitation(co-IP) assays for the interaction of 14-3-3ω with MPK3/MPK6 and MKK4/MKK5. Protoplasts co-transfected with the MPK3/MPK6-GFP or MKK4/MKK5-GFP construct and the 14-3-3ω-FLAG construct were treated with 1 mM CaCl2, 5 mM EGTA or 10 μg ml−1 R18 for 1 h, and total protein extracts were subjected to immunoprecipitation. (d) Effect of 14-3-3ω on MAPK activation triggered by MKK4DD or MKK5DD. Protoplasts were transfected with MKK4DDor MKK5DD in the presence or absence of 14-3-3ω-FLAG constructs, and total protein extracts were subjected to immunoblot analysis with phosphor-p44/42 MAPK (Erk1/2) antibody. Equal loading was verified by immunoblot analysis with an anti-MPK6 antibody. (e) Phosphorylation status of ABI4 in wild-type, 14-3-3ωOE and 14-3-3ωRNAi plants expressing 35S:ABI4-GFP after NF treatment. Protein extracts from ABI4OE, ABI4WT/14-3-3ωOE and ABI4WT/14-3-3ωRNAi seedlings at 0.5 h after NF treatment were separated by Phos-tag SDS–PAGE followed by immunoblot analysis with anti-GFP antibody (top). Another immunoblot analysis was done at the same time to detect the ABI4 protein (middle). Ponceau S staining was used to demonstrate equal loading (bottom). (f) Effect of NF and Lin treatments on the relative activity of the LUC reporter in protoplasts. Protoplasts isolated from wild-type, abi4, 14-3-3ωRNAi and cas-1 mutant seedlings were cotransformed with both LHCBp:LUC and 35S:GUS constructs. Relative LUC activities after treatments are the ratio of LUC to GUS (internal control) normalized to the value of control (without treatment). Data represent means±s.d. from five biological replicates. Asterisks indicate significant differences from the value of wild-type at *P<0.05 or **P<0.01 using Student's t test.
The association of 14-3-3ω with MKK4/MKK5 and MPK3/MPK6 suggests that it may function as a MAPK scaffold protein enabling spatial proximity. MAPK scaffold proteins would additionally increase the efficiency and specificity of MAPK activation by bringing MAPK cascade components into close proximity for physical assembly. We subsequently tested whether 14-3-3ω would have a similar stimulatory function in MAPK signalling in transfected protoplasts. As shown in Fig. 6d, expression of MKK4DD/MKK5DD, the constitutively active form of MKK4/MKK5 (ref. 49), can activate endogenous MPK3 and MPK6. As expected, the activation of MPK3 and MPK6 by MKK4DD or MKK5DD was significantly enhanced when the construct expressing epitope-tagged 14-3-3ω was cotransfected into protoplasts (Fig. 6d). By contrast, 14-3-3ω was unable to promote MAPK activation triggered by the constitutively active MKK9DD (Supplementary Fig. 10), suggesting that such promotive effect of 14-3-3ω on MAPK activation was dependent on the interaction with its client protein. We next examined the effect of disrupting such 14-3-3 interaction via R18 treatment on MAPK activation and observed that the treatment with R18 peptide, but not with the inactive R18Lys peptide, diminished the NF-mediated activation of MPK3 and MPK6 (Supplementary Fig. 11a). Similarly, the R18 peptide markedly suppressed the activation of MPK3 and MPK6 triggered by MKK4DD/MKK5DD, as well as the enhancing effect by14-3-3ω on MPK3 and MPK6 activation (Supplementary Fig. 11b), further supporting the notion that the enhancement of MAPK activation by 14-3-3ω was dependent on the interaction between 14-3-3ω and the MKK4/MKK5–MPK3/MPK6 module. Consistent with the protoplast transient assay, overexpression of 14-3-3ω significantly enhances MPK3/MPK6 activation, whereas downregulation of 14-3-3ω expression reduced MPK3/MPK6 activation as compared with wild-type plants on NF and Lin treatment (Supplementary Fig. 12). Accordingly, the NF-induced phosphorylation status of ABI4 was also increased by 14-3-3ω overexpression but attenuated in 14-3-3ω silencing background (Fig. 6e). These results collectively suggest that 14-3-3ω facilitates MAPK activation by functioning as a Ca2+-dependent scaffold for the MKK4/MKK5–MPK3/MPK6 module.
Finally, we examined the physiological significance of 14-3-3ω-mediated Ca2+-dependent scaffolding for MAPK signalling on the retrograde response. To this end, we monitored the activity of the LHCB1.2 promoter (LHCBp) by transforming WT and 14-3-3ωRNAi protoplasts with a LHCBp:LUC construct and measured LUC activity. In fact, NF or Lin treatments significantly suppressed LUC activity in WT but not in 14-3-3ωRNAi protoplasts (Fig. 6f). Similarly, the extent of repression of LUC activity observed in the cas-1 mutant or after pretreatment with Ca2+ blockers was much weaker(Supplementary Fig. 13), providing further evidence that CAS-generated cytosolic Ca2+ transients and Ca2+-dependent MAPK scaffolding function of 14-3-3ω are both required to repress the LHCB expression in response to retrograde signalling.
Discussion
Coordinated communication between chloroplast and the nucleus are critical for proper chloroplast biogenesis and maintenance. Several components involved in the generation and transmission of retrograde signalling have been identified4,5. ABI4 has been proposed to competitively bind to promoter sequences and block the expression of photosynthetic genes in response to plastid signals11. Therefore, the involvement of ABI4 in plastid retrograde signalling is likely to be associated with a further activation step. So far, to our knowledge, no interacting partner has been identified to modulate the activity or stability of ABI4. In this study, we have elucidated the detailed mechanisms of ABI4 activation in retrograde signalling through MPK3/MPK6-mediated phosphorylation. Such phosphorylation-dependent activation is positively regulated by CAS-mediated cytosolic Ca2+ signalling and involves 14-3-3ω-mediated Ca2+-dependent scaffolding of the MAPK cascades.
Arabidopsis MAPK signalling pathways are involved in the regulation of various cellular processes. This includes responses related to biotic and abiotic stresses, hormone signalling and development programs19. Our results show that MPK3 and MPK6 play a prominent role during retrograde signalling by repressing the transcription of LHCB gene. Such multifunctionality of MPK3/MPK6 in different biological processes are considered to be conferred by their different substrates50. However, until now, only a few substrates have been reported with functional data51. Here we provided several lines of evidence supporting that ABI4 is a substrate of MPK3/MPK6. Both MPK3 and MPK6 interacted with ABI4 in yeast two-hybrid, pull-down and LIC assays. In vitro phosphorylation assays together with site-directed mutagenesis of the phosphorylated residues indicated that ABI4 can be phosphorylated by activated MPK3/MPK6. This suggestion was further corroborated by the Phos-tag mobility shift assays. Phosphorylation of the substrates of MPKs has been associated with changes in their stability and/or subcellular localization19,52. Recent studies have shown that ABI4 is regulated post-transcriptionally and protein stability is controlled through protein degradation involving the AP2-associated and the PEST motifs at the N-terminal region53,54. In our study, both the mutated ABI4AAA-GFP and wild-type ABI4WT-GFP were exclusively observed in the nucleus, suggesting that the subcellular localization of ABI4 was unaffected by phosphorylation (Supplementary Fig. 14). However, we observed that ABI4AAA-GFP is degraded much more rapidly compared with ABI4WT-GFP after addition of the protein synthesis inhibitor (Supplementary Fig. 15), implying that T111, S114 and S130 also contribute to the stability of ABI4. In our study, in the presence of NF or Lin, LHCB expression was much less repressed in mpk3 and mpk6 than in the wild type and more pronounced derepression of LHCB was observed in MPK6KRmpk3 mutants. These results suggest that MPK3 and MPK6 play redundant roles, but both are required for mediating retrograde response. MPK3/MPK6 phosphorylation sites in ABI4 are within the DNA-binding domain. It has been proposed that a competition for the binding of the LHCB promoter between ABI4 and activators such as G-box binding factors (GBFs) occurs in the regulation of LHCB transcription11. Our EMSA and ChIP assay show that the binding activity of ABI4 to the LHCB promoter is positively regulated by phosphorylation. Further genetic analysis revealed that phosphorylation of ABI4 enhances its repression of LHCB transcription, because a non-phosphorylated mutant of ABI4 failed to rescue the gun phenotype of the abi4 mutant. Based on these results, we conclude that ABI4 is activated by phosphorylation and that the phosphorylated active form of ABI4 predominantly binds the promoter of LHCB resulting in its repression during the retrograde response. A recent study has shown that a set of AP2/ERF transcriptional factors including ERF6, ERF104 and ERF105 exhibit rapid transcriptional upregulation after MPK6 activation downstream of the triose phosphate/phosphate translocator during high light-mediated retrograde signalling55. However, the transcription factors involved in these processes are unknown. Analyses of the promoters of these AP2/ERF genes revealed that the core element of the ABI4 binding motif (CCAC) was repeatedly present. The possibility of the involvement of phosphorylation of ABI4 in the transcriptional activation during this fast retrograde response to high light exposure can therefore not be excluded.
The involvement of Ca2+ in the retrograde response is supported by the finding that NF and Lin induce a rapid and transient Ca2+ increase in the cytosol, but this cytosolic Ca2+ transient was strongly diminished in cas-1 mutants. These data suggest that perturbations of plastid tetrapyrrole biosynthesis or inhibition of plastid gene expression can elicit a transient increase in the cytosolic Ca2+ concentration. Such modulation of cellular Ca2+ signalling by chloroplasts during the retrograde response rely on chloroplast-localized CAS, possibly as a result of Ca2+ mobilization from chloroplasts in response to Lin and NF treatment. CAS-mediated Ca2+ signalling has previously been implicated in the retrograde control of immune responses and photoacclimation56,57. Although the results obtained in our study highlight that calcium released from chloroplasts contributes to MAPK activation during the retrograde response, the contribution of other internal Ca2+ stores for cytoslic Ca2+ transient cannot be excluded, because cytosolic Ca2+ transients were not completely abolished in cas-1 mutants during the retrograde response. Meanwhile, the possibility that other tetrapyrrole intermediates mediate MAPK activation cannot be excluded. Impairment of chloroplast development might result in the generation of an inhibitory signal associated with the accumulation of certain chlorophyll intermediates. Thus, Mg-protoporphyrin IX (Mg-ProtoIX) has been reported to be involved in stress signalling and regulation of the activation of cyclin-dependent kinase A (CDKA) during the cell proliferation cycle in algae58,59.
Our study additionally suggests that a MAPK cascade links the CAS-mediated intracellular Ca2+ signalling to the nucleus to mediate the activation of ABI4 via the Ca2+-binding protein 14-3-3ω through its Ca2+-dependent scaffolding function.14-3-3 proteins have been implicated in the regulation of almost every plant cellular process through binding to a great variety of client proteins. The Arabidopsis genome contains at least 13 expressed 14-3-3 isoforms57. Our gene expression analysis showed that 14-3-3ω, but not other 14-3-3 family members, is dramatically induced by NF and Lin treatment, implying a distinct role for 14-3-3ω in the regulation of the retrograde response. We further demonstrated that calcium can promote the association between 14-3-3ω and the MKK4/MKK5–MPK3/MPK6 module. Because chelation of intracellular-free Ca2+ by EGTA abolishes such an interaction and the enhancement of the interaction in response to retrograde signalling was significantly reduced in the cas-1 mutant, we postulate that 14-3-3ω acts as a Ca2+-dependent scaffold/anchor protein in MAPK signalling to facilitate MAPK activation by interacting with MKK4/MKK5 and MPK3/MPK6. Such an idea is further supported by the observation that 14-3-3ω overexpression results in the stimulation of MAPK signalling, whereas downregulation of 14-3-3ω by RNAi decreases MAPK activation. The findings collectively suggest that 14-3-3ω couples Ca2+ signalling to MAPK signalling during the retrograde response through Ca2+-dependent scaffolding, which may bring MPK3/MPK6 in close proximity to its activator kinase MKK4/MKK5, thereby enabling the efficient activation of MPK3/MPK6 and the subsequent phosphorylation of ABI4. Consistent with this notion, a transient expression assay revealed that LHCB repression upon Lin and NF treatment was less marked in CAS-deficient and 14-3-3ω-silenced background relative to wild type. Therefore, it seems that CAS-generated cytosolic Ca2+ transients and 14-3-3ω-mediated MAPK scaffolding both are essential events for the promotion of activation of ABI4 and subsequent suppression of LHCB during the retrograde signalling. In our study, because the MPK3/MPK6 do not contain canonical binding sites of the mode I [Arg-Ser-X-(pSer)-X-Pro] and mode II [Arg-X-X-X-(pSer)-X-Pro] for 14-3-3s interactions60, the interaction of 14-3-3ω with MPK3/MPK6 is likely mediated through non-canonical binding sites. The MKK4 amino-acid sequence of residues 2–8, RPIQSPP, and the MKK5 amino-acid sequence of residues 21–27, RPDLSLP, resemble the mode II recognition site. Replacing the Ser-6 of MKK4 and Ser-25 of MKK5 with Ala markedly reduced the interaction with 14-3-3ω (Supplementary Fig. 16), indicating that the Ser residue may serve as a 14-3-3ω recognition site in the yeast two-hybrid assay. However, these particular Ser residues have not been reported to be phosphorylated in vivo, so it cannot be excluded that Ser-6 of MKK4 and Ser-25 of MKK5 may have some structural function needed for signal transduction rather than act through their phosphorylation.
In summary, we have identified several components of retrograde signal pathway involving ABI4 activation post-transcriptionally. These results, combined with our previous study on the role of PTM in the activation of ABI4 expression24, suggest that activation of ABI4 at both the transcriptional (by PTM) and post-translational levels (by MPK3/MPK6) leads to the repression of LHCB expression, as depicted in our working model (Fig. 7). Similar to PTM, disruption of MPK3/MPK6 also compromises the retrograde response. It is likely that both events are necessary for the repression of LHCB and that they act together in one regulatory pathway to coordinate photosynthesis-associated nuclear genes expression in response to retrograde signals.
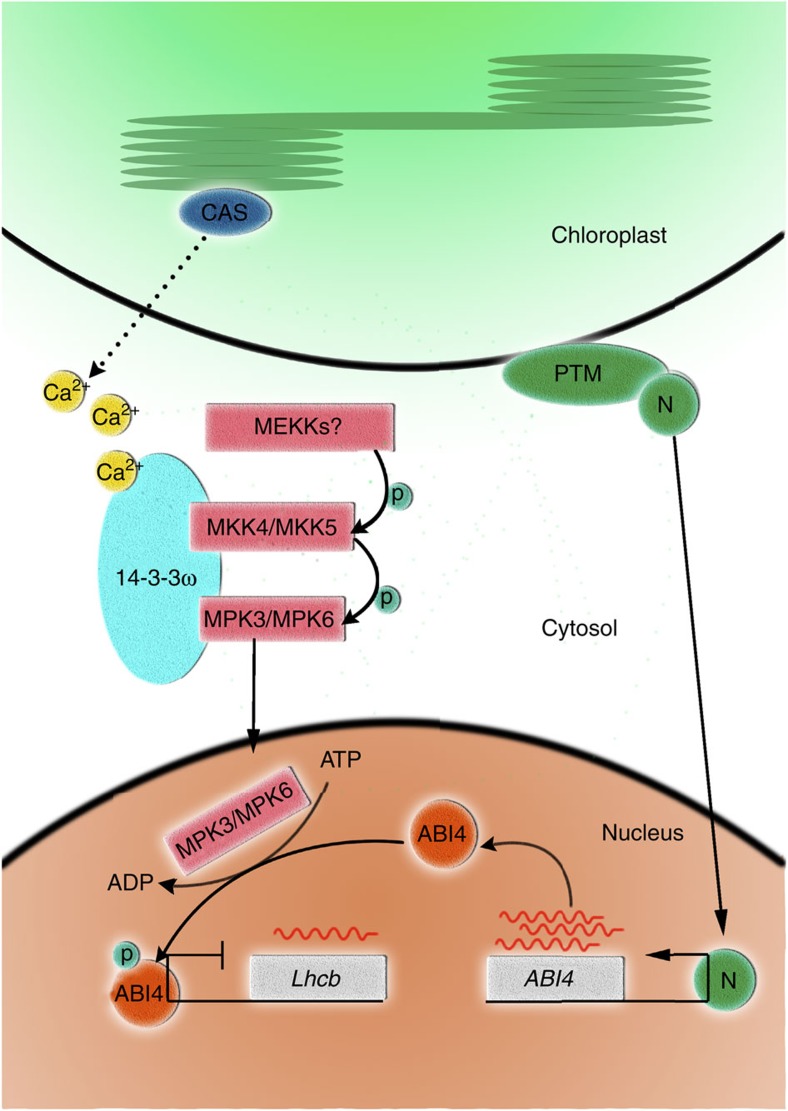
Figure 7. A model illustrating the transcriptional and posttranslational regulation of ABI4 by PTM and MPK3/MPK6 in retrograde signalling.
Scheme illustrating the regulation of ABI4 activity during retrograde signalling by transcriptional (PTM) and post-translational (MPK3/MPK6) mechanisms. Perturbations of plastid tetrapyrrole biosynthesis or inhibition of plastid gene expression can elicit a transient increase in the cytosolic Ca2+ concentration, and thylakoid-localized CAS protein is involved in the generation of cytosolic calcium transients, possibly as a result of the Ca2+ mobilization from chloroplast in response to lincomycin and NF treatments. Subsequently, elevation of cytosolic calcium promotes the interaction between MKK4/MKK5–MPK3/MPK6 and the Ca2+-binding protein 14-3-3ω. 14-3-3ω functions as Ca2+-dependent scaffolding protein to bring MPK3/MPK6 in close proximity to its activator kinase MKK4/MKK5, thereby facilitating the efficient activation of MPK3/MPK6. Thus, relocation of activated MPK3/MPK6 into the nucleus together with phosphorylation of ABI4 is induced. In parallel, cleavage of PTM occurs and the released N-terminal fragment (designated as ‘N') moves to the nucleus where it activates the transcription of ABI4. Activation of ABI4 at both the transcriptional and posttranslational levels leads to the repression of LHCB.
Methods
Plant materials and growth conditions
The abi4, mpk3 (Salk_100651), mpk6 (Salk_127507), cas-1(Salk_070416) and MKK5DD transgenic plants were described previously24,28,35. Homozygous mutant lines were identified by PCR analysis using specific primers (Supplementary Table 2). All the mutants used in this study are in the Columbia background. Wild type and mutant seeds were sterilized with 10% sodium hypochlorite for 10 min, washed five times with sterile water, and sown onto MS medium. To ensure synchronized germination, the seeds were kept in the dark at 4 °C for 2 days. All the plants were grown at 22 °C in a growth chamber under a 14 h light/10 h dark photoperiod at a photon flux density of 100 μmol photons m−2 s−1.
For NF treatment, the seeds were surface-sterilized and placed on half-strength MS media with or without the addition of 5 μM norflurazon (NF, Sandoz Pharmaceuticals). Plants were grown in the dark for 4 days and then transferred to light for 3 more days. For Lin treatment, the seeds were surface-sterilized and placed on half-strength MS media with or without the addition of 500 μM Lin (Sigma-Aldrich). After 7 days, the seedlings were harvested. For MAP kinase activity assays, 7-day-old seedlings were treated with 5 μM NF or 500 μM Lin for the indicated times. For DEX treatment, 7-day-old seedlings were collected for the indicated times following treatment with 2 mM DEX (Sigma-Aldrich). For LaCl3 and EGTA treatments, 7-day-old wild-type seedlings were treated with 2 mM LaCl3 (Sigma-Aldrich), 10 mM EGTA (Amresco) for 30 min prior to elicitation with NF or Lin treatment. For Tiron and DMTU treatments, the dark-grown 4-day-old wild-type seedlings were pretreated with 10 mM Tiron (Sigma-Aldrich) and 5 mM DMTU (Sigma-Aldrich) for 8 h, and then exposed to NF treatment in darkness for the time indicated.
Generation of transgenic plants and double mutants
To construct the ABI4WT and ABI4AAA overexpression binary vector, the coding region of ABI4 was amplified from wild-type complementary DNA using primers containing SalI and NcoI sites and inserted into the pGEM-T Easy vector (Promega) to produce pGEM-ABI4WT. To mutagenize the Thr-111, Ser-114 and Ser-130 residues to Ala, the fragments were amplified from pGEM-ABI4WT template using the Fast Mutagenesis System Kit (TransGen Biotech) according to the manufacturer's protocol with primers listed in Supplementary Table 2. To generate ABI4AAA, all three Ser/Thr residues mutated to Ala by three successive mutagenesis steps to produce pGEM-ABI4AAA. After confirmation by sequencing, the wild-type and mutant fragments were released from pGEM-ABI4WT and pGEM-ABI4AAA with Sal1I and NcoI and ligated into the same restriction sites of the pCAMBIA2300 vector containing GFP as a marker under the control of the cauliflower mosaic virus 35 S promoter. The resulting constructs were electroporated into Agrobacterium strain C58C1 and then introduced into the WT or abi4 background via the floral infiltration method61. Screening for kanamycin resistance was used to identify transformants. Independent lines were identified based on qPCR and immunoblot analysis with the anti-GFP antibody (G1544, Sigma-Aldrich) at a dilution of 1:4,000. The secondary antibody (goat anti-rabbit IgG, Sigma-Aldrich, A0545) conjugated to horseradish peroxidase (HRP) was used at a dilution of 1/10,000 for detection by the enhanced chemiluminescence assay.
For the generation of MPK6KRmpk3 plants, the coding region of MPK6 was amplified from wild-type cDNA using primers containing BamHI and XmaI sites and inserted into the pGEM-T Easy vector to produce pGEM-MPK6. To mutagenize the Lys to Arg mutation on the Lys-92 residue, the fragment was amplified from pGEM-MPK6 template using the Fast Mutagenesis System Kit according to the manufacturer's protocol with primers listed in Supplementary Table 2 to generate pGEM-MPK6KR. After confirmation by sequencing, the MPK6KR fragments were released from pGEM-MPK6KR with BamHI and XmaI and ligated together with a C-terminal FLAG epitope into the same restriction sites of pSN1301 binary vector to produce 35S:MPK6KR. In addition, a 2,000-bp fragment upstream of ATG start codon of MPK6 was amplified from Arabidopsis genomic DNA, digested with HindIII and BamH1 and ligated into the same restriction sites of pSN1301 to replace the 35S promoter, resulting in MPK6p: MPK6KR. The resulting constructs were introduced into Agrobacterium strain C58C1 and transformed into mpk3 background by floral infiltration. Screening for hygromycin resistance was used to identify transformants and expression of MPK6KR proteins was identified based on qPCR and immunoblot analysis with the anti-FLAG M2 antibody(A8592, Sigma-Aldrich) covalently conjugated to HRPat a 1/4,000 dilution.
To generate MKK5KR transgenic plants, the coding region of MKK5 was amplified from wild-type cDNA using primers containing XhoI and SpeI sites and inserted into the pGEM-T Easy vector to produce pGEM-MKK5. To generate the Lys to Arg mutation on the Lys-99 residue, the fragment was amplified from pGEM-MKK5 template using the Fast Mutagenesis System Kit according to the manufacturer's protocol with primers listed in Supplementary Table 2, giving rise to pGEM-MKK5KR. After sequencing confirmation, the MKK5KR fragments were released from pGEM-MKK5KR with XhoI and SpeI and ligated into the same restriction sites of the steroid-inducible pTA7002 binary vector28. The resulting constructs were introduced into Agrobacterium strain C58C1 and transformed into wild-type Arabidopsis by floral infiltration. The transformants were identified by screening for hygromycin resistance.
To generate MKK5DD/ABI4WT and MKK5KR/ABI4WT transgenic plants, MKK5DD and MKK5KR were crossed into an ABI4WT background, respectively. MKK5DD and MKK5KR were identified using hygromysin resistance, whereas the ABI4WT were identified by kanamycin resistance and transgene expression. To generate ABI4OE/MPK6KR/mpk3 transgenic plants, MPK6KR/mpk3 was crossed into an ABI4WT background. MPK6KR was identified using hygromycin resistance, whereas ABI4WT was identified by kanamycin resistance and transgene expression, and T-DNA insertions of MPK3 were identified by PCR of genomic DNA. Homozygous F3 plants from both crosses were used for the experiments. To generate mpk3abi4 and mpk6abi4 double mutants, the mpk6 and mpk3 mutants were crossed into abi4 background. For genotyping of the progeny, the T-DNA insertions of MPK3 and MPK6 were identified by PCR of genomic DNA, whereas the abi4 allele was identified on the basis of the ABA-insensitive phenotype. Homozygous F3 plants were used for the experiments.
To generate 14-3-3ω overexpressing transgenic plants, the fragments encoding full-length of 14-3-3ω fused with an N-terminal Flag epitope were amplified from wild-type cDNA, digested with XmaI and SacI and ligated into the same restriction sites of the pSN1301 binary vector under the control of a CaMV35S promoter. The resulting constructs were introduced into Agrobacterium strain C58C1 and transformed into wild-type Arabidopsis by floral infiltration. The transformants were identified by screening for hygromycin resistance and independent lines with the expression of FLAG-tagged 14-3-3ω were identified based on immunoblot analysis with the anti-FLAG M2 antibody (A8592, Sigma-Aldrich) covalently conjugated to HRP at a 1/4,000 dilution.
To generate 14-3-3ω RNAi transgenic plants, a 423-bp cDNA fragment containing the last exon and 3′untranslated region of 14-3-3ω was amplified by RT-PCR from total RNA extracted from wild-type seedling using primers containing NcoI and SwaI sites. After digesting with NcoI and SwaI, the resulting DNA fragment was ligated into the NcoI-SwaI sites pFGC5941 in sense orientation. Subsequently, the same fragment was amplified and inserted into downstream of chalcone synthase intron of pFGC5941 already containing the sense repeat using BamHI and SmaI sites. The resulting pFGC5941 vector contained the CaMV35S promoter-driven423-bp cDNA fragment of 14-3-3ω in both sense and antisense orientations separated by a chalcone synthase intron. This resulting RNAi construct was electroporated into the Agrobacterium tumefaciens strain C58C1 and then introduced into the wild-type via the floral dip method. Transgenic plants were selected on MS plates in the presence of 50 μg ml−1 basta.
To generate ABI4WT/14-3-3ωOE and ABI4WT/14-3-3ωRNAi transgenic plants, 14-3-3ωOE and 14-3-3ωRNAi were crossed into an ABI4WT background, respectively. 14-3-3ωOE and 14-3-3ωRNAi were identified using hygromycin and basta resistance, respectively, whereas the ABI4WT were identified by kanamycin resistance. Homozygous F3 plants from both crosses were used for the experiments.
RNA extraction and quantitative real-time PCR
Total RNA was extracted from seedlings by RNA extraction kit (Tiangen). After DNase I treatment, reverse transcription was conducted using PrimeScript II 1st Strand cDNA Synthesis Kit (Takara) according to the manufacturer's protocol. Real-time PCR was performed using an Mx3000p Real-time PCR System (Agilent, Stratagene) with the SYBR Premix ExTaq kit (Takara) in accordance with the manufacturer's instructions. For each sample, three replicates were performed and the expression levels were normalized to those of UBQ. All primers sequences were listed in Supplementary Table 2.
Protein extraction and immunoblot analysis
Total protein was extracted from Arabidopsis using extraction buffer as described previously62. Briefly, plant material was ground in the Eppendorf tube using extraction buffer (125 mM Tris-HCl, pH 8.8, 1% (w/v) SDS, 10% (v/v) glycerol, 50 mM Na2S2O5), centrifuged at 13,000g for 10 min, and the supernatant was saved. Protein concentrations were determined using the Bio-Rad DC protein assay (Bio-Rad) following the manufacturer's instructions. For immunoblot analysis, total protein was separated by SDS–polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes. The membranes were incubated with specific primary antibodies(GFP antibody, G1544, Sigma-Aldrich; FLAG M2 antibody, A8592, Sigma-Aldrich; MPK6 antibody, A7104, Sigma-Aldrich; Firefly Luciferase antibody, ab181640, Abcam). The secondary antibody (goat anti-rabbit IgG, Sigma-Aldrich, A0545) conjugated to HRP was used at a dilution of 1/10,000 for detection by the enhanced chemiluminescence assay. Uncropped images of blots/gels are shown in Supplementary Fig. 17.
Assay of MAP kinase activity
For MAPK assay with seedlings, total protein was extracted from 7-day-old Arabidopsis seedlings using extraction buffer as described previously63. MAPK activation was monitored by immunoblot analysis using Phospho-p44/42 MAPK (Erk1/2) antibodies (#4370, Cell Signalling) at a dilution of 1/4000. The proteins were separated by 10% SDS–PAGE gels and immunodetected with antibody against MPK6 (A7104, Sigma-Aldrich) at a dilution of 1/4,000 to verify the same loading. The secondary antibody (goat anti-rabbit IgG, Sigma-Aldrich, A0545) conjugated to HRP was used at a dilution of 1/10,000 for detection by the enhanced chemiluminescence assay.
For MAPK assay in protoplasts, protoplasts were isolated from 4-week-old plants according to the protocol by Yoo et al.64. Briefly, 0.5–1 mm leaf strips were cut from fully expanded young leaves of 4-week-old Arabidopsis plants, and then transferred into the enzyme solution (20 mM MES, pH 5.7, 1.5% cellulase R10, 0.4% macerozyme R10, 0.4 M mannitol, 20 mM KCl, 10 mM CaCl2, 0.1% BSA) in the dark for at least 3 h at room temperature. After digestion, equal volume of W5 solution (2 mM MES, pH 5.7, 154 mM NaCl, 125 mM CaCl2, 5 mM KCl) was added and the enzyme solution was filtered using a 75-μm nylon mesh. The protoplasts were recovered through centrifugation at 100g for 1–2 min. The MKK5DDconstruct for protoplast expression was made by PCR amplifying the pET28-MKK5DD plasmid49 and in-frame inserted between NcoI and XmaI sites of pSAT6-EYFP-N1, resulting pSAT6-MKK5DD, in which the stop codon was introduced at the N terminus of GFP. To generate MKK4DD construct for protoplast assay, open reading frames of MKK4 were PCR amplified from Arabidopsis cDNA, cloned into pGEM-T vector (Promega), resulting in pGEM-MKK4. After confirmation by sequencing, the constitutively active form of MKK4 was generated by changing both putative phosphorylation sites to Asp residues (T224D and S230D) using the Fast Mutagenesis System Kit (TransGen Biotech) according to the manufacturer's protocol with primers listed in Supplementary Table 2, giving rise to pGEM-MKK4DD. After sequencing confirmation, the pGEM-MKK4DD was digested with NcoI and XmaI and ligated into the same restriction sites of pSAT6-EYFP-N1, resulting pSAT6-MKK4DD, in which the stop codon was introduced at the N terminus of GFP. 14-3-3ω construct is the same as used in the CoIP assay. The combinations of the indicated plasmids were co-transformed into Arabidopsis protoplasts using PEG-calcium-mediated transformation. After overnight incubation at 23 °C, the protoplasts were lysed by vigorously vortexing for 1 min. The resultant clear lysate was centrifuged at 12,000g for 10 min at 4 °C, and the supernatant was separated on a 10% SDS–PAGE gel and subjected to immunoblot analysis using the phosphor-p44/42 MAPK (Erk1/2) antibody at a dilution of 1/4,000. The secondary antibody (goat anti-rabbit IgG, Sigma-Aldrich, A0545) conjugated to horseradish peroxidase was used at a dilution of 1/10,000 for detection by the enhanced chemiluminescence assay. For R18 peptide treatment, R18 peptide was added to the protoplasts at a final concentration of 10 μg ml−1 for 2 h prior to protein extraction.
Measurements of Ca2+ concentrations
Stable transgenic Arabidopsis plants expressing cytosol-targeted apoaequorin under control of the 35S promoter with comparable amount of apoaequorin in wild-type and cas-1 mutants background, respectively35, were used. Ca2+ concentration was determined using the method described by Knight et al.65. Briefly, 6-day-old seedlings were transferred from the Petri dish to an eppendorf tube containing 200 μl of reconstitution buffer (2 mM MES–NaOH, pH 5.7) containing 5 μM coelenterazine (Promega) and incubated overnight in the dark at room temperature. After overnight incubation, the aequorin luminescence was recorded every 0.2 s in GLOMAX20/20 Luminometer (Promega). After 3 min of counting for the base level, NF or Lin was injected into the tube at a final concentration of 5 μM and 500 μM, respectively. The luminescence was recorded immediately for the indicated time. At the end of the experiments, the remaining aequorin was discharged by the addition of an equal amount of discharging solution (2 M CaCl2 dissolved in 20% (v/v) ethanol). Calibration of aequorin luminescence was performed according to Alvarez et al.66
Yeast two-hybrid assay
For yeast two-hybrid screening, experiments were performed using Matchmaker Gold Yeast Two-Hybrid System (Clontech) according to the manufacturer's instructions. Briefly, the bait, N-terminal fragments of ABI4 (amino acids 1–160), was cloned into NdeI and PstI sites of pGBT7 vector (Clontech) and introduced into the yeast strain Y2H Gold (Clontech). The bait strain was grown in SD/-Trp liquid medium overnight and prepared for mating with library strain (Universal Normalized Arabidopsis cDNA library, Clontech) at 30 °C for 24 h. Then, yeast cells were pelleted, washed twice with 0.5 × YPDA and resuspended in 10 ml of 0.5 × YPDA. The resuspension culture was spread out on SD/-Leu/-Trp/-His plates in the presence of 125 ng ml−1 Aureobasidin A. After 4 days, the positive clones were picked up for a α-galactosidase activity assay and blue colonies were further used for plasmid extraction and sequencing.
To assay the interaction of ABI4 with the MAPKs, the N-terminal fragments of ABI4 (amino acids 1–160) and the coding regions of each member of Arabidopsis MAPKs were amplified by PCR with primers containing appropriate restriction sites and inserted into vectors of pGBK and pGAD, respectively. To assay the interaction of 14-3-3ω with the components of the MAPK pathway, fragments encoding full-length 14-3-3ω were amplified by PCR from wild-type cDNA and digested with EcoRI and PstI. The resulting fragments were inserted into the same restriction sites of pGBK plasmid. Full-length cDNAs corresponding to MKK4, MKK5, MKK9 and MEKK1 were amplified by PCR. The resulting fragments were digested by the appropriate restriction enzymes and subcloned in pGAD vector, to yield the vectors pGAD-MKK4, pGAD-MKK5, pGAD-MKK9, pGAD-MKK4, and pGAD-MEKK1. pGAD-MPK3, pGAD-MPK6 and pGAD-MPK4 constructs are the same as used in the ABI4–MAPKs interaction assay. To assay the interaction of 14-3-3Φ with the components of the MAPK pathway, full-length cDNA14-3-3Φ was amplified by PCR from wild-type cDNA and digested with NdeI and PstI. The resulting fragments were inserted into the same restriction sites of pGBK plasmid. PGAD-MPK3, pGAD-MPK6, pGAD-MKK4 and pGAD-MKK5 constructs are the same as used in the 14-3-3ω interaction assay. The MKK4S6A-pGAD and MKK5S25A-pGAD was generated from pGAD-MKK4 and pGAD-MKK5 vectors by changing indicated Ser to Ala residues (S6A and S25A) using the Fast Mutagenesis System Kit (TransGen Biotech) according to the manufacturer's protocol with primers listed in Supplementary Table 2, giving rise toMKK4S6A-pGAD and MKK5S25A-pGAD. All constructs were validated by sequencing and the combination of the indicated constructs were co-transformed yeast strainY2H Gold (Clontech). The yeast two-hybrid transformation and interaction assays were performed in accordance with Yeastmaker Yeast Transformation System 2 User Manual.
Pull-down assay
Fragments encoding full-length MPK3 and MPK6 were amplified by PCR from wild-type cDNA and digested with BamHI and SalI. The resulting fragments were inserted into the same restriction sites of PGEX5X-1 plasmid and transformed into Escherichia coli (E. coli) BL21 (DE3). The resulting strains were grown in LB medium containing 100 μg ml−1 ampicillin and expression of recombinant proteins was induced by the addition of 0.4 mM isopropyl-β-D-thiogalactopyranoside at 25 °C. After 5 h, cells were collected and resuspended in PBS (135 mM NaCl, 2.7 mM KCl, 1.5 mM KH2PO4 and 8 mM K2HPO4, pH 7.4). After sonication and centrifugation, the GST fusion proteins were purified from supernatant with GST-Bind Resin (Novagen). The beads bound with GST-MPK3/MPK6 and GST were washed with PBS for GST pull-down assays. Fifty microlitre aliquots of glutathione-agarose beads were incubated with 4 μg of recombinant His-tagged ABI4 protein at 4 °C for 4 h. The beads were washed four times with 50 mM Tris-HCl, pH 7.4, 100 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40 by centrifugation at 4 °C and boiled with SDS–PAGE sample buffer for 5 min. The eluted proteins were separated on a 15% SDS–PAGE gel and subjected to immunoblot analysis using the antibody against His-tag (CW0083M, CWBIO) at a dilution of 1/1,000. The secondary antibody (goat anti-rabbit IgG, Sigma-Aldrich, A0545) conjugated to horseradish peroxidase was used at a dilution of 1/10,000 for signal detection by the enhanced chemiluminescence assay.
Co-immunoprecipitation assay
CoIP assay was performed in protoplasts generated from wild-type and cas-1 mutants. Briefly, the coding sequences of 14-3-3ω were amplified by PCR amplified using primers containing a FLAG epitope and stop codon at the 3′ end and in-frame inserted into the Nco1-BamH1 sites of pSAT6-EYFP-N1 after digestion with NcoI and BamHI, resulting in FLAG-tagged 14-3-3ω construct. The coding regions of MPK3, MKK4 and MKK5 were amplified by PCR and in-frame ligated to the NcoI-XmaI sites of GFP fusion vector pSAT6-EYFP-N1with the GFP at C terminus after digestion to produce MPK3-GFP, MKK4-GFP and MKK5-GFP, respectively. The coding regions of MPK6 were amplified were amplified by PCR using primers containing HindIII and BamHI sites and in-frame inserted into the same restriction sites of pSAT4A-cEYFP-N1 after digestion to generate MPK6-GFP. The combinations of the indicated plasmids were cotransformed into Arabidopsis protoplasts using PEG-calcium-mediated transformation. After overnight incubation at 23 °C, the protoplasts were harvested and lysed in 300 μl of IP buffer (10 mMHEPES–KOH, pH 7.5, 0.5% Nonidet P-40, 2 mM EDTA, 150 mM NaCl, 10% glycerol, 1 mM NaVO3, 5 mM NaF and 1 × protease inhibitor cocktail (Roche)) by vigorous vortexing for 1 min. The sample was centrifuged at 12,000g for 10 min at 4 °C to remove cellular debris. Twenty microlitres of the supernatant was collected as the input fraction. The rest of the supernatant (280 μl) was mixed with 320 μl IP buffer containing 20 μl of anti-GFP antibody coupled to protein A/G agarose beads, and incubated for 2 h at 4 °C with gentle mixing. After incubation, the beads were washed thoroughly four to five times with 1 ml of IP buffer per wash. The binging protein was eluated by boiling the beads in 30 μl of 2 × SDS–PAGE loading buffer and the presence of co-immunoprecipitated proteins was detected by immunoblotting analysis using the anti-FLAG M2 antibody(A8592, Sigma-Aldrich) covalently conjugated to horseradish peroxidase (HRP)at a 1/4,000 dilution.
Luciferase complementation imaging assay
LCI assay was performed according to Chen et al.67. Briefly, the fragments encoding full-length ABI4 were amplified by PCR and ligated to the KpnI-PstI site of pCAMBIA-CLuc vector after digestion to produce ABI4-CLuc. The coding regions of MPK3 and MPK6 were amplified by PCR and ligated to the BamHI-SalI site of pCAMBIA-NLuc vector after digestion to produce MPK3-NLuc and MPK6-NLuc. The resulting constructs were transformed into Agrobacterium strain GV3101. Agrobacterium bacteria containing indicated constructs were grown in LB medium at 30 °C overnight. After centrifugation and washing two times by infiltration buffer (10 mM MgCl2, 10 mM MES, pH 5.8, 100 μM acetosyringone), the bacterial suspensions were re-suspended in the same infiltration buffer, mixed with an equal volume and co-infiltrated into fully expanded leaves of the 3-week-old Nicotianabenthamiana using a needleless syringe. After 3 days, the leaves were sprayed with luciferin solution (100 μM luciferin, 0.1% Triton X-100) and kept in the dark for 5 min to quench the fluorescence. The LUC image was acquired by a low-light cooled CCD imaging apparatus (CHEMIPROHT 1300B/LND, 16 bits; Roper Scientific).
BiFC assay
BiFC assays were performed essentially according to Walter et al.68 Briefly, the fragments encoding full-length 14-3-3ω were amplified by PCR from wild-type cDNA and digested with SalI and XmaI. The resulting fragments were inserted into the same restriction sites of pSAT4A-nEYFP-N1 plasmid to produce 14-3-3ω-nYFP. The coding regions of MPK3, MKK4 and MKK5 were amplified by PCR and ligated into the EcoRI-XmaI site of pSAT4A-cEYFP-N1 after digestion to produce MPK3-cYFP, MKK4-cYFP and MKK5-cYFP. The coding regions of MPK6 were amplified by PCR using primers containing SalI and BamHI sites and inserted into the same restriction sites of pSAT4A-cEYFP-N1 after digestion to produce MPK6-cYFP. The combinations of the indicated plasmids were co-transformed into Arabidopsis protoplasts using PEG-calcium-mediated transformation. YFP fluorescence was visualized 12–18 h after transformation using a confocal laser scanning microscope (LSM 510 Meta; Zeiss).
In vitro phosphorylation assay
For in vitro phosphorylation assays, the ABI4cDNA was amplified and inserted into the XhoI-PstI sites of the pCold vector (Takara). Mutations of ABI4 were introduced by Fast Mutagenesis System Kit (TransGen Biotech) according to the manufacturer's instructions and verified by sequencing. Primers used for mutagenesis are listed in Supplementary Table 2. The constructs were transformed into E. coli BL21 (DE3). E. coliBL21 (DE3) cells harbouring the plasmids encoding His-tagged MPK3, His-tagged MPK6 and Flag-tagged MKK9DD were the same as those used previously60. The BL21 (DE3) cells were collected after the application of 0.4 mM isopropyl-β-D-thiogalactopyranoside at 25 °C for 6 h and resuspended in 0.1 M Hepes–KOH, pH 7.5. After sonication and centrifugation, His-tagged wild type and mutant ABI4 proteins, His-tagged MPK3 and His-tagged MPK6 were purified through affinity chromatography by using Ni-NTA affinity agarose (Novagen) and Flag-tagged MKK9DD was immunoprecipitated with anti-FLAG M2 Affinity Gel (A2220, Sigma-Aldrich) according to the manufacturer's instructions.
The in vitro kinase assay was performed as described previously49. Activated recombinant MPK3/MPK6 was generated by incubating His-tagged MPK3/MPK6 (10 μg) with recombinant MKK9DD (1 μg) at 27 °C for 30 min in 50 μl of reaction buffer (20 mM HEPES–KOH, pH 7.5, 10 mM MgCl2, 1 mM DTT and 50 mM ATP). Then, recombinant ABI4 was phosphorylated by activated MPK3/MPK6 in the same reaction buffer containing [γ-32P]ATP (0.1 μCi per reaction). Addition of SDS sample buffer stopped the reactions after 30 min. After PAGE analysis (10% SDS polyacrylamide gel), phosphorylated ABI4 was detected by autoradiography. For proteins used in the immunoblot assay, phosphorylation was performed without the addition of [γ-32P]ATP. Uncropped images of autoradiography/immunoblots are shown in Supplementary Fig. 17.
Mobility shift assay
Mobility shift assays to detect phosphorylated ABI4 in vivo were performed essentially according to Guan et al.69. Briefly, total protein was extracted from seedlings and separated on a 10% SDS–PAGE gel containing 50 μM Phos-tag reagent (NARD Institute) and 100 μM MnCl2. For alkaline phosphatase treatment, the protein extracts were incubated with calf intestinal alkaline phosphatase (Sigma-Aldrich) at 37 °C for 1 h and subsequently subjected to Phos-tag-SDS–PAGE. The separated proteins were then transferred to a nitrocellulose membrane and blotted with anti-GFP antibody (G1544, Sigma-Aldrich) at a dilution of 1/4,000. The secondary antibody (goat anti-rabbit IgG, Sigma-Aldrich, A0545) conjugated to HRP was used at a dilution of 1/10,000 for signal detection by the enhanced chemiluminescence assay.
Chromatin immunoprecipitations
Seven-day-old seedlings of WT, ABI4WT/abi4 and ABI4AAA/abi4 grown on 1/2 MS media in the presence or absence of NF were harvested for ChIP assays following the procedure previously described24. Briefly, the seedlings were cross-linked with 1% formaldehyde in MC buffer (10 mM sodium phosphate, pH 7, 50 mM NaCl, 0.1 M sucrose) for 0.5 h under vacuum and washed thoroughly with MC buffer. Then, liquid nitrogen was added and the seedlings were ground to powder. Next, M1 buffer (10 mM potassium phosphate, pH 7.0, 0.1 M NaCl. 10 mM β-mercaptoethanol, 1 M 2-methyl 2,4-pentanediol) was added and the resulting slurry was filtered (Miracloth) and centrifuged (1,000g, for 10 min at 4 °C). The pellet obtained was washed five times with M2 buffer (M1 buffer containing 10 mM MgCl2, 0.5% Triton X-100), and one time with M3 buffer (M1 buffer without 2-methyl 2,4-pentanediol). The resulting crude nuclear pellet was then resuspended and sonicated in 10 mM sodium phosphate, pH 7, 0.1 M NaCl, 0.5% Sarkosyl, 10 mM EDTA, Complete Protease Inhibitor Cocktail (Roche). After centrifugation, the supernatant was mixed with an equal amount of IP buffer (50 mM Hepes–KOH, pH 7.5, 150 mM KCl, 5 mM MgCl2, 10 μM ZnSO4, 1% Triton X-100, 0.05% SDS) and precleared with Protein A/G-Agarose beads (Abmart) for 1 h at 4 °C. After centrifugation, the supernatant of chromatin complexes were incubated with anti-GFP antibody (Sigma-Aldrich) at 4 °C overnight with gentle mixing. The samples were subsequently centrifuged at 14,000g, for 2 min and the supernatant incubated with Protein A/G-Agarose beads (Abmart) for 1 h at 4 °C. Protein A/G-Agarose beads were subsequently pelleted, washed five times with IP buffer and vortexed for 30 s with cold glycine elution buffer (0.1 M glycine, 0.5 M NaCl, 0.05% Tween-20, pH 2.8). The supernatant was collected, neutralized with 50 μl of 1 M Tris-HCl, pH 9 and treated with RNase A at 37 °C for 15 min. After proteinase K digestion at 37 °C for 1 h, the immunoprecipitated DNA was recovered from the elutant by phenol–chloroform extraction and ethanol precipitations and analysed by quantitative real-time PCR with primers shown in Supplementary Table 2. The precipitated DNA from wild type with GFP antibody served as negative control for the PCR amplification.
Electrophoresis mobility shift assay
The PCR fragments of ABI4WT and ABI4AAA, which were amplified from pCold vectors containing wild-type and mutant ABI4 proteins for phosphorylation assays, were cloned into the EcoRI and XhoI sites of a maltose-binding protein fusion vector (pETMALc-H vector), resulting in pETMALc-H-ABI4WT and pETMALc-H-ABI4AAA. Plasmids were then transformed into the E. coli strain BL21 (DE3). The transgenic bacteria were grown at 25 °C and induced by adding isopropyl β-D-thiogalactopyranoside (final concentration of 0.4 mM) during logarithmic growth. After cell lysis in 20 mM Tris-HCl, pH 7.4, 1 mM EDTA and 200 mM NaCl using an ultrasonic cell crusher, followed by centrifugation, the supernatant was purified by amylose resin (NEB). For labelling of the synthetic DNA oligonucleotides (5′-GACTAGAGACTGCCACATAAGAATAGTAAAGTTA-3′), the Biotin 3′ End DNA Labeling Kit (Pierce) was employed. EMSA assay was performed using Light Shift Chemiluminescent EMSA Kit (Pierce). Each binding mixtures (10 μl) containing 2 μg recombinant proteins, 1 μl of poly (dI/dC), 1 μl 10 × binding buffer, 1 μl 50% glycerol and 200 fmol of labelled probe with or without excess cold competitor double-stranded oligonucleotides were incubated at room temperature for 20 min. The DNA–protein complexes were separated on 6% native polyacrylamide gels and transferred to a positive nylon membrane. Membranes were subjected to ultraviolet light for crosslinking and probed with a streptavidin conjugated with HRP. To detect biotin-labelled DNA probes, chemiluminescence was monitored with the Light Shift Chemiluminescent EMSA Kit (Pierce). To test the impact of MAPK phosphorylation on the capacity of ABI4 to bind DNA, recombinant ABI4 was incubated with equally mixed activated MPK3 and MPK6 in the kinase reaction buffer prior to the EMSA assay. Uncropped scans of X-ray films used in figures are included in Supplementary Fig. 17.
LUC activity assay
The reporter plasmids (LHCBp:LUC) and the 35S:GUS internal control constructs were co-transformed into Arabidopsis protoplasts isolated from 4-week-old wild-type and the indicated genotype seedlings. After transformation, the protoplasts were incubated under light for 12–15 h. The activity of LUC and GUS was quantified using a Modulus Luminometer/Fluometer (Promega) as described previously24. Briefly, the protoplasts were pelleted and resuspended in Luciferase Cell Culture Lysis 5X Reagent (Promega). For GUS enzymatic assay, 10 μl of the protoplast lysate were added into 100 μl of MUG substrate mix (10 mM Tris-HCl, pH 8, 1 mM MUG, 2 mM MgCl2) and incubated at 37 °C for 30–180 min. The reaction was stopped by adding 0.9 ml, 0.2 M Na2CO3. For luciferase activity assay, 5 μl of the lysate was mixed with 50 μl of luciferase assay substrate (Promega). The fluorescence was measured using a GloMax 20/20 luminometer(Promega) and relative reporter gene expression was expressed as the ratio of LUC to GUS.
Data availability
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files or are available from the corresponding author upon request.
Additional information
How to cite this article: Guo, H. et al. Plastid-nucleus communication involves calcium-modulated MAPK signalling. Nat. Commun. 7:12173 doi: 10.1038/ncomms12173 (2016).
Supplementary Material
Supplementary Figures 1 - 17 and Supplementary Tables 1 and 2
Acknowledgments
We are grateful to Li Legong (Capital Normal University) and Takashi Shiina (Kyoto Prefectural University) for providing us with transgenic Arabidopsis plants expressing cytoplasm-targeted aequorin in wild-type and cas mutants background, respectively. The work in our laboratory was supported by the Major State Basic Research Development Program (grant 2015CB150100), and the National Natural Science Foundation of China (grant 31370273) and funds from Chinese Academy of Sciences.
Footnotes
Author contributions This project was initiated and supervised by L.Z., H.G. and X.S. designed the research. H.G., P.F. and Y.L. performed the research. X.S., W.C., X.X., D.R. and C.L. joined in the discussion and data analysis. L.Z. and H.G. analysed data and wrote the manuscript together. J.D.R. and D.L. assisted in writing and discussion.
References
- Pogson B. J. & Albrecht V. Genetic dissection of chloroplast biogenesis and development: an overview. Plant Physiol. 155, 1545–1551 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H.-S. & Chory J. Signalling between chloroplasts and the nucleus: can a systems biology approach bring clarity to a complex and highly regulated pathway? Plant Physiol. 152, 453–459 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leister D. Chloroplast research in the genomic age. Trends Genet. 19, 47–56 (2003). [DOI] [PubMed] [Google Scholar]
- Nott A., Jung H.-S., Koussevitzky S. & Chory J. Plastid-to-nucleus retrograde signalling. Annu. Rev. Plant Biol. 57, 739–759 (2006). [DOI] [PubMed] [Google Scholar]
- Chi W., Sun X. & Zhang L. Intracellular signalling from plastid to nucleus. Annu. Rev. Plant Biol. 64, 559–582 (2013). [DOI] [PubMed] [Google Scholar]
- Fernandez A. P. & Strand A. Retrograde signalling and plant stress: plastid signals initiate cellular stress responses. Curr. Opin. Plant Biol. 11, 509–513 (2008). [DOI] [PubMed] [Google Scholar]
- Oelmüller R., Levitan I., Bergfeld R., Rajasekhar V. & Mohr H. Expression of nuclear genes as affected by treatments acting on the plastids. Planta 168, 482–492 (1986). [DOI] [PubMed] [Google Scholar]
- Strand Å., Asami T., Alonso J., Ecker J. R. & Chory J. Chloroplast to nucleus communication triggered by accumulation of Mg-protoporphyrinIX. Nature 421, 79–83 (2003). [DOI] [PubMed] [Google Scholar]
- Fey V. et al. Retrograde plastid redox signals in the expression of nuclear genes for chloroplast proteins of Arabidopsis thaliana. J. Biol. Chem. 280, 5318–5328 (2005). [DOI] [PubMed] [Google Scholar]
- Lee K. P., Kim C., Landgraf F. & Apel K. EXECUTER1- and EXECUTER2-dependent transfer of stress-related signals from the plastid to the nucleus of Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 104, 10270–10275 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koussevitzky S. et al. Signals from chloroplasts converge to regulate nuclear gene expression. Science 316, 715–719 (2007). [PubMed] [Google Scholar]
- Estavillo G. M. et al. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signalling in Arabidopsis. Plant Cell 23, 3992–4012 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodson J. D., Perez-Ruiz J. M. & Chory J. Heme synthesis by plastid ferrochelatase I regulates nuclear gene expression in plants. Curr. Biol. 21, 897–903 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramel F. et al. Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc. Natl Acad. Sci. USA 109, 5535–5540 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y. et al. Retrograde signalling by the plastidial metabolite MEcPP regulates expression of nuclear stress-response genes. Cell 149, 1525–1535 (2012). [DOI] [PubMed] [Google Scholar]
- Chi W., Feng P., Ma J. & Zhang L. Metabolites and chloroplast retrograde signalling. Curr. Opin. Plant Biol. 25, 32–38 (2015). [DOI] [PubMed] [Google Scholar]
- Fiil B. K., Petersen K., Petersen M. & Mundy J. Gene regulation by MAP kinase cascades. Curr. Opin. Plant Biol. 12, 615–621 (2009). [DOI] [PubMed] [Google Scholar]
- Lampard G. R., MacAlister C. A. & Bergmann D. C. Arabidopsis stomatalinitiation is controlled by MAPK-mediated regulation of the bHLH SPEECHLESS. Science 322, 1113–1116 (2008). [DOI] [PubMed] [Google Scholar]
- Rodriguez M. C., Petersen M. & Mundy J. Mitogen-activated protein kinase signalling in plants. Annu. Rev. Plant Biol. 61, 621–649 (2010). [DOI] [PubMed] [Google Scholar]
- Meng X. et al. A MAPK cascade downstream of ERECTA receptor-like protein kinase regulates Arabidopsis inflorescence architecture by promoting localized cell proliferation. Plant Cell 24, 4948–4960 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K. Scaffold proteins in mammalian MAP kinase cascades. J. Biochem. 135, 657–661 (2004). [DOI] [PubMed] [Google Scholar]
- Witzel F., Maddison L. & Bluthgen N. How scaffolds shape MAPK signalling: what we know and opportunities for systems approaches. Front. Physiol. 3, 475 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J. G., Li Z. & Sacks D. B. IQGAP1 integrates Ca2+/calmodulin and B-Raf signalling. J. Biol. Chem. 283, 22972–22982 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X. et al. A chloroplast envelope-bound PHD transcription factor mediates chloroplast signals to the nucleus. Nat. Commun. 2, 477 (2011). [DOI] [PubMed] [Google Scholar]
- Andreasson E. & Ellis B. Convergence and specificity in the Arabidopsis MAPK nexus. Trends Plant Sci. 15, 106–113 (2010). [DOI] [PubMed] [Google Scholar]
- Sharrocks A. D., Yang S. H. & Galanis A. Docking domains and substrate-specificity determination for MAP kinases. Trends Biochem. Sci. 25, 448–453 (2000). [DOI] [PubMed] [Google Scholar]
- Jacobs D., Glossip D., Xing H. M., Muslin A. J. & Kornfeld K. Multiple docking sites on substrate proteins form a modular system that mediates recognition by ERK MAP kinase. Genes Dev. 13, 163–175 (1999). [PMC free article] [PubMed] [Google Scholar]
- Ren D. et al. A fungal-responsive MAPK cascade regulates phytoalexin biosynthesis in Arabidopsis. Proc. Natl Acad. Sci. USA 105, 5638–5643 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita E., Kinoshita-Kikuta E. & Koike T. Separation and detection of large phosphoproteins using Phos-tag SDS-PAGE. Nat. Protoc. 4, 1513–1521 (2009). [DOI] [PubMed] [Google Scholar]
- Wang H., Ngwenyama N., Liu Y., Walker J. C. & Zhang S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell. 19, 63–73 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho S. K. et al. Regulation of floral organ abscission in Arabidopsis thaliana. Proc. Natl Acad. Sci. USA 105, 15629–15634 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agell N., Bachs O., Rocamora N. & Villalonga P. Modulation of the Ras/Raf/MEK/ERK pathway by Ca2+, and calmodulin. Cell. Signal. 14, 649–654 (2002). [DOI] [PubMed] [Google Scholar]
- Chuderland D. & Seger R. Calcium regulates ERK signalling by modulating its protein-protein interactions. Commun. Integr. Biol. 1, 4–5 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H. & Shiina T. Calcium signalling in plant endosymbiotic organelles: mechanism and role in physiology. Mol. Plant 7, 1094–1104 (2014). [DOI] [PubMed] [Google Scholar]
- Nomura H., Komori T., Kobori M., Nakahira Y. & Shiina T. Evidence for chloroplast control of external Ca2+-induced cytosolic Ca2+ transients and stomatal closure. Plant J. 53, 988–998 (2008). [DOI] [PubMed] [Google Scholar]
- Weinl S. et al. A plastid protein crucial for Ca2+-regulated stomatal responses. New Phytol. 179, 675–686 (2008). [DOI] [PubMed] [Google Scholar]
- Zhang A., Jiang M., Zhang J., Tan M. & Hu X. Mitogen-activated protein kinase is involved in abscisic acid-induced antioxidant defense and acts downstream of reactive oxygen species production in leaves of maize plants. Plant Physiol. 141, 475–487 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z. et al. Pathogen-secreted proteases activate a novel plant immune pathway. Nature 521, 213–216 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Wang P., Shao W., Zhu J. K. & Dong J. The BASL polarity protein controls a MAPK signalling feedback loop in asymmetric cell division. Dev. Cell 33, 136–149 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S. et al. 14-3-3 facilitates Ras-dependent Raf-1 activation in vitro and in vivo. Mol. Cell. Biol. 18, 3947–3955 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalle M. et al. ZmMPK6, a novel maize MAP kinase that interacts with 14-3-3 proteins. Plant Mol. Biol. 59, 713–722 (2005). [DOI] [PubMed] [Google Scholar]
- Oh C. S., Pedley K. F. & Martin G. B. Tomato 14-3-3 protein 7 positively regulates immunity-associated programmed cell death by enhancing protein abundance and signalling ability of MAPKKKα. Plant Cell 22, 260–272 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLille J. M., Sehnke P. C. & Ferl R. J. The Arabidopsis 14-3-3 family of signalling regulators. Plant Physiol. 126, 35–38 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A. L., Sehnke P. C. & Ferl R. J. Isoform-specific subcellular localization among 14-3-3 proteins in Arabidopsis seems to be driven by client interactions. Mol. Biol. Cell 16, 1735–1743 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.-D., Cho Y.-H., Tena G., Xiong Y. & Sheen J. Dual control of nuclear EIN3 by bifurcate MAPK cascades in C2H4 signalling. Nature 451, 789–795 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athwal G. S., Huber J. L. & Huber S. C. Biological significance of divalent metal ion binding to 14-3-3 proteins in relationship to nitrate reductase inactivation. Plant Cell Physiol. 39, 1065–1072 (1998). [DOI] [PubMed] [Google Scholar]
- Abarca D., Madueno F., Martinez-Zapater J. M. & Salinas J. Dimerization of Arabidopsis 14-3-3 proteins: structural requirements within the N-terminal domain and effect of calcium. FEBS Lett. 462, 377–382 (1999). [DOI] [PubMed] [Google Scholar]
- Wang B. et al. Isolation of high-affinity peptide antagonists of 14-3-3 proteins by phage display. Biochemistry 38, 12499–12504 (1999). [DOI] [PubMed] [Google Scholar]
- Liu Y. & Zhang S. Phosphorylation of 1-aminocyclopropane-1-carboxylic acid synthase by MPK6, a stress-responsive mitogen-activated protein kinase, induces ethylene biosynthesis in Arabidopsis. Plant Cell 16, 3386–3399 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao G. et al. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 23, 1639–1653 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X. & Zhang S. MAPK cascades in plant disease resistance signalling. Annu. Rev. Phytopathol. 51, 245–266 (2013). [DOI] [PubMed] [Google Scholar]
- Djamei A., Pitzschke A., Nakagami H., Rajh I. & Hirt H. Trojan horse strategy in Agrobacterium transformation: abusing MAPK defense signalling. Science 318, 453–456 (2007). [DOI] [PubMed] [Google Scholar]
- Finkelstein R., Lynch T., Reeves W., Petitfils M. & Mostachetti M. Accumulation of the transcription factor ABA-insensitive (ABI)4 is tightly regulated post-transcriptionally. J. Exp. Bot. 62, 3971–3979 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorio J., Hernandez-Bernal A. F., Cordoba E. & Leon P. Characterization of evolutionarily conserved motifs involved in activity and regulation of the ABA-INSENSITIVE (ABI) 4 transcription factor. Mol. Plant 7, 422–436 (2014). [DOI] [PubMed] [Google Scholar]
- Vogel M. O. et al. Fast retrograde signalling in response to high light involves metabolite export, MITOGEN-ACTIVATED PROTEIN KINASE6, and AP2/ERF transcription factors in Arabidopsis. Plant Cell 26, 1151–1165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroutsos D. et al. The chloroplast calcium sensor CAS is required for photoacclimation in Chlamydomonasreinhardtii. Plant Cell 23, 2950–2963 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura H. et al. Chloroplast-mediated activation of plant immune signalling in Arabidopsis. Nat. Commun. 3, 926 (2012). [DOI] [PubMed] [Google Scholar]
- Kobayashi Y. et al. Tetrapyrrole signal as a cell-cycle coordinator from organelle to nuclear DNA replication in plant cells. Proc. Natl Acad. Sci. USA 106, 803–807 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Imamura S., Hanaoka M. & Tanaka K. A tetrapyrrole-regulated ubiquitin ligase controls algal nuclear DNA replication. Nat. Cell Biol. 13, 483–487 (2011). [DOI] [PubMed] [Google Scholar]
- Chevalier D., Morris E. R. & Walker J. C. 14-3-3 and FHA domains mediate phosphoprotein interactions. Annu. Rev. Plant Biol. 60, 67–91 (2009). [DOI] [PubMed] [Google Scholar]
- Clough S. J. & Bent A. F. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 (1998). [DOI] [PubMed] [Google Scholar]
- Martinez-Garcia J. F., Monte E. & Quail P. H. A simple, rapid and quantitative method for preparing Arabidopsis protein extracts for immunoblot analysis. Plant J. 20, 251–257 (1999). [DOI] [PubMed] [Google Scholar]
- Wang P., Du Y., Li Y., Ren D. & Song C. P. Hydrogen peroxide-mediated activation of MAP kinase 6 modulates nitric oxide biosynthesis and signal transduction in Arabidopsis. Plant Cell 22, 2981–2998 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S. D., Cho Y. H. & Sheen J. Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat. Protoc. 2, 1565–1572 (2007). [DOI] [PubMed] [Google Scholar]
- Knight H., Trewavas A. J. & Knight M. R. Cold calcium signalling in Arabidopsis involves two cellular pools and a change in calcium signature after acclimation. Plant Cell 8, 489–503 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J. & Montero M. Measuring [Ca2+] in the endoplasmic reticulum with aequorin. Cell Calcium 32, 251–260 (2002). [DOI] [PubMed] [Google Scholar]
- Chen H. et al. Firefly luciferase complementation imaging assay for protein-protein interactions in plants. Plant Physiol. 146, 368–376 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M. et al. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation. Plant J. 40, 428–438 (2004). [DOI] [PubMed] [Google Scholar]
- Guan Q., Yue X., Zeng H. & Zhu J. The protein phosphatase RCF2 and its interacting partner NAC019 are critical for heat stress-responsive gene regulation and thermotolerance in Arabidopsis. Plant Cell 26, 438–453 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1 - 17 and Supplementary Tables 1 and 2
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the article and its Supplementary Information files or are available from the corresponding author upon request.