Abstract
Considering the global popularity and also the various biological and medicinal properties of saffron, this study was conducted to assess the influence of its aqueous extracts administration on blood pressure, pressure-rate product (PRP) and electrocardiogram (ECG) indices of rat. Animals were divided to control (CTL), SAF50, SAF100, and SAF200 groups that orally received tap water, aqueous extracts of saffron 50, 100 and 200 mg/kg/day respectively for seven days. On day 8, data were recorded.
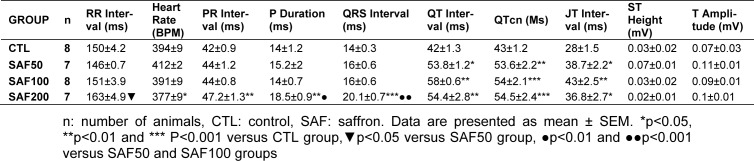
Different doses of saffron had no significant effect on blood pressure and also PRP. Higher dose (200 mg/kg) of saffron significantly increased the PR interval, P duration, QT interval (p<0.01), QRS interval, QTcn (normalized corrected QT) (p<0.001), and JT interval (p<0.05) of ECG compared to the CTL group. In addition, the two other doses only significantly prolonged the QT, QTcn and JT intervals of ECG versus the CTL group. The SAF200 group also showed a notable increase in RR interval which only was significant versus to the SAF50. There was no significant difference among ST height and T amplitude ranges of different groups.
The results suggest that high dose of saffron definitely slows the electrical conduction velocity in both atrium and ventricle.
Keywords: Crocus sativus L. (Saffron), electrocardiogram (ECG), heart electrical conduction, blood pressure, PRP
Introduction
The traditional consumption of saffron as a seasoning for some foods and also for medicinal purposes in the west and east societies is well known. In recent years, many studies reported some pharmacological and therapeutical aspects of saffron such as its protective effects on heart (Sachdeva et al., 2012[20]; Joukar et al., 2010[14]), brain (Ghadrdoost et al., 2011[8]; Hosseinzadeh et al., 2012[11]) and kidney (Ajami et al., 2010[1]) whenever these organs encountered to the stressful conditions. In vitro studies showed stimulatory effect of saffron on IFN-γ and IL-4 secretion from peripheral blood mononuclear cells (Boskabady et al., 2011[4]) and also pro-apoptotic effect of this substance in a lung cancer-derived cell line is reported (Samarghandian et al., 2010[21]). In addition, human studies demonstrated the antidepressants effects (Akhondzadeh et al., 2005[3]; Akhondzadeh Basti et al., 2007[2]) and alleviative role of saffron for women menstrual distress (Fukui et al., 2011[7]). Despite the beneficial effect of saffron, Mohajeri et al. (2007[18]) indicated that high doses of Crocus sativus L. stigma is associated with hepatotoxic and nephrotoxic effects.
Electrical properties of heart are considered as an integral part of heart function. Impairment of the electrical rhythm and electrical conductivity of the heart can lead to life-threatening cardiac arrhythmia.
Annual sudden cardiac death due to ventricular arrhythmia has been estimated at about six million people (Mehra, 2007[17]). In previous study we indicated the cardioprotective effect of saffron on isoproterenol-induced heart injury that partly applies by improvement of antioxidant system (Joukar et al., 2010[14]). Based on best knowledge, so far only one in vitro study investigated the effect of saffron on heart electrical conductivity and reported that saffron has a depressant effect on AV nodal rate-dependent properties (Khori et al., 2012[15]). Considering the global popularity of saffron and the discovery of new pharmacological properties of this spice in recent years and also the lack of adequate information regarding its effects on cardiac electrophysiology, the present study was designed to investigate the effect of saffron extract administration on blood pressure, myocardial oxygen demand index (PRP) and ECG properties of rat.
Materials and Methods
All experiments were conducted in strict concordance with the guidelines of animal studies provided by Ethic committee permission No 86/123KA-Kerman University of Medical Sciences and performed on 30 male Wistar rats weighed 250-300 g, purchased from the laboratory animal breeding center of medical school, Kerman University of Medical Sciences, Kerman, Iran.
Preparation of saffron extract
Saffron stigmas were collected from Zarand (Kerman province, Iran), identified and confirmed by Botany Department of Bahonar, University of Kerman, Iran. Dried stigmas were chopped and macerated in tap water for 3 days. Then the mixture was filtered and different concentrations of saffron solution i.e 50 mg/ml, 100 mg/ml and 200 mg/ml were prepared separately (Joukar et al., 2010[14]). This method was chosen based on the current using pattern among consumers.
Animals
Animals were housed in a temperature-controlled room and 12-h light/12-h dark cycle and were randomized to four groups. The control group (CTL) received 1 cc/kg of tap water by gastric tube for 7 days. Saffron groups including SAF50, SAF100 and SAF200 were fed with saffron extract, the similar volume of water used for the control group, with doses of 50, 100, and 200 mg/kg /daily respectively for seven days.
Surgical and experimental protocol and ECG recording
On day 8, animals were deeply anesthetized by intraperitoneal injection of sodium thiopental (50 mg/kg i.p.) (Joukar et al., 2011[13]). Animals had spontaneously breathing throughout the experiment. A heparinized saline filled (15 units/ml) cannula inserted into the right carotid artery and connected to a pressure transducer and a PowerLab analog to digital converter (AD Instruments, Australia) for recording of heart rate and arterial blood pressure (BP). Time window for animal recovery from surgery was 15 min. After this time, ECG and BP were recorded for analyzing. Mean arterial pressure (MAP) was calculated by “MAP=Pd+(Ps−Pd)/3 formula”, where Pd is the diastolic arterial pressure and Ps is the systolic arterial pressure. Pressure-rate product (PRP), an indirect measure of myocardial oxygen demand, was determined as the product of the heart rate and mean arterial pressure ((MAP*heart rate)*1000-1) (Joukar et al., 2012[12]). The ECG was recorded with standard limb lead II using a PowerLab data acquisition system and the duration and voltage of waves and also intervals of ECG were determined by mean of 5 min ECG recorded strip. In order to obviate the dependence of QT interval on heart rate, corrected QT (QTc) intervals were measured using Bazett's formula normalized as QTcn-B=QT/(RR/ f)1/2, where RR is R-R interval and f =150 ms (Kmecova and Klimas, 2010[16]).
Statistical analysis
The data are presented as the mean ± S.E.M. Comparisons were performed among different groups by one-way ANOVA and post-hoc Tukey's test. P-values less than 0.05 were considered as statistically significant.
Results
Heart rate, arterial blood pressure and PRP
Administration of different doses of saffron for one-week had no significant effect on systolic, diastolic, and mean arterial pressure and also PRP (Figure 1(Fig. 1) and Figure 2(Fig. 2)). However the dose of 50 mg/kg/day was associated with a significant enhancement of the heart rate whenever compared with the group of SAF200. A non-significant increase of PRP also was observed in SAF50 group than other groups (Table 1(Tab. 1) and Figure 2(Fig. 2)).
Figure 1. Arterial blood pressure in different experimental groups. Values are mean ± SEM. n=7-8, CTL control, SAF: saffron, S: systolic pressure, D: diastolic pressure, MAP: mean arterial pressure.
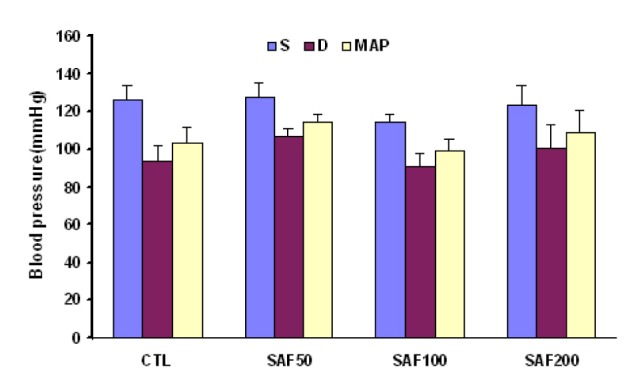
Figure 2. Pressure - rate product (PRP) as mmHg*beats/min*1000-1 in different animal groups. SAF: saffron.
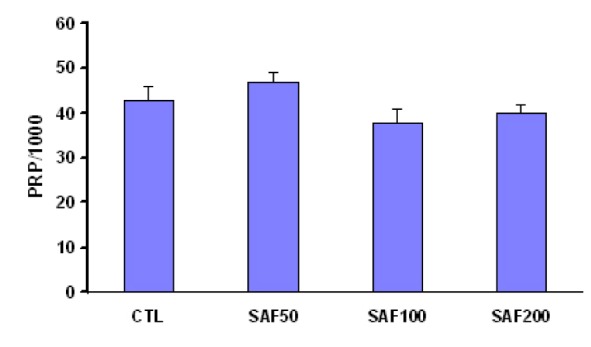
Table 1. ECG parameters of different groups of the study.
ECG parameters
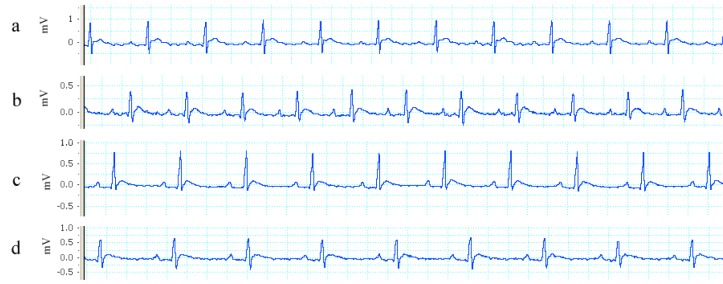
Figure 3(Fig. 3) shows some sample traces of ECGs recorded from different animal groups with increased QT intervals in saffron groups along with gradual reduction in heart rate at doses higher than 50 mg/kg. Trace "a" is from CTL group, trace "b" is from SAF50 group, trace "c" is from SAF100 group and trace "d" is from SAF200 group.
Figure 3. The sample traces of ECG normal sinus rhythm recorded from different animal groups. Trace "a" (CTL group), trace "b" (SAF50 group), trace "c" (SAF100) group and trace "d" is from SAF200 group.
As shown in Table 1(Tab. 1), the dose of 200 mg/kg/day of saffron induced more disturbance on ECG than that of other two doses (50 and 100 mg/kg/day). Administration of the higher dose (200 mg/kg) significantly increased the PR Interval, P Duration, QRS Interval, QT Interval, QTcn and JT interval of ECG compared to CTL group. In addition, lower (50 mg/kg/day) and intermediate (100 mg/kg/day) doses of saffron only significantly prolonged the QT, QTcn and JT intervals of ECG versus CTL group. On the other hand, P duration and QRS interval were more prolonged in higher dose than intermediate and lower doses of saffron (p≤0.01 and p≤0.001 respectively) (Table 1(Tab. 1)). Although the RR interval showed a notable increase in SAF200 group, but it was significant only relative to SAF50 group (Table 1(Tab. 1) and Figure 3(Fig. 3)). There was no significant difference in the ST height and T amplitude ranges of all groups.
Discussion
The aim of the current study was to elucidate whether saffron consumption has any effect on blood pressure, myocardial oxygen demand and electrophysiological conduction of rat heart. The results of this experiment indicated that one-week administration of saffron had no significant effect on blood pressure and myocardial oxygen demand of normal rat. Saffron apparently has no effect on P wave and T wave amplitudes and ST height but especially at higher doses significantly slowed the electrical conductivity of heart as prolongation of PR interval, P duration, QRS interval, QTc interval and JT interval. This means that saffron consumption significantly reduces the atrial, nodal and ventricular impulse conduction. Previous studies were considered a cardiovascular protective role for saffron (Sachdeva et al., 2012[20]; Joukar et al., 2010[14]) and some of its constituents (Goyal et al., 2010[9]). Saffron can exert a cardioprotective effect by preserving hemodynamics and left ventricular functions and also attenuation of myocardial degeneration in stressful condition (Sachdeva et al., 2012[20]). Other study demonstrated the cardioprotective effect of crocin as a pharmacologically active component of saffron in isoproterenol-induced cardiac toxicity (Goyal et al., 2010[9]). The results of these studies emphasized that saffron and crocin can improve the reduction of arterial blood pressure induced by isoproterenol that is consistent with our findings concerning the stability of blood pressure in the presence of saffron. Maintain the normal blood pressure along with no significant alteration in heart rate in all groups with different doses of saffron is a reason to justify the lack of significant change in PRP as an index of myocardial oxygen consumption. The PR interval indicates the atrio-ventricular conduction velocity pattern. The results showed that saffron consumption was associated with increase in PR interval that implies impairment in atrio-ventricular conduction. In agreement with our study, others demonstrated the depressant effect of saffron on AV nodal rate-dependent properties and also increasing the Wenckebach block cycle length and nodal functional refractory period in vitro (Khori et al., 2012[15]). Based on PR interval prolongation induced by saffron, it is possible that saffron can slow nodal conductivity through the blockade of calcium current and reduction of the slope of pacemaker potential similar to the actions of class 4 of antiarrhythmic drugs. In confirmation of this possibility, it is reported that aqueous-ethanol extract of saffron has a potent inhibitory effect on the calcium channel of guinea-pig isolated heart (Boskabady et al., 2008[5]).
The QT interval, the period from QRS complex onset to the end of T wave, is considered as the electrocardiographic manifestation of ventricular depolarization and repolarization. Generally, QT interval shortens with tachycardia and lengthens with bradycardia. For exact assessment of this index a rate corrected QT (QTc) should be calculated for eliminating its dependence on heart rate (Gupta et al., 2007[10]). QTc interval increased at different doses of saffron. Interestingly, this effect was raised during both the depolarization, as the wider QRS wave, and repolarization, as the JT interval prolongation, phases of action potential. This means that similar to atrio-nodal conduction, saffron can reduce ventricular conduction velocity and also can exert a delay on ventricular cells repolarization and hence increase action potential duration. Myocardial depolarization is mediated by influx of sodium ions; however, myocardial repolarization primarily is induced by efflux of potassium ions. The QRS prolongation may result from blockade of sodium channels, like to the actions of Class 1 antiarrhythmic drugs, and JT interval prolongation can induce by blockade of potassium channel similar to the actions of Class 3 antiarrhythmic drugs (Brunton et al., 2011[6]). Therefore, saffron could potentially be an antiarrhythmics agent due to its ability for reduction of electrical conductivity and also for prolongation of action potential. On the other hand, long QT interval may cause early afterdepolarizations (EADs) due to activation of inward depolarizing currents. This in turn can cause ventricular extrasystoles and finally increase the risk of reentry and a polymorphic ventricular tachycardia known as torsade de pointes (TdP) (Gupta et al., 2007[10]; Roden et al., 1996[19]).
In summary, this study showed for the first time that oral administration of saffron, as commonly used in people, can cause changes in the ECG of rat. The alterations include prolongation of the P wave, QRS complex, PR, QTc and JT intervals. These electrophysiological disturbances may be due to change in expression or permeability of ion channels which are responsible for the production and propagation of the action potential in cardiac cells. Future studies are needed to clarify the exact mechanisms as well as long-term consequences of saffron consumption on the electrical characteristics of the human heart.
Acknowledgement
The author would like to thank Professor Hamid Najafipour and the personnel of the Department of Physiology and Pharmacology, Kerman University of Medical Sciences for their good cooperation and assistance.
References
- 1.Ajami M, Eghtesadi S, Pazoki-Toroudi H, Habibey R, Ebrahimi SA. Effect of crocus sativus on gentamicin induced nephrotoxicity. Biol Res. 2010;43:83–90. [PubMed] [Google Scholar]
- 2.Akhondzadeh Basti A, Moshiri E, Noorbala AA, Jamshidi AH, Abbasi SH, Akhondzadeh S. Comparison of petal of Crocus sativus L. and fluoxetine in the treatment of depressed outpatients: a pilot double-blind randomized trial. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:439–442. doi: 10.1016/j.pnpbp.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Akhondzadeh S, Tahmacebi-Pour N, Noorbala AA, Amini H, Fallah-Pour H, Jamshidi AH, et al. Crocus sativus L. in the treatment of mild to moderate depression: a double-blind, randomized and placebo-controlled trial. Phytother Res. 2005;19:148–151. doi: 10.1002/ptr.1647. [DOI] [PubMed] [Google Scholar]
- 4.Boskabady MH, Seyedhosseini Tamijani SM, Rafatpanah H, Rezaei A, Alavinejad A. The effect of Crocus sativus extract on human lymphocytes' cytokines and T helper 2/T helper 1 balance. J Med Food. 2011;14:1538–1545. doi: 10.1089/jmf.2011.1697. [DOI] [PubMed] [Google Scholar]
- 5.Boskabady MH, Shafei MN, Shakiba A, Sang Sefidi H. Effect of aqueous-ethanol extract from Crocus sativus (Saffron) on Guinea-pig isolated heart. Phytother Res. 2008;22:330–4. doi: 10.1002/ptr.2317. [DOI] [PubMed] [Google Scholar]
- 6.Brunton L, Chabner B, Knollman B. Goodman & Gilmans The pharmacological basis of therapeutics. 12th. New York: McGraw–Hill; 2011. pp. 828–832. [Google Scholar]
- 7.Fukui H, Toyoshima K, Komaki R. Psychological and neuroendocrinological effects of odor of saffron (Crocus sativus) Phytomedicine. 2011;18:726–730. doi: 10.1016/j.phymed.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Ghadrdoost B, Vafaei AA, Rashidy-Pour A, Hajisoltani R, Bandegi AR, Motamedi F, et al. Protective effects of saffron extract and its active constituent crocin against oxidative stress and spatial learning and memory deficits induced by chronic stress in rats. Eur J Pharmacol. 2011;667:222–229. doi: 10.1016/j.ejphar.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 9.Goyal SN, Arora S, Sharma AK, Joshi S, Ray R, Bhatia J, et al. Preventive effect of crocin of Crocus sativus on hemodynamic, biochemical, histopathological and ultrastuctural alterations in isoproterenol-induced cardiotoxicity in rats. Phytomedicine. 2010;17:227–232. doi: 10.1016/j.phymed.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Lawrence AT, Krishnan K, Kavinsky CJ, Trohman RG. Current concepts in the mechanisms and management of drug-induced QT prolongation and torsade de pointes. Am Heart J. 2007;153:891–899. doi: 10.1016/j.ahj.2007.01.040. [DOI] [PubMed] [Google Scholar]
- 11.Hosseinzadeh H, Sadeghnia HR, Ghaeni FA, Motamedshariaty VS, Mohajeri SA. Effects of saffron (Crocus sativus L.) and its active constituent, crocin, on recognition and spatial memory after chronic cerebral hypoperfusion in rats. Phytother Res. 2012;26:381–386. doi: 10.1002/ptr.3566. [DOI] [PubMed] [Google Scholar]
- 12.Joukar S, Bashiri H, Dabiri S, Ghotbi P, Sarveazad A, Divsalar K, et al. Cardiovascular effects of black tea and nicotine alone or in combination against experimental induced heart injury. J Physiol Biochem. 2012;68:271–279. doi: 10.1007/s13105-011-0141-z. [DOI] [PubMed] [Google Scholar]
- 13.Joukar S, Najafipour H, Dabiri S, Sheibani V, Esmaeili-Mahani S, Ghotbi P, et al. The effect of chronic co-administration of morphine and verapamil on isoproterenol-induced heart injury. Cardiovasc Hematol Agents Med Chem. 2011;9:218–224. doi: 10.2174/187152511798120930. [DOI] [PubMed] [Google Scholar]
- 14.Joukar S, Najafipour H, Khaksari M, Sepehri G, Shahrokhi N, Dabiri S, et al. The effect of saffron consumption on biochemical and histopathological heart indices of rats with myocardial infarction. Cardiovasc Toxicol. 2010;10:66–71. doi: 10.1007/s12012-010-9063-1. [DOI] [PubMed] [Google Scholar]
- 15.Khori V, Alizadeh AM, Yazdi H, Rakhshan E, Mirabbasi A, Changizi S, et al. Frequency-dependent electrophysiological remodeling of the AV node by hydroalcohol extract of Crocus sativus L. (Saffron) during experimental atrial fibrillation: the role of endogenous nitric oxide. Phytother Res. 2012;26:826–832. doi: 10.1002/ptr.3643. [DOI] [PubMed] [Google Scholar]
- 16.Kmecova J, Klimas J. Heart rate correction of the QT duration in rats. Eur J Pharmacol. 2010;641:187–92. doi: 10.1016/j.ejphar.2010.05.038. [DOI] [PubMed] [Google Scholar]
- 17.Mehra R. Global public health problem of sudden cardiac death. J Electrocardiol. 2007;40:S118–S122. doi: 10.1016/j.jelectrocard.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 18.Mohajeri D, Mousavi Gh, Mesgari M, Doustar Y, Khayat Nouri MH. Subacute toxicity of Crocus sativus L. (saffron) stigma ethanolic extract in rats. Am J Pharmacol Toxicol. 2007;2:189–193. [Google Scholar]
- 19.Roden DM, Lazzara R, Rosen M, Schwartz PJ, Towbin J, Vincent GM. Multiple mechanisms in the long-QT syndrome. Current knowledge, gaps, and future directions. The SADS Foundation Task Force on LQTS. Circulation. 1996;94:1996–2012. doi: 10.1161/01.cir.94.8.1996. [DOI] [PubMed] [Google Scholar]
- 20.Sachdeva J, Tanwar V, Golechha M, Siddiqui KM, Nag TC, Ray R, et al. Crocus sativus L. (saffron) attenuates isoproterenol-induced myocardial injury via preserving cardiac functions and strengthening antioxidant defense system. Exp Toxicol Pathol. 2012;64:557–564. doi: 10.1016/j.etp.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 21.Samarghandian S, Boskabady MH, Davoodi S. Use of in vitro assays to assess the potential antiproliferative and cytotoxic effects of saffron (Crocus sativus L.) in human lung cancer cell line. Pharmacogn Mag. 2010;6:309–314. doi: 10.4103/0973-1296.71799. [DOI] [PMC free article] [PubMed] [Google Scholar]