Abstract
β-lactams are the most widely used group of antimicrobials. However, increasing resistance to these valuable drugs in uropathogens, mediated principally by β-lactamases, has become a major concern. The present study was undertaken to determine the prevalence of Extended Spectrum β-Lactamase (ESBL) producers in clinical isolates of urine specimens, collected from various healthcare centres across south Mumbai. A total of 195 gram negative urine isolates were identified as Pseudomonas aeruginosa (13), Proteus mirabilis (21), Klebsiella pneumoniae (29), Escherichia coli (96), Enterobacter aerogenes (1), Enterobacter cloacae (1), Enterococcus fecalis (1), Morganella morganii (1), Citrobacter diversus (16), Citrobacter amalonaticus (5) and Proteus vulgaris (11). Antimicrobial Susceptibility Testing (AST) by Kirby-Bauer method showed 43.07 % (84/195) of the isolates were resistant to more than 70 % of the antibiotics used. Confirmatory screening using a combination of Double Disk Synergy Test (DDST), Phenotypic Confirmatory Disc Diffusion Test (PCDDT) and E-test revealed the overall prevalence of ESBL producers to be 34.71 % (68/195). The study showed 72.05 % of the ESBL producers to be resistant to fluoroquinolones, highlighting its extensive use in the region of south Mumbai. All ESBL producers were found to be sensitive to Imipenem whereas 82.36 % showed susceptibility to Amikacin making these 2 antibiotics the most effective choice of drug against ESBLs. In order to ensure rational treatment of highly resistant pathogens, the occurrence of ESBL and its primary studies may serve as a base for further research and findings.
Keywords: ESBL, drug resistance, beta-lactam, uropathogens
Introduction
With over 150 million annual diagnoses of Urinary Tract Infections (UTIs) worldwide, it remains one of the most common community-acquired as well as nosocomial infections (Gonzalez and Schaeffer, 1999[19]). The latter are frequently complicated by structural or functional alterations in the urinary tract, impaired renal function or diseases which impair the immunity, in contrast to community-acquired UTIs (Mazzuli, 2002[33]; Truls and Bjerklund, 2006[44]), which are often free of any sort of complications. What adds to the problem is the emergence of drug resistance among uropathogens in the form of Extended Spectrum β-Lactamase (ESBL) production. ESBLs are chromosomal or plasmid-mediated β-lactamases (enzymes that cleave the β-lactam ring) which have mutated from pre-existing broad-spectrum β-lactamases (TEM-1, TEM-2, SHV-1) as a consequence of widespread use of 3rd generation Cephalosporins as well as Aztreonam (Shukla et al., 2004[42]; Giriyapur et al., 2011[18]). ESBLs may be inhibited by β-lactamase inhibitors such as Clavulanic acid or Sulbactam (Bradford, 2001[7]). However, several inhibitor-resistant ESBL producers are also encountered, by virtue of Amp-C lactamase hyper production or loss of porin (Ananthan and Subha, 2005[4]).
ESBLs were first identified in the 1980s and have gradually spread throughout the world by nosocomial routes. Hospitals (Bradford, 2001[7]; Kassis-Chikhani et al., 2004[28]) and nursing homes (Arpin et al., 2003[5]; Wiener et al., 1999[45]) have been identified as important reservoirs of ESBL-producing Enterobacteriaceae. Patients admitted to hospitals are more likely to serve as reservoirs for these resistant organisms and eventually, the patients in the community acquire ESBL-producing strains (Arpin et al., 2003[5]).
The study of drug resistance in UTI causing pathogens is gaining more importance because the resistance mechanism of ESBL producers differs from one species to another. Moreover the vast number of species included in the family Enterobacteriaceae further adds to the diagnostic and clinical complications associated with UTIs. ESBL-producing genes are normally harboured on plasmids 80 kb in size or larger, and most often carry resistance determinants for aminoglycosides, fluoroquinolones, tetracyclines, Chloramphenicol and even Cotrimoxizole, making the micro-organisms resist a wide variety of drugs (Chaudhary and Aggarwal, 2004[10]).
The effectiveness of an antibiotic administered to a patient depends on the site and severity of the infection, liver and renal function, presence of implants and local (geographic) resistance patterns. It is also believed that the age, pregnancy and lactation in the patient determine the effectiveness of the antibiotic used (Chaudhary and Aggarwal, 2004[10]). Amoxycillin (β-lactam antibiotic) was traditionally used in the first line therapy for UTIs, but with the spread of drug resistance, other treatment options now include Amoxycillin-Clavulanate and Cephalosporins like Cefixime, Cefotaxime, and Ceftazidime. Fluoroquinolones, though used in the treatment of UTIs, are not regarded as an acceptable form of antibiotic prophylaxis given their cost-ineffectiveness and the risk of emergence of organisms resistant to this class of antimicrobials (Cendron, 2008[9]).
Since β-lactam antibiotics are still widely used, emergence of β-lactamase producers has become a matter of serious concern. The various mechanisms of drug resistance in gram-negative bacilli include production of β-lactamases (Jarlier et al., 1988[24]), Amp C lactamases (Phillippon et al., 2002[40]), efflux mechanisms (Fukuda and Hiramatsu, 1997[16]) and porin deficiency (Ananthan and Subha, 2005[4]). ESBL producers may exhibit more than one such resistance mechanism, further complicating the situation.
This study attempts to investigate the prevalence of ESBL production among gram negative uropathogens and its antibiogram pattern using isolates from urine samples collected from various hospitals and pathological laboratories across south Mumbai.
Material and Methods
Collection of samples from south Mumbai
A total of 225 isolates from urine samples were collected from 3 government Tertiary care hospitals, 2 private hospitals and 4 pathological laboratories situated in south Mumbai over a period of 6 months (September 2011 to February 2012). These isolates were maintained on Luria-Bertani (LB) Agar slants and stored at refrigerated conditions.
Isolation and identification
The cultures were isolated on CLED (Cystiene Lactose Electrolyte Deficient) Agar and MacConkey's (MAC) Agar to study their cultural characteristics. A single isolated colony was considered for further studies and identification was done using standard conventional, morphological, cultural and biochemical tests (Collee et al., 1996[15]).
Determination of antimicrobial susceptibility to generate an antibiogram pattern of the identified pathogens
Antimicrobial Susceptibility Testing (AST) was performed using disk diffusion method as described by the Clinical and Laboratory Standard Institute (CLSI) using Kirby-Bauer method (CLSI, 2012[11]). Dodeca discs (PBL-Bio-Disc- code # 612, PBL-Bio-Disc- code # 212, Pathoteq biological laboratories) were used for performing AST.
PBL Biodisc 612 contained Ticarcillin (85 mcg), Oxytetracycline (30 mcg), Ceftriaxone (30 mcg), Cefipime (30 mcg), Cefuroxime (30 mcg), Nalidixic acid (30 mcg), Norfloxacin (10 mcg), Amoxycillin (30 mcg), Cefadroxil (30 mcg), Cefoperazone (75 mcg), Ceftazidime (30 mcg), Polymixin-B (300 mcg) and PBL Biodisc 212 contained Ampicillin (20 mcg), Co-trimoxazole (25 mcg), Cefotaxime (30 mcg), Piperacillin (100 mcg), Chloramphenicol (30 mcg), Ciprofloxacin (5 mcg), Ceftizoxime (30 mcg), Tetracycline (30 mcg), Ofloxacin (5 mcg), Gentamicin (10 mcg), Amikacin (30 mcg), Gatifloxacin (10 mcg).
E. coli ATCC 25922 was used as a standard quality control strain.
ESBL screening
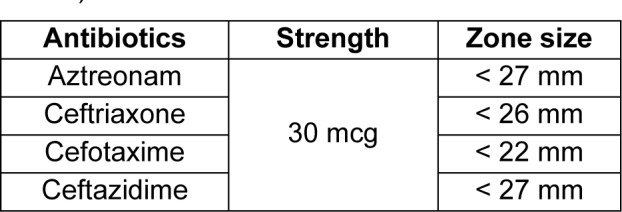
All the isolates showing resistance to 3rd generation cephalosporins, namely Ceftazdime, Ceftriaxone and Cefotaxime, were further tested for confirmation of β-lactamase production by phenotypic methods. The Optical Density (O.D.) of the cultures were adjusted to 0.1 (at 530 nm) and swabbed on Mueller-Hinton (MH) Agar plates for further studies. The screening was done as per CLSI guidelines (CLSI, 2012[11]) mentioned in Table 1(Tab. 1).
Table 1. Standard zone sizes of antibiotics for ESBL screening as per CLSI guidelines (CLSI, 2012).
Phenotypic confirmatory disk diffusion test (PCDDT) for ESBL detection
Ceftazidime (30 mcg) was used alone as well as in combination with Clavulanic acid (10 mcg). Both the disks were placed on MH Agar plates pre-swabbed with the respective culture and incubated at 37 °C for 24 h. An increase in the zone diameter for Ceftazidime-Clavulanic acid by ≥ 5mm was considered positive for ESBL production (CLSI, 2010[13]).
Double disk synergy test (DDST)
DDST was carried out using 5 antibiotics, namely Amoxycillin-Clavulanic acid (20/10 mcg), Aztreonam (30 mcg), Cefotaxime (30 mcg), Ceftriaxone (30 mcg) and Ceftazidime (30 mcg). The disks were placed at a distance of 1.5 cm from a centrally-placed Amoxycillin-Clavulanic acid disk. Enhancement of the zone of inhibition towards the Clavulanate disk after 24 hrs incubation at 37 °C was considered indicative of a potential ESBL producer (Jarlier et al., 1988[24]).
Confirmation of ESBL production by E-test
Confirmation of ESBL production was done using Multi-Ezy MICTM Strips (HiMedia Laboratories Pvt. Ltd.). These strips differ from the conventional E-strips in that they contain a gradient of 3 antibiotics with and without Clavulanic acid on either sides of the strip respectively instead of one antibiotic. The Multi-Ezy MICTM Strips used contained Cefotaxime, Ceftazidime and Cefipime (MIX) on one side in a two-fold gradient and the same antibiotics with Clavulanic acid (MIX+) on the other side. A ratio of inhibition zones for MIX and MIX+ of ≥ 8 was considered as a positive E-test (CLSI, 2011[12]). Reference cultures used in the study were E. coli ATCC 25922 as negative control and Klebsiella pneumoniae ATCC 700603 as positive control.
Results
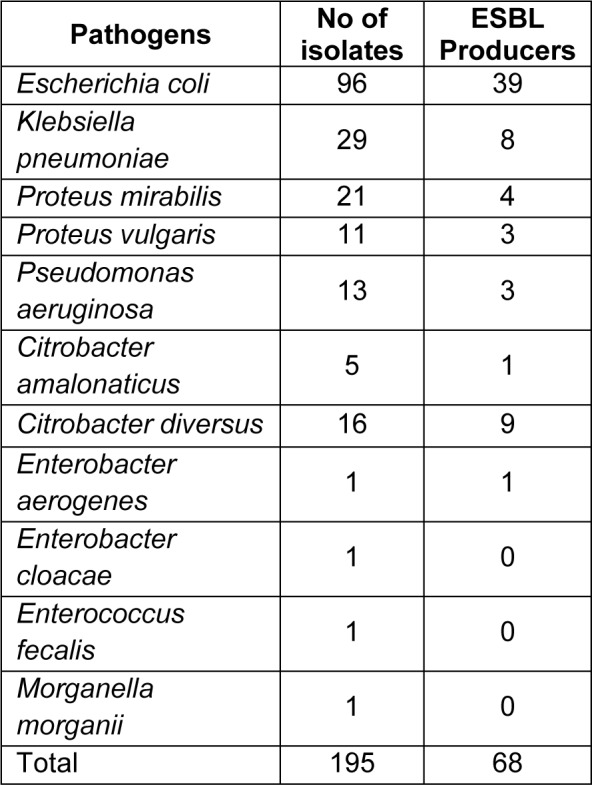
In the present study, 225 isolates were collected from various healthcare centres, out of which 195 isolates were characterised as gram negative. These isolates were identified using conventional cultural, biochemical and morphological tests, and comprised of Pseudomonas aeruginosa (13), Proteus mirabilis (21), Klebsiella pneumoniae (29), Escherichia coli (96), Enterobacter aerogenes (1), Enterobacter cloacae (1), Enterococcus fecalis (1), Morganella morganii (1), Citrobacter diversus (16), Citrobacter amalonaticus (5) and Proteus vulgaris (11).
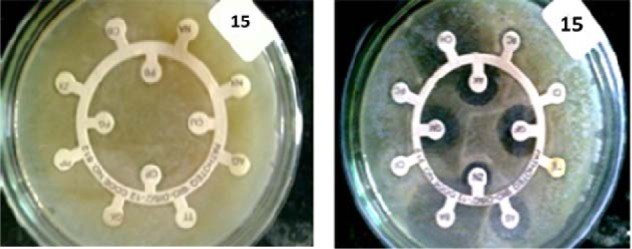
Antibiotic sensitivity test was carried out using Kirby Bauer method (Figure 1(Fig. 1)). Around 88.20 % (172/195) isolates were found to be resistant to more than 3 antibiotics. Among these cultures, 43.07 % (84/195) isolates were resistant to over 70 % antibiotics used in the study including 3rd generation Cephalosporins (Ceftazidime, Cefotaxime and Ceftriaxone).
Figure 1. Antibiotic sensitivity test (AST) showing highly resistant strain of Citrobacter diversus (resistant to 20 of the 24 tested antibiotics).
These cultures were further checked for ESBL production by PCDDT, DDST and E-test (Figure 2(Fig. 2)). Clavulanic acid was used as a β-lactamase inhibitor in the above tests. Confirmatory tests for detection of ESBL producers using Ezy-MICTM strips showed 34.87 % (68/195) of the isolates to be ESBL producers (Table 2(Tab. 2)).
Figure 2. Isolate 70 (E. coli.) showing: (a) Positive PCDDT when swabbed on MH Agar and incubated with Ceftazidime (left) and Ceftazidime-Clavulanate (right). (b) Positive DDST when swabbed on MH Agar and incubated with (clockwise from top-left) Ceftazidime, Ceftriaxone, Cefotaxime, Aztreonam, and Amoxycillin-Clavulanate at the centre. (c) E-test showing positive ESBL production, with a ratio of Antibiotic: Antibiotic-Clavulanic acid > 8.
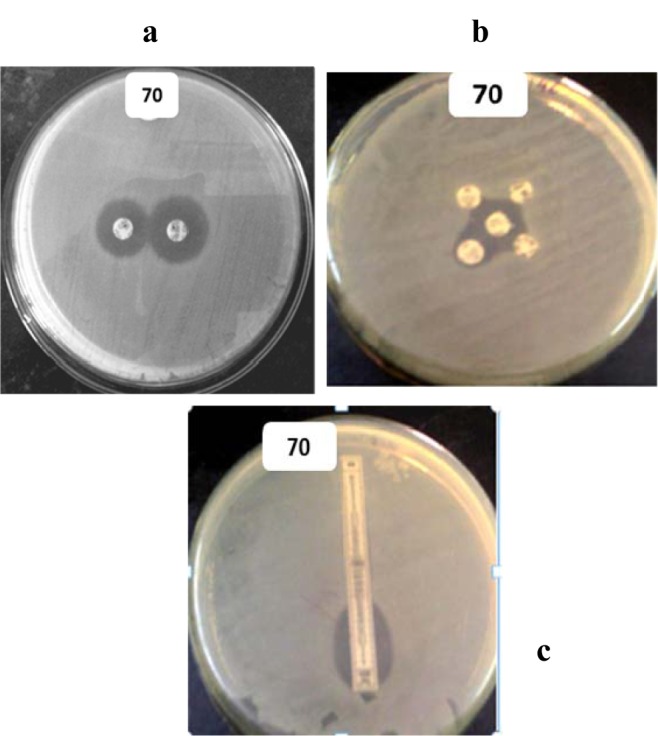
Table 2. ESBL producing uropathogens.
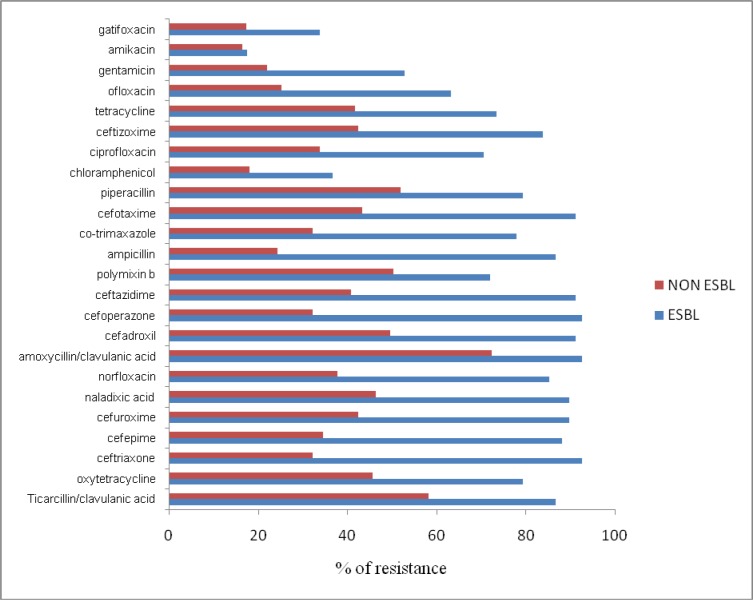
The antibiogram pattern for the isolates analysed showed ESBL-producing isolates to possess a higher degree of resistance towards antibiotics that are routinely prescribed against urinary tract infections as compared to non-ESBL-producing isolates (Figure 3(Fig. 3)). ESBL producers showed around 90 % resistance to antibiotics like Ceftazidime, Ceftriaxone, Cefotaxime, Naladixic acid and Ampicillin (Figure 3(Fig. 3)). Resistance to Ciprofloxacin was exhibited by 72.05 % (48/68) of the ESBL producers.
Figure 3. Antibiogram showing resistance pattern of ESBL and non-ESBL-producing uropathogens towards different antibiotics.
Resistance to Amikacin was found to be comparatively lower in both ESBL and non-ESBL producers. Gentamicin, Chloramphenicol and Ampicillin were found to be more effective on non-ESBLs as compared to ESBL producing uropathogens (Figure 3(Fig. 3)).
Discussion
In India, the prevalence of ESBLs has been reported since the 1990s (Revathi and Singh, 1997[41]; Karim et al., 2001[27]; Mathai et al., 2002[30]; Gupta et al., 2007[21]; Aggarwal et al., 2009[2]; Narayanaswamy and Mallika, 2011[35]) with very few reports from south India published (Padmini and Raju, 2004[36]; Menon et al., 2006[34]; Jemima and Verghese, 2008[25]; Mano and Vasanthi, 2008[29]; Kamatchi et al., 2009[26]; Narayanaswamy and Mallika, 2011[35]; Gururajan et al., 2011[22]).
The phenotypic data generated in the current study indicates a high prevalence of ESBL producers in the region of south Mumbai (situated in the western part of India), where 68 out of 195 (34.87 %) uropathogens were found to be ESBL producers. With a hot and humid climate prevalent in Mumbai, incidences of UTIs are constantly on the rise. Statistics have shown that ESBL-producing E. coli are found to be the highest in India (60 %), followed by Hong Kong (48 %) and Singapore (33 %) (Hsueh et al., 2011[23]).
In the current study, 96 of the 195 isolates (49.23 %) were identified as E. coli, of which 36 isolates (49.32 %) were ESBL producers. General studies have revealed E. coli to be one of the most frequently encountered drug-resistant uropathogens (Gupta et al., 1999[20], 2007[21]; Tambekar et al., 2006[43]); a fact clearly reflected in the current study carried out in south Mumbai. The study however showed only 8 out of 29 (27.5 %) Klebsiella pneumoniae isolates to be ESBL producers, which is generally the second most common uropathogen and a frequent ESBL producer (Gales et al., 2002[17]; Mathur et al., 2002[31]). Overall, 88.20 % (172/195) isolates were found to be resistant towards multiple drugs.
A recent study in Chennai reported ESBL production among 47 % E. coli and 37 % Klebsiella pneumoniae in a tertiary care centre (Gururajan et al., 2011[22]). Another study conducted in the suburbs of Chennai showed ESBL production among 75.5 % E. coli isolates (Maya et al., 2011[32]). Frequency of ESBL production in Islamabad and Rawalpindi was found to be 48 and 35 % respectively among E. coli isolates (Zaman et al., 1999[46]).
Other countries, in comparison with India, show a reduced incidence of ESBL producers. A study has shown that the prevalence of ESBL producing isolates of E. coli is 13.3 % in Lebanon, 9.2 % in Korea, 10.3 % in Arabia and 17 % in Turkey (Ananthan and Subha, 2005[4]). However there is a constant increase in the population of ESBL producing strains in communities of other countries (Arpin et al., 2003[5]; Borer et al., 2002[6]; Wiener et al., 1999[45]; Paterson et al., 2003[39]).
Mumbai has witnessed a gradual rise in the number of antibiotic-resistant microorganisms, primarily stemming from administering drugs without any preceding isolation or sensitivity tests which is coupled with poverty and illiteracy among the masses. This has given way to improper dosing, lack of completion of the prescribed course and the use of leftover drugs (Akram et al., 2007[3]). In concurrence, this study reflects a comparatively high percentage of ESBL producers (34.87 %) in the region under study.
The antibiogram pattern (Figure 3(Fig. 3)) of uropathogens shows a higher degree of resistance in ESBL producers as compared to non-ESBL producers. It also revealed that 49 of 68 (72.05 %) ESBL producers were resistant to Ciprofloxacin, one of the most commonly used fluoroquinolone drugs which might be indicative of fluoroquinolones being prescribed at a high frequency in south Mumbai. Although fluoroquinolones should not be prescribed routinely for UTIs (Burka et al., 2005[8]; Park-Wyllie et al., 2006[38]; Coleman et al., 2002[14]), such a high degree of resistance clearly shows the misuse of antibiotics by healthcare professionals. Several of the commonly-used fluoroquinolones have been known to exhibit a host of side effects and are, for this reason, banned in India (Adams and Tavakoli, 2006[1]).
Gatifloxacin, a fluoroquinolone, was banned in India on the 18th of March 2011, with a statement issued by the health ministry stating “the use of the following drugs is likely to involve certain risks to human beings, whereas safer alternatives to the said drugs are available”. This move comes after international studies revealed Gatifloxacin to pose a 17 times higher risk of developing serious hyperglycaemia (high blood sugar) in comparison to other antibiotics in elderly patients (Adams and Tavakoli, 2006[1]).
An individual is at a significantly higher risk of being infected by ESBL-producing uropathogens if he/she is exposed to antibiotics for a long period of time, is kept in the ICU for a long time period, suffers from any severe illness, undergoes instrumentation or catheterization procedures or is a resident in nursing homes or institutes which frequently use 3rd generation cephalosporins (Chaudhary and Aggarwal, 2004[10]). Under such circumstances, fluoroquinolones become the drug of choice. However, Ciprofloxacin also shows many side effects if given in slightly higher doses and hence its extensive use should be avoided. It is hence advised that it should be used only in cases where the bacterial load is extremely high (Cendron, 2008[9]). Since the data in the current study is not confined to any specific healthcare centre, it is evident that fluoroquinolones are extensively used throughout south Mumbai.
A low degree of resistance to Amikacin (an aminoglycoside drug) was observed for both ESBL and non-ESBL producers and hence may be helpful in combating severe infections. ESBL producers showed resistance to β-lactam antibiotics as well as non-β-lactam antibiotics. All ESBL producers were sensitive to carbapenems which are used as last resort drugs. These results are in accordance with the findings in a tertiary care hospital based in south India (Padmini et al., 2008[37]).
The current study, which examines the spread of drug resistance among uropathogens in south Mumbai, is, to our awareness, the first of its kind as it is not restricted to a specific healthcare centre. Additional studies can be carried out with a larger sample size to obtain a more conclusive scenario and possibly extend its implications to other regions of Mumbai city. The results obtained can be confirmed using molecular studies to check whether the widespread resistance is due to a specific gene or multiple genes.
Acknowledgement
This study is a Major Research project assigned and funded by the University Grants Commission (F. No. 40-111/2011 (SR) dated 4th July 2011. We are also thankful to Dr. Gupte, Consultant, HiMedia Laboratories Pvt. Ltd for his guidance and help with E-Test and in obtaining the standard cultures.
References
- 1.Adams M, Tavakoli H. Gatifloxacin-induced hallucinations in a. 19-year-old man. Psychosomatics. 2006;47:360. doi: 10.1176/appi.psy.47.4.360. [DOI] [PubMed] [Google Scholar]
- 2.Aggarwal R, Chaudhary U, Sikka R. Detection of extended spectrum beta-lactamase production among uropathogens. J Lab Physicians. 2009;1:7–10. doi: 10.4103/0974-2727.44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akram M, Shahid M, Khan AU. Etiology and antibiotic resistance patterns of community acquired urinary tract infections in JNMC Hospital Aligarh. Ann Clin Microbiol Antimicrob. 2007;6:4. doi: 10.1186/1476-0711-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ananthan S, Subha A. Cefoxitin resistance mediated by loss of a porin in clinical strains of Klebsiella pneumoniae and Escherichia coli. Indian J Med Microbiol. 2005;23:20–23. doi: 10.4103/0255-0857.13867. [DOI] [PubMed] [Google Scholar]
- 5.Arpin C, Dubois V, Coulange L, André C, Fischer I, Noury P, et al. Extended-spectrum ß-lactamase producing Enterobacteriaceae in community and private health care centres. Antimicrob Agents Chemother. 2003;47:3506–3514. doi: 10.1128/AAC.47.11.3506-3514.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borer A, Gilad J, Menashe G, Peled N, Riesenberg K, Schlaeffer F. Extended-spectrum beta-lactamase producing Enterobacteriacea strains in community-acquired bacteremia in Southern Israel. Med Sci Monit. 2002;8(1):CR44–CR47. [PubMed] [Google Scholar]
- 7.Bradford PA. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001;48:933–951. doi: 10.1128/CMR.14.4.933-951.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burka JM, Bower K, Vanroekel R, Stutzman R, Kuzmowych C, Howard R. The effect of fourth-generation fluoroquinolones gatifloxacin and moxifloxacin on epithelial healing following photorefractive keratectomy. Am J Ophthalmol. 2005;140:83–7. doi: 10.1016/j.ajo.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 9.Cendron M. Antibiotic prophylaxis in the management of vesicoureteral reflux. Adv Urol. 2008 doi: 10.1155/2008/825475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhary U, Aggarwal R. Extended spectrum -lactamases (ESBL) - an emerging threat to clinical therapeutics. Indian J Med Microbiol. 2004;22:75–80. [PubMed] [Google Scholar]
- 11.CLSI. CLSI; 2012. Performance standards for antimicrobial disc susceptibility tests;approved standard – 11th ed., document M02-A11. [Google Scholar]
- 12.CLSI. CLSI; 2011. Performance standards for antimicrobial disc susceptibility tests;Vol. 31 No. 1. document M100-S21. [Google Scholar]
- 13.CLSI. CLSI; 2010. Performance standards for antimicrobial susceptibility tests. Twentieth informational supplemented. document M100-S20. [Google Scholar]
- 14.Coleman C, Spencer J, Chung J, Reddy P. Possible gatifloxacin-induced fulminant hepatic failure. Ann Pharmacother. 2002;36:1162–7. doi: 10.1345/aph.1A414. [DOI] [PubMed] [Google Scholar]
- 15.Collee JG, Fraser AG, Marmion BP, Simmons A. Mackie & McCartney practical medical microbiology. 14th. London: Churchill Livingstone; 1996. pp. 361–381. [Google Scholar]
- 16.Fukuda H, Hiramatsu K. Mechanisms of endogenous drug resistance acquisition by spontaneous chromosomal gene mutation [in Japanese] Nihon Rinsho. 1997;55:1185–1190. [PubMed] [Google Scholar]
- 17.Gales A, Sader H, Jones R. Urinary tract infection’s trends in the American hospitals: reports from the SENTRY Antimicrobial Surveillance programme (1997-2000) Diagn Microbial Infect Dis. 2002;44:289–299. doi: 10.1016/s0732-8893(02)00470-4. [DOI] [PubMed] [Google Scholar]
- 18.Giriyapur R, Nandihal N, Krishna B, Patil A, Chandrasekhar M. Comparison of disc-diffusion methods for the detection of extended-spectrum beta lactamase-producing Enterobacteriaceae. J Lab Physicians. 2011;3:33–36. doi: 10.4103/0974-2727.78561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez C, Schaeffer A. Treatment of urinary tract infection: what’s old, what’s new, and what works. World J Urol. 1999;17:372–382. doi: 10.1007/s003450050163. [DOI] [PubMed] [Google Scholar]
- 20.Gupta K, Scholes D, Stamm WE. Increasing prevalence of antimicrobial resistance among uropathogens causing acute uncomplicated cystitis in women. JAMA. 1999;281:736–738. doi: 10.1001/jama.281.8.736. [DOI] [PubMed] [Google Scholar]
- 21.Gupta N, Kundra S, Sharma A, Gautam V, Arora DR. Antimicrobial susceptibility of uropathogens in India. J Infect Dis Antimicrob Agents. 2007;24:13–18. [Google Scholar]
- 22.Gururajan G, Kathireshan A, Kaliyaperumal, Balagurunathan R. Prevalence of extended spectrum beta lactamases in uropathogenic Escherichia coli and Klebsiella species in a Chennai suburban tertiary care hospital and its antibiogram pattern. Res J Microbiol. 2011;6:796–804. [Google Scholar]
- 23.Hsueh PR, Hoban DJ, Carmeli Y, Chen SY, Desikan S, Alejandria M, et al. Consensus review of epidemiology and appropriate antimicrobial therapy of complicated urinary tract infections in Asia–Pacific region. J Infect. 2011;63:114–123. doi: 10.1016/j.jinf.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Jarlier V, Nicolas MH, Fournier G, Philippon A. Extended broad-spectrum β-lactamases conferring transferable resistance to newer β-lactam agents in Enterobacteriaceae: Hospital prevalence and susceptibility patterns. Rev Infect Dis. 1988;10:867–878. doi: 10.1093/clinids/10.4.867. [DOI] [PubMed] [Google Scholar]
- 25.Jemima SA, Verghese S. Molecular characterization of nosocomial CTX-M type beta-lactamases producing Enterobacteriaceae from a tertiary care hospital in South India. Indian J Med Microbiol. 2008;26:356–358. doi: 10.4103/0255-0857.43581. [DOI] [PubMed] [Google Scholar]
- 26.Kamatchi C, Magesh H, Sekhar U, Vaidyanathan R. Identification of clonal clusters of Klebsiella pneumoniae isolates from Chennai by extended spectrum beta lactamase genotyping and antibiotic resistance phenotyping analysis. Am J Infect Dis. 2009;5:74–82. [Google Scholar]
- 27.Karim A, Poirel S, Nagarajan, Nordmann P. Plasmid mediated extended-spectrum beta-lactamase from India and gene association with insertion sequence ISEcp1. FEMS Microbiol Lett. 2001;201:237–241. doi: 10.1111/j.1574-6968.2001.tb10762.x. [DOI] [PubMed] [Google Scholar]
- 28.Kassis-Chikhani N, Vimont S, Asselat K, Trivalle C, Minassian B, Sengelin C, et al. CTX-M β-lactamase- producing Escherichia coli in long-term care facilities, France. Emerg Infect Dis. 2004;10:1697–1698. doi: 10.3201/eid1009.030969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mano JV, Vasanthi S. Detection of extended spectrum beta lactamases (ESBL) in the family of enterobacteriaceae at rural hospital. Indian J Med Res. 2008;127:671. [Google Scholar]
- 30.Mathai D, Rhomberg PR, Biedenbach DJ, Jones RN. Evaluation of the in vitro activity of six broad-spectrum beta-lactam antimicrobial agents tested against recent clinical isolates from India: A survey of ten medical center laboratories. Diagn Microbiol Infect Dis. 2002;44:367–377. doi: 10.1016/s0732-8893(02)00466-2. [DOI] [PubMed] [Google Scholar]
- 31.Mathur P, Tatman A, Das B, Dhawan B. Prevalence of extended spectrum beta lactamase producing gram negative bacteria in a tertiary care hospital. Indian J Med Res. 2002;115:153–157. [PubMed] [Google Scholar]
- 32.Maya AS, Prabhakar K, Hanna LE, Sarayu YL. Phenotypic and genotypic characterization of extended-spectrum beta-lactamase producing Escherichia coli clinical isolates from Semi urban Area. J Pharm Res. 2011;4:6–10. [Google Scholar]
- 33.Mazzuli T. Resistance trends in urinary tract pathogens and impact on management. J Urol. 2002;168:1720–1722. doi: 10.1097/01.ju.0000028385.10311.c9. [DOI] [PubMed] [Google Scholar]
- 34.Menon T, Bindu D, Kumar CP, Nalini S, Thirunarayan MA. Comparison of double disc and three dimensional methods to screen for ESBL producers in a tertiary care hospital. Indian J Med Microbiol. 2006;24:117–120. doi: 10.4103/0255-0857.25192. [DOI] [PubMed] [Google Scholar]
- 35.Narayanaswamy A, Mallika M. Prevalence of extended spectrum beta lactamases in urinary isolates of Escherichia coli in a tertiary care hospital, Chennai, South India. Internet J Med. 2011;6:39–43. [Google Scholar]
- 36.Padmini SB, Raju BA. Extended spectrum β-lactamases in urinary isolates of Escherichia coli and Klebsiella pneumoniae - prevalence and susceptibility pattern in a tertiary care hospital. Indian J Med Microbiol. 2004;22:172–174. [PubMed] [Google Scholar]
- 37.Padmini SB, Raju BA, Mani KR. Detection of Enterobacteriacea producing CTX-M extended spectrum β-lactamases from a tertiary care hospital in South India. Indian J Med Microbiol. 2008;26:163–166. doi: 10.4103/0255-0857.40534. [DOI] [PubMed] [Google Scholar]
- 38.Park-Wyllie LY, Juurlink DN, Kopp A, Shah BR, Stukel TA, Stumpo C, et al. Outpatient gatifloxacin therapy and dysglycemia in older adults. N Engl J Med. 2006;354:1352–1361. doi: 10.1056/NEJMoa055191. [DOI] [PubMed] [Google Scholar]
- 39.Paterson DL, Hujer KM, Hujer AM, Yeiser B, Bonomo MD, Rice LB. Extended-spectrum beta-lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: dominance and widespread prevalence of SHV- and CTX-M-type beta-lactamases. Antimicrob Agents Chemother. 2003;47:3554–3560. doi: 10.1128/AAC.47.11.3554-3560.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Philippon A, Arlet G, Jacoby GA. Plasmid-determined AmpC type β-lactamases. Antimicrob Agents Chemother. 2002;46:1–11. doi: 10.1128/AAC.46.1.1-11.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Revathi G, Singh S. Detection of expanded spectrum cephalosporin resistance due to inducible lactamases in hospital isolates. Indian J Med Microbiol. 1997;15:113–115. [Google Scholar]
- 42.Shukla I, Tiwari R, Agrawal M. Prevalence of extended spectrum β-lactamase producing Klebsiella pneumoniae in a tertiary care hospital. Indian J Med Microbiol. 2004;22:87–89. [PubMed] [Google Scholar]
- 43.Tambekar D, Dhanorkar D, Gulhane S, Khandelwal V, Dudhane M. Antibacterial susceptibility of some urinary tract pathogens to commonly used antibiotics. Afr J Biotechnol. 2006;5:1562–1565. [Google Scholar]
- 44.Truls E, Bjerklund J. Hospital acquired urinary tract infections in urology departments: Pathogens, susceptibility and use of antibiotics. Int J Antimicrob Agents. 2006;28S:S91–107. doi: 10.1016/j.ijantimicag.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Wiener J, Quinn J, Bradford P, Goering R, Nathan C, Bush K. Multiple antibiotic-resistant Klebsiella and Escherichia coli in nursing homes. JAMA. 1999;281:517–523. doi: 10.1001/jama.281.6.517. [DOI] [PubMed] [Google Scholar]
- 46.Zaman G, Karamat KA, Abbasi S, Rafi S, Ikram A. Prevalence of extended-spectrum beta-lactamase (ESBL) producing enterobacteriaceae in nosocomial isolates. Pak Armed Forces Med J. 1999;49:91–96. [Google Scholar]