Abstract
Stenotrophomonas maltophilia is an opportunistic pathogen that causes respiratory and urinary tract infections, as well as wound infections in immunocompromised patients. This pathogen is difficult to treat due to increased resistance to many antimicrobial agents. We investigated the in vitro biofilm formation of S. maltophilia, including effects of fluoroquinolones (FQs) and azithromycin on biofilm formation. The organism initiated attachment to polystyrene surfaces after a 4 h incubation period, and reached maximal growth at 18–24 h. In the presence of FQs (moxifloxacin, levofloxacin or ciprofloxacin), the biofilm biomass was significantly reduced (P < 0.05). A lower concentration of moxifloxacin (10 μg/mL) exhibited a better inhibiting effect on biofilm formation than 100 μg/mL (P < 0.01), but with no difference in effect compared to the 50 μg/mL concentration (P > 0.05). However, the inhibitory effects of 10 μg/mL of levofloxacin or ciprofloxacin were slightly less pronounced than those of the higher concentrations. A combination of azithromycin and FQs significantly reduced the biofilm inhibiting effect on S. maltophilia preformed biofilms compared to azithromycin or FQs alone. We conclude that early use of clinically acceptable concentrations of FQs, especially moxifloxacin (10 μg/mL), may possibly inhibit biofilm formation by S. maltophilia. Our study provides an experimental basis for a possible optimal treatment strategy for S. maltophilia biofilm-related infections.
Stenotrophomonas maltophilia (S. maltophilia) is an opportunistic pathogen that is widely distributed in nature. It is detected in rivers, sewage, as well as in pharyngeal, sputum, and fecal samples of healthy individuals1,2. S. maltophilia often causes respiratory and urinary tract infections in immunocompromised patients. This pathogen, which is the third most common infectious non-fermentative bacterium after Pseudomonas aeruginosa and Acinetobacter baumannii3,4,5, can also cause severe bacteremia and endocarditis2,6. Risk factors for S. maltophilia infections include immune dysfunction and invasive clinical treatments to combat diseases, including endotracheal intubation and malignant tumors2,7,8.
A bacterial biofilm refers to a group of bacterial cells that adhere to one another on a surface. These adherent cells are often embedded within a self-produced matrix of an extracellular polymeric substance. Many infectious bacteria, such as S. maltophilia, Pseudomonas aeruginosa and Staphylococcus aureus, are capable of forming biofilms9. Biofilms exhibit greater resistance to antimicrobial drugs than non-biofilm forming bacteria, and are therefore more difficult to treat clinically10,11. It has been reported that high concentrations (500 μg/mL) of moxifloxacin, levofloxacin, or ciprofloxacin can significantly inhibit S. maltophilia biofilm formation, and that the inhibition is significantly reduced when the concentration is lowered to 50 μg/mL12. Treatment of biofilm-related infections using a combination of fluoroquinolone (FQ) and azithromycin has also been reported and appear to have different efficacies for different bacteria; the effect is synergetic for Pseudomonas aeruginosa and Legionella but antergic for Mycobacterium avium and Salmonella typhi13,14,15,16,17,18. However, the effect of azithromycin on S. maltophilia biofilm formation is still not clearly defined, requiring further investigation.
In this study, we investigated biofilm formation by S. maltophilia using microtiter plate staining system plus scanning electron microscopy (SEM), and observed the in vitro effects of three FQs (moxifloxacin, levofloxacin, and ciprofloxacin) on different stages of biofilm formation for this organism. In addition, we investigated the effect of combined azithromycin and FQ treatment on the biofilm formation of S. maltophilia. Our study provides an experimental basis for possible clinical treatment of biofilm related infections caused by S. maltophilia.
Results
In vitro biofilm formation by S. maltophilia
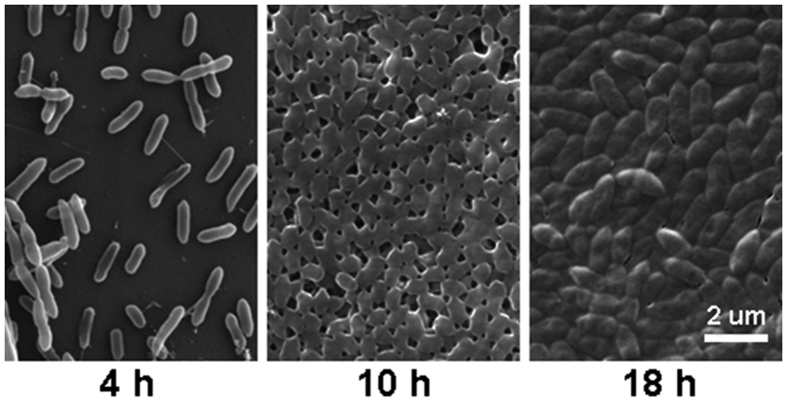
Among 45 S. maltophilia strains studied, biofilm formation was weak in two (4.4%) strains, moderate in seven (15.6%) strains, and strong in 35 (77.8%) strains. There was no biofilm formation in one strain (Fig. 1). SEM analysis on biofilm formation by S. maltophilia strain 10275 (a strong biofilm producer randomly selected), showed that after 4 h incubation, the bacterial cells were clearly attached to the polystyrene surface and occupied approximately 30% of the surface. After 10 h of incubation, almost the entire polystyrene surface was occupied by cells, with little space in between attached cells. Bacterial cells began to secrete extracellular polymeric substances after 10 h, and completely filled the surface at 18 h (Fig. 2).
Figure 1. OD values of 45 S. maltophilia strains, indicating their capacity to form biofilms.

Results are means ± SDs. **P < 0.01.
Figure 2. SEM images of one S. maltophilia strain* (10275) biofilms after 4 h (left), 10 h (middle) and 18 h (right).
To further understand the dynamics of biofilm formation, we measured optical density (OD) values at different time points (2 hourly) for 15 randomly selected S. maltophilia strains (7 strong, 6 moderate and 2 weak biofilm producers). The average OD values are shown in Fig. 3, and reveal that the organism was in the initial adhesive stage after 4 h of incubation, and in the exponential phase of growth after 8 h of incubation. Maximum growth was achieved after about 18 h of incubation (plateau phase).
Figure 3. Average ODs at different time points (hours) of 15 randomly* selected S. maltophilia isolates.
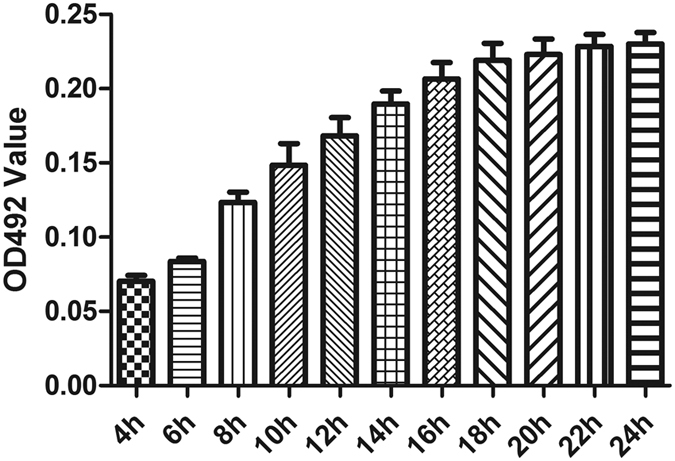
Results are means ± SDs. *Included 7 strong, 6 moderate and 2 weak biofilm producers.
Antimicrobial susceptibility of planktonic and biofilm
A planktonic antimicrobial susceptibility test showed that approximately 90%, 80% and 70%, of the 45 S. maltophilia strains were susceptible to moxifloxacin, levofloxacin, and ciprofloxacin, respectively, and the results were consistent with previous studies19,20,21,22 (Table 1). We then compared the antibiotic susceptibility results between the planktonic and biofilm forms of 15 strains (7 strong, 6 moderate and 2 weak biofilm producers) randomly selected, for the antibiotics moxifloxacin, levofloxacin and ciprofloxacin. As shown in Table 2, the minimum inhibitory concentration (MIC) of the biofilm form (MIC-b) of each organism was much higher than that of the planktonic (MIC-p) form in all the 15 strains. Furthermore, the concentration of FQs required to inhibit re-growth of the biofilm cells was up to 256 times the MIC-b. These results indicate that the biofilms were more resistant to moxifloxacin, levofloxacin or ciprofloxacin than the planktonic culture (Table 3).
Table 1. Planktonic antimicrobial susceptibility of 45 S. maltophilia isolates.
| MIC Range | Mode | MIC50a | MIC90b | %c | |
|---|---|---|---|---|---|
| Moxifloxacin | 0.03125–8 | 0.25 | 0.25 | 4 | 88.64 |
| Levofloxacin | 0.0625– > 8 | 0.25 | 0.5 | 4 | 79.55 |
| Ciprofloxacin | 0.5– > 16 | 1 | 1 | 16 | 65.91 |
aMIC50, MIC inhibiting 50% of the isolates tested.
bMIC90, MIC inhibiting 90% of the isolates tested.
cPercentages of susceptibility were calculated on the basis of interpretative breakpoints suggested by CLSI guidelines35.
Table 2. Comparison of antimicrobial susceptibility between planktonic and biofilm forms of the organism among 15 randomly selected S. maltophilia isolates.
| ID of isolates | MIC-pa |
MIC-bb |
||||
|---|---|---|---|---|---|---|
| MXF | LVX | CIP | MXF | LVX | CIP | |
| 2443 | 0.5 | 0.5 | 1 | 2 | >2048 | 32 |
| 10275 | 0.5 | 2 | 2 | >1024 | >2048 | >2048 |
| 2322 | 4 | 8 | 16 | >1024 | >2048 | >2048 |
| 7225 | 0.0625 | 0.5 | 2 | 2 | 16 | 32 |
| 2782 | 4 | 8 | 16 | 16 | 32 | 32 |
| 2618 | 0.5 | 2 | 4 | 16 | 32 | 32 |
| 6414 | 0.0625 | 0.25 | 1 | 1 | 4 | 32 |
| 7387 | 0.25 | 0.5 | 1 | 1 | 2 | 2 |
| 7249 | 0.0625 | 0.5 | 2 | 2 | 8 | 32 |
| 11252 | 0.25 | 0.0625 | 1 | 4 | 16 | 32 |
| 11829 | 0.25 | 0.5 | 1 | 4 | 16 | 32 |
| 2579 | 0.125 | 0.25 | 1 | 4 | 32 | 32 |
| 283 | 0.25 | 0.5 | 2 | 4 | 16 | 16 |
| 2629 | 2 | 4 | 8 | 128 | 16 | 32 |
| 11757 | 0.25 | 0.0625 | 0.5 | 0.5 | 2 | 2 |
MIC-pa, minimum inhibitory concentration of floating bacteria.
MIC-bb, minimum inhibitory concentration in the biofilm plate.
Values represent the geometric mean of three independent experiments.
MXF: Moxifloxacin; LVX: Levofloxacin; CIP: Ciprofloxacin.
Table 3. Comparison of antimicrobial susceptibility between MRC and MBEC among 15 randomly selected S. maltophilia isolates.
| ID of isolates | MRCa (μg/ml) |
MBECb(μg/ml) |
||||
|---|---|---|---|---|---|---|
| MXF | LVX | CIP | MXF | LVX | CIP | |
| 2443 | >1024 | >2048 | >2048 | >1024 | >2048 | >2048 |
| 10275 | >1024 | >2048 | >2048 | >1024 | >2048 | >2048 |
| 2322 | >1024 | >2048 | >2048 | >1024 | >2048 | >2048 |
| 7225 | >1024 | >2048 | 2048 | >1024 | >2048 | >2048 |
| 2782 | 1024 | >2048 | >2048 | >1024 | >2048 | >2048 |
| 2618 | 1024 | >2048 | >2048 | >1024 | >2048 | >2048 |
| 6414 | 1024 | >2048 | >2048 | >1024 | >2048 | >2048 |
| 7387 | 1024 | >2048 | 2048 | >1024 | >2048 | >2048 |
| 7249 | >1024 | >2048 | >2048 | >1024 | 2048 | >2048 |
| 11252 | >1024 | >2048 | 2048 | >1024 | >2048 | 1024 |
| 11829 | >1024 | >2048 | >2048 | >1024 | >2048 | 2048 |
| 2579 | 1024 | >2048 | 2048 | >1024 | >2048 | >2048 |
| 283 | >1024 | >2048 | >2048 | >1024 | 2048 | >2048 |
| 2629 | >1024 | >2048 | >2048 | >1024 | >2048 | >2048 |
| 11757 | >1024 | >2048 | >2048 | >1024 | >2048 | 2048 |
MRCa: minimum regrowth concentration.
MBECb: minimum biofilm eradication concentration.
MXF: Moxifloxacin; LVX: Levofloxacin; CIP: Ciprofloxacin.
Effects of fluoroquinolones (FQ) on S. maltophilia preformed biofilm of 15 randomly selected strains
SEM was used to examine the effects of three different concentrations of moxifloxacin (10 μg/mL, 50 μg/mL and 100 μg/mL) on preformed biofilm of 15 randomly selected strains over three time points (4 hr, 10 hr and 18 hr). These three time points were selected based on findings from the 15 strains representing strong, moderate and weak biofilm producers (as previously mentioned), as well as the stages of biofilm production as determined by SEM (as mentioned above). Due to cost constrains, SEM was performed only on moxifloxacin, chosen because this FQ exhibited the most distinct inhibitory effect on S. maltophilia preformed biofilm as outlined below. Compared to the control (no antibiotic treatment, Fig. 2), moxifloxacin treatment significantly reduced the number of bacterial cells that adhered to the coverslip for the 4 hr, 10 hr and 18 hr incubation periods (Fig. 4). Moxifloxacin concentrations of 50 μg/mL or 100 μg/mL resulted in greater numbers of abnormal bacterial cells, especially at the early stages of biofilm formation (4 h). However, these biofilms were able to recover over time (Fig. 4).
Figure 4. Scanning electron microscopy images of biofilms of one S. maltophilia strain (10275), treated with moxifloxacin for 4 h (A–C), 10 h (D–F) and 18 h (G–I).
The concentrations of moxifloxacin were: 10 μg/mL (A,D,G), 50 μg/mL (B,E,H), 100 μg/mL (C,F,I). Magnification: ×20,000.
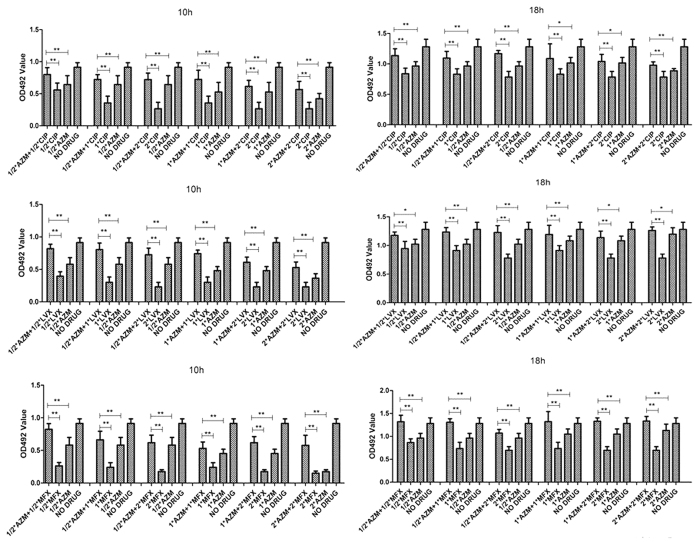
We further quantified, by measuring biofilm biomass, the effect of 3 different concentrations (10 μg/mL, 50 μg/mL and 100 μg/mL) of each of the three FQs (moxifloxacin, levofloxacin, ciprofloxacin), on preformed biofilm (biofilm allowed to form after 4 hr, 10 hr and 18 hr incubation before antibiotic addition) using 15 randomly selected S. maltophilia strains (7 strong, 6 moderate and 2 weak biofilm producers). As shown in Fig. 5, moxifloxacin treatment had a greater inhibitory effect on biofilm formation of immature preformed biofilms (4 h and 10 h) than mature preformed biofilms (18 h) as evidenced by significant differences in the biofilm biomass quantity. Specifically, the biomass for the immature (4 h or 10 h) preformed biofilm treated with moxifloxacin was only approximately 39% compared to that of untreated biofilm, but reached 69% for the mature (18 h) preformed biofilm treated with moxifloxacin. Similar results were observed following incubation with levofloxacin and ciprofloxacin (Fig. 5). All three FQs effectively inhibited biofilm formation (P < 0.05). However, in contrast to levofloxacin and ciprofloxacin, in which the level of inhibition of biofilm formation in preformed biofilm was more pronounced in the higher concentration (100 μg/mL), an opposite effect was observed for moxifloxacin. Specifically, the lower moxifloxacin concentration (10 and 50 μg/mL) produced higher inhibition levels for biofilm production in the 10 hr and mature (18 hr) preformed biofilms, than the 100 μg/mL concentration.
Figure 5. In vitro effects of fluoroquinolones on preformed biofilm of 15 S. maltophilia strains randomly selected.
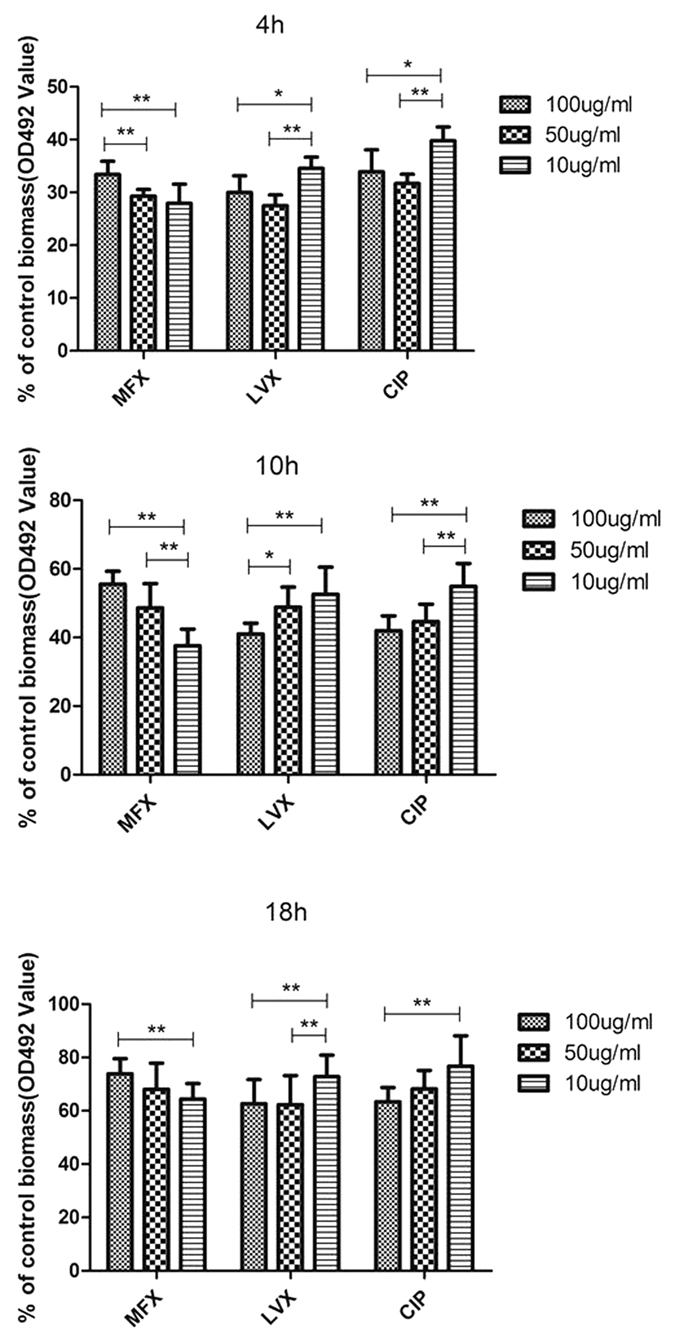
Results are means ± SDs. MXF: Moxifloxacin; LVX: Levofloxacin; CIP: Ciprofloxacin. *P < 0.05; **P < 0.01.
In vitro effects of moxifloxacin on viability of S. maltophilia on preformed biofilms
To further confirm our findings on the effect of moxifloxacin on biofilm formation using biomass measurements on preformed biofilms, we studied the effect of the 3 different moxifloxacin concentrations on the viability of 15 randomly selected S. maltophilia strains using a plate count technique23. As shown in Fig. 6, moxifloxacin (all concentrations) had a far greater effect on the viability of the organism in premature (biofilms formed after 4 h, 10 h incubation) (<10%) than in the mature (18 hr) biofilms (>28%) (P < 0.001). Furthermore, in both the premature and mature biofilms, the percentage survival of cells was greater in the higher concentration of the antibiotic. Overall, a greater proportion of cells survived (>28%) for the mature (18 hr preformed biofilms) than the immature (4 and 10 hr preformed biofilms) (<10%) (P < 0.001).
Figure 6. In vitro effects of moxifloxacin on viability of biofilms for 15 S. maltophilia strains randomly selected.
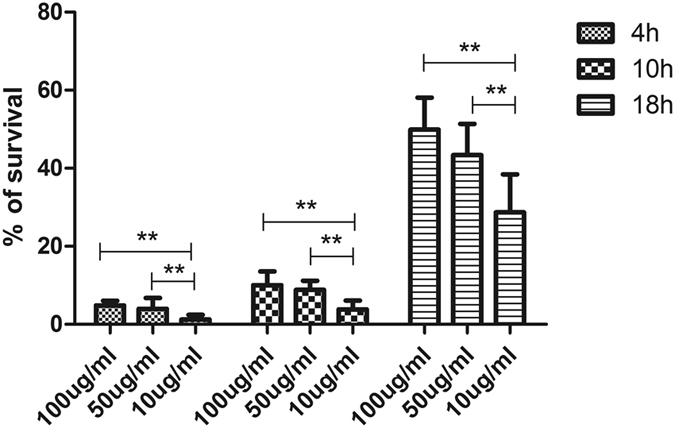
**P < 0.01.
Effects of a combination of FQs and azithromycin on S. maltophilia preformed biofilms
To further examine the effects of antibiotics on biofilm formation by S. maltophilia, a combination of FQs and azithromycin was tested on the 15 randomly selected S. maltophilia strains. Interestingly, compared with FQs or azithromycin alone, the combination of FQs and azithromycin reduced the inhibiting effect on S. maltophilia preformed biofilms (P < 0.05). We tested several combinations of different drug concentrations, and none of the combinations increased the associated inhibition (Fig. 7). The worst inhibiting effect was observed when azithromycin was combined with moxifloxacin (Fig. 7).
Figure 7. In vitro effects of fluoroquinolones combined with azithromycin on biofilms of 15 randomly selected S. maltophilia strains after 10 h or 18 h.
Results are means ± SDs. MXF: Moxifloxacin; LVX: Levofloxacin; CIP: Ciprofloxacin; AZM: azithromycin. *P < 0.05; **P < 0.01.
Effect of a combination of ciprofloxacin and azithromycin on preformed biofilm of Pseudomonas aeruginosa
Several studies have indicated that a combination of FQs and azithromycin has a synergetic effect on Pseudomonas aeruginosa biofilm13,14. To validate our findings regarding the antagonistic effect of combined FQs and azithromycin on S. maltophilia, we also tested the effect of a combination of ciprofloxacin and azithromycin on 15 randomly selected Pseudomonas aeruginosa biofilm14. As depicted in Fig. 8, our results confirm the previously reported antagonistic effect.
Figure 8. In vitro effects of ciprofloxacin combined with azithromycin on Pseudomonas aeruginosa biofilms after 24 h.
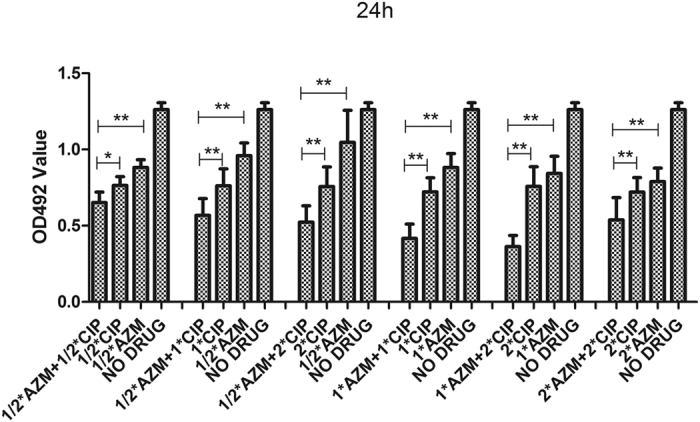
Results are means ± SDs. CIP: Ciprofloxacin; AZM: azithromycin. *P < 0.05; **P < 0.01.
Discussion
Biofilms are products of bacterial adherence to a natural or living surface. The starting point of various chronic infections is often the formation of a biofilm by the infecting organism. Thus targeting the biofilm phase of the organism is an important consideration for effective treatment of most chronic infections. Selection of antibiotics to treat S. maltophilia infections is often based on conventional antimicrobial susceptibility results of organisms grown planktonically in liquid media. However, it is known that organisms such as S. maltophilia actually grow as biofilms which exhibit higher resistance to antibiotics compared to floating bacteria on epithelial cells of the airway. This suggests that selection of antibiotics for S. maltophilia infections based on biofilm susceptibility testing may provide an accurate and effective guide on appropriate treatment11. Consequently, it is critical to understand the dynamics of biofilm formation by S. maltophilia which could give insights into the treatment of infections caused by this organism.
In this study, using a microplate assay, we demonstrated the ability of the majority of S. maltophilia clinical strains studied to adhere to abiotic surface, which is in agreement to previous studies8,24,25. Furthermore, using scanning electron microscopy (SEM) and microplate plate assay, we observed dynamic biofilm formation of S. maltophilia in vitro, including initial attachment of the organism to polystyrene surface after 4 hrs incubation, and achieving maximal biofilm growth after approximately 24 hrs.
S. maltophilia is naturally resistant to many antimicrobial agents. In this study, we demonstrated that S. maltophilia biofilms are more resistant to three fluoroquinolone (FQ) antibiotics, which is consistent with previous results21,26. Thus caution must be exercised when treating S. maltophilia infections based on antibiotic susceptibility testing of planktonic forms of the organism. The enhanced resistance to antimicrobial agents by S. maltophilia is due to several factors, including chromosomally encoded multidrug resistance, efflux pump and antibiotic modifying enzymes24.
It is widely believed that sub-MICs of FQs have inhibitory effects on bacterial adhesion12,25,27. It is thus possible that this class of antibiotics may also have inhibitory effects on S. maltophilia biofilm formation12, and this inhibition may be dose-dependent25. Based on our findings showing that S. maltophilia is in the initial adhesive stage at 4–6 h of incubation, exponential phase of biofilm formation at 10–16 h, and forms mature biofilm at 18–24 h, we chose the 4, 10 and 18 h incubation periods for further study of this organism.
The three FQs studied (moxifloxacin, levofloxacin, and ciprofloxacin) each at 3 different concentrations (10, 50, 100 μg/ml), exhibited greater inhibitory effects on preformed S. maltophilia non-mature biofilms (when FQs were applied at 4 h or 10 h incubations). However, the inhibitory effect was significantly reduced for mature biofilms (when FQs were applied on biofilms formed after 18 h incubation). These findings which were confirmed by SEM (Fig. 4), may be explained by differences in antibiotic permeability of the biofilm in the early and mature stages of development, heterogeneity associated with cell growth, quorum sensing systems, and other mechanisms9. Thus based on our findings, early identification (and treatment) of organisms causing infections may be important for controlling biofilm formation.
Interestingly, for mature preformed biofilms (18 h), the inhibitory effect of moxifloxacin at 10 μg/mL (and 50 μg/mL) concentration was surprisingly even greater than at 100 μg/mL. This is consistent with a previous report that showed that the inhibitory effect of moxifloxacin on biofilms is different from that of other FQs25. It is not clear however, why lower concentrations of moxifloxacin has a greater inhibitory effect than higher concentrations. Our results suggest that the conventional dose of moxifloxacin (400 mg, qd), which is equivalent to 10 μg/mL in human alveolar lining fluid, can be used as an alternative treatment for S. maltophilia biofilm-related lung infection or colonization.
Although the effect of a combination of FQs and azithromycinon on biofilm formation has been studied previously24, results have been mixed. Specifically, this combination therapy has been shown to have a synergistic effect on Pseudomonas aeruginosa and Legionella biofilm formation, but an antagonistic effect on Mycobacterium avium and Salmonella typhi13,14,15,16,17. In this study, we found that azithromycin somehow weakened the antibacterial effect of FQs, especially when moxifloxacin was involved. We speculate that azithromycin may interfere with FQs, resulting in a reduction in their antibacterial effectiveness against some bacteria.
Previous studies have also reported that clarithromycin reduces the effects of gatifloxacin and levofloxacin on Mycobacterium avium. This may be due to clarithromycin-induced inhibition of protein synthesis interfering with the bactericidal activity of FQs28. A similar mechanism may be involved with azithromycin. Wang et al.29 also reported that ica (intercellular adhesin) operon encoded enzymes may mediate intercellular adherence of bacteria and the accumulation of multilayer biofilms through the production of polysaccharides that promote intercellular adhesion. A sub-inhibitory concentration of erythromycin has been shown to induce ica expression, suggesting a possible role for macrolides in biofilm formation29. Further studies are required to determine whether a combination of moxifloxacin and azithromycin has a similar mechanism of action.
This study has several limitations. First, due to cost implications, we mainly relied on biomass measurement (spectrophotometric microtiter assay) for biofilm quantification, and this method measures both viable and unviable bacteria, including the extracellular polymeric substance. However, in agreement with several studies12,30, we demonstrated in one experiment the high correlation between the microtiter plate assay (OD readings) and the viable cell enumerations (plate count) in the measurement of biofilm production by bacteria.
Second, most of the assays to examine the effects of the 3 FQs on biofilm formation were performed on a limited number of isolates (15; 7 strong, 6 moderate and 2 weak biofilm producers) for the biofilm assay and 3 time periods (4 h, 10 h and 18 hr incubation periods). It can be argued that the small number of isolates used and the selected time points, may not be representative enough to give sufficient statistical confidence to the findings. However, the time points selected were based on our findings involving 15 strains chosen randomly to represent strong, moderate and weak biofilm producers, as well as SEM analysis showing different stages of biofilm formation. Furthermore, SEM and the biofilm viability plate assay were performed on 15 isolates only. It is arguable that studies based on different S. maltophilia strains and on higher numbers of isolates would yield different results. However, our findings are in agreement with previous studies, and give some insight into biofilm formation by S. maltophilia in the presence of a selected number of FQs. Nevertheless, we consider our findings as exploratory and hypothesis generating, requiring confirmation in the future.
Finally, we didn’t study in detail the suppressive effects of levofloxacin and ciprofloxacin on preformed biofilm and also did not explore in-depth the effects of azithromycin on biofilm formation, as the devices commonly used for SEM study of biofilms are expensive.
In conclusion, findings from this study suggest that early use of clinically acceptable concentrations of selected FQs (10 μg/mL), especially for moxifloxacin, can inhibit biofilm formation by S. maltophilia. Our study provides an experimental basis for a possible optimal treatment strategy for biofilm-related infections caused by S. maltophilia.
Materials and Methods
Strains and antibiotics
Forty-five S. maltophilia strains were collected and stored in our laboratory as previously described31. Antimicrobial drugs (including azithromycin, moxifloxacin, levofloxacin and ciprofloxacin) ordered from the National Institute for the Control of Pharmaceutical and Biological Products, Beijing, or Bayer company, Germany, were used. Pseudomonas aeruginosa ATCC 27853 was used for quality control in all experiments.
Scanning electron microscopy (SEM) observation of biofilm formation
SEM was used to observe biofilm formation and effects of antimicrobial drugs on biofilm formation12. In order to observe the morphological changes associated with biofilms following moxifloxacin treatment, biofilms were allowed to grow on sterile flat-bottom 6-well polystyrene tissue culture plates (LabServ, Thermo Fisher Scientific, USA) in the presence or absence of moxifloxacin treatment. Fresh Cation-Adjusted Mueller Hinton Broth (CAMHB) without any antibiotic was used as a control. The samples were fixed with 2.5% glutaraldehyde for at least 4 h at 4 °C. After washing with phosphate buffered saline (PBS), the samples were dehydrated in a series of ethanol solutions of increasing concentration (50 to 100%). The cells were subsequently washed with pure acetone, followed by isoamyl acetate. They were then dried with a critical point dryer (Hitachi, Japan). The dried samples were coated with 15-nm gold using an argon automatic sputter coater (Hitachi, Japan). After processing, the samples were viewed with a Philips XL30CP scanning electron microscope.
Biofilm formation assay
S. maltophilia isolates (n = 45) and S. maltophilia ATCC13637 were used in the biofilm formation assay. Microplate assays were performed as previously described with some modifications12,32,33. Briefly, overnight cultures of S. maltophilia were standardized to a 0.5 MacFarland standard (equivalent to 1.5 × 108 CFU/mL), and then diluted (1:100) with fresh Luria Bertani broth. Aliquots (200 μL) of standardized inocula were added to the wells of sterile flat-bottom 96-well polystyrene tissue culture plates (Thermo Fisher Scientific, USA) and incubated at 37 °C over a series of time-points (2, 4, 6, 8, 10, 12, 14, 16, 18, 20, 22, and 24 h) in a closed and humidified plastic container. The medium was then discarded and non-adherent cells were removed by washing three times in sterilized ultrapure water. The cells were stained with 0.01% (w/v) crystal violet for 20 min. The excess stain was then removed by washing with water and the stained biofilms were dried for 30 min at ambient room temperature and extracted with 33% (v/v) glacial acetic acid. The amount of biofilm produced was quantified by measuring the optical density at 492 nm (OD492) using a plate reader (Varioskan Flash; Thermo Fisher Scientific, USA). Non-inoculated media were used as a control. The low cut-off point for biofilm production was chosen according to the criteria described by Christensen et al.34, i.e. the cut-off point was defined as three standard deviations above the mean optical density of the control (ODc) wells12. Based on this cut-off, strains were classified into the following categories: no biofilm producer (OD ≤ ODc), weak biofilm producer (ODc < OD ≤ 2 × ODc), moderate biofilm producer (2 × ODc < OD ≤ 4 × ODc), and strong biofilm producer (4 × ODc < OD)31. Each isolate was assayed three times and the results were presented as the average of the three assays.
Planktonic antimicrobial susceptibility test
The minimum inhibitory concentrations (MICs) of moxifloxacin, levofloxacin, ciprofloxacin were determined by the CLSI broth microdilution technique (M100-S22)35. Escherichia coli ATCC 25922 was used as the quality control strain.
Biofilm antimicrobial susceptibility test for 15 randomly selected strains
The minimum inhibitory concentration in the biofilm plate (MIC-b) was defined as the lowest concentration of an antibiotic in which a planktonic bacterial population could not be established by shedding of bacteria from the biofilm23. MIC-b was assessed as previously described, with minor modifications. In brief, aliquots (75 μL) of the standardized inocula were added to the wells of a 96-well microplate and incubated for 24 h at 37 °C for production of biofilm. The medium was then discarded, wells washed with sterilized saline water, and 100 μL of 1:2 dilutions of moxifloxacin, levofloxacin or ciprofloxacin (from 2048 μg/mL to 0.50 μg/mL) prepared in fresh Mueller Hinton (MH) broth, was added to the established biofilms. The 96-well plates were wrapped with aluminum foil and placed in an incubator at 37 °C for 18–20 h. The MIC-b was determined as the minimum antibiotic concentration in which a planktonic bacterial population could not be established by shedding of bacteria from the biofilm. All samples were assessed in quadruplicate.
Determination of minimum regrowth concentration (MRC)
MRC, which is defined as the minimum antibiotic concentration (μg/ml) required to inhibit regrowth of the cells, was determined as previously described36. Briefly, 100 μL of appropriate dilutions of each of the three FQs in MH broth, were transferred into the 96-well tissue culture microtiter plate wells with established biofilms and incubated for 18–20 h at 37 °C. Each well was washed three times with PBS under aseptic conditions, 100 μL TSB added, and the samples were incubated for 24 h at 37 °C. Minimum antibiotic concentration which inhibited regrowth of the cells (mg/L) was determined.
Determination of minimum biofilm eradication concentration (MBEC)
MBEC was defined as the lowest antimicrobial concentration which prevented bacteria regrowth after antimicrobial exposure. In other words, the lowest antibiotic concentration required to eradicate the biofilm. This assay was performed on 15 randomly selected strains. The MBEC assay was performed as previously described37, with slight modifications. The 24 h biofilms in a 96-well tissue culture microtiter plate were washed three times with 250 μL PBS and air dried. Serial 2-fold dilutions ranging from 2048 to 0.5 μg/ml for LVX and CIP, and from 1024 to 0.25 μg/ml for MFX, were prepared in CAMHB. A 200 μL sample of each concentration for each antibiotic was added to a corresponding well, and plates were incubated for 24 h at 37 °C. After the incubation, antibiotic solutions were aspirated gently, plates were washed two times with sterile PBS solution, and wells were scraped thoroughly, with particular attention for the well edges. Well contents were transferred to 1 ml of PBS solution and placed in a sonicating water bath (Bandelin sonopuls HD 2200) for 5 min to disrupt the biofilm, and 100 μL samples were plated on TSA. Colonies were counted after 24 h at 37 °C. The MBEC was defined as the lowest concentration of antibiotic that prevented bacterial regrowth.
Effects of FQs on S. maltophilia preformed biofilm
This test, which was done on 15 randomly selected strains (7 strong, 6 moderate and 2 weak biofilm producers), was performed as previously described with minor modifications12. Biofilm was allowed to form after 4 h, 10 h, and 18 h incubation periods (which were defined as the preformed biofilm) at 37 °C. After this, the supernatant from each well was gently discarded, and wells washed gently three times with sterile saline water. Different concentrations of moxifloxacin, levofloxacin or ciprofloxacin (100, 50 and 10 μg/mL) prepared in 200 μL of fresh CAMHB was then added to the wells. Fresh CAMHB without any antibiotic was used as a control. The plates were incubated at 37 °C for 6 h, and the quantity of biofilm produced determined as previously described at OD492.
Effects of moxifloxacin on viability of S. maltophilia on preformed biofilms
This test was performed as previously described with minor modifications23. The viability of the biofilm was determined by plate counts and was performed on 15 randomly selected strains. Following the effect of different concentrations of moxifloxacin (100, 50 and 10 μg/mL) on the S. maltophilia preformed biofilm allowed to form after 4 h, 10 h, and 18 h incubation periods, supernatant was discarded, the wells were washed and the surfaces of the wells were scraped with sterile cotton swabs (three wells per dilution). Swabs were transferred into tubes containing 2 mL of PBS, sonicated (Transonic SB-120DT, Shanghai, China) for 3 min, and vortexed vigorously to aid dissolution of bacterial clumps. The number of viable cells was estimated by plating serial dilutions of these suspensions on Mueller Hinton agar (Oxoid Ltd.) plates.
Effect of combined FQs and azithromycin on S. maltophilia preformed biofilm
This test was performed on the 15 randomly selected S. maltophilia strains and Pseudomonas aeruginosa strains. After 10 and 18 h (for S. maltophilia strains) and 24 h (for Pseudomonas aeruginosa strains) incubation periods at 37 °C, the supernatant from each well was gently discarded. Each well was then washed three times with sterile saline water without destroying the attached biofilm. Different concentrations of moxifloxacin, levofloxacin or ciprofloxacin (0.5×, 1× or 2× of the MIC) were prepared following dilution in 100 μL of fresh CAMHB in the wells of the micro-titer plate. Additionally, various concentrations of azithromycin (0.5×, 1×, or 2× of the MIC) were added to wells on the same plate. Fresh CAMHB without antibiotics was added to the control wells. The plates were incubated at 37 °C for 6 h and the OD492 was measured.
Statistical analysis
All assays were performed in triplicate (at a minimum) and repeated three times. Multiple comparisons of responses to antibiotics and the inhibition of biofilm formation were performed using multiple factor variance analysis. A P-value < 0.05 was considered statistically significant. Statistical analysis was conducted with SPSS Statistics 17.0 software.
Additional Information
How to cite this article: Wang, A. et al. Effects of Fluoroquinolones and Azithromycin on Biofilm Formation of Stenotrophomonas maltophilia. Sci. Rep. 6, 29701; doi: 10.1038/srep29701 (2016).
Acknowledgments
We thank all the members of China Antimicrobial Resistance Surveillance System for supply of the isolates.
Footnotes
Author Contributions Conceived and designed the experiments: C.Z. Performed the experiments: A.W. Analyzed the data: A.W. Contributed reagents/materials/analysis tools: A.W. and S.X. Wrote the paper: C.Z., A.W., Q.W. and T.K.
References
- Gilardi G. L. Pseudomonas maltophilia infections in man. American journal of clinical pathology 51, 58–61 (1969). [DOI] [PubMed] [Google Scholar]
- Denton M. & Kerr K. G. Microbiological and clinical aspects of infection associated with Stenotrophomonas maltophilia. Clinical microbiology reviews 11, 57–80 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safdar A. & Rolston K. V. Stenotrophomonas maltophilia: changing spectrum of a serious bacterial pathogen in patients with cancer. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 45, 1602–1609 (2007). [DOI] [PubMed] [Google Scholar]
- Sader H. S. & Jones R. N. Antimicrobial susceptibility of uncommonly isolated non-enteric Gram-negative bacilli. International journal of antimicrobial agents 25, 95–109 (2005). [DOI] [PubMed] [Google Scholar]
- Crossman L. C. et al. The complete genome, comparative and functional analysis of Stenotrophomonas maltophilia reveals an organism heavily shielded by drug resistance determinants. Genome biology 9, R74 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway S. P., Brownlee K. G., Denton M. & Peckham D. G. Antibiotic treatment of multidrug-resistant organisms in cystic fibrosis. American journal of respiratory medicine: drugs, devices, and other interventions 2, 321–332 (2003). [DOI] [PubMed] [Google Scholar]
- Huang T. P. & Wong A. C. A cyclic AMP receptor protein-regulated cell-cell communication system mediates expression of a FecA homologue in Stenotrophomonas maltophilia. Applied and environmental microbiology 73, 5034–5040 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T. P., Somers E. B. & Wong A. C. Differential biofilm formation and motility associated with lipopolysaccharide/exopolysaccharide-coupled biosynthetic genes in Stenotrophomonas maltophilia. Journal of bacteriology 188, 3116–3120 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah T. F. & O’Toole G. A. Mechanisms of biofilm resistance to antimicrobial agents. Trends in microbiology 9, 34–39 (2001). [DOI] [PubMed] [Google Scholar]
- Romero R. et al. Detection of a microbial biofilm in intraamniotic infection. American journal of obstetrics and gynecology 198, 135 e131–135 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K., Yau Y. C., Matukas L. & Waters V. Biofilm compared to conventional antimicrobial susceptibility of Stenotrophomonas maltophilia Isolates from cystic fibrosis patients. Antimicrobial agents and chemotherapy 57, 1546–1548 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Bonaventura G., Spedicato I., D’Antonio D., Robuffo I. & Piccolomini R. Biofilm formation by Stenotrophomonas maltophilia: modulation by quinolones, trimethoprim-sulfamethoxazole, and ceftazidime. Antimicrobial agents and chemotherapy 48, 151–160 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols D. P. et al. Effects of azithromycin in Pseudomonas aeruginosa burn wound infection. The Journal of surgical research 183, 767–776 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini H., Chhibber S. & Harjai K. Azithromycin and ciprofloxacin: a possible synergistic combination against Pseudomonas aeruginosa biofilm-associated urinary tract infections. International journal of antimicrobial agents 45, 359–367 (2015). [DOI] [PubMed] [Google Scholar]
- Martin S. J., Pendland S. L., Chen C., Schreckenberger P. & Danziger L. H. In vitro synergy testing of macrolide-quinolone combinations against 41 clinical isolates of Legionella. Antimicrobial agents and chemotherapy 40, 1419–1421 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E. et al. Activity of moxifloxacin by itself and in combination with ethambutol, rifabutin, and azithromycin in vitro and in vivo against Mycobacterium avium. Antimicrobial agents and chemotherapy 45, 217–222 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno Y. et al. In vitro and in vivo activities of novel fluoroquinolones alone and in combination with clarithromycin against clinically isolated Mycobacterium avium complex strains in Japan. Antimicrobial agents and chemotherapy 51, 4071–4076 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry C. M. et al. Randomized controlled comparison of ofloxacin, azithromycin, and an ofloxacin-azithromycin combination for treatment of multidrug-resistant and nalidixic acid-resistant typhoid fever. Antimicrobial agents and chemotherapy 51, 819–825 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellos-Bourboulis E. J., Karnesis L., Galani I. & Giamarellou H. In vitro killing effect of moxifloxacin on clinical isolates of Stenotrophomonas maltophilia resistant to trimethoprim-sulfamethoxazole. Antimicrobial agents and chemotherapy 46, 3997–3999 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss K., Restieri C., De Carolis E., Laverdiere M. & Guay H. Comparative activity of new quinolones against 326 clinical isolates of Stenotrophomonas maltophilia. The Journal of antimicrobial chemotherapy 45, 363–365 (2000). [DOI] [PubMed] [Google Scholar]
- Gesu G. P., Marchetti F., Piccoli L. & Cavallero A. Levofloxacin and ciprofloxacin in vitro activities against 4,003 clinical bacterial isolates collected in 24 Italian laboratories. Antimicrobial agents and chemotherapy 47, 816–819 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senol E. Stenotrophomonas maltophilia: the significance and role as a nosocomial pathogen. The Journal of hospital infection 57, 1–7 (2004). [DOI] [PubMed] [Google Scholar]
- Passerini de Rossi B., Garcia C., Calenda M., Vay C. & Franco M. Activity of levofloxacin and ciprofloxacin on biofilms and planktonic cells of Stenotrophomonas maltophilia isolates from patients with device-associated infections. International journal of antimicrobial agents 34, 260–264 (2009). [DOI] [PubMed] [Google Scholar]
- Brooke J. S. Stenotrophomonas maltophilia: an emerging global opportunistic pathogen. Clinical microbiology reviews 25, 2–41 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompilio A. et al. Subinhibitory concentrations of moxifloxacin decrease adhesion and biofilm formation of Stenotrophomonas maltophilia from cystic fibrosis. Journal of medical microbiology 59, 76–81 (2010). [DOI] [PubMed] [Google Scholar]
- Schmitz F. J., Sadurski R., Verhoef J., Milatovic D. & Fluit A. C. Typing of 154 clinical isolates of Stenotrophomonas maltophilia by pulsed-field gel electrophoresis and determination of the in vitro susceptibilities of these strains to 28 antibiotics. The Journal of antimicrobial chemotherapy 45, 921–923 (2000). [DOI] [PubMed] [Google Scholar]
- Vidigal P. G. et al. Effects of green tea compound epigallocatechin-3-gallate against Stenotrophomonas maltophilia infection and biofilm. PloS one 9, e92876 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka H., Sano C., Sato K. & Shimizu T. Antimicrobial activities of clarithromycin, gatifloxacin and sitafloxacin, in combination with various antimycobacterial drugs against extracellular and intramacrophage Mycobacterium avium complex. International journal of antimicrobial agents 19, 139–145 (2002). [DOI] [PubMed] [Google Scholar]
- Wang Q. et al. Enhancement of biofilm formation by subinhibitory concentrations of macrolides in icaADBC-positive and -negative clinical isolates of Staphylococcus epidermidis. Antimicrobial agents and chemotherapy 54, 2707–2711 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almshawit H., Macreadie I. & Grando D. A simple and inexpensive device for biofilm analysis. Journal of microbiological methods 98, 59–63 (2014). [DOI] [PubMed] [Google Scholar]
- Zhuo C., Zhao Q. Y. & Xiao S. N. The impact of spgM, rpfF, rmlA gene distribution on biofilm formation in Stenotrophomonas maltophilia. PloS one 9, e108409 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Toole G. A. & Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Molecular microbiology 30, 295–304 (1998). [DOI] [PubMed] [Google Scholar]
- Passerini de Rossi B., Calenda M., Vay C. & Franco M. Biofilm formation by Stenotrophomonas maltophilia isolates from device-associated nosocomial infections. Revista Argentina de microbiologia 39, 204–212 (2007). [PubMed] [Google Scholar]
- Christensen G. D. et al. Adherence of coagulase-negative staphylococci to plastic tissue culture plates: a quantitative model for the adherence of staphylococci to medical devices. Journal of clinical microbiology 22, 996–1006 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Ssusceptibility Testing; Twenty-Second Informational Supplement. CLSI approved standard M100-S22. Clinical and Laboratory Standards Institute Wayne, PA (2012).
- Cernohorska L. & Votava M. Determination of minimal regrowth concentration (MRC) in clinical isolates of various biofilm-forming bacteria. Folia microbiologica 49, 75–78 (2004). [DOI] [PubMed] [Google Scholar]
- Mataraci E. & Dosler S. In vitro activities of antibiotics and antimicrobial cationic peptides alone and in combination against methicillin-resistant Staphylococcus aureus biofilms. Antimicrobial agents and chemotherapy 56, 6366–6371 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]