Abstract
Spatially distributed sequential stimulation (SDSS) has demonstrated substantial power output and fatigue benefits compared to single electrode stimulation (SES) in the application of functional electrical stimulation (FES). This asymmetric electrode setup brings new possibilities but also new questions since precise placement of the electrodes is one critical factor for good muscle activation. The aim of this study was to compare the power output, fatigue and activation properties of proximally versus distally placed SDSS electrodes in an isokinetic knee extension task simulating knee movement during recumbent cycling. M. vastus lateralis and medialis of seven able-bodied subjects were stimulated with rectangular bi-phasic pulses of constant amplitude of 40 mA and at an SDSS frequency of 35 Hz for 6 min on both legs with both setups (i.e. n=14). Torque was measured during knee-extension movement by a dynamometer at an angular velocity of 110 deg/s. Mean power, peak power and activation time were calculated and compared for the initial and final stimulation phases, together with an overall fatigue index. Power output values (Pmean, Ppeak) were scaled to a standardised reference input pulse width of 100 μs (Pmean,s, Ppeak,s). The initial evaluation phase showed no significant differences between the two setups for all outcome measures. Ppeak and Ppeak,s were both significantly higher in the final phase for the distal setup (25.4 ± 8.1 W vs. 28.2 ± 6.2 W, p=0.0062 and 34.8 ± 9.5 W vs. 38.9 ± 6.7 W, p=0.021, respectively). With distal SDSS, there was modest evidence of higher Pmean and Pmean,s (p=0.071, p=0.14, respectively) but of longer activation time (p=0.096). The rate of fatigue was similar for both setups. For practical FES applications, distal placement of the SDSS electrodes is preferable.
Key Words: functional electrical stimulation, spatially distributed sequential stimulation, knee dynamometer, fes cycling
Volitionally-activated healthy muscles can maintain a moderate constant power output in an isometric or dynamic cyclical task without fatiguing over time. This is possible through selective, non-synchronous motor unit recruitment and activation rates that are continuously adjusted by the central nervous system to maintain precisely the targeted force level.1-5 Artificially-activated muscles, in contrast, have low fatigue resistance, which limits the long-term power output of a muscle during isometric or dynamic tasks.6 Current FES technology employs a relatively crude approach to muscle stimulation. The muscular power output is mainly increased by modulating the intensity of the stimulation parameters, such as higher frequency, higher amplitude or longer pulse width.7-9 Unlickely, increasing power is strongly linked with increased fatigue. A general problem is that motor units of different types are recruited synchronously in a non-selective manner.10 Due to the spatially fixed electrodes, a higher stimulation intensity can compensate fatigue only with a limited number of new fibres.11 Previous studies have shown reductions in the rate of fatigue through variation of frequency by a simple frequency reduction or with initial frequency bursts (i.e. doublets). Nevertheless, decreased overall power output was observed with either sustained or repeated contractions, which in the end is a critical factor for the applicability of FES.12-15 These problems are fundamental for muscle activation through electrical stimulation since electrodes are spatially fixed and the activation of the same fibres results in a drop in force output when they become fatigued.11,16
Nguyen et al.17 addressed this by replacing one large electrode with four smaller ones. Stimulation frequency was reduced from 40Hz to 10Hz per electrode and a small time shift was implemented between the electrodes (spatially distributed sequential stimulation, SDSS). The total stimulation frequency thus remained at 40Hz. This temporally and spatially distributed stimulation gave better fatigue resistance, more physiological muscle activation in EMG recordings, and higher power output in dynamic knee extension tasks (Laubacher 2016, unpublished data). This asymmetric electrode setup brings new possibilities but also new questions since precise placement of the electrodes is one critical factor for good muscle activation.18-20 Active electrodes are usually placed on the motor point. But, with SDSS electrodes, none of the four electrodes is exactly on this crucial point. This raises the question: what happens when the four electrodes are used as reference electrodes and positioned proximally?
The aim of this study was to compare the power output, fatigue and activation properties of proximally versus distally placed SDSS electrodes in an isokinetic knee extension task simulating knee movement during recumbent cycling.
Materials and Methods
Subjects
Seven able-bodied male subjects (age 30.7 ± 4.2 yrs; height 178.9 ± 10.2 cm; mass 73.9 ± 12.2 kg, mean ± SD) participated in this study. None of the subjects had any known history of neurological or musculoskeletal problems. Each participant gave written informed consent. The study was approved by the local ethics committee (Ethics committee of the Swiss Canton of Bern, KEK Bern).
Device
The knee dynamometer is a custom made measurement device, which moves one leg at a constant velocity and measures the isokinetic torque produced during stimulated knee extension. The lower leg is fixed with a brace to an aluminium load cell (LCB130, ME-Meßsysteme GmbH, Germany), which moves, via a lever arm, a chain drive system connected to a magnetostrictive torque sensor (S-2220-75, NCTE AG Germany). The torque sensor and the load cell are used to bi-directionally measure the effective torque on the gauge bar in real time. A brushless motor (EC45, 250 W, Maxon Motor AG, Switzerland) is used with a planetary gear head (Gear Ratio: 156:1, GP42, Maxon Motor AG, Switzerland). The actuator can generate a maximum continuous torque of 90 Nm. A position sensor (Vert-X 28, Contelec Gmbh, Switzerland) is used for angle measurement with a resolution of 0.648 deg to control the motor torque.
The measurements were performed at a constant angular velocity of ~110 °/s, which is equivalent to a cycling cadence of 50 rpm, and the range of motion was set from 45° to 130° knee extension (where 180° is full extension).
Stimulation
The electrical pulses were generated with an eight channel stimulator (RehaStim, Hasomed GmbH, Germany). Self-adhesive active electrodes (Pals Platinum, Axelgaard Mfg. Co., LTD, USA) were placed on the motor points of the m. quadriceps lateralis and medialis and dispersive electrodes were placed 10 – 15 cm proximal to the corresponding muscle motor point (Fig. 1). The skin was cleaned and the body hair shaved at the position of the electrodes. Muscle motor points were detected for each stimulated muscle prior to measurement with a stimulation pen (Motor Point Pen, Compex, Switzerland). Subjects were stimulated with rectangular bi-phasic pulses at a constant amplitude of 40 mA. Current was applied using an SDSS electrode setup, which consists of four small electrodes with a surface area of 4.5 × 2.5 cm each and one large electrode (9 × 5 cm) with the same total area. Each of the four small electrodes used a frequency of 8.75 Hz and a phase shift of 90 °, which corresponds to an overall stimulation frequency of 35 Hz. The pulse width was individually set for each subject during a familiarization. Two different SDSS electrode placements were investigated: distal versus proximal SDSS electrodes. In both cases the active part was placed on the previously detected motor point.
Fig 1.
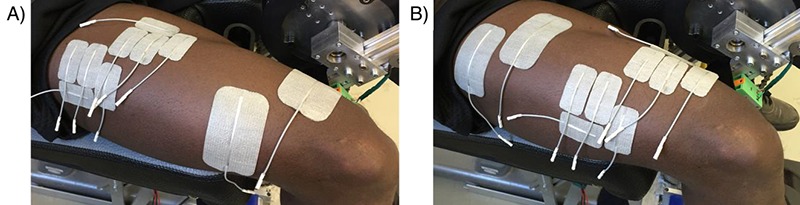
The right leg of a subject with A) proximal SDSS and B) distal SDSS electrode setups.
The mean pulse widths were 73.3 ± 14.2 μs for the proximal and 73.3 ± 14.4 μs for the distal SDSS setup.
Procedure
Each subject participated in two sessions with only one electrode placement tested for each leg within each session. Between the two measurements in each session, subjects had a break of 15 minutes. Stimulation setup order and the leg were chosen randomly. Before each measurement a familiarization was conducted. Subjects were placed on the dynamometer and individual adjustments to body proportions were made. Then a two-minute phase was started in which the measured leg was moved by the device without stimulation (non-stimulation [ns] phase). Then the pulse width was manually increased after every third extension, starting at 0 μs. Pulse width was increased up to the subjects’ pain threshold or up to the point they were no longer able to stay relaxed. 80 % of this maximal pulse width (PWmax) was then used for the test measurements.
After a rest period of 10 minutes, the measurement started with an ns-phase of two minutes followed by a stimulation phase (st-phase) of 6 minutes. Finally, there was a second two-minute ns-phase. Range of motion and speed were equal for the ns and st-phases. Each session was conducted on a different day with at least one day of rest in between. Electrode positions were marked to ensure identical placement each day
Outcomes and statistical analysis
Only the extension phase of joint motion was evaluated, as the setup was to simulate cycling motion. The measured torque (τ), together with the angular speed was used to calculate the instantaneous output power (Pm). The power used to move the leg during the ns-phase was denoted as Pns. The net effective power output of one stimulation cycle is thus Pstim = Pns – Pm. For every knee extension the following outcomes were calculated: (a) mean power output during one extension (Pmean), (b) peak power output (Ppeak) and (c) the time from onset of the stimulation to 80% of Ppeak (tpeak80). To compare the different stimulation setups, Pstim was scaled using a reference pulse width of 100 μs (Pstim,s), e.g. subject A had a pulse width of 80 μs, so the scaled mean power output Pmean,s of that subject is Pmean * (100/80) and the scaled peak power output Ppeak,s is Ppeak * (100/80).
All outcomes were calculated for the initial 15 knee extensions and for the final 15 knee extensions. A fatigue index (FI) describes the percentage reduction in Pmean from the initial phase (Pinit) to the final phase (Pfinal): FI=1-(Pinit-Pfinal)/Pinit. The higher the value, the higher the fatigue resistance; FI=1 means no fatigue.
Each leg delivered one dataset for each electrode setup. The data were tested for normality using the Shapiro-Wilk-Test and then a paired t-test for normally distributed data and a Wilcoxon test for non-normal data was applied to test differences of means. The significance level was set at α = 0.05 for all tests. Statistical analyses were carried out using the Matlab
Results
Figure 2 shows the development of Pmean,s, Ppeak,s and tpeak80 over the 6-minute stimulation phase. The corresponding outcome measures for the initial and final stimulation phases are summarised in Tab. 1. No significant differences between distal and proximal electrode placement were found for any outcome measures during the initial stimulation phase. In the final stimulation phase, Ppeak and Ppeak,s showed significantly higher values for the distal SDSS setup: 25.4 ± 8.1 W vs. 28.2 ± 6.2 W, p=0.0062 and 34.8 ± 9.5 W vs. 38.9 ± 6.7 W, p=0.021, respectively. In the final phase, there was modest evidence of higher Pmean (Fig. 3) and Pmean,s with the distal SDSS placement (11.8 ± 3.8 W vs. 12.7 ± 3.3 W, p=0.071 and 16.2 ± 4.5 W vs. 17.4 ± 3.4 W, p=0.14), and of longer tpeak80 for distal SDSS (347.6 ± 29.2 ms vs. 359.4 ± 38.2 ms, p=0.096). The modestly higher mean power output in the final stage with distal SDSS, and a lower dispersion of power values, can be conveniently visualised (Fig. 3). Fatigue resistance was not different between the two stimulation setups (FI 0.61 ± 0.14 vs. 0.64 ± 0.9, p=0.38).
Fig 2.
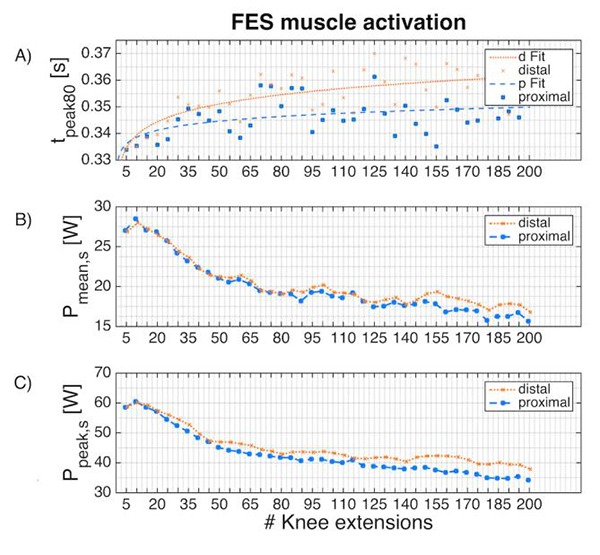
Each point represents the mean of all subjects. (A) Activation time to 80% peak power output per extension, including a power curve fitting (f(x)=axb, RMSE = 0.0064 for proximal placement and RMSE = 0.0062 for distal placement). (B) Scaled mean power output (Pmean,s) and (C) scaled peak power output (Ppeak,s) per extension during the 6-minute stimulation phase.
Table 1.
Outcome Measures for paired comparisons and p-values for comparisons of means
| Phase | Parameter | Mean ± SD | MD (95% CI) | p-Value | ||
|---|---|---|---|---|---|---|
| proximal SDSS | distal SDSS | |||||
| initial | Pmean [W] | 19.4 ± 4.8 | 19.9 ± 4.8 | -0.43 (-2.73,1.87) | 0.69 | |
| Pmean,s [W] | 27.5 ± 8.6 | 27.4 ± 4.7 | 0.16 (-3.38,3.70) | 0.92 | ||
| Ppeak [W] | 41.6 ± 10.2 | 42.9 ± 7.9 | -1.29 (-6.67,4.09) | 0.61 | ||
| Ppeak,s [W] | 59.2 ± 20.9 | 59.4 ± 8.9 | -0.16 (-9.44,9.13) | 0.97 | ||
| tpeak80 [ms] | 336.1 ± 38.3 | 335.8 ± 34.8 | 0.28 (-20.00,20.50) | 0.98 | ||
| final | Pmean [W] | 11.8 ± 3.8 | 12.7 ± 3.3 | -0.91 (-1.88,0.08) | 0.071 | |
| Pmean,s [W] | 16.2 ± 4.5 | 17.4 ± 3.4 | -1.23 (-2.92,0.45) | 0.14 | ||
| Ppeak [W] | 25.4 ± 8.1 | 28.2 ± 6.2 | -2.81 (-4.65,-0.98) | 0.0062 | ||
| Ppeak,s [W] | 34.8 ± 9.5 | 38.9 ± 6.7 | -4.08 (-7.43,-0.73) | 0.021 | ||
| tpeak80 [ms] | 347.6 ± 29.2 | 359.4 ± 38.2 | -11.80 (-26.00,2.50) | 0.096 | ||
| Fatigue Index | 0.61 ± 0.14 | 0.64 ± 0.09 | -0.03 (-0.11,0.04) | 0.38 | ||
| Pulse width [μs] | 73.3 ± 14.2 | 73.3 ± 14.4 | 0.00 (-2.34,2.34) | 1.00 | ||
| SDSS: Spatially Distributed Sequential Stimulation, MD: Mean Difference, SD: Standard Deviation, CI: Confidence Interval | ||||||
Fig 3.
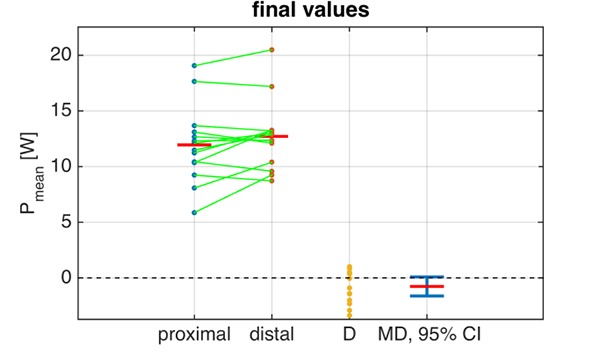
Data samples for Pmean for the final stimulation phase for both setups; the green lines link the sample pairs from each subject; the red bars depict mean values. D is the difference between the paired samples. MD is the mean difference (red bar) with its 95% confidence interval (CI). Inclusion of the value 0 within the 95 % CI signifies a non-significant difference between the means; this conforms with p>0.05 (cf. Tab. 1)
Discussion
The aim of this study was to compare the power output, fatigue and activation properties of proximally versus distally placed SDSS electrodes in an isokinetic knee extension task simulating knee movement during recumbent cycling.
Power output. Overall, especially in the final phase of stimulation, the distal SDSS setup showed higher power outputs (the only exception was Pmean,s in the initial phase, which was minimally lower for distal SDSS). This might be regarded as surprising, since the active electrode was placed exactly on the motor point in the proximal SDSS setup.
Splitting one large electrode into four small ones of the same overall size, and using a sequential stimulation strategy, was previously shown to give increased power output and better fatigue resistance compared to a standard electrode setup. The temporal and spatial shift had a significant impact on muscle activation in a dynamic knee extension task.17-21
Using a standard electrode setup (SES), it should not make a difference which electrode (active, dispersive) is positioned at the motor point: the electrical field is the same and the current direction should not activate the muscle fibres differently or change the number of recruited motor units. On the other hand, the electrical field changes with the size of the electrodes,22-24 and the distance between the active and dispersive electrodes has a substantial influence on the torque.25 To control for these factors in the two setups investigated in the present study, the distance was not changed and the active electrode was placed distally. That no substantial or significant difference was observed for any power output parameter in the initial phase of the task may indicate that both setups recruited and activated a similar number of motor units.26
The similar development over time of Pmean and Ppeak, as well as the different development of tpeak80 (Fig. 2A) between setups, shows that the muscle fibre recruitment and the power curve of a single extension is not the same over time. Although FI was not significantly different, the examination of power development over time (Fig. 2B, C) shows that distal placement seems to have a slower power decrease and a higher power output in the final stimulation phase. Smaller electrodes increase the current density compared to larger electrodes using the same amplitude and pulse width.23,24 Thus, the non-equal size of the electrodes (active, dispersive) leads to an asymmetric electrical field, which seems to influence muscle activation. In contrast to the distal placement, where four small active electrodes are placed around the motor point, in the proximal setup one large active electrode is placed exactly on the motor point. In consequence, the change of the electrical field at this sensitive position is lower in this setup. Less change over time in the pattern of activation has been shown to favourably affect fatigue and power output development (13, 14).
Activation time. Activation time, i.e. the time from stimulation onset to 80% of peak power, plays a crucial role when electrical stimulation is used to produce a functional movement. Usually, more than just one muscle group is involved, so that both the coordination of the force and the activation of the different muscles are of importance. In FES-cycling, often only three major muscle groups are involved (m. quadriceps, m. hamstrings and m. gluteus) and the coordination of these muscles is one factor for achievement of high power output.25
During repetitive activation, muscles not only fatigue, but their activation becomes more delayed.21 During the initial phase, no difference was observed in tpeak80 between the two electrode setups. tpeak80 for the proximal placement starts to flatten out after about 30 extensions, whereas tpeak80 for the distal placement is still increasing at this time and only begins to flatten out after about 65 extensions (Fig. 2A). The mean difference for tpeak80 of 11.8 ms in the final phase corresponds to a phase shift of 6° when cycling 50 rpm, which might have an influence on the overall cycling performance.27,28 By positioning the electrodes more precisely in relation to the motor points (proximal setup), the activation becomes more efficient and the muscle activation time is less affected by the duration of the task.21
In conclusion, the SDSS approach to muscle stimulation seems to provide substantial performance benefits, but the placement of the electrodes is still a crucial factor. Distal placement of the SDSS electrodes showed higher power output values in the final stimulation phase but also a slightly increased activation time. The development of new array electrodes, specifically for SDSS, where the initial pulse is applied directly on the motor point and the following pulses are randomly distributed, may combine the positive effects of the proximal and distal electrode placements. Based on the evidence presented here, for practical FES applications, distal placement of the SDSS electrodes appears to be preferable.
Acknowledgement
The authors thank Prof. Dr. Robert Riener of ETH Zurich for contributions to the protocol development.
Research supported by the Swiss National Science Foundation (SNF-Nr.: 320030_150128/1)
Contributor Information
Efe A. Aksöz, Email: efeanil.aksoez@bfh.ch.
Stuart Binder-Macleod, Email: sbinder@udel.edu.
Kenneth J. Hunt, Email: kenneth.hunt@bfh.ch.
References
- 1.Adam A, De Luca CJ. Recruitment order of motor units in human vastus lateralis muscle is maintained during fatiguing contractions. J Neurophysiol. 2003;90(5):2919-27. [DOI] [PubMed] [Google Scholar]
- 2.Carpentier A, Duchateau J, Hainaut K. Motor unit behaviour and contractile changes during fatigue in the human first dorsal interosseus. J Physiol. 2001;534(Pt 3):903-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Contessa P, Adam A, De Luca CJ. Motor unit control and force fluctuation during fatigue. J Appl Physiol (1985). 2009;107(1):235-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maladen RD, Perumal R, Wexler AS, Binder-Macleod SA. Effects of activation pattern on nonisometric human skeletal muscle performance. J Appl Physiol (1985). 2007;102(5):1985-91. [DOI] [PubMed] [Google Scholar]
- 5.Heckman CJ, Enoka RM. Motor unit. Compr Physiol. 2012;2(4):2629-82. [DOI] [PubMed] [Google Scholar]
- 6.Doucet BM, Lam A, Griffin L. Neuromuscular electrical stimulation for skeletal muscle function. Yale J Biol Med. 2012;85(2):201-15. [PMC free article] [PubMed] [Google Scholar]
- 7.Baldwin ER, Klakowicz PM, Collins DF. Wide-pulse-width, high-frequency neuromuscular stimulation: implications for functional electrical stimulation. J Appl Physiol (1985). 2006;101(1):228-40. [DOI] [PubMed] [Google Scholar]
- 8.Gorgey AS, Mahoney E, Kendall T, Dudley GA. Effects of neuromuscular electrical stimulation parameters on specific tension. Eur J Appl Physiol. 2006;97(6):737-44. [DOI] [PubMed] [Google Scholar]
- 9.Gregory CM, Dixon W, Bickel CS. Impact of varying pulse frequency and duration on muscle torque production and fatigue. Muscle Nerve. 2007;35(4):504-9. [DOI] [PubMed] [Google Scholar]
- 10.Jubeau M, Gondin J, Martin A, Sartorio A, Maffiuletti NA. Random motor unit activation by electrostimulation. Int J Sports Med. 2007;28(11):901-4. [DOI] [PubMed] [Google Scholar]
- 11.Bickel CS, Gregory CM, Dean JC. Motor unit recruitment during neuromuscular electrical stimulation: a critical appraisal. Eur J Appl Physiol. 2011;111(10):2399-407. [DOI] [PubMed] [Google Scholar]
- 12.Binder-Macleod SA, Guerin T. Preservation of force output through progressive reduction of stimulation frequency in human quadriceps femoris muscle. Phys Ther. 1990;70(10):619-25. [DOI] [PubMed] [Google Scholar]
- 13.Chou LW, Binder-Macleod SA. The effects of stimulation frequency and fatigue on the force-intensity relationship for human skeletal muscle. Clin Neurophysiol. 2007;118(6):1387-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kesar T, Chou L-W, Binder-Macleod SA. Effects of stimulation frequency versus pulse duration modulation on muscle fatigue. J Electromyogr Kinesiol. 2008;18(4):662-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorgey AS, Black CD, Elder CP, Dudley GA. Effects of electrical stimulation parameters on fatigue in skeletal muscle. J Orthop Sports Phys Ther. 2009;39(9):684-92. [DOI] [PubMed] [Google Scholar]
- 16.Keller T, Lawrence M, Kuhn A, Morari M. New multi-channel transcutaneous electrical stimulation technology for rehabilitation. Conf Proc IEEE Eng Med Biol Soc. 2006;1:194-7. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen R, Masani K, Micera S, Morari M, Popovic MR. Spatially distributed sequential stimulation reduces fatigue in paralyzed triceps surae muscles: a case study. Artif Organs. 2011;35(12):1174-80. [DOI] [PubMed] [Google Scholar]
- 18.Maffiuletti NA. Physiological and methodological considerations for the use of neuromuscular electrical stimulation. Eur J Appl Physiol. 2010;110(2):223-34. [DOI] [PubMed] [Google Scholar]
- 19.Gobbo M, Gaffurini P, Bissolotti L, Esposito F, Orizio C. Transcutaneous neuromuscular electrical stimulation: influence of electrode positioning and stimulus amplitude settings on muscle response. Eur J Appl Physiol. 2011;111(10):2451-9. [DOI] [PubMed] [Google Scholar]
- 20.Gobbo M, Maffiuletti NA, Orizio C, Minetto MA. Muscle motor point identification is essential for optimizing neuromuscular electrical stimulation use. J Neuroeng Rehabil. 2014;11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sayenko DG, Nguyen R, Popovic MR, Masani K. Reducing muscle fatigue during transcutaneous neuromuscular electrical stimulation by spatially and sequentially distributing electrical stimulation sources. Eur J Appl Physiol. 2014;114(4):793-804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alon G, Kantor G, Ho HS. Effects of electrode size on basic excitatory responses and on selected stimulus parameters. J Orthop Sports Phys Ther. 1994;20(1):29-35. [DOI] [PubMed] [Google Scholar]
- 23.Kuhn A, Keller T, Lawrence M, Morari M. The influence of electrode size on selectivity and comfort in transcutaneous electrical stimulation of the forearm. IEEE Trans Neural Syst Rehabil Eng. 2010;18(3):255-62. [DOI] [PubMed] [Google Scholar]
- 24.Livshitz LM, Mizrahi J, Einziger PD. Interaction of array of finite electrodes with layered biological tissue: effect of electrode size and configuration. IEEE Trans Neural Syst Rehabil Eng. 2001;9(4):355-61. [DOI] [PubMed] [Google Scholar]
- 25.Vieira TM, Potenza P, Gastaldi L, Botter A. Electrode position markedly affects knee torque in tetanic, stimulated contractions. Eur J Appl Physiol. 2016,116(2):335-42. [DOI] [PubMed] [Google Scholar]
- 26.Hodson-Tole EF, Wakeling JM. Motor unit recruitment for dynamic tasks: current understanding and future directions. J Comp Physiol B. 2009;179(1):57-66. [DOI] [PubMed] [Google Scholar]
- 27.Gföhler M, Lugner P. Dynamic simulation of FES-cycling: influence of individual parameters. IEEE Trans Neural Syst Rehabil Eng. 2004;12(4):398-405. [DOI] [PubMed] [Google Scholar]
- 28.Hunt KJ, Fang J, Saengsuwan J, Grob M, Laubacher M. On the efficiency of FES cycling: A framework and systematic review. Technol Health Care. 2012;20(5):395-422. [DOI] [PubMed] [Google Scholar]


