Our study presents important new information about the relationship between type 2 diabetes and primary multidrug resistant tuberculosis (MDR TB). Using a well-characterized patient population, we found diabetes was associated with delayed culture conversion among patients with and MDR TB.
Keywords: culture conversion, diabetes, Georgia, MDR TB, socioeconomic status
Abstract
Background. Diabetes is a risk factor for active tuberculosis (TB), but little is known about the relationship between diabetes and multidrug-resistant (MDR) TB. We aimed to assess risk factors for primary MDR TB, including diabetes, and determine whether diabetes reduced the rate of sputum culture conversion among patients with MDR TB.
Methods. From 2011 to 2014, we conducted a cohort study at the National Center for Tuberculosis and Lung Diseases in Tbilisi, Georgia. Adult (≥35 years) patients with primary TB were eligible. Multidrug-resistant TB was defined as resistance to at least rifampicin and isoniazid. Patients with capillary glycosylated hemoglobin (HbA1c) ≥ 6.5% or previous diagnosis were defined to have diabetes. Polytomous regression was used to estimate the association of patient characteristics with drug resistance. Cox regression was used to compare rates of sputum culture conversion in patients with and without diabetes.
Results. Among 318 patients with TB, 268 had drug-susceptibility test (DST) results. Among patients with DST results, 19.4% (52 of 268) had primary MDR TB and 13.4% (36 of 268) had diabetes. In multivariable analyses, diabetes (adjusted odds ratio [aOR], 2.51; 95% confidence interval [CI], 1.00–6.31) and lower socioeconomic status (aOR, 3.51; 95% CI, 1.56–8.20) were associated with primary MDR TB. Among patients with primary MDR TB, 44 (84.6%) converted sputum cultures to negative. The rate of sputum culture conversion was lower among patients with diabetes (adjusted hazard ratio [aHR], 0.34; 95% CI, .13–.87) and among smokers (aHR, 0.16; 95% CI, .04–.61).
Conclusions. We found diabetes was associated with an increased risk of primary MDR TB; both diabetes and smoking were associated with a longer time to sputum culture conversion.
There are an estimated 9.5 million new cases of active tuberculosis (TB) disease annually, including 480 000 cases of multidrug-resistant (MDR) TB, defined as resistance to at least rifampicin and isoniazid [1]. More importantly, the global reported incidence of MDR TB has increased substantially over the previous 5 years [2–4]. Simultaneous to the increase of MDR TB, the global prevalence of type 2 diabetes mellitus (diabetes) has increased greatly and in 2015 an estimated of 415 million adults had prevalent diabetes [5]. Although diabetes is an established risk factor for active TB disease and an estimated 15%–25% of global active TB cases are attributable to diabetes [6], whether diabetes is associated with MDR TB remains unclear.
Previous studies reported that diabetes is associated with having MDR TB [7, 8], whereas others found no increased prevalence of MDR TB among patients with diabetes compared with those without diabetes [9, 10]. The majority of global MDR TB cases are thought to be due to primary infection with a resistant strain [11], but whether the increased risk of primary infection for MDR TB is associated with diabetes remains understudied. Although less in known about risk factors for primary MDR TB, studies have reported that close contact with MDR TB patients, either household [12, 13] or nosocomial contact [14], was associated with primary MDR TB. Improved global control of TB will require improved prevention of primary MDR TB including a better understanding of the relationship between diabetes and risk of MDR TB.
Several studies have reported diabetes to be associated with poor TB treatment outcomes [15–17]. For example, diabetes was reported to increase the risk of death [18], relapse [15, 17], and failure in TB treatment [19]. However, whether diabetes impacts MDR TB treatment outcomes such as delayed culture conversion is not known. Given the paucity of information on the relationship between diabetes and MDR TB, the main objective of this study was to determine the relationship between diabetes and TB treatment outcomes (including time to culture conversion) among patients with primary MDR TB.
METHODS
Setting and Study Design
This work was part of the Hemoglobin A1c Levels among TB patients in Tbilisi (HALT) study, a prospective cohort observed between 2011 and 2014 at the National Center for Tuberculosis and Lung Diseases (NCTLD), the primary center for the National Tuberculosis Program in Tbilisi, Georgia [20]. Patients aged 35 and older with new pulmonary TB either laboratory confirmed (by molecular diagnostic tests, acid-fast bacilli [AFB] smear, and/or culture positive for Mycobacterium tuberculosis [(MTB]) or clinically diagnosed (based on clinical symptoms and chest x-ray findings) were eligible. Retreatment cases or patients with prior history of TB were excluded. Patients with MDR TB were observed during treatment to evaluate time to sputum culture conversion.
Definitions
The primary difference of this study with our previously reported findings [20] is in the study outcomes. The primary outcomes in this study were TB drug resistance profile (primary MDR TB defined as a case with no prior history of TB treatment) and time to sputum culture conversion among patients with MDR TB. We classified drug resistance pattern into 3 categories: fully susceptible, any resistance (resistant to 1 or more but not MDR TB or extensively drug-resistant [XDR] TB), and MDR or XDR TB (M/XDR). Drug-susceptibility tests (DSTs) were performed at the Georgia National Tuberculosis Reference Laboratory using LJ absolute concentration method, as previously described [20]. Fully susceptible TB was defined as TB that was susceptible to first-line anti-TB drugs (isoniazid, rifampin, ethambutol, and streptomycin). Any resistance was defined as TB resistant to at least 1 first-line TB drug but not MDR TB or XDR TB. MDR TB was defined as resistance to at least isoniazid and rifampin. Multidrug-resistant TB with additional resistance to any fluoroquinolone and at least one of 3 injectable second-line drugs was classified as XDR TB. Patients with missing drug-susceptibility results for all first-line anti-TB drugs were excluded from analyses.
Time to culture conversion was defined as time (in days) from the beginning of TB treatment until the date of the first of 2 consecutive negative sputum culture results that were at least 30 days apart. We classified MDR TB treatment outcome based on World Health Organization criteria as favorable and poor outcome. The favorable outcome group included patients who were cured and completed treatment; whereas patients who were lost to follow up, failed therapy, or died were defined as having a poor treatment outcome [21]. Diabetes status was determined by capillary glycosylated hemoglobin (HbA1c) measured within 60 days after the TB treatment initiation. Diabetes status was defined according to the 2014 American Diabetes Association clinical guidelines [22]; patients with HbA1c ≥6.5% and/or with a history of diabetes diagnosis were considered to have diabetes.
Demographic and behavioral risk factor information was collected using a questionnaire at the time of enrollment. Participants were interviewed in Kartuli (official language of Georgia) or Russian. Patients were asked to self-report their highest education level, socioeconomic status (SES), smoking status, and alcohol use. Education level, SES, and smoking status were classified as previously reported [20]. Alcohol use was classified as never, intermediate (≤4 drinks per occasion), and heavy (≥5 drinks per occasion).
Statistical Analyses
All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC). A 2-sided P value <.05 was considered significant in all analyses. To examine the bivariate association between diabetes and drug resistance profile, we used χ2 for categorical variables and Kruskal-Wallis for continuous nonnormally distributed variables. Multivariable polytomous logistic models were used to estimate the association of diabetes with drug resistance profile. Hazard assessment was performed using Cox proportional hazard models to estimate the rate of culture conversion among MDR TB patients with and without diabetes. Patients were censored if at the end of MDR TB treatment they did not have documented culture conversion, were lost to follow up, or died (with no documentation of prior conversion). Proportional hazard assumptions were assessed graphically, with goodness-of-fit tests, and using time-dependent models [23]. Covariates included in multivariable models were based on previous literature and observed bivariate associations with the primary exposure and outcomes.
Institutional Review Board Approval
This study was reviewed and approved by the institutional review boards at the NCTLD and Emory University.
RESULTS
Study Cohort and Baseline Characteristics
During the study period, 586 newly diagnosed TB patients were treated at NCLTD in Tbilisi; 324 had HbA1c measured and 318 were enrolled in this study. Of the 318 patients, 268 (84.3%) had DST results and were included in final analyses. We compared basic demographic and clinical characteristics among patients with and without DST results and found that patients with missing DST were similar to patients with available DST with respect to gender, SES, baseline AFB, human immunodeficiency virus status (HIV), and median HbA1c (data not shown). Compared with those with DST, those with missing DST were significantly older (55.5 vs 49.0 years) and more likely to be current smokers (62.0% vs 48.9%) (P < .05 for both comparisons).
Among 268 included patients with TB, 52 (19.4%) had M/XDR TB, the median HbA1c was 5.4% (interquartile range [IQR], 5.1%–5.7%), and the prevalence of diabetes was 13.4% (36 of 268). Among patients with diabetes, 25% (9 of 36) were newly diagnosed and 75% (27 of 36) were previously known to have diabetes. Most patients were male (75.4%), and the median age was 49 years (IQR, 42–58). Most participants were in the lower and middle SES (61.9% combined) with the median monthly income of $118.00 (IQR, $41–$412) (Table 1). Self-reported current smoking was high (49.1%), and any alcohol use was reported among 70.0%.
Table 1.
Baseline Characteristics and Drug-Resistant Profile Among Newly Diagnosed Adult TB Patients in Tbilisi, Georgia, 2011–2014a
| Variable | Type of Resistance |
Total N = 268 |
P Value | ||
|---|---|---|---|---|---|
| Fully Susceptiblea N% = 137 (51.1) |
Any Resistance (but not M/XDR)b N% = 79 (29.5) |
MDR and XDR TBc N% = 52 (19.4) |
|||
| N% | N% | N% | N% | ||
| Age | |||||
| Median (IQR) | 50 (41–58) | 48 (42–54) | 47 (42.5–58) | 49 (42–58) | .82 |
| 35–54 | 89 (65.0) | 60 (76.0) | 35 (67.3) | 184 (68.7) | .24 |
| ≥55 | 48 (35.0) | 19 (24.0) | 17 (32.7) | 84 (31.3) | |
| Sex | |||||
| Female | 37 (27.0) | 14 (17.7) | 15 (28.9) | 66 (24.6) | .24 |
| Male | 100 (73.0) | 65 (82.3) | 37 (71.1) | 202 (75.4) | |
| Education (formal years) | |||||
| Median (IQR) | 11 (10–14) | 11 (10–14) | 10 (10–11) | 11 (10–14) | .07 |
| <High School completed (≤9) | 16 (11.8) | 10 (12.7) | 4 (7.7) | 30 (11.2) | <.01d |
| High school (10–11) | 68 (50.0) | 42 (53.2) | 42 (80.8) | 152 (56.9) | |
| >High School (≥12) | 52 (38.2) | 27 (34.1) | 6 (11.5) | 85 (31.9) | |
| Household Income (USD/month) | |||||
| Median (IQR) | 176.47 (58.8–529.4) | 117.65 (5.88–411.76) | 62.94 (0–205.88) | 117.65 (41.18–411.77) | <.01 |
| ≤$59 | 36 (26.2) | 27 (34.2) | 25 (48.1) | 88 (32.8) | .07 |
| $60–$176 | 42 (30.7) | 22 (27.9) | 14 (26.9) | 78 (29.1) | |
| ≥$177 | 59 (43.1) | 30 (37.9) | 13 (25.0) | 102 (38.1) | |
| Internally Displaced | |||||
| No | 124 (90.5) | 76 (96.2) | 46 (88.5) | 246 (91.8) | .24 |
| Yes | 13 (9.5) | 3 (3.8) | 6 (11.5) | 22 (8.2) | |
| Imprisonment | |||||
| No | 118 (86.1) | 66 (83.5) | 45 (86.5) | 229 (85.5) | .85 |
| Yes | 19 (13.9) | 13 (16.5) | 7 (13.5) | 39 (14.5) | |
| Smoking Status | |||||
| Never smoker | 39 (28.7) | 8 (10.1) | 14 (26.9) | 61 (22.9) | .04 |
| Past smoker | 36 (26.5) | 24 (30.4) | 15 (28.9) | 75 (28.1) | |
| Current smoker | 61 (44.8) | 47 (59.5) | 23 (44.2) | 131 (49.0) | |
| Alcohol Use | |||||
| Never | 45 (33.1) | 16 (20.3) | 19 (36.5) | 80 (30.0) | .18 |
| Intermediate | 35 (25.7) | 20 (25.3) | 10 (19.2) | 65 (24.3) | |
| Heavy | 56 (41.2) | 43 (54.4) | 23 (44.2) | 122 (45.7) | |
| Contact with MDR TB Patient | |||||
| No | 126 (92.6) | 68 (90.7) | 47 (92.2) | 241 (92.0) | .88 |
| Yes | 10 (7.4) | 7 (9.3) | 4 (7.8) | 21 (8.0) | |
| BMI | |||||
| Median (IQR) | 21.19 (19.2–22.9) | 21.55 (20.2–23.4) | 21.48 (19.6–24.6) | 21.30 (19.7–23.6) | .34 |
| <18.5 | 28 (21.1) | 14 (18.4) | 8 (15.4) | 50 (19.2) | .44 |
| 18.5–24.9 | 91 (68.4) | 51 (67.1) | 33 (63.5) | 175 (67.1) | |
| ≥25 | 14 (10.5) | 11 (14.5) | 11 (21.1) | 36 (13.7) | |
| Diabetes | |||||
| Median Hba1c (IQR) | 5.4 (5.1–5.7) | 5.5 (5.2–5.7) | 5.3 (5.2–5.85) | 5.4 (5.1–5.7) | .61 |
| No diabetes | 120 (87.6) | 71 (89.9) | 41 (78.8) | 232 (86.6) | .18 |
| Diabetes | 17 (12.4) | 8 (10.1) | 11 (21.2) | 36 (13.4) | |
| HIV Status | |||||
| Negative | 128 (97.7) | 75 (94.9) | 49 (94.2) | 252 (96.2) | .45 |
| Positive | 3 (2.3) | 4 (5.1) | 3 (5.8) | 10 (3.8) | |
| Hypertension | |||||
| No | 113 (82.5) | 68 (86.1) | 41 (78.8) | 222 (82.8) | .56 |
| Yes | 24 (17.5) | 11 (13.9) | 11 (21.2) | 46 (17.2) | |
| Liver Disease | |||||
| No | 118 (86.8) | 70 (88.6) | 39 (75.0) | 227 (85.0) | .08 |
| Yes | 18 (13.2) | 9 (11.4) | 13 (25.0) | 40 (15.0) | |
| Kidney Disease | |||||
| No | 126 (92.0) | 69 (90.8) | 44 (84.6) | 239 (90.2) | .32 |
| Yes | 11 (8.0) | 7 (9.2) | 8 (15.4) | 26 (9.8) | |
| Cavitary disease | |||||
| None | 105 (81.4) | 57 (73.1) | 31 (59.6) | 193 (74.5) | .01 |
| Any Cavity | 24 (18.6) | 21 (26.9) | 21 (40.4) | 66 (25.5) | |
Abbreviations: BMI, body mass index; HbA1c, hemoglobin A1c; HIV, human immunodeficiency virus; IQR, interquartile range; MDR, multidrug resistant; MTB, Mycobacterium tuberculosis; M/XDR, MDR or XDR tuberculosis; TB, tuberculosis; USD, US dollars; XDR, extensively drug resistant.
a Patients with MTB susceptible to first-line anti-TB drugs (isoniazid, rifampin, ethambutol, and streptomycin).
b Patients with MTB resistant to at least 1 first-line TB drug but not MDR TB (including patients with monodrug-resistant TB, polydrug-resistant TB, and patients with missing no more than 3 first-line drug-susceptibility test results).
c Patients with MTB resistant to isoniazid and rifampicin; there were 49 patients of MDR TB and 3 patients of XDR TB.
d Bold indicates that the finding is statistically significant at level of confidence of 5% (P < .05).
Diabetes and Drug Resistance Profile
Among the 268 patients with available DST results, 137 (51.1%) were fully susceptible to all first-line anti-TB drugs, 79 patients (29.5%) had any resistance (resistant to 1 or more but not M/XDR TB), and the remaining had M/XDR TB. The prevalence of MDR TB among patients with diabetes was 30.6% vs 17.7% among patients without diabetes (P = .07). In univariate analysis, lower education attainment, lower monthly household income, and cavitary disease were significantly associated with MDR TB, whereas smoking and heavy alcohol use were associated with any drug resistance (resistant to 1 or more but not M/XDR TB) (Table 2). In multivariable analysis, independent risk factors for having MDR included diabetes (adjusted odds ratio [aOR], 2.51; 95% confidence interval [CI], 1.00–6.31) and lower household income (aOR, 3.51; 95% CI, 1.56–8.20). The risk of any drug resistance (resistant to 1 or more but not M/XDR TB) was significantly higher among past smokers (aOR, 3.94; 95% CI, 1.25–12.47) and current smokers (aOR, 4.56; 95% CI, 1.49–14.02).
Table 2.
Polytomous Regression for Type of Resistance Among Newly Diagnosed Adult TB Patients in Tbilisi, Georgia, 2011–2014
| Variable | Type of Resistance |
|||
|---|---|---|---|---|
| Any Resistance (but not M/XDR) bvs Fully Susceptiblea |
M/XDRc vs Fully Susceptible |
|||
| cOR (95% CI) | aOR (95% CI)d | cOR (95% CI) | aOR (95% CI)d | |
| Age | ||||
| 35–54 | 1 | 1 | 1 | 1 |
| ≥55 | 0.59 (.32–1.10) | 0.72 (.36–1.42) | 0.90 (.46–1.77) | 0.94 (.44–2.00) |
| Sex | ||||
| Female | 1 | 1 | 1 | 1 |
| Male | 1.72 (.86–3.42) | 0.71 (.25–2.04) | 0.91 (.45–1.85) | 0.84 (.27–2.61) |
| Education (formal years) | ||||
| <High School completed (≤9) | 1.20 (.48–3.01) | 2.17 (.54–8.64) | ||
| High school (10–11) | 1.19 (.65–2.18) | 5.35 (2.12–13.55)e | ||
| >High School (≥12) | 1 | 1 | ||
| Household Income (USD/month) | ||||
| ≤$59 | 1.48 (.76–2.87) | 1.65 (.81–3.36) | 3.15 (1.43–6.93) | 3.51 (1.56–8.20) |
| $60–$176 | 1.03 (.52–2.03) | 1.14 (.54–2.43) | 1.51 (.65–3.55) | 1.78 (.72–4.41) |
| ≥$177 | 1 | 1 | 1 | 1 |
| Internally Displaced | ||||
| No | 1 | 1 | ||
| Yes | 0.38 (.10–1.36) | 1.24 (.45–3.47) | ||
| Prior History of Incarceration | ||||
| No | 1 | 1 | ||
| Yes | 1.22 (.57–2.63) | 0.97 (.38–2.45) | ||
| Smoking Status | ||||
| Never smoker | 1 | 1 | 1 | 1 |
| Past smoker | 3.25 (1.30–8.15) | 3.94 (1.25–12.47) | 1.16 (.49–2.73) | 1.52 (48–4.76) |
| Current smoker | 3.76 (1.60–8.79) | 4.56 (1.49–14.02) | 1.05 (.48–2.28) | 1.52 (.50–4.59) |
| Alcohol Use | ||||
| Never | 1 | 1 | 1 | 1 |
| Intermediate | 1.61 (.73–3.55) | 1.14 (.42–3.07) | 0.68 (.28–1.64) | 0.82 (.27–2.47) |
| Heavy | 2.16 (1.08–4.33) | 1.28 (.48–3.44) | 0.97 (.47–2.01) | 0.93 (.32–2.72) |
| Contact with MDR TB Patient | ||||
| No | 1 | 1 | ||
| Yes | 1.30 (.47–3.56) | 1.07 (.32–3.59) | ||
| BMI | ||||
| <18.5 | 0.89 (.43–1.85) | 0.79 (.33–1.90) | ||
| 18.5–24.9 | 1 | 1 | ||
| ≥25 | 1.40 (.59–3.312) | 2.17 (.90–5.25) | ||
| Diabetes | ||||
| No Diabetes | 1 | 1 | 1 | 1 |
| Diabetes | 0.80 (.33–1.94) | 1.20 (.46–3.14) | 1.89 (.82–4.38) | 2.51 (1.00–6.31) |
| HIV Status | ||||
| Negative | 1 | 1 | 1 | 1 |
| Positive | 2.28 (.50–10.45) | 1.86 (.39–8.89) | 2.61 (.51–13.38) | 2.57 (.47–14.05) |
| Hypertension | ||||
| No | 1 | 1 | ||
| Yes | 0.76 (.35–1.65) | 1.26 (.57–2.81) | ||
| Liver Disease | ||||
| No | 1 | 1 | ||
| Yes | 0.84 (.36–1.98) | 2.19 (.98–4.86) | ||
| Kidney Disease | ||||
| No | 1 | 1 | 1 | 1 |
| Yes | 1.16 (.43–3.13) | 1.01 (.35–2.91) | 2.08 (.79–5.51) | 1.65 (.59–4.63) |
| Cavitary Disease | ||||
| No | 1 | 1 | ||
| Yes | 1.61 (.83–3.15) | 2.96 (1.48–6.03) | ||
Abbreviations: aOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; cOR, crude odds ratio; HIV, human immunodeficiency virus; MDR, multidrug resistant; MTB, Mycobacterium tuberculosis; M/XDR, MDR or XDR tuberculosis; TB, tuberculosis; XDR, extensively drug resistant.
a Patients with MTB susceptible to all of the first-line TB drugs used in Georgia (isoniazid, rifampin, ethambutol, and streptomycin).
b Patients with MTB resistant to at least 1 first-line TB drug but not MDR TB (including patients with monodrug-resistant TB, polydrug-resistant TB, and patients with missing no more than 3 first-line drug-susceptibility test results).
c Patients with MTB resistant to isoniazid and rifampicin; there were 49 patients of MDR TB and 3 patients of XDR TB.
d Adjusted odds ratio after controlling for age, sex, socioeconomic status, smoking status, alcohol use, HIV status, diabetes status, and kidney disease. Empty cells mean that the variables were not included in the multivariate model.
e Bold indicates that the finding is statistically significant at level of confidence of 5% (P < .05).
Diabetes and Time to Culture Conversion Time Among Patients With Multidrug-Resistant Tuberculosis
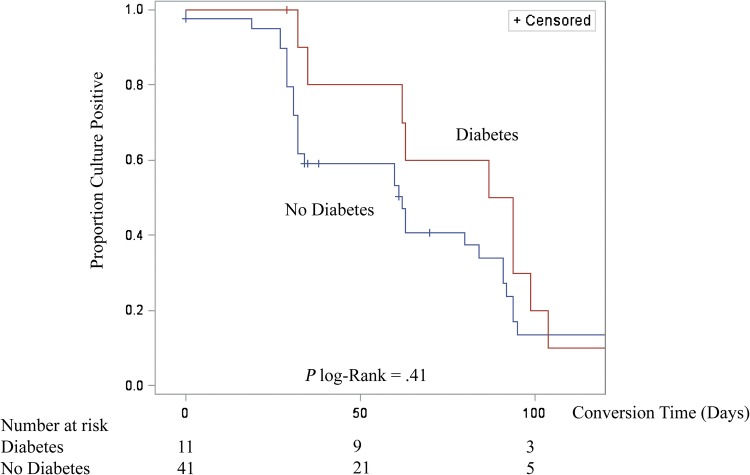
Among 52 patients with M/XDR TB, 44 (84.6%) converted sputum cultures to negative during follow up. Among M/XDR TB patients who converted, the median culture conversion time was 62 days (IQR, 32–94) (Table 3). The prevalence of diabetes among patients MDR TB was 21.2% (11 of 52), and median time to culture conversion among MDR TB patients with diabetes was greater than those without diabetes (91 days [IQR, 62–99] vs 60 days [IQR, 31–84]), and this difference was on the borderline of statistical significance (P = .06) (Figure 1). In univariate analysis, the hazard rate of culture conversion was nonsignificantly lower among patients with diabetes (crude hazard ratio [cHR], 0.75; 95% CI, .36–1.53). In multivariable analysis, after adjusting for age, sex, SES, HIV status, cavitary disease, and grade of AFB smear, the rate of sputum culture conversion was significantly lower (ie, took longer to convert sputum culture from positive to negative) among MDR TB patients with diabetes (adjusted HR [aHR], 0.34; 95% CI, .13–.87) compared with MDR TB patients without diabetes. Using various combinations of covariates, we ran additional adjusted hazard models for rate of sputum culture conversion (Supplementary Table 1). Multidrug-resistant TB patients with diabetes had consistently lower rates of conversion (aHR range, 0.34–0.66; 95% CI range, .17–1.42) than MDR TB patients without diabetes. The time to sputum culture conversion was nonsignificantly longer among patients with poorer control diabetes (HbA1c ≥ 8.0%) compared with those with HbA1c < 8.0% (median culture conversion 87 days vs 65 days, respectively; P = .58) (Table 4). Nearly all MDR TB patients with diabetes (10 of 11) were receiving diabetes medications at the start of their TB treatment. The rate of sputum culture conversion was significantly lower among patients who were current smokers in the univariate analysis (cHR, 0.44; 95% CI, .19–.98) with the median time to culture conversion of 84 (IQR, 32–104). In multivariable analysis, the rate of culture conversion was also lower among current smokers (aHR, 0.16; 95%, CI .04–.61) compared with nonsmokers.
Table 3.
Univariate and Multivariate Hazard Rate Ratio Analysis of Patient Characteristics and Sputum Culture Conversion Among Adult Patients With Newly Diagnosed MDR TB in Tbilisi, Georgia, 2011–2014
| Characteristic | Converted 44/52 (84.6%) |
Median (IQR)a | cHR (95% CI) | aHRb (95% CI) |
|---|---|---|---|---|
| N % | ||||
| Age | ||||
| 35–54 | 30/35 (85.7) | 60 (31–92) | 1 | 1 |
| ≥55 | 14/17 (82.4) | 82 (32–94) | 0.90 (.47–1.72) | 0.45 (.19–1.06) |
| Sex | ||||
| Female | 12/15 (80.0) | 61 (32–63) | 1 | 1 |
| Male | 32/37 (86.5) | 74 (32–95) | 0.50 (.24–1.02) | 1.45 (.49–4.28) |
| Household Income | ||||
| ≤$59 | 22/25 (88.0) | 63 (32–94) | 0.97 (.45–2.09) | 0.66 (.25–1.72) |
| $60–$176 | 12/14 (85.7) | 61 (30–77) | 1.66 (.71–3.87) | 0.93 (.32–2.69) |
| ≥$177 | 10/13 (76.9) | 74 (31–95) | 1 | 1 |
| Smoking Status | ||||
| Never smoker | 12/14 (85.7) | 62 (32–89) | 1 | 1 |
| Past smoker | 15/15 (100.0) | 60 (29–92) | 0.97 (.45–2.10) | 0.60 (.20–1.79) |
| Current smoker | 17/23 (73.9) | 84 (32–104) | 0.44 (.19–0.98)c | 0.16 (.04–0.61) |
| Alcohol Use | ||||
| Never | 17/19 (89.5) | 63 (32–87) | 1 | |
| Intermediate | 9/10 (90.0) | 34 (29–94) | 1.06 (.46–2.41) | |
| Heavy | 18/23 (78.3) | 72 (32–99) | 0.58 (.29–1.15) | |
| Imprisonment | ||||
| No | 38/45 (84.4) | 63 (32–94) | 1 | |
| Yes | 6/7 (85.7) | 30 (29–84) | 1.89 (.78–4.57) | |
| Contact with MDR TB Patient | ||||
| No | 41/47 (87.2) | 62 (32–94) | 1 | |
| Yes | 2/4 (50.0) | 17 (0–34) | 3.54 (.80–15.62) | |
| Diabetes | ||||
| No diabetes | 34/41 (82.9) | 60 (31–91) | 1 | 1 |
| Diabetes | 10/11 (90.9) | 91 (62–99) | 0.75 (.36–1.53) | 0.34 (.13–0.87) |
| HIV Status | ||||
| Negative | 42/49 (85.7) | 62 (32–94) | 1 | 1 |
| Positive | 2/3 (66.7) | 78 (29–126) | 0.65 (.15–2.73) | 0.51 (.09–3.06) |
| BMI | ||||
| <18.5 | 7/8 (87.5) | 32 (27–80) | 2.82 (1.18–6.75) | |
| 18.5–24.9 | 29/33 (87.9) | 62 (32–94) | 1 | |
| ≥25 | 8/11 (72.7) | 63 (62–89) | 1.19 (.53–2.68) | |
| Cavitary Disease | ||||
| None | 25/31 (80.7) | 62 (32–94) | 1 | 1 |
| Any cavity | 19/21 (90.5) | 63 (31–92) | 1.31 (.71–2.40) | 2.48 (1.04–5.90) |
| AFB Smear (among culture positive) | ||||
| Negative | 5/8 (62.5) | 32 (29–80) | 1 | 1 |
| Positive | 35/39 (89.7) | 62 (31–94) | 0.66 (.25–1.73) | 0.56 (.17–1.88) |
Abbreviations: AFB, acid-fast bacilli; aHR, adjusted hazard rate ratio; BMI, body mass index; cHR, crude hazard rate ratio; CI, confidence interval; HIV, human immunodeficiency virus; IQR, interquartile range; MDR, multidrug resistant; TB, tuberculosis.
a Among patients who converted, median time (measured in days) from the initial MDR treatment until the first 2 consecutive negative culture results (≥30 days apart).
b Hazard rate ratio after controlling for age, sex, socioeconomic, smoking status, diabetes status, HIV status, cavitary disease, and AFB smear. Empty cells mean that the variables were not included in the multivariate model.
c Bold indicates that the finding is statistically significant at level of confidence of 5% (P < .05).
Figure 1.
Time to sputum culture conversion among 52 primary multidrug-resistant tuberculosis patients with and without diabetes in Tbilisi, Georgia, 2011–2014.
Table 4.
Information of Patients With MDR TB Complicated With Diabetes in the Country of Georgia, 2011–2014
| No | Gender | HbA1c Levela (%) | Conversion Timeb (Days) | MDR TB Treatment Outcome | Diabetes Medication | Diagnosed With Diabetes Before |
|---|---|---|---|---|---|---|
| 1 | Female | 5.6 | 29 | Defaulted | Yes | Yes |
| 2 | Male | 6.5 | 99 | Cured | Yes | Yes |
| 3 | Male | 6.5 | 35 | Defaulted | Yes | Yes |
| 4 | Female | 6.9 | 94 | Cured | Yes | Yes |
| 5 | Female | 8.0 | 32 | Defaulted | Yes | Yes |
| 6 | Female | 8.4 | 62 | Cured | Yes | Yes |
| 7 | Male | 8.5 | 126 | Completed | Yes | Yes |
| 8 | Male | 9.0 | 94 | Defaulted | Yes | Yes |
| 9 | Male | 9.1 | 87 | Cured | Yes | Yes |
| 10 | Female | 10.4 | 63 | Cured | Yes | Yes |
| 11 | Male | 11.5 | 104 | Defaulted | Unknown | No |
Abbreviations: HbA1c, hemoglobin A1c; MDR, multidrug resistant; TB, tuberculosis.
a HbA1c level was classified into 2 categories (HbA1c ≥ 8.0% vs <8.0%) to indicate poorer control of diabetes.
b Median culture conversion was 87 days (HbA1c ≥ 8.0%) vs 65 days (HbA1c < 8.0%) (P = .58).
At the end of follow-up, 50 (96.2%) of 52 patients with MDR TB had treatment outcome information; 1 patient had missing treatment outcome data, and 1 patient remained on treatment. Twenty-seven (54%) patients had a favorable treatment outcome (cured or completed), whereas 46% (23 of 50) of patients had poor treatment outcome (died or lost to follow up). The risk of poor treatment outcome was similar among those with diabetes (45.5%) and those without diabetes (46.2%) (P = .96). The risk of poor treatment outcome among those who reported current use of tobacco was 72.7% compared with 25.0% among those who reported past or no history of tobacco use (P ≤ .01).
DISCUSSION
We found that diabetes was a significant risk factor for primary MDR TB and that among a small subset of patients with MDR TB (N = 52), the rate of sputum culture conversion was lower in those with diabetes. The associations with MDR TB and culture conversion remained significant even after adjusting for key confounding factors. Although we only observed 11 patients with MDR TB and diabetes, we found a nonsignificant trend toward an increased median time to sputum culture conversion among MDR TB patients with diabetes. Our findings suggest that diabetes may have a more important role in MDR TB and response to MDR TB treatment than previously indicated.
Whether diabetes is associated with increased prevalence or risk of MDR TB has been inconsistently reported in previous studies. Consistent with our findings that patients with diabetes have more than twice the odds of MDR TB, a retrospective cohort study conducted on the Texas-Mexico border also found that the risk of developing MDR TB was higher among patients with diabetes among both Texas (aOR, 2.14; 95% CI, 1.10–4.17) and Mexican patients (aOR, 1.80; 95% CI, 1.13–4.17) [7]. A case-control study conducted in Bangladesh also reported greater risk of MDR TB among patients with diabetes (OR, 2.25; 95% CI, 1.4–3.6) [24]. However, both Texas and Bangladesh studies included patients with previously treated TB, whereas our study only included patients with newly diagnosed TB. Few studies have previously examined the association between diabetes and primary MDR TB. A cross-sectional study conducted in Taiwan reported that diabetes did not increase the odds of prevalent MDR TB among newly diagnosed patients (aOR, 0.95; 95% CI, .34–2.68) [9]. However, unlike our study, the Taiwanese study did not adjust for potential confounders such as HIV status, smoking, and alcohol use. A case-control study conducted in China reported that comorbidity factors (including diabetes) were associated with an increased risk of primary transmission of MDR TB (aOR, 57.1; 95% CI, 8.6–424.2) [25]. However, this study categorized diabetes with cardiovascular disease, respiratory disease, and cancer as general comorbidity factors, consequently the association between diabetes and primary MDR TB was indeterminate.
Diabetes is associated with altered immune function likely leading to increased susceptibility to bacterial infections such as MTB [7], but whether altered immune function also leads to increased risk of primary MDR TB or other drug resistance is not known. Although our data demonstrated an association between diabetes and MDR TB, the biologic basis for this observation is speculative. Whether resistant strains of MTB are more infectious than the susceptible strains is unknown [26]; however, a previous animal model reported that mutations in bacterial genes may increase TB pathogenicity [27]. For example, a molecular analysis of isoniazid-resistant strains found that katG gene mutations in isogenic MTB resulted in increased coding of catalase-peroxidase, an enzyme that may prevent bacterial susceptibility to oxidative stress during the host infection process [28]. However, observations from population-based studies do not support the animal model. A 3-year prospective household contact study conducted in Peru reported that resistant MTB strains (MDR TB) resulted in fewer secondary TB cases compared with susceptible strains (TB incidence 3.3% vs 4.8%; P < .05), suggesting that the fitness (defined as the ability of pathogen to infect, reproduce, cause disease, and be transmitted) of resistant strains may be lower than the drug-susceptible strains [12]. Studies are needed to determine whether household contact members with diabetes are at increased risk of developing active TB from MDR TB index patients.
Time to sputum culture conversion is a strong predictor of MDR TB treatment success [29], but few studies have examined whether diabetes is associated with lower rates of sputum culture conversion. Our previous work in the country of Georgia reported a nonstatistically significant, lower culture conversion rate among patients with diabetes (aHR, 0.95; 95% CI, .71–1.28) [30]. However, our previous work used self-reported diabetes status, whereas the present study used level of glycosylated hemoglobin (HbA1c) to determine patients' diabetes status. A multinational cohort of patients with MDR TB also reported lower but nonsignificant unadjusted rate of sputum culture conversion among patients with diabetes (HR, 0.76; 95% CI, .54–1.06) [31]. A retrospective cohort study of MDR TB patients from Latvia reported that concurrent diabetes was associated with longer time to culture conversion (P = .02) [32]. Our findings are consistent with the previous studies that suggested diabetes does importantly impact response to MDR TB treatment. Although not statistically significant, we found that patients with controlled diabetes (HbA1c < 8.0%) had faster culture conversion times than those with uncontrolled diabetes (HbA1c ≥ 8.0%). Screening for diabetes and glucose control at the beginning of MDR TB treatment may help to identify patients who have higher risk of delayed sputum culture conversion.
Although not the primary objective of this study, we observed that lower household income was associated with MDR TB and that current smoking was associated with any resistance (resistant to 1 or more but not M/XDR). We also reported that patients with MDR TB who reported to be current smokers had a significantly lower rate of sputum culture conversion. Similar to this study's findings, previous studies consistently reported that low SES and smoking play critical roles in risk of TB and response to TB treatment [33–37]. Consistent with our finding that patients with lower SES had 3 times greater odds of MDR TB, a study in Turkey reported that the risk of MDR TB is increased by 6-fold among patients with low SES [38]. Although the association between smoking and poor MDR TB treatment outcomes is under studied, this study's finding that smoking lowers the rate of sputum culture conversion is consistent with our previous study in Georgia (aHR, 0.82; 95% CI, .71–.95) [30]. Smoking is thought to be associated with compromised immune mechanisms including reduced phagocytic function of alveolar macrophage [39]. Therefore, in regions with high rates of MDR TB such as Georgia, expanded surveillance and TB prevention programs should be targeted in low SES settings where smoking rates are typically higher. A new approach on TB control that includes smoking cessation program is also suggested to prevent poor TB treatment outcome.
This study was subject to limitations. First, our sample size was limited; of all patients seeking for treatment at NCTLD, only 55.3% (324 of 586) were eligible to participate and only 318 were enrolled. However, patients enrolled in the present study were similar with respect to demographic and clinical characteristics when being compared with all patients with TB in the country of Georgia during the study time period. Likewise, we only had 52 patients with MDR TB in our study and 11 patients with MDR TB and diabetes. Studies with greater power are needed to improve precision of estimated effects. Second, our study population came from 1 TB reference center in Tbilisi, and the generalizability to other settings may be limited. Nonetheless, the NCTLD (where our study was conducted) is a referral center for patients from the entire country of Georgia, and consequently our findings are likely relevant to other former Soviet Republics and other low- and middle-income settings with high rates of MDR TB. Third, 16% of enrolled patients in our study did not have complete DST results available and were excluded from analyses. However, we found that patients with missing DST had similar distribution of gender, SES, baseline AFB status, HIV, and HbA1c when compared with patients with available DST. Among patients who were included in the analysis, only 52 had MDR TB and 11 of 52 had diabetes. Nonetheless, in our study, there were only 2 patients with MDR TB who had a missing treatment outcome, providing an exceptional follow-up rate in prospective analyses (96.2%). Fourth, we used a capillary point-of-care HbA1c test to measure diabetes status at 1 time point among study participants. In an ideal setting, a diabetes diagnosis should not be based solely on the result of 1 point-of-care HbA1c test because it may result in misclassification. However, the majority of patients with diabetes in our study were previously diagnosed with diabetes; therefore, if misclassification of diabetes occurred, it was likely due to classifying patients as not having diabetes. Fifth, some of the key covariates in our analysis, such as smoking status and alcohol use, were self-reported and may have resulted in misclassification. However, previous studies have reported high validity of self-reported smoking and alcohol use behaviors compared with biomarker measurement [40]; therefore, we do not believe the misclassification led to substantial bias in our reported measures of association.
CONCLUSIONS
Previous studies assessing the relationship between diabetes and MDR TB principally were among patients with prior history of TB treatment, whereas in our study we found that diabetes was associated with primary infection with MDR TB and reduced rate of sputum culture conversion during MDR TB treatment. Expanding our understanding of the risk factors for primary infection of MDR TB, including the role of diabetes, will help improve effective MDR TB prevention efforts.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
Disclaimer. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Financial support. This study was supported in part by the National Institutes of Health (NIH) Fogarty International Center (D43TW007124), NIH National Institute of Allergy and Infectious Diseases (K23AI103044 and K24AI114444), Bethesda, Maryland; and the Atlanta Clinical and Translational Science Institute (NIH/NCATS UL1TR000454), the Laney Graduate School of Emory University, and the Emory Global Health Institute (Atlanta, GA).
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.World Health Organization. Global Tuberculosis Report 2014. Geneva: World Health Organization; 2014. [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Report 2011. Geneva: World Health Organization; 2011. [Google Scholar]
- 3.World Health Organization. Global Tuberculosis Report 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- 4.World Health Organization. Global Tuberculosis Report 2013. Geneva: World Health Organization; 2013. [Google Scholar]
- 5.International Diabetes Federation. IDF Diabetes Atlas. 7 ed Brussels, Belgium: International Diabetes Federation; 2015. [Google Scholar]
- 6.Lönnroth K, Roglic G, Harries AD. Improving tuberculosis prevention and care through addressing the global diabetes epidemic: from evidence to policy and practice. Lancet Diabetes Endocrinol 2014; 2:730–9. [DOI] [PubMed] [Google Scholar]
- 7.Fisher-Hoch SP, Whitney E, McCormick JB et al. . Type 2 diabetes and multidrug-resistant tuberculosis. Scand J Infect Dis 2008; 40:888–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tanrikulu AC, Hosoglu S, Ozekinci T et al. . Risk factors for drug resistant tuberculosis in southeast Turkey. Trop Doct 2008; 38:91–3. [DOI] [PubMed] [Google Scholar]
- 9.Hsu AH, Lee JJ, Chiang CY et al. . Diabetes is associated with drug-resistant tuberculosis in Eastern Taiwan. Int J Tuberc Lung Dis 2013; 17:354–6. [DOI] [PubMed] [Google Scholar]
- 10.Duangrithi D, Thanachartwet V, Desakorn V et al. . Impact of diabetes mellitus on clinical parameters and treatment outcomes of newly diagnosed pulmonary tuberculosis patients in Thailand. Int J Clin Pract 2013; 67:1199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gandhi NR, Nunn P, Dheda K et al. . Multidrug-resistant and extensively drug-resistant tuberculosis: a threat to global control of tuberculosis. Lancet 2010; 375:1830–43. [DOI] [PubMed] [Google Scholar]
- 12.Grandjean L, Gilman RH, Martin L et al. . Transmission of multidrug-resistant and drug-susceptible tuberculosis within households: a prospective cohort study. PLoS Med 2015; 12:e1001843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teixeira L, Perkins MD, Johnson JL et al. . Infection and disease among household contacts of patients with multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2001; 5:321–8. [PubMed] [Google Scholar]
- 14.Breathnach AS, de Ruiter A, Holdsworth GM et al. . An outbreak of multi-drug-resistant tuberculosis in a London teaching hospital. J Hospital Infect 1998; 39:111–7. [DOI] [PubMed] [Google Scholar]
- 15.Dooley KE, Tang T, Golub JE et al. . Impact of diabetes mellitus on treatment outcomes of patients with active tuberculosis. Am J Trop Med Hyg 2009; 80:634–9. [PMC free article] [PubMed] [Google Scholar]
- 16.Viswanathan AA, Gawde NC. Effect of type II diabetes mellitus on treatment outcomes of tuberculosis. Lung India 2014; 31:244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baker MA, Harries AD, Jeon CY et al. . The impact of diabetes on tuberculosis treatment outcomes: a systematic review. BMC Med 2011; 9:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magee MJ, Foote M, Maggio DM et al. . Diabetes mellitus and risk of all-cause mortality among patients with tuberculosis in the state of Georgia, 2009–2012. Ann Epidemiol 2014; 24:369–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morsy AM, Zaher HH, Hassan MH, Shouman A. Predictors of treatment failure among tuberculosis patients under DOTS strategy in Egypt. East Mediterr Health J 2003; 9:689–701. [PubMed] [Google Scholar]
- 20.Magee MJ, Kempker RR, Kipiani M et al. . Diabetes mellitus is associated with cavities, smear grade, and multidrug-resistant tuberculosis in Georgia. Int J Tuberc Lung Dis 2015; 19:685–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.World Health Organization. Definitions and Reporting Framework for Tuberculosis–2013 Revision. Geneva: World Health Organization; 2013. [Google Scholar]
- 22.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014; 37:S81–90. [DOI] [PubMed] [Google Scholar]
- 23.Kleinbaum DG, Klein M. Survival Analysis: A Self-Learning Text. New York: Springer-Verlag; 1996. [Google Scholar]
- 24.Rifat M, Milton AH, Hall J et al. . Development of multidrug resistant tuberculosis in Bangladesh: a case-control study on risk factors. PLoS One 2014; 9:e105214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li WB, Zhang YQ, Xing J et al. . Factors associated with primary transmission of multidrug-resistant tuberculosis compared with healthy controls in Henan Province, China. Infect Dis Poverty 2015; 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Borrell S, Gagneux S. Infectiousness, reproductive fitness and evolution of drug-resistant Mycobacterium tuberculosis. Int J Tuberc Lung Dis 2009; 13:1456–66. [PubMed] [Google Scholar]
- 27.Ordway DJ, Sonnenberg MG, Donahue SA et al. . Drug-resistant strains of Mycobacterium tuberculosis exhibit a range of virulence for mice. Infect Immun 1995; 63:741–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gagneux S, Burgos MV, DeRiemer K et al. . Impact of bacterial genetics on the transmission of isoniazid-resistant Mycobacterium tuberculosis. PLoS Pathog 2006; 2:e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurbatova EV, Cegielski JP, Lienhardt C et al. . Sputum culture conversion as a prognostic marker for end-of-treatment outcome in patients with multidrug-resistant tuberculosis: a secondary analysis of data from two observational cohort studies. Lancet Respir Med 2015; 3:201–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magee MJ, Kempker RR, Kipiani M et al. . Diabetes mellitus, smoking status, and rate of sputum culture conversion in patients with multidrug-resistant tuberculosis: a cohort study from the country of Georgia. PloS One 2014; 9:e94890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kurbatova EV, Gammino VM, Bayona J et al. . Predictors of sputum culture conversion among patients treated for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2012; 16:1335–43. [DOI] [PubMed] [Google Scholar]
- 32.Holtz TH, Sternberg M, Kammerer S et al. . Time to sputum culture conversion in multidrug-resistant tuberculosis: predictors and relationship to treatment outcome. Ann Intern Med 2006; 144:650–9. [DOI] [PubMed] [Google Scholar]
- 33.Sharma SK, Mohan A. Multidrug-resistant tuberculosis. Indian J Med Res 2004; 120:354–76. [PubMed] [Google Scholar]
- 34.He GX, Wang HY, Borgdorff MW et al. . Multidrug-resistant tuberculosis, People's Republic of China, 2007–2009. Emerg Infect Dis 2011; 17:1831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang JY, Hsueh PR, Jan IS et al. . The effect of smoking on tuberculosis: different patterns and poorer outcomes. Int J Tuberc Lung Dis 2007; 11:143–9. [PubMed] [Google Scholar]
- 36.Tachfouti N, Nejjari C, Benjelloun MC et al. . Association between smoking status, other factors and tuberculosis treatment failure in Morocco. Int J Tuberc Lung Dis 2011; 15:838–43. [DOI] [PubMed] [Google Scholar]
- 37.d'Arc LyraBatista J, Albuquerque MD, Ximenes RA, Rodrigues LC. Smoking increases the risk of relapse after successful tuberculosis treatment. Int J Epidemiol 2008; 37:841–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tanrikulu AC, Abakay A, Abakay O. Risk factors for multidrug-resistant tuberculosis in Diyarbakir, Turkey. Med Sci Monit 2010; 16:PH57–62. [PubMed] [Google Scholar]
- 39.Lin HH, Ezzati M, Chang HY, Murray M. Association between tobacco smoking and active tuberculosis in Taiwan. Am J Respir Crit Care Med 2009; 180:475–80. [DOI] [PubMed] [Google Scholar]
- 40.Brener ND, Billy JO, Grady WR. Assessment of factors affecting the validity of self-reported health-risk behavior among adolescents: evidence from the scientific literature. J Adolesc Health 2003; 33:436–57. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.