Abstract
Stenotrophomonas maltophilia is an inherently multidrug resistant (MDR) opportunistic pathogen with many mechanisms of resistance. SENTRY studies reveal decreasing sensitivities of S. maltophilia to trimethoprim-sulfamethoxazole and fluoroquinolones. Ceftolozane-tazobactam (Zerbaxa, Merck & Co., Inc.) a novel intravenous combination agent of a third-generation cephalosporin and β-lactamase inhibitor was demonstrated to have in vitro activity against many Gram-positive, Gram-negative, and MDR organisms. Data for ceftolozane-tazobactam's use outside of Food and Drug Administration (FDA) approved indications has been limited thus far to two case reports which demonstrated its efficacy in pan-resistant Pseudomonas aeruginosa pneumonia. Herein, we describe the first published case of treatment of MDR S. maltophilia in polymicrobial osteomyelitis with long-term (>14 days) ceftolozane-tazobactam and metronidazole. Ceftolozane-tazobactam may offer a possible alternative for clinicians faced with limited options in the treatment of resistant pathogens including MDR S. maltophilia.
1. Introduction
Stenotrophomonas maltophilia is an inherently multidrug resistant (MDR) opportunistic pathogen with many mechanisms of resistance which may challenge clinicians to find safe and effective treatment regimens. Antimicrobial resistance mechanisms include β-lactamase production, the presence of class 1 integrons and ISCR elements (resistance to trimethoprim-sulfamethoxazole, TMP-SMX), expression of quinolone resistance (Qnr) genes, and multidrug efflux pumps [1].
Although TMP-SMX is often regarded as the drug of choice with fluoroquinolones (FQs) as reasonable alternatives, SENTRY studies reveal decreasing sensitivities of S. maltophilia to TMP-SMX (96.0% to 94.5%) and levofloxacin (83.4% to 77.3%) [2–4]. Other options with historically good susceptibility profiles but rising resistance rates include ceftazidime, ticarcillin-clavulanate, and tetracyclines [1]. Therefore, knowledge of the activity of other compounds, including new agents, which might be effective in treating S. maltophilia is desirable.
Ceftolozane-tazobactam (Zerbaxa, Merck & Co., Inc.) is a novel intravenous combination agent of a third-generation cephalosporin and β-lactamase inhibitor Food and Drug Administration (FDA) approved in 2014 for the treatment of complicated intra-abdominal (cIAI) (when combined with metronidazole) and complicated urinary tract infection (cUTI) (Figure 1) [5]. Ceftolozane-tazobactam has demonstrated in vitro activity against many Gram-positive, Gram-negative, and MDR organisms. It retains activity against ESBL-producing Enterobacteriaceae (TEM-SHV, CTX-M, and OXA) and MDR Pseudomonas aeruginosa with resistance mechanisms including chromosomal AmpC, loss of outer membrane porin (OprD), and upregulation of efflux pumps (MexY and MexAB). Its activity against MDR P. aeruginosa is surpassed only by colistin [6].
Figure 1.
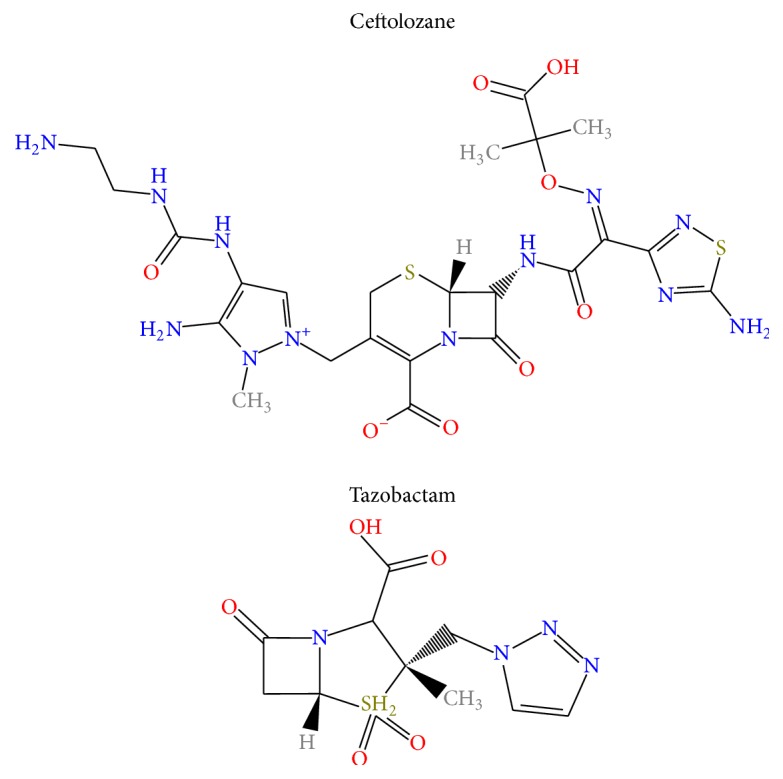
Chemical structure of ceftolozane-tazobactam.
Data for ceftolozane-tazobactam's use outside of the FDA approved indications (cIAI and cUTI) has been limited thus far to two case reports which demonstrated its efficacy in pan-resistant P. aeruginosa pneumonia [7, 8].
Herein, we describe the first published case of treatment of MDR S. maltophilia in polymicrobial osteomyelitis with long-term (>14 days) ceftolozane-tazobactam and metronidazole.
2. Case Presentation
A 20-year-old male with no significant past medical history presented to the emergency department after suffering a crush injury to his right foot. After incision and drainage (I&D) of the wound and open reduction internal fixation of the navicular, tarsal, and metatarsals, he was discharged on cephalexin 500 mg orally (PO) every 6 hours.
Subsequent clinic visits revealed delayed wound healing and moderate-to-severe edema. By postoperative week six, the wound had dehisced with signs of necrosis and abscess formation. He underwent surgical intervention the following day where the wound was incised and drained, hardware was removed, and cultures were obtained. He was sent home on levofloxacin 750 mg PO daily and told to follow up in one week. Upon return, inspection of his foot showed exposed metatarsal bone which was dark and foul smelling. He was admitted for further management.
Wound cultures taken at surgery the week prior resulted in Klebsiella pneumoniae, Enterobacter cloacae, Streptococcus anginosus, and Bacteroides ovatus. Piperacillin-tazobactam 3.375 g IV every 6 hours was started. Another I&D was performed with cultures taken from necrotic bone. A PICC line was placed to initiate a prolonged course of antimicrobials.
On day 4, bone cultures returned S. anginosus, Granulicatella adiacens, and MDR S. maltophilia, resistant to TMP-SMX and levofloxacin. Etests were ordered to explore alternative antimicrobial options: ceftolozane-tazobactam, minimum inhibitory concentration (MIC) 0.5 mg/L; tigecycline MIC 2 mg/L; ceftazidime MIC 2 mg/L; and colistin MIC 0.5 mg/L. Due to concerns that monotherapy would not suffice and the need for simplified outpatient parenteral antimicrobial therapy to facilitate patient discharge, ceftolozane-tazobactam was favored [9–11].
The antibiotic regimen was therefore changed to ceftolozane-tazobactam 1.5 g IV every 8 hours plus metronidazole 500 mg PO every 8 hours (for coverage against Bacteroides ovatus) for six weeks with wound VAC to be changed every other day. At six- and ten-week follow-up, his wound was noted to be healing nicely with no purulence or serous drainage; inflammatory symptoms were also absent. At week 14, granulation tissue had failed to completely cover exposed bone, so patient underwent reconstruction of right foot defect with a fasciocutaneous flap. Cultures were obtained from the excised subcutaneous tissue which resulted in pan-sensitive coagulase positive Staphylococcus, Staphylococcus lugdunensis, and Gemella morbillorum; however, antimicrobial therapy was forgone as no overt signs of infection were present. Also of note, this culture was negative for the previously cultured organisms (S. anginosus, Granulicatella adiacens, and S. maltophilia). At 30-week follow-up, fasciocutaneous graft had taken well and no signs of infection were present.
3. Discussion
Ceftolozane-tazobactam's many unique properties including the presence of a 7-aminothiadiazole (activity against Gram-negative organisms), alkoximino group (stability against β-lactamases), dimethylacetic acid moiety (activity against P. aeruginosa), and a bulky pyrazole ring (stability in the presence of AmpC β-lactamase) allow for increased activity against broad-spectrum Gram-negative organisms including some ESBL-producing Enterobacteriaceae and MDR P. aeruginosa [5, 6].
Ceftolozane-tazobactam's activity against these MDR Gram-negative pathogens in cIAI and cUTI is promising to clinicians. However, further information regarding its utility for off-label indications is speculative at best and based mostly upon unpublished manufacturer data from Phase I and II trials. In the case of osteomyelitis, variable bone to plasma ratio has been seen in animal data ranging from 5.2% to 9.0% in rabbit model and up to 40.0% in rat model femur concentration (Table 1). Nonetheless these ranges of results are comparable to cephalosporins that are widely used in osteomyelitis such as cefazolin and cefepime which have bone concentrations at 17.9% and 46%–76%, respectively (Table 2) [12]. While there are no Clinical & Laboratory Standards Institute (CLSI) approved MIC to predict sensitivity to S. maltophilia, one may speculate that a breakpoint of 0.5 mg/L may offer a reasonable chance of treatment success.
Table 1.
Ratio of ceftolozane concentrations between bone tissue and plasma.
| Dose | Time after last dose (hours) | Bone : serum concentration ratio | |
|---|---|---|---|
| Rabbit model | 1 g q 8 h | 1.5 |
Marrow: 14.1%–17.5% Bone: 6.2%–9.0% |
| Rat model | 20 mg/kg | 2 | 27% |
| 8 | 40% |
Source: unpublished manufacturer data.
Table 2.
Bone penetration of cephalosporins and β-lactamase inhibitors [12].
| Time after last dose (hours) | Bone (mg/kg): serum concentration (mg/L) ratioa | Method | |
|---|---|---|---|
| Cephalosporins | |||
| Ceftriaxone | 0.2–8 | 0.07–0.17 | HPLC |
| Cefotaxime | 0.75–4 | 0.02–0.28 | Bioassay |
| Cefuroxime (osteomyelitis) | 1 | 0.04–0.08 | HPLC |
| Cefazolin | 0.9 | 0.179 | HPLC |
| Cefepime | 1-2 | 0.46–0.76 | HPLC |
| Ceftazidime (ischemic bone) | 1-2 | 0.04–0.08 | HPLC |
| Ceftazidime | 2 | 0.54 | Bioassay |
|
| |||
| β-Lactamase inhibitors | |||
| Clavulanic acid | 1 | 1.14–1.76 | Bioassay |
| Sulbactam | 0.25–4 | 0.17–0.71 | Gas chromatography |
| Tazobactam | 1.5 | 0.22–0.26 | HPLC |
aAssumed bone density of 1 kg/L was assumed if not reported.
HPLC: high-performance liquid chromatography.
In this patient case, ceftolozane-tazobactam was demonstrated in vitro to be active against the offending pathogens including MDR S. maltophilia. The wound evidenced healing during and after completing antibiotic therapy and at posttreatment follow-up visits. Tolerability to ceftolozane-tazobactam beyond 14 days of treatment has previously not been demonstrated but was well tolerated in this case with no adverse drug reactions.
While further investigations are needed to examine ceftolozane-tazobactam's utility in off-label indication, the authors felt that it was important to share this experience with ceftolozane-tazobactam in this case of polymicrobial osteomyelitis. Ceftolozane-tazobactam may offer a possible alternative for clinicians faced with limited options in the treatment of resistant pathogens including MDR S. maltophilia.
Competing Interests
The authors declare that they have no competing interests.
References
- 1.Chang Y.-T., Lin C.-Y., Chen Y.-H., Hsueh P.-R. Update on infections caused by Stenotrophomonas maltophilia with particular attention to resistance mechanisms and therapeutic options. Frontiers in Microbiology. 2015;6, article 893 doi: 10.3389/fmicb.2015.00893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Falagas M. E., Valkimadi P.-E., Huang Y.-T., Matthaiou D. K., Hsueh P.-R. Therapeutic options for Stenotrophomonas maltophilia infections beyond co-trimoxazole: a systematic review. Journal of Antimicrobial Chemotherapy. 2008;62(5):889–894. doi: 10.1093/jac/dkn301. [DOI] [PubMed] [Google Scholar]
- 3.Farrell D. J., Sader H. S., Jones R. N. Antimicrobial susceptibilities of a worldwide collection of Stenotrophomonas maltophilia isolates tested against tigecycline and agents commonly used for S. maltophilia infections. Antimicrobial Agents and Chemotherapy. 2010;54(6):2735–2737. doi: 10.1128/aac.01774-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sader H. S., Flamm R. K., Jones R. N. Tigecycline activity tested against antimicrobial resistant surveillance subsets of clinical bacteria collected worldwide (2011) Diagnostic Microbiology and Infectious Disease. 2013;76(2):217–221. doi: 10.1016/j.diagmicrobio.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Cho J. C., Fiorenza M. A., Estrada S. J. Ceftolozane/tazobactam: a novel cephalosporin/β-lactamase inhibitor combination. Pharmacotherapy. 2015;35(7):701–715. doi: 10.1002/phar.1609. [DOI] [PubMed] [Google Scholar]
- 6.Cluck D., Lewis P., Stayer B., Spivey J., Moorman J. Ceftolozane-tazobactam: a new-generation cephalosporin. American Journal of Health-System Pharmacy. 2015;72(24):2135–2146. doi: 10.2146/ajhp150049. [DOI] [PubMed] [Google Scholar]
- 7.Soliman R., Lynch S., Meader E., et al. Successful ceftolozane/tazobactam treatment of chronic pulmonary infection with pan-resistant Pseudomonas aeruginosa . JMM Case Reports. 2015 doi: 10.1099/jmmcr.0.000025. [DOI] [Google Scholar]
- 8.Alqaid A., Dougherty C. K., Ahmad S. Triple antibiotic therapy with ceftolozane/tazobactam, colistin and rifampin for pan-resistant Pseudomonas aeruginosa ventilator-associated pneumonia. SWRCCC. 2015;3(11):35–39. doi: 10.12746/swrccc2015.0311.144. [DOI] [Google Scholar]
- 9.Garcia Sanchez J. E., Vazquez Lopez M. L., Blazquez De Castro A. M., et al. Aztreonam/clavulanic acid in the treatment of serious infections caused by Stenotrophomonas maltophilia in neutropenic patients: case reports. Journal of Chemotherapy. 1997;9(3):238–240. doi: 10.1179/joc.1997.9.3.238. [DOI] [PubMed] [Google Scholar]
- 10.Leung C., Drew P., Azzopardi E. A. Extended multidrug-resistant Stenotrophomonas maltophilia septicemia in a severely burnt patient. Journal of Burn Care and Research. 2010;31(6):p. 966. doi: 10.1097/bcr.0b013e3181f93b46. [DOI] [PubMed] [Google Scholar]
- 11.Pérez P. N., Ramírez M. A., Fernández J. A., de Guevara L. L. A patient presenting with cholangitis due to Stenotrophomonas maltophilia and Pseudomonas aeruginosa successfully treated with intrabiliary colistine. Infectious Disease Reports. 2014;6(2):17–19. doi: 10.4081/idr.2014.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landersdorfer C. B., Bulitta J. B., Kinzig M., Holzgrabe U., Sörgel F. Penetration of antibacterials into bone: pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clinical Pharmacokinetics. 2009;48(2):89–124. doi: 10.2165/00003088-200948020-00002. [DOI] [PubMed] [Google Scholar]


