Abstract
The psychology of extinction has been studied for decades. Approximately 10 years ago, however, there began a concerted effort to understand the neural circuits of extinction of fear conditioning, in both animals and humans. Progress during this period has been facilitated by an unusual degree of coordination between rodent and human researchers examining fear extinction. This successful research program could serve as a model for translational research in other areas of behavioral neuroscience. Here we review the major advances and highlight new approaches to understanding and exploiting fear extinction.
Keywords: Conditioning, prefrontal, cingulate, amygdala, fMRI, anxiety
Introduction
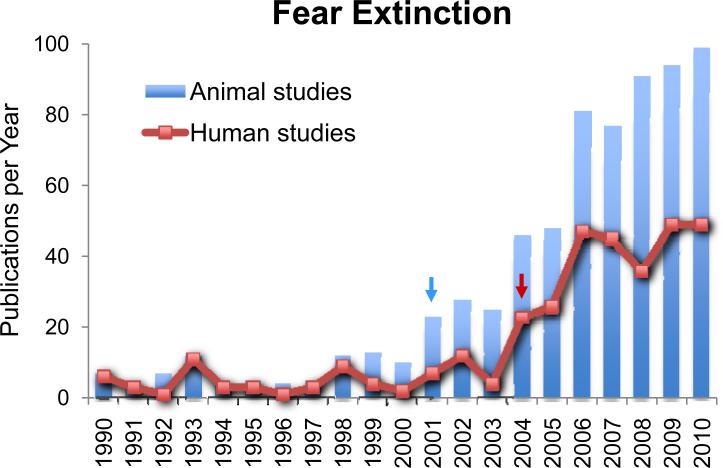
Fear extinction refers to the decrement in conditioned fear responses that occurs with repeated presentation of a conditioned fear stimulus that is unreinforced. Because the neural mechanisms of fear extinction have been examined since the 1960s, a comprehensive review of the psychological and neurobiological basis of fear extinction is not possible in this venue. Numerous reviews on molecular mechanisms of extinction, clinical relevance, and inhibition circuitry within the amygdala have recently appeared (Pape & Paré 2010, Herry et al 2010, Sotres-Bayon & Quirk 2010, Myers et al 2011, Etkin et al 2011). In this review, we start with a brief overview of fundamental psychological concepts of extinction, and then review the key factors prior to 2000 that lead to the recent increase in fear extinction studies. Figure 1 shows the number of publications using the key words “fear extinction” since 1990. Note that both animal and human studies exhibited a sharp increase after 2000, with animal studies preceding human studies by several years. We focus on fear extinction research during this last decade, which was facilitated by a rodent-to-human translational approach. Lastly, we disucss future directions for fear extinction research in the next decade.
Figure 1.
Exponential increase in fear extinction studies within the last decade. Number of peer-reviewed studies identified with key terms “fear” and “extinction” in PubMed, in the past 20 years. Note that the increase in animal studies (blue bars, blue arrow) began several years prior to the increase in human studies (orange line, orange arrow).
Overview of fear extinction and its psychological basis
Initial attempts to understand fear extinction focused on psychological and behavioral phenomenon. In the 1920's, Pavlov observed that extinguished appetitive responses in dogs would spontaneously recover with the passage of time, and proposed that extinction was a special form of inhibition (Pavlov 1927). Despite early theoretical formulations of extinction-related inhibition (Konorski 1967), the search for inhibitory circuits had been largely unsuccessful (Kimble & Kimble 1970, Chan et al 2001). Research focusing on the behavioral and psychological aspects of conditioning and extinction provided key data upon which contemporary studies on the neural mechanisms of fear extinction were based. In addition to the passage of time, it was shown that extinguished responding could be renewed with a change in context (Bouton & King 1983), or reinstated with unconditioned stimuli (Rescorla & Heth 1975). The phenomena of spontaneous recovery, fear renewal, reinstatement, as well accelerated re-acquisition (Rescorla 2001) have been described in detail over the past decades (reviewed in Rescorla 1988, Bouton & Moody 2004). Together, they constitute strong evidence that extinction does not erase the initial association between the CS and US but forms a new association (CS-No US) that inhibits expression of the conditioned memory.
Context, in particular, is able to gate expression of conditioning vs. extinction memory. That is, when an animal is conditioned in one context (context A), and then extinguished in a different context (context B), the extinction memory can only be expressed if the CS is presented in context B. Though “context” is often defined as the physical place (and all sensory cues present within), it is proposed that the internal state of the animal can also be considered a “context”. Also, the passage of time can be viewed as a contextual shift (Bouton et al 2006).
Setting the stage for fear extinction mechanisms
From avoidance to fear conditioning: the amygdala as a hub of fear
Initial animal work on fear extinction in the 1960-80's used the active avoidance paradigms popular at the time. Systemically administered drugs were used to implicate stress hormones, benzodiazepines, and monoamines in extinction (Buresova et al 1964, Bohus & De Wied 1966, Kokkinidis 1983, Koob et al 1986). Setting the stage for later molecular work, it was shown that extinction learning required protein synthesis (Flood et al 1977) and cortical norepinephrine (Mason et al 1979). Direct manipulations of the brain were few, but lesions and electrical stimulation implicated the septum, prefrontal cortex, striatum, and hippocampus in extinction of avoidance responses (Lovely 1975, Sanberg et al 1979, Brennan & Wisniewski 1982, Gralewicz & Gralewicz 1984).
Our knowledge of the fear learning circuits advanced rapidly during the 1980-90's. Study of avoidance gave way to more ethologically relevant conditioned responses such as freezing and potentiation of startle responses (Chi 1965, Blanchard & Blanchard 1969, Davis & Astrachan 1978). The amygdala became the centerpiece of the fear conditioning circuit when it was shown that discrete lesions could block the acquisition and expression of conditioned fear responses (LeDoux et al 1984, Hitchcock & Davis 1986), and that the lateral amygdala received direct input from sensory areas of the thalamus (LeDoux et al 1985). Neurobiological evidence began to detail how the association between tone and shock is formed and expressed within different subnuclei of the amygdala (LeDoux 2000, Davis 2000, Maren 2005, Sigurdsson et al 2007).
During the same time, anatomical studies described the connections of the amygdala central nucleus with downstream structures implicated in the expression of conditioned fear responses, including the hypothalamus, periaquaductal gray, pons, and other brainstem regions (Kapp et al 1979, Applegate et al 1982, LeDoux et al 1988, Romanski & LeDoux 1993, Pitkanen et al 1997). Studies during this period also described the inhibitory circuits within the amygdala that were later found to be involved in fear extinction, such as the GABAergic intercalated cells (Nitecka & Ben Ari 1987, Paré & Smith 1993), lateral division of the central nucleus (Sun & Cassell 1993), and inhibitory cells within the lateral and basolateral nuclei (Mahanty & Sah 1998). Thus, the advances in understanding the fear conditioning provided the framework against which to investigate extinction-induced reduction of fear.
Advent of human neuroimaging tools
Paralleling advances in animal work on neural circuits of fear, the field of human neuroimaging was established with the first functional magnetic resonance imaging (fMRI) study published in 1991 (Belliveau et al 1991). The initial wave of fMRI studies focused on paradigms involving functional activation of the visual and motor cortices (reviewed in Rosen et al 1998). With respect to emotional learning, early fMRI studies sought to determine whether rodent models of the amygdala in conditioned fear were valid in the human brain. Using a simple differential conditioning paradigm in healthy humans (a blue square as the CS and a mild shock as the US), LaBar et al (1998) (1998) and later Büchel et al. (1999) reported increased amygdala activation in response to the CS+ (CS that is paired with the US), as compared to CS- (CS that is not paired with the shock). Subsequent fMRI studies using fearful faces as stimuli also resulted in significant amgydala activation in healthy humans (Breiter et al 1996, Whalen et al 1998). These observations were critical for two reasons: 1) they provided unequivocal evidence that amygdala function was conserved across species, and 2) they validated the use of fMRI and cross-species experimental designs for studying fear learning.
Initial research on fear extinction mechanisms: In search of the inhibitor
In contrast to fear learning, fear extinction research was only just beginning in the 1990s. Harris and Westbrook (1998) confirmed that extinction was a form of inhibition by showing that a beta-carboline antagonist of GABA-A receptors could block the development and expression of extinction. A prescient study by Falls and coworkers (1992) showed that fear extinction required NMDA receptors in the BLA, confirming that extinction was an active form of learning similar to conditioning itself. Initial hints of the involvement of the prefrontal cortex in emotion regulation came from earlier studies showing compulsive-like behavior (disinhibition) in dogs after lesions of the subgenual region of the medial prefrontal cortex (Brutkowski & Mempel 1961), and from monkeys with lesions of orbitofrontal cortex (Butter et al 1963). Inspired by these older studies, LeDoux and coworkers found that lesions of sensory cortices impaired fear extinction (Teich et al 1989, LeDoux et al 1989), and reasoned that sensory areas interacted with frontal or hippocampal cortices to mediate extinction (for review see Sotres-Bayon et al 2004). Anatomical studies appeared showing direct projections from the ventral medial prefrontal cortex (vmPFC) to the amygdala (Hurley et al 1991, McDonald 1991, McDonald 1998), in particular to inhibitory areas such as the intercalated cells (Vertes 2004). The first direct evidence for the involvement of the vmPFC in fear extinction came from Morgan et al. (1993) The authors showed that pre-training lesions of the vmPFC had no effect on the acquisition of conditioned fear but impaired fear extinction across days. Davis and coworkers were unable to replicate extinction deficits with lesions of visual cortex or vmPFC (Falls & Davis 1993, Gewirtz et al 1997), suggesting a possible difference between extinction of freezing vs. potentiated startle, or the specific location of the lesions (Sotres-Bayon et al 2004). Nevertheless, Morgan et al. (1993) generated interest in prefrontal cortex as an inhibitor in extinction.
A clinical connection to fear extinction
It was first proposed in the late 1980's that fear conditioning may serve as an animal model for anxiety disorders, such as posttraumatic stress disorder (PTSD), and could be useful for understanding the underlying psychopathology of anxiety disorders. Pitman and colleagues proposed that PTSD patients hyper-condition; i.e. form strong associations between traumatic events and sensory cues present at the time trauma (Pitman 1988). These strong associations later become resistant to extinction. Moreover, neuroimaging data that emerged during the late 1990's implicated the amygdala and the vmPFC in the psychopathology of anxiety disorders, which matched the clinical profile of perseverative fear and anxiety. Positron emission tomography (PET) studies showed decreased prefrontal blood flow in PTSD patients (Semple et al 1996, Bremner et al 1999). PTSD patients also showed reduced activation of vmPFC, as indicated by fMRI, when recalling traumatic events with the help of script-driven imagery (Shin et al 1999). Thus, an improved understanding of fear learning circuits provided hypotheses which could be tested in humans with contemporary neuroimaging tools, which in turn provided a framework for translational research on fear extinction.
The inhibitor found?
Rodent infralimbic prefrontal cortex
In an attempt to resolve the apparent conflict in previous studies assessing vmPFC's role in extinction (Morgan et al 1993, Gewirtz et al 1997), Quirk et al. (2000) made lesions of the vmPFC, focusing on the infralimbic subregion (IL), as opposed to the prelimbic subregion (PL)(see figure 2). IL lesions did not impair the ability of rats to extinguish conditioned freezing responses within an extinction session, indicating that prefrontal circuits were not necessary for the initial learning of extinction. The following day, however, rats with vmPFC lesions were unable to retrieve their extinction memory at the start of the testing session. Thus, a distinction was made between the acquisition of extinction, and its subsequent retrieval, reflecting behavioral studies showing that retrieval of extinction was regulated by contextual and temporal factors (Bouton 1993, Rescorla 2004). Thus, dissecting extinction into separate phases of acquisition, consolidation, and retrieval, similar to other types of learning, would be necessary for understanding the neurobiology of extinction (Quirk & Mueller 2008). It would no longer be sufficient to simply view extinction as the reversal of a primary conditioning process, but rather a separate process that interacts with conditioning memory.
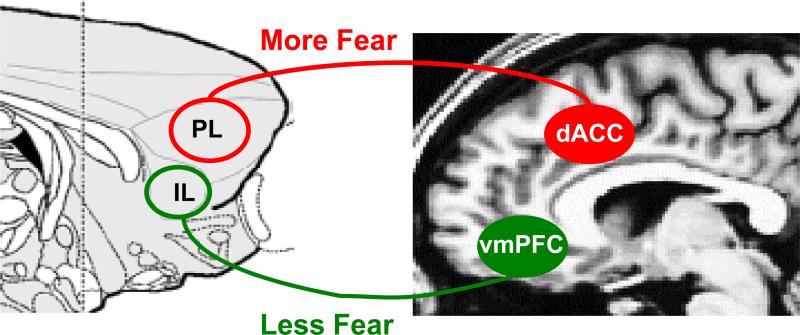
Figure 2.
Homologous prefrontal structures in rodent and human brain that modulate fear expression. The rodent prelimbic cortex (PL) and human dorsal anterior cingulate cortex (dACC) increase fear expression and oppose extinction, while the rodent infralimbic cortex (IL) and human ventromedial prefrontal cortex inhibit fear expression and promote extinction.
Because lesion studies are often difficult to interpret, additional approaches were needed to test the vmPFC hypothesis. Accordingly, Milad and Quirk (2002) used single cell recording to determine the phase of conditioning/extinction vmPFC might signal. Paralleling lesion findings, cells in IL did not signal tones during conditioning or extinction phases, but did signal tones during the retrieval phase. Furthermore, the magnitude of IL tone responses was inversely correlated with freezing at the retrieval test, consistent with a safety signal. Similar findings have been reported with the activity marker cFos (Hefner et al 2008, Knapska & Maren 2009) and metabolic mapping methods (Barrett et al 2003). Moving closer to a test of causality, Milad and Quirk (2002) showed that mimicking IL tone responses with microstimulation reduced fear and strengthened extinction. Both recording and stimulation findings were specific to IL, and were not observed in the adjacent prelimbic cortex. Taken together, these studies redefined the role of vmPFC in extinction, from a general notion of prefrontal involvement to a specific role of IL plasticity in the retrieval of previously learned extinction. Additional support for a role of vmPFC in extinction came from Garcia and colleagues who showed that extinction potentiated thalamic and hippocampal inputs to vmPFC (Herry & Garcia 2003), and extinction memory could be modulated by administering potentiating or depressing trains of stimulation to vmPFC inputs (Herry & Garcia 2002).
Subsequent studies have confirmed the role of IL in the retrieval of extinction, using lesions, drug infusions, and stimulation approaches (see figure 3) (Akirav & Maroun 2007, Quirk & Mueller 2008, Holmes & Wellman 2009, for recent reviews, see Herry et al 2010, Sotres-Bayon & Quirk 2010). For a listing of studies implicating vmPFC prior to 2008, see Quirk and Mueller (2008). More recent studies specifically of IL are listed here in table 1. Extinction memory requires IL activation of NMDA receptors (Burgos-Robles et al 2007, Sotres-Bayon et al 2007), protein kinase A (Mueller et al., 2008), MAP kinase (Hugues et al 2004), cannabinoid receptors (Lin et al 2009), and protein synthesis (Santini et al 2004, Mueller et al 2008). Together, these studies suggest that a calcium-mediated cascade in IL triggers protein kinases and protein synthesis necessary for long-term extinction memory. In addition to increased tone responding, extinction increased burst-type firing of IL neurons (Burgos-Robles et al 2007, Chang et al 2010), and reversed conditioning-induced depression of intrinsic excitability (Santini et al 2008). This suggests that extinction-induced potentiation of intrinsic and synaptic mechanisms in IL could increase local plasticity and increase the impact of IL on its targets. In support of this, the degree of IL bursting is correlated with extinction retrieval (Santini et al 2008), and pharmacologically augmenting IL excitability strengthens extinction memory (Santini & Porter 2010). Thus, IL rodent data confirmed early observations (Pavlov 1927, Konorski 1967) that extinction does not return the brain to its pre-conditioning state, but rather potentiates inhibitory circuits.
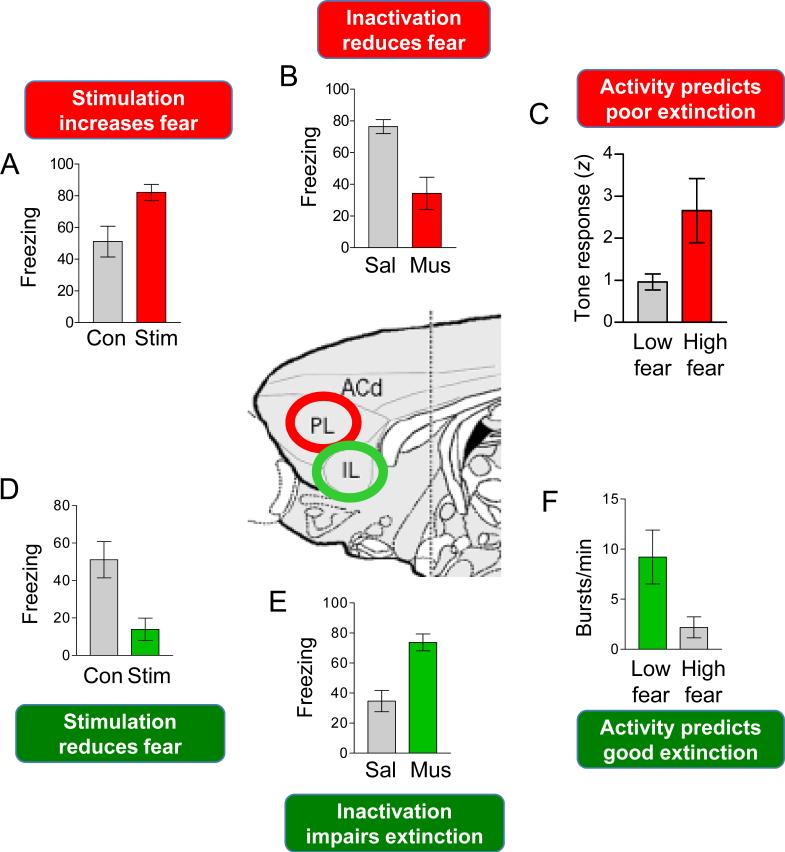
Figure 3.
Summary of rodent findings for PL and IL prefrontal cortex. A-C: Divergent findings suggest that PL activity increases fear expression. D-F: Parallel data suggest that IL inhibits fear expression and strengthens extinction recall (D,E,F). Data adapted from previously published studies: A, D: Vidal-Gonzalez et al., 2006: see also (Kim et al 2010); B, E: Sierra-Mercado et al., 2011, see also (Laurent & Westbrook 2009); C: Burgos-Robles et al.,2009; F: Burgos-Robles et al., 2007, see also (Chang et al 2010).
Table 1.
Effects of infralimbic cortex manipulations on memory for fear extinction
| Method | Task | Ext. memory | Reference | |
|---|---|---|---|---|
| Facilitators | ||||
| CB1 receptor agonist | cued fc | enhanced | Lin et al., 2009 | |
| BDNF | cued fc | enhanced | Peters et al., 2010 | |
| M-type K(+) channel blocker | cued fc | enhanced | Santini & Porter, 2010 | |
| GABAa antagonist picrotoxin | cued fc | enhanced | Thompson et al., 2010 | |
| microstimulation | cued fc | enhanced | Kim et al., 2010 | |
| GABAa antagonist picrotoxin | cued fc | enhanced | Chang & Maren, 2011 | |
| histone acetyltransferase | cued fc | enhanced | Marek et al., 2011 | |
| Inhibitors | ||||
| CB1 receptor antagonist | cued fc | impaired | Lin et al., 2009 | |
| inactivation with muscimol | context fc | impaired | Laurent & Westbrook, 2009 | |
| D2 antagonist raclopride | cued fc | impaired | Mueller et al., 2010 | |
| mGluR5 antagonist MPEP | cued fc | impaired | Fontanez-Nuin et al., 2011 | |
| inactivation with muscimol | cued fc | impaired | Sierra-Mercado et al., 2011 |
Translating IL findings to humans
Spurred by rodent data on extinction, researchers developed numerous ingenious paradigms for assessing fear conditioning and extinction in healthy humans. These included examinations of fear potentiated startle (Jovanovic et al 2005, Jovanovic et al 2006), return of fear phenomena: renewal, reinstatement, and the context dependency of extinction (Vervliet et al 2005, Vansteenwegen et al 2005, Hermans et al 2005, LaBar & Phelps 2005, Milad et al 2005a, Norrholm et al 2006) extinction in adolescents (Pine et al 2001), and the use of virtual reality (Baas et al 2004, Grillon et al 2006, Huff et al 2010). Regarding brain imaging, initial studies focused mostly on within-session extinction learning (i.e. LaBar et al 1998), and found increased activation of the amygdala and orbitofrontal cortex during extinction training (Gottfried & Dolan 2004, Knight et al 2004). However, the involvement of rodent IL in extinction recall, but not its initial learning, called for a multi-day conditioning paradigm in humans that would distinguish between extinction learning and its subsequent recall.
The homolog of IL in the human brain: Ventromedial prefrontal cortex (vmPFC)
Phelps and colleagues conducted the first fMRI study to identify a functional homolog of IL in the human brain in a two-day paradigm that would assess extinction recall (Phelps et al 2004). They showed that vmPFC increased its activation during recall of extinction in healthy humans, a finding that was later replicated (Kalisch et al 2006). This suggested that vmPFC might be a functional homologue of the rodent IL (see figure 2). We developed and validated a human fear conditioning and extinction paradigm that allowed for contextual manipulation of extinction recall, and would compare recall of extinction with recall of conditioning (via the use of extinguished vs. unextinguished stimuli), in order to better translate rodent data (Milad et al 2005a, Rauch et al 2006). Using this paradigm, we observed vmPFC deactivation during conditioning, which converted to significant activation by the end of extinction learning (Milad et al 2007b). During extinction recall, the magnitude vmPFC activation to the extinguished stimulus (relative to the unextinguished stimulus) was positively correlated with the magnitude of extinction retention (Milad et al 2007b). That is, the stronger the activation of the vmPFC, the better the ability to inhibit conditioned responding during extinction recall. Analysis of a different cohort showed that, in addition to activation, the thickness of the vmPFC was also correlated with extinction recall (Milad et al 2005b, Hartley et al 2011) Thus, both structure and function of the human vmPFC positively correlate with the magnitude of extinction memory, similar to rat IL (see figure 4).
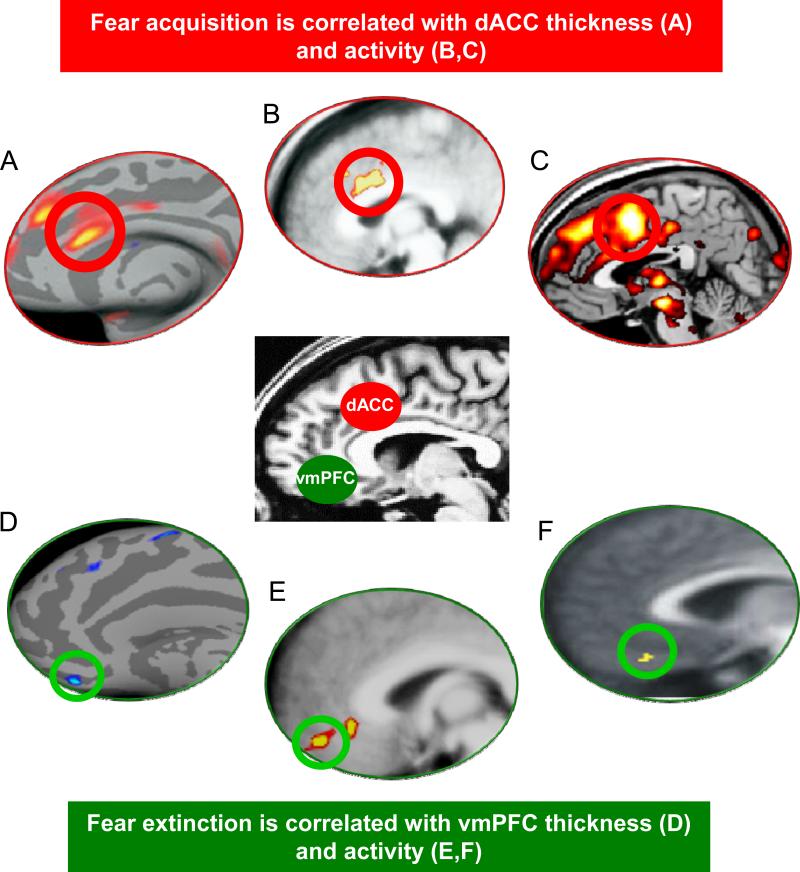
Figure 4.
Summary of neuroimaging research demonstrating that the dACC (A-C) regulates fear acquisition, and vmPFC (D-F) regulates fear extinction, in healthy humans. A,B: Milad et al. (2007a), C: Linnman et al. (2011b); D: Milad et al. (2005b), E: Milad et al. (Milad et al 2007b), F: Kalisch et al., 2006.
Opposing extinction
Rodent prelimbic prefrontal cortex
Lying just dorsal to the IL is the prelimbic cortex (PL). The idea that PL may be important for conditioned fear has historic precedents in studies with rabbits (McLaughlin et al 2002) and rodents, using a trace conditioning paradigm (delay between CS and US) (Runyan et al 2004). For classical auditory fear conditioning (without a trace), inactivation of PL reduces fear expression (Corcoran & Quirk 2007, Laurent & Westbrook 2009, Sierra-Mercado et al 2011). Paralleling these inactivation findings, single PL neurons showed sustained increases in firing rate in response to conditioned tones (Burgos-Robles et al 2009). The time course of PL activity mirrored that of freezing (see figure 5A), and was excessive in rats showing poor retrieval of extinction. Unlike lateral amygdala, PL neurons show sustained tone responses lasting the duration of freezing response, further implicating PL in the generation of freezing. Thus, PL receives a transient fear signal from the amygdala, and converts it into a sustained signal that returns to the amygdala to sustain fear (see figure 5B). Finally, microstimulation of PL increased freezing responses to conditioned tones and impaired extinction (Vidal-Gonzalez et al 2006). These and other recent studies (see table 2), indicate that PL drives conditioned freezing responses and opposes extinction. Thus, the prefrontal cortex is not simply an inhibitor, but is able to exert dual control over fear expression via separate modules, each with access to separate sets of inputs and outputs (Sotres-Bayon & Quirk 2010).
Figure 5.
Prefrontal-amygdala interaction in rodents and humans. A. The tone response of a single PL neuron (blue line, z-score) superimposed upon the rat's freezing to the tone (gray bars). Adapted from Burgos-Robles et al., 2009. Note the high correlation between sustained PL activity and freezing (bin: 3 sec). B. Left: Schematic illustrating reciprocal connections between PL and the BLA. Transient tone response emanating from BLA trigger sustained tone responses in PL that feedback to BLA and drive fear. Right: perievent time histogram showing a conditioned tone response of a PL neuron under control conditions (black bars), and after inactivation of BLA with infusion of muscimol (red bars). Fear signals in BLA drive PL. C. Psychophysiological interaction (PPI) analysis during extinction recall in healthy humans. During recall of a non-extinguished stimulus, increased coupling of the neural activity of the amgydala (seed) with the dACC (red circle) was observed, and reduced functional coupling between the amgydala (seed) and vmPFC (green circle) was observed (adapted from Linnman et al. (2011c).
Table 2.
Effects of prelimbic cortex manipulations on fear expression
| Method | Task | Retrieval | Reference | |
|---|---|---|---|---|
| Facilitators | ||||
| microstimulation | cued fc | enhanced | Vidal-Gonzalez et al., 2006 | |
| CB1 receptor agonist | cued fc | enhanced | Lin et al., 2009 | |
| Inhibitors | ||||
| inactivation with TTX | cued fc | impaired | Corcoran & Quirk, 2007 | |
| inactivation with muscimol | context fc | impaired | Laurent & Westbrook, 2009 | |
| cannabidiol | context fc | impaired | Lemos et al., 2010 | |
| cannabinoid antagonist AM-251 | cued fc | impaired | Tan et al., 2010 | |
| Site specific BDNF knockout | cued fc | impaired | Choi et al., 2010 | |
| inactivation with muscimol | cued fc | impaired | Sierra-Mercado et al., 2011 |
Translating PL findings to humans: Dorsal anterior cingulate (dACC)
Spurred by the rodent findings in PL, we examined our structural and functional data for evidence of cortical areas involved in fear expression. As with vmPFC, both cortical thickness and activation of the dorsal anterior cingulate (dACC) was positively correlated with SCR during the conditioning phase (Milad et al 2007a, but see Hartley et al 2011)(see figure 4). The increased responsiveness to conditioned stimuli during fear acquisition was recently replicated in a separate cohort of healthy subjects (Linnman et al 2011a). Activation of dACC has been noted in previous studies of fear conditioning (Buchel et al 1998, Cheng et al 2003, Phelps et al 2004, Knight et al 2004) but its significance as a predictor of fear levels was not emphasized. dACC activation has been observed in response to unconditioned stimuli as well as conditioned stimuli. dACC was activated by USs consisting of loud noise (Dunsmoor et al 2008, Knight et al 2010) and electric shock (Linnman et al 2011a). Interestingly, when the subject expected the shock but its delivery was omitted, dACC was also activated (Linnman et al 2011a). These data further support the role of dACC in the expression of conditioned fear in humans, similar to the rodent PL.
Prefrontal connectivity with amygdala and hippocampus
Rodent connectivity
PL and IL cortices can modulate fear expression through descending projections to the amygdala. Whereas PL targets the basal nucleus of the amygdala, IL targets inhibitory areas such as the lateral division of the central nucleus (CeL) and intercalated (ITC) neurons (McDonald 1998, Vertes 2004). Physiological studies support excitatory and inhibitory effects for PL and IL, respectively. PL stimulation excites BLA neurons, which tend to fire at short latencies following PL spikes (Likhtik et al 2005). In contrast, IL stimulation drives ITC neurons (Amir et al 2011), which then inhibits central nucleus output neurons (Royer & Paré 2002). This circuit is consistent with the finding that IL stimulation reduces the responsiveness of central nucleus output neurons to BLA or cortical stimulation (Quirk et al 2003). Thus, via divergent projections, PL and IL can bidirectionally gate the expression of amygdala-dependent fear memories.
In addition to gating fear expression, recent evidence suggests that IL contributes to extinction-induced iplasticity within the amygdala. ITC cells are essential for fear extinction (Likhtik et al 2008, Jungling et al 2008), and show extinction-induced potentiation of BLA inputs (Amano et al 2010). IL activity is essential for the development of this extinction-induced plasticity in ITC (Amano et al 2010). The cooperativity between IL and inhibitory circuits within the amygdala suggests that successful extinction will require correlated activity between these areas. Pharmacological inactivation of either IL or BLA (including ITCs) prevents the development of stable extinction memory (Laurent et al 2008, Sierra-Mercado et al 2011). Indeed, unit recording data suggests that BLA neurons processes extinction via reciprocal connectivity with prefrontal and hippocampal areas (Herry et al 2008, Herry et al 2010),. BLA input is responsible for conditioned fear signaling in PL (Laviolette et al 2005, Sotres-Bayon et al 2010) suggesting that neural activity of these two regions may be correlated during conditioned fear expression (see figure 5C).
The hippocampus plays an essential role in contextual gating of extinction to an explicit CS (Bouton et al 2006, Ji & Maren 2007), as well as conditioning and extinction of context conditioning (Radulovic & Tronson 2010). The ventral hippocampus (vHPC) projects directly to both PL/IL and the BLA and therefore is in a position to modulate fear responses (Hugues & Garcia 2007). While it is tempting to ask if hippocampal output excites fear or inhibits fear, there is evidence that hippocampus may have either effect, depending on the experimental condition examined. Hippocampal inactivation reduces the expression of conditioned fear(Sierra-Mercado et al 2011), and prevents the renewal of fear after extinction (Ji & Maren 2005, Hobin et al 2006), both suggesting fear excitation. However, inactivation during extinction training leads to poor recall of extinction (Corcoran et al 2005, Sierra-Mercado et al 2011), and low-frequency stimulation of vHPC disrupts extinction memory (Hugues & Garcia 2007), suggesting that plasticity in the hippocampal system inhibits fear. The necessity of both IL and vmPFC activity for extinction memory suggests that the two structures may work together during recall of extinction.
Human connectivity
Given the striking homology between rodent IL/PL and human vmPFC/dACC one might also predict cross-species parallels in connectivity. In humans, however, it is more challenging to study subregional connectivity. Limits in the spatial and temporal resolution of fMRI make it difficult to subdivide a small structure like the amygdala. Furthermore, the extent to which BOLD signals represent excitatory vs. inhibitory inputs, spiking activity, or local processing is only beginning to be explored (Angenstein et al 2009). Thus, the understanding of the complex nature of the neural circuits within the amygdala in the human brain is very limited. For the hippocampus, its classical role in contextual conditioning and extinction is studied in animals with multi-modal shifts and contexts changes. Such manipulations are technically challenging within an fMRI scanner. Indeed, an initial study did not report hippocamal activation to manipulations of visual contexts during auditory conditioning (Armony & Dolan 2001). More recent studies using different visual contextual manipulations were able to observe hippocampal activations during extinction recall (Kalisch et al 2006, Milad et al 2007b, Lang et al 2009)
Despite these challenges, emerging data show hippocampal and amygdala involvement similar to rodent findings, in the context of fear extinction (Kalisch et al 2006, Milad et al 2007b, Lang et al 2009). Importantly, the hippocampus is activated together with the vmPFC during recall of extinction, and is sensitive to changes in visual context (Milad et al 2007b). In addition to the conventional method of analyzing activations and deactivations, various analytic tools are being used in fMRI to examine the functional connectivity between different brain regions. One such tool is resting-state functional connectivity (fcMRI), which examines the temporal oscillations in spontaneous BOLD signal between a selected seed region and the rest of the brain (Greicius et al 2003, Buckner & Vincent 2007). This analysis is conducted while subjects are not performing any tasks in the fMRI scanner. While a positive correlation between a seed region and a given structure does not directly indicate anatomical connectivity, some recent studies indicate that results from fcMRI studies are fairly constrained by anatomical connections (Greicius et al 2009, Van Dijk et al 2010). Using this tool, recent reports have shown that applying specific seeds to the approximate location of the central (Ce) and basal (BL) nuclei of the amygdala allows for an estimation of connectivity with that region. Accordingly, BL shows greater resting connectivity with vmPFC than with dACC, and Ce shows greater connectivity with dACC than with vmPFC (Etkin et al 2009, Roy et al 2009) Consistent with a role in reducing fear, the hippocampus shows greater connectivity with the BL than with Ce (Roy et al 2009).
Another important fMRI tool used to examine interactions between different brain regions is known as psychophysiological interaction (PPI), an analysis conducted on task-driven BOLD activations (Friston et al 1997). This method examines how a “behavioral” component of the task can modulate inter-regional coupling during the same task. Using this tool, we recently showed that there was decreased coupling between the amygdala and the vmPFC, and increased coupling between the amygdala and dACC, when the subjects were shown the unextinguished stimulus (relative to the unextinguished stimulus) during extinction recall (Linnman et al 2011c)(see figure 5C). These findings support inter-structural relationships observed in rodent studies, and demonstrate the need for PPI analysis of extinction circuits throughout different phases of extinction training.
In addition to functional connectivity, recent studies are beginning to employ diffusion tensor imaging (DTI) as a tool to examine the integrity of the structural connectivity between the amygdala and different subregions of the medial prefrontal cortex. This tool examines white matter fiber tracts based on the diffusion of water molecules along the tracts. The use of DTI to assess the integrity of prefrontal-amygdala connections during fear extinction has yet to be examined. Nonetheless, DTI, as well as fcMRI and PPI, are already being used in conjunction with various experimental paradigms that examined the neurobiological basis of other types of emotion regulation (i.e. instructing the participants to evaluate and suppress their emotions in response to a given stimulus). These studies revealed very similar findings to those observed in fear extinction research, namely, the success of emotion regulation appears to be associated with reduced amygdala activation together with increased activation of various prefrontal regions, including the vmPFC (for reviews, see Hartley & Phelps 2010, Kim et al 2011b).
Testing the circuit in an anxiety disorder
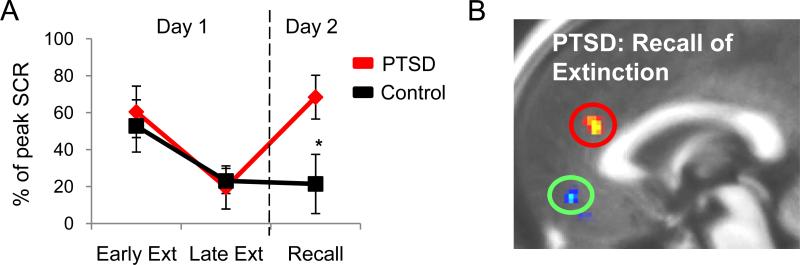
The combined rodent-human extinction findings predicted that fear extinction recall and its associated network would be impaired in PTSD patients (Milad et al 2006). This hypothesis was recently tested (Milad et al 2008, Milad et al 2009b). Consistent with vmPFC dysfunction, PTSD patients showed normal conditioning and within-session extinction, but were unable to recall extinction memory the following day (see figure 6). Furthermore, this deficit in extinction recall was associated with hypoactivation in the vmPFC and hyperactivation in the dACC in PTSD subjects (Milad et al 2009b), providing direct support for the prefrontal-amygdala extinction model. Similar observations have been made for schizophrenia (Holt et al 2009), suggesting that dysfunction in fear extinction circuits might cut across disorders (Graham and Milad, in press), potentially explaining anxious features of multiple mood and anxiety disorders (Insel et al 2010). The model could be further exploited by recent observations that dACC resting metabolism was able to predict fear conditioning and its subsequent extinction (Linnman et al 2011b, Linnman et al 2011c), and perigenual prefrontal activity was able to predict clinical response to extinction-based therapy for PTSD (Bryant et al 2008).
Figure 6.
In PTSD, reduced extinction recall is associated with failure to activate vmPFC and increased activation of dACC. A. Skin conductance responses normalized to peak acquisition levels show intact fear learning (indexed in early extinction [early ext]), intact extinction learning (late ext), but impaired extinction recall (day 2) relative to controls. B. fMRI data using a contrast of extinguished vs. unextinguished conditioned stimuli in PTSD vs. Controls during extinction recall, showinge hypoactivation of the vmPFC (green circle) and a trend toward hyperactivation of dACC (red circle) in PTSD relative to controls. Adapted from Milad et al. (2009b)
The next ten years: where do we go from here?
The last decade witnessed an expansive growth in the field of fear extinction with hundreds of publications, providing a rich understanding of the neural mechanisms in both rodents and humans. Nonetheless, there is still much to be learned, and new lines of research are extending fear extinction further into psychological and clinical domains.
Extinction across the lifespan
Like other forms of learning, the capacity to extinguish varies across the lifespan, and age-related changes in extinction reflect developmental changes in prefrontal-amygdala circuitry. Despite early investigations with development of avoidance learning (Myslivecek & Hassmannova 1979), much has been recently learned with auditory fear conditioning. Richardson and colleagues have shown that extinction in pre-weanling rats violates the “rules” of extinction: it is not context-dependent, does not require NMDA receptors, and does not require the prefrontal cortex (reviewed in Kim & Richardson 2010). Instead of potentiating inhibitory systems, early life extinction appears to erase fear memories from the amygdala (Kim & Richardson 2008, Gogolla et al 2009). During adolescence, extinction again becomes compromised (Esmoris-Arranz et al 2008), as twice the number of training trials are needed to learn extinction and activate the IL (Kim et al 2011a). Finally, aged rats show impaired extinction coupled with a shift of excitability from IL to PL (Kaczorowski et al 2011). Developmental aspects of fear extinction have not yet been studied in humans, but it is known that older individuals show decreased awareness of CS-US contingencies that support conditioned fear responses (LaBar et al 2004). These findings highlight the presence of windows of vulnerability with respect to extinction, but also windows for therapeutic intervention, if early extinction can erase fear memories before they become a clinical problem.
Sex differences in fear extinction: from basic mechanisms to clinical relevance
Sexual dimorphism in the vmPFC, amygdala, hippocampus, and dACC is well documented (Goldstein et al 2001). Sex hormones such as estradiol and progesterone are known modulators of synaptic plasticity, LTP, and NMDA receptor function (for review, see Gillies & McArthur 2010). Sex hormones also modulate dendritic spine density within the prefrontal cortex (Hao et al 2006) and modulate how stress influences the function of the vmPFC and the hippocampus (Maeng et al 2010, Shansky et al 2010). From a clinical prospective, women are twice as likely to develop anxiety and mood disorders (Kessler et al., 2005a,b). In addition, damage to human vmPFC differentially affects men and women: unilateral right damage produces severe emotional defects in men, whereas unilateral left damage produces severe defects in women (Tranel et al 2005). Despite these findings, the majority of human studies combined data from male and female subjects, and the majority of animal studies used males. Few studies have directly examined the influence of sex differences and the role of sex hormones on fear extinction.
There is substantial evidence to suggest that fear extinction may differ between the sexes, and that extinction consolidation may be modulated by sex hormones. Indeed, sex differences in emotional memories have consistently been documented in rodents and humans (reviewed in Andreano & Cahill 2009). Recent evidence from rodents and naturally cycling women suggests that fluctuations in the menstrual cycle alter extinction retention (Zeidan et al 2011). (Milad et al 2009a, Milad et al 2010). Moreover, exogenous estradiol administration facilitates extinction (Chang et al 2009, Milad et al 2009a, Zeidan et al 2011). Future research in this domain could be informative in describing the mechanistic differences between ‘his and her’ brains in processing fear extinction. Such research could potentially explain the increased prevalence of PTSD in women, and potentially lead to sex-specific treatments for mood and anxiety disorders.
Individual variability in fear extinction: Biomarkers
Traditionally, behavioral neuroscience has emphasized average behavior, however, individual variability in fear extinction could potentially explain why some individuals are prone to develop anxiety disorders (Bush et al 2007). Failure to recall extinction in rats is correlated with decreased excitability in IL neurons (Burgos-Robles et al 2007, Santini et al 2008), and decreased BDNF in the hippocampus (Heldt et al 2007, Peters et al 2010). Consistent with animal models, humans expressing a SNP correlated with decreased BDNF release (Val66Met) show impaired fear extinction (Soliman et al 2010), and increased response to social stressors (Shalev et al 2009). The serotonin transporter short allele is also associated with increased risk for anxiety, as well as decreased vmPFC – amygdala connectivity (Pezawas et al 2005, Hariri et al 2006). In both of these cases, a reverse translation approach was used to develop mouse models of the human polymorphism (Wellman et al 2007, Soliman et al 2010). Both of these mice exhibited impaired fear extinction, as well as deficits in prefrontal cortex. Such a combined rodent-human approach will be particularly useful in investigating the newest genetic biomarkers of PTSD: the stress-related gene FKBP5 (Binder et al 2008), and the estrogen-sensitive pituitary adenylate cyclase-activating polypeptide (PACAP) (Ressler et al 2011).
Another emerging approach for understanding individual differences in extinction is to compare existing strains of inbred mice or rats. Fear extinction deficits in one particular strain of mouse (129S1) can be reversed with a dietary manipulation that restored the balance of activity in IL vs.PL (Hefner et al 2008, Whittle et al 2010). A subset of Lewis rats shows impaired fear extinction, but only after exposure to a predator (Goswami et al 2010), providing an animal model of how traumatic experiences can reveal underlying susceptibilities to extinction failure. Gene knockout techniques have been used to discover new molecules involved in fear extinction, including zinc transporter (Martel et al 2010), protease nexin-1 (Meins et al 2010), and metabotropic glutamate receptors (Goddyn et al 2008, Xu et al 2009). Thus, the function of candidate genes derived from patients, inbred rodents strains, or knockout studies can be assessed within the prefrontal-amygdala circuits that control fear extinction. Of particular interest will be the extent to which genetic factors contribute to dACC resting metabolism, which can predict subsequent extinction recall (Linnman et al 2011c).
Conclusion
We have shown how translational research in the past 10 years has moved fear extinction from a primarily psychological concept to a specific circuit conserved across species. Human neuroimaging is sometimes criticized as being too descriptive and lacking mechanistic explanations. Animal models, while mechanistic, are often viewed as too simplistic for modeling psychiatric disorders, and therefore not relevant. Though there is some validity to these criticisms, we have shown how combining both approaches in a translational research program mitigates the limitations of each approach alone. Perhaps more than other fields of psychology, conditioned fear provides for specific hypotheses in both rodents and humans, which can be tested with new techniques to correlate behavior with neural structure and function. The animal studies allowed us to identify the circuits of fear extinction, characterize molecular machinery, and test ways to manipulate the circuit. The neuroimaging approach allowed for translation of those findings to the human brain, to test specific hypotheses about extinction and its role in anxiety disorders. Continuing parallel lines of fear extinction research in rodents and humans could lead to novel therapeutic approaches for strengthening extinction, and help us learn how our brains overcome fear.
BOX 1 Facilitating extinction in rodents.
The advantage of the rodent model is the ability to deliver drugs systemically or directly into the extinction circuit, in an attempt to facilitate extinction. Some of these can then be translated for possible use in humans. Systemic drugs that facilitate extinction (without altering fear expression) include growth factor FGF-2 (Graham & Richardson 2011), adrenergic antagonist yohimbine (Morris & Bouton 2007), NMDA agonist d-cycloserine (Walker et al 2002), cannabinoids (Lafenetre et al 2007), histamine (Bonini et al 2011), estrogen (Milad et al 2009a), a BDNF receptor agonist (Andero et al 2011), and corticosterone (Gourley et al 2009). Many of these act within prefrontal-amygdala extinction circuit (see table 1). The facilitating effect of electrical stimulation of IL (Vidal-Gonzalez et al 2006, Kim et al 2010) can be followed up with new optogenetic techniques for activating specific cell types with focal lasers, which is beginning to be used to dissect inhibitory circuits within the amygdala (Ciocchi et al 2010, Tye et al 2011). Facilitation of fear extinction may also explain some of the therapeutic effects of electrical deep brain stimulation (DBS), which is increasingly used to treat obsessive compulsive disorder (Greenberg et al 2008, Rodriguez-Romaguera et al 2010).
BOX 2 Impact of fear extinction in the clinic.
Research on fear extinction is beginning to show some potential clinical applications. It is now well established that the NMDA receptor partial agonist d-cycloserine, which facilitates extinction in rodents (Davis et al 2006), augments the clinical response to cognitive behavioral therapy (CBT) for a number of anxiety disorders (for review, see Ganasen et al 2010). We may soon see additional classes of CBT adjuncts, such as cannabinoids, noradrenergic drugs, and neurotrophic factors, based on promising rodent studies (see table 1). Non-pharmacological approaches to facilitating extinction could come from transcranial magnetic stimulation (TMS) (Boggio et al 2010), or simply modifying the timing of extinction sessions. Extinction performed shortly after reactivation of fear memory results in a stronger extinction memory that resists return of fear manipulations, in both rats and humans (Monfils et al 2009, Schiller et al 2009). Assessment of fear extinction, or activity in extinction structures, may identify people at risk for anxiety disorders, such as firefighters (Guthrie & Bryant 2006), or those likely to respond to CBT (Bryant et al 2008, Lonsdorf et al 2010).
Summary points.
-
1-
Rodent research on fear extinction in the past 10 years has made great progress in generating specific hypotheses about neural circuits, which are being tested in humans with neuroimaging.
-
2-
The rodent infralimbic cortex is important for fear extinction recall, and is homologous to the human ventromedial prefrontal cortex. The rodent prelimbic cortex is important for fear expression, and is homologous to the human dorsal anterior cingulate cortex.
-
3-
IL/vmPFC connect to inhibitory centers within the amygdala, whereas PL/dACC connect to excitatory areas within the amygdala.
-
4-
Activity in PL (rodents) or dACC (humans) can predict the extent of subsequent extinction learning.
-
5-
Failure to properly activate the vmPFC and dACC in humans results in inappropriate fear expression, and is observed in anxiety disorders.
Future directions.
-
1-
How does extinction circuitry and psychology change as we age?
-
2-
How are the neural mechanisms that mediate fear extinction different in “his” and “her” brains?
-
3-
How does sleep (or the lack thereof) affect with the consolidation of the fear extinction memory?
-
4-
Do successful treatments of psychiatric disorders return fear extinction circuits to a healthy state?
-
5-
Can the capacity to extinguish predict clinical response to CBT, or itself be predicted with biological markers?
-
6-
Do deficits in fear extinction cut across existing classifications of mood and anxiety disorders?
Footnotes
Disclosure Statement
MRM has received consulting fees from MircoTransponder Inc. The authors are not aware of any other affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Mohammed R. Milad, Dept. of Psychiatry, Massachusetts General Hospital, Harvard Medical School, 149 13th St., Room 2614, Boston, MA 02129.
Gregory J. Quirk, Depts. of Psychiatry, and Anatomy & Neurobiology, University of Puerto Rico School of Medicine, P.O. Box 365067, San Juan, PR 00936.
Literature Cited
- Akirav I, Maroun M. The role of the medial prefrontal cortex-amygdala circuit in stress effects on the extinction of fear. Neural Plast. 2007:30873. doi: 10.1155/2007/30873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano T, Unal CT, Paré D. Synaptic correlates of fear extinction in the amygdala. Nat. Neurosci. 2010;13:489–94. doi: 10.1038/nn.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir A, Amano T, Paré D. PHYSIOLOGICAL IDENTIFICATION AND INFRALIMBIC RESPONSIVENESS OF RAT INTERCALATED AMYGDALA NEURONS. J. Neurophysiol. 2011 doi: 10.1152/jn.00136.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andero R, Heldt SA, Ye K, Liu X, Armario A, Ressler KJ. Effect of 7,8-dihydroxyflavone, a small-molecule TrkB agonist, on emotional learning. Am. J. Psychiatry. 2011;168:163–72. doi: 10.1176/appi.ajp.2010.10030326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreano JM, Cahill L. Sex influences on the neurobiology of learning and memory. Learn. Mem. 2009;16:248–66. doi: 10.1101/lm.918309. [DOI] [PubMed] [Google Scholar]
- Angenstein F, Kammerer E, Scheich H. The BOLD response in the rat hippocampus depends rather on local processing of signals than on the input or output activity. A combined functional MRI and electrophysiological study. J. Neurosci. 2009;29:2428–39. doi: 10.1523/JNEUROSCI.5015-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Applegate CD, Frysinger RC, Kapp BS, Gallagher M. Multiple unit activity recorded from amygdala central nucleus during Pavlovian heart rate conditioning in rabbit. Brain Res. 1982;238:457–62. doi: 10.1016/0006-8993(82)90123-8. [DOI] [PubMed] [Google Scholar]
- Armony JL, Dolan RJ. Modulation of auditory neural responses by a visual context in human fear conditioning. Neuroreport. 2001;12:3407–11. doi: 10.1097/00001756-200110290-00051. [DOI] [PubMed] [Google Scholar]
- Baas JM, Nugent M, Lissek S, Pine DS, Grillon C. Fear conditioning in virtual reality contexts: a new tool for the study of anxiety. Biol. Psychiatry. 2004;55:1056–60. doi: 10.1016/j.biopsych.2004.02.024. [DOI] [PubMed] [Google Scholar]
- Barrett D, Shumake J, Jones D, Gonzalez-Lima F. Metabolic mapping of mouse brain activity after extinction of a conditioned emotional response. J. Neurosci. 2003;23:5740–9. doi: 10.1523/JNEUROSCI.23-13-05740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belliveau JW, Kennedy DN, Jr., McKinstry RC, Buchbinder BR, Weisskoff RM, Cohen MS, Vevea JM, Brady TJ, Rosen BR. Functional mapping of the human visual cortex by magnetic resonance imaging. Science. 1991;254:716–9. doi: 10.1126/science.1948051. [DOI] [PubMed] [Google Scholar]
- Binder EB, Bradley RG, Liu W, Epstein MP, Deveau TC, Mercer KB, Tang Y, Gillespie CF, Heim CM, Nemeroff CB, Schwartz AC, Cubells JF, Ressler KJ. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard RJ, Blanchard DC. Crouching as an index of fear. J. Comp Physiol Psychol. 1969;67:370–5. doi: 10.1037/h0026779. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Rocha M, Oliveira MO, Fecteau S, Cohen RB, Campanha C, Ferreira-Santos E, Meleiro A, Corchs F, Zaghi S, Pascual-Leone A, Fregni F. Noninvasive brain stimulation with high-frequency and low-intensity repetitive transcranial magnetic stimulation treatment for posttraumatic stress disorder. J. Clin. Psychiatry. 2010;71:992–9. doi: 10.4088/JCP.08m04638blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohus B, De Wied D. Inhibitory and facilitatory effect of two related peptides on extinction of avoidance behavior. Science. 1966;153:318–20. doi: 10.1126/science.153.3733.318. [DOI] [PubMed] [Google Scholar]
- Bonini JS, Da Silva WC, Da Silveira CK, Kohler CA, Izquierdo I, Cammarota M. Histamine facilitates consolidation of fear extinction. Int. J. Neuropsychopharmacol. 2011:1–9. doi: 10.1017/S1461145710001501. [DOI] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol. Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME, King DA. Contextual control of the extinction of conditioned fear: tests for the associative value of the context. J. Exp. Psychol. Anim Behav. Process. 1983;9:248–65. [PubMed] [Google Scholar]
- Bouton ME, Moody EW. Memory processes in classical conditioning. Neurosci. Biobehav. Rev. 2004;28:663–74. doi: 10.1016/j.neubiorev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and temporal modulation of extinction: behavioral and biological mechanisms. Biol. Psychiatry. 2006;60:352–60. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, Kennedy WA, Rauch SL, Buckner RL, Strauss MM, Hyman SE, Rosen BR. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17:875–87. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Staib LH, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol. Psychiatry. 1999;45:806–16. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan JF, Wisniewski C. The efficacy of response prevention on avoidance behavior in young and adult rats with prefrontal cortical injury. Behav. Brain Res. 1982;4:117–31. doi: 10.1016/0166-4328(82)90068-7. [DOI] [PubMed] [Google Scholar]
- Brutkowski S, Mempel E. Disinhibition of inhibitory conditioned responses following selective brain lesions in dogs. Science. 1961;134:2040–1. doi: 10.1126/science.134.3495.2040. [DOI] [PubMed] [Google Scholar]
- Bryant RA, Felmingham K, Whitford TJ, Kemp A, Hughes G, Peduto A, Williams LM. Rostral anterior cingulate volume predicts treatment response to cognitive-behavioural therapy for posttraumatic stress disorder. J. Psychiatry Neurosci. 2008;33:142–6. [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Dolan RJ, Armony JL, Friston KJ. Amygdala-hippocampal involvement in human aversive trace conditioning revealed through event-related functional magnetic resonance imaging. J. Neurosci. 1999;19:10869–76. doi: 10.1523/JNEUROSCI.19-24-10869.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: an event-related fMRI study. Neuron. 1998;20:947–57. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Vincent JL. Unrest at rest: default activity and spontaneous network correlations. Neuroimage. 2007;37:1091–6. doi: 10.1016/j.neuroimage.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Buresova O, Bures J, Bohdanecky Z, Weiss T. Effect of atropine on learning, extinction, retention and retrieval in rats. Psychopharmacologia. 1964;5:255–63. doi: 10.1007/BF02341258. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Quirk GJ. Sustained conditioned responses in prelimbic prefrontal neurons are correlated with fear expression and extinction failure. J. Neurosci. 2009;29:8474–82. doi: 10.1523/JNEUROSCI.0378-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–80. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Bush DE, Sotres-Bayon F, LeDoux JE. Individual differences in fear: isolating fear reactivity and fear recovery phenotypes. J. Trauma Stress. 2007;20:413–22. doi: 10.1002/jts.20261. [DOI] [PubMed] [Google Scholar]
- Butter CM, Mishkin M, Rosvold HE. Conditioning and extinction of a food-rewarded response after selective ablation of frontal cortex in Rhesus monkeys. Exp. Neurol. 1963;7:65–75. doi: 10.1016/0014-4886(63)90094-3. [DOI] [PubMed] [Google Scholar]
- Chan KH, Morell JR, Jarrard LE, Davidson TL. Reconsideration of the role of the hippocampus in learned inhibition. Behav. Brain Res. 2001;119:111–30. doi: 10.1016/s0166-4328(00)00363-6. [DOI] [PubMed] [Google Scholar]
- Chang CH, Berke JD, Maren S. Single-unit activity in the medial prefrontal cortex during immediate and delayed extinction of fear in rats. PLoS. One. 2010;5:e11971. doi: 10.1371/journal.pone.0011971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CH, Maren S. Medial prefrontal cortex activation facilitates re-extinction of fear in rats. Learn. Mem. 2011;18:221–5. doi: 10.1101/lm.2070111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YJ, Yang CH, Liang YC, Yeh CM, Huang CC, Hsu KS. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor beta. Hippocampus. 2009;19:1142–50. doi: 10.1002/hipo.20581. [DOI] [PubMed] [Google Scholar]
- Cheng DT, Knight DC, Smith CN, Stein EA, Helmstetter FJ. Functional MRI of human amygdala activity during Pavlovian fear conditioning: stimulus processing versus response expression. Behav. Neurosci. 2003;117:3–10. doi: 10.1037//0735-7044.117.1.3. [DOI] [PubMed] [Google Scholar]
- Chi CC. The effect of amobarbital sodium on conditioned fear as measured by the potentiated startle response in rats. Psychopharmacologia. 1965;7:115–22. doi: 10.1007/BF00403634. [DOI] [PubMed] [Google Scholar]
- Choi DC, Maguschak KA, Ye K, Jang SW, Myers KM, Ressler KJ. Prelimbic cortical BDNF is required for memory of learned fear but not extinction or innate fear. Proc. Natl. Acad. Sci. U. S. A. 2010;107:2675–80. doi: 10.1073/pnas.0909359107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciocchi S, Herry C, Grenier F, Wolff SB, Letzkus JJ, Vlachos I, Ehrlich I, Sprengel R, Deisseroth K, Stadler MB, Muller C, Luthi A. Encoding of conditioned fear in central amygdala inhibitory circuits. Nature. 2010;468:277–82. doi: 10.1038/nature09559. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Desmond TJ, Frey KA, Maren S. Hippocampal inactivation disrupts the acquisition and contextual encoding of fear extinction. J. Neurosci. 2005;25:8978–87. doi: 10.1523/JNEUROSCI.2246-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Activity in prelimbic cortex is necessary for the expression of learned, but not innate, fears. J. Neurosci. 2007;27:840–4. doi: 10.1523/JNEUROSCI.5327-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in conditioned and unconditioned fear and anxiety. In: Aggleton JP, editor. The Amygdala. Oxford University Press; Oxford: 2000. pp. 213–288. 213-288. [Google Scholar]
- Davis M, Astrachan DI. Conditioned fear and startle magnitude: effects of different footshock or backshock intensities used in training. J. Exp. Psychol. Anim Behav. Process. 1978;4:95–103. doi: 10.1037//0097-7403.4.2.95. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol. Psychiatry. 2006;60:369–75. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Bandettini PA, Knight DC. Neural correlates of unconditioned response diminution during Pavlovian conditioning. Neuroimage. 2008;40:811–7. doi: 10.1016/j.neuroimage.2007.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmoris-Arranz FJ, Mendez C, Spear NE. Contextual fear conditioning differs for infant, adolescent, and adult rats. Behav. Processes. 2008;78:340–50. doi: 10.1016/j.beproc.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, Greicius MD. Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Arch. Gen. Psychiatry. 2009;66:1361–72. doi: 10.1001/archgenpsychiatry.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falls WA, Davis M. Visual cortex ablations do not prevent extinction of fear-potentiated startle using a visual conditioned stimulus. Behav. Neural Biol. 1993;60:259–70. doi: 10.1016/0163-1047(93)90504-b. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J. Neurosci. 1992;12:854–63. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood JF, Jarvik ME, Bennett EL, Orme AE, Rosenzweig MR. Protein synthesis inhibition and memory for pole jump active avoidance and extinction. Pharmacol. Biochem. Behav. 1977;7:71–7. doi: 10.1016/0091-3057(77)90013-2. [DOI] [PubMed] [Google Scholar]
- Fontanez-Nuin DE, Santini E, Quirk GJ, Porter JT. Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons. Cereb. Cortex. 2011;21:727–35. doi: 10.1093/cercor/bhq147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Ganasen KA, Ipser JC, Stein DJ. Augmentation of cognitive behavioral therapy with pharmacotherapy. Psychiatr. Clin. North Am. 2010;33:687–99. doi: 10.1016/j.psc.2010.04.008. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Falls WA, Davis M. Normal conditioned inhibition and extinction of freezing and fear-potentiated startle following electrolytic lesions of medial prefrontal cortex in rats. Behav. Neurosci. 1997;111:712–26. doi: 10.1037//0735-7044.111.4.712. [DOI] [PubMed] [Google Scholar]
- Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol. Rev. 2010;62:155–98. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goddyn H, Callaerts-Vegh Z, Stroobants S, Dirikx T, Vansteenwegen D, Hermans D, van der PH, D'Hooge R. Deficits in acquisition and extinction of conditioned responses in mGluR7 knockout mice. Neurobiol. Learn. Mem. 2008;90:103–11. doi: 10.1016/j.nlm.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Gogolla N, Caroni P, Luthi A, Herry C. Perineuronal nets protect fear memories from erasure. Science. 2009;325:1258–61. doi: 10.1126/science.1174146. [DOI] [PubMed] [Google Scholar]
- Goldstein JM, Seidman LJ, Horton NJ, Makris N, Kennedy DN, Caviness VS, Jr., Faraone SV, Tsuang MT. Normal sexual dimorphism of the adult human brain assessed by in vivo magnetic resonance imaging. Cereb. Cortex. 2001;11:490–7. doi: 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Goswami S, Cascardi M, Rodriguez-Sierra OE, Duvarci S, Paré D. Impact of predatory threat on fear extinction in Lewis rats. Learn. Mem. 2010;17:494–501. doi: 10.1101/lm.1948910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nat. Neurosci. 2004;7:1144–52. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Gourley SL, Kedves AT, Olausson P, Taylor JR. A history of corticosterone exposure regulates fear extinction and cortical NR2B, GluR2/3, and BDNF. Neuropsychopharmacology. 2009;34:707–16. doi: 10.1038/npp.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BM, Richardson R. Memory of fearful events: the role of fibroblast growth factor-2 in fear acquisition and extinction. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.05.041. [DOI] [PubMed] [Google Scholar]
- Gralewicz K, Gralewicz S. Effects of hippocampal stimulation on retention and extinction of one way active avoidance response in cats. Acta Neurobiol. Exp. (Wars. ) 1984;44:61–72. [PubMed] [Google Scholar]
- Greenberg BD, Gabriels LA, Malone DA, Jr., Rezai AR, Friehs GM, Okun MS, Shapira NA, Foote KD, Cosyns PR, Kubu CS, Malloy PF, Salloway SP, Giftakis JE, Rise MT, Machado AG, Baker KB, Stypulkowski PH, Goodman WK, Rasmussen SA, Nuttin BJ. Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol. Psychiatry. 2008 doi: 10.1038/mp.2008.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:253–8. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb. Cortex. 2009;19:72–8. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Baas JM, Cornwell B, Johnson L. Context conditioning and behavioral avoidance in a virtual reality environment: effect of predictability. Biol. Psychiatry. 2006;60:752–9. doi: 10.1016/j.biopsych.2006.03.072. [DOI] [PubMed] [Google Scholar]
- Guthrie RM, Bryant RA. Extinction learning before trauma and subsequent posttraumatic stress. Psychosom. Med. 2006;68:307–11. doi: 10.1097/01.psy.0000208629.67653.cc. [DOI] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, Morrison JH. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J. Neurosci. 2006;26:2571–8. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Drabant EM, Weinberger DR. Imaging genetics: perspectives from studies of genetically driven variation in serotonin function and corticolimbic affective processing. Biol. Psychiatry. 2006;59:888–97. doi: 10.1016/j.biopsych.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Harris JA, Westbrook RF. Evidence that GABA transmission mediates context-specific extinction of learned fear. Psychopharmacology (Berl) 1998;140:105–15. doi: 10.1007/s002130050745. [DOI] [PubMed] [Google Scholar]
- Hartley CA, Fischl B, Phelps EA. Brain Structure Correlates of Individual Differences in the Acquisition and Inhibition of Conditioned Fear. Cereb. Cortex. 2011 doi: 10.1093/cercor/bhq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley CA, Phelps EA. Changing fear: the neurocircuitry of emotion regulation. Neuropsychopharmacology. 2010;35:136–46. doi: 10.1038/npp.2009.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J. Neurosci. 2008;28:8074–85. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Stanek L, Chhatwal JP, Ressler KJ. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry. 2007;12:656–70. doi: 10.1038/sj.mp.4001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermans D, Dirikx T, Vansteenwegen D, Baeyens F, Van den BO, Eelen P. Reinstatement of fear responses in human aversive conditioning. Behav. Res. Ther. 2005;43:533–51. doi: 10.1016/j.brat.2004.03.013. [DOI] [PubMed] [Google Scholar]
- Herry C, Ciocchi S, Senn V, Demmou L, Muller C, Luthi A. Switching on and off fear by distinct neuronal circuits. Nature. 2008;454:600–6. doi: 10.1038/nature07166. [DOI] [PubMed] [Google Scholar]
- Herry C, Ferraguti F, Singewald N, Letzkus JJ, Ehrlich I, Luthi A. Neuronal circuits of fear extinction. Eur. J. Neurosci. 2010;31:599–612. doi: 10.1111/j.1460-9568.2010.07101.x. [DOI] [PubMed] [Google Scholar]
- Herry C, Garcia R. Prefrontal cortex long-term potentiation, but not long-term depression, is associated with the maintenance of extinction of learned fear in mice. J. Neurosci. 2002;22:577–83. doi: 10.1523/JNEUROSCI.22-02-00577.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herry C, Garcia R. Behavioral and paired-pulse facilitation analyses of long-lasting depression at excitatory synapses in the medial prefrontal cortex in mice. Behav. Brain Res. 2003;146:89–96. doi: 10.1016/j.bbr.2003.09.017. [DOI] [PubMed] [Google Scholar]
- Hitchcock J, Davis M. Lesions of the amygdala, but not of the cerebellum or red nucleus, block conditioned fear as measured with the potentiated startle paradigm. Behav. Neurosci. 1986;100:11–22. doi: 10.1037//0735-7044.100.1.11. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–82. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci. Biobehav. Rev. 2009;33:773–83. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, Cassidy BS, Walsh JP, Goff DC. Extinction memory is impaired in schizophrenia. Biol. Psychiatry. 2009;65:455–63. doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Zeilinski DJ, Fecteau ME, Brady R, LaBar KS. Human fear conditioning conducted in full immersion 3-dimensional virtual reality. J. Vis. Exp. 2010 doi: 10.3791/1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugues S, Deschaux O, Garcia R. Postextinction infusion of a mitogen-activated protein kinase inhibitor into the medial prefrontal cortex impairs memory of the extinction of conditioned fear. Learn. Mem. 2004;11:540–3. doi: 10.1101/lm.77704. [DOI] [PubMed] [Google Scholar]
- Hugues S, Garcia R. Reorganization of learning-associated prefrontal synaptic plasticity between the recall of recent and remote fear extinction memory. Learn. Mem. 2007;14:520–4. doi: 10.1101/lm.625407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J. Comp Neurol. 1991;308:249–76. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, Heinssen R, Pine DS, Quinn K, Sanislow C, Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167:748–51. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Ji J, Maren S. Electrolytic lesions of the dorsal hippocampus disrupt renewal of conditional fear after extinction. Learn. Mem. 2005;12:270–6. doi: 10.1101/lm.91705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Maren S. Hippocampal involvement in contextual modulation of fear extinction. Hippocampus. 2007;17:749–58. doi: 10.1002/hipo.20331. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, Duncan EJ. Fear potentiation and fear inhibition in a human fear-potentiated startle paradigm. Biol. Psychiatry. 2005;57:1559–64. doi: 10.1016/j.biopsych.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Keyes M, Fiallos A, Jovanovic S, Myers KM, Davis M, Duncan EJ. Contingency awareness and fear inhibition in a human fear-potentiated startle paradigm. Behav. Neurosci. 2006;120:995–1004. doi: 10.1037/0735-7044.120.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jungling K, Seidenbecher T, Sosulina L, Lesting J, Sangha S, Clark SD, Okamura N, Duangdao DM, Xu YL, Reinscheid RK, Pape HC. Neuropeptide S-mediated control of fear expression and extinction: role of intercalated GABAergic neurons in the amygdala. Neuron. 2008;59:298–310. doi: 10.1016/j.neuron.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski CC, Davis SJ, Moyer JR., Jr Aging redistributes medial prefrontal neuronal excitability and impedes extinction of trace fear conditioning. Neurobiol. Aging. 2011 doi: 10.1016/j.neurobiolaging.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Kalisch R, Korenfeld E, Stephan KE, Weiskopf N, Seymour B, Dolan RJ. Context-dependent human extinction memory is mediated by a ventromedial prefrontal and hippocampal network. J. Neurosci. 2006;26:9503–11. doi: 10.1523/JNEUROSCI.2021-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapp BS, Frysinger RC, Gallagher M, Haselton JR. Amygdala central nucleus lesions: effect on heart rate conditioning in the rabbit. Physiol Behav. 1979;23:1109–17. doi: 10.1016/0031-9384(79)90304-4. [DOI] [PubMed] [Google Scholar]
- Kim JH, Li S, Richardson R. Immunohistochemical analyses of long-term extinction of conditioned fear in adolescent rats. Cereb. Cortex. 2011a;21:530–8. doi: 10.1093/cercor/bhq116. [DOI] [PubMed] [Google Scholar]
- Kim JH, Richardson R. The effect of temporary amygdala inactivation on extinction and reextinction of fear in the developing rat: unlearning as a potential mechanism for extinction early in development. J. Neurosci. 2008;28:1282–90. doi: 10.1523/JNEUROSCI.4736-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Richardson R. New findings on extinction of conditioned fear early in development: theoretical and clinical implications. Biol. Psychiatry. 2010;67:297–303. doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN, Whalen PJ. The structural and functional connectivity of the amygdala: From normal emotion to pathological anxiety. Behav. Brain Res. 2011b doi: 10.1016/j.bbr.2011.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SC, Jo YS, Kim IH, Kim H, Choi JS. Lack of medial prefrontal cortex activation underlies the immediate extinction deficit. J. Neurosci. 2010;30:832–7. doi: 10.1523/JNEUROSCI.4145-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble DP, Kimble RJ. The effect of hippocampal lesions on extinction and “hypothesis” behavior in rats. Physiol Behav. 1970;5:735–8. doi: 10.1016/0031-9384(70)90271-4. [DOI] [PubMed] [Google Scholar]
- Knapska E, Maren S. Reciprocal patterns of c-Fos expression in the medial prefrontal cortex and amygdala after extinction and renewal of conditioned fear. Learn. Mem. 2009;16:486–93. doi: 10.1101/lm.1463909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn Affect. Behav. Neurosci. 2004;4:317–25. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- Knight DC, Waters NS, King MK, Bandettini PA. Learning-related diminution of unconditioned SCR and fMRI signal responses. Neuroimage. 2010;49:843–8. doi: 10.1016/j.neuroimage.2009.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinidis L. The effects of chronic amphetamine administration on the acquisition and extinction of an active and passive avoidance response in mice. Pharmacol. Biochem. Behav. 1983;19:593–8. doi: 10.1016/0091-3057(83)90333-7. [DOI] [PubMed] [Google Scholar]
- Konorski J. Integrative Activity of the Brain. University of Chicago Press; Chicago: 1967. [Google Scholar]
- Koob GF, Dantzer R, Bluthe RM, Lebrun C, Bloom FE, Le Moal M. Central injections of arginine vasopressin prolong extinction of active avoidance. Peptides. 1986;7:213–8. doi: 10.1016/0196-9781(86)90215-9. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Cook CA, Torpey DC, Welsh-Bohmer KA. Impact of healthy aging on awareness and fear conditioning. Behav. Neurosci. 2004;118:905–15. doi: 10.1037/0735-7044.118.5.905. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: a mixed-trial fMRI study. Neuron. 1998;20:937–45. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Phelps EA. Reinstatement of conditioned fear in humans is context dependent and impaired in amnesia. Behav. Neurosci. 2005;119:677–86. doi: 10.1037/0735-7044.119.3.677. [DOI] [PubMed] [Google Scholar]
- Lafenetre P, Chaouloff F, Marsicano G. The endocannabinoid system in the processing of anxiety and fear and how CB1 receptors may modulate fear extinction. Pharmacol. Res. 2007;56:367–81. doi: 10.1016/j.phrs.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Lang S, Kroll A, Lipinski SJ, Wessa M, Ridder S, Christmann C, Schad LR, Flor H. Context conditioning and extinction in humans: differential contribution of the hippocampus, amygdala and prefrontal cortex. Eur. J. Neurosci. 2009;29:823–32. doi: 10.1111/j.1460-9568.2009.06624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Marchand AR, Westbrook RF. The basolateral amygdala is necessary for learning but not relearning extinction of context conditioned fear. Learn. Mem. 2008;15:304–14. doi: 10.1101/lm.928208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent V, Westbrook RF. Inactivation of the infralimbic but not the prelimbic cortex impairs consolidation and retrieval of fear extinction. Learn. Mem. 2009;16:520–9. doi: 10.1101/lm.1474609. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. J. Neurosci. 2005;25:6066–75. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Iwata J, Cicchetti P, Reis DJ. Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J. Neurosci. 1988;8:2517–29. doi: 10.1523/JNEUROSCI.08-07-02517.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Ruggiero DA, Reis DJ. Projections to the subcortical forebrain from anatomically defined regions of the medial geniculate body in the rat. J. Comp Neurol. 1985;242:182–213. doi: 10.1002/cne.902420204. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Sakaguchi A, Reis DJ. Subcortical efferent projections of the medial geniculate nucleus mediate emotional responses conditioned to acoustic stimuli. J. Neurosci. 1984;4:683–98. doi: 10.1523/JNEUROSCI.04-03-00683.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE, Xagoraris A, Romanski LM. Indelibility of subcortical emotional memories. J. Cog. Neurosci. 1989;1:238–43. doi: 10.1162/jocn.1989.1.3.238. [DOI] [PubMed] [Google Scholar]
- Lemos JI, Resstel LB, Guimaraes FS. Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav. Brain Res. 2010;207:105–11. doi: 10.1016/j.bbr.2009.09.045. [DOI] [PubMed] [Google Scholar]
- Likhtik E, Pelletier JG, Paz R, Paré D. Prefrontal control of the amygdala. J. Neurosci. 2005;25:7429–37. doi: 10.1523/JNEUROSCI.2314-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Likhtik E, Popa D, Apergis-Schoute J, Fidacaro GA, Paré D. Amygdala intercalated neurons are required for expression of fear extinction. Nature. 2008;454:642–5. doi: 10.1038/nature07167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HC, Mao SC, Su CL, Gean PW. The role of prefrontal cortex CB1 receptors in the modulation of fear memory. Cereb. Cortex. 2009;19:165–75. doi: 10.1093/cercor/bhn075. [DOI] [PubMed] [Google Scholar]
- Linnman C, Rougemont-Bucking A, Beucke JC, Zeffiro TA, Milad MR. Unconditioned responses and functional fear networks in human classical conditioning. Behav. Brain Res. 2011a;221:237–45. doi: 10.1016/j.bbr.2011.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C, Zeidan MA, Pitman RK, Milad MR. Cingulate and amygdala resting metabolism correlate with autonomic and functional activation during fear conditioning. Biol. Psychiatry. 2011b in press. [Google Scholar]
- Linnman C, Zeidan MA, Pitman RK, Quirk GJ, Milad MR. Resting amygdala and medial prefrontal metabolism predict functional activation of the fear extinction circuit. Am. J. Psychiatry. 2011c doi: 10.1176/appi.ajp.2011.10121780. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Ruck C, Bergstrom J, Andersson G, Ohman A, Lindefors N, Schalling M. The COMTval158met polymorphism is associated with symptom relief during exposure-based cognitive-behavioral treatment in panic disorder. BMC. Psychiatry. 2010;10:99. doi: 10.1186/1471-244X-10-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovely RH. Hormonal dissociation of limbic lesion effects on shuttle box avoidance in rats. J. Comp Physiol Psychol. 1975;89:224–30. doi: 10.1037/h0076807. [DOI] [PubMed] [Google Scholar]
- Maeng LY, Waddell J, Shors TJ. The prefrontal cortex communicates with the amygdala to impair learning after acute stress in females but not in males. J. Neurosci. 2010;30:16188–96. doi: 10.1523/JNEUROSCI.2265-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–7. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- Marek R, Coelho CM, Sullivan RK, Baker-Andresen D, Li X, Ratnu V, Dudley KJ, Meyers D, Mukherjee C, Cole PA, Sah P, Bredy TW. Paradoxical Enhancement of Fear Extinction Memory and Synaptic Plasticity by Inhibition of the Histone Acetyltransferase p300. J. Neurosci. 2011;31:7486–91. doi: 10.1523/JNEUROSCI.0133-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S. Building and burying fear memories in the brain. Neuroscientist. 2005;11:89–99. doi: 10.1177/1073858404269232. [DOI] [PubMed] [Google Scholar]
- Martel G, Hevi C, Friebely O, Baybutt T, Shumyatsky GP. Zinc transporter 3 is involved in learned fear and extinction, but not in innate fear. Learn. Mem. 2010;17:582–90. doi: 10.1101/lm.1962010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason ST, Roberts DC, Fibiger HC. Interaction of brain noradrenaline and the pituitary-adrenal axis in learning and extinction. Pharmacol. Biochem. Behav. 1979;10:11–6. doi: 10.1016/0091-3057(79)90161-8. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience. 1991;44:1–14. doi: 10.1016/0306-4522(91)90247-l. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Prog. Neurobiol. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McLaughlin J, Skaggs H, Churchwell J, Powell DA. Medial prefrontal cortex and pavlovian conditioning: trace versus delay conditioning. Behav. Neurosci. 2002;116:37–47. [PubMed] [Google Scholar]
- Meins M, Herry C, Muller C, Ciocchi S, Moreno E, Luthi A, Monard D. Impaired fear extinction in mice lacking protease nexin-1. Eur. J. Neurosci. 2010;31:2033–42. doi: 10.1111/j.1460-9568.2010.07221.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009a;164:887–95. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J. Psychiatr. Res. 2008;42:515–20. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Pitman RK, Rauch SL. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005a;42:456–64. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol. Psychiatry. 2009b;66:1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quinn BT, Pitman RK, Orr SP, Fischl B, Rauch SL. Thickness of ventromedial prefrontal cortex in humans is correlated with extinction memory. Proc. Natl. Acad. Sci. U. S. A. 2005b;102:10706–11. doi: 10.1073/pnas.0502441102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role of the human dorsal anterior cingulate cortex in expression of learned fear. Biol. Psychiatry. 2007a;62:1191–4. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biol. Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry. 2007b;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, Goldstein JM. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–8. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: key to persistent attenuation of fear memories. Science. 2009;324:951–5. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MA, Romanski LM, LeDoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci. Lett. 1993;163:109–13. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- Morris RW, Bouton ME. The effect of yohimbine on the extinction of conditioned fear: a role for context. Behav. Neurosci. 2007;121:501–14. doi: 10.1037/0735-7044.121.3.501. [DOI] [PubMed] [Google Scholar]
- Mueller D, Bravo-Rivera C, Quirk GJ. Infralimbic D2 receptors are necessary for fear extinction and extinction-related tone responses. Biol. Psychiatry. 2010;68:1055–60. doi: 10.1016/j.biopsych.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller D, Porter JT, Quirk GJ. Noradrenergic signaling in infralimbic cortex increases cell excitability and strengthens memory for fear extinction. J. Neurosci. 2008;28:369–75. doi: 10.1523/JNEUROSCI.3248-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Carlezon WA, Jr., Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology. 2011;36:274–93. doi: 10.1038/npp.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myslivecek J, Hassmannova J. Ontogeny of active avoidance in the rat: learning and memory. Dev. Psychobiol. 1979;12:169–86. doi: 10.1002/dev.420120209. [DOI] [PubMed] [Google Scholar]
- Nitecka L, Ben Ari Y. Distribution of GABA-like immunoreactivity in the rat amygdaloid complex. J. Comp Neurol. 1987;266:45–55. doi: 10.1002/cne.902660105. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, Duncan EJ. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learn. Mem. 2006;13:681–5. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape HC, Paré D. Plastic synaptic networks of the amygdala for the acquisition, expression, and extinction of conditioned fear. Physiol Rev. 2010;90:419–63. doi: 10.1152/physrev.00037.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paré D, Smith Y. The intercalated cell masses project to the central and medial nuclei of the amygdala in cats. Neuroscience. 1993;57:1077–90. doi: 10.1016/0306-4522(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Pavlov I. Conditioned Reflexes. Oxford University Press; London: 1927. [Google Scholar]
- Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–90. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat. Neurosci. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pine DS, Fyer A, Grun J, Phelps EA, Szeszko PR, Koda V, Li W, Ardekani B, Maguire EA, Burgess N, Bilder RM. Methods for developmental studies of fear conditioning circuitry. Biol. Psychiatry. 2001;50:225–8. doi: 10.1016/s0006-3223(01)01159-3. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–23. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Pitman RK. Post-traumatic stress disorder, conditioning, and network theory. Psychiatric Annals. 1988;18:182–9. [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Paré D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J. Neurosci. 2003;23:8800–7. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J. Neurosci. 2000;20:6225–31. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radulovic J, Tronson NC. Molecular specificity of multiple hippocampal processes governing fear extinction. Rev. Neurosci. 2010;21:1–17. doi: 10.1515/revneuro.2010.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research--past, present, and future. Biol. Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Behavioral studies of Pavlovian conditioning. Annu. Rev Neurosci. 1988;11:329–52. doi: 10.1146/annurev.ne.11.030188.001553. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Retraining of extinguished Pavlovian stimuli. J. Exp. Psychol. Anim Behav. Process. 2001;27:115–24. [PubMed] [Google Scholar]
- Rescorla RA. Spontaneous recovery. Learn. Mem. 2004;11:501–9. doi: 10.1101/lm.77504. [DOI] [PubMed] [Google Scholar]
- Rescorla RA, Heth CD. Reinstatement of fear to an extinguished conditioned stimulus. J. Exp. Psychol. Anim Behav. Process. 1975;1:88–96. [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, Norrholm SD, Kilaru V, Smith AK, Myers AJ, Ramirez M, Engel A, Hammack SE, Toufexis D, Braas KM, Binder EB, May V. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–7. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Romaguera J, Do Monte FH, Rodriguez-Resto JO, Quirk GJ. Deep brain stimulation within the nucelus accumbens of rats strengthens memory for fear extinction. Soc. Neurosci. Abstr. 2010:809.3. [Google Scholar]
- Romanski LM, LeDoux JE. Information cascade from primary auditory cortex to the amygdala: corticocortical and corticoamygdaloid projections of temporal cortex in the rat. Cereb. Cortex. 1993;3:515–32. doi: 10.1093/cercor/3.6.515. [DOI] [PubMed] [Google Scholar]
- Rosen BR, Buckner RL, Dale AM. Event-related functional MRI: past, present, and future. Proc. Natl. Acad. Sci. U. S. A. 1998;95:773–80. doi: 10.1073/pnas.95.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–26. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royer S, Paré D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115:455–62. doi: 10.1016/s0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- Runyan JD, Moore AN, Dash PK. A role for prefrontal cortex in memory storage for trace fear conditioning. J. Neurosci. 2004;24:1288–95. doi: 10.1523/JNEUROSCI.4880-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanberg PR, Pisa M, Fibiger HC. Avoidance, operant and locomotor behavior in rats with neostriatal injections of kainic acid. Pharmacol. Biochem. Behav. 1979;10:137–44. doi: 10.1016/0091-3057(79)90179-5. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena DO, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J. Neurosci. 2004;24:5704–10. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Porter JT. M-type potassium channels modulate the intrinsic excitability of infralimbic neurons and regulate fear expression and extinction. J. Neurosci. 2010;30:12379–86. doi: 10.1523/JNEUROSCI.1295-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J. Neurosci. 2008;28:4028–36. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Monfils MH, Raio CM, Johnson DC, LeDoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2009;XX:XX. doi: 10.1038/nature08637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple WE, Goyer PF, McCormick R, Compton-Toth B, Morris E, Donovan B, Muswick G, Nelson D, Garnett ML, Sharkoff J, Leisure G, Miraldi F, Schulz SC. Attention and regional cerebral blood flow in posttraumatic stress disorder patients with substance abuse histories. Psychiatry Res. 1996;67:17–28. doi: 10.1016/0925-4927(96)02735-7. [DOI] [PubMed] [Google Scholar]
- Shalev I, Lerer E, Israel S, Uzefovsky F, Gritsenko I, Mankuta D, Ebstein RP, Kaitz M. BDNF Val66Met polymorphism is associated with HPA axis reactivity to psychological stress characterized by genotype and gender interactions. Psychoneuroendocrinology. 2009;34:382–8. doi: 10.1016/j.psyneuen.2008.09.017. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb. Cortex. 2010;20:2560–7. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. Am. J. Psychiatry. 1999;156:575–84. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–38. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdsson T, Doyere V, Cain CK, LeDoux JE. Long-term potentiation in the amygdala: a cellular mechanism of fear learning and memory. Neuropharmacology. 2007;52:215–27. doi: 10.1016/j.neuropharm.2006.06.022. [DOI] [PubMed] [Google Scholar]
- Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, Jing D, Tottenham N, Amso D, Somerville LH, Voss HU, Glover G, Ballon DJ, Liston C, Teslovich T, Van Kempen T, Lee FS, Casey BJ. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–6. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Emotional perseveration: an update on prefrontal-amygdala interactions in fear extinction. Learn. Mem. 2004;11:525–35. doi: 10.1101/lm.79504. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Diaz-Mataix L, Bush DE, LeDoux JE. Consolidation of fear extinction requires NR2B-containing NMDA receptors in the ventromedial prefrontal cortex, but not in the lateral amygdala. Soc. Neurosci. Abstr. 2007:307.5. [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr. Opin. Neurobiol. 2010;20:231–5. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Sierra-Mercado D, Quirk GJ. Ventral hippocampus gates fear-related signals in prelimbic cortex. Soc. Neurosci. Abstr. 2010:809.15. [Google Scholar]
- Sun N, Cassell MD. Intrinsic GABAergic neurons in the rat central extended amygdala. J. Comp Neurol. 1993;330:381–404. doi: 10.1002/cne.903300308. [DOI] [PubMed] [Google Scholar]
- Tan H, Lauzon NM, Bishop SF, Bechard MA, Laviolette SR. Integrated cannabinoid CB1 receptor transmission within the amygdala-prefrontal cortical pathway modulates neuronal plasticity and emotional memory encoding. Cereb. Cortex. 2010;20:1486–96. doi: 10.1093/cercor/bhp210. [DOI] [PubMed] [Google Scholar]
- Teich AH, McCabe PM, Gentile CC, Schneiderman LS, Winters RW, Liskowsky DR, Schneiderman N. Auditory cortex lesions prevent the extinction of Pavlovian differential heart rate conditioning to tonal stimuli in rabbits. Brain Res. 1989;480:210–8. doi: 10.1016/0006-8993(89)91584-9. [DOI] [PubMed] [Google Scholar]
- Thompson BM, Baratta MV, Biedenkapp JC, Rudy JW, Watkins LR, Maier SF. Activation of the infralimbic cortex in a fear context enhances extinction learning. Learn. Mem. 2010;17:591–9. doi: 10.1101/lm.1920810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–81. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, Thompson KR, Gradinaru V, Ramakrishnan C, Deisseroth K. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–62. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KR, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J. Neurophysiol. 2010;103:297–321. doi: 10.1152/jn.00783.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansteenwegen D, Hermans D, Vervliet B, Francken G, Beckers T, Baeyens F, Eelen P. Return of fear in a human differential conditioning paradigm caused by a return to the original acquistion context. Behav. Res. Ther. 2005;43:323–36. doi: 10.1016/j.brat.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51:32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Vervliet B, Vansteenwegen D, Baeyens F, Hermans D, Eelen P. Return of fear in a human differential conditioning paradigm caused by a stimulus change after extinction. Behav. Res. Ther. 2005;43:357–71. doi: 10.1016/j.brat.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Vidal-Gonzalez I, Vidal-Gonzalez B, Rauch SL, Quirk GJ. Microstimulation reveals opposing influences of prelimbic and infralimbic cortex on the expression of conditioned fear. Learn. Mem. 2006;13:728–33. doi: 10.1101/lm.306106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. J. Neurosci. 2002;22:2343–51. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL, Izquierdo A, Garrett JE, Martin KP, Carroll J, Millstein R, Lesch KP, Murphy DL, Holmes A. Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J. Neurosci. 2007;27:684–91. doi: 10.1523/JNEUROSCI.4595-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PJ, Rauch SL, Etcoff NL, McInerney SC, Lee MB, Jenike MA. Masked presentations of emotional facial expressions modulate amygdala activity without explicit knowledge. J. Neurosci. 1998;18:411–8. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle N, Hauschild M, Lubec G, Holmes A, Singewald N. Rescue of impaired fear extinction and normalization of cortico-amygdala circuit dysfunction in a genetic mouse model by dietary zinc restriction. J. Neurosci. 2010;30:13586–96. doi: 10.1523/JNEUROSCI.0849-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhu Y, Contractor A, Heinemann SF. mGluR5 has a critical role in inhibitory learning. J. Neurosci. 2009;29:3676–84. doi: 10.1523/JNEUROSCI.5716-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, Goldstein JM, Milad MR. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biol. Psychiatry. 2011 doi: 10.1016/j.biopsych.2011.05.016. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]