We evaluated the positivity of (1-3)-β-D-glucan, a serum marker of invasive fungal diseases (IFD), at diagnosis and during treatment. (1-3)-β-D-Glucan may not be an early marker but could prove useful for diagnosis of chronic IFD.
Keywords: (1-3)-β-d-glucan, diagnostic tool, invasive fungal diseases, kinetics
Abstract
Background. Early diagnosis and treatment are crucial in invasive fungal diseases (IFD). Serum (1-3)-β-d-glucan (BG) is believed to be an early IFD marker, but its diagnostic performance has been ambiguous, with insufficient data regarding sensitivity at the time of IFD diagnosis (TOD) and according to outcome. Whether its clinical utility is equivalent for all types of IFD remains unknown.
Methods. We included 143 patients with proven or probable IFD (49 invasive candidiasis, 45 invasive aspergillosis [IA], and 49 rare IFD) and analyzed serum BG (Fungitell) at TOD and during treatment.
Results. (1-3)-β-d-glucan was undetectable at TOD in 36% and 48% of patients with candidemia and IA, respectively; there was no correlation between negative BG results at TOD and patients' characteristics, localization of infection, or prior antifungal use. Nevertheless, patients with candidemia due to Candida albicans were more likely to test positive for BG at TOD (odds ratio = 25.4, P = .01) than patients infected with other Candida species. In 70% of the patients with a follow-up, BG negativation occurred in >1 month for candidemia and >3 months for IA. A slower BG decrease in patients with candidemia was associated with deep-seated localizations (P = .04). Thirty-nine percent of patients with rare IFD had undetectable BG at TOD; nonetheless, all patients with chronic subcutaneous IFD tested positive at TOD.
Conclusions. Undetectable serum BG does not rule out an early IFD, when the clinical suspicion is high. After IFD diagnostic, kinetics of serum BG are difficult to relate to clinical outcome.
The incidence of invasive fungal diseases (IFDs) has increased in the past decades as a consequence of the ever-growing population of immunocompromised patients (with a solid organ transplant [SOT] or hematopoietic stem cell transplant) or patients receiving advanced critical care [1–4]. Aside from Pneumocystis jirovecii, the most common threats are Candida spp and Aspergillus spp, but a large variety of other opportunistic fungal pathogens can cause severe diseases in vulnerable patients. A key to a favorable prognosis of these deadly infections is early initiation of an accurate antifungal therapy, which itself relies on early diagnosis. Nonetheless, diagnosis of both common and rare IFD remains challenging, partly due to the limited availability of sensitive early diagnostic markers.
Among possible markers, (1-3)-β-d-glucan (BG), an abundant cell wall polysaccharide found in a majority of fungi [5], has been used more and more frequently in the last 5 years [6, 7]. It has been proposed as an early biomarker of IFD and is included in diagnostic criteria of IFD in the 2008 version of European Organization for Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) [6]. However, this marker cannot discriminate the various fungi that can cause IFD.
Different studies have evaluated clinical performance of the BG assay. Some focused on specific target populations (ie, hematological patients [8], intensive care unit [ICU] patients [9, 10], or the pediatric population [11]) or on specific IFD types (candidemia [10, 12] or invasive aspergillosis [IA] [8]), whereas others included all kinds of patients with all kinds of IFD [13, 14]. They reported wide discrepancies on sensitivity (40%–100%) and specificity (45%–99%) of the assay [7, 15, 16], probably due to the heterogeneity of their designs. Other studies have analyzed diagnostic utility of serial BG sampling before diagnosis of IFD [17–20], with some of them emphasizing its value as an early IFD marker, especially in invasive candidiasis (IC) [10, 17, 18, 20]. Finally, a few other research groups have attempted to interpret postdiagnosis kinetics of BG levels [18, 20–23]. Nonetheless, the use of this marker in clinical practice remains problematic [24].
In the present study, we sought to assess BG serum levels in patients who received a diagnosis of various types of IFD (proven or probable; including IC or IA, as well as a panel of rare IFD). To obtain useful clinical information, we evaluated the rate of BG positivity when the diagnosis was made (time of diagnosis [TOD]) and analyzed the kinetics of serum BG levels during antifungal treatment and its relation to prognosis.
PATIENTS AND METHODS
Hospital and Patients
Necker-Enfants Malades is a teaching hospital, where adults and children at high risk for IFD receive medical care. From January 2011 to July 2015, we carried out an observational study on BG testing performed in the mycology laboratory at the request of clinicians from various hospital wards. In the logs of the laboratory, we identified all patients (adults and children) with documented fungal infections (except for pneumocystosis) who had at least 1 sample analyzed for BG at the TOD (TOD being a time interval around the day of diagnosis, ie, the date of collection of the sample allowing diagnostic; see below).
From this list, we first selected a group of patients with IC, which included (1) patients with a positive culture from a normally sterile site (including blood cultures, joint fluid aspiration, vertebral or cardiac biopsy) and a BG sample collected between 1 day before and 7 days after the first positive culture was drawn and (2) patients with chronic disseminated candidiasis (ie, hepatosplenic candidiasis) diagnosed as possible IFD on the basis of a typical computed tomography (CT) scan, according to EORTC/MSG diagnostic criteria (6) and for whom a BG sample was taken between 7 days before and after the CT scan, which first showed microabscesses compatible with the diagnosis.
From the same original list, we then selected patients with probable or proven IA diagnosed according to the EORTC/MSG criteria (6), except that we extended the definition of probable IA to patients in whom real-time PCR detected deoxyribonucleic acid of Aspergillus fumigatus in a normally sterile sample (ie, serum, cerebrospinal fluid, or biopsy) [25, 26]. For IA, the patients selected had a BG sample taken between 3 days before and 7 days after the day of diagnosis. In case of slowly evolving IA, such as bone infections, the delay was extended to 15 days after the day of diagnosis.
Finally, from the same list, we selected patients with rare IFD defined as patients with deep-seated, invasive, or chronic infections due to fungi other than Candida and Aspergillus, with histopathological evidence or a positive culture of normally sterile material (biopsy or needle aspiration of a sterile site, or blood culture). For rare IFD, the patients selected had a BG sample taken between 7 days before and 10 days after the day of diagnosis.
For all 3 types of IFD cases, all BG test results (that were requested by the clinicians after the initial BG assay) were also analyzed and defined as “BG follow-up.” Patients known to have another IFD diagnosed within 1 month before or after the diagnosis of the IFD under study, or those receiving intravenous immunoglobulins (IVIG), were excluded from the analysis. This study was approved by the local ethics committee (record 20160310).
(1-3)-β-d-Glucan Antigen Detection
All BG testing was performed at the request of treating physicians by means of the Fungitell assay (Associates of Cape Cod, Inc., Falmouth, MA). Each sample was tested in duplicate, and the mean of 2 values was considered a definitive result, except when duplicates differed by more than 20%, in which case the test was repeated. We used the recommended positive cutoff value of 80 pg/mL and recorded all positive results up to 523 pg/mL. Higher results were recorded as >523 pg/mL. None of the samples were diluted.
Data Collection and Analysis
Patients' charts were reviewed, and we collected patients' demographic and general data (hospital ward, immunocompromised status) and clinical data related to the IFD (use of antifungal drugs 2 weeks before IFD, antifungal treatment after diagnosis, neutropenia [ie, neutrophil count <1500 cells/µL], the presence of a central venous catheter, assessment of IFD extent, outcome, and medical procedures or treatments known as a possible cause of false-positive BG results [albumin use, a surgical procedure, mucositis, digestive graft-versus-host disease, or hemodialysis]).
We performed the statistical analyses using the R software (version 3.2.2; http://www.cran.r-project.org). Univariate analysis was conducted using 2-sided Pearson's, Fisher's exact, and Student's t tests (α = 0.05). Variables with univariate P values <.25 were included in multivariate analyses, which were conducted using descending stepwise logistic regression. The logistic models were tested for statistical stability using the RVAideMemoire package (http://cran.r-project.org/web/packages/RVAideMemoire/). To analyze BG negativation kinetics, we performed survival analysis using Kaplan–Meier estimates.
RESULTS
Invasive Candidiasis
Forty-nine patients with candidiasis were included. As shown in Table 1, 41 had candidemia, with associated deep-seated localizations in 11. The remaining 8 had IC (endocarditis, osteoarthritis, or chronic disseminated candidiasis) without concomitant candidemia. Overall, cultures were positive in 43 of 49 patients, with Candida albicans being the most common (19 of 43, 44%) of the 11 species isolated (Table 1). Among the 41 cases of candidemia, 15 (36.5%) tested negative for BG (<80 pg/mL) at the TOD (Table 1). There were no differences between patients with negative or positive BG test results at the TOD in terms of the patients' category (see Table 1), time of BG sampling, antifungal drug use before candidemia, vascular catheterization at the TOD, outcome, and a possible cause of false-positive BG (Table 2). By contrast, patients with C. albicans candidemia were significantly more likely to have a positive BG result at the TOD (15 of 16) than did patients with candidemia due to another Candida species (9 of 25; multivariate odds ratio = 25.4; 95% confidence interval, 3.6–560.3; P <.01) (Table 2). Of note, patients with C. albicans candidemia had deep-seated localizations (8 of 16) more frequently than those with non-C. albicans candidemia (4 of 25), but the difference was not significant (data not shown).
Table 1.
Demographics, Clinical Characteristics, and Mycological Data of the Patients With Invasive Candidiasis at the Time of Diagnosis
| Patient ID | Age | Gender | Category of Patient | Sample Providing Diagnosis | Candida Species/Diagnostic Element | Location of Candidiasis | Duration of Candidemia (Days) | BG at TOD |
|
|---|---|---|---|---|---|---|---|---|---|
| Time Interval Between Sample Providing Diagnosis and BG Sample (Days) | BG Value (pg/mL) | ||||||||
| 1 | 23 | M | Hematology | Blood culture | Candida lusitaniae | Blood | 3 | 2 | 267 |
| 2 | 72 | M | Hematology | Blood culture | Candida krusei | Blood | 1 | −1 | >523 |
| 3 | 41 | F | Hematology | Blood culture | Candida glabrata | Blood | 3 | 3 | <80 |
| 4 | 46 | M | Hematology | Blood culture | Candida tropicalis | Blood | 1 | 2 | >523 |
| 5 | 59 | M | Hematology | Blood culture | C. glabrata | Blood | 4 | 3 | <80 |
| 6 | 61 | M | Hematology | Blood culture | Candida guilliermondii | Blood | 5 | −1 | <80 |
| 7 | 76 | M | Hematology | Blood culture | C. glabrata | Blood | 2 | 0 | 471 |
| 8 | 64 | F | Hematology | Blood culture | Candida albicans | Blood | 1 | 0 | 156 |
| 9 | 62 | F | Hematology | Blood culture | C. guilliermondii | Blood | 1 | −1 | <80 |
| 10 | 51 | F | Hematology | Blood culture | C. krusei | Blood | 6 | 3 | 116 |
| 11 | 46 | M | Hematology | Blood culture | C. guilliermondii | Blood | 2 | 2 | <80 |
| 12 | 68 | M | Hematology | Blood culture | Candida norvegensis | Blood | 5 | −1 | <80 |
| 13 | 74 | M | Hematology | Blood culture | C. albicans | Blood | 4 | 1 | 278 |
| 14 | 61 | F | Hematology | Blood culture | C. norvegensis | Blood | 3 | 2 | <80 |
| 15 | 75 | M | Hematology | Blood culture | C. albicans | Blood | 1 | 3 | <80 |
| 16 | 77 | M | Hematology | Blood culture | C. albicans | Blood | 1 | 2 | 233 |
| 17 | 30 | M | Hematology | Blood culture | C. albicans | Blood | 4 | 1 | >523 |
| 18 | 56 | M | SOT | Blood culture | Candida parapsilosis | Blood | 1 | 2 | 131 |
| 19 | 65 | M | SOT | Blood culture | C. parapsilosis | Blood | 4 | 3 | >523 |
| 20 | 70 | F | SOT | Blood culture | C. parapsilosis | Blood | 1 | 5 | 87 |
| 21 | 44 | F | SOT | Blood culture | C. tropicalis | Blood | 1 | 0 | 260 |
| 22 | 14 | F | Pediatric ICU | Blood culture | Kodamaea ohmeri | Blood | 3 | 6 | <80 |
| 23 | 0 | M | Pediatric ICU | Blood culture | C. albicans | Blood | 1 | 2 | 372 |
| 24 | 17 | F | Pediatric ICU | Blood culture | C. albicans | Blood | 1 | 4 | 100 |
| 25 | 2 | M | Pediatric ICU | Blood culture | C. albicans | Blood | 2 | 5 | >523 |
| 26 | 2 | M | Pediatric ICU | Blood culture | C. parapsilosis | Blood | 1 | 3 | <80 |
| 27 | 6 | F | Pediatric ICU | Blood culture | Hyphopichia burtonii | Blood | 2 | 2 | <80 |
| 28 | 1 | F | Pediatric ICU | Blood culture | C. lusitaniae | Blood | 2 | 7 | <80 |
| 29 | 1 | F | Pediatric ICU | Blood culture | C. tropicalis | Blood | 1 | 3 | 216 |
| 30 | 2 | M | Pediatric ICU | Blood culture | C. guilliermondii | Blood | 1 | 2 | <80 |
| 31 | 75 | M | Hematology | Blood culture | C. albicans | Blood, Urine | 1 | 2 | 428 |
| 32 | 12 | M | Pediatric ICU | Blood culture | C. lusitaniae | Blood, Urine | 1 | 3 | <80 |
| 33 | 2 | M | Pediatric ICU | Blood culture | C. albicans | Blood, Urine | 4 | 2 | 179 |
| 34 | 7 | M | Pediatric ICU | Blood culture | C. krusei | Blood, Ascites | 1 | 7 | <80 |
| 35 | 64 | F | Hematology | Blood culture | C. glabrata; C. krusei | Blood, Abdominal abscess | 11 | −1 | 367 |
| 36 | 4 | F | Pediatric ICU | Blood culture, Pericardial fluid | C. albicans | Blood, Sternum, Mediastinum, | 3 | 7 | 97 |
| 37 | 42 | F | Hematology | Blood culture | C. albicans | Blood, Brain | 6 | 2 | >523 |
| 38 | 66 | M | Hematology | Blood culture | C. albicans | Blood, Brain | 5 | 0 | 411 |
| 39 | 63 | F | Hematology | Blood culture | C. albicans; Candida kefyr | Blood, Intestine | 3 | 4 | >523 |
| 40 | 34 | M | Hematology | Blood culture | C. albicans | Blood, Liver, Spleen, Kidney | 1 | 2 | >523 |
| 41 | 31 | M | Hematology | Blood culture | C. albicans | Blood, Liver, Spleen, Kidney | 1 | 0 | 460 |
| 42 | 0 | F | Preterm neonate | Joint fluid aspiration | C. albicans | Multifocal osteoarthritis, Eye | na | 7 | >523 |
| 43 | 81 | M | Auto-immune disorder | Bone biopsy | C. albicans | Spondylodiscitis | na | 4 | 274 |
| 44 | 27 | M | Sickle Cell Anemia | Heart valve | C. albicans | Heart | na | −6a | 117 |
| 45 | 55 | M | Hematology | CT scanb | Microabscesses | Liver, Spleen | na | 1 | 260 |
| 46 | 55 | F | Hematology | CT scanb | Microabscesses | Liver, Spleen | na | 3 | >523 |
| 47 | 53 | M | Hematology | Liver MRI | Microabscesses | Liver | na | −4 | 348 |
| 48 | 56 | M | Hematology | CT scanb | Microabscesses | Liver, Spleen, Kidney | na | 2 | >523 |
| 49 | 23 | F | Hematology | CT scanb | Microabscesses | Liver | na | 1 | 373 |
Abbreviations: BG, (1-3)-β-d-glucan antigens; CT, computed tomography; ICU, intensive care unit; ID, identification; MRI, magnetic resonance imaging; na, not applicable; SOT, solid organ transplant; TOD, time of diagnosis.
a The diagnosis of endocarditis was made 2 months after fungemia due to C. albicans without deep-seated localization.
b Abdominal CT scan.
Table 2.
Comparison of Demographic and Clinical Characteristics of the Patients With Candidemia Who Tested Positive vs Negative for BG at the Time of Diagnosis
| Total (N = 41) | Patients With Negative BG at TOD (n = 15) (%) | Patients With Positive BG at TOD (n = 26) (%) | P Valuea | Multivariate ORb | Pab | |
|---|---|---|---|---|---|---|
| Socio-demographic data | ||||||
| Gender | ||||||
| Female | 16 | 6 (40.0) | 10 (38.5) | 1.00 | ||
| Male | 25 | 9 (60.0) | 16 (61.5) | |||
| Average age (years) [range] | 40.6 [0; 77] | 34.5 [1–75] | 44.2 [0–77] | .28 | ||
| Medical data | ||||||
| Category of patient | ||||||
| ICU pediatric patients | 13 | 7 (46.7) | 6 (23.1) | .17 | 1.0 | 0.09 |
| Hematology or Renal transplant adult patients | 28 | 8 (53.3) | 20 (76.9) | 4.7 [0.9–36.4] | ||
| Candida species responsible for candidemia | ||||||
| Non-albicans Candida species | 25 | 14 (93.3) | 11 (42.3) | <.01 | 1.0 | 0.01 |
| Candida albicans | 16 | 1 (6.7) | 15 (57.7) | 25.4 [3.6–560.3] | ||
| Median time interval between blood culture sampling and yeast growth (hours) [range] | 31 [10–67] | 29 [15–60] | 34 [10–67] | .67 | ||
| Median duration of candidemia (days) [range] | 2 [1–11] | 2 [1–5] | 1.5 [1–11] | .64 | ||
| Use of catheter at time of 1st positive blood culture | 39 | 14 (93.3) | 25 (96.2) | 1.00 | ||
| Positive culture of catheter | 11 | 4 (26.7) | 7 (28.0) | .75 | ||
| Systemic antifungal drugs before candidemia | 17 | 6 (40.0) | 11 (42.3) | 1.00 | ||
| Outcome | ||||||
| Positive | 25 | 13 (86.7) | 12 (46.2) | .02 | — | |
| Worsening (invasive candidiasis and/or death within 30 d) | 16 | 2 (13.3) | 14 (53.8) | |||
| Median time interval between candidemia and 1st BG sampling (day) [range] | 2 [−3;7] | 3 [−1;7] | 2 [−3;7] | .21 | — | |
| Neutropenia at time of candidemia | 22 | 6 (40.0) | 16 (61.5) | .21 | — | |
| Albumin during 1 mo before candidemia | 3 | 1 (6.7) | 2 (8.0) | 1.00 | ||
| Surgery within 15 d before candidemia | 8 | 2 (13.3) | 6 (23.1) | .69 | ||
| Mucitis or digestive GVH disease within 15 d before candidemia | 15 | 5 (33.3) | 10 (38.5) | 1.00 | ||
| Hemodialysis within 15 d before candidemia | 8 | 1 (6.7) | 7 (26.9) | .22 | — | |
Abbreviations: BG, (1-3)-β-d-glucan antigens; GVH, graft-vs-host; ICU, intensive care unit; OR, odds ratio; TOD, time of diagnosis.
a Univariate analyses were performed using Pearson χ2, Fisher exact, Wilcoxon, or Student t tests (α = 0.05).
b All variables with P values <.25 were included for multivariate descending stepwise logistic regression.
In 21 of 41 (51%) patients with candidemia, BG were also tested 2 to 7 days before the first positive blood culture was drawn. Seven (33%) tested positive for BG, 5 of whom had an associated deep-seated localization (data not shown). (1-3)-β-d-glucan follow-up was available for 27 of 41 (66%) patients, for 1–9 weeks (Supplementary Table 1). Of those 27, 5 had persistently negative BG test results (“negative profile”, Supplementary Table 1) and 6 alternating negative or low BG results (“unreliable kinetic profile”). Among the 16 of 27 (59%) remaining patients, who had positive BG kinetics, 5 (31%) had a rapid decrease in the BG level after the diagnosis (over half decrease in <7 days, and negativation in less than 1 month, “rapid BG decrease profile”), whereas 11 (69%) had persistently high BG test results (over half BG decrease in more than 15 days, and negativation in more than 1 month, “slow BG decrease profile”). Examples of these BG postdiagnosis kinetic profiles are presented in Supplementary Figure 1.
We observed a trend (P = .04) toward more frequent deep-seated localizations (central nervous system, kidney, liver, mediastinum, deep abscesses) associated with candidemia in patients with slow decrease profiles versus those with rapid decrease profiles (data not shown).
All 8 patients with IC without candidemia (1 case of endocarditis relapse, 2 osteoarthritis, and 5 chronic disseminated candidiasis) had positive BG at the TOD (Table 1). In 3 of the patients for whom a BG follow-up was available, BG values remained high for more than 6 weeks (Supplementary Table 1).
Invasive Aspergillosis
Forty-five patients with IA (32 [71%] probable and 13 [29%] proven IA) were included in the study. Thirty-two (71%) had a pulmonary IA, in 8 of them, associated with secondary locations (sinus, brain, skin, or pharynx), and 13 (29%) had extrapulmonary IA (ear, sinus, brain, meninges, cranium, skin, aorta, or vertebra). The patients' characteristics are given in Table 3.
Table 3.
Demographics, Clinical Characteristics, and Mycological Data of the Patients With Invasive Aspergillosis at the Time of Diagnosis
| Patient ID | Age | Gender | Category of Patient | Sample (Test) Providing Initial Diagnosis | Aspergillus Species | GM Ag at TOD (Index) | Location of IA | EORTC/MSG IA Classification | BG at TOD |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Time Interval Between Sample Providing Diagnosis and BG Sample (Days) | BG Value (pg/mL) | |||||||||
| 1 | 28 | F | Hematology | Serum (PCR) | Aspergillus fumigatus | 0.55 | Lung | Probable | 0 | 80 |
| 2 | 38 | M | Hematology | Serum (GM Ag) | A. fumigatus | 0.72 | Lung | Probable | 0 | <80 |
| 3 | 73 | M | Hematology | Serum (GM Ag) | — | 0.73 | Lung | Probable | 0 | <80 |
| 4 | 80 | F | Hematology | Serum (GM Ag) | — | 1 | Lung | Probable | 1 | <80 |
| 5 | 29 | F | Hematology | Serum (GM Ag) | — | 1.18 | Lung | Probable | 0 | <80 |
| 6 | 63 | F | Hematology | Serum (GM Ag) | A. fumigatus | 0.56 | Lung | Probable | −3 | 467 |
| 7 | 69 | F | Hematology | Serum (GM Ag) | — | 0.67 | Lung | Probable | 0 | <80 |
| 8 | 30 | F | Hematology | Serum (GM Ag) | — | 0.54 | Lung | Probable | 0 | <80 |
| 9 | 63 | M | Hematology | Serum (GM Ag) | A. fumigatus | 0.51 | Lung | Probable | −3 | <80 |
| 10 | 54 | F | Hematology | Serum (PCR) | A. fumigatus | 1.15 | Lung | Probable | 0 | 99 |
| 11 | 63 | F | Hematology | BAL (Culture) | A. fumigatus | 0.5 | Lung | Probable | 3 | <80 |
| 12 | 20 | M | Hematology | Serum (GM Ag) | A. fumigatus | 0.64 | Lung | Probable | 0 | 131 |
| 13 | 23 | M | Hematology | Serum (PCR) | A. fumigatus | 0.27 | Lung | Probable | −2 | >523 |
| 14 | 0 | M | Hematology | Lung biopsy (Culture) | A. fumigatus | Nd | Lung | Proven | −2 | <80 |
| 15 | 51 | M | Hematology | Serum (GM Ag) | — | 1.69 | Lung | Probable | 4 | <80 |
| 16 | 61 | M | Hematology | Serum (GM Ag) | A. fumigatus | 0.84 | Lung | Probable | 0 | 129 |
| 17 | 46 | F | Hematology | Serum (GM Ag) | A. fumigatus | 1.13 | Lung | Probable | 0 | <80 |
| 18 | 76 | M | Hematology | Serum (PCR) | A. fumigatus | 5.63 | Lung | Probable | 0 | <80 |
| 19 | 67 | M | Hematology | Serum (GM Ag) | — | 0.61 | Lung | Probable | 0 | <80 |
| 20 | 73 | F | Hematology | Serum (PCR) | A. fumigatus | 1.9 | Lung | Probable | 0 | 176 |
| 21 | 53 | M | SOT, Hematology | Bronchoaspiration (Culture) | A. fumigatus | 0.06 | Lung | Probable | −5a | 113 |
| 22 | 60 | M | SOT | Lung biopsy (Culture) | A. fumigatus | Nd | Lung | Probable | 7 | <80 |
| 23 | 71 | F | SOT | Serum (GM Ag) | — | 1.3 | Lung | Probable | 5 | 349 |
| 24 | 62 | M | Hematology | Serum (PCR) | A. fumigatus | 0.44 | Lung | Probable | 0 | <80 |
| 25 | 22 | M | Hematology | Uvula biopsy (Culture) | A. fumigatus | 0.23 | Lung, Pharynx | Proven | 1 | <80 |
| 26 | 47 | F | Hematology | Serum (GM Ag) | — | 1.8 | Lung, Sinus | Probable | 0 | 83 |
| 27 | 57 | M | Hematology | Serum (GM Ag) | A. fumigatus | 0.64 | Lung, Sinus | Probable | 0 | 257 |
| 28 | 55 | F | Hematology | Serum (GM Ag) | — | 4.61 | Lung, Sinus | Probable | 0 | >523 |
| 29 | 42 | M | SOT | Serum (PCR) | A. fumigatus | Nd | Lung, Brain | Probable | 3 | >523 |
| 30 | 74 | M | SOT, Hematology | CSF (PCR) | A. fumigatus | Nd | Lung, Sinus, Brain | Probable | 0 | <80 |
| 31 | 65 | M | Hematology | Serum (GM Ag) | — | 2.04 | Lung, Sinus, Brain | Probable | 0 | <80 |
| 32 | 51 | M | SOT | Skin biopsy (Culture) | A. fumigatus | 0.09 | Lung, Brain, Skin | Proven | 4 | 377 |
| 33 | 56 | M | SOT | CSF (PCR) | A. fumigatus | Nd | Aorta, Skin, Brain | Probable | 1 | >523 |
| 34 | 61 | M | Hematology | Serum (GM Ag) | — | 1.44 | Brain | Probable | 0 | 95 |
| 35 | 63 | F | Hematology | Sinus biopsy (Culture) | Aspergillus flavus | 0.22 | Sinus | Proven | 0 | <80 |
| 36 | 74 | M | Non-IC | Sinus biopsy (Culture) | A. fumigatus | 0.17 | Sinus, Eye socket | Proven | 2 | <80 |
| 37 | 72 | M | SOT | CSF (PCR) | A. fumigatus | 0.11 | Sinus, Brain | Probable | 6 | 278 |
| 38 | 56 | M | Non-IC | Sinus biopsy (Culture) | A. fumigatus | Nd | Sinus, Brain | Proven | −3 | <80 |
| 39 | 19 | M | Non-IC | Sinus biopsy (Culture) | A. flavus | 0.1 | Sinus, Cranium, Brain | Proven | 11 | >523 |
| 40 | 76 | M | Diabetes | Sinus biopsy (Culture) | A. fumigatus | Nd | Ear, Meninges | Proven | 7 | 328 |
| 41 | 71 | M | Diabetes | Sinus biopsy (Culture) | A. flavus | 0.3 | Ear, Sinus, Cranium | Proven | 14 | <80 |
| 42 | 74 | F | Diabetes | Clivus biopsy (Culture) | A. flavus | 0.18 | Ear, Cranium | Proven | 12 | 160 |
| 43 | 68 | F | Diabetes | Meningeal biopsy (Culture) | A. flavus | 0.06 | Ear, Cranium, Meninges | Proven | 13 | 118 |
| 44 | 68 | M | Non-IC | Vertebral biopsy (Culture) | Aspergillus terreus | 1.06 | Vertebra | Proven | 3 | >523 |
| 45 | 19 | M | Non-IC | Vertebral biopsy (Culture) | A. terreus | 0.18 | Vertebra | Proven | 0 | 440 |
Abbreviations: BG, (1-3)-β-d-glucan; CSF, cerebral spinal fluid; EORTC/MS, European Organization for Research and Treatment of Cancer/Mycoses Study Group; GM Ag, galactomannan antigens; IA, invasive aspergillosis; Nd, not determined; Non-IC, non-immunocompromised; PCR, polymerase chain reaction; SOT, solid organ transplant; TOD, time of diagnosis.
a An exception was made regarding the inclusion of this patient, even though his/her first BG was sampled 5 days before mycological diagnosis.
At the TOD, 22 of 45 (48%) were BG negative (Table 3). When comparing the groups of patients with negative and positive BG test results at the TOD, we did not observe differences in the categories of the patients (see Table 3), the location or classification of IA, the time of BG sampling, the antifungal drug use before the diagnosis, neutropenia status of the patients, 6- or 12-week survival, or the possible cause of false-positive BG results (Supplementary Table 2).
In 14 of 45 (31%) IA patients, BG were measured 4 to 15 days before TOD, and the results were positive in 5 cases (data not shown). (1-3)-β-d-glucan follow-up was available for 37 of 45 (82%) patients for periods ranging from 1.5 to 29 weeks (Supplementary Table 3). Of the 37 patients with BG follow-up, 7 were persistently BG negative, 5 had variable positive/negative BG test results, and 25 were repeatedly BG positive (Supplementary Table 3). Among these 25, BG levels decreased rapidly in 7 (28%), with negativation within 1 month after the TOD, whereas in 14 (56%) patients, BG results were still positive after 3 months (Supplementary Figure 2). Examples of BG postdiagnosis kinetic profiles are presented in Figure 1. When comparing patients with rapid versus slow BG negativation in terms of type and location of IA and survival, we did not observe significant differences, except for a trend toward slower BG negativation in patients with sinus and/or brain IA (P = .05; data not shown).
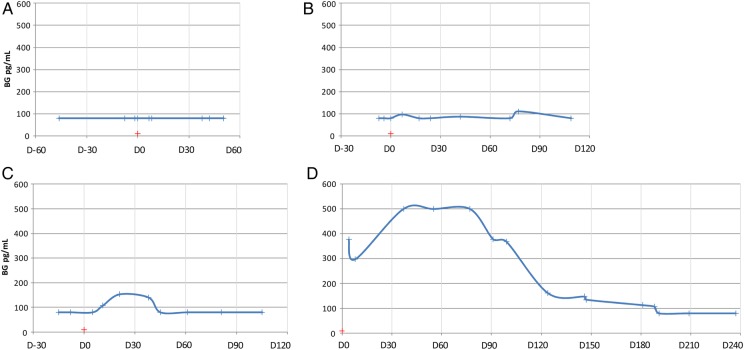
Figure 1.
Examples of the 4 main categories of (1-3)-β-d-glucan (BG) postdiagnosis kinetic profiles observed after a diagnosis of invasive aspergillosis (IA). For each example, the blue curve represents the BG kinetics before, at the time, and after a diagnosis of IA. The red cross represents the day of collection (D0) of the first biological sample that led to the diagnosis of IA: y-axis, BG values in pg/mL; x-axis, time relative to the day (D0) of collection of the sample that led to the diagnosis of IA, expressed in weeks. (A) Negative BG profile (N = 8 of 37): patients showing persistently negative BG test results after IA diagnosis. The kinetics shown are those of Patient 19 (hematology patient), who received a diagnosis of probable pulmonary IA, for whom 3 BG assays were performed before diagnosis and 6 BG assays were performed within 12 weeks of diagnosis. The first mycological result allowing for the diagnosis of probable IA was the detection of serum galactomannan antigens. (B) Unreliable BG profile (N = 5 of 37): patients showing alternating negative or low BG test results after IA diagnosis. The kinetics shown are those of Patient 2 (hematology patient), who received a diagnosis of probable pulmonary IA, for whom 2 BG assays were performed before diagnosis and 8 BG assays were performed within 16 weeks of diagnosis. The first mycological result allowing for diagnosis of probable IA was the detection of serum galactomannan antigens. (C) Rapid BG decrease profile (N = 6 of 37): patients with positive BG kinetics after IA diagnosis, who showed a rapid decrease in the BG level, with BG negativation in less than 1 month. The kinetics shown are those of Patient 7 (hematology patient), who received a diagnosis of probable pulmonary IA, for whom 2 BG assays were performed before diagnosis and 8 assays were performed within 13 weeks of diagnosis. The first mycological result allowing for diagnosis of probable IA was the detection of serum galactomannan antigens. (D) Slow BG decrease profile (N = 14 of 37): patients with positive BG kinetics after IA diagnosis, who showed persistently elevated BG levels for more than 3 months. The kinetics shown are those of Patient 32 (solid organ transplant), who received a diagnosis of proven multisite IA, for whom 15 BG assays were performed within 33 weeks of diagnosis. The first mycological result allowing for diagnosis of proven IA was a culture of a skin biopsy that tested positive for Aspergillus fumigatus.
Rare Invasive Fungal Diseases
The 49 patients with rare IFD were classified into 18 diagnoses including cryptococcosis (N = 10); mucormycosis (N = 6); fusariosis, scedosporiosis, and trichosporonosis (N = 4 each); eumycotic mycetoma (N = 5); and invasive subcutaneous phaeohyphomycosis (N = 3). The patients' characteristics are presented in Table 4.
Table 4.
Demographics, Clinical Characteristics, and Mycological Data of Patients With Rare Invasive Fungal Diseases
| IFD due to Rare Fungi | Age | Gender | Category of Patient | Sample Providing Diagnosis | Diagnostic Element | Location of Fungal Infection | BG at TOD |
|
|---|---|---|---|---|---|---|---|---|
| Time Interval Between Sample Providing Diagnosis and BG Sample (Days) | BG Value (pg/mL) | |||||||
| Cryptococcosis | 5 | F | Non-IC | Blood culture | Cryptococcus neoformans | Blood, Meninges, Lymph nodes | 6 | <80 |
| 55 | F | Hematology | CSF | C. neoformans | Meninges | 1 | 244 | |
| 57 | M | HIV | CSF | C. neoformans | Blood, Meninges, Urine | 4 | <80 | |
| 56 | F | SOT | Serum | Positive cryptococcal EIA antigen | Meninges, Skin, Urine | 0 | 89 | |
| 43 | M | SOT | Sputum | C. neoformans | Lung | 5 | <80 | |
| 48 | F | SOT | CSF | C. neoformans | Meninges | 1 | <80 | |
| 60 | M | HIV | Pulmonary biopsy | Encapsulated yeasts at Grocott staining | Lung | −1 | 342 | |
| 18 | F | Hematology | Blood culture | C. neoformans | Blood | −1 | <80 | |
| 62 | M | SOT | CSF | C. neoformans | Meninges | 0 | <80 | |
| 17 | F | SOT | CSF | C. neoformans | Meninges | 8 | <80 | |
| Zygomycosis | 69 | F | SOT | Cutaneous biopsy | Mucor irregularis | Skin and subcutaneous tissue | 6 | 144 |
| 4 | F | SOT | Gastric biopsy | Lichtheimia sp | Gastrointestinal tract | 1 | 158 | |
| 22 | M | Hematology | Sinus biopsy | Rhizopus sp | Sinus | 0 | 134 | |
| 66 | F | Hematology | Sinus biopsy | Lichtheimia ramosa | Sinus | −1 | <80 | |
| 8 | F | SOT | Cutaneous biopsy; Serum | Rhizopus oryzae | Skin and subcutaneous tissue | 10 | <80 | |
| IFD due to Fusarium sp | 70 | F | Hematology | Blood culture | Fusarium solanii | Blood | 2 | 99 |
| 61 | F | SOT | Sputum | Fusarium sp | Lung | 0 | <80 | |
| 42 | M | Hematology | Blood culture | Fusarium sp | Blood | 10 | <80 | |
| 62 | F | Hematology | Blood culture | Fusarium proliferatum | Blood | 0 | <80 | |
| IFD due to Trichosporon sp | 33 | M | Hematology | Blood culture | Trichosporon sp | Blood | 1 | >523 |
| 67 | F | Hematology | Blood culture | Trichosporon sp | Blood | 9 | >523 | |
| 10 | F | ICU | Blood culture | Trichosporon asahii | Blood | 6 | <80 | |
| IFD due to Rhodotorula sp | 3 | M | ICU | Blood culture | Rhodotorula mucilaginosa | Blood | 4 | 184 |
| 2 | M | Hematology | Blood culture | R. mucilaginosa | Blood | 5 | <80 | |
| IFD due to Geotrichum sp | 72 | M | Hematology | Blood culture | Geotrichum capitatum | Blood | 0 | 287 |
| IFD due to Malassezia sp | 4 | M | Hematology | Blood culture | Malassezia furfur | Blood | 3 | <80 |
| IFD due to Acremonium sp | 63 | M | Hematology | Blood culture | Acremonium strictum | Blood | 0 | <80 |
| Infection due to Trichoderma sp | 67 | F | SOT | BAL | Trichoderma longibrachiatum | Lung | 3 | 489 |
| IFD due to Exophiala sp | 8 | F | Hematology | CSF | Exophiala dermatitidis | Brain, Meninges | −3 | >523 |
| IFD due to Cladophialophora sp | 6 | M | Non-IC | CSF | Cladophialophora bantiana | Brain | 6 | 145 |
| IFD due to Rasamsonia sp | 29 | M | Hematology | Cutaneous abscess | Rasamsonia argillacea | Lung, Skin, Subcutaneous tissue | 1 | <80 |
| Histoplasmosis | 43 | F | HIV | Bone marrow aspiration | Histoplasma capsulatum | Bone marrow, Lymph nodes, Skin | 2 | 233 |
| 36 | F | HIV | Bone marrow aspiration | H. capsulatum | Disseminated | 3 | 255 | |
| Coccidioidomycosis | 32 | M | Non-IC | Sputum | Coccidioides immitis | Lung (relapse) | 0a | 150 |
| IFD due to Scedosporium sp | 69 | F | SOT | Bone biopsy | Scedosporium apiospermum | Lung, Bone, Eye, Blood | 2 | >523 |
| 14 | M | Hematology | Sputum | Scedosporium prolificans | Lung, Blood | 1 | <80 | |
| 32 | M | Cystic fibrosis | Sputum | Scedosporium sp | Bone (relapse), Disseminated | 7b | 270 | |
| 52 | F | SOT | Cutaneous sample | S. apiospermum | Subcutaneous tissue, Bone | 0 | <80 | |
| Invasive cutaneous phaeohyphomycosis | 62 | M | SOT | Cutaneous biopsy | Alternaria infectoria | Skin, Subcutaneous tissue | 0 | >523 |
| 58 | F | SOT | Cutaneous biopsy | Alternaria rosea | Skin, Subcutaneous tissue | −4 | 298 | |
| 63 | M | SOT | Cutaneous biopsy | Phialemonium dimorphosporum | Skin, Subcutaneous tissue | 3 | >523 | |
| Deep dermatophytosis | 72 | M | SOT | Cutaneous biopsy | Trichophyton rubrum | Skin, Subcutaneous tissue | −2 | 151 |
| 56 | M | SOT | Cutaneous biopsy | T. rubrum | Skin, Subcutaneous tissue | 1 | >523 | |
| Eumycotic mycetoma | 34 | M | Non-IC | Cutaneous biopsy | Madurella mycetomatis | Lumbar subcutaneous tissue, Lung, Pleura, Retroperitoneum | 0c | 254 |
| 59 | M | SOT | Cutaneous biopsy | Nonidentified black fungus | Subcutaneous tissue | 1d | 304 | |
| 69 | M | Diabetes | Cutaneous biopsy | Exophiala jeanselmei | Subcutaneous tissue | 0 | 305 | |
| 68 | M | SOT | Cutaneous biopsy | F. solanii | Subcutaneous tissue | 0 | 337 | |
| 54 | M | SOT | Grains in seropurulent exudate | Nonidentified fungus | Subcutaneous tissue | 0 | 237 | |
Abbreviations: BG, (1-3)-β-d-glucan; BAL, bronchoalveolar lavage; CSF, cerebral spinal fluid; EIA, enzyme immunoassay; HIV, human immunodeficiency virus; ICU, intensive care unit; IFD, invasive fungal diseases; Non-IC, non-immunocompromised; SOT, solid organ transplant; TOD, time of diagnosis.
a The initial diagnosis of pulmonary coccidioidomycosis was performed 21 months before the current relapse.
b The initial diagnosis of spondylodiscitis due to Scedosporium sp was performed 4 years before the current relapse.
c The initial diagnosis of the eumycotic mycetoma was performed 13 years before the current onset.
d The initial diagnosis of the eumycotic mycetoma was performed 24 years before the current onset.
Overall, 19 (39%) of the patients with rare IFD were BG negative at the TOD. We observed great variability of BG positivity at the TOD in almost every category of rare IFD, except for the 10 patients with chronic subcutaneous IFD (including phaeohyphomycosis, deep dermatophytosis, or eumycotic mycetoma) who were all BG positive at the TOD. A BG follow-up was available for 4 of these 10 patients, and their BG results remained positive for over 25 weeks after the TOD. Unexpectedly, 3 of 10 patients with cryptococcosis (1 pulmonary case and 2 meningeal cases) and 3 of 6 patients with mucormycosis (1 subcutaneous case, 1 intestinal, and 1 sinus case) were BG positive at the TOD.
DISCUSSION
Our study, which aimed at assessing BG serum levels at TOD and BG follow-up kinetics in patients treated for documented IFD, has resulted in several significant findings.
First, we observed a relatively low BG positivity rate at the TOD in the 2 most common IFD, candidemia and IA, as well as in rare IFD. Indeed, only 64%, 52%, and 61% of patients who received a diagnosis of candidemia, probable/proven IA, or rare IFD tested positive for BG at the TOD, respectively. These findings confirmed the results of other studies reporting low BG sensitivity in different IFD and various patient populations [13–15, 19]. For instance, Koo et al [13] and Ostrosky-Zeichner et al [14], who both analyzed the BG assay performance at the TOD regardless of the category of patients or type of IFD, observed an average sensitivity of 64%. Koo et al [13] reported even lower BG sensitivity at the TOD among patients receiving a hematopoietic stem cell transplant or patients with febrile neutropenia (43% and 38%, respectively) [13]. It is noteworthy that the BG positivity rate observed at the TOD in patients who received a diagnosis of candidemia in our study was somehow lower than the ones previously reported; other authors, including Ostrosky-Zeichner et al [14], reported sensitivity rates above 80% [27, 28]. This lack of positive BG test results at the TOD observed in every type of IFD is casting doubt on the usefulness of BG detection for early diagnosis of IFD. Thus, the BG serial sampling proposed in some works [17, 20, 29, 30] to help anticipate IFD diagnosis in at-risk patients might be effective only for a minority of patients, which remains to be determined.
Among patients with candidemia, we did not find medical risk factors associated with BG positivity at the TOD (eg, vascular catheterization, prior antifungal drug use, or an already known possible cause of false-positive BG results), but we observed differences strongly related to the Candida species involved. Indeed, we found that BG is a more reliable diagnostic marker in cases of candidemia due to C. albicans than to other Candida species. To our knowledge, this finding has not been reported previously. Ostrosky-Zeichner et al [14] stated that the BG assay may be less sensitive for Candida parapsilosis than for other Candida species, but this notion was not confirmed by Martínez-Jiménez [27], who observed good sensitivity of BG testing in cases of candidemia due to C. parapsilosis, even better than for C. albicans. Because most Candida infections are caused by endogenous Candida strains colonizing the patients before the infection [31, 32], a procedure of BG serial sampling may thus be worthwhile (targeting patients at risk of candidemia and known to be colonized by C. albicans or hospitalized in wards reporting high prevalence of C. albicans infections). Regarding the BG evolution in patients with candidemia who tested positive for BG at the TOD, we observed very different patterns of BG dynamics. In one third of the cases, the BG levels decreased rapidly after initiation of treatment (a significant decrease in 1 week), whereas in the other cases (two thirds), the BG level was persistently elevated during at least 2 weeks after the IFD diagnosis. It is worth mentioning that patients with slow BG decrease profiles were more likely to have candidemia associated with deep-seated infected sites. Furthermore, patients with IC not associated with candidemia, in particular patients with chronic disseminated candidiasis, also showed persistently elevated serum BG levels. These findings are consistent with the results of other studies suggesting that a decrease in BG levels after treatment initiation is a marker of a favorable prognosis, especially in IC [20, 22, 23, 33]. Jaijakul et al [22] observed a decrease in BG levels among patients who received successful treatment of candidemia, whereas patients with tissue infection or treatment failure showed persistently elevated BG levels. Altogether, these results should encourage clinicians to evaluate BG kinetics in patients with positive BG test results at the TOD of candidemia or in patients with suspected deep-seated candidiasis, in particular chronic disseminated candidiasis. Complementary prospective studies focusing on BG kinetics during treatment would be useful in this regard.
As for IA, we did not find a significant association between the level of BG at TOD and the status of the patient (immunocompromised or not), the type of aspergillosis (location or EORTC/MSG classification), or the outcome. On the other hand, as in candidemia, only one third of the patients with positive BG at the TOD had a rapid BG decrease profile (negativation within 1 month), whereas two thirds showed a very slow BG decrease, with negativation often observed more than 100 days after the diagnosis. This very slow BG evolution, which might be as lengthy as or even longer than the therapeutic protocol itself, may limit the use of BG serial sampling for monitoring the clinical course in cases of IA [18, 19]. Nevertheless, in the absence of a tangible improvement after treatment initiation, persistently elevated or rising BG levels 1 month after the diagnosis may warn clinicians about a possible treatment failure [18, 19]. Further studies in this area are necessary to evaluate the real efficacy of such a strategy.
Regarding BG detection in rare IFD, we observed a relatively low frequency of positive BG results at the TOD, but we noticed an exception. In cases of chronic subcutaneous IFD (phaeohyphomycosis, deep dermatophytosis, or eumycotic mycetoma), all patients tested positive for BG at the TOD, and we observed extremely slow BG decrease after the diagnosis (BG persistently positive for more than 25 weeks). This result may be linked to the chronic and relatively indolent progression of this type of infections, with often delayed diagnosis [34]. Thus, our findings suggest that BG may be a useful diagnostic marker of chronic subcutaneous IFD. Although we expected all patients with cryptococcosis and mucormycosis to test negative for BG at the TOD [6, 35], some of them tested positive. Such findings have already been reported regarding cryptococcosis [36, 37]. Rhein et al [37], who observed 89% sensitivity of a BG assay in cerebrospinal fluid of patients with cryptococcal meningitis, suggested that due to the low reactivity of Cryptococcus species with the BG test [38] (presumably due to BG's low concentration in the Cryptococcus cell wall), the success of BG detection during cryptococcosis may depend on the fungal burden during the infection. For mucormycosis, however, none of the studies involving patients with this type of IFD showed BG positivity at the TOD [13, 14, 19, 36]. Positive BG test results in our cases of mucormycosis may be associated, as suggested above, with the substantial fungal burden. These positive test results may also be a consequence of a yet unknown cross-reactivity of the BG assay. Indeed, if we had assessed the main causes of BG false-positivity in our study (IVIG, albumin injection, hemodialysis, or a recent surgical procedure), we might have missed less frequent causes, such as concomitant infection due to Nocardia [39] or even unknown causes.
CONCLUSIONS
Overall, our study, which included many categories of patients (hematology, SOT, ICU, and even non-immunocompromised patients), suggests that BG detection in the serum of at-risk patients is not as effective as an early diagnostic marker of IFD. Nonetheless, our findings also indicate that this marker may help to assess the risk of chronic infection after the initial IFD diagnosis, and, in particular, serial BG sampling may facilitate the evaluation of the risk of disseminated candidiasis associated with candidemia. Taken together, our results offer valuable information on clinical utility of the BG assay in various IFD and underscore the difficulties with interpretation of BG data in clinical settings.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
Author contributions. All coauthors have contributed significantly to the work. C. A. and M.-E. B. wrote the first draft of the manuscript, and all of the coauthors participated in revision and approved the final version.
Financial support. This work was supported by the Association pour la Promotion de l′Enseignement et de la Recherche en Mycologie.
Potential conflicts of interest. C. A. received a research grant from Pfizer. P. F. received grants from SIDACTION and the French National Agency for Research on AIDS and Viral Hepatitis; consultancy, or lecture fees, and travel grants from ViiV Healthcare, Bristol-Myers Squibb, Janssen Cilag, Gilead Sciences, and MSD. D. C. has received fees for board membership of MSD. O. L. has received consultancy or lecture fees from Gilead Sciences, Pfizer, Astellas, and Merck. M.-E. B. has received institutional grant funding from MSD and Astellas and lecture fees or travel grants from MSD, Astellas, and Gilead Sciences. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Kontoyiannis DP, Marr KA, Park BJ et al. . Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) database. Clin Infect Dis 2010; 50:1091–100. [DOI] [PubMed] [Google Scholar]
- 2.Pappas PG, Alexander BD, Andes DR et al. . Invasive fungal infections among organ transplant recipients: results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin Infect Dis 2010; 50:1101–11. [DOI] [PubMed] [Google Scholar]
- 3.Pfaller MA, Diekema DJ. Epidemiology of invasive mycoses in North America. Crit Rev Microbiol 2010; 36:1–53. [DOI] [PubMed] [Google Scholar]
- 4.Bitar D, Lortholary O, Le Strat Y et al. . Population-based analysis of invasive fungal infections, France, 2001–2010. Emerg Infect Dis 2014; 20:1149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manners DJ, Masson AJ, Patterson JC. The structure of a β-(1->3)-d-glucan from yeast cell walls. Biochem J 1973; 135:19–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Pauw B, Walsh TJ, Donnelly JP et al. . Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008; 46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Theel ES, Doern CD. β-d-glucan testing is important for diagnosis of invasive fungal infections. J Clin Microbiol 2013; 51:3478–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sulahian A, Porcher R, Bergeron A et al. . Use and limits of (1-3)-β-d-glucan assay (Fungitell), compared to galactomannan determination (Platelia Aspergillus) for diagnosis of invasive aspergillosis. J Clin Microbiol 2014; 52:2328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Posteraro B, De Pascale G, Tumbarello M et al. . Early diagnosis of candidemia in intensive care unit patients with sepsis: a prospective comparison of (1->3)-β-d-glucan assay, Candida score, and colonization index. Crit Care 2011; 15:R249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Del Bono V, Delfino E, Furfaro E et al. . Clinical performance of the (1,3)-β-d-glucan assay in early diagnosis of nosocomial Candida bloodstream infections. Clin Vaccine Immunol 2011; 18:2113–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mularoni A, Furfaro E, Faraci M et al. . High levels of β-d-glucan in immunocompromised children with proven invasive fungal disease. Clin Vaccine Immunol 2010; 17:882–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mokaddas E, Khan ZU, Ahmad S et al. . Value of (1-3)-β-d-glucan, Candida mannan and Candida DNA detection in the diagnosis of candidaemia. Clin Microbiol Infect 2011; 17:1549–53. [DOI] [PubMed] [Google Scholar]
- 13.Koo S, Bryar JM, Page JH et al. . Diagnostic performance of the (1→3)-β-d-glucan assay for invasive fungal disease. Clin Infect Dis 2009; 49:1650–9. [DOI] [PubMed] [Google Scholar]
- 14.Ostrosky-Zeichner L, Alexander BD, Kett DH et al. . Multicenter clinical evaluation of the (1→3) β-d-glucan assay as an aid to diagnosis of fungal infections in humans. Clin Infect Dis 2005; 41:654–9. [DOI] [PubMed] [Google Scholar]
- 15.Karageorgopoulos DE, Vouloumanou EK, Ntziora F et al. . β-d-glucan assay for the diagnosis of invasive fungal infections: a meta-analysis. Clin Infect Dis 2011; 52:750–70. [DOI] [PubMed] [Google Scholar]
- 16.Marchetti O, Lamoth F, Mikulska M et al. . ECIL recommendations for the use of biological markers for the diagnosis of invasive fungal diseases in leukemic patients and hematopoietic SCT recipients. Bone Marrow Transplant 2012; 47:846–54. [DOI] [PubMed] [Google Scholar]
- 17.Mohr JF, Sims C, Paetznick V et al. . Prospective survey of (1->3)-β-d-glucan and its relationship to invasive candidiasis in the surgical intensive care unit setting. J Clin Microbiol 2011; 49:58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Senn L, Robinson JO, Schmidt S et al. . 1,3-β-d-glucan antigenemia for early diagnosis of invasive fungal infections in neutropenic patients with acute leukemia. Clin Infect Dis 2008; 46:878–85. [DOI] [PubMed] [Google Scholar]
- 19.Hachem RY, Kontoyiannis DP, Chemaly RF et al. . Utility of galactomannan enzyme immunoassay and (1,3)-β-d-glucan in diagnosis of invasive fungal infections: low sensitivity for Aspergillus fumigatus infection in hematologic malignancy patients. J Clin Microbiol 2009; 47:129–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tissot F, Lamoth F, Hauser PM et al. . β-glucan antigenemia anticipates diagnosis of blood culture-negative intraabdominal candidiasis. Am J Respir Crit Care Med 2013; 188:1100–9. [DOI] [PubMed] [Google Scholar]
- 21.Koo S, Baden LR, Marty FM. Post-diagnostic kinetics of the (1 → 3)-β-d-glucan assay in invasive aspergillosis, invasive candidiasis and Pneumocystis jirovecii pneumonia. Clin Microbiol Infect 2012; 18:E122–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaijakul S, Vazquez JA, Swanson RN, Ostrosky-Zeichner L. (1,3)-β-d-glucan as a prognostic marker of treatment response in invasive candidiasis. Clin Infect Dis 2012; 55:521–6. [DOI] [PubMed] [Google Scholar]
- 23.Mikulska M, Furfaro E, Del Bono V et al. . Persistence of a positive (1,3)-β-d-glucan test after clearance of candidemia in hematopoietic stem cell transplant recipients. Clin Vaccine Immunol 2011; 18:518–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Azoulay E, Guigue N, Darmon M et al. . (1, 3)-β-d-glucan assay for diagnosing invasive fungal infections in critically ill patients with hematological malignancies. Oncotarget 2016; 7:21484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White PL, Barnes RA, Springer J et al. . Clinical performance of Aspergillus PCR for testing serum and plasma: a study by the European Aspergillus PCR Initiative. J Clin Microbiol 2015; 53:2832–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suarez F, Lortholary O, Buland S et al. . Detection of circulating Aspergillus fumigatus DNA by real-time PCR assay of large serum volumes improves early diagnosis of invasive aspergillosis in high-risk adult patients under hematologic surveillance. J Clin Microbiol 2008; 46:3772–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martínez-Jiménez MC, Muñoz P, Valerio M et al. . Candida biomarkers in patients with candidaemia and bacteraemia. J Antimicrob Chemother 2015; 70:2354–61. [DOI] [PubMed] [Google Scholar]
- 28.Giacobbe DR, Esteves P, Bruzzi P et al. . Initial serum (1,3)-β-d-glucan as a predictor of mortality in proven candidaemia: findings from a retrospective study in two teaching hospitals in Italy and Brazil. Clin Microbiol Infect 2015; 21:954.e9–17. [DOI] [PubMed] [Google Scholar]
- 29.Koltze A, Rath P, Schöning S et al. . β-d-glucan screening for detection of invasive fungal disease in children undergoing allogeneic hematopoietic stem cell transplantation. J Clin Microbiol 2015; 53:2605–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schwarzinger M, Sagaon-Teyssier L, Cabaret O et al. . Performance of serum biomarkers for the early detection of invasive aspergillosis in febrile, neutropenic patients: a multi-state model. PLoS One 2013; 8:e65776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miranda LN, van der Heijden IM, Costa SF et al. . Candida colonisation as a source for candidaemia. J Hosp Infect 2009; 72:9–16. [DOI] [PubMed] [Google Scholar]
- 32.Eggimann P, Garbino J, Pittet D. Epidemiology of Candida species infections in critically ill non-immunosuppressed patients. Lancet Infect Dis 2003; 3:685–702. [DOI] [PubMed] [Google Scholar]
- 33.Sims CR, Jaijakul S, Mohr J et al. . Correlation of clinical outcomes with β-glucan levels in patients with invasive candidiasis. J Clin Microbiol 2012; 50:2104–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crabol Y, Poiree S, Bougnoux ME et al. . Last generation triazoles for imported eumycetoma in eleven consecutive adults. PLoS Negl Trop Dis 2014; 8:e3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Associates of Cape Cod Incorporated. Fungitell Assay. Available at: http://www.acciusa.com/clinical/fungitell/index.html. Accessed 28 September 2015. [Google Scholar]
- 36.Obayashi T, Negishi K, Suzuki T, Funata N. Reappraisal of the serum (1-->3)-beta-d-glucan assay for the diagnosis of invasive fungal infections--a study based on autopsy cases from 6 years. Clin Infect Dis 2008; 46:1864–70. [DOI] [PubMed] [Google Scholar]
- 37.Rhein J, Bahr NC, Morawski BM et al. . Detection of high cerebrospinal fluid levels of (1→3)-β-d-glucan in cryptococcal meningitis. Open Forum Infect Dis 2014; 1:ofu105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odabasi Z, Paetznick VL, Rodriguez JR et al. . Differences in beta-glucan levels in culture supernatants of a variety of fungi. Med Mycol 2006; 44:267–72. [DOI] [PubMed] [Google Scholar]
- 39.Koncan R, Favuzzi V, Ligozzi M et al. . Cross-reactivity of Nocardia spp. in the fungal (1-3)-β-d-glucan assay performed on cerebral spinal fluid. Diagn Microbiol Infect Dis 2015; 81:94–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.