Abstract
Tapentadol is a novel oral analgesic with a dual mode of action as an agonist of the µ-opioid receptor (MOR), and as a norepinephrine reuptake inhibitor (NRI) all in a single molecule. Immediate release (IR) tapentadol shows its analgesic effect quickly, at around 30 minutes. Its MOR agonistic action produces acute nociceptive pain relief; its role as an NRI brings about chronic neuropathic pain relief. Absorption is rapid, with a mean maximal serum concentration at 1.25-1.5 h after oral intake. It is present primarily in the form of conjugated metabolites after glucuronidation, and excretes rapidly and completely via the kidneys. The most common adverse reactions are nausea, dizziness, vomiting, and somnolence. Constipation is more common in use of the ER formulation. Precautions against concomitant use of central nervous system depressants, including sedatives, hypnotics, tranquilizers, general anesthetics, phenothiazines, other opioids, and alcohol, or use of tapentadol within 14 days of the cessation of monoamine oxidase inhibitors, are advised. The safety and efficacy have not been established for use during pregnancy, labor, and delivery, or for nursing mothers, pediatric patients less than 18 years of age, and cases of severe renal impairment and severe hepatic impairment. The major concerns for tapentadol are abuse, addiction, seeking behavior, withdrawal, and physical dependence. The presumed problem for use of tapentadol is to control the ratio of MOR agonist and NRI. In conclusion, tapentadol produces both nociceptive and neuropathic pain relief, but with worries about abuse and dependence.
Keywords: Acute pain, Addictive behavior, Adverse drug reactions, Alpha adrenergic receptors, Chronic pain, Hyperalgesia, Mode of action, Mu opioid receptor, N-desmethyltapentadol, Neuropathic pain, Nociceptive pain, Tapentadol
INTRODUCTION
Tramadol has become a familiar weak opioid analgesic using a µ-opioid receptor agonist and a serotonin and norepinephrine reuptake inhibitors mechanisms of action [1]. Acetaminophen added to the tramadol is also commonly prescribed to improve the efficacy of central analgesia. The mixture is unique in its action on the central nervous system (CNS), but is only effective in cases with no peripheral inflammatory reaction, in which cases non-steroidal anti-inflammatory drugs (NSAIDs) are preferable. However, tramadol has a significant abuse potential, an increased risk of convulsions of serotonin syndrome in chronic administration, as well as frequently causing initial dizziness, nausea, and somnolence [2].
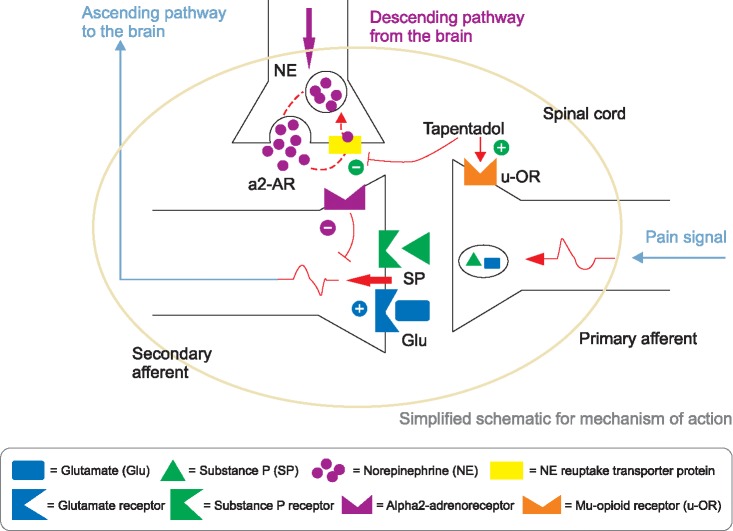
Tapentadol, in a single molecule, is a novel analgesic with a dual mode of action as an agonist of the µ-opioid receptor (MOR), and as a norepinephrine reuptake inhibitor (NRI). Its analgesic merits begin quickly, at around 30 m for both acute nociceptive and chronic neuropathic pain [3]. The drug has a representative default, with the tablet containing both an MOR agonist and an NRI. This is similar to a mixture of salt and pepper making it difficult to season your food satisfactorily (Fig. 1).
Fig. 1. A dual mode of action mechanism for tapentadol. (Modified from Tzschentke TM, Christoph T, Kögel B, Schiene K, Hennies HH, Englberger W, et al. (-)-(1R,2R)-3-(3-dimethylamino-1-ethyl-2-methyl-propyl)-phenol hydrochloride (tapentadol HCl): a novel mu-opioid receptor agonist/norepinephrine reuptake inhibitor with broad-spectrum analgesic properties. J Pharmacol Exp Ther 2007; 323: 265-76. Pertovaara A. Noradrenergic pain modulation. Prog Neurobiol 2006; 80: 53-83).
After the launch of tapentadol IR in United States, in June 2009, nonmedical diversion by college students was reported, using different administration routes and methods such as chewing and swallowing, as well as inhalation, instead of intact swallowing [4]. Some of the same authors, affiliated with the manufacturer, reported that the rates of tapentadol IR abuse and diversion were lower than those of oxycodone and hydrocodone, but similar to those of tramadol [5].
This review focuses on the pros and cons of dual analgesic mode and concern related to abuse/diversion, along with general drug information.
MAIN BODY
1. Brief history and clinical profile of tapentadol
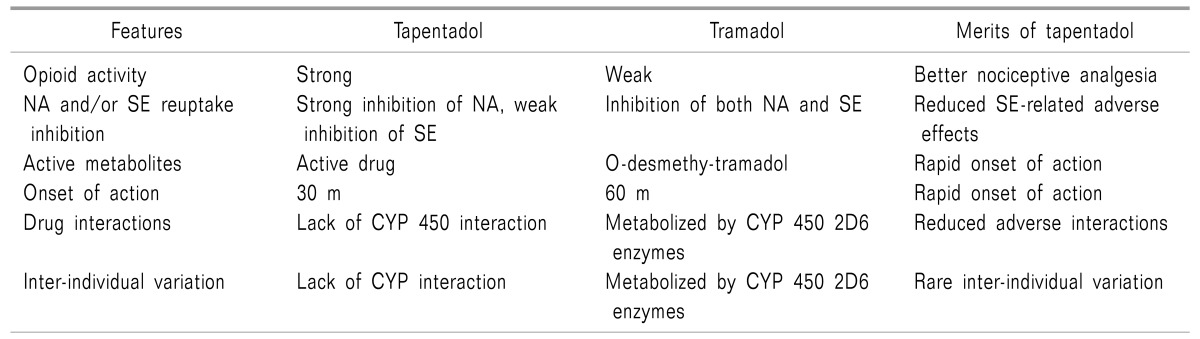
Tapentadol [(-)-(R, R)] was discovered in the late 1980s from morphine, tramadol, and a metabolite of tramadol [(-)-(S,S)-M-1] sequentially. The development of tapentadol from tramadol included these 4 steps: 1) opening of the cyclohexane ring; 2) changing from a prodrug to a direct acting drug; 3) selecting 1 enantiomer; and 4) replacement of the tertiary hydroxyl group (Table 1) [6].
Table 1. Better Properties of Tapentadol Compared with Those of Tramadol [6,15].
CYP: cytochrome, NA: norepinephrine, SE: serotonin.
Tapentadol hydrochloride IR was approved by the United States Food and Drug Administration (FDA) in 2008, for the treatment of moderate to severe acute pain [7]. Tapentadol ER also obtained FDA approval in the United States for management of moderate to severe chronic pain and neuropathic pain in 2011 and 2012, respectively.
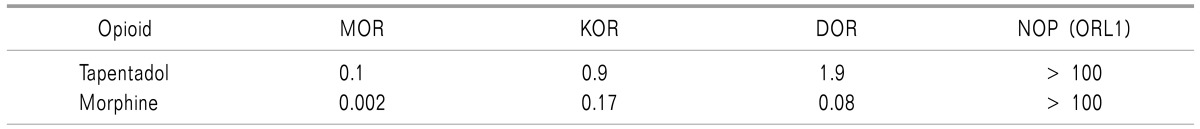
Tapentadol binds to mu, delta, and kappa opiate receptors (Table 2) [7]. The smaller the inhibitory constant (Ki), the greater the binding affinity and the smaller amount of medication needed in order to inhibit the activity of the enzyme. It shows Ki values of 0.1 µM in a rat mu-opioid receptor binding assay and 0.5 µM in a rat synaptosomal norepinephrine reuptake assay [6,8]. In conclusion, the affinity of tapentadol for mu-opiate receptors is about 1/50 fold lower than that of morphine. In addition, tapentadol shows dose-dependent inhibition of norepinephrine reuptake, but only moderate increase in serotonin activity [7,9].
Table 2. Comparison of the Affinity [Ki (µM)] of Tapentadol and Morphine at Various Opioid Receptor Subtypes in Rat Brain Membranes or Human Recombinant Receptors [6,7].
MOR: mu-opiate receptor, KOR: kappa-opiate receptor, DOR: delta-opiate receptor, NOP (ORL1): nociception receptor (opioid receptor-like receptor).
2. Various preparation and conversion of opioids
Currently available doses are 50, 75, and 100 mg of the IR form taken 4 to 6 times in a day and 50, 100, 150, 200, and 250 mg of the extended release (ER) form taken twice a day. Recommended maximal daily doses are 700 mg on the first day and 600 mg on subsequent days for IR, and 500 mg per day for ER formulations.
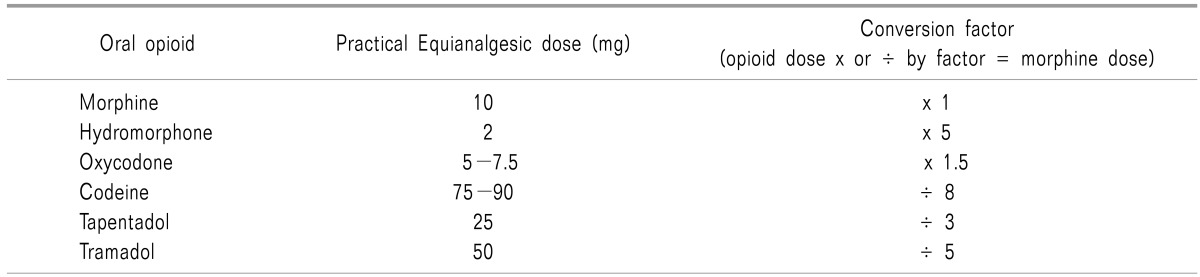
Fifty mg of tapentadol IR 4 times in a day can convert into100 mg of tapentadol ER twice a day. Based on 10 mg of oral morphine, an equianalgesic dose of oral tapentadol is 25 mg, compared to 5 mg of oxycodone. Twenty mg of oral morphine ER twice a day can be equivalent to 50 mg of tapentadol ER twice a day (Table 3).
Table 3. Equianalgesic Dose of Oral Opioids by Opioid Conversion Guideline by Government of Western Australia.
3. Pharmacodynamics
Tapentadol has a dual mechanism of action: 1) inhibition of the ascending pain pathway related to agonists of MORs in the peripheral tissues, spinal cord, and brain; 2) potentiation of descending pain modulation related to NRI. It has 50 times less affinity to mu-opioid receptors than morphine; however, it is only 2-3 times less potent in its analgesic effect due to the NRI opioid-sparing effect [10].
In addition to primary tricyclic antidepressants (TCAs), the representative agents to potentiate inhibition of the descending pain pathway related to NRI and serotonin receptor inhibition. Nefopam acts as a triple neurotransmitter (norepinephrine, serotonin, and dopamine) reuptake inhibition [11,12]. All these drugs, such as TCAs, tramadol [13], nefopam, and tapentadol, can be applied to neuropathic pain.
4. Pharmacokinetics
Absorption of tapentadol is rapid with a mean maximal serum concentration of 1.25-1.5 h after oral intake. It is present primarily in the form of conjugated metabolites, and excretes rapidly and completely up to 95% within 24 h and 99.9% within 5 d via the kidneys [14,15]. Unlike tramadol, tapentadol is a single enantiomer with no active metabolites [16].
5. Contraindications
The absolute contraindications are impaired pulmonary function and paralytic ileus due to its opioid property, and concomitant use within 14 days of monoamine oxidase inhibitors (MAOI), due to its NRI property [15].
The safety and efficacy have not been established for use during pregnancy, labor, and delivery, or for nursing mothers, pediatric patients less than 18 years of age, and cases of severe renal impairment (creatinine clearance < 30 ml/m) and severe hepatic impairment (Child-Pugh score 10 to 15) [17].
6. Adverse events
The most common adverse events, reported by ≥ 10% in any immediate-release (IR) oral tablets were nausea, dizziness, vomiting, and somnolence. Constipation is less common than in users of oxycodone [18].
7. Abuse, diversion, and opioid doctor shopping
The risks of abuse and opioid doctor shopping were lower in the tapentadol immediate release than in the oxycodone immediate release group [19,20]. However, these 2 studies were performed by pharmaceutical company, and it is difficult to ignore the relatively lower risk of abuse and shopping behavior.
The population-based rates of diversion were 0.03/100,000 for tapentadol IR and 0.001/100,000 for tapentadol ER compared to 1.495 for the other schedule II tablets, defined by the "United States Controlled Substances Act". Diversion rates based on drug availability were 0.03/1,000 (tapentadol IR), 0.016/1,000 (tapentadol ER), and 0.172/1,000 (other Schedule II opioid tablets) per prescriptions dispensed [21].
In another study for tapentadol abuse potential, prescription volume-adjusted relative risk for tapentadol ER was lowest compared to other comparatives, including fentanyl ER, tramadol ER, morphine ER, oxycodone ER, and oxymorphone ER, with the exception of hydromorphone ER. Tapentadol IR abuse prevalence was lower than all comparators except fentanyl IR [22].
The population-based rates and drug availability of the diversion of tapentadol ER were slightly lower than that of tapentadol IR, and much lower than those of other Schedule II opioid medications [21].
The endorsement ratio of recreational abusers for tapentadol was lower that of oxymorphone, but slightly higher than that of tramadol [23].
In summary, tapentadol shows a relatively lower risk for abuse, diversion, and opioid doctor shopping than those of the other opioids, but slightly higher than that of tramadol. Tamper-resistant technology may be a promising method to reduce diversion of tapentadol [24].
CONCLUSIONS
Tapentadol has a unique synthetic opioid compound with an NRI property. It produces both acute nociceptive and chronic neuropathic analgesia. However, it is present primarily in the form of conjugated metabolites after glucuronidation, and excretes rapidly and completely via the kidneys.
The most common adverse reactions are nausea, dizziness, vomiting, and somnolence. Precautions against concomitant use of central nervous system depressants, or use of tapentadol within 14 days of the cessation of monoamine oxidase inhibitors, are advised.
The major concerns for tapentadol are abuse, addiction, seeking behavior, withdrawal, and physical dependence. The presumed problem for use of tapentadol is to control the ratio of MOR agonist and NRI.
In conclusion, tapentadol produces both nociceptive and neuropathic pain relief, but with worries about abuse and dependence.
ACKNOWLEDGEMENTS
This study was supported by a 2016 research grant from Pusan National University Yangsan Hospital.
References
- 1.Lassen D, Damkier P, Brøsen K. The pharmacogenetics of tramadol. Clin Pharmacokinet. 2015;54:825–836. doi: 10.1007/s40262-015-0268-0. [DOI] [PubMed] [Google Scholar]
- 2.Farquhar-Smith P, Gubbay A. Tramadol and acetaminophen combination for chronic non-cancer pain. Expert Opin Pharmacother. 2013;14:2297–2304. doi: 10.1517/14656566.2013.839985. [DOI] [PubMed] [Google Scholar]
- 3.Kress HG. Tapentadol and its two mechanisms of action: is there a new pharmacological class of centrally-acting analgesics on the horizon? Eur J Pain. 2010;14:781–783. doi: 10.1016/j.ejpain.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 4.Dart RC, Bartelson BB, Adams EH. Nonmedical use of tapentadol immediate release by college students. Clin J Pain. 2014;30:685–692. doi: 10.1097/AJP.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 5.Dart RC, Cicero TJ, Surratt HL, Rosenblum A, Bartelson BB, Adams EH. Assessment of the abuse of tapentadol immediate release: the first 24 months. J Opioid Manag. 2012;8:395–402. doi: 10.5055/jom.2012.0139. [DOI] [PubMed] [Google Scholar]
- 6.Buschmann H. Chapter 12 Tapentadol – from morphine and tramadol to the discovery of tapentadol. In: Fischer J, Ganellin CR, Rotella DP, editors. Analogue-based drug discovery III. Weinheim: Wiley-VCH; 2013. pp. 295–318. [Google Scholar]
- 7.Wade WE, Spruill WJ. Tapentadol hydrochloride: a centrally acting oral analgesic. Clin Ther. 2009;31:2804–2818. doi: 10.1016/j.clinthera.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Smyth LA, Collins I. Measuring and interpreting the selectivity of protein kinase inhibitors. J Chem Biol. 2009;2:131–151. doi: 10.1007/s12154-009-0023-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tzschentke TM, Folgering JH, Flik G, De Vry J. Tapentadol increases levels of noradrenaline in the rat spinal cord as measured by in vivo microdialysis. Neurosci Lett. 2012;507:151–155. doi: 10.1016/j.neulet.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 10.Ramaswamy S, Chang S, Mehta V. Tapentadol--the evidence so far. Anaesthesia. 2015;70:518–522. doi: 10.1111/anae.13080. [DOI] [PubMed] [Google Scholar]
- 11.Ok YM, Cheon JH, Choi EJ, Chang EJ, Lee HM, Kim KH. Nefopam reduces dysesthesia after percutaneous endoscopic lumbar discectomy. Korean J Pain. 2016;29:40–47. doi: 10.3344/kjp.2016.29.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim KH, Abdi S. Rediscovery of nefopam for the treatment of neuropathic pain. Korean J Pain. 2014;27:103–111. doi: 10.3344/kjp.2014.27.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barber J. Examining the use of tramadol hydrochloride as an antidepressant. Exp Clin Psychopharmacol. 2011;19:123–130. doi: 10.1037/a0022721. [DOI] [PubMed] [Google Scholar]
- 14.Terlinden R, Ossig J, Fliegert F, Lange C, Göhler K. Absorption, metabolism, and excretion of 14C-labeled tapentadol HCl in healthy male subjects. Eur J Drug Metab Pharmacokinet. 2007;32:163–169. doi: 10.1007/BF03190478. [DOI] [PubMed] [Google Scholar]
- 15.Singh DR, Nag K, Shetti AN, Krishnaveni N. Tapentadol hydrochloride: a novel analgesic. Saudi J Anaesth. 2013;7:322–326. doi: 10.4103/1658-354X.115319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park CH. Comparison of morphine and tramadol in transforaminal epidural injections for lumbar radicular pain. Korean J Pain. 2013;26:265–269. doi: 10.3344/kjp.2013.26.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu XS, Smit JW, Lin R, Stuyckens K, Terlinden R, Nandy P. Population pharmacokinetics of tapentadol immediate release (IR) in healthy subjects and patients with moderate or severe pain. Clin Pharmacokinet. 2010;49:671–682. doi: 10.2165/11535390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Afilalo M, Etropolski MS, Kuperwasser B, Kelly K, Okamoto A, Van Hove I, et al. Efficacy and safety of Tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo- and active-controlled phase III study. Clin Drug Investig. 2010;30:489–505. doi: 10.2165/11533440-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Cepeda MS, Fife D, Ma Q, Ryan PB. Comparison of the risks of opioid abuse or dependence between tapentadol and oxycodone: results from a cohort study. J Pain. 2013;14:1227–1241. doi: 10.1016/j.jpain.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Cepeda MS, Fife D, Vo L, Mastrogiovanni G, Yuan Y. Comparison of opioid doctor shopping for tapentadol and oxycodone: a cohort study. J Pain. 2013;14:158–164. doi: 10.1016/j.jpain.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 21.Dart RC, Surratt HL, Le Lait MC, Stivers Y, Bebarta VS, Freifeld CC, et al. Diversion and illicit sale of extended release tapentadol in the United States. Pain Med. 2015:pii: pnv032. doi: 10.1093/pm/pnv032. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Butler SF, McNaughton EC, Black RA. Tapentadol abuse potential: a postmarketing evaluation using a sample of individuals evaluated for substance abuse treatment. Pain Med. 2015;16:119–130. doi: 10.1111/pme.12524. [DOI] [PubMed] [Google Scholar]
- 23.McNaughton EC, Black RA, Weber SE, Butler SF. Assessing abuse potential of new analgesic medications following market release: an evaluation of internet discussion of tapentadol abuse. Pain Med. 2015;16:131–140. doi: 10.1111/pme.12547. [DOI] [PubMed] [Google Scholar]
- 24.Vosburg SK, Jones JD, Manubay JM, Ashworth JB, Shapiro DY, Comer SD. A comparison among tapentadol tamperresistant formulations (TRF) and OxyContin® (non-TRF) in prescription opioid abusers. Addiction. 2013;108:1095–1106. doi: 10.1111/add.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]