We observed high mortality and liver disease progression associated primarily driven by untreated HIV and chronic hepatitis C as well as alcohol use in a cohort of PWID in India. Interventions to reduce HIV and HCV burden are needed.
Keywords: hepatitis C virus, HIV/AIDS, liver stiffness, low- and middle-income settings, mortality
Abstract
Background. There are limited data on clinical outcomes of hepatitis C virus (HCV) infection from low- and middle-income countries. We characterize mortality and liver disease progression in a cohort of people who inject drugs (PWID) with high HCV burden.
Methods. In a cohort of PWID in Chennai, India, 851 persons were observed semiannually. Information on death was obtained through verbal autopsy and liver disease progression, which was defined as an incident liver stiffness measurement of ≥12.3 kPa if it was <12.3 at baseline. Poisson and Cox regression were used to identify factors associated with mortality and disease progression, respectively.
Results. At baseline, 36.9% of cases were infected with HCV, 16.7% were infected with human immunodeficiency virus (HIV), 71.6% had no or mild stiffness, 14.9% had moderate stiffness, and 13.5% had severe stiffness or cirrhosis. Mortality was significantly higher among those with moderate (mortality rate ratio [MRR] = 2.31) and severe stiffness (MRR = 4.86) at baseline, those with ongoing substance use, those who were HIV monoinfected and not on antiretroviral therapy (ART) (MRR = 6.59), and those who were HIV/HCV coinfected regardless of ART status (MRR for no ART = 5.34; MRR for ART = 4.51). Of those with no or mild stiffness, 25.9% and 6.4% had evidence of progression to moderate and severe stiffness or cirrhosis, respectively; 38.3% of those with moderate stiffness had evidence of progression to severe stiffness or cirrhosis. Factors associated with progression included age, alcohol use, body mass index, and chronic HCV infection.
Conclusions. We observed significant morbidity and mortality primarily driven by untreated HIV, HIV/HCV coinfection, and alcohol use. Even with improved access to HIV treatment, in the absence of HCV treatment, outcomes are unlikely to improve for HIV/HCV-coinfected persons.
Hepatitis C virus (HCV) infection affects 185 million persons worldwide, with the vast majority living in low- and middle-income countries (LMICs) [1]. Hepatitis C virus infection becomes persistent in approximately 75% of those infected and can lead to progressive fibrosis, cirrhosis, hepatocellular carcinoma, and premature mortality. Although newly available direct-acting antivirals have dramatically improved cure rates, especially in individuals with limited disease (>95%) [2–8], these will not be accessible to all immediately [9, 10].
Progression of HCV has been consistently associated with older age at infection, male sex, obesity, insulin resistance and diabetes, heavy alcohol consumption, hepatitis B virus (HBV) coinfection, and human immunodeficiency virus (HIV)-associated immunosuppression [11–13]. Other more inconsistently associated factors include HCV genotype, cigarette smoking, and cannabis smoking [13, 14]. However, most longitudinal studies have been conducted in high-income settings among white subjects [11–15]. Little data exist from LMICs and Asian subjects who have different comorbidities, coinfections, and environmental characteristics, all of which may accelerate liver fibrosis progression and/or complicate treatment. Understanding factors associated with disease progression and mortality will be critical for prioritizing treatment.
India has an estimated population prevalence of HCV of 1%–2% [16]. We have previously demonstrated high burden of HCV, HIV, and HBV infection in a cohort of current and former PWID in southern India [17, 18]. In this study, we characterize long-term outcomes of HCV and associated coinfections and comorbidities in this same cohort.
METHODS
Study Population
As previously described, the Chennai HIV, HCV and Eeral (Liver Disease) Study (CHHEERS) was established to characterize the burden and cofactors of liver disease among HIV- and HCV-infected PWID in Chennai, India [18]. In brief, we recruited a convenience sample of PWID through community outreach. Participants had to be ≥18 years old, provide informed consent, report a history of drug injection in the prior 5 years, and have no intention of migrating for 2 years. Between February 2012 and July 2015, 1324 participants were screened, 1062 were eligible, and 1042 were enrolled. In the latter cohort, a convenience sample of 860 (83%) were enrolled in longitudinal follow up, and 778 have thus far completed at least 1 follow-up visit (90.5%). Compared to those enrolled in follow up, those not enrolled were significantly less likely to be infected with HIV and HCV and more likely to report heavy alcohol consumption.
Study Procedures
Human immunodeficiency virus-negative and HIV-positive participants not on antiretroviral therapy (ART) were observed semiannually, and HIV-positive participants on ART had additional quarterly visits. At semiannual visits, participants underwent a blood draw and a questionnaire that collected information on sociodemographics, substance use, and HIV, HCV, and HBV testing and treatment history. A nurse collected information on medications, clinical history, and vital signs, and a physician looked for signs of liver disease, conducted anthropometric measurements, and performed a liver stiffness assessment. At baseline, participants also underwent liver ultrasound for steatosis and a fasting blood draw.
Assessments
Transient elastography was performed using FibroScan (EchoSens, Paris, France) [19]. In brief, this technology involves pulse-echo ultrasonography acquisitions, which measure the velocity of a shear wave propagated through the liver. Results are instantaneously received as a single, quantitative variable of liver stiffness measurement (LSM), reported in kilopascals (kPa). Three operators who were trained and certified by the manufacturer performed assessments with a single device. Examinations with ≥10 discrete valid measurements, success rate ≥60% (number of valid divided by the total number of measurements), and limited variability (interquartile range [IQR] of measures divided by the median value ≤30%) were considered valid [20]. Of the 4666 FibroScan assessments, 4568 (97.9%) were valid. Ultrasound was performed by a radiologist using a high-resolution B-mode ultrasonography system (Logic 400; GE, Milwaukee, WI) as previously described [18] and graded according to standard criteria into mild, moderate, and severe [21].
Human immunodeficiency virus serostatus was determined using double enzyme-linked immunosorbent assay (ELISA) testing (Murex HIV-1.2.O, Abbott Murex, UK and Vironostika HIV Uni-form II Ag/Ab, Biomérieux, The Netherlands). CD4+ count was estimated using the FlowCARE PLG CD4 (CD45-FITC/CD4-PE) assay (Beckman Coulter, Brea, California) and HIV-1-ribonucleic acid (RNA) with RealTime HIV-1 assay (Abbott Laboratories, Abbott Park, Illinois) in samples from HIV-positive participants. HCV antibody testing was performed using the Genedia HCV ELISA 3.0 (Green Cross Medical Science, Chungbuk, Korea). Hepatitis C virus RNA was ascertained using the RealTime HCV assay (Abbott Molecular Inc., Des Plaines, Illinois) in samples from HCV-positive participants. Chronic HBV infection was measured by presence of hepatitis B surface antigen (Hepanostika HBsAg Uniform II; Biomérieux, Zaltbommel, The Netherlands). Plasma glucose, triglycerides, total cholesterol, including high-density lipoprotein cholesterol and low-density lipoprotein cholesterol, were measured using an enzymatic methods (Olympus AU400; Olympus Diagnostica, Tokyo, Japan). Plasma insulin was measured by immunoassay (ELISA) using a Bioscience kit (Monobind kit; Monobind Inc., Lake Forest, California). Homeostasis model assessment-estimated insulin resistance score was calculated as (fasting plasma glucose [mmol/L] × fasting insulin [μU/mL]/22.5).
Mortality
Information on death and cause was obtained through reports from other participants and field workers who actively tracked participants within 1 month of a missed visit through locator information. Self-reported information from family or network members (verbal autopsy) was used to classify cause of death; no death registry is available in India. Cause was grouped into the following categories: acquired immune deficiency syndrome (AIDS) related, drug related, accidental, liver related, other chronic disease related, and nonspecific (cause not known).
Statistical Analysis
The mortality analysis included persons enrolled in longitudinal follow up who had a valid baseline LSM (n = 851). Mortality rates per 100 person-years (PY) were calculated. Person-time at risk was calculated between enrollment and either date of death or the last contact date. For participants with whom staff were able to maintain regular contact (even if visits were missed), this date was May 1, 2016 (analysis date). For participants who could not be contacted (n = 54, 6.3%), person-time at risk was time between enrollment and the last study visit. Characteristics of persons were compared by liver stiffness using Fisher's exact tests for categorical variables and Kruskal-Wallis tests for continuous variables. Persons were classified as either having no/mild stiffness (LSM < 8.5 kPA), moderate stiffness (8.5–12.3 kPA), or severe stiffness/cirrhosis (≥12.3 kPA) based on established cutoffs [22–24]. Factors associated with all-cause mortality were examined using Poisson regression. We considered fixed (eg, demographics, liver stiffness at baseline) and time-varying (HIV, HCV, drug, alcohol use, and liver stiffness) covariates. Factors were considered for multivariable models based on prior evidence and statistical associations in univariable models (P < .10). Variables were retained in final models if associated with either outcome at P < .05. Population attributable fractions (PAFs) were calculated for a simplified multivariate model [25].
To characterize liver disease progression among those with no/mild or moderate stiffness, we used survival analysis methods (n = 663). Persons were observed until they developed the outcome, severe stiffness/cirrhosis (≥12.3 kPA), or were censored at their last study visit. Sensitivity analyses were performed (1) removing the additional quarterly visits for HIV-positive persons on ART, (2) excluding LSM where a concomitant liver enzyme measurement was elevated >200 IU/L, and (3) restricting analysis to only individuals with no/mild stiffness at baseline and defining no/mild stiffness as having 2 LSM < 8.5 kPA. In previous studies, it has been demonstrated that having 2 consistent low measurements is associated with higher negative predictive value [23]. The purpose of this final analysis was to rule out misclassification of disease progression. Analyses were conducted in Stata, version 13.0 (StataCorp, College Station, Texas).
Ethical Clearances
This study was approved by the Johns Hopkins and YRGCARE Institutional Review Boards.
RESULTS
At baseline, the median age was 39. All patients were male, and 55.8% had primary school education or less. Although 94.5% of patients reported a history of injecting heroin, only 14.3% reported injection in the 6 months before enrollment. A total of 90.6% of patients reported a lifetime history of marijuana use, and 95.4% reported a history of cigarette smoking. At baseline, the median number of cigarettes smoked daily was 10; 34.6% reported drinking 7 or more drinks per day, and 53.6% had evidence of alcohol dependence on Alcohol Use Disorders Identification Test (AUDIT). One hundred forty-two (16.7%) patients were infected with HIV, 47.9% of whom were on ART. Three hundred fourteen (36.9%) patients were HCV antibody positive: 78.0% were chronically infected and 11 (3.4%) reported HCV treatment.
At baseline, 609 (71.6%) had no/mild stiffness, 127 (14.9%) had moderate stiffness, and 115 (13.5%) had severe stiffness/cirrhosis. Those with higher stiffness were older, had longer injection duration, were more likely to have a history of pharmaceutical drug injection, and consumed more alcohol per day (P < .05 for all) (Table 1). Those with severe stiffness/cirrhosis were significantly more likely to have chronic HCV and HIV/HCV coinfection (P < .0001). This group was also significantly more likely to have signs and/or symptoms of liver disease, elevated liver enzymes, insulin resistance, steatosis, and lower cholesterol levels.
Table 1.
Baseline Characteristics of Study Population by Liver Stiffness (n = 851)a
| Characteristics | No/Mild Stiffness (n = 609) | Moderate Stiffness (n = 127) | Severe Stiffness (n = 115) | P Valueb | Total |
|---|---|---|---|---|---|
| Median age | 37 (31–44) | 40 (34–45) | 44 (39–48) | <.001 | 39 (32–45) |
| Male | 609 (100) | 127 (100) | 115 (100) | — | 851 (100) |
| Education | |||||
| None or primary | 350 (57.5) | 67 (52.8) | 58 (50.4) | .503 | 475 (55.8) |
| Secondary | 95 (15.6) | 18 (14.2) | 16 (13.9) | 129 (15.2) | |
| High School | 103 (16.9) | 24 (18.9) | 26 (22.6) | 153 (18.0) | |
| Vocational/University/Graduate | 61 (10.0) | 18 (14.2) | 15 (13.0) | 94 (11.1) | |
| Median monthly income, rupees | 6000 (4000–9000) | 6000 (4000–8000) | 6000 (3000–10 000) | .409 | 6000 (4000–9000) |
| Injection duration (years) | 12 (7–19) | 15 (9–20) | 18 (13–23) | <.001 | 14 (8–20) |
| Lifetime drug injection | |||||
| Heroin | 576 (94.6) | 118 (92.9) | 110 (95.7) | .642 | 804 (94.5) |
| Stimulants | 3 (0.5) | 2 (1.6) | 0 (0) | .291 | 5 (0.6) |
| Buprenorphine | 342 (56.2) | 81 (63.8) | 93 (80.9) | <.001 | 516 (60.6) |
| Other prescription opiates | 129 (21.2) | 38 (29.9) | 37 (32.2) | .010 | 204 (24.0) |
| Sedatives | 270 (44.3) | 64 (50.4) | 71 (61.7) | .002 | 405 (47.6) |
| Injection drug use in prior 6 mo | 97 (15.9) | 15 (11.8) | 10 (8.7) | .089 | 122 (14.3) |
| Lifetime noninjection drug use | |||||
| Marijuana | |||||
| Heroin | 547 (89.8) | 116 (91.3) | 108 (93.9) | .41 | 771 (90.6) |
| Stimulants | 326 (53.5) | 75 (59.0) | 91 (79.1) | <.001 | 492 (57.8) |
| Buprenorphine | 111 (18.2) | 27 (21.3) | 33 (28.7) | .038 | 171 (20.1) |
| Other prescription opiates | 70 (11.5) | 11 (8.7) | 13 (11.3) | .696 | 94 (11.1) |
| Sedatives | 321 (52.7) | 72 (56.7) | 67 (58.3) | .444 | 460 (54.1) |
| Median cigarettes smoked daily | 8 (4–15) | 10 (4–12) | 10 (5–16) | .226 | 10 (4–15) |
| Drinks per day | |||||
| None | 96 (15.8) | 19 (15.0) | 14 (12.2) | .02 | 129 (15.2) |
| 1–4 | 156 (25.6) | 30 (23.6) | 34 (29.6) | 220 (25.9) | |
| 5–6 | 166 (27.3) | 23 (18.1) | 19 (16.5) | 208 (24.4) | |
| 7 or more | 191 (31.4) | 55 (43.3) | 48 (41.7) | 294 (34.6) | |
| AUDIT category | |||||
| No/mild alcohol use | 191 (31.4) | 33 (26.0) | 26 (22.6) | .089 | 250 (29.4) |
| Harmful/hazardous alcohol use | 110 (18.1) | 19 (15.0) | 16 (13.9) | 145 (17.0) | |
| Alcohol dependence | 308 (50.6) | 75 (59.0) | 73 (63.5) | 456 (53.6) | |
| HIV and treatment status | |||||
| Negative | 509 (83.6) | 104 (81.9) | 90 (78.3) | .131 | 703 (82.6) |
| Positive and no ART | 45 (7.4) | 16 (12.6) | 13 (11.3) | 74 (8.7) | |
| Positive on ART | 51 (8.4) | 6 (4.7) | 11 (9.6) | 68 (8.0) | |
| HIV infection/CD4 count (cells/mm3) | |||||
| HIV negative | 509 (83.6) | 104 (81.9) | 90 (78.3) | .047 | 703 (82.6) |
| HIV positive, ≥200 | 14 (2.3) | 2 (1.6) | 9 (7.8) | 25 (2.9) | |
| HIV positive, <200 | 82 (13.5) | 20 (15.8) | 15 (13.0) | 117 (13.8) | |
| HCV infection | |||||
| HCV antibody negative | 414 (68.0) | 73 (57.5) | 33 (28.7) | <.001 | 520 (61.1) |
| HCV antibody positive, RNA neg | 56 (9.2) | 5 (3.9) | 8 (7.0) | 69 (8.1) | |
| HCV antibody positive, RNA pos | 129 (21.2) | 47 (37.0) | 69 (60.0) | 245 (28.8) | |
| HIV/HCV infection | |||||
| HIV negative, HCV RNA negative | 418 (68.6) | 69 (54.3) | 37 (32.2) | <.001 | 524 (61.6) |
| HIV positive, HCV RNA negative | 49 (8.1) | 9 (7.1) | 4 (3.5) | 62 (7.3) | |
| HIV negative, HCV RNA positive | 83 (13.6) | 34 (26.8) | 50 (43.5) | 167 (19.6) | |
| HIV positive, HCV RNA positive | 46 (7.6) | 13 (10.2) | 19 (16.5) | 78 (9.2) | |
| Ever HCV treatmentc | 5 (2.6) | 2 (3.8) | 4 (4.9) | .569 | 11 (3.4) |
| Alternative medications for HIVd | 3 (3.1) | 0 (0) | 0 (0) | 1.000 | 3 (2.1) |
| Alternative medications for HCVc | 3 (1.6) | 2 (3.8) | 2 (2.5) | .429 | 7 (2.2) |
| HBsAg positive | 34 (5.6) | 12 (9.5) | 12 (10.4) | .061 | 58 (6.8) |
| History of tuberculosis | 99 (16.3) | 15 (11.8) | 23 (20.0) | .224 | 137 (16.1) |
| History of dengue | 4 (0.7) | 0 (0) | 0 (0) | 1.000 | 4 (0.5) |
| History of chikungunya | 47 (7.7) | 8 (6.3) | 10 (8.7) | .834 | 65 (7.6) |
| History of malaria | 63 (10.3) | 13 (10.2) | 14 (12.2) | .882 | 90 (10.6) |
| Signs or symptoms of liver disease | 84 (13.8) | 15 (11.8) | 35 (30.4) | <.001 | 134 (15.8) |
| Median body mass index, kg/m2 | 19.5 (17.7–22.5) | 20.9 (18.2–23.6) | 20.6 (18.4–24.6) | .007 | 19.8 (17.9–23.0) |
| Median ALT, mg/dL | 23 (15–39) | 42 (24–82) | 62 (31–103) | <.001 | 28 (16–54) |
| Median AST, mg/dL | 29 (23–47) | 61 (32–107) | 84 (56–157) | <.001 | 35 (24–71) |
| Median GGT, mg/DL | 32 (21–64) | 69 (35–197) | 176 (77–335) | <.001 | 40 (23–109) |
| Median platelet count | 221 (183–273) | 202 (171–243) | 158 (112–210) | <.001 | 213 (170–261) |
| Median total cholesterol, mg/dL | 170 (140–199) | 168 (138–203) | 151 (123–188) | .006 | 169 (138–198) |
| Median HOMA IR | 1.12 (0.60–2.11) | 1.33 (0.59–2.55) | 1.9 (0.72–4.15) | <.001 | 1.22 (0.59–2.42) |
| Steatosis | |||||
| Normal | 295 (48.4) | 44 (34.7) | 42 (36.5) | .006 | 381 (44.8) |
| Mild | 241 (39.6) | 59 (46.5) | 51 (44.4) | 351 (41.3) | |
| Moderate | 73 (12.0) | 24 (18.9) | 22 (19.1) | 119 (14.0) | |
Data are presented as n (%) or median (interquartile range); percentages may not add to 100% due to missing data.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUDIT, Alcohol Use Disorders Identification Test; GGT, gamma-glutamyl transferase; ART, antiretroviral therapy; HBsAg, surface antigen of the hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HOMA IR, homeostasis model assessment-estimated insulin resistance score; neg, negative; pos, positive; RNA, ribonucleic acid.
a 851 with valid FibroScan scores at baseline among those enrolled in longitudinal follow-up.
b Fisher's exact test for categorical variables and Kruskal–Wallis for continuous variables.
c Among HCV antibody positives.
d Among HIV antibody positives.
Mortality
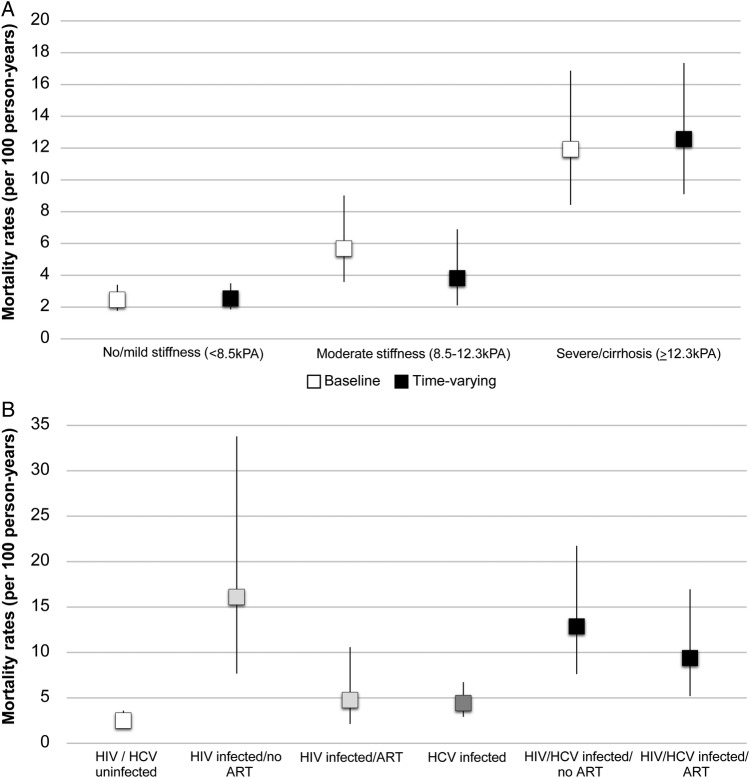
Across a median of 2.16 years (IQR, 1.33–3.53), 91 persons died (mortality rate, 4.36 per 100 PY; 95% confidence interval [CI], 3.55–5.35). Among the 91 deaths, cause of death could not be determined in 58 (63.7%) patients. Among the 33 patients with a cause determination, 10 (30.3%) died of a liver-related cause, 9 (27.3%) died of some other chronic condition, 3 (9.1%) died of an HIV/AIDS-related cause, 8 (24.2%) died of an ‘accidental' cause, and 3 (9.1%) died of a drug-related cause. Mortality was significantly higher among those with baseline severe stiffness/cirrhosis (11.93 per 100 PY vs 2.45 per 100 PY, P < .001; mortality rate ratio [MRR], 4.86; 95% CI, 3.02–7.82) (Figure 1) and moderate stiffness (5.68 per 100 PY; MRR, 2.31; 95% CI, 1.31–4.08) compared with those with no/mild stiffness. When considering time-varying liver stiffness, mortality was significantly higher among those with severe but not moderate stiffness (Table 2, Figure 1A).
Figure 1.
(A) Mortality rates per 100 person-years by liver stiffness at baseline and over follow-up. (B) Mortality rates per 100 person-years by human immunodeficiency virus (HIV), hepatitis C virus (HCV), and antiretroviral therapy (ART) status.
Table 2.
Correlates of Mortality Among People Who Inject Drugs in Chennai, India (n = 851)
| Characteristics | Unadjusted MRR (95% CI) | Multivariable Model 1: aMRR (95% CI) | Multivariable Model 2: aMRR (95% CI) |
|---|---|---|---|
| Age (scaled by 10 y) | 1.25 (.98–1.58) | 1.34 (1.02–1.75) | 1.17 (.87–1.56) |
| Education | |||
| None | REF | ||
| Primary school | 0.81 (.47–1.39) | ||
| Secondary school | 0.85 (.42–1.73) | ||
| High school | 0.78 (.42–1.45) | ||
| Vocational/University/Graduate school | 0.50 (.20–1.25) | — | — |
| Injection duration (scaled by 10 y) | 1.17 (.90–1.51) | — | — |
| Injection drug use in prior 6 mo | 2.71 (1.31–5.59) | 2.68 (1.28–5.62) | 3.26 (1.54–6.90) |
| AUDIT category | |||
| No/mild alcohol use | REF | REF | REF |
| Harmful/hazardous alcohol use | 1.07 (.63–1.81) | 1.44 (.82–2.54) | 1.41 (.78–2.52) |
| Alcohol dependence | 2.68 (1.68–4.30) | 3.33 (2.03–5.46) | 3.01 (1.80–5.03) |
| Hepatitis B antibody positive | 1.40 (.70–2.79) | — | — |
| HCV infection | 2.26 (1.46–3.50) | — | — |
| HCV infection, RNA | |||
| HCV antibody negative | REF | ||
| HCV antibody positive, RNA negative | 1.83 (.94–3.57) | ||
| HCV antibody positive, RNA positive | 2.37 (1.50–3.75) | — | — |
| HIV positive | 3.15 (2.08–4.77) | — | — |
| HIV infection/HIV RNA (copies/mL) | |||
| HIV negative | REF | ||
| HIV positive, undetectable | 0.63 (.15–2.60) | ||
| HIV positive, 40–1000 | 3.16 (1.43–6.95) | ||
| HIV positive, 1000–10 000 | 3.93 (1.69–9.14) | ||
| HIV positive, >10 000 | 4.39 (2.71–7.13) | — | — |
| HIV infection/CD4 count (cells/mm3) | |||
| HIV negative | REF | ||
| HIV positive, ≥200 | 2.30 (1.43–3.71) | ||
| HIV positive, <200 | 9.23 (5.12–16.7) | — | — |
| HIV infection and ART use/ HCV RNA | |||
| HIV negative, HCV RNA negative | REF | REF | REF |
| HIV positive not on ART, HCV RNA negative | 6.45 (2.83–14.7) | 6.59 (2.87–15.1) | 7.58 (3.29–17.5) |
| HIV positive on ART, HCV RNA negative | 1.91 (.79–4.59) | 2.15 (.87–5.35) | 2.15 (.85–5.41) |
| HIV negative, HCV RNA positive | 1.78 (1.02–3.09) | 1.76 (.99–3.10) | 0.87 (.46–1.68) |
| HIV positive not on ART, HCV RNA positive | 5.16 (2.73–9.76) | 5.34 (2.82–10.1) | 3.37 (1.69–6.69) |
| HIV positive on ART, HCV RNA positive | 3.76 (1.88–7.53) | 4.51 (2.20–9.25) | 2.70 (1.26–5.77) |
| Baseline liver stiffness | |||
| No/mild | REF | ||
| Moderate | 2.31 (1.31–4.08) | ||
| Severe | 4.86 (3.02–7.82) | — | — |
| Liver stiffness | |||
| No/mild | REF | REF | |
| Moderate | 1.50 (.77–2.94) | 1.34 (.67–2.68) | |
| Severe | 4.95 (3.15–7.78) | — | 5.46 (3.24–9.20) |
| Steatosis | |||
| Normal | REF | ||
| Mild | 0.95 (.60–1.49) | ||
| Moderate | 1.43 (.79–2.60) | — | — |
Abbreviations: aMRR, adjusted mortality ratio; AUDIT, Alcohol Use Disorders Identification Test; CI, confidence interval; ART, antiretroviral therapy; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MRR, mortality rate ratio; REF, reference; RNA, ribonucleic acid.
In univariable analysis, mortality was also associated with ongoing drug injection (MRR, 2.71; 95% CI, 1.31–5.59), alcohol dependence (MRR, 2.68; 95% CI, 1.68–4.30) (Table 2), HCV infection regardless of whether the persons had cleared (MRR, 2.26; 95% CI, 1.46–3.50) or were chronically infected (MRR, 2.37; 95% CI, 1.50–3.75), and HIV (MRR, 3.15; 95% CI, 2.08–4.77). Associations among chronic HCV, HIV, and mortality differed by whether an individual was on ART. Among those who were HIV negative, HCV infection was associated with higher mortality (MRR, 1.78; 95% CI, 1.02–3.09) (Table 2, Figure 1B). Being HIV positive and not on ART was associated with significant mortality risk that did not differ by whether a person was HCV coinfected (MRR, 5.16; 95% CI, 2.73–9.76) or not (MRR, 6.45; 95% CI, 2.83–14.7). However, among HIV-positive persons on ART, mortality was significantly increased among those who were HCV coinfected (MRR, 3.76; 95% CI, 1.88–7.53) but not those who were HIV monoinfected (MRR, 1.91; 95% CI, .79–4.59). All associations persisted in multivariable analysis (Table 2). Associations between chronic HCV infection and mortality were attenuated after adjustment for severe stiffness/cirrhosis, which remained an independent predictor (adjusted MRR, 5.46; 95% CI, 3.24–9.20). In a multivariate model that included independent effects of these factors (Table 3), the PAFs were as follows: active injection drug use (6.3%), alcohol use (32.5%), HIV (32.3%), and cirrhosis (35.3%). The association of HCV with mortality in this model was almost entirely explained by cirrhosis. In a model that did not include cirrhosis, the PAF for HCV was 21.3%.
Table 3.
Population Attributable Fractions (PAF) for Mortality
| Characteristics | Model 1 |
Model 2 |
||
|---|---|---|---|---|
| MRR (95% CI) | PAF | MRR (95% CI) | PAF | |
| Age (scaled by 10 y) | 1.10 (.83–1.46) | — | 1.29 (.99–1.69) | — |
| Alcohol usea | 2.31 (1.47–3.64) | 32.5% | 2.36 (1.53–3.64) | 33.0% |
| Injection drug use in prior 6 mo | 2.96 (1.42–6.17) | 6.3% | 2.64 (1.27–5.48) | 5.6% |
| Chronic HCV | 0.95 (.58–1.55) | −2.8% | 1.68 (1.08–2.60) | 21.3% |
| HIV positive | 3.75 (2.38–5.91) | 32.3% | 3.19 (2.05–4.99) | 29.3% |
| Cirrhosis | 5.02 (3.12–8.10) | 35.3% | — | — |
Abbreviations: AUDIT, Alcohol Use Disorders Identification Test; CI, confidence interval; HCV, hepatitis C virus; HIV, human immunodeficiency virus; MRR, mortality rate ratio.
a At least harmful/hazardous on AUDIT.
Liver Stiffness Progression
Over a median of 2.35 years, among 548 of those with baseline no/mild stiffness, 142 (25.9%) had at least 1 subsequent measurement consistent with moderate stiffness and 35 (6.4%) had 1 measurement consistent with severe stiffness/cirrhosis. Among 115 of those with baseline moderate stiffness, 44 (38.3%) had a subsequent measurement consistent with severe stiffness/cirrhosis. Among 101 of those with baseline severe stiffness/cirrhosis, the median maximal stiffness score over follow up was 26.3 kPA (IQR, 16.8–37.4 kPA). In univariable analysis, among 663 patients with no/mild or moderate stiffness at baseline, factors associated with progression to severe stiffness/cirrhosis included longer injection duration (hazard ratio [HR], 1.44; 95% CI, 1.14–1.82), longer cigarette smoking duration (HR, 1.35; 95% CI, 1.10–1.66), higher body mass index (BMI) (HR per 2 kg/m2, 1.15; 95% CI, 1.05–1.25), mild (HR vs none, 2.06; 95% CI, 1.25–3.39) and moderate steatosis (HR vs none, 2.24; 95% CI, 1.10–4.55), and chronic HCV (HR, 3.85; 95% CI, 2.40–6.19) (Table 4). Among those not HCV coinfected, HIV was not significantly associated with progression risk even among those not on ART (HR, 2.13; 95% CI, .52–8.68). Risk was elevated among those with chronic HCV and was comparable among those who were HIV uninfected (HR, 4.26; 95% CI, 2.50–7.26), HIV infected not on ART (HR, 3.95; 95% CI, 1.70–9.14), and HIV infected on ART (HR, 5.49; 95% CI, 2.74–11.0). The risk of progression also increased with higher baseline stiffness (HR, 1.61; 95% CI, 1.44–1.79). In multivariable adjustment, all associations persisted. Moreover, after adjustment, the association between alcohol dependence and cirrhosis risk became statistically significant (HR, 2.14; 95% CI, 1.22–3.75). These associations were attenuated after adjustment for baseline stiffness measures, and associations rendered with BMI and alcohol use were nonstatistically significant. Results were similar in all sensitivity analyses (Supplementary Tables).
Table 4.
Predictors of Progression to Severe Stiffness/Cirrhosis Among People Who Inject Drugs (PWID) in Chennai, India (n = 663)
| Characteristics | Unadjusted HR (95% CI) | Adjusted HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|
| Age (scaled by 10 y) | 1.33 (1.08–1.64) | 1.21 (.91–1.61) | 1.08 (.79–1.49) |
| AUDIT category | |||
| No/mild alcohol use | REF | REF | |
| Harmful/hazardous alcohol use | 0.68 (.38–1.20) | 0.98 (.50–1.91) | — |
| Alcohol dependence | 1.60 (.94–2.71) | 2.14 (1.22–3.75) | |
| Injection duration (scaled by 10 y) | 1.44 (1.14–1.82) | — | — |
| Injection drug use in prior 6 mo | 1.33 (.41–4.31) | — | — |
| Cigarette smoking duration (scaled by 10 y) | 1.35 (1.10–1.66) | — | — |
| Cigarette smoking in prior 6 mo | 0.88 (.54–1.44) | — | — |
| Used marijuana in prior 6 mo | 0.64 (.40–1.03) | — | — |
| HBsAg positive | 0.67 (.25–1.82) | — | — |
| HCV infection, RNA | |||
| HCV antibody negative | REF | ||
| HCV antibody positive, RNA negative | 0.54 (.16–1.83) | — | — |
| HCV antibody positive, RNA positive | 3.85 (2.40–6.19) | ||
| HIV infection/CD4 count (cells/mm3) | |||
| HIV negative | REF | ||
| HIV positive, ≥200 | 1.54 (.93–2.56) | — | — |
| HIV positive, <200 | 2.61 (.91–7.50) | ||
| HIV infection and ART use/ HCV RNA | |||
| HIV negative, HCV RNA negative | REF | REF | REF |
| HIV positive not on ART, HCV RNA negative | 2.13 (.52–8.68) | 2.21 (.51–9.53) | 1.44 (.32–6.49) |
| HIV positive on ART, HCV RNA negative | 1.15 (.35–3.80) | 1.11 (.30–4.03) | 1.04 (.28–3.88) |
| HIV negative, HCV RNA positive | 4.26 (2.50–7.26) | 3.83 (2.26–6.50) | 2.58 (1.46–4.56) |
| HIV positive not on ART, HCV RNA positive | 3.95 (1.70–9.14) | 4.49 (1.98–10.2) | 2.05 (.85–4.97) |
| HIV positive on ART, HCV RNA positive | 5.49 (2.74–11.0) | 6.28 (3.16–12.5) | 5.79 (2.67–12.6) |
| Baseline liver stiffness score (by 1 unit) | 1.61 (1.44–1.79) | — | 1.61 (1.43–1.81) |
| Body mass index (by 2 kg/m2) | 1.15 (1.05–1.25) | 1.18 (1.08–1.28) | — |
| HOMA IR > 2 | 1.52 (.96–2.39) | — | — |
| Steatosis | |||
| Normal | REF | ||
| Mild | 2.06 (1.25–3.39) | — | — |
| Moderate | 2.24 (1.10–4.55) | ||
Abbreviations: AUDIT, Alcohol Use Disorders Identification Test; ART, antiretroviral therapy; HBsAg, surface antigen of the hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HOMA IR, homeostasis model assessment-estimated insulin resistance score; HR, hazard ratio from Cox proportional hazards model; REF, reference; RNA, ribonucleic acid.
DISCUSSION
In this relatively young population with high HIV and HCV prevalence and substantial liver disease burden, we observed high mortality over a relatively short period of time. Moreover, in this same time span, a large proportion of individuals with mild stiffness appeared to experience at least some disease progression. We identified several modifiable and nonmodifiable factors associated with both mortality and disease progression reflecting a combination of behavioral factors and disease status.
Longitudinal cohorts characterizing clinical outcomes among PWID derive primarily from the United States and Western Europe. In a meta-analysis of 67 cohort studies of PWID, 14 of which were from LMICs [15], mortality was 2.35 per 100 PY and mortality among PWID was approximately 15 times higher than comparable general population settings. Cohorts from Asia demonstrated the highest mortality at 5.25 per 100 PY [15]. Although AIDS and drug overdose represented the primary drivers of mortality [15], non-AIDS mortality was higher among LMIC than non-LMIC settings; however, only 5 studies from LMICs had cause of death data [26]. In a previous study, we demonstrated that mortality among a cohort of PWID in Chennai, India from 2005 to 2008 was 4.25 per 100 PY [27]. In this prior cohort, mortality risk was overwhelmingly driven by HIV infection; those with a CD4 < 200 cells/mm3 had mortality rates that were 34.5 times higher than those who were HIV-uninfected.
Seven years later in a comparable cohort, mortality has not declined appreciably and drug use and HIV infection remain important drivers; however, we are now seeing increased mortality associated with HIV/HCV coinfection and alcohol use. Overall, 51.3% of those with a CD4 < 350 cells/mm3 (current eligibility threshold for India) reported using ART. Mortality risk for those on ART differed by whether individuals were HCV coinfected. On the one hand, those without HCV had comparable mortality rates with those who were HIV/HCV uninfected. On the other hand, those on ART with HCV coinfection had a >4-fold increased mortality risk—a risk that was comparable to HIV monoinfected persons not on ART. Efforts need to continue to focus on engaging and retaining PWID in HIV care through strategies such as patient navigators, incentives, and other supportive interventions. However, these data suggest that even if ART access improves, mortality will remain elevated among those with HIV/HCV coinfection in the absence of efforts to reduce HCV burden.
Some of this association between HIV/HCV and mortality was explained by higher prevalence of severe stiffness/cirrhosis. Not surprisingly, severe stiffness/cirrhosis was associated with higher mortality risk as was moderate stiffness at baseline, in part due to high risk of progression to severe stiffness/cirrhosis among this group. Moreover, hepatitis C treatment in this cohort was negligible. In India, as in many countries, access to curative therapies remains challenging. Sofosbuvir, ledipasvir, and daclatasvir are licensed in India, and generic versions are being produced by pharmaceutical companies. The cost of therapy, although lower than that in developed country settings (approximately $1000), is still too high for most. Although the Indian government provides free ART, there are no systems in place to provide free HCV therapy.
Hepatitis C virus treatment will be critical for those who have already progressed to severe stiffness/cirrhosis. However, the fact that even those with moderate stiffness had elevated mortality is concerning. Overall, 38% of those with moderate stiffness at baseline had evidence of progression to severe stiffness/cirrhosis, and 28% of those with mild stiffness at baseline had evidence of progression to moderate or severe stiffness/cirrhosis. There are few longitudinal data on liver stiffness, particularly from LMICs [28], but our data are suggestive of more rapid disease progression in some subgroups than what has been seen in developed settings. In studies among persons infected with HCV or HBV in Europe, progression ranged from 11% to 17.5% over 4–5 years [29], which is substantially lower than that found in our study. Although some of this progression might reflect misclassification of initial disease status, it still points to a substantial disease burden.
Aside from HIV and HCV, a key driver of adverse outcomes in this cohort was alcohol use. We have previously demonstrated declines in drug injection in Chennai with reciprocal increases in alcohol use [30]. The burden in this population is striking; 53.6% had evidence of dependence, and 34.6% reported drinking >7 drinks per day at baseline. Across follow up with repeated counseling on the harms of alcohol use, 41.9% of those with evidence of dependence at baseline reported some reduction in intake. However, relapse into dependency was not infrequent. At the most recent follow-up visit, 7.3% of patients reported alcohol dependence and 7.8% reported drinking more than 7 drinks a day. Additional efforts are clearly needed to promote sustained reductions in alcohol use particularly among those with HCV infection. Even with expanded HCV treatment, persons will still be at risk for adverse outcomes if they do not reduce alcohol intake.
Several limitations should be acknowledged. Approximately 10% of individuals enrolled in the longitudinal follow-up component of this study were lost, and there were differences in those lost with respect to HIV and HCV status and alcohol use, factors associated with mortality and disease progression. However, these rates (approximately 5% per year) are comparable to other PWID cohort studies, and there did not appear to be differences with respect to the stage of liver disease among those who were lost versus those who had some follow up. Misclassification of liver stiffness is also possible given that we did not have biopsies for confirmation and that we observed substantial progression particularly among those with moderate stiffness at baseline. However, sensitivity analyses that focused on those with no/mild stiffness at baseline revealed comparable results. FibroScan has been validated previously in India [24, 31, 32]; moreover, the strong associations between increasing stiffness and mortality represent further validation. We did not have information on HCV genotype infection, and although we have previously demonstrated a predominance of genotype 3 infection in a comparable cohort in India, it is unclear whether the genotype of infection is somehow related to progression [33]. Finally, we did not have complete data on cause of death because no death registries are available in India. Moreover, because there are limitations to using verbal autopsy to define cause, the data on cause of death should be interpreted with caution.
CONCLUSIONS
In conclusion, we observed significant mortality in this cohort of PWID that was primarily driven by untreated HIV, HIV/HCV coinfection, and alcohol use. Efforts are needed to engage PWID in HIV care and treatment. However, in the absence of HCV treatment, morbidity and mortality outcomes are unlikely to improve dramatically for persons coinfected with HIV and HCV.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank our staff and participants, without whom this research would not have been possible.
Financial support. This work was supported by the National Institutes of Health (grants R01DA026727 and T32AI102623) and the Johns Hopkins Center for AIDS Research (grant P30 AI094189).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology 2013; 57:1333–42. [DOI] [PubMed] [Google Scholar]
- 2.Sulkowski MS, Gardiner DF, Rodriguez-Torres M et al. . Daclatasvir plus sofosbuvir for previously treated or untreated chronic HCV infection. N Engl J Med 2014; 370:211–21. [DOI] [PubMed] [Google Scholar]
- 3.Afdhal N, Zeuzem S, Kwo P et al. . Ledipasvir and sofosbuvir for untreated HCV genotype 1 infection. N Engl J Med 2014; 370:1889–98. [DOI] [PubMed] [Google Scholar]
- 4.Lawitz E, Gane E, Pearlman B et al. . Efficacy and safety of 12 weeks versus 18 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin for hepatitis C virus genotype 1 infection in previously untreated patients with cirrhosis and patients with previous null response with or without cirrhosis (C-WORTHY): a randomised, open-label phase 2 trial. Lancet 2015; 385:1075–86. [DOI] [PubMed] [Google Scholar]
- 5.Sulkowski M, Hezode C, Gerstoft J et al. . Efficacy and safety of 8 weeks versus 12 weeks of treatment with grazoprevir (MK-5172) and elbasvir (MK-8742) with or without ribavirin in patients with hepatitis C virus genotype 1 mono-infection and HIV/hepatitis C virus co-infection (C-WORTHY): a randomised, open-label phase 2 trial. Lancet 2015; 385:1087–97. [DOI] [PubMed] [Google Scholar]
- 6.Curry MP, O'Leary JG, Bzowej N et al. . Sofosbuvir and velpatasvir for HCV in patients with decompensated cirrhosis. N Engl J Med 2015; 373:2618–28. [DOI] [PubMed] [Google Scholar]
- 7.Foster GR, Afdhal N, Roberts SK et al. . Sofosbuvir and velpatasvir for HCV genotype 2 and 3 infection. N Engl J Med 2015; 373:2608–17. [DOI] [PubMed] [Google Scholar]
- 8.Feld JJ, Jacobson IM, Hezode C et al. . Sofosbuvir and velpatasvir for HCV genotype 1, 2, 4, 5, and 6 infection. N Engl J Med 2015; 373:2599–607. [DOI] [PubMed] [Google Scholar]
- 9.Solomon SS, Mehta SH, Srikrishnan AK et al. . Burden of hepatitis C virus disease and access to hepatitis C virus services in people who inject drugs in India: a cross-sectional study. Lancet Infect Dis 2015; 15:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jayasekera CR, Barry M, Roberts LR, Nguyen MH. Treating hepatitis C in lower-income countries. N Engl J Med 2014; 370:1869–71. [DOI] [PubMed] [Google Scholar]
- 11.Poynard T, Bedossa P, Opolon P. Natural history of liver fibrosis progression in patients with chronic hepatitis C. The OBSVIRC, METAVIR, CLINIVIR, and DOSVIRC groups. Lancet 1997; 349:825–32. [DOI] [PubMed] [Google Scholar]
- 12.Alberti A. What are the comorbidities influencing the management of patients and the response to therapy in chronic hepatitis C? Liver Int 2009; 29(suppl 1):15–8. [DOI] [PubMed] [Google Scholar]
- 13.Rosen HR. Clinical practice. Chronic hepatitis C infection. N Engl J Med 2011; 364:2429–38. [DOI] [PubMed] [Google Scholar]
- 14.Mallat A, Hezode C, Lotersztajn S. Environmental factors as disease accelerators during chronic hepatitis C. J Hepatol 2008; 48:657–65. [DOI] [PubMed] [Google Scholar]
- 15.Mathers BM, Degenhardt L, Bucello C et al. . Mortality among people who inject drugs: a systematic review and meta-analysis. Bull World Health Organ 2013; 91:102–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sievert W, Altraif I, Razavi HA et al. . A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int 2011; 31(suppl 2):61–80. [DOI] [PubMed] [Google Scholar]
- 17.Solomon SS, Srikrishnan AK, Mehta SH et al. . High prevalence of HIV, HIV/hepatitis C virus coinfection, and risk behaviors among injection drug users in Chennai, India: a cause for concern. J Acquir Immune Defic Syndr 2008; 49:327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solomon SS, Srikrishnan AK, McFall AM et al. . Burden of liver disease among community-based people who inject drugs (PWID) in Chennai, India. PLoS One 2016; 11:e0147879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sandrin L, Fourquet B, Hasquenoph JM et al. . Transient elastography: a new noninvasive method for assessment of hepatic fibrosis. Ultrasound Med Biol 2003; 29:1705–13. [DOI] [PubMed] [Google Scholar]
- 20.Castera L, Foucher J, Bernard PH et al. . Pitfalls of liver stiffness measurement: a 5-year prospective study of 13,369 examinations. Hepatology 2010; 51:828–35. [DOI] [PubMed] [Google Scholar]
- 21.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986; 292:13–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirk GD, Astemborski J, Mehta SH et al. . Assessment of liver fibrosis by transient elastography in persons with hepatitis C virus infection or HIV-hepatitis C virus coinfection. Clin Infect Dis 2009; 48:963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta SH, Kirk GD, Astemborski J et al. . Stability of liver fibrosis among HCV-infected injection drug users. Antivir Ther 2012; 17:813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das K, Sarkar R, Ahmed SM et al. . “Normal” liver stiffness measure (LSM) values are higher in both lean and obese individuals: a population-based study from a developing country. Hepatology 2012; 55:584–93. [DOI] [PubMed] [Google Scholar]
- 25.Newson RB. Attributable and unattributable risks and fractions and other scenario comparisons. Stata J 2013; 13:672–98. [Google Scholar]
- 26.Mathers BM, Degenhardt L. Examining non-AIDS mortality among people who inject drugs. AIDS 2014; 28(suppl 4):S435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solomon SS, Celentano DD, Srikrishnan AK et al. . Mortality among injection drug users in Chennai, India (2005-2008). AIDS 2009; 23:997–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith DJ, Combellick J, Jordan AE, Hagan H. Hepatitis C virus (HCV) disease progression in people who inject drugs (PWID): a systematic review and meta-analysis. Int J Drug Policy 2015; 26:911–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez FA, Van den Eynde E, Perez-Hoyos S et al. . Liver stiffness and aspartate aminotransferase levels predict the risk for liver fibrosis progression in hepatitis C virus/HIV-coinfected patients. HIV Med 2015; 16:211–8. [DOI] [PubMed] [Google Scholar]
- 30.Solomon SS, Celentano DD, Srikrishnan AK et al. . Low incidences of human immunodeficiency virus and hepatitis C virus infection and declining risk behaviors in a cohort of injection drug users in Chennai, India. Am J Epidemiol 2010; 172:1259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goyal R, Mallick SR, Mahanta M et al. . Fibroscan can avoid liver biopsy in Indian patients with chronic hepatitis B. J Gastroenterol Hepatol 2013; 28:1738–45. [DOI] [PubMed] [Google Scholar]
- 32.Sharma P, Dhawan S, Bansal R et al. . The usefulness of transient elastography by FibroScan for the evaluation of liver fibrosis. Indian J Gastroenterol 2014; 33:445–51. [DOI] [PubMed] [Google Scholar]
- 33.Mehta SH, Vogt SL, Srikrishnan AK et al. . Epidemiology of hepatitis C virus infection & liver disease among injection drug users (IDUs) in Chennai, India. Indian J Med Res 2010; 132:706–14. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.