Abstract
Redox homeostasis is tightly controlled in cells as it is critical for most cellular functions. Iron-Sulfur centers (Fe-S) are metallic cofactors with electronic properties that are associated with proteins and allow fine redox tuning. Following the observation that altered Fe-S biosynthesis is correlated with a high sensitivity to hydroxyurea (HU), a potent DNA replication blocking agent, we identified that oxidative stress response pathway under the control of the main regulator Yap1 attenuates HU deleterious effects, as it significantly increases resistance to HU, Fe-S biosynthesis and DNA replication kinetics in the presence of HU. Yap1 effect is mediated at least in part through up-regulation of two highly conserved genes controlling cytosolic Fe-S biosynthesis and oxidative stress, Dre2 and Tah18. We next observed that HU produces deleterious effects on cytosolic Fe-S clusters in proteins in vivo but not in vitro, suggesting that HU’s impact on Fe-S in vivo is mediated by cellular metabolism. Finally, we evidenced that HU exposure was accompanied by production of reactive oxygen species intracellularly. Altogether, this study provides mechanistic insight on the initial observation that mutants with altered Fe-S biosynthesis are highly sensitive to HU and uncovers a novel mechanism of action of this widely used DNA replication inhibitor.
Hydroxyurea (HU) is a chemical compound first synthesized in 1869 in Germany by Dressler and Stein, but which antiproliferative activity was only reported in the 1960–70’s1,2,3. Early studies had also shown HU impact on nucleosides incorporation4,5 mainly attributed the inactivation of ribonucleotide reductase (RNR)6,7,8. Indeed HU can scavenge the tyrosyl radical in the R2 subunit of RNR9, which is essential for enzyme activity and is otherwise very stable10. Because the radical mechanism is conserved among different RNRs, HU proved to be active in many organisms. HU is still widely used in clinics for treating several disorders such as chronic myeloid leukemia, sickle cell disease, AIDS and others. If HU’s DNA replication blocking properties are easily related to antitumor activity which is of benefit in myeloid leukemia and HIV proliferation, it is not so clear how HU can help against sickle cell anemia. Noteworthy, HU treatment provokes a boost in the levels of fetal hemoglobin (Hb F,α2γ2) possibly due to nitric oxide production11. HU is also very commonly used for cell cycle studies, as it efficiently and reversibly blocks DNA replication. In accordance, cells with mutated alleles involved in DNA metabolism often turn out to be sensitive to HU. In contrast, many genetic screening based on HU hypersensitivity identified players that are not always directly related to DNA metabolism12,13.
Dre2-Tah18 is a protein complex that has been identified in yeast by different methods14,15,16. Tah18 is a diflavin oxido-reductase, exhibiting three redox domains: a flavodoxin-like domain binding flavin mononucleotide (FMN), a flavin adenine dinucleotide (FAD) and a nicotinamide adenine dinucleotide (NAD) binding domains; this organization is reminiscent of P450 reductases16,17. N-terminus of Tah18 including FMN- and FAD-binding domains, is able to bind C-terminus of Dre2 in vivo and in vitro, and this interaction is essential for yeast viability17. Dre2 C-terminus is highly conserved between all species except bacteria, as identified by sequence alignment studies16,18. When exogenously expressed in bacteria, Dre2 was shown to include one Iron-Sulfur (Fe-S) cluster18 and possibly two. In vitro, Tah18 is capable of reducing the Fe-S cluster in Dre2, which controls downstream synthesis of Fe-S clusters in cytosolic proteins19.
Studies on Dre2-Tah18 physiological role are pretty recent; the complex is implicated in cytosolic Fe-S-containing proteins biosynthesis18,19, in controlling hydrogen peroxide (H2O2)-induced cell death16 and in ribonucleotide reductase metallocofactor assembly20. Interestingly, Fe-S clusters were identified in B-type DNA polymerases that include Pol3 in yeast21, showing that functional DNA replication is indeed conditioned by cytosolic Fe-S biogenesis, as initially suggested by genetic data22. In the absence of stress, Dre2-Tah18 complex is cytosolic. After exposure of the cells to lethal doses of H2O2, the complex dissociates and Tah18-dependent mitochondria damages and cell death occur16. The complex was suggested to act as a biosensor for intracellular oxidative stress, promoting cell death when the redox balance is severely affected, a role in opposition to its essential function for survival in the absence of stress16.
Several tah18 mutants were generated in our laboratory among which the previously described tah18-5I5 mutant16. Mutated cells are not affected for DNA repair after camptothecin or gamma rays exposure16, but Fe-S biosynthesis is decreased23 and growth is severely impaired after chronic exposure to HU. These phenotypes are in accordance with a role for Tah18 in DNA replication, as also suggested by genetic links between Tah18 and Pol3 that encodes DNA polymerase delta22, and the presence of Fe-S clusters in Pol321. In order to identify new regulators of the Dre2-Tah18 complex, we performed a multicopy suppressor screen for genes that suppress tah18-5I5 sensitivity to HU when overexpressed. Two genes, YAP1 and DRE2 were identified, and their roles in HU resistance are studied through this work.
Dre2 physically interacts with Tah18 in vivo16 and this interaction is essential for yeast viability17. However, no link between YAP1 and TAH18 had been anticipated previously. YAP1 is a transcription factor that has been identified in yeast on the basis of its sequence homology with human AP-1 transcription factor24,25 and is considered as a key player in regulating the response to oxidative stress26, activating the transcription of a whole set of genes encoding antioxidant activities called the “Yap1 regulon”27. In response to H2O2 treatment, activation of transcription by Yap1 overlaps with activation by another transcription factor, Skn7, which allows fine tuning of the response to oxidative stress27,28. Transcription activating activity of Yap1 is regulated by its nuclear localization. In the absence of stress, Yap1 operates a continuous cycle between cytosolic and nuclear compartments, due to the dual presence of a nuclear localization signal (NLS) in its N-terminal region and a nuclear export signal (NES) in its C-terminal region29,30. In unstressed conditions, Yap1 is primarily located in the cytoplasm. In the presence of oxidative stress such as H2O2, Gpx3 catalyzes an intramolecular disulfide bond between the redox-sensitive cysteines of Yap1, which in turn prevents the binding of the exportin Crm1 to Yap1 C-terminus. As a consequence, Yap1 is not exported efficiently in the cytosol and accumulates in the nucleus30, permitting increased transcription of Yap1-controlled genes. In such oxidizing conditions, Yap1 degradation is also accelerated, restricting the duration of Yap1 effect in response to oxidative stress31. Yap1 was also suggested to be reduced by thioredoxins as the absence of thioredoxins provokes constitutive Yap1 oxidation32,33.
The present study reports an unexpected genetic interaction between YAP1 and TAH18 in the presence of HU. We identified that HU alters Fe-S centers in vivo through the production of reactive oxygen species (ROS). We further demonstrate that YAP1 alleviates yeast sensitivity to HU and DNA replication kinetics in the presence HU both by decreasing ROS and improving Fe-S biosynthesis.
Results
YAP1 or DRE2 overexpression improves tah18-5I5 mutant’s growth in the presence of hydroxyurea
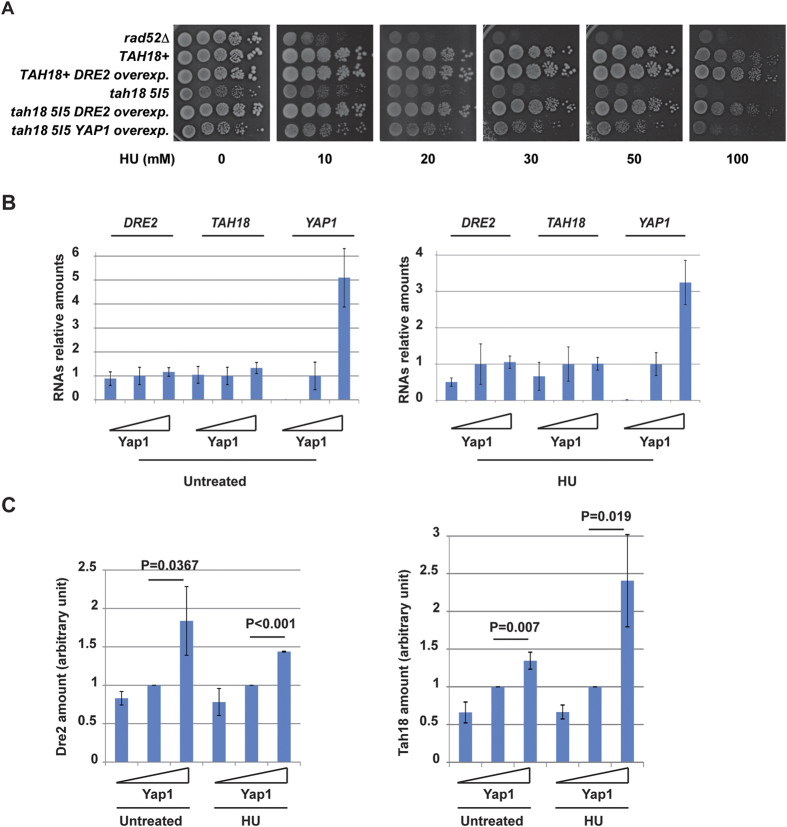
A tah18 mutated allele, namely tah18-5I5, was generated previously using mutagenic PCR16 and contains several point mutations. This allele confers impaired growth over 32 °C to the yeast cells. This allele did not confer any particular sensitivity when yeasts were exposed to the DNA-damaging agent camptothecin, creating lesions in a topoisomerase I-dependent manner, nor to gamma rays that generates DNA lesions mainly in the form of base damage and double and single strand breaks16. In contrast, tah18-5I5 mutant revealed to be highly sensitive to chronic exposure to HU, as described also by other authors20. Growth was strongly inhibited from 20 mM for both tah18-5I5 mutant and the rad52Δ mutant (control), known as very sensitive to this drug (Fig. 1A). We performed a multicopy suppressor screen to identify suppressor genes of tah18-5I5 mutant sensitivity to HU, as this might also help to understand the link of Tah18 with DNA replication. Tah18-5I5 mutant was transformed by a multicopy 2 μ-based yeast DNA library constructed in YEp13 vector (LEU2 marker) and cells were plated SC–LEU medium at 28 °C to select yeasts containing plasmids. Transformed cells were then replica plated on 50 mM HU-containing plates at 28 °C and growing clones were selected. Plasmids were retrieved and tested by re-transformation of the tah18-5I5 mutant strain. Finally, DNA sequencing revealed that two genes, DRE2 and YAP1, were able to suppress tah18-5I5 sensitivity to HU when overexpressed (Fig. 1A).
Figure 1. Tah18 and Dre2 are regulated by Yap1.
(A) DRE2 and YAP1 overexpression can rescue viability of tah18 cells upon HU chronic exposure. 10-fold serial dilutions of yeast cells grown until log phase were spotted on YPD medium containing increasing concentration of HU as indicated. Growth was assessed after 4 days at 28 °C. (B) Average expression of DRE2 and TAH18 mRNA levels in three strains with increasing YAP1 expression levels. mRNA levels have been quantified in three strains: no YAP1 expression (yap1Δ), wild-type YAP1 expression (WT) and YAP1 overexpression (YAP1 carried out on a multicopy plasmid). Increasing expression of YAP1 in the three strains is illustrated as a triangle. mRNA level of each gene was calculated as ratio relative to that of the wild-type (WT) strain (set as 1). (C) Dre2 and Tah18 protein levels in three strains with increasing YAP1 expression levels. Yeast cells were treated with 100 mM HU for 1 hour and collected for protein analysis. Dre2 and Tah18 were detected with specific antibodies and actin was used as an internal control. Odyssey infrared imaging system was used for quantification. Relative Dre2 or Tah18 amounts were calculated as follow: Dre2 or Tah18 amounts = [Dre2 or Tah18 band intensity]/[Act1 band intensity]. Amounts in WT were set as 1. The reported values are the means of three independent experiments ± SEM. P values were calculated using unpaired one-tailed t-test, n = 3.
Identifying DRE2 as a multicopy suppressor of tah18-5I5 could be reasonably expected, given that DRE2 overexpression is known to stabilise the Dre2-Tah18 complex by mass action and to suppress tah18-5I5 mutant thermosensitive growth16. In contrast, functional links between TAH18 and YAP1 had not been previously anticipated. Identification of DRE2 and YAP1 through this new screen indicates that the Dre2-Tah18 complex plays a role in response to HU chronic exposure, and that oxidative stress response might be involved in this process as well.
YAP1 overexpression increases Tah18 and Dre2 levels
Yap1 has been extensively studied as a transcription factor required for oxidative stress response (for review see26,34). We thus assessed whether Yap1 may regulate the transcription of TAH18 or/and DRE2 in the absence of stress as both proteins form a highly stable cytosolic complex16. In order to mimic increasing levels of Yap1 in vivo, we used a set of yeast strains that are respectively deleted for yap1 (yap1Δ), expressing YAP1 at the endogenous level (YAP1), or overexpressing YAP1 from a multicopy plasmid (YAP1 overexp.). These three strains were challenged with HU and RNAs were quantified. Quantitative RT-PCR analysis indicated that absence, presence or overexpression of YAP1 had little effect on TAH18 and DRE2 mRNA levels in treated or untreated cells (Fig. 1B). Challenging cells with HU did not change this result. We next quantified Dre2 and Tah18 intracellular protein amounts using western blotting and polyclonal antibodies against Dre2 and Tah18 that can recognize their target with a high specificity (Ref. 16 and Supplementary. Fig. 1). Dre2 and Tah18 amounts increased as Yap1 levels increased (Fig. 1C), independently of the presence or absence of HU, showing that Yap1 levels influences Dre2 and Tah18 levels in vivo in a HU-independent manner. Taking these data together, we conclude that Tah18 and Dre2 levels are controlled by Yap1 in vivo to a moderate extent, as YAP1 overexpression results in a subtle increase in the amount of Dre2-Tah18 complex. Thus, part of Yap1 activity in HU resistance might occur through increasing amounts of Dre2-Tah18 complex. These results might also indicate that Yap1, by decreasing oxidative stress, protects Dre2 and Tah18 from degradation and stabilizes the complex.
YAP1 overexpression improves cytosolic Fe-S proteins synthesis and release from hydroxyurea block in tah18 cells
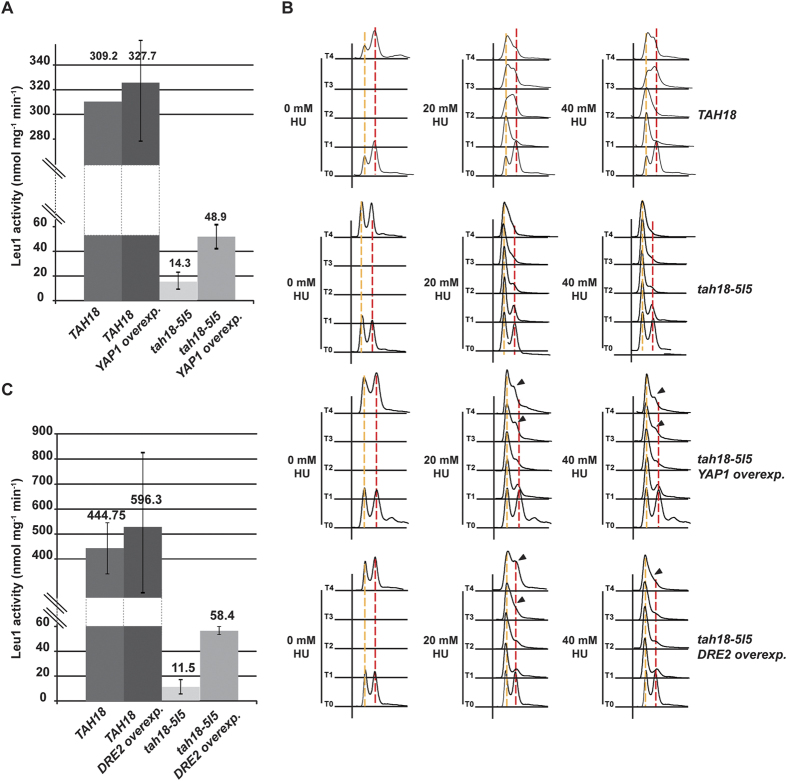
Consistent with its role in Fe-S biogenesis, tah18-5I5 mutant exhibits decreased cytosolic Fe-S proteins biosynthesis17,19. To test the hypothesis that overexpressing YAP1 can improve Dre-Tah18 activity, we next examined whether overexpressing YAP1 can rescue decreased cytosolic Fe-S biosynthesis in tah18 cells. We measured the activity of Leu1, a cytosolic isopropylmalate isomerase containing Fe-S, which catalyzes the second step in the leucine biosynthesis pathway. Its activity can be measured by monitoring the consumption of the artificial substrate of Leu1, citraconate, and is considered as a good reporter of cytosolic Fe-S proteins biosynthesis35. As previously noted17, Leu1 activity was strongly reduced in tah18-5I5 mutants but found indeed significantly increased in presence of overexpressed YAP1, reflecting an improved cytosolic Fe-S biosynthesis (Fig. 2A).
Figure 2. YAP1 or DRE2 overexpression improves tah18-5I5 cytosolic Fe-S biosynthesis and hydroxyurea block release.
(A,C) Isopropylmalate isomerase (Leu1) activity measurement. Citraconate was used as a substrate of Leu1. Leu1 activity (nmol mg−1 min−1) was calculated from the decrease in OD at 235 nm due to citraconate isomerization. The genotype of yeast strains used is indicated. The reported values are the means of three independent experiments ± SEM. (B) Flow cytometry analysis of yeast cells incubated in the presence of HU (20 and 40 mM as indicated). The genotype of yeast strains used is indicated. Time points at 0, 1, 2, 3 and 4 hour exposure are shown. S phase restart is indicated by an arrow in tah18-5I5 cells overexpressing YAP1 or DRE2.
HU is a chemical compound known to scavenge tyrosyl free radicals in RNR; as a consequence, it decreases deoxyribonucleotides (dNTPs) biosynthesis and inhibits DNA replication36. In accordance, cells exposed to HU block their cell cycle at the very beginning of S phase before progressively returning to cycling after adapting to the drug. We examined whether YAP1 overexpression might influence cell cycle arrest in the presence of HU. Cells were subjected to low doses of HU (20 and 40 mM) and consequently blocked their cell cycle after 2 hours as shown by the G1-S pick (Fig. 2B). Kinetics in tah18-5I5 cells is slower than that usually observed in wild-type cells, and is actually consistent with slow growth phenotype of tah18-5I5 cells (doubling time >3 hours). After 3 hours in the presence of HU, tah18-5I5 overexpressing YAP1 escaped the block as S phase cells were visible, in contrast to control tah18-5I5, indicating that YAP1 overexpression permits the cells to bypass HU-induced cell cycle arrest (Fig. 2B). Therefore, activation of the oxidative stress response by overexpressing YAP1 in tah18-5I5 mutant improves cytosolic Fe-S cluster biosynthesis and release from HU-induced cell cycle arrest.
DRE2 overexpression also improves cytosolic Fe-S cluster biosynthesis and release from hydroxyurea block in tah18 cells
As described above, we observed that DRE2 overexpression improves growth of tah18-5I5 mutants in the presence of HU (Fig. 1A). We verified whether overexpressing DRE2 also rescues decreased cytosolic Fe-S biosynthesis in tah18 cells.
As found with YAP1 overexpressed, Leu1 activity was partially restored in case of DRE2 overexpression, indicating that DRE2 overexpression improves cytosolic Fe-S cluster biosynthesis (Fig. 2C). We also analysed whether DRE2 overexpression influences DNA replication by measuring kinetics of yeast cells release from an HU block. tah18-5I5 cells were grown in presence of mild doses of HU, as previously described. DRE2 overexpression in tah18-5I5 cells allowed bypassing HU effects (Fig. 2B), as an early exit from the block is visible from 3 and 4 hours after the block. Taken these observations together, we conclude that DRE2 overexpression significantly improves cytosolic Fe-S biosynthesis and release from HU block in tah18-5I5 cells, comparable to YAP1 overexpression. These results indicate that improving Fe-S biosynthesis is of benefit for yeast cells in the presence of HU.
Hydroxyurea decreases Fe-S levels in yeast cells but not in purified Leu1
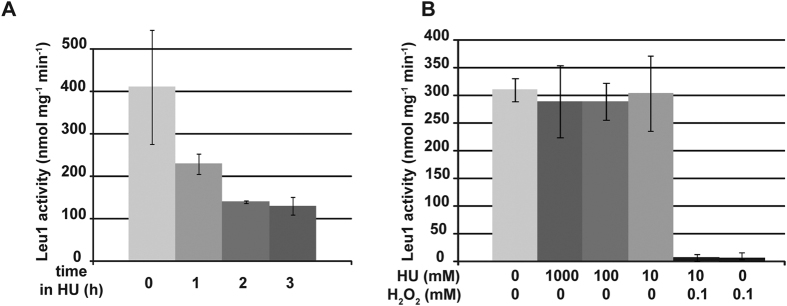
HU is well known for inhibiting dNTPs synthesis by scavenging tyrosyl free radicals involved in NDPs reduction into dNDPs37,38. As a consequence, HU has been mainly regarded as a DNA replication blocking agent, but less as a redox-interfering drug. Because tah18-5I5 mutant (this work) and a dre2 mutant (not shown) are highly sensitive to HU, and because increasing cytosolic Fe-S biosynthesis improves yeast resistance to HU, we examined whether HU might have deleterious effects on cytosolic Fe-S. Wild-type yeast cells were incubated in the presence of HU for 1, 2 and 3 hours, before being collected and assessed for cytosolic Fe-S level using Leu1 assay. In the presence of HU, Leu1 activity was found significantly decreased by 3- to 4-fold, showing that indeed cytosolic Fe-S are altered in cells that have been cultivated in the presence of HU (Fig. 3A).
Figure 3. Hydroxyurea targets Fe-S centers in vivo.
(A) Leu1 activity measurement of semi-purified protein extract from cells exposed to 200 mM HU for 1, 2 and 3 hours. (B) Leu1 activity measurement of semi-purified protein extract in the presence of HU and/or H2O2. HU and/or H2O2 have been added in vitro to partially purified protein extract as indicated, and activity was measured. The reported values are the means of three independent experiments ± SEM.
We next assessed whether HU acts directly on Fe-S clusters. Semi-purified Leu1 extracts from untreated cells were prepared, and HU was then added directly into the extracts before assessing Leu1 activity. H2O2 was used as a control, as it directly oxidizes Fe-S39. As shown in Fig. 3B, direct addition of HU within the extracts did not affect Leu1 activity even using high HU concentration (1 M as compared to 200 mM classically used in yeast cells media). In contrast, semi-purified Leu1 retained no activity in the presence of micromolar amounts of H2O2 (Fig. 3B), arguing that deleterious effects of HU on Fe-S centers in yeast cells are not due to direct interaction between HU and Fe-S centers. We conclude that, different to H2O2, HU is not capable of altering Fe-S centers directly and that the previously characterized, deleterious consequences of HU on Fe-S centers might depend on cell metabolism.
Detection of H2O2 in yeast cells exposed to hydroxyurea using sensitive redox sensor HyPer
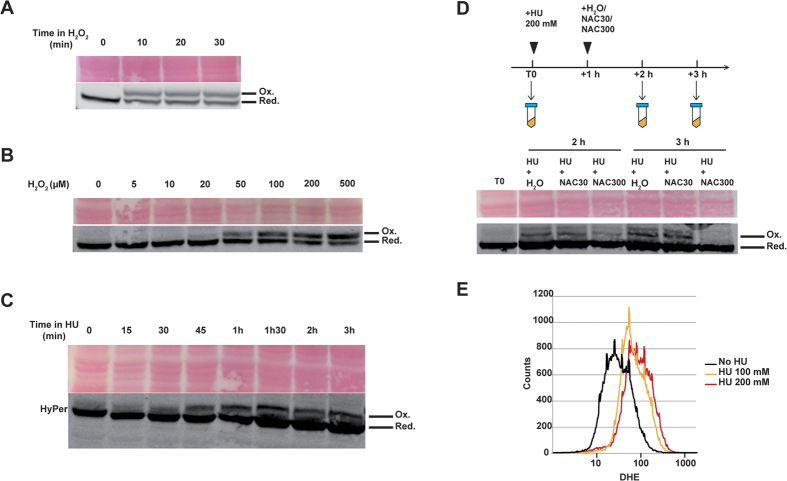
Because activating the oxidative stress response through Yap1 relieves HU phenotypes, we hypothesised that ROS could be generated in the presence of HU. Detecting low level of ROS in vivo is technically challenging but recently developed, genetically encoded biosensors provide an alternative way to overcome the limitations of conventional redox measurements40. HyPer is a genetically encoded fluorescent sensor that consists of circularly permuted yellow fluorescent protein (cpYFP) inserted into the regulatory domain of prokaryotic H2O2-sensing protein OxyR41. HyPer is capable of detecting production and local bursts of H2O2 in the cytoplasm and mitochondria of HeLa cells after various stimulations such as Apo2L/TRAIL treatment or in the cytoplasm of PC-12 cells stimulated by nerve growth factor41. HyPer construct was designed similarly to previously published redox-sensitive YFP (rxYFP) as a sensor, and stably integrated within yeast chromosomes to guarantee comparable expression between yeast cells in culture (see Methods and42). Redox western blot conditions were set up so that oxidized and reduced forms of HyPer could be separated on SDS-PAGE, and detected using an anti-GFP antibody. Cells were exposed to 1 mM H2O2 for increasing times (10, 20 and 30 min) to evaluate HyPer’s response time to treatment (Fig. 4A). An oxidized band appeared as soon as 10 min after bolus addition indicating fast kinetics of H2O2 detection by the probes. Finally we evaluated H2O2 detection threshold by treating cells with increasing concentrations of exogenous H2O2 for 20 min. Oxidized forms of HyPer were detected from the 20–50 μM range and over (Fig. 4B), making HyPer a sensitive tool to detect subtle H2O2 changes within yeast cells.
Figure 4. Hydrogen peroxide is detected in yeast cells after hydroxyurea exposure.
(A–D) HyPer detection using redox western blotting. (A) Exponentially growing yeast cells expressing HyPer cultured in YPD were exposed to H2O2 (1 mM) for increasing times as indicated. Oxidized (Ox.) and reduced (Red.) HyPer bands are indicated, and Ponceau red staining is shown as loading control. (B) Yeast cells were treated for 20 min with increasing H2O2 concentrations as indicated. (C) Yeast cells were treated with HU (200 mM) for increasing times as indicated. (D) Yeast cells were treated with HU (200 mM) for 1 hour. NAC (30 mM or 300 mM) was then added to cultures for 1 hour and 2 hours. H2O was also used as a control. Samples were processed at various times as indicated. (E) ROS detection using DHE. Exponentially growing yeast cells cultured in YPD were exposed to HU (100 and 200 mM) for 1 hour, before staining with DHE to evidence ROS production.
Yeast cells were treated with HU 200 mM for increasing times, and redox state of HyPer was monitored. An extra band appeared 30–45 min after treatment, with comparable size to oxidized HyPer (Fig. 4C), suggesting that HyPer is oxidized in response to HU. To further assess HyPer modification, the antioxidant N-Acetyl-Cysteine (NAC) was used. Yeast cells were treated for 1 hour with HU 200 mM, before adding NAC to the medium for an additional one or two hours (Fig. 4D). The intensity of the modified HyPer band decreased after treatment with NAC 300 mM for 1 hour, and the band totally disappeared after two hours, in contrast to water- or NAC 30 mM- treated samples. This confirmed that HU indeed provokes HyPer oxidation in vivo.
Increased ROS level following HU treatment was further evidenced using dihydroethidium (DHE). In the presence of ROS, DHE is rapidly oxidized to form a red fluorescent 2-hydroxyethidium detectable by flow cytometry (FACS). Yeast cells were treated with HU 100 mM or HU 200 mM for 1 hour and then stained by DHE for 2 hours. FACS analysis revealed increased fluorescence in the treated samples (Fig. 4E), indicative of ROS production in yeast cells after HU treatment. Taken together, these experiments show that ROS, including H2O2, are generated after HU treatment in vivo.
Discussion
Thanks to a genetic screen for multicopy suppressors we identified that oxidative stress response under the control of Yap1 improves HU resistance of yeast cells that are defective for Fe-S biosynthesis (tah18-5I5 mutant). We further demonstrate that YAP1 overexpression actually improves Fe-S biosynthesis and release from an HU block, and that this occurs at least in part through stabilizing the Dre2-Tah18 complex. Finally, we demonstrate that HU per se alters Fe-S centers in vivo through a mechanism that involves the production of ROS. This sheds light on the role of oxidative stress response in the resistance to HU. As summarized in Fig. 5, we propose that Yap1 acts in HU resistance by different means. First, it is involved in eliminating ROS that are produced during HU exposure due to its transcriptional activity controlling numerous antioxidant proteins. TRX2 encodes a cytosolic thioredoxin that is a key player in the response to oxidative stress, and which expression is finely regulated by Yap143. Overexpressing TRX2 slightly improved tah18-5I5 mutants’ growth on HU (Supplementary Fig. 2) in accordance with the fact that increased antioxidant activity is of benefit during HU exposure. This result also suggests that at least part of Yap1 effect in HU resistance is mediated by Trx2. Second, Yap1 increases Dre2-Tah18 complex, which improves Fe-S biogenesis and thus counteracts Fe-S alteration in the presence of HU. This result is consistent with previous data showing that Dre2, as an Fe-S-containing protein, is highly sensitive to oxidation in vitro17. It is thus possible that Dre2 is sensitive to oxidative stress also in vivo, so that YAP1 overexpression, by slightly increasing Dre2 levels, stabilizes Dre2-Tah18 complex. It is interesting that, in addition to ROS production, HU might also perturb Fe-S formation by scavenging tyrosyl radicals of proteins involved in Fe-S biosynthesis. This could then decrease even more Fe-S levels in vivo.
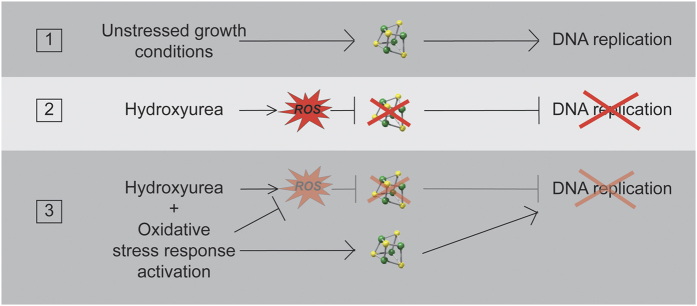
Figure 5. Hydroxyurea induces DNA replication arrest by producing ROS and targeting Fe-S centers.
The model presented here does not show the known effects of HU on RNR. HU targets Fe-S centers in vivo by producing ROS, which delay DNA replication (1–2). Activation of Yap1 decreases ROS levels and increases Fe-S biosynthesis, which in turn attenuates HU-induced Fe-S alteration and improves DNA replication (3).
It is well documented, particularly in bacteria, that many Fe-S centers are sensitive to oxidative agents. Fe-S proteins are inactivated by superoxide anion (O2•−)44 and by very low levels of H2O239,45. Mice lacking the mitochondrial superoxide dismutase SOD2 exhibit chronically increased levels of oxidative stress (O2•−) associated with decreased activities of aconitase and succinate deshydrogenase, two Fe-S-containing enzymes46. In this study, we evidenced ROS production occurs after HU treatment in yeast cells, which provides mechanistic insights to the deleterious effects of HU toward Fe-S. Several arguments in literature support our finding. Notably, catalase, which eliminates H2O2 in vivo47 was identified as a target of HU in plants12. It is thus possible that catalase inhibition by HU participates to the H2O2 boost we evidenced. Also, respiration-defective mutants with notably reduced endogenous ROS production were found significantly more resistant to HU in bacteria. Furthermore, addition of the OH• scavenger thiourea in the presence HU suppressed bacteria sensitivity to HU, suggesting that ROS generation is the direct cause of HU-induced cell death13. Actually it is unknown whether H2O2 is the only ROS produced in HU. O2•− might also be produced, as indicated by DHE sensor (Fig. 4E), even though O2•− is not a strong oxidant, but it is highly deleterious to some Fe-S centers44. O2•− is rapidly converted to H2O2 either by spontaneous dismutation or by the action of superoxide dismutases (SODs)48. Other byproducts might also include nitroxide, as shown in rats49.
Fe-S centers are also targeted by metallic cations in vitro and in vivo50,51. Beside its well known property to inhibit RNR activity in vivo, HU is reported to interact with metallic ions such as Cu2+, Fe2+ and Fe3+ and form complexes, and that may affect its pharmacological properties52. In vitro, the interaction between HU and Fe3+ in aqueous solutions forms a HU-Fe complex which decomposes through redox processes, forming Fe2+, NH4+, CO2 and NO2; HU radical species H2N-CO-NHO• are also detected in the course of the reaction53. HU may interfere with electron transfer processes in biological systems and might induce damage to cellular macromolecules54. A few studies reported that indeed HU is a Fe3+ reducing agent in vivo as well, and was even suggested to be a clinically relevant iron chelator55. In yeast, interplay between HU and iron mobilization has been studied56. Strikingly, the authors identified that the transcriptional response to HU in yeast is partially overlapping with the low iron stress response. Part of the transcriptional response to HU is dependent on the Aft1 and Aft2 transcription factors, known to control iron homeostasis in response to changes in iron availability57. This is consistent with results obtained in bacteria, as HU stress also activates iron uptake58. Altogether, these studies are in agreement with the fact that HU may act as an iron chelator in cells, as suggested above. For these reasons, we tested whether iron homeostasis might be compromised in tah18-5I5 mutants, as this could influence sensitivity to HU. Excess iron or iron depletion did not influence tah18-5I5 growth in the presence of HU (Supplementary Fig. 3), indicating that iron homeostasis is not severely affected and is probably not the major cause of HU sensitivity in tah18-5I5 mutants.
HU has been used in clinics to block cell proliferation and is known to inhibit DNA replication by decreasing intracellular dNTP pool59. Interestingly, it is now known that Dre2-Tah8 is involved in RNR metallocofactor assembly as an electron donor, playing a critical role in the formation of Fe3+ 2-Y• cofactor in RNR and in supplying dNTPs20. In accordance, synthetic lethality had been originally observed between a mutated allele of the Fe-S cluster-containing DNA polymerase delta subunit pol3, and dre2 or tah18 mutants22. It is thus probable that RNR is compromised in the mutated tah18-5I5 strain; Dre2 and Tah18 increased expression resulting from YAP1 overexpression might then also improve dNTP content and thus counteract HU effects.
Several studies pointed out that dNTPs exhaust is not complete after exposure to HU, and that basal dNTPs levels are maintained in a checkpoint-independent manner59,60. These authors suggested that the persistence of a certain dNTPs level after HU-induced DNA replication arrest might be helpful for other DNA metabolisms such as DNA repair. Independently, it is well known that numerous DNA-transacting proteins contain Fe-S centers, among which Rad361, DNA primase62,63, DNA264, XPD and FancJ65, Pol321, this very last being necessary for the formation of an active replication complex, at least in vitro. Because Fe-S centers in Leu1 are altered when cells are exposed to HU, we speculate that Fe-S centers in Pol3 and in other Fe-S DNA replication proteins might be altered as well. This could account for the fact that HU arrests DNA replication even though the dNTP pool is not fully depleted59.
Methods
Strains and media
All strains used in this study are isogenic, except EGY48 from Clontech, 3B9 (BY4741) and 21A5 (double mutant fet3Δ fet4Δ) (Table 1). Gene disruptions were made by standard PCR-based methods. Yeast strains were grown in YPD medium (1% yeast extract, 2% bacto peptone, 2% glucose). For YPD plates, 2% agar was added. For URA3-containing plasmid selection, yeasts were grown on Synthetic Complete (SC) medium lacking uracil (SC–URA) (0.67% yeast nitrogen base, adenine, histidine, lysine, leucine, tyrosine, methionine, tryptophan, 40 mg/liter for each, 2% glucose, 3% agar). HU, iron citrate and bathophenantroline (BPS) were purchased from Sigma. 30 mM stock solution of BPS was prepared in ethanol. For cell cycle experiments, 20 and 40 mM HU were used in exponential yeast cultures. 1 ml of cell culture was collected at indicated time points, and processed for flow cytometry. Detailed information regarding strain construction is provided as Supplementary Methods.
Table 1. Yeast strains used in this study.
| Strain | Genotype | Source |
|---|---|---|
| 3B9 | MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0 rad52Δ::KANR | Euroscarf |
| 5C4 | MATa ura3 leu2 LYS2 his3 dre2Δ::TRP1-CYH2S pRS416-DRE2-URA3 | 16 |
| 5I5 | MATa his3 leu2 LYS2 trp1 ura3 cyh2R tah18-5I5 | 16 |
| 8C2 | MATalpha HIS3 leu2 lys2 trp1 ura3 cyh2R | 17 |
| 8C3 | MATa his3 leu2 LYS2 trp1 ura3 cyh2R | Gérard Faye |
| 8C4 | MATa/alpha HIS3/his3 leu2/leu2 LYS2/lys2 trp1/trp1 ura3/ura3 cycloR/cycloR | Gérard Faye |
| 10F3 | MATa HIS3 leu2 lys2 trp1 ura3 cyh2R dre2::DRE2-eGFP-KANR | This study |
| 11B3 | MATa his3 LEU2-pRS305-TAH18Promoter-GFP-TAH18 LYS2 tah18Δ::TRP1-CYH2S ura3 | 16 |
| 11B8 | MATalpha HIS3 leu2 lys2 trp1 ura3 cyh2R yap1Δ::HisG-URA3-HisG | This study |
| 12A6 | MATa HIS3 leu2 lys2 trp1 ura3 cyh2R dre2::DRE2-eGFP-KANR pRS425-YAP1-LEU2 | This study |
| 12G2 | MATalpha HIS3 leu2 lys2 trp1 ura3 cyh2R tah18-5I5 | 16 |
| 12G3 | MATalpha his3 leu2 LYS2 trp1 ura3 cyh2R tah18-5I5 | 16 |
| 13E2 | MATalpha HIS3 leu2 lys2 ura3 cyh2R TRP1-pRS304-TEF1p-DRE2 | This study |
| 13E5 | MATa his3 leu2 LYS2 ura3 cyh2R TRP1-pRS304-TEF1p-DRE2 dre2Δ::TRP1-CYH2S | This study |
| 13E6 | MATalpha HIS3 leu2 lys2 ura3 cyh2R TRP1-pRS304-TEF1p-DRE2 dre2Δ::TRP1-CYH2S | This study |
| 14C8 | MATa HIS3 leu2 lys2 ura3 cyh2R TRP1-pRS304 TEF1p-DRE2 dre2Δ::TRP1-CYH2S tah18-5I5 | This study |
| 14D6 | MATalpha HIS3 leu2 lys2 trp1 ura3 cyh2R pRS426-YAP1-URA3 | This study |
| 17D1 | MATa his3 leu2 LYS2 trp1 ura3 tah18-5I5 pRS425-YAP1-LEU2 | This study |
| 18H6 | MATalpha HIS3 leu2 lys2 trp1 ura3 tah18-5I5 pRS425-TRX2-LEU2 #1 | This study |
| 18H8 | MATalpha his3 leu2 LYS2 trp1 ura3 pRS425-TRX2-LEU2 | This study |
| 18I1 | MATalpha his3 leu2 LYS2 trp1 ura3 tah18-5I5 pRS425-TRX2-LEU2 #2 | This study |
| 19H4 | MATalpha his3 leu2 LYS2 trp1 ura3 cyh2R tah18-5I5 pRS426-YAP1-URA3 | This study |
| 19I4 | MATa HIS3-pRS303-Hyper leu2 LYS2 trp1 ura3 cyh2R | This study |
| 21A5 | MATa ade6 can1 his3 leu2 trp1 ura3 fet3-2::HIS3 fet4-1::LEU2 | 69 |
Cloning
All pRS vectors used in this study have been described66. Multicopy 2 μ-based yeast DNA library made in YEp13 vector (LEU2 marker) was a kind gift from B. Daignan-Fornier. For construction of pRS303-HyPer, plasmid MEHp9742 was restricted using SacII and NotI, generating an insert containing rxYFP coding sequence under the control of the PGK promoter. This insert was ligated into pR30366 digested by SacII and NotI, generating plasmid pRS303-Cyto-rxYFP. Plasmid pRS303-Cyto-rxYFP was digested with NotI-XbaI, yielding a 785 bp-DNA fragment containing PGK1 promoter. DNA fragment was subcloned into pRS303, yielding pRS303-PromPGK. HyPer coding region was amplified by PCR with oligo 733 and oligo 735 containing Nuclear Localization Signal, using pHyPer-cyto vector (Evrogen) as a template. PCR product was digested with BamHI-EcoRI before subcloning into pRS303-PromPGK, yielding pRS303-HyPer. For construction of pRS425-Trx2, Trx2 coding region was amplified by PCR with oligo 670 and oligo 671, using genomic DNA as a template. PCR product was digested with BamHI-HindIII before subcloning into pRS425, yielding pRS425-Trx2. pRS425-Trx2 #2 and #12 are two independent constructs in E. coli.
RNA purification and quantitative RT-PCR
Yeast RNAs were purified as described previously67. All buffers were added with 0.1% v/v diethylpyrocarbonate (DEPC) before being autoclaved. 50 mL of exponentially growing yeasts were pelleted and washed in cold water, before resuspending in TES buffer (10 mM Tris-HCl pH 7.5, 10 mM EDTA, 0.5% SDS) and adding equal volume of acid phenol. The mix was incubated during 1 hour at 65 °C, and aqueous phase was submitted to 3 consecutive phenol extractions. RNAs from aqueous phase were precipitated by centrifugation after addition of ethanol and 0.5 M sodium acetate. RNA were then washed with 70% ethanol and dried before resupending in H2O. RNAs were incubated in presence of RNAse-free DNAse I (New England Biolabs) according to the manufacturer’s instructions. The absence of contaminating DNA was further confirmed using classical PCR and 20-bp standard oligos. Next, cDNA were obtained using an iScript cDNA synthesis Kit (Bio-Rad). cDNA were amplified using iQ SYBR Green Supermix (Bio-Rad) and a set of primer pairs (Table 2). ACT1 expression was used as an internal control. Quantitative RT-PCR reactions were carried out in the CFX96 Bio-Rad Thermal Cycler and mRNA relative levels were calculated using Bio-Rad CFX Manager software. mRNA levels of wild-type cells were normalized to 1 for each gene and each condition tested.
Table 2. Oligonucleotides used for quantitative RT-PCR.
| forward ACT1 #440 | CTGCCGGTATTGACCAAACT |
| reverse ACT1 #441 | CGGTGATTTCCTTTTGCATT |
| forward DRE2 #478 | AATGGCCACTGAACCAAAAG |
| reverse DRE2 #479 | CATCGCTGGTATCCACTTGA |
| forward TAH18 #482 | GGTGTCGGTCTAGCACCATT |
| reverse TAH18 #483 | AATTTTGCCCTTACGGAACC |
| forward YAP1 #480 | ACACCAATCCCAGCCTACTG |
| reverse YAP1 #481 | GAATTGTCGTCTGGGAAAGC |
Western blotting
Protein extracts were prepared using TCA method68. About 50 μg of proteins were separated on a 4–12% gradient NuPAGE Novex Bis-Tris gel with MOPS SDS buffer (Life Technologies). After transfer to a nitrocellulose ECL membrane (Amersham), Dre2 and Tah18 were probed with a rabbit anti-Dre2 antibody designed against Dre2 1–133 N-terminus peptide16 or a rabbit anti-Tah18 antibody (1/2500, described below), and a fluorescent secondary anti-rabbit IgG (IRDye, LI-COR). Proteins were quantified using Odyssey infrared imaging system (LI-COR) using Act1 as an internal control. Act1 was probed with a goat anti-Act1 antibody (sc-1616, Santa Cruz Biotechnology), and a fluorescent secondary anti-goat (IRDye, LI-COR). Amounts of proteins were calculated as follow: [protein band intensity]/[Act1 intensity]. Amounts in wild-type cells were set as 1.
Antibody against Tah18
TAH18 1-600 (FMN binding domain) was subcloned into pET19b, and the plasmid obtained was transformed into E. coli BL21 strain. Overexpressed 6xHis-tagged, 1–200 N-terminus Tah18 peptide, was purified using NiNTA technology (Qiagen). Antisera were raised in rabbit (Agrobio Company, France) and tested for Tah18 detection by western blotting as shown in Supplementary Fig. 1.
Redox western blotting
Cell extracts were prepared as for classic western blot using the TCA method68 until the step of TCA removal from pelleted broken cells. From there, pellets were washed three times with acetone. Dry pellets were resuspended in 50 μl of TES buffer (100 mM Tris-Cl pH 8.8, 10 mM EDTA, 1% SDS) containing 50 mM N-Ethylmaleimide (NEM). Following 1 hour incubation at 30 °C with strong agitation in the dark, insoluble protein was removed by centrifugation at 13000 g for 10 min at room temperature. Each sample containing about 30 μg of protein was prepared with NuPAGE LDS sample buffer without reductant and was separated on a 12% NuPAGE Novex Bis-Tris gel with MOPS SDS buffer (Life Technologies). After transfer to a nitrocellulose membrane, oxidized and reduced forms of HyPer were probed with anti-GFP (A-11122, Life Technologies).
Isopropylmalate isomerase (Leu1) activity measurement
Leu1 activity was measured on semi-purified protein extract by successive ammonium sulphate precipitation following a method previously described by Kohlhaw et al.35. Cells were grown until mid-log phase and were harvested. Crude extracts were prepared in 50 mM phosphate buffer (pH 7.1) containing 50 mM NaCl and the 50% to 65% ammonium sulphate fraction was resuspended in 50 mM phosphate buffer (pH 7.1) containing 1.6 M ammonium sulphate and 30% glycerol. The artificial substrate citraconate was used to determine total Leu1 activity by monitoring consumption of citraconate at 235 nm using increasing amounts of protein. Protein concentrations were measured using a Quant-iT protein assay kit in a Qubit fluorimeter (Life Technologies). For evaluating direct effect of HU on Leu1 activity, semi-purified Leu1 was pre-incubated on ice for 60 min in presence of HU (1 M, 100 mM and 10 mM) or H2O2 (100 μM), before monitoring consumption of citraconate at 235 nm.
Flow cytometry for cell cycle
1 ml of yeast cells culture was spined down for 5 min at maximum speed (14000 rpm) and supernatant was removed. Cells were immediately resuspended in 1 ml 75% ethanol and kept overnight at 4 °C. Fixed cells were spined down and pellet was washed in 1 ml Tris 50 mM (pH 7.5) before resuspension in 1 ml Tris 50 mM (pH 7.5) containing RNase mg/ml (Sigma). Suspensions were incubated 4 hours at 37 °C, before resuspending cells in 500 μL PBS buffer containing propidium iodide 0.05 mg/ml. Cell suspension was sonicated for 5 sec at medium power, before diluting cells at 1/10th in Tris 50 mM (pH 7.5) and analysing samples on a Bekton Dickinson FACS Calibur. 50000 cells were counted for each acquisition.
Flow cytometry for ROS detection
2 ml of yeast cells culture treated for 45 min with HU 100 mM or HU 200 mM were spined down for 5 min at maximum speed (14000 rpm) and supernatant was removed. Cells were washed three times with 2 ml PBS, and lastly resuspended in 1 ml PBS containing DHE 50 μg/ml. Suspensions were gently agitated in the dark for 1 hour. Cells were then washed again three times with PBS. 20 μL of cell suspension was diluted in 1 ml PBS and samples were analyzed on a Bekton Dickinson FACS Calibur using FL1 channel. 50000 cells were counted for each acquisition.
Statistics
Unpaired one-tailed Student’s t-test was used for comparisons of protein levels in yeast strains (Fig. 1).
Additional Information
How to cite this article: Huang, M.-E. et al. DNA replication inhibitor hydroxyurea alters Fe-S centers by producing reactive oxygen species in vivo. Sci. Rep. 6, 29361; doi: 10.1038/srep29361 (2016).
Supplementary Material
Acknowledgments
We thank Dr Nicolas Soler for precious help in purifying the Tah18 peptide used for making polyclonal anti-Tah18 antibody. We are grateful to Sarah Flocan, Mirella Miredin and colleagues for their expertise in media preparation, and to members from the Huang’s lab, Gérard Faye, Roland Chanet, Elie Hatem and Amélie Heneman for helpful discussions and support during the course of study. We thank J.M. Camadro for yeast strain 21A5. This work was made possible by continuous financial support from INSERM, CNRS and the Institut Curie. This study was also supported by a 2-year grant from “La Fondation ARC” (No. PJA 20131200136). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication. L.V. is a research scientist at INSERM.
Footnotes
Author Contributions L.V. conceived the experiment(s), C.F., Z.F., D.B., S.B. and L.V. conducted the experiment(s), M.-E.H., C.F., Z.F., D.B., S.B. and L.V. analyzed the results, and M.-E.H. and L.V. wrote the manuscript. All authors reviewed the manuscript.
References
- Young C. W. & Hodas S. Hydroxyurea: Inhibitory Effect on DNA Metabolism. Science 146, 1172–1174 (1964). [DOI] [PubMed] [Google Scholar]
- Rosenkranz H. S., Garro A. J., Levy J. A. & Carr H. S. Studies with hydroxyurea. I. The reversible inhibition of bacterial DNA synthesis and the effect of hydroxyurea on the bactericidal action of streptomycin. Biochim Biophys Acta 114, 501–515 (1966). [PubMed] [Google Scholar]
- Timson J. Hydroxyurea. Mutat Res 32, 115–132 (1975). [DOI] [PubMed] [Google Scholar]
- Gale G. R. Effect of Hydroxyurea on the Incorporation of Thymidine into Ehrlich Ascites Tumor Cells. Biochem Pharmacol 13, 1377–1382 (1964). [DOI] [PubMed] [Google Scholar]
- Schwartz H. S., Garofalo M., Sternberg S. S. & Philips F. S. Hydroxyurea: inhibition of deoxyribonucleic acid synthesis in regenerating liver of rats. Cancer Res 25, 1867–1870 (1965). [PubMed] [Google Scholar]
- Krakoff I. H., Brown N. C. & Reichard P. Inhibition of ribonucleoside diphosphate reductase by hydroxyurea. Cancer Res 28, 1559–1565 (1968). [PubMed] [Google Scholar]
- Graslund A., Ehrenberg A. & Thelander L. Characterization of the free radical of mammalian ribonucleotide reductase. J Biol Chem 257, 5711–5715 (1982). [PubMed] [Google Scholar]
- Reichard P. & Ehrenberg A. Ribonucleotide reductase--a radical enzyme. Science 221, 514–519 (1983). [DOI] [PubMed] [Google Scholar]
- Ehrenberg A. & Reichard P. Electron spin resonance of the iron-containing protein B2 from ribonucleotide reductase. J Biol Chem 247, 3485–3488 (1972). [PubMed] [Google Scholar]
- Nordlund P., Sjoberg B. M. & Eklund H. Three-dimensional structure of the free radical protein of ribonucleotide reductase. Nature 345, 593–598 (1990). [DOI] [PubMed] [Google Scholar]
- Cokic V. P. et al. Hydroxyurea induces fetal hemoglobin by the nitric oxide-dependent activation of soluble guanylyl cyclase. J Clin Invest 111, 231–239 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juul T. et al. The in vivo toxicity of hydroxyurea depends on its direct target catalase. J Biol Chem 285, 21411–21415 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayashiki T. & Mori H. Genome-wide screening with hydroxyurea reveals a link between nonessential ribosomal proteins and reactive oxygen species production. J Bacteriol 195, 1226–1235 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogan N. J. et al. Global landscape of protein complexes in the yeast Saccharomyces cerevisiae. Nature 440, 637–643 (2006). [DOI] [PubMed] [Google Scholar]
- Yu H. et al. High-quality binary protein interaction map of the yeast interactome network. Science 322, 104–110 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernis L. et al. A newly identified essential complex, Dre2-Tah18, controls mitochondria integrity and cell death after oxidative stress in yeast. PLoS ONE 4, e4376 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler N. et al. Interaction between the reductase Tah18 and highly conserved Fe-S containing Dre2 C-terminus is essential for yeast viability. Mol Microbiol 82, 54–67 (2011). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. Dre2, a Conserved Eukaryotic Fe/S Cluster Protein, Functions in Cytosolic Fe/S Protein Biogenesis. Mol Cell Biol 28, 5569–5582 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz D. J. et al. Tah18 transfers electrons to Dre2 in cytosolic iron-sulfur protein biogenesis. Nat Chem Biol 6, 758–765 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang Y. et al. Conserved electron donor complex Dre2-Tah18 is required for ribonucleotide reductase metallocofactor assembly and DNA synthesis. Proc Natl Acad Sci USA 111, E1695–1704 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netz D. J. et al. Eukaryotic DNA polymerases require an iron-sulfur cluster for the formation of active complexes. Nat Chem Biol 8, 125–132 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanet R. & Heude M. Characterization of mutations that are synthetic lethal with pol3-13, a mutated allele of DNA polymerase delta in Saccharomyces cerevisiae. Curr Genet 43, 337–350 (2003). [DOI] [PubMed] [Google Scholar]
- Soler N. et al. A S-adenosylmethionine methyltransferase-like domain within the essential, Fe-S-containing yeast protein Dre2. Febs J 279, 2108–2119 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moye-Rowley W. S., Harshman K. D. & Parker C. S. YAP1 encodes a yeast homolog of mammalian transcription factor AP-1. Cold Spring Harb Symp Quant Biol 53 Pt 2, 711–717 (1988). [DOI] [PubMed] [Google Scholar]
- Moye-Rowley W. S., Harshman K. D. & Parker C. S. Yeast YAP1 encodes a novel form of the jun family of transcriptional activator proteins. Genes Dev 3, 283–292 (1989). [DOI] [PubMed] [Google Scholar]
- Temple M. D., Perrone G. G. & Dawes I. W. Complex cellular responses to reactive oxygen species. Trends Cell Biol 15, 319–326 (2005). [DOI] [PubMed] [Google Scholar]
- Lee J. et al. Yap1 and Skn7 control two specialized oxidative stress response regulons in yeast. J Biol Chem 274, 16040–16046 (1999). [DOI] [PubMed] [Google Scholar]
- Brombacher K., Fischer B. B., Rufenacht K. & Eggen R. I. The role of Yap1p and Skn7p-mediated oxidative stress response in the defence of Saccharomyces cerevisiae against singlet oxygen. Yeast 23, 741–750 (2006). [DOI] [PubMed] [Google Scholar]
- Kuge S., Jones N. & Nomoto A. Regulation of yAP-1 nuclear localization in response to oxidative stress. EMBO J 16, 1710–1720 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge S., Toda T., Iizuka N. & Nomoto A. Crm1 (XpoI) dependent nuclear export of the budding yeast transcription factor yAP-1 is sensitive to oxidative stress. Genes Cells 3, 521–532 (1998). [DOI] [PubMed] [Google Scholar]
- Gulshan K., Thommandru B. & Moye-Rowley W. S. Proteolytic degradation of the Yap1 transcription factor is regulated by subcellular localization and the E3 ubiquitin ligase Not4. J Biol Chem 287, 26796–26805 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izawa S. et al. Thioredoxin deficiency causes the constitutive activation of Yap1, an AP-1-like transcription factor in Saccharomyces cerevisiae. J Biol Chem 274, 28459–28465 (1999). [DOI] [PubMed] [Google Scholar]
- Carmel-Harel O. et al. Role of thioredoxin reductase in the Yap1p-dependent response to oxidative stress in Saccharomyces cerevisiae. Mol Microbiol 39, 595–605 (2001). [DOI] [PubMed] [Google Scholar]
- Ikner A. & Shiozaki K. Yeast signaling pathways in the oxidative stress response. Mutat Res 569, 13–27 (2005). [DOI] [PubMed] [Google Scholar]
- Kohlhaw G. B. Isopropylmalate dehydratase from yeast. Methods Enzymol 166, 423–429 (1988). [DOI] [PubMed] [Google Scholar]
- Elford H. L. Effect of hydroxyurea on ribonucleotide reductase. Biochem Biophys Res Commun 33, 129–135 (1968). [DOI] [PubMed] [Google Scholar]
- Larsen I. K., Sjoberg B. M. & Thelander L. Characterization of the active site of ribonucleotide reductase of Escherichia coli, bacteriophage T4 and mammalian cells by inhibition studies with hydroxyurea analogues. Eur J Biochem 125, 75–81 (1982). [DOI] [PubMed] [Google Scholar]
- Atta M. et al. Escherichia coli and herpes-simplex-virus ribonucleotide reductase R2 subunit. Compared reactivities of the redox centres. Biochem J 290 (Pt 3), 807–810 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang S. & Imlay J. A. Micromolar intracellular hydrogen peroxide disrupts metabolism by damaging iron-sulfur enzymes. J Biol Chem 282, 929–937 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer A. J. & Dick T. P. Fluorescent protein-based redox probes. Antioxid Redox Signal 13, 621–650 (2010). [DOI] [PubMed] [Google Scholar]
- Belousov V. V. et al. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat Methods 3, 281–286 (2006). [DOI] [PubMed] [Google Scholar]
- Dardalhon M. et al. Redox-sensitive YFP sensors monitor dynamic nuclear and cytosolic glutathione redox changes. Free Radic Biol Med 52, 2254–2265 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge S. & Jones N. YAP1 dependent activation of TRX2 is essential for the response of Saccharomyces cerevisiae to oxidative stress by hydroperoxides. EMBO J 13, 655–664 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint D. H., Tuminello J. F. & Emptage M. H. The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J Biol Chem 268, 22369–22376 (1993). [PubMed] [Google Scholar]
- Jang S. & Imlay J. A. Hydrogen peroxide inactivates the Escherichia coli Isc iron-sulphur assembly system, and OxyR induces the Suf system to compensate. Mol Microbiol 78, 1448–1467 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinerfeld D. et al. Endogenous mitochondrial oxidative stress: neurodegeneration, proteomic analysis, specific respiratory chain defects, and efficacious antioxidant therapy in superoxide dismutase 2 null mice. J Neurochem 88, 657–667 (2004). [DOI] [PubMed] [Google Scholar]
- Grant C. M., Perrone G. & Dawes I. W. Glutathione and catalase provide overlapping defenses for protection against hydrogen peroxide in the yeast Saccharomyces cerevisiae. Biochem Biophys Res Commun 253, 893–898 (1998). [DOI] [PubMed] [Google Scholar]
- Turrens J. F. Mitochondrial formation of reactive oxygen species. J Physiol 552, 335–344 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. et al. In vivo production of nitric oxide in rats after administration of hydroxyurea. Mol Pharmacol 52, 1081–1086 (1997). [DOI] [PubMed] [Google Scholar]
- Macomber L. & Imlay J. A. The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity. Proc Natl Acad Sci USA 106, 8344–8349 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F. F. & Imlay J. A. Silver(I), mercury(II), cadmium(II), and zinc(II) target exposed enzymic iron-sulfur clusters when they toxify Escherichia coli. Appl Environ Microbiol 78, 3614–3621 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstantinou E., Pashalidis I., Kolnagou A. & Kontoghiorghes G. J. Interactions of hydroxycarbamide (hydroxyurea) with iron and copper: implications on toxicity and therapeutic strategies. Hemoglobin 35, 237–246 (2011). [DOI] [PubMed] [Google Scholar]
- Kujundžic N., Nigovic B. & Sankovic K. i. Reaction of Hydroxyurea with Iron(III): Products and the Stoichiometry of the Redox Reaction. Zeitschrift für anorganische und allgemeine Chemie 630, 2749–2753 (2004). [Google Scholar]
- Nigovic B., Kujundzic N. & Sankovic K. Electron transfer in N-hydroxyurea complexes with iron(III). Eur J Med Chem 40, 51–55 (2005). [DOI] [PubMed] [Google Scholar]
- Italia K., Colah R. & Ghosh K. Hydroxyurea could be a good clinically relevant iron chelator. PLoS One 8, e82928 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubacq C. et al. Role of the iron mobilization and oxidative stress regulons in the genomic response of yeast to hydroxyurea. Mol Genet Genomics 275, 114–124 (2006). [DOI] [PubMed] [Google Scholar]
- Shakoury-Elizeh M. et al. Transcriptional remodeling in response to iron deprivation in Saccharomyces cerevisiae. Mol Biol Cell 15, 1233–1243 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies B. W. et al. Hydroxyurea induces hydroxyl radical-mediated cell death in Escherichia coli. Mol Cell 36, 845–860 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koc A., Wheeler L. J., Mathews C. K. & Merrill G. F. Hydroxyurea arrests DNA replication by a mechanism that preserves basal dNTP pools. J Biol Chem 279, 223–230 (2004). [DOI] [PubMed] [Google Scholar]
- Bianchi V., Pontis E. & Reichard P. Changes of deoxyribonucleoside triphosphate pools induced by hydroxyurea and their relation to DNA synthesis. J Biol Chem 261, 16037–16042 (1986). [PubMed] [Google Scholar]
- Pugh R. A. et al. The iron-containing domain is essential in Rad3 helicases for coupling of ATP hydrolysis to DNA translocation and for targeting the helicase to the single-stranded DNA-double-stranded DNA junction. J Biol Chem 283, 1732–1743 (2008). [DOI] [PubMed] [Google Scholar]
- Klinge S., Hirst J., Maman J. D., Krude T. & Pellegrini L. An iron-sulfur domain of the eukaryotic primase is essential for RNA primer synthesis. Nat Struct Mol Biol 14, 875–877 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner B. E. et al. An iron-sulfur cluster in the C-terminal domain of the p58 subunit of human DNA primase. J Biol Chem 282, 33444–33451 (2007). [DOI] [PubMed] [Google Scholar]
- Pokharel S. & Campbell J. L. Cross talk between the nuclease and helicase activities of Dna2: role of an essential iron-sulfur cluster domain. Nucleic Acids Res 40, 7821–7830 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolf J., Makrantoni V., Ingledew W. J., Stark M. J. & White M. F. The DNA repair helicases XPD and FancJ have essential iron-sulfur domains. Mol Cell 23, 801–808 (2006). [DOI] [PubMed] [Google Scholar]
- Sikorski R. S. & Hieter P. A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collart M. A. & Oliviero S. Preparation of yeast RNA. Curr Protoc Mol Biol Chapter 13, Unit13 12 (2001). [DOI] [PubMed] [Google Scholar]
- Foiani M., Marini F., Gamba D., Lucchini G. & Plevani P. The B subunit of the DNA polymerase alpha-primase complex in Saccharomyces cerevisiae executes an essential function at the initial stage of DNA replication. Mol Cell Biol 14, 923–933 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dix D., Bridgham J., Broderius M. & Eide D. Characterization of the FET4 protein of yeast. Evidence for a direct role in the transport of iron. J Biol Chem 272, 11770–11777 (1997). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.