Abstract
Background: National Football League (NFL) players are exposed to multiple head collisions during their careers. Increasing awareness of the adverse long-term effects of repetitive head trauma has raised substantial concern among players, medical professionals, and the general public.
Objective: To determine whether low perfusion in specific brain regions on neuroimaging can accurately separate professional football players from healthy controls.
Method: A cohort of retired and current NFL players (n = 161) were recruited in a longitudinal study starting in 2009 with ongoing interval follow up. A healthy control group (n = 124) was separately recruited for comparison. Assessments included medical examinations, neuropsychological tests, and perfusion neuroimaging with single photon emission computed tomography (SPECT). Perfusion estimates of each scan were quantified using a standard atlas. We hypothesized that hypoperfusion particularly in the orbital frontal, anterior cingulate, anterior temporal, hippocampal, amygdala, insular, caudate, superior/mid occipital, and cerebellar sub-regions alone would reliably separate controls from NFL players. Cerebral perfusion differences were calculated using a one-way ANOVA and diagnostic separation was determined with discriminant and automatic linear regression predictive models.
Results: NFL players showed lower cerebral perfusion on average (p < 0.01) in 36 brain regions. The discriminant analysis subsequently distinguished NFL players from controls with 90% sensitivity, 86% specificity, and 94% accuracy (95% CI 95-99). Automatic linear modeling achieved similar results. Inclusion of age and clinical co-morbidities did not improve diagnostic classification.
Conclusion: Specific brain regions commonly damaged in traumatic brain injury show abnormally low perfusion on SPECT in professional NFL players. These same regions alone can distinguish this group from healthy subjects with high diagnostic accuracy. This study carries implications for the neurological safety of NFL players.
Keywords: Brain imaging, cognition, concussion, football, NFL, SPECT, traumatic brain injury
INTRODUCTION
The current public dialogue regarding the possible risks of repetitive head trauma playing football is not unlike the sport itself, with intense exchanges of opposing perspectives [1]. The genesis of this recent debate was the discovery of neuropsychiatric symptoms linked to a distinct neuropathological entity in former National Football League (NFL) players, chronic traumatic encephalopathy (CTE) [2, 3]. Noticeably lacking in this dialogue are assertions weighted in data from living professional football players. Several small studies have described structural and functional brain abnormalities in this group. One study of 28 former NFL players found lower hippocampal volumes in those with a history of concussion leading to loss of consciousness compared to 27 controls [4]. This same cohort was found to have white matter tract abnormalities on diffusion tensor imaging correlating to increased symptoms of depression [5]. A separate functional MRI study of 54 retired NFL players compared to 53 controls found hypoconnectivity and hyperactivation in the frontal lobes suggestive of executive dysfunction [6].
Other studies have shown increased structural abnormalities [7] and neuropsychiatric deficits in NFL players. A study of 30 retired players found a lifetime history of concussions correlated to increased depressive symptoms on the Beck Depression Inventory II test [8]. In a larger group of 1,044 former players surveyed, the increased risk of depression persists at least up to 9 years after retirement [9] and this association was independent of declining physical health. Currently, there is a relative lack of data on i) What regions are neurophysiologically impaired in living NFL players compared to healthy controls; ii) How well these brain abnormalities distinguish possible repetitive concussive and subconcussive pathology in NFL players from healthy individuals; and iii) What specific brain areas are most predictive of such a classification. The purpose of this work is to address these questions in a large NFL cohort with functional imaging data and predictive data analytics. We specifically hypothesize that areas of abnormally low cerebral perfusion on single photon emission computed tomography (SPECT) imaging in NFL players compared to controls will reliably separate these groups with high accuracy.
METHODS
Study participants
Starting in 2009, we recruited a cohort of retired and current NFL players (n = 161; mean age = 52 ± 14.2 years) as described in prior work [10] along with a control group of healthy individuals (n = 124; mean age = 44 ± 16.6 years, M:F 44%:56%). Players were recruited from 27 teams and all positions (Integ Review IRB Certificate Number: 004). All recruitment was done in accordance with Institutional Review Board approval. Inclusion criteria were being on an active NLF roster for a minimum of 1 year. Exclusion criteria were any subjects that could not discontinue psychoactive medications for an appropriate period prior to functional neuroimaging. Each study participant was interviewed by a board certified psychiatrist, completed a 315 intake questionnaire of mental and physical health, and was given either the Microcog Assessment of Cognitive Functioning [11] or WebNeuro [12] computerized neuropsychological battery. Concussion history from high school through NFL play, including loss of consciousness, was obtained from each study participant. Concussions were defined using the Center for Disease Control and Prevention (CDC) [13] criteria “conditions of temporarily altered mental status as a result of head trauma” that may or may not involve a loss of consciousness.
Functional perfusion neuroimaging
All subjects (n = 285) received functional perfusion neuroimaging with SPECT as described in previous studies [14, 15]. Briefly, each participant received an appropriate dose of technetium-99 m hexamethylpropyleneamine (HMPAO) intravenously. The injection was done in normal room lighting while each subject performed the Connors’ Continuous Performance Test II [16]. Regional cerebral blood flow was modeled in 128 regions using a standard neuroanatomical atlas [17] as previously described [14, 15]. Age, gender, and race corrections were done in the analyses.
Predictive data analytics
All statistical analyses were performed in Statistical Package for Social Science (SPSS, version 23, IBM, Armonk, NY) and were controlled for both age and gender. To identify specific brain regions we hypothesize are predictive in distinguishing NFL players from controls, a one-way ANOVA was conducted to identify regions of low perfusion in NFL players compared to controls. In all, 36 regions out of the 128 tested met our cutoff level for statistical significance (p < 0.01). These regions were then used to classify NFL players from controls with two separate predictive data analytic methods. The first was discriminant analysis using a leave-one-out cross validation [18] followed by extraction of predicted probabilities from this model. These probabilities were then used to determine diagnostic sensitivity, specificity, and accuracy from input into receiver operating characteristic (ROC) and Area Under the Curve (AUC) analyses. The second approach was the automatic linear modeling (ALM) approach in SPSS [19] done to verify the diagnostic accuracy metrics obtained from the discriminant analysis and to provide a ranking of the most predictive features from the 36 brain regions initially selected for the analysis.
RESULTS
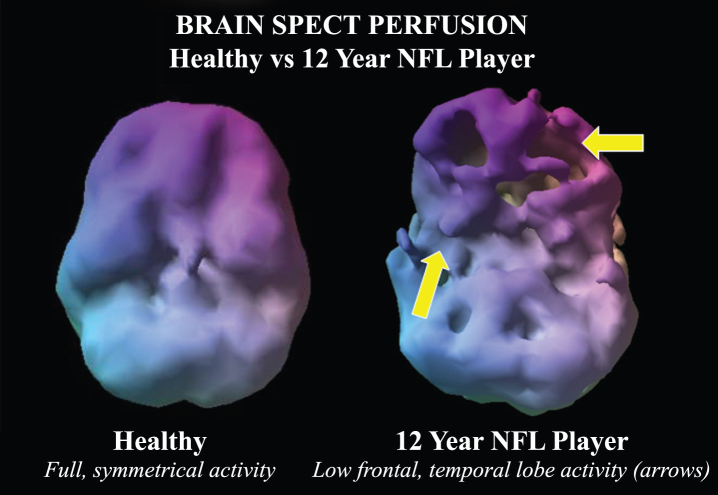
All NFL players were male, while 56% of the control group were women. The control group was 56% Caucasian, while the NFL group was 53%. In the NFL group, baseline neuropsychological assessments showed 92% of players had decreased general cognitive proficiency, 86% had decreased information processing speed, 83% had memory loss, 83% had attentional deficits, and 85% had executive function impairment. The most common psychiatric co-morbidities in the NFL players were depression (29%), anxiety (22%), and attention deficit hyperactivity disorder (ADHD) (13%). In the NFL cohort, 3% had a history of substance abuse. In total, 36 brain regions were found to have abnormally low blood flow on functional perfusion neuroimaging on ANOVA (p < 0.01, average F-value 29.51 ± 16.76. These regions are listed in Table 1. Figure 1 shows a visual 3-D rendering of a SPECT scan of a 12-year NFL player compared to a control subject.
Table 1.
Brain regions with hypoperfusion in NFL players compared to healthy controls
| Brain Area | F-value | p-value |
| Amygdala Left | 26.676 | 0.0000 |
| Amygdala Right | 24.279 | 0.0000 |
| Cingulum Ant Left | 38.716 | 0.0000 |
| Cingulum Ant Right | 14.611 | 0.0000 |
| Cingulum Mid Left | 8.101 | 0.0050 |
| Cingulum Post Right | 13.308 | 0.0000 |
| Caudate Left | 16.417 | 0.0000 |
| Caudate Right | 12.437 | 0.0000 |
| Frontal Inferior Opercular Left | 33.461 | 0.0000 |
| Frontal Inferior Opercular Right | 23.21 | 0.0000 |
| Frontal Inferior Orbital Left | 22.678 | 0.0000 |
| Frontal Inferior Orbital Right | 25.082 | 0.0000 |
| Hippocampus Left | 9.739 | 0.0020 |
| Hippocampus Right | 17.07 | 0.0000 |
| Insula Left | 22.26 | 0.0000 |
| Insula Right | 59.181 | 0.0000 |
| Paracentral Lobule Left | 29.503 | 0.0000 |
| Paracentral Lobule Right | 26.7 | 0.0000 |
| Postcentral Left | 26.032 | 0.0000 |
| Postcentral Right | 19.48 | 0.0000 |
| Precuneus Left | 40.399 | 0.0000 |
| Precuneus Right | 40.882 | 0.0000 |
| Rectus Left | 8.556 | 0.0040 |
| Rectus Right | 14.49 | 0.0000 |
| Temporal Pole Sup Left | 62.102 | 0.0000 |
| Temporal Pole Sup Right | 51.886 | 0.0000 |
| Temporal Sup Ant Left | 50.16 | 0.0000 |
| Temporal Sup Ant Right | 70.902 | 0.0000 |
| Vermis 10 | 37.222 | 0.0000 |
| Vermis 45 | 7.501 | 0.0070 |
| Cerebellum 10 Left | 17.694 | 0.0000 |
| Cerebellum 10 Right | 31.926 | 0.0000 |
| Rolandic Opercular Left | 40.464 | 0.0000 |
| Rolandic Opercular Right | 60.956 | 0.0000 |
| Olfactory Left | 19.853 | 0.0000 |
| Olfactory Right | 38.418 | 0.0000 |
Fig.1.
Example of a volume rendered of the undersurface of the brain in normal control compared to a 12-year NFL player. While the control subject has symmetric activity, the NFL player has multiple defects in the inferior frontal and temporal lobes suggestive of concussion related hypoperfusion.
Predictive modeling
The discriminant analysis yielded a correct classification rate of 87% with the leave-one-out cross validation at 82%. The corresponding accuracy of the linear discriminant probabilities in identifying NFL players from controls was 94.4% based upon AUC analysis with 90% sensitivity and 86% specificity. The automatic linear modeling analysis produced essentially identical results with 95% accuracy (95% CI: 92.6-97.6), 90% sensitivity and 87% specificity. The diagnostic metrics of these two models do not change when adding demographic co-variates such as age, gender, and race or co-morbidities such as depression or ADHD. Based upon the automated linear modeling, the most important regions in predicting NFL players from controls were the i) anterior superior temporal lobes, ii) rolandic operculum, iii) insula, iv) superior temporal poles, v) precuneus, and vi) cerebellar vermis. The accuracy estimates from these models did not change when accounting for age, gender, or co-morbidities such as anxiety, depression, or ADHD by including them as co-variates in both discriminant and automatic linear regression models. Additionally, the results of these predictive models were not significantly different when female subjects were entirely excluded from the analysis. For example, in the discriminant analysis, the accuracy was 92%, sensitivity was 86%, and specificity was 81%. The correct classification was 86% and 80% with a leave-one-out validation with females completely excluded from the analysis.
DISCUSSION
Former and current NFL players have lower cerebral perfusion and impaired neuropsychological function compared to healthy controls. The regional perfusion abnormalities alone reliably separate these individuals from controls with high accuracy in two separate predictive analytic models. The result of our study advances the literature in living NFL players by characterizing regional abnormalities and their ability to distinguish players from controls in the largest known imaging study of this population. The main caveats to our data are its retrospective design and lack of NFL players with no traumatic brain injury (TBI) to serve as an NFL control group thus necessitating our use of a non-NFL control group.
Playing professional football has been correlated with alterations on structural and molecular neuroimaging [20] and our study adds several key insights. First results of low perfusion in the frontal and temporal lobes match what is known from a prior systematic review of the literature identifying the most common abnormalities on SPECT in mild TBI [21]. Second, involvement of the hippocampus and precuneus suggest neural correlates for poor memory function in our NFL subjects and increased risk for dementia [22]. Abnormally low flow in the insula is consistent with literature correlating perfusion decreases in this structure with depression, a common co-morbidity in our study [23]. Low flow in the cerebellar vermis is relevant as mild TBI involving this structure in diffusion tensor imaging is related to anxiety, also a common co-morbidity in our population [24]. From a network perspective, the abnormal brain regions in this study are most commonly found in the default mode network and frontal-parietal network, both of which are implicated in Alzheimer’s disease and psychiatric disorders [25]. We had previously used default mode network regions to separate veterans with histories of TBI from healthy controls with 94% accuracy [15]. Also of note is the correlation between the functional abnormalities seen in our cohort and the in vivo characterization of CTE on PET tau imaging with increased deposits in frontal regions, amygdala, and anterior cingulate [26, 27].
There are several implications of our study to consider. In this and other studies, it is becoming increasingly suggestive that playing football at the professional level is associated with brain abnormalities in players. Longitudinal studies are required at the pre-teen, high school, and college level to better characterize the time course of such alternations.
DISCLOSURE STATEMENT
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-0207r1).
REFERENCES
- [1]. Robbins L, Conidi F (2013) Stop football … save brains: A point counterpoint discussion. Headache 53, 817–823. [DOI] [PubMed] [Google Scholar]
- [2]. Omalu BI, DeKosky ST, Minster RL, Kamboh MI, Hamilton RL, Wecht CH (2005) Chronic traumatic encephalopathy in a National Football League player. Neurosurgery 57, 128–134; discussion 128–134. [DOI] [PubMed] [Google Scholar]
- [3]. Omalu BI, DeKosky ST, Hamilton RL, Minster RL, Kamboh MI, Shakir AM, Wecht CH (2006) Chronic traumatic encephalopathy in a national football league player: Part II. Neurosurgery 59, 1086–1092; discussion 1092–1083. [DOI] [PubMed] [Google Scholar]
- [4]. Strain JF, Womack KB, Didehbani N, Spence JS, Conover H, Hart J Jr, Kraut MA, Cullum CM (2015) Imaging correlates of memory and concussion history in retired national football league athletes. JAMA Neurol 72, 773–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Strain J, Didehbani N, Cullum CM, Mansinghani S, Conover H, Kraut MA, Hart J Jr, Womack KB (2013) Depressive symptoms and white matter dysfunction in retired NFL players with concussion history. Neurology 81, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6]. Hampshire A, MacDonald A, Owen AM (2013) Hypoconnectivity and hyperfrontality in retired American football players. Sci Rep 3, 2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Koerte IK, Hufschmidt J, Muehlmann M, Tripodis Y, Stamm JM, Pasternak O, Giwerc MY, Coleman MJ, Baugh CM, Fritts NG, Heinen F, Lin A, Stern RA, Shenton ME (2016) Cavum septi pellucidi in symptomatic former professional football players. J Neurotrauma 33, 346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Didehbani N, Munro Cullum C, Mansinghani S, Conover H, Hart J Jr (2013) Depressive symptoms and concussions in aging retired NFL players. Arch Clin Neuropsychol 28, 418–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Kerr ZY, Marshall SW, Harding HP Jr, Guskiewicz KM (2012) Nine-year risk of depression diagnosis increases with increasing self-reported concussions in retired professional football players. Am J Sports Med 40, 2206–2212. [DOI] [PubMed] [Google Scholar]
- [10]. Amen DG, Newberg A, Thatcher R, Jin Y, Wu J, Keator D, Willeumier K (2011) Impact of playing American professional football on long-term brain function. J Neuropsychiatry Clin Neurosci 23, 98–106. [DOI] [PubMed] [Google Scholar]
- [11]. Raymond PD, Hinton-Bayre AD, Radel M, Ray MJ, Marsh NA (2006) Test-retest norms and reliable change indices for the MicroCog Battery in a healthy community population over 50 years of age. Clin Neuropsychol 20, 261–270. [DOI] [PubMed] [Google Scholar]
- [12]. Silverstein SM, Berten S, Olson P, Paul R, Willams LM, Cooper N, Gordon E (2007) Development and validation of a World-Wide-Web-based neurocognitive assessment battery: WebNeuro. Behav Res Methods 39, 940–949. [DOI] [PubMed] [Google Scholar]
- [13]. Centers for Disease Control and Prevention: Sports-Related Recurrent Brain Injuries—United States., http://www.cdc.gov/mmwr/preview/mmwrhtml/00046702.htm.
- [14]. Amen DG, Raji CA, Willeumier K, Taylor D, Tarzwell R, Newberg A, Henderson TA (2015) Functional neuroimaging distinguishes posttraumatic stress disorder from traumatic brain injury in focused and large community datasets. PLoS One 10, e0129659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Raji CA, Willeumier K, Taylor D, Tarzwell R, Newberg A, Henderson TA, Amen DG (2015) Functional neuroimaging with default mode network regions distinguishes PTSD from TBI in a military veteran population. Brain Imaging Behav 9, 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Conners’ CPT II V. 5, Conners, K, MHS, Toronto, Canada.
- [17]. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289. [DOI] [PubMed] [Google Scholar]
- [18]. Moller C, Pijnenburg YA, van der Flier WM, Versteeg A, Tijms B, de Munck JC, Hafkemeijer A, Rombouts SA, van der Grond J, van Swieten J, Dopper E, Scheltens P, Barkhof F, Vrenken H, Wink AM (2015) Alzheimer disease and behavioral variant frontotemporal dementia: Automatic classification based on cortical atrophy for single-subject diagnosis.. Radiology, doi: 10.1148/radiol.2015150220 [DOI] [PubMed] [Google Scholar]
- [19]. Yang H (2013) The case for being automatic: Introducing the automatic linear modeling (LINEAR) procedure in SPSS statistics. Mult Linear Regression Viewp 92, 27–37. [Google Scholar]
- [20]. Coughlin JM, Wang Y, Munro CA, Ma S, Yue C, Chen S, Airan R, Kim PK, Adams AV, Garcia C, Higgs C, Sair HI, Sawa A, Smith G, Lyketsos CG, Caffo B, Kassiou M, Guilarte TR, Pomper MG (2015) Neuroinflammation and brain atrophy in former NFL players: An in vivo multimodal imaging pilot study. Neurobiol Dis 74, 58–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Raji CA, Tarzwell R, Pavel D, Schneider H, Uszler M, Thornton J, van Lierop M, Cohen P, Amen DG, Henderson T (2014) Clinical utility of SPECT neuroimaging in the diagnosis and treatment of traumatic brain injury: A systematic review. PLoS One 9, e91088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Grothe MJ, Teipel SJ, Alzheimer’s Disease Neuroimaging I (2016) Spatial patterns of atrophy, hypometabolism, and amyloid deposition in Alzheimer’s disease correspond to dissociable functional brain networks. Hum Brain Mapp 37, 35–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Richieri R, Boyer L, Faget-Agius C, Farisse J, Mundler O, Lancon C, Guedj E (2015) Determinants of brain SPECT perfusion and connectivity in treatment-resistant depression. Psychiatry Res 231, 134–140. [DOI] [PubMed] [Google Scholar]
- [24]. Alhilali LM, Delic JA, Gumus S, Fakhran S (2015) Evaluation of white matter injury patterns underlying neuropsychiatric symptoms after mild traumatic brain injury. Radiology 277, 793–800. [DOI] [PubMed] [Google Scholar]
- [25]. Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE (2011) Functional network organization of the human brain. Neuron 72, 665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Barrio JR, Small GW, Wong KP, Huang SC, Liu J, Merrill DA, Giza CC, Fitzsimmons RP, Omalu B, Bailes J, Kepe V (2015) In vivo characterization of chronic traumatic encephalopathy using [F-18]FDDNP PET brain imaging. Proc Natl Acad Sci U S A 112, E2039–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Small GW, Kepe V, Siddarth P, Ercoli LM, Merrill DA, Donoghue N, Bookheimer SY, Martinez J, Omalu B, Bailes J, Barrio JR (2013) PET scanning of brain tau in retired national football league players: Preliminary findings. Am J Geriatr Psychiatry 21, 138–144. [DOI] [PubMed] [Google Scholar]