Summary
Co-inhibitory receptors, such as CTLA-4 and PD-1, have an important role in regulating T cell responses and have proven to be effective targets in the setting of chronic diseases where constitutive co-inhibitory receptor expression on T cells dampens effector T cell responses. Unfortunately, many patients still fail to respond to therapies that target CTLA-4 and PD-1. The next wave of co-inhibitory receptor targets that are being explored in clinical trials include Lag-3, Tim-3, and TIGIT. These receptors while belonging to the same class of receptors as PD-1 and CTLA-4 exhibit unique functions especially at tissue sites where they regulate distinct aspects of immunity. Increased understanding of the specialized functions of these receptors will inform the rational application of therapies that target these receptors to the clinic.
Introduction
Co-inhibitory or immune checkpoint receptors have a critical role in the maintenance of immune homeostasis: their expression on effector T cells ensures the proper contraction of effector T cell responses while their expression on regulatory T (Treg) cells guarantees the proper function of Treg cells to control effector T cells. Co-inhibitory receptors play a central role in regulating autoimmune disease. Indeed, many co-inhibitory receptors have been genetically linked to autoimmune diseases (Kasagi et al., 2011; Qu et al., 2009; Song et al., 2011; Wang et al., 2014). [Au: Would like to call out the Vignali review on this topic in this issue here? We will update the details during production.] Accordingly, their function in regulating pro-inflammatory T cell responses and the maintenance of self-tolerance has been most widely studied in this context. More recently, the role of co-inhibitory receptors has come to the forefront in cancer (Wolchok, 2016 this issue) and chronic viral infection (Wherry, 2016; this issue) where these receptors are highly expressed and are being targeted clinically to improve anti-tumor and anti-viral T cell responses (Mahoney et al., 2015; Pauken and Wherry, 2015). While current immunotherapies directed against the co-inhibitory receptors CTLA-4 and PD-1 are exhibiting unprecedented efficacy in several cancer indications and in some chronic viral infections, there are still many patients that do not respond to these therapeutic approaches and some tumor types remain largely refractory to these therapies. This has prompted intense investigation into the targeting of other co-inhibitory receptors in order to broaden the therapeutic repertoire. Lag-3, Tim-3, and TIGIT comprise the next generation of co-inhibitory receptors to be translated to the clinic. This review will highlight the unique aspects of each of these molecules in regulating immune responses, specifically at tissue sites.
Lag-3
Discovery, ligands, and function
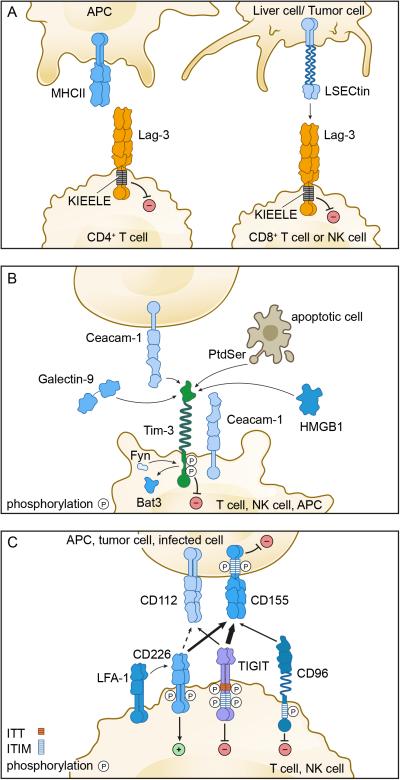
Lymphocyte activation gene-3 (Lag-3) was discovered 25 years ago as a molecule that is up-regulated on activated CD4+ and CD8+ T cells and a subset of natural killer (NK) cells (Triebel et al., 1990) (Table I). Lag-3 structurally resembles the CD4 co-receptor and, indeed, binds to MHC class II with a higher affinity than CD4 (Huard et al., 1995) (Figure 1A). The fact that Lag-3 impacts on the function of CD8+ T cells and NK cells, neither of which interact with MHC Class II, has led to speculation about the existence of alternate ligands for Lag-3. In this regard, it has been suggested that LSECtin, a member of the DC-SIGN family of molecules, is another ligand for Lag-3 (Xu et al., 2014). LSECtin is expressed in the liver and also on many tumors (Xu et al., 2014), thus providing a potential mechanism by which Lag-3-expressing CD8+ T cells and NK cells can be regulated in these tissues (Figure 1A).
Table I.
Comparison of Lag-3, Tim-3, and TIGIT.
| Lag-3 | Tim-3 | TIGIT | |
|---|---|---|---|
| Expression | |||
| CD41 | Tr1, nTreg, iTreg | Th1, Tr1, nTreg2 | Tr1,Tfh, nTreg3 |
| CD81 | dysfunctional T cells | Tc1, dysfunctional T cells | dysfunctional T cells |
| Natural Killer Cells | + | + | + |
| Dendritic Cells | - | + | - |
| Monocytes/Macrophages | - | +/−4 | - |
| Signaling Motifs | KIEELE | 5Tyrosine | ITT/ITIM |
| Ligands | MHC II, LSECtin | Galectin-9, Ceacam-1, HMGB-1, Phosphatidyl Serine | CD112, CD155 |
Lag-3,Tim-3, and TIGIT are transiently up-regulated on activated CD4+ and CD8+ T cells.
In both mouse and human, Tim-3 is either not expressed or expressed on a very small fraction of CD4+Foxp3+ Treg in the normal circulation but is highly expressed on Treg at sites of tissue inflammation
In both mouse and human, TIGIT is expressed on about one-third of CD4+FoxP3+ Treg in the normal circulation and is highly up-regulated on Treg at sites of tissue inflammation
In the mouse, Tim-3 is expressed on monocytes/macrophages only in inflammatory conditions. In humans, Tim-3 is expressed on peripheral blood monocytes and on macrophages.
Tim-3 has 5 tyrosines in its cytoplasmic tail but no known signaling motif.
Figure 1. Co-inhibitory receptor pathways.
A) The Lag-3 pathway. Left panel, Lag-3 is expressed on CD4+ T cells and binds to MHC class II on antigen presenting cells. Right panel, Lag-3 is expressed on CD8+ T cells and NK cells and binds to L-SECtin on tumor cells or liver cells. The cytoplasmic tail of Lag-3 contains a unique KIEELE motif that is essential for the inhibitory function of Lag-3. B) The Tim-3 pathway. Tim-3 is expressed on T cells, NK cells, and some APC. Tim-3 ligands include soluble ligands (galectin-9 and HMGB1) and cell surface ligands (Ceacam-1 and Phosphatidyl serine – PtdSer). Bat-3 and Fyn bind to the same region on the cytoplasmic tail of Tim-3. Ligand binding triggers the dissociation of Bat-3 from the cytoplasmic tail of Tim-3, thus allowing Fyn to bind and promote the inhibitory function of Tim-3. C) The CD226/TIGIT Pathway. CD226, TIGIT, and CD96 are expressed on T cells and NK cells and share the ligands CD112 and CD155, which are expressed on APCs and other cells such as tumor cells. CD226 associates with the integrin LFA-1 and delivers a positive signal. TIGIT, CD96, and CD155 contain ITIM motifs in their cytoplasmic tails and can deliver inhibitory signals. TIGIT further contains an ITT-like motif. CD155 and TIGIT exist as homodimers on the cell surface, and dimerization is essential for their proper function.
Although initial examination of Lag-3 deficient mice revealed no T cell defects (Miyazaki et al., 1996), subsequent careful examination both in vitro and in vivo revealed that Lag-3 deficient T cells exhibit defects consistent with Lag-3 being a negative regulator of T cell expansion (Workman et al., 2004; Workman and Vignali, 2003). Administration of the superantigen Staphylococcal enterotoxin B (SEB) in Lag-3 deficient mice was shown to result in uncontrolled expansion of Vβ8+ T cells and splenomegaly. Similarly, OVA-specific Lag-3 deficient CD4+ T cells were shown to exhibit uncontrolled expansion after immunization with OVA.
In addition to effector CD4+ T cells, Lag-3 is also expressed on CD4+ T cells that have regulatory functions. Lag-3 is expressed on both activated natural Treg (nTreg) and induced CD4+ FoxP3+ Treg (iTreg) cells (Table I), where expression levels are higher than that observed on activated effector CD4+ T cells (Huang et al., 2004). Blockade of Lag-3 on Treg cells abrogates Treg cell suppressor function while ectopic expression of Lag-3 in non-Treg CD4+ T cells confers suppressive activity. In addition, Lag-3 is required for Treg cell-mediated control of T cell homeostasis (Workman and Vignali, 2005). Together these data support a functional role for Lag-3 in Treg cell function. Lag-3 is further expressed on CD4+ FoxP3− IL-10-secreting Type 1 regulatory (Tr1) T cells. Indeed, Tr1 cells can be identified in both humans and mice by expression of Lag-3 together with CD49b (Gagliani et al., 2013); however, whether Lag-3 is required for Tr1-mediated suppression of immune responses has not been addressed.
Signaling
The association of Lag-3 with inhibition of effector T cells and promotion of Treg cell-mediated suppression raises the important question of how Lag-3 signals in these different T cell subsets to achieve its inhibitory effects. To date, most of what we know about Lag-3 signaling addresses its role in effector T cells where Lag-3 has been shown to associate with CD3 and crosslinking of Lag-3 together with CD3 inhibits T cell proliferation, cytokine production, and calcium flux (Hannier et al., 1998). The signaling pathway downstream of Lag-3 responsible for these effects is still not clear. In fact, the cytoplasmic tail of Lag-3 is unique among all known immune receptors. The Lag-3 cytoplasmic tail has three regions that are conserved between human and mouse. The first region contains a serine-phosphorylation site, the second region contains a unique KIEELE motif, and the third region contains glutamic acid-proline (EP) repeats (Workman et al., 2002). Of these three regions, the KIEELE motif has been shown to be essential for the inhibitory function of Lag-3 in effector CD4+ T cells (Workman et al., 2002); however, the intracellular proteins that bind to this motif have not been identified. Moreover, whether this motif is required for the effects of Lag-3 in Treg cells is not known.
Role in disease
The association of Lag-3 with T cell regulation via its roles in effector T cells, Treg, and potentially Tr1 cells position Lag-3 as a potential target for modulating T cell responses in disease (Figure 2A and 3). Indeed, current data support that modulating Lag-3 can impact autoimmunity, cancer, chronic viral infection, and parasitic infection. The role of Lag-3 in these different disease contexts will be discussed below.
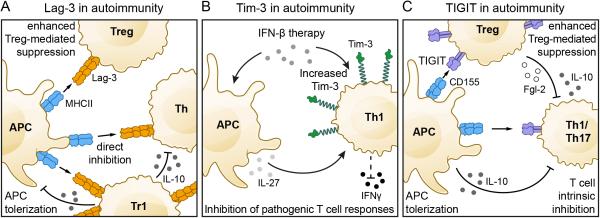
Figure 2. The Lag-3, Tim-3, and TIGIT pathways in autoimmunity.
A) Lag-3 plays a protective role in autoimmunity by dampening T helper (Th) cell responses directly through engagement of MHCII. In addition, Lag-3 indirectly inhibits effector T cell responses via promotion of Treg and Tr1-mediated suppression. B) In autoimmune diseases such as MS, Tim-3 is under-expressed on pathogenic Th1 cells. IFN-β therapy can increase Tim-3 on antigen specific T cells directly or indirectly via promotion of IL-27 production from local antigen presenting cells. Increased expression of Tim-3 is associated with reduction in disease relapses. C) TIGIT inhibits auto-pathogenic Th1/Th17 T cell responses through three different pathways: 1) TIGIT directly inhibits T cell activation and expansion; 2) TIGIT expressing effector and regulatory T cells engage CD155 on APCs thereby inducing tolerogenic APCs that secrete IL-10; 3) TIGIT promotes Treg-mediated suppression through the induction of IL-10 and Fgl2, which potently and selectively suppress Th1 and Th17 responses.
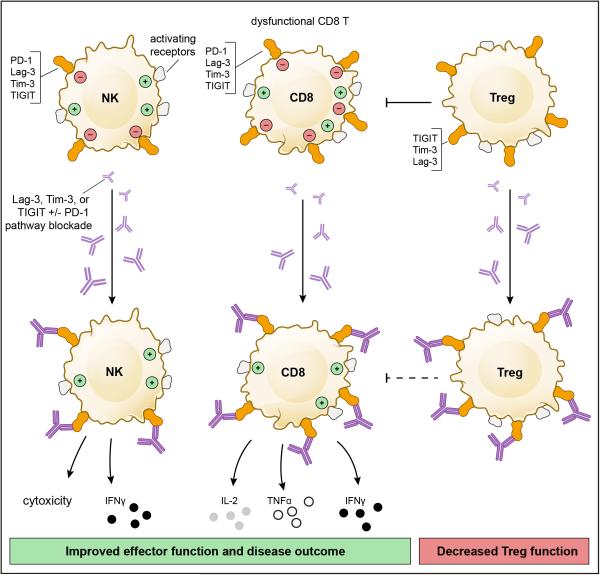
Figure 3. The Lag-3, Tim-3, and TIGIT pathways in chronic diseases.
Lag-3, Tim-3, and TIGIT are highly expressed on dysfunctional or exhausted T cells in chronic diseases such as chronic viral infection and cancer. In these diseases, combinatorial receptor blockade has strong synergistic effects, resulting in improved effector CD8+ T cell and NK cell function as well as decreased Treg-mediated suppression. These combined actions improve disease outcome.
Autoimmunity
Deficiency in co-inhibitory receptor expression can promote autoimmunity. This is most notable for CTLA-4 deficiency and PD-1 deficiency both of which result in the development of spontaneous autoimmunity even on genetic backgrounds that normally don't develop disease (Nishimura et al., 1999; Nishimura et al., 2001; Tivol et al., 1995; Waterhouse et al., 1995). In this regard, Lag-3 deficiency alone does not predispose towards autoimmunity unless the mice are on a permissive genetic background. Lag-3 deficiency on the NOD background results in accelerated Type 1 diabetes with 100% of Lag-3 deficient mice developing diabetes before age-matched wild-type controls (Bettini et al., 2011; Okazaki et al., 2011). In wild-type NOD mice, administration of blocking anti-Lag-3 antibody also accelerates Type 1 diabetes (Bettini et al., 2011). Furthermore, Lag-3 deficiency on the B6.SJL background results in increased susceptibility to Hg-induced autoimmunity and defects in antigen-specific tolerance induction (Jha et al., 2014). The importance of Lag-3 for antigen specific tolerance and its impact on autoimmunity indicated by the aforementioned studies is further reinforced by a recent study showing that auto-antigen specific tolerance drives the generation of regulatory T cells that express Lag-3 together with PD-1, Tim-3, TIGIT, and the immunosuppressive cytokine IL-10 (Burton et al., 2014). This last observation points to the co-operative function of Lag-3 with other co-inhibitory molecules for achieving optimal T cell regulation.
Cancer and chronic viral infections
Co-inhibitory receptors are highly expressed on the dysfunctional or exhausted T cells that develop in chronic diseases such as chronic viral infections and cancer. Dysfunctional or exhausted T cells are characterized by variable deficits in their ability to proliferate and elicit effector functions (cytotoxicity, cytokine production) upon stimulation through the TCR (Wherry and Kurachi, 2015). Chronic infection with the clone 13 strain of LCMV is the gold standard experimental model for studies of T cell dysfunction or exhaustion. In this model, Lag-3 expression was shown to correlate strongly with the severity of infection (Blackburn et al., 2009) and to be co-expressed with PD-1 on dysfunctional or exhausted virus-specific CD8+ T cells (Richter et al., 2010). Interestingly, while blockade of Lag-3 alone had little effect (Blackburn et al., 2009; Richter et al., 2010), blockade of Lag-3 synergized with blockade of PD-L1 to improve CD8+ T cell responses and reduce viral load (Blackburn et al., 2009). Similarly, Lag-3 is expressed on dysfunctional or exhausted parasite-specific CD4+ T cells during malaria infection and Lag-3 blockade synergizes with PD-L1 blockade to improve CD4+ T cell function and parasite clearance (Butler et al., 2012).
Cancer and chronic infections share common features, notably chronic exposure to antigen and the development of dysfunctional or exhausted effector T cells. Indeed, Lag-3 and PD-1 are co-expressed on both CD4+ and CD8+ tumor infiltrating lymphocytes (TILs) in several pre-clinical murine models of cancer and co-blockade of the Lag-3 and PD-1 pathways has been shown to synergize to improve anti-tumor CD8+ T cell responses (Woo et al., 2012). Lag-3 blockade has also been shown to synergize with anti-tumor vaccination to improve tumor-specific CD8+ T cell activation. Interestingly, this effect did not require CD4+ T cells, thus supporting a direct role for Lag-3 in regulating CD8+ T cells (Grosso et al., 2007). In humans, Lag-3 and PD-1 co-expression has been noted to mark dysfunctional or exhausted CD8+ T cells in ovarian cancer and, as observed in pre-clinical cancer models, Lag-3 and PD-1 co-blockade improved the proliferation and cytokine production of tumor-antigen specific CD8+ T cells (Matsuzaki et al., 2010). Thus, in both chronic infections and cancer, Lag-3 and PD-1 signaling functionally cooperate to dampen T cell responses (Figure 3).
As mentioned above, Lag-3 is highly expressed on Treg cells. Lag-3+ FoxP3+ Treg cells have been shown to be expanded in the PBMC, tumor-infiltrated lymph node, and tumor tissue of both melanoma and colorectal cancer patients (Camisaschi et al., 2010). These Lag-3+ Treg cells exhibit an activated phenotype, producing high IL-10 and TGF-β1. Lastly, the presence of Lag-3+ CD49b+ IL-10-producing Tr1 cells has been associated with poor prognosis in colorectal cancer (Chen and Chen, 2014). Together these data support a role for Lag-3 in suppressing immunity via its role in Treg cells.
Lag-3 has thus been shown to be an important immune regulator in autoimmunity, chronic viral infection, parasitic infection, and cancer; however, whether Lag-3-driven regulation in these different disease contexts stems from its function in modulating effector T cell, regulatory T cell (Treg and/or Tr1), or NK cell responses has not been determined. Resolution of this important issue will require careful examination of mice harboring conditional deletion of Lag-3 in different immune cell subsets. Furthermore, which ligand (MHC class II or LSECtin) is operational in promoting T cell inhibition via Lag-3 has not been addressed.
Clinical trials
The potential for Lag-3-driven regulation of immune responses is now being explored in clinical trials for cancer. Early trials have focused on using a soluble Lag-3-Ig and are based on the observation that administration of soluble Lag-3-Ig together with irradiated wild type tumor cells inhibits the growth of established tumors (Prigent et al., 1999). Phase I studies of soluble Lag-3-Ig (IMP321) have been completed in advanced renal cell carcinoma (Brignone et al., 2009) and advanced pancreatic adenocarcinoma, where IMP321 was combined with the chemotherapeutic gemcitabine (Wang-Gillam et al., 2013). In both trials, IMP321 was well tolerated with no treatment related adverse events. In renal cell carcinoma, tumor growth reduction and stable disease were observed at high treatment doses. In pancreatic adenocarcinoma, lack of activity was attributed to suboptimal dosing. IMP321 has also been tested in combination with MART-1 peptide vaccination in advanced melanoma in a phase I trial (Romano et al., 2014). Although positive responses were not observed as per RECIST (response evaluation criteria in solid tumors) criteria, increased frequencies of MART-1-reactive CD8+ T cells along with decreased frequencies of Treg cells were observed, thus supporting further exploration of IMP321. Lastly, a Phase I/II trial of IMP321 in combination with the chemotherapeutic paclitaxel in metastatic breast carcinoma has shown an objective response rate of 50% (Brignone et al., 2010).
Although Lag-3-Ig has shown some efficacy in the clinic, the mechanism by which Lag-3-Ig modulates anti-tumor responses remains unclear. IMP321 was initially described as an activator of antigen presenting cells (APC); however, MHC Class II crosslinking by Lag-3 in dendritic cells (DC) has been shown to suppress the maturation and antigen presenting function of DC (Liang et al., 2008). Moreover, IMP321 has a human IgG1 tail and thus can mediate Fc-dependent functions.
In contrast to Lag-3-Ig, the targeting of Lag-3 with antibodies is more straightforward. Antibodies that block Lag-3 binding to MHC Class II are now being explored in the clinic. These trials are exploring the use of anti-Lag-3 antibodies either alone or in combination with anti-PD-1 in both solid and hematologic cancers. These trials are still recruiting patients and thus it will be some time before trial data are available.
Tim-3
Discovery, ligands, and function
T cell immunoglobulin-3 (Tim-3) was identified 13 years ago as a cell surface molecule selectively expressed on IFN-γ- producing CD4+ T helper 1 (Th1) and CD8+ T cytotoxic 1 (Tc1) T cells (Monney et al., 2002) (Figure 1B and Table I). In addition to its expression on T cells, Tim-3 has now been identified on Treg cells and on innate immune cells (DC, NK cells, monocytes). The discovery of Tim-3 led to the identification of the Tim family of genes, which is encoded both in mice and in humans in loci that have been repeatedly associated with immune-mediated diseases such as asthma, allergy, and atopy (Meyers et al., 2005). In the mouse there are eight Tim genes; however, only three of these, Havcr1 [Tim-1], Havcr2 [Tim-3], and Timd4 [Tim-4], are conserved in humans.
Initial examination of Tim-3 function suggested that Tim-3 is a negative regulator of Type 1 immunity. Anti-Tim-3 antibody was shown to exacerbate experimental autoimmune encephalomyelitis (EAE) (Monney et al., 2002), a T cell mediated autoimmune disease of the central nervous system that serves as an animal model for multiple sclerosis (MS). Subsequent studies using Tim-3 deficient mice and wild type mice treated with Tim-3-Ig fusion protein showed that Tim-3 signaling is required for the induction of antigen-specific tolerance and that Tim-3 blockade enhances the development of spontaneous autoimmunity (Sabatos et al., 2003; Sanchez-Fueyo et al., 2003). The C-Type lectin galectin-9 was later discovered as a Tim-3 ligand (Zhu et al., 2005). This discovery solidified the inhibitory function of Tim-3 as galectin-9-triggering of Tim-3 was shown to induce cell death in Tim-3+ Th1 cells and ameliorate EAE.
In addition to galectin-9, several other ligands have been subsequently identified for Tim-3 (Figure 1B and Table I), some of which primarily have a role in innate immune cells. One of these, phosphatidyl serine (PtdSer), is not a unique ligand for Tim-3 as Tim-1, Tim-3, and Tim-4 all bind to PtdSer (Cao et al., 2007; Santiago et al., 2007a; Santiago et al., 2007b). The binding of Tim-3 to PtdSer is rather weak (at least 5 fold lower) relative to the binding of Tim-1 and Tim-4 to PtdSer (DeKruyff et al., 2010). The binding of Tim-3 to PtdSer has been implicated in the uptake of apoptotic cells and cross-presentation of antigen by dendritic cells, which constitutively express high levels of Tim-3 (Nakayama et al., 2009); however, this is not a mechanism that would be operative in T cells, which are not phagocytic.
Another Tim-3 ligand that impacts on innate immune responses is high mobility group protein B1 (HMGB1) (Chiba et al., 2012). HMGB1 binding to Tim-3 was identified by examination of the mechanisms underlying the defective responses of Tim-3+ dendritic cells (DC) to nucleic acid stimulation. HMGB1 binds DNA released from dying cells and facilitates delivery to innate cells via binding to receptor for advanced glycation end products (RAGE), and Toll-like receptors (TLRs) (2,4, and 9), thereby triggering innate cell activation and production of pro-inflammatory cytokines. The binding of Tim-3 to HMGB1 can interfere with this process, thus suppressing activation of the innate immune response.
Most recently, Ceacam-1 was identified as a novel cell surface ligand for Tim-3 (Huang et al., 2015). Ceacam-1 co-immunoprecipitates with Tim-3 and is co-expressed with Tim-3 on CD4+ T cells upon tolerance induction and on CD8+ TILs that exhibit dysfunctional/exhausted phenotype. Importantly, the negative regulatory function of Tim-3 is defective in the absence of Ceacam-1, suggesting a requirement for Ceacam-1:Tim-3 interaction for proper Tim-3 function. Notably, Ceacam-1 binds to Tim-3 in both cis and trans, where the cis interaction promotes the stability of mature Tim-3 glycoprotein on the cell surface and both the cis and trans interactions drive the inhibitory function of Tim-3. Whether triggering of Tim-3 by Ceacam-1, galectin-9, or both ligands together differentially impacts on Tim-3 function remains to be determined.
Signaling
That Tim-3 is a key regulator of effector T cell function underscores the importance of elucidating the signaling pathway downstream of Tim-3. Similar to Lag-3, Tim-3 does not have a classical signaling motif in its cytoplasmic tail (Table I). Rather the cytoplasmic tails of both mouse and human Tim-3 contain five conserved tyrosine residues among which Y256 and Y263 can be phosphorylated by either Src kinases (Lee et al., 2011) or ITK (van de Weyer et al., 2006). Y256 and Y263 are involved in the binding of Bat3 (HLA-B associated transcript 3), p85 PI3K, Fyn, and Lck to the C-terminal tail of Tim-3 (Lee et al., 2011; Rangachari et al., 2012). In the absence of ligand-mediated Tim-3 signaling, Bat3 is bound to Tim-3 and blocks SH2 domain-binding sites in the Tim-3 tail. In this state, Bat3 recruits the catalytically active form of Lck, thereby forming an intracellular molecular complex with Tim-3 that preserves and potentially promotes T cell signaling (Rangachari et al., 2012). In contrast, Bat3 deficient T cells exhibit elevated pY505 Lck, the catalytically inactive form of Lck (Rangachari et al., 2012). Galectin-9 and Ceacam-1 binding to Tim-3 leads to phosphorylation of Y256 and Y263 and release of Bat-3 from the Tim-3 tail, thereby promoting Tim-3 mediated T cell inhibition by allowing binding of SH2 domain-containing Src kinases and subsequent regulation of TCR signaling (Huang et al., 2015; Rangachari et al., 2012) (Figure 1B). Interestingly, Fyn binds to the same region on the Tim-3 tail as Bat3. Fyn has been implicated in the induction of T cell anergy (Davidson et al., 2007) and is known to be a key kinase to activate phosphoprotein associated with glycosphingolipid microdomains (PAG), which recruits Csk to suppress Lck function (Salmond et al., 2009; Smida et al., 2007). Since Fyn and Bat3 bind to the same domain in the Tim-3 cytoplasmic tail, it is possible that a switch between Tim-3-Bat3 and Tim-3-Fyn may trigger the switch of Tim-3 function from being permissive to TCR signaling to inhibition of upstream TCR signaling. In line with these data, loss of Bat-3 has been shown to result in dephosphorylation and degradation of TCRζ (Rangachari et al., 2012).
Overall, current data show that the Tim-3 cytoplasmic tail has the potential to interact with multiple components of the TCR complex and that the balance of Bat-3 versus Fyn bound to the Tim-3 intracellular tail may be a key determinant of Tim-3 function. As Tim-3 has many ligands, it will be important to determine how different ligands affect the binding of Bat-3 versus Fyn to the Tim-3 tail. This will be crucial to unraveling how Tim-3 functions to determine effector T cell responses.
Role of Tim-3 in disease
The role of Tim-3 was initially investigated in autoimmunity where highly activated and uncontrolled responses directed to self-antigens are key drivers of disease. More recently, Tim-3 function has been examined in two diseases that serve as good counterpoints to autoimmunity, namely chronic viral infection and cancer. Indeed, Tim-3 can be protective in autoimmunity but is often poorly expressed (Figure 2B), while in both cancer and chronic viral infection, Tim-3 is highly expressed and contributes to the dampening of protective immunity (Figure 3). The function of Tim-3 in these different settings will be discussed below.
Autoimmunity
As Th1 cells predominantly express Tim-3 (Monney et al., 2002) and Th1 cells are considered to be an important player in tissue-specific autoimmunity, the function of Tim-3 was initially probed in models of autoimmunity and tolerance. Animals treated with anti-Tim-3 antibody were shown to develop hyper-acute EAE accompanied by uncontrolled macrophage activation (Monney et al., 2002). Furthermore, administration of soluble Tim-3-Ig resulted in T cell hyperactivation, IFN-γ production, and loss of high dose tolerance (Sabatos et al., 2003). These data gave the first indication that Tim-3 might function as an inhibitory molecule that serves to contract IFN-γ-driven inflammation. Indeed, it was later shown that administration of galectin-9 ameliorated EAE while knock-down of galectin-9 in vivo with siRNA exacerbated disease (Zhu et al., 2005). In keeping with its expression on IFN-γ-secreting Th1 T cells, Tim-3-deficiency regulates Th1- but not Th17 cell-driven EAE (Lee and Goverman, 2013). Collectively, these observations suggested that Tim-3 expression is an important determinant of autoimmunity. Indeed, in humans, Tim-3 expression is low on T cells in the peripheral blood and cerebrospinal fluid of patients with MS (Koguchi et al., 2006; Yang et al., 2008), in the peripheral blood of patients with rheumatoid arthritis (RA) (Liu et al., 2010), and in the peripheral blood of patients with psoriasis (Kanai et al., 2012). Importantly, Tim-3 expression is regained in MS patients that exhibit stable disease and reduced relapses after IFN-β therapy (Yang et al., 2008) (Figure 2B). Moreover, preliminary genome-wide analysis of mRNA expression data from the peripheral blood of MS patients show that Tim-3 is significantly induced in responders to IFN-β therapy whereas non-responders show lower or no Tim-3 induction (Ottoboni et al., 2012).
The promotion of Tim-3 expression in MS patients after IFN-β therapy is in line with a recent study showing that IL-27, which is potently induced by IFN-β, promotes not only Tim-3, but also IL-10 expression on murine T cells (Zhu et al., 2015). In addition, a recent study shows that IFN–β can induce Tim-3 directly on murine Th1 cells (Boivin et al., 2015). Thus, IFN-β can promote Tim-3 expression both directly and indirectly. Similarly, increased Tim-3 expression on peripheral blood T cells is also associated with responsiveness to treatment (Methotrexate or Tocilizumab (anti-IL6R)) and decreased disease activity in RA (Liu et al., 2010), although the mechanism underlying the increased Tim-3 expression in this setting has not been elucidated.
Cancer and chronic viral infections
In recent years, the role of Tim-3 in the T cell dysfunction that arises in chronic viral infection and cancer has been heavily investigated. In the LCMV chronic infection model, Tim-3 marks virus-specific CD8+ T cells that exhibit the biggest defects in pro-inflammatory cytokine (IL-2, TNF-α, IFN-γ) production (Jin et al., 2010). All virus-specific CD8+ Tim-3+ T cells co-express PD-1 and co-blockade of Tim-3 and PD-1 is more effective at restoring anti-viral immunity than blockade of either receptor alone (Figure 3 and Table I). In humans, Tim-3 is similarly expressed on dysfunctional or exhausted CD8+ T cells during chronic viral infection. In HIV, Tim-3 marks virus-specific CD8+ T cells in the peripheral blood that exhibit dysfunctional/exhausted phenotype and Tim-3 blockade restores proliferation in response to stimulation with HIV-1 peptides (Jones et al., 2008). Tim-3+ T cells that exhibit dysfunctional or exhausted phenotype are also found in the peripheral blood and liver of patients chronically infected with Hepatitis C Virus (HCV) and in the peripheral blood of patients with Hepatitis B virus (HBV). Importantly, blockade of Tim-3 restores effector function in T cells in these chronic viral infections (Golden-Mason et al., 2009; McMahan et al., 2010; Nebbia et al., 2012; Wu et al., 2012). Interestingly, increased frequencies of Tim-3+ T cells in HIV, HCV, and HBV patients positively correlate with increasing viral load and disease progression while reduced frequencies of Tim-3+ T cells correlate with anti-viral treatment and resolution of viral infection (Jones et al., 2008; McMahan et al., 2010; Wu et al., 2011). The positive correlation of Tim-3 with disease in chronic viral infections is diametrically opposed to the negative correlation of Tim-3 with disease activity in autoimmunity discussed above. Together these observations support the value of Tim-3 as a prognostic indicator of disease course in both chronic viral infections and autoimmunity.
The observation that CD8+ Tim-3+ T cells exhibit dysfunctional or exhausted phenotype in chronic viral infection has called into question the reliability of using PD-1 expression as the sole hallmark for identifying dysfunctional or exhausted CD8+ T cells in chronic disease. Indeed, in HIV-infection, Tim-3 is found on dysfunctional/exhausted T cells that lack PD-1 expression (Jones et al., 2008). Moreover, Tim-3 expression marks the most dysfunctional/exhausted population within CD8+ PD-1+ T cells in multiple chronic viral infections in humans (HCV, HBV) and also in experimental models of chronic viral infections (LCMV, HBV, Friend virus) (Jin et al., 2010; Ju et al., 2009; McMahan et al., 2010; Takamura et al., 2010). Importantly, co-blockade of the Tim-3 and PD-1 pathways results in greater restoration of T cell responses in HCV, HBV, and LCMV than PD-1 pathway blockade alone (Jin et al., 2010; McMahan et al., 2010; Nebbia et al., 2012) (Figure 3). Collectively, these observations underscore the importance of the Tim-3 pathway in promoting T cell dysfunction and suggest that Tim-3 and PD-1 have non-redundant and synergistic functions in inhibiting effector T cell responses.
Tim-3 also marks dysfunctional or exhausted CD8+ T cells in cancer (Figure 3). Indeed, it was first shown here that expression of Tim-3 and PD-1 could be used to stratify populations of CD8+ TILs that exhibit different functional phenotypes (Sakuishi et al., 2010). Specifically, CD8+ Tim-3+ PD-1+ double positive TILs exhibit severe dysfunctional or exhausted phenotype while CD8+ Tim-3−PD-1+ single positive TILs exhibit weak dysfunction/exhaustion and CD8+ Tim-3−PD-1− double negative TILs exhibit good effector function. In line with these observations, co-blockade of the Tim-3 and PD-1 pathways is superior to PD-1 pathway blockade alone at improving anti-tumor effector function and suppressing tumor growth in preclinical models of both solid and hematologic cancer (Ngiow et al., 2011; Sakuishi et al., 2010; Zhou et al., 2011) (Figure 3). Importantly, in patients with advanced metastatic melanoma (Fourcade et al., 2010), non-small cell lung cancer (NSCLC) (Gao et al., 2012), or follicular B cell non-Hodgkin lymphoma (FL) (Yang et al., 2012), Tim-3 expression also marks dysfunctional/exhausted T cells and Tim-3 blockade improves function with Tim-3/PD-1 co-blockade showing greater effects (Fourcade et al., 2010; Yang et al., 2012). Notably, as observed in HIV, HBV, and HCV, the frequency of Tim-3+ T cells positively correlates with cancer severity and poor prognosis in both NSCLC (Gao et al., 2012) and FL (Yang et al., 2012). Interestingly, in FL, the frequency of PD-1+ T cells in tumors does not correlate with disease even though Tim-3 and PD-1 expression are correlated (Yang et al., 2012). This is likely due to the presence of PD-1 on other T cells that may not be dysfunctional and again underscores the value of Tim-3 expression as a marker for T cell dysfunction/exhaustion and the presence of Tim-3+ cells as a prognostic indicator of disease course.
In addition to its role in regulating effector T cell responses, Tim-3 may also have a role in regulating the function of FoxP3+ Treg cells. Tim-3 is up-regulated on the FoxP3+ Treg that are present at tissue sites in different pathological settings (Table I). In a model of allograft rejection, up to 40 % of graft-infiltrating FoxP3+ cells express Tim-3 (Gupta et al., 2012). In cancer, Tim-3+ Treg cells constitute the majority (>50%) of Treg cells present in tumor tissue in both experimental tumor models and human tumors (Gao et al., 2012; Sakuishi et al., 2013; Yan et al., 2013). Notably, Tim-3+ Treg cells are infrequent in the peripheral blood and in peripheral lymphoid tissues. These observations suggest that Tim-3 marks tissue Treg cells and that Tim-3+ Treg cells may have a specialized role in suppressing immune responses at peripheral tissue sites. Although, the Tim-3+ Treg cells in tissue allografts appear to be short-lived (Gupta et al., 2012), several lines of data show that Tim-3+ Treg cells have superior suppressive function when compared to Tim-3− Treg cells. Tim-3+ Treg cells have higher expression of known Treg cell effector molecules such as IL-10, Granzymes, and perforin as well as higher FoxP3 compared to Tim-3− Treg cells. Furthermore, Tim-3+ Treg cells exhibit superior suppressor function in vitro relative to Tim-3− Treg cells (Gautron et al., 2014; Gupta et al., 2012; Sakuishi et al., 2013). Lastly, the presence of Tim-3+ Treg cells has been found to correlate with poor clinical parameters such as the presence of nodal metastases and advanced disease stage in lung cancer (Gao et al., 2012), further supporting the value of Tim-3 as a prognostic indicator of disease course.
IL-27, a heterodimeric immunosuppressive cytokine, is a potent inducer of Tr1 cells (Awasthi et al., 2007) and, as mentioned above, also drives the expression of Tim-3 on CD4+ T cells (Zhu et al., 2015). Given that Tr1 cells also express Lag-3 (Gagliani et al., 2013), these observations raise the possibility that Tim-3, Lag-3, and potentially other co-inhibitory receptors have an important regulatory role in Tr1 cells and are in line with the demonstrated role of IL-27 in promoting resolution of autoimmune tissue inflammation (Fitzgerald et al., 2007a; Fitzgerald et al., 2007b) and suppressing anti-tumor immunity (Zhu et al., 2015). Thus, Tim-3 functions in both effector and regulatory T lymphocyte subsets to regulate immune responses at sites of tissue inflammation.
Tim-3 in innate immunity
Recent studies have shown that Tim-3 is expressed on mature resting CD56dim NK cells and is further up-regulated upon activation in response to cytokine stimulation (Gleason et al., 2012; Ndhlovu et al., 2012). High expression of Tim-3 marks effector NK that are producing IFN-γ and are undergoing degranulation (Ndhlovu et al., 2012). However, in the context of metastatic melanoma, Tim-3 marks NK cells that exhibit a functional phenotype reminiscent of T cell dysfunction or exhaustion and Tim-3 blockade similarly restores function (da Silva et al., 2014). Furthermore, the level of Tim-3 expression on NK cells correlates with disease stage and poor prognostic factors. These observations extend the co-inhibitory function of Tim-3 to NK cells and further underscore the prognostic value of Tim-3 in cancer.
As mentioned above, Tim-3 may inhibit DC activation by acting as a molecular sink for HMGB1 (Chiba et al., 2012). While this mechanism can inhibit DC responses, it is not known whether it depends on the ability of Tim-3 to signal into DC. Examination of the role of Tim-3 in mice bearing conditional deletion of Tim-3 in DC will help resolve this important issue.
In recent years, it has further been discovered that Tim-3 has a role in regulating monocyte and/or macrophage function in both humans and mice (Yang et al., 2013; Zhang et al., 2012). Down-modulation of Tim-3 signaling either by Tim-3 antibody blockade or by Tim-3 knock-down with small-interfering RNA in monocytes and macrophages increases the production of IL-1β, IL-6, IL-10, IL-12, TNF-α, and HMGB1 in response to activation via TLRs (Yang et al., 2013; Zhang et al., 2012). The modulation of responses to TLR stimulation by Tim-3 has important implications for sepsis where Tim-3 expression has been shown to be up-regulated on PBMC in patients with acute sepsis but suppressed in patients with severe sepsis (Yang et al., 2013). These observations are consistent with a model where during acute sepsis Tim-3 is up-regulated on macrophages in order to attenuate the massive inflammatory response and prevent unwanted tissue pathology but that during the progression of sepsis the expression of Tim-3 becomes down-regulated, thereby resulting in uncontrolled macrophage activation. The fact that Tim-3 expression is dynamically regulated in response to LPS, initially increasing and then decreasing after either prolonged stimulation with LPS or stimulation with increasing doses of LPS supports this model (Yang et al., 2013). Indeed, Tim-3 may have a specialized role in regulating the response to LPS through TLR4 as Tim-3 blockade exacerbates sepsis after cecal ligation and puncture in wild type but not TLR4−/− mice. Collectively, these data support that Tim-3 is an inhibitory receptor on monocytes/macrophages and that increasing Tim-3 signals may be of therapeutic value in treating severe sepsis.
Tim-3: promotion of Myeloid-Derived Suppressor Cells
Tim-3 can also suppress the immune response indirectly via its promotion of myeloid-derived suppressor cells (MDSC). MDSC are a heterogeneous population of immature myeloid cells that exhibit features of both granulocytes and monocytes and are important suppressors of the T cell response in many pathologic conditions but most notably in cancer where they expand to large numbers (reviewed in (Gabrilovich and Nagaraj, 2009)). In mice, MDSC express CD11b and high levels of the granulocyte marker Gr-1. Transgenic over-expression of Tim-3 on T cells promotes expansion of CD11b+Gr-1+ cells (Dardalhon et al., 2010). Accordingly, Tim-3 transgenic mice exhibit accelerated tumor growth and decreased autoimmunity (Dardalhon et al., 2010). The Tim-3-galectin-9 interaction drives expansion of MDSC as galectin-9 transgenic mice also exhibit expansion of MDSC and introduction of Tim-3 deficiency reverses this expansion. Together, these observations indicate that Tim-3 can suppress the adaptive immune response indirectly via promotion of MDSC.
It is important to note that therapeutic blockade of Tim-3 in order to enhance immune responses will affect multiple targets including CD4+ T cells, CD8+ T cells, FoxP3+ Treg, FoxP3− Tr1 cells, NK cells, DCs, and MDSCs and that in all of these cell types Tim-3 acts to inhibit immune responses and promote tolerance.
TIGIT
Discovery, ligands, and function
TIGIT (T cell immunoglobulin and ITIM domain) was first identified by bioinformatic algorithm as a novel member of the CD28 family and was given the HUGO designation Vsig9, which was later changed to Vstm3 and then to TIGIT. A number of groups simultaneously identified the molecule to be expressed on NK cells, effector, and memory T cells and Treg cells and each group gave it a different name including Vstm3 (Levin et al., 2011), TIGIT (Stanietsky et al., 2009; Yu et al., 2009) and WUCAM (Boles et al., 2009). TIGIT is a receptor of the Ig superfamily that is specifically expressed in immune cells where it functions as a co-inhibitory receptor (Boles et al., 2009; Stanietsky et al., 2009; Yu et al., 2009). TIGIT is expressed on activated T cells and is also found on NK cells, memory T cells, a subset of Treg cells as well as follicular T helper (Tfh) cells (Boles et al., 2009; Joller et al., 2011; Joller et al., 2014; Levin et al., 2011; Stanietsky et al., 2009; Yu et al., 2009). TIGIT binds two ligands CD155 (PVR) and CD112 (PVRL2, nectin-2), which are expressed on APCs, T cells and a variety of non-hematopoietic cell types including tumor cells (Casado et al., 2009; Levin et al., 2011; Mendelsohn et al., 1989; Stanietsky et al., 2009; Yu et al., 2009). However, TIGIT binds with much higher affinity to CD155 than to CD112 (Table II) and whether the TIGIT:CD112 interaction has functional relevance in mediating inhibitory functions still needs to be addressed. CD226 (DNAM-1) and CD96 (Tactile) bind to the same ligands and together with TIGIT form a pathway in which CD226 delivers a positive co-stimulatory signal (Bottino et al., 2003), while CD96 and TIGIT deliver inhibitory signals (Chan et al., 2014) (Figure 1C).
Table II.
Ligand binding affinities for TIGIT, CD226, and CD96
| Affinity (nM) | ||
|---|---|---|
| Receptor | CD155 | CD112 |
| TIGIT | 1-3 | Not measurable |
| CD226 | 114-199 | 8790 |
| CD96 | 37.6 | Not tested |
The pathway formed by CD226, TIGIT, and their ligands is reminiscent of the B7-CD28-CTLA-4 pathway in that both pathways are formed by a positive and negative receptor expressed on T and NK cells that share ligands expressed on APCs. Like CTLA-4, TIGIT binds its ligands with much higher affinity than CD226 (Figure 1C and Table II) and can inhibit the interaction between CD226 and CD155 in a dose-dependent manner in competition assays (Levin et al., 2011; Stanietsky et al., 2009; Yu et al., 2009). A study suggests that TIGIT not only competes with CD226 for its ligands but that it can also directly bind to CD226 in cis and disrupt its homodimerization and hence its costimulatory function (Johnston et al., 2014). However, to what degree TIGIT and CD226 are co-expressed on T cells at inflamed tissue sites is unclear. Thus, a careful examination of CD226 and TIGIT co-expression together with visualization of CD226 homodimers in disease settings is needed to determine whether this mechanism is operative in vivo.
In addition to directly acting on T and NK cells, TIGIT also indirectly suppresses immune responses through the triggering of CD155 on DCs. TIGIT engagement of CD155, which itself contains an immunoreceptor tyrosine-based inhibitory motif (ITIM) in its cytoplasmic tail, inhibits IL-12p40 production and instead induces IL-10 from treated DCs, rendering them tolerogenic (Yu et al., 2009). Why the engagement of CD155 by CD226 or CD96 would not similarly induce tolerance is not completely understood. TIGIT binds to its ligand CD155 by forming a heterotetramer with a core TIGIT homodimer (Stengel et al., 2012), reminiscent of what was observed with the CTLA-4/B7-1 crystal structure (Stamper et al., 2001). This clustering of TIGIT is essential for the observed back-signaling into DCs as disruption of the TIGIT-TIGIT interface abrogates CD155 phosphorylation (Stengel et al., 2012). The differential ability of TIGIT vs. CD226 and CD96 to induce a tolerizing signal in DCs could therefore be linked to their ability to induce CD155 clustering.
Signaling
TIGIT shares structural similarities with the larger PVR-nectin family of molecules and is composed of an extracellular IgV domain, a type 1 transmembrane region, and a cytoplasmic tail containing an ITIM and an immunoglobulin tail tyrosine (ITT)-like motif, which are highly conserved between mouse and human (Boles et al., 2009; Levin et al., 2011; Stanietsky et al., 2009; Stengel et al., 2012; Yu et al., 2009) (Figure 1C and Table I). However, which of the two motifs is important for the inhibitory function of TIGIT seems to differ between the species. Biochemical aspects of TIGIT signaling have only been studied in NK cells, where in mice, the function of the two motifs seems redundant. Phosphorylation of the tyrosine residue in either the ITIM motif (Y233) or the ITT-like motif (Y227) is sufficient for signal transduction and the inhibitory activity of TIGIT is only lost when both residues are mutated (Stanietsky et al., 2013). In human NK cells, different groups have reported an essential role for the phosphorylation of the tyrosine residue in either the ITIM motif (Y231) (Stanietsky et al., 2009) or the ITT-like motif (Y225) (Li et al., 2014; Liu et al., 2013). Hence, the contribution of the ITIM vs. ITT-like motif in mediating the inhibitory signal of human TIGIT remains unclear. As these studies were performed in cell lines overexpressing TIGIT, investigating the role of the ITIM vs. ITT-like motifs in primary cells may bring more clarity.
Engagement of TIGIT through CD155 induces its phosphorylation through Fyn and Lck and the recruitment of SHIP1 (SH2 domain-containing inositol-5-phosphatase 1) through the cytosolic adaptor Grb2 (growth factor receptor-bound protein 2) (Liu et al., 2013). Recruitment of SHIP1 to the TIGIT tail blocks signal transduction through the PI3K (phosphoinositide 3-kinase) and MAPK (mitogen-activated protein kinase) pathways and results in NK cell inhibition (Li et al., 2014; Liu et al., 2013). In addition, upon phosphorylation, the ITT-like motif of TIGIT binds β-arrestin 2 and recruits SHIP1 to limit NF-κB (nuclear factor-κB) signaling (Li et al., 2014; Liu et al., 2013). The combined effects of TIGIT on these three signaling pathways lead to a strong reduction of NK cytotoxicity, granule polarization, and cytokine secretion in NK cells (Li et al., 2014; Liu et al., 2013; Stanietsky et al., 2009). Although the inhibitory effects of TIGIT on T cell responses were initially believed to be indirect via CD155 ligation on DCs, we (Joller et al., 2011), and others (Levin et al., 2011), later showed that TIGIT also directly induces T cell inhibition in a cell intrinsic manner (Joller et al., 2011; Levin et al., 2011). In T cells, TIGIT blocks productive T cell activation, proliferation, and acquisition of effector functions by targeting molecules in the TCR signaling pathway. TIGIT engagement downregulates components of the TCR complex itself (e.g. TCRα, CD3ε) as well as central regulators of the TCR signaling cascade such as PLCγ (Joller et al., 2011). At the same time, however, TIGIT engagement induces anti-apoptotic molecules such as Bcl-xL as well as upregulation of the receptors for IL-2, IL-7, and IL-15, which promote T cell survival. Thus, while TIGIT inhibits T cell activation, it also actively contributes to their maintenance and ensures that the T cells that have been functionally inhibited are not deleted from the repertoire.
Role of TIGIT in disease
Autoimmunity
Genome wide association studies have linked a SNP in the positive regulator CD226 (Gly307Ser) of the TIGIT-CD226 pathway to multiple autoimmune diseases in humans including type 1 diabetes, multiple sclerosis, and rheumatoid arthritis (Hafler et al., 2009; Maiti et al., 2010). As a consequence, the function of TIGIT was initially investigated in models of autoimmunity and tolerance (Figure 2C). Although TIGIT deficient mice do not develop spontaneous autoimmunity, they display augmented T cell responses upon immunization (Joller et al., 2011). A series of EAE experiments demonstrated that TIGIT has an inhibitory function in regulating CNS autoimmunity. As observed for Tim-3, blocking of TIGIT exacerbates autoimmune disease (Levin et al., 2011). TIGIT-deficient mice were shown to be highly susceptible to EAE with higher frequencies of encephalitogenic T cells and elevated levels of pro-inflammatory cytokines relative to wild type controls (Joller et al., 2011). Furthermore, when crossed to MOG35–55-specific TCR transgenic 2D2 mice, TIGIT deficient mice developed spontaneous atypical EAE that was marked by signs of neurologic dysfunction reminiscent of Th17 cell-driven disease (Jager et al., 2009; Joller et al., 2011). In addition to EAE, TIGIT also plays a protective role in collagen-induced arthritis (CIA) and graft versus host disease (GvHD). In both models, blocking of TIGIT resulted in an exacerbation of the disease driven by enhanced pro-inflammatory T cell responses (Levin et al., 2011). Collectively, these data suggest that TIGIT plays an important role in maintaining peripheral tolerance by dampening T cell activation.
In addition to its direct inhibitory role in NK and effector T cells, TIGIT also inhibits immune responses through promoting Treg function (Figure 2C). TIGIT is a direct target gene of Foxp3, the master transcription factor in Treg (Zhang et al., 2013). In Treg cells, TIGIT expression correlates with markers for natural, rather than peripherally induced Treg cells and TIGIT+ Treg cells show enhanced demethylation in Treg cell-specific demethylated regions compared to their TIGIT− Treg cell counterparts, leading to higher lineage stability (Fuhrman et al., 2015; Joller et al., 2014). TIGIT+ Treg cells further express higher levels of Treg cell signature genes, such as Foxp3, CD25, and CTLA-4 and engagement of TIGIT on Treg cells leads to an upregulation of the suppressive mediator Fgl2, which confers superior suppressive function to TIGIT+ Treg cells (Joller et al., 2014). Importantly, TIGIT-dependent induction of Fgl2 results in selective sparing of Th2 cell responses, while potently suppressing pro-inflammatory Th1 and Th17 cell responses, which are the dominant responses driving autoimmune tissue inflammation. Thus, TIGIT+ Treg cells not only inhibit autoreactive T cells by suppressing their proliferation, but also by shifting the cytokine balance away from a Th1 and Th17 cell dominated response and towards a Th2 cell-like response.
Cancer and chronic viral infections
In addition to its protective role in autoimmune diseases, TIGIT has also gained attention in the context of cancer and chronic infections (Figure 3). The TIGIT ligands CD155 and CD112 are widely expressed on tumor cells. CD226, the positive counterpart of this costimulatory pathway, promotes cytotoxicity and enhances anti-tumor responses (Gilfillan et al., 2008; Iguchi-Manaka et al., 2008). In contrast, TIGIT negatively regulates anti-tumor responses as TIGIT-deficient mice show significantly delayed tumor growth in two different tumor models (Kurtulus et al., 2015). Interestingly, TIGIT does not seem to affect metastasis formation as the number of lung nodules found after intravenous injection of B16 melanoma cells was comparable in TIGIT deficient and in wild type mice (Chan et al., 2014; Kurtulus et al., 2015). The suppressive function of TIGIT is also exploited by Fusobacterium nucleatum, a bacterium often found within the tumor microenvironment, to inhibit protective immune responses (Gur et al., 2015). TIGIT directly binds to the Fap2 protein of F. nucleatum and its engagement inhibits NK cell cytotoxicity in vitro.
Within the tumor microenvironment, TIGIT is highly expressed on human and murine TILs across a broad range of tumors (Chauvin et al., 2015; Johnston et al., 2014; Kurtulus et al., 2015). In murine tumors, CD8+ TIGIT+ TILs co-express PD-1, Tim-3, and Lag3 and exhibit the most dysfunctional phenotype among CD8+ TILs (Kurtulus et al., 2015). TIGIT further marks tumor tissue Treg. Importantly, TIGIT expression is relatively poor in the peripheral lymphoid organs of tumor-bearing mice but highly enriched in tumor tissue, indicating a specialized role for TIGIT in regulating immune responses in tumor tissue (Kurtulus et al., 2015).
As has been mentioned earlier, blockade of the PD-1-PD-L1 pathway is able to restore function in exhausted CD8+ T cells and co-blockade with Tim-3 is able to further enhance this effect (Ngiow et al., 2011; Sakuishi et al., 2010; Zhou et al., 2011). A number of recent publications indicate that TIGIT might have similar effects. In CD8+ TILs from melanoma patients, co-blockade of TIGIT with PD-1 additively improved proliferation, cytokine production, and degranulation (Chauvin et al., 2015). Similarly, co-blockade of TIGIT with PDL1 showed synergistic effects in the murine CT26 tumor model, leading to enhanced CTL effector function and reversal of CD8+ T cell exhaustion. Combined treatment resulted in complete tumor rejection and induced tumor antigen-specific protective memory responses (Johnston et al., 2014). Interestingly, TIGIT not only synergizes with PD-1 but also with Tim-3 in impairing protective anti-tumor responses (Kurtulus et al., 2015). Therefore, co-blockade of either TIGIT with PD-1 or TIGIT with Tim-3 promotes anti-tumor immunity and induces tumor regression. Collectively, these data indicate that TIGIT synergizes with other co-inhibitory molecules to dampen effector T cell responses and promote T cell dysfunction.
As mentioned above, TIGIT is highly enriched on tumor-infiltrating Treg. The TIGIT+ Treg in tumor tissue exhibit a highly active and suppressive Treg phenotype. Importantly, dissection of the functional role of TIGIT in CD8+ T cells and Treg suggests that TIGIT plays a key role in driving suppression in the tumor environment via its function in Treg (Kurtulus et al., 2015). Thus, TIGIT can suppress anti-tumor immunity by multiple mechanisms that include direct suppression of effector CD8+ T cell function and indirect suppression via promotion of Treg function.
The chronic exposure to antigen and the functional exhaustion of effector T cells are hallmarks of both cancer and chronic infections. Similar to its role in anti-tumor responses, CD226 was shown to enhance CTL and NK functions during persistent viral infection and thus promote viral clearance (Nabekura et al., 2014; Welch et al., 2012). Recent data showed that exhausted CD8+ T cells induced in chronic LCMV infection also co-express TIGIT with PD-1, Tim-3, and Lag-3 (Doering et al., 2012; Johnston et al., 2014) (Figure 3). Parallel to its role in TILs, co-blockade of TIGIT with PDL1 restored cytokine production in exhausted CD8+ T cells in chronic LCMV infection (Johnston et al., 2014). Whether in this context TIGIT also has synergistic effects with Tim-3, as seen in cancer, remains to be determined.
TIGIT shifts the cytokine balance
As observed for all co-inhibitory receptors, TIGIT has a general dampening effect on the immune response as exemplified by the hyperproliferative phenotype of T cells from TIGIT deficient mice (Joller et al., 2011; Levin et al., 2011). In addition to this general regulatory role, TIGIT and its costimulatory counterpart CD226 have differential effects on the cytokine environment elicited following immunization. In mouse and human effector T cells, CD226 is expressed on Th1 and Th17, but not on Th2 cells, and promotes IFN-γ and IL-17 production (Dardalhon et al., 2005; Lozano et al., 2013). In contrast, TIGIT inhibits production of IFN-γ and IL-17 while enhancing Th2 cell cytokines and IL-10 (Burton et al., 2014; Joller et al., 2011; Joller et al., 2014; Lozano et al., 2012; Yu et al., 2009). Thus, TIGIT shifts the balance away from Type 1 and Type 17 immunity towards Type 2 immunity and IL-10.
TIGIT mediates this shift in the cytokine balance by targeting the immune response at multiple levels, namely through its action on APCs, effector T cells, and Treg cells. In DCs, TIGIT ligation of CD155 inhibits IL-12p40 production and instead induces IL-10 production, thus generating tolerogenic DCs that suppress T cell proliferation and IFN-γ production from responding T cells (Yu et al., 2009). Hence, TIGIT dampens Type 1 immunity indirectly via its interaction with APCs.
TIGIT further acts directly in effector T cells to induce a shift from a Type 1 or Type 17-dominated to an IL-10-dominated immune response. TIGIT deficient mice exhibit increased frequencies of IFN-γ+ and IL-17+ CD4+ T cells while simultaneously showing a near complete loss in IL-10 production after immunization with antigen in complete Freund's adjuvant (Joller et al., 2011). Importantly, this further holds true for human effector T cells, where TIGIT knock down results in upregulation of T-bet and IFN-γ with a concomitant decrease in IL-10 (Lozano et al., 2012). In addition, in a model of antigen-specific tolerance induction, where reduction in IFN-γ+ T cells goes along with an increase in IL-10+Foxp3− Tr1 cells, IL-10 expression is correlated with TIGIT expression (Burton et al., 2014). Therefore, the direct action of TIGIT on effector T cells further contributes to shift the cytokine balance by inhibiting pro-inflammatory Type 1 and Type 17 immunity while favoring IL-10 induction.
In Foxp3+ Treg cells, TIGIT expression marks a functionally distinct subset that selectively suppresses pro-inflammatory Type 1 and Type 17 responses (Joller et al., 2014). TIGIT ligation in Treg cells directly induces the suppressive mediator Fgl2 in a CEBPα-dependent manner. Fgl2 inhibits differentiation of IFN-γ-secreting Th1 cells but promotes secretion of IL-4 and IL-10 (Chan et al., 2003). Co-culture of TIGIT+ Treg cells with effector T cells stimulated under polarizing conditions suppresses Th1 and Th17 cell differentiation but not Th2 cell differentiation. This effect is entirely dependent on Fgl2 as loss of Fgl2 in TIGIT+ Treg cell restores their ability to suppress Th2 cell responses. Importantly, this differential suppression can also be recapitulated in vivo as TIGIT+ Treg cells are capable of inhibiting Th1 or Th17 cell responses elicited upon immunization with peptide in complete Freund's adjuvant. In contrast, TIGIT+ Treg cells are unable to suppress disease in a Th2 cell -driven asthma model (Joller et al., 2014). In addition, TIGIT ligation in Treg cells directly induces IL-10 and IL-10+ Treg cells are almost exclusively found within the TIGIT+ Treg cell subset. Thus, TIGIT+ Treg cell shift the cytokine balance by selectively suppressing Type 1 and Type 17 immunity while favoring Type 2 immunity and secretion of IL-10. TIGIT therefore targets different players in the immune response that work together to dampen pro-inflammatory Type 1 and Type 17 immunity and instead shift the cytokine balance towards an IL-10 dominated or Type 2 immunity dominant environment.
Conclusion
The current landscape of co-inhibitory receptor pathways has expanded from CTLA-4 and PD-1 to include Lag-3, Tim-3, and most recently, TIGIT. This growing landscape of co-inhibitory receptor pathways raises the important question of why there are so many pathways that seemingly perform the same function? A simplistic answer would be that the immune system has built-in a high order of functional redundancy to ensure the preservation of immune homeostasis and self-tolerance in the event that one or more co-inhibitory receptor pathways are compromised. While this may be true, it seems that such an immune fail-safe could be achieved with fewer pathways. We propose an alternative model, namely that CTLA-4 and PD-1 represent a first tier of co-inhibitory receptors that are primarily responsible for maintaining self-tolerance and restricting T cell clonotypes in lymphoid organs and that Lag-3, Tim-3, and TIGIT represent a second tier of co-inhibitory molecules that has distinct and specific roles in regulating immune responses, particularly at sites of tissue inflammation. Indeed, although Lag-3, Tim-3, and TIGIT have partially overlapping expression patterns (Table I), their unique signalling tails provides a basis for both their unique regulatory functions as well as for the synergistic effects of therapies targeting these molecules in disease (Figure 3).
Our proposed model fits with the dominant function of CTLA-4 and then PD-1 in maintaining self-tolerance relative to Tim-3, Lag-3, and TIGIT (Figure 4). Indeed, the first and second tier co-inhibitory receptors can be ranked in a hierarchy. CTLA-4 sits at the top of this hierarchy given its critical role in maintaining self-tolerance as demonstrated by the massive lymphoproliferation and early lethality that occurs in mice deficient in CTLA-4 (Tivol et al., 1995; Waterhouse et al., 1995). In line with these observations, CTLA-4 blockade in cancer patients has been shown to result in significant grade 3-5 autoimmune-like toxicities in a fraction of treated patients (Hodi et al., 2010; Robert et al., 2015). PD-1 ranks second in the hierarchy. Mice deficient in PD-1 develop spontaneous autoimmunity but with lower penetrance and at a much later age than CTLA-4 deficient mice (Nishimura et al., 1999; Nishimura et al., 2001). Indeed, cancer patients undergoing anti-PD-1 immunotherapy exhibit less toxicity than patients treated with anti-CTLA-4 (Robert et al., 2015). Based on current data, Tim-3, Lag-3, and TIGIT would equally rank next in the hierarchy. Mice deficient in these molecules do not develop spontaneous autoimmunity and their inhibitory function only becomes evident in susceptible backgrounds or upon active induction of disease. Accordingly, interference with these pathways would be predicted to be associated with less toxicity than has been observed with either CTLA-4 or PD-1.
Figure 4. Hierarchy of co-inhibitory receptors.
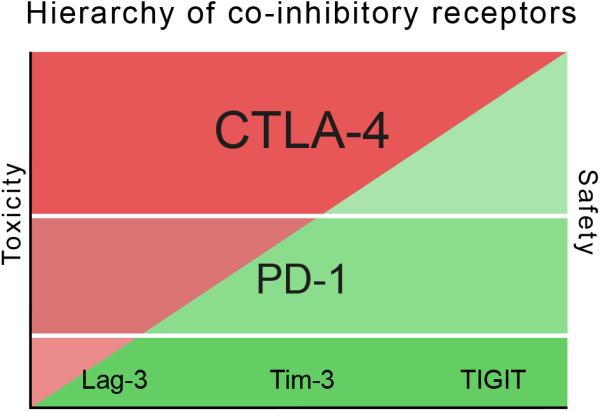
Co-inhibitory receptors are ranked from top to bottom according to their impact on the maintenance of self-tolerance. The impact of a given co-inhibitory receptor on self-tolerance is directly proportional to the amount of autoimmune toxicity observed when the receptor is deficient either as a result of genetic loss or therapeutic modulation. Genetic and/or therapeutic modulation of co-inhibitory receptors at the top of the hierarchy (Tier 1) is predicted to be associated with more autoimmune-like toxicity than modulation of co-inhibitory receptors at the bottom of the hierarchy (Tier 2). Accordingly, Tier 2 co-inhibitory receptors are predicted to have a better safety profile in the clinic.
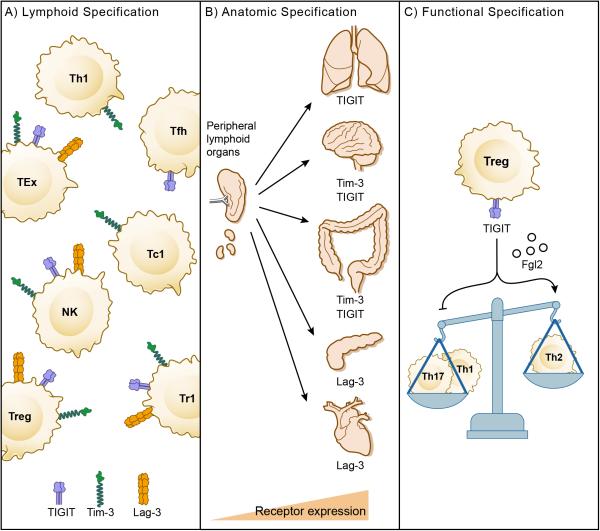
According to our model, the second tier of co-inhibitory receptors provides specificity to the regulation of immune responses in tissue, where their ligands may be expressed and function to maintain tissue tolerance and inhibit immunopathology (Figure 4). This concept of specification can operate at multiple levels. The first is at the level of the lymphocyte and is exemplified by the expression of different co-inhibitory receptors on distinct lymphocyte subsets. One example is the preferential expression of Tim-3 on IFN-γ-secreting effector T cells that infiltrate inflamed tissues (Monney et al., 2002). Another example is the specific up-regulation of Tim-3 and TIGIT on tissue Treg (Kurtulus et al., 2015; Sakuishi et al., 2013) (Figure 5A). Anatomic specification operates at the level of tissue sites. One example of anatomic specification is the Tim-3 pathway. Two of the known ligands for Tim-3, galectin-9 and Ceacam-1, are highly expressed in the gut, thus positioning the Tim-3 pathway as having a dominant role in regulating immune responses in the gut. Other pathways may have dominant roles in other organs (Figure 5B). Functional specification holds that some pathways may regulate distinct features of the immune response. Here, the TIGIT pathway seems to have evolved to shift the cytokine balance and specifically suppress Type 1 and Type 17 immunity while sparing or even promoting Type 2 immunity (Joller et al., 2014) (Figure 5C).
Figure 5. Specification of checkpoint-receptor pathways.
A) Lymphoid specification. Some co-inhibitory receptors are preferentially expressed on distinct lymphocyte subsets. B) Anatomic specification. Some co-inhibitory receptor pathways may dominate in different tissue sites where ligands and/or receptors are highly expressed. C) Functional specification. Some co-inhibitory receptors may regulate distinct aspects of immunity such as the regulation of the balance between Type 1/Type 17 immunity and Type 2 immunity by TIGIT.
As therapies that target Lag-3, Tim-3, and TIGIT move forward in clinical development, it is important to deepen our understanding of the specialized roles of each of these molecules in regulating the immune response and their tissue-specific functions. The insight gained into the specialized functions of these molecules will inform as to how to best apply therapies that interfere with these pathways in the clinic, particularly in the context of combinatorial strategies with existing therapies (Figure 6).
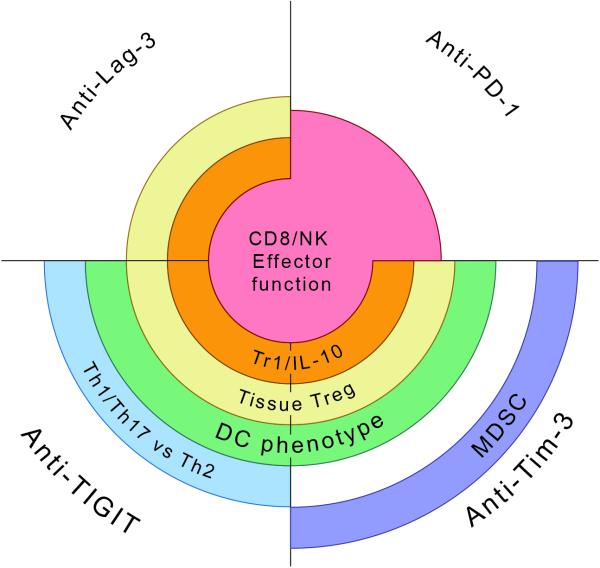
Figure 6. Immunological effects of checkpoint receptor blockades.
Schematic representation showing the effects of PD-1, Lag-3, Tim-3, and TIGIT blockades on the immune response. While all checkpoint receptor blockades have some effect on CD8+ T cell and NK cell effector function, the effect of PD-1 blockade is proportionally larger than that of Lag-3, Tim-3, or TIGIT blockade alone. Lag-3, Tim-3, and TIGIT blockades will preferentially affect tumor tissue Treg and IL-10 producing Tr1 cells. Tim-3 and TIGIT blockades will additionally affect DC phenotype. A unique effect of TIGIT blockade is shifting the balance in favor of Type 1/17 immunity versus Type 2 immunity while a unique effect of Tim-3 blockade is to dampen MDSC. Thus, different checkpoint receptor blockades can be combined to achieve distinct effects on the immune response.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- Bettini M, Szymczak-Workman AL, Forbes K, Castellaw AH, Selby M, Pan X, Drake CG, Korman AJ, Vignali DA. Cutting edge: accelerated autoimmune diabetes in the absence of LAG-3. Journal of immunology. 2011;187:3493–3498. doi: 10.4049/jimmunol.1100714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2009;10:29–37. doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin N, Baillargeon J, Doss PM, Roy AP, Rangachari M. Interferon-beta suppresses murine Th1 cell function in the absence of antigen-presenting cells. PloS one. 2015;10:e0124802. doi: 10.1371/journal.pone.0124802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles KS, Vermi W, Facchetti F, Fuchs A, Wilson TJ, Diacovo TG, Cella M, Colonna M. A novel molecular interaction for the adhesion of follicular CD4 T cells to follicular DC. European journal of immunology. 2009;39:695–703. doi: 10.1002/eji.200839116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottino C, Castriconi R, Pende D, Rivera P, Nanni M, Carnemolla B, Cantoni C, Grassi J, Marcenaro S, Reymond N, et al. Identification of PVR (CD155) and Nectin-2 (CD112) as cell surface ligands for the human DNAM-1 (CD226) activating molecule. The Journal of experimental medicine. 2003;198:557–567. doi: 10.1084/jem.20030788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brignone C, Escudier B, Grygar C, Marcu M, Triebel F. A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:6225–6231. doi: 10.1158/1078-0432.CCR-09-0068. [DOI] [PubMed] [Google Scholar]
- Brignone C, Gutierrez M, Mefti F, Brain E, Jarcau R, Cvitkovic F, Bousetta N, Medioni J, Gligorov J, Grygar C, et al. First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. Journal of translational medicine. 2010;8:71. doi: 10.1186/1479-5876-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton BR, Britton GJ, Fang H, Verhagen J, Smithers B, Sabatos-Peyton CA, Carney LJ, Gough J, Strobel S, Wraith DC. Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nature communications. 2014;5:4741. doi: 10.1038/ncomms5741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler NS, Moebius J, Pewe LL, Traore B, Doumbo OK, Tygrett LT, Waldschmidt TJ, Crompton PD, Harty JT. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–195. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camisaschi C, Casati C, Rini F, Perego M, De Filippo A, Triebel F, Parmiani G, Belli F, Rivoltini L, Castelli C. LAG-3 expression defines a subset of CD4(+)CD25(high)Foxp3(+) regulatory T cells that are expanded at tumor sites. Journal of immunology. 2010;184:6545–6551. doi: 10.4049/jimmunol.0903879. [DOI] [PubMed] [Google Scholar]
- Cao E, Zang X, Ramagopal UA, Mukhopadhaya A, Federov A, Federov E, Zencheck WD, Lary JW, Cole JL, Deng H, et al. T cell immunoglobulin mucin-3 crystal structure reveals a novel ligand binding surface. Immunity. 2007;26:311–321. doi: 10.1016/j.immuni.2007.01.016. [DOI] [PubMed] [Google Scholar]
- Casado JG, Pawelec G, Morgado S, Sanchez-Correa B, Delgado E, Gayoso I, Duran E, Solana R, Tarazona R. Expression of adhesion molecules and ligands for activating and costimulatory receptors involved in cell-mediated cytotoxicity in a large panel of human melanoma cell lines. Cancer Immunol Immunother. 2009;58:1517–1526. doi: 10.1007/s00262-009-0682-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CJ, Martinet L, Gilfillan S, Souza-Fonseca-Guimaraes F, Chow MT, Town L, Ritchie DS, Colonna M, Andrews DM, Smyth MJ. The receptors CD96 and CD226 oppose each other in the regulation of natural killer cell functions. Nat Immunol. 2014;15:431–438. doi: 10.1038/ni.2850. [DOI] [PubMed] [Google Scholar]
- Chan CW, Kay LS, Khadaroo RG, Chan MW, Lakatoo S, Young KJ, Zhang L, Gorczynski RM, Cattral M, Rotstein O, Levy GA. Soluble fibrinogen-like protein 2/fibroleukin exhibits immunosuppressive properties: suppressing T cell proliferation and inhibiting maturation of bone marrow-derived dendritic cells. Journal of immunology. 2003;170:4036–4044. doi: 10.4049/jimmunol.170.8.4036. [DOI] [PubMed] [Google Scholar]
- Chauvin JM, Pagliano O, Fourcade J, Sun Z, Wang H, Sander C, Kirkwood JM, Chen TH, Maurer M, Korman AJ, Zarour HM. TIGIT and PD-1 impair tumor antigen-specific CD8(+) T cells in melanoma patients. The Journal of clinical investigation. 2015;125:2046–2058. doi: 10.1172/JCI80445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Chen Z. The effect of immune microenvironment on the progression and prognosis of colorectal cancer. Medical oncology. 2014;31:82. doi: 10.1007/s12032-014-0082-9. [DOI] [PubMed] [Google Scholar]
- Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, Fujioka Y, Ohba Y, Gorman JV, Colgan JD, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13:832–842. doi: 10.1038/ni.2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, Osman I, Bhardwaj N. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer immunology research. 2014;2:410–422. doi: 10.1158/2326-6066.CIR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Anderson AC, Karman J, Apetoh L, Chandwaskar R, Lee DH, Cornejo M, Nishi N, Yamauchi A, Quintana FJ, et al. Tim-3/galectin-9 pathway: regulation of Th1 immunity through promotion of CD11b+Ly-6G+ myeloid cells. Journal of immunology. 2010;185:1383–1392. doi: 10.4049/jimmunol.0903275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardalhon V, Schubart AS, Reddy J, Meyers JH, Monney L, Sabatos CA, Ahuja R, Nguyen K, Freeman GJ, Greenfield EA, et al. CD226 is specifically expressed on the surface of Th1 cells and regulates their expansion and effector functions. Journal of immunology. 2005;175:1558–1565. doi: 10.4049/jimmunol.175.3.1558. [DOI] [PubMed] [Google Scholar]
- Davidson D, Schraven B, Veillette A. PAG-associated FynT regulates calcium signaling and promotes anergy in T lymphocytes. Mol Cell Biol. 2007;27:1960–1973. doi: 10.1128/MCB.01983-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeKruyff RH, Bu X, Ballesteros A, Santiago C, Chim YL, Lee HH, Karisola P, Pichavant M, Kaplan GG, Umetsu DT, et al. T cell/transmembrane, Ig, and mucin-3 allelic variants differentially recognize phosphatidylserine and mediate phagocytosis of apoptotic cells. Journal of immunology. 2010;184:1918–1930. doi: 10.4049/jimmunol.0903059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doering TA, Crawford A, Angelosanto JM, Paley MA, Ziegler CG, Wherry EJ. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity. 2012;37:1130–1144. doi: 10.1016/j.immuni.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. Journal of immunology. 2007a;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- Fitzgerald DC, Zhang GX, El-Behi M, Fonseca-Kelly Z, Li H, Yu S, Saris CJ, Gran B, Ciric B, Rostami A. Suppression of autoimmune inflammation of the central nervous system by interleukin 10 secreted by interleukin 27-stimulated T cells. Nat Immunol. 2007b;8:1372–1379. doi: 10.1038/ni1540. [DOI] [PubMed] [Google Scholar]
- Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. The Journal of experimental medicine. 2010;207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman CA, Yeh WI, Seay HR, Saikumar Lakshmi P, Chopra G, Zhang L, Perry DJ, McClymont SA, Yadav M, Lopez MC, et al. Divergent Phenotypes of Human Regulatory T Cells Expressing the Receptors TIGIT and CD226. Journal of immunology. 2015;195:145–155. doi: 10.4049/jimmunol.1402381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini F, et al. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nature medicine. 2013;19:739–746. doi: 10.1038/nm.3179. [DOI] [PubMed] [Google Scholar]
- Gao X, Zhu Y, Li G, Huang H, Zhang, Wang F, Sun J, Yang Q, Zhang X, Lu B. TIM-3 expression characterizes regulatory T cells in tumor tissues and is associated with lung cancer progression. PloS one. 2012;7:e30676. doi: 10.1371/journal.pone.0030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautron AS, Dominguez-Villar M, de Marcken M, Hafler DA. Enhanced suppressor function of TIM-3+ FoxP3+ regulatory T cells. European journal of immunology. 2014;44:2703–2711. doi: 10.1002/eji.201344392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilfillan S, Chan CJ, Cella M, Haynes NM, Rapaport AS, Boles KS, Andrews DM, Smyth MJ, Colonna M. DNAM-1 promotes activation of cytotoxic lymphocytes by nonprofessional antigen-presenting cells and tumors. The Journal of experimental medicine. 2008;205:2965–2973. doi: 10.1084/jem.20081752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason MK, Lenvik TR, McCullar V, Felices M, O'Brien MS, Cooley SA, Verneris MR, Cichocki F, Holman CJ, Panoskaltsis-Mortari A, et al. Tim-3 is an inducible human natural killer cell receptor that enhances interferon gamma production in response to galectin-9. Blood. 2012;119:3064–3072. doi: 10.1182/blood-2011-06-360321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden-Mason L, Palmer BE, Kassam N, Townshend-Bulson L, Livingston S, McMahon BJ, Castelblanco N, Kuchroo V, Gretch DR, Rosen HR. Negative immune regulator Tim-3 is overexpressed on T cells in hepatitis C virus infection and its blockade rescues dysfunctional CD4+ and CD8+ T cells. J. Virol. 2009;83:9122–9130. doi: 10.1128/JVI.00639-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosso JF, Kelleher CC, Harris TJ, Maris CH, Hipkiss EL, De Marzo A, Anders R, Netto G, Getnet D, Bruno TC, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self-and tumor-tolerance systems. The Journal of clinical investigation. 2007;117:3383–3392. doi: 10.1172/JCI31184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Thornley TB, Gao W, Larocca R, Turka LA, Kuchroo VK, Strom TB. Allograft rejection is restrained by short-lived TIM-3+PD-1+Foxp3+ Tregs. The Journal of clinical investigation. 2012;122:2395–2404. doi: 10.1172/JCI45138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur C, Ibrahim Y, Isaacson B, Yamin R, Abed J, Gamliel M, Enk J, Bar-On Y, Stanietsky-Kaynan N, Coppenhagen-Glazer S, et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity. 2015;42:344–355. doi: 10.1016/j.immuni.2015.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafler JP, Maier LM, Cooper JD, Plagnol V, Hinks A, Simmonds MJ, Stevens HE, Walker NM, Healy B, Howson JM, et al. CD226 Gly307Ser association with multiple autoimmune diseases. Genes and immunity. 2009;10:5–10. doi: 10.1038/gene.2008.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. Journal of immunology. 1998;161:4058–4065. [PubMed] [Google Scholar]
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CT, Workman CJ, Flies D, Pan X, Marson AL, Zhou G, Hipkiss EL, Ravi S, Kowalski J, Levitsky HI, et al. Role of LAG-3 in regulatory T cells. Immunity. 2004;21:503–513. doi: 10.1016/j.immuni.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature. 2015;517:386–390. doi: 10.1038/nature13848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huard B, Prigent P, Tournier M, Bruniquel D, Triebel F. CD4/major histocompatibility complex class II interaction analyzed with CD4-and lymphocyte activation gene-3 (LAG-3)-Ig fusion proteins. European journal of immunology. 1995;25:2718–2721. doi: 10.1002/eji.1830250949. [DOI] [PubMed] [Google Scholar]
- Iguchi-Manaka A, Kai H, Yamashita Y, Shibata K, Tahara-Hanaoka S, Honda S, Yasui T, Kikutani H, Shibuya K, Shibuya A. Accelerated tumor growth in mice deficient in DNAM-1 receptor. The Journal of experimental medicine. 2008;205:2959–2964. doi: 10.1084/jem.20081611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. Journal of immunology. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha V, Workman CJ, McGaha TL, Li L, Vas J, Vignali DA, Monestier M. Lymphocyte Activation Gene-3 (LAG-3) negatively regulates environmentally-induced autoimmunity. PloS one. 2014;9:e104484. doi: 10.1371/journal.pone.0104484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Comps-Agrar L, Hackney J, Yu X, Huseni M, Yang Y, Park S, Javinal V, Chiu H, Irving B, et al. The Immunoreceptor TIGIT Regulates Antitumor and Antiviral CD8(+) T Cell Effector Function. Cancer cell. 2014;26:923–937. doi: 10.1016/j.ccell.2014.10.018. [DOI] [PubMed] [Google Scholar]
- Joller N, Hafler JP, Brynedal B, Kassam N, Spoerl S, Levin SD, Sharpe AH, Kuchroo VK. Cutting edge: TIGIT has T cell-intrinsic inhibitory functions. Journal of immunology. 2011;186:1338–1342. doi: 10.4049/jimmunol.1003081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joller N, Lozano E, Burkett PR, Patel B, Xiao S, Zhu C, Xia J, Tan TG, Sefik E, Yajnik V, et al. Treg Cells Expressing the Coinhibitory Molecule TIGIT Selectively Inhibit Proinflammatory Th1 and Th17 Cell Responses. Immunity. 2014;40:569–581. doi: 10.1016/j.immuni.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J. Exp. Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju Y, Hou N, Zhang XN, Zhao D, Liu Y, Wang JJ, Luan F, Shi W, Zhu FL, Sun WS, et al. Blockade of Tim-3 pathway ameliorates interferon-gamma production from hepatic CD8+ T cells in a mouse model of hepatitis B virus infection. Cell. Mol. Immunol. 2009;6:35–43. doi: 10.1038/cmi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Satoh T, Igawa K, Yokozeki H. Impaired Expression of Tim-3 on Th17 and Th1 Cells in Psoriasis. Acta Derm Venereol. 2012 doi: 10.2340/00015555-1285. [DOI] [PubMed] [Google Scholar]
- Kasagi S, Kawano S, Kumagai S. PD-1 and autoimmunity. Critical reviews in immunology. 2011;31:265–295. doi: 10.1615/critrevimmunol.v31.i4.10. [DOI] [PubMed] [Google Scholar]
- Koguchi K, Anderson DE, Yang L, O'Connor KC, Kuchroo VK, Hafler DA. Dysregulated T cell expression of TIM3 in multiple sclerosis. J. Exp. Med. 2006;203:1413–1418. doi: 10.1084/jem.20060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtulus S, Sakuishi K, Ngiow SF, Joller N, Tan DJ, Teng MWL, Smyth MJ, Kuchroo VK, Anderson AC. TIGIT predominantly regulates the immune response via regulatory T cells. The Journal of clinical investigation. 2015;125 doi: 10.1172/JCI81187. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lee J, Su EW, Zhu C, Hainline S, Phuah J, Moroco JA, Smithgall TE, Kuchroo VK, Kane LP. Phosphotyrosine-dependent coupling of Tim-3 to T-cell receptor signaling pathways. Mol Cell Biol. 2011;31:3963–3974. doi: 10.1128/MCB.05297-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Goverman JM. The influence of T cell Ig mucin-3 signaling on central nervous system autoimmune disease is determined by the effector function of the pathogenic T cells. Journal of immunology. 2013;190:4991–4999. doi: 10.4049/jimmunol.1300083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin SD, Taft DW, Brandt CS, Bucher C, Howard ED, Chadwick EM, Johnston J, Hammond A, Bontadelli K, Ardourel D, et al. Vstm3 is a member of the CD28 family and an important modulator of T-cell function. European journal of immunology. 2011;41:902–915. doi: 10.1002/eji.201041136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Xia P, Du Y, Liu S, Huang G, Chen J, Zhang H, Hou N, Cheng X, Zhou L, et al. T-cell immunoglobulin and ITIM domain (TIGIT) receptor/poliovirus receptor (PVR) ligand engagement suppresses interferon-gamma production of natural killer cells via beta-arrestin 2-mediated negative signaling. The Journal of biological chemistry. 2014;289:17647–17657. doi: 10.1074/jbc.M114.572420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B, Workman C, Lee J, Chew C, Dale BM, Colonna L, Flores M, Li N, Schweighoffer E, Greenberg S, et al. Regulatory T cells inhibit dendritic cells by lymphocyte activation gene-3 engagement of MHC class II. Journal of immunology. 2008;180:5916–5926. doi: 10.4049/jimmunol.180.9.5916. [DOI] [PubMed] [Google Scholar]
- Liu S, Zhang H, Li M, Hu D, Li C, Ge B, Jin B, Fan Z. Recruitment of Grb2 and SHIP1 by the ITT-like motif of TIGIT suppresses granule polarization and cytotoxicity of NK cells. Cell death and differentiation. 2013;20:456–464. doi: 10.1038/cdd.2012.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Shu Q, Gao L, Hou N, Zhao D, Liu X, Zhang X, Xu L, Yue X, Zhu F, et al. Increased Tim-3 expression on peripheral lymphocytes from patients with rheumatoid arthritis negatively correlates with disease activity. Clin Immunol. 2010;137:288–295. doi: 10.1016/j.clim.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Lozano E, Dominguez-Villar M, Kuchroo V, Hafler DA. The TIGIT/CD226 Axis Regulates Human T Cell Function. Journal of immunology. 2012 doi: 10.4049/jimmunol.1103627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano E, Joller N, Cao Y, Kuchroo V, Hafler DA. The CD226/CD155 Interaction Regulates the Proinflammatory (Th1/Th17)/Anti-Inflammatory (Th2) Balance in Humans. Journal of immunology. 2013 doi: 10.4049/jimmunol.1300945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nature reviews. Drug discovery. 2015;14:561–584. doi: 10.1038/nrd4591. [DOI] [PubMed] [Google Scholar]
- Maiti AK, Kim-Howard X, Viswanathan P, Guillen L, Qian X, Rojas-Villarraga A, Sun C, Canas C, Tobon GJ, Matsuda K, et al. Non-synonymous variant (Gly307Ser) in CD226 is associated with susceptibility to multiple autoimmune diseases. Rheumatology (Oxford) 2010;49:1239–1244. doi: 10.1093/rheumatology/kep470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, Eppolito C, Qian F, Lele S, Shrikant P, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7875–7880. doi: 10.1073/pnas.1003345107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan RH, Golden-Mason L, Nishimura MI, McMahon BJ, Kemper M, Allen TM, Gretch DR, Rosen HR. Tim-3 expression on PD-1+ HCV-specific human CTLs is associated with viral persistence, and its blockade restores hepatocyte-directed in vitro cytotoxicity. The Journal of clinical investigation. 2010;120:4546–4557. doi: 10.1172/JCI43127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelsohn CL, Wimmer E, Racaniello VR. Cellular receptor for poliovirus: molecular cloning, nucleotide sequence, and expression of a new member of the immunoglobulin superfamily. Cell. 1989;56:855–865. doi: 10.1016/0092-8674(89)90690-9. [DOI] [PubMed] [Google Scholar]
- Meyers JH, Sabatos CA, Chakravarti S, Kuchroo VK. The TIM gene family regulates autoimmune and allergic diseases. Trends Mol Med. 2005;11:362–369. doi: 10.1016/j.molmed.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Dierich A, Benoist C, Mathis D. Independent modes of natural killing distinguished in mice lacking Lag3. Science. 1996;272:405–408. doi: 10.1126/science.272.5260.405. [DOI] [PubMed] [Google Scholar]
- Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA, et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415:536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- Nabekura T, Kanaya M, Shibuya A, Fu G, Gascoigne NR, Lanier LL. Costimulatory molecule DNAM-1 is essential for optimal differentiation of memory natural killer cells during mouse cytomegalovirus infection. Immunity. 2014;40:225–234. doi: 10.1016/j.immuni.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Akiba H, Takeda K, Kojima Y, Hashiguchi M, Azuma M, Yagita H, Okumura K. Tim-3 mediates phagocytosis of apoptotic cells and cross-presentation. Blood. 2009;113:3821–3830. doi: 10.1182/blood-2008-10-185884. [DOI] [PubMed] [Google Scholar]
- Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood. 2012;119:3734–3743. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebbia G, Peppa D, Schurich A, Khanna P, Singh HD, Cheng Y, Rosenberg W, Dusheiko G, Gilson R, ChinAleong J, et al. Upregulation of the Tim-3/galectin-9 pathway of T cell exhaustion in chronic hepatitis B virus infection. PloS one. 2012;7:e47648. doi: 10.1371/journal.pone.0047648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngiow SF, von Scheidt B, Akiba H, Yagita H, Teng MW, Smyth MJ. Anti-TIM3 antibody promotes T cell IFN-{gamma}-mediated anti-tumor immunity and suppresses established tumors. Cancer research. 2011;71:3540–3551. doi: 10.1158/0008-5472.CAN-11-0096. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11:141–151. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- Nishimura H, Okazaki T, Tanaka Y, Nakatani K, Hara M, Matsumori A, Sasayama S, Mizoguchi A, Hiai H, Minato N, Honjo T. Autoimmune dilated cardiomyopathy in PD-1 receptor-deficient mice. Science. 2001;291:319–322. doi: 10.1126/science.291.5502.319. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Okazaki IM, Wang J, Sugiura D, Nakaki F, Yoshida T, Kato Y, Fagarasan S, Muramatsu M, Eto T, et al. PD-1 and LAG-3 inhibitory co receptors act synergistically to prevent autoimmunity in mice. The Journal of experimental medicine. 2011;208:395–407. doi: 10.1084/jem.20100466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottoboni L, Keenan BT, Tamayo P, Kuchroo M, Mesirov JP, Buckle GJ, Khoury SJ, Hafler DA, Weiner HL, De Jager PL. An RNA profile identifies two subsets of multiple sclerosis patients differing in disease activity. Science translational medicine. 2012;4:153ra131. doi: 10.1126/scitranslmed.3004186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauken KE, Wherry EJ. Overcoming T cell exhaustion in infection and cancer. Trends in immunology. 2015;36:265–276. doi: 10.1016/j.it.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigent P, El Mir S, Dreano M, Triebel F. Lymphocyte activation gene-3 induces tumor regression and antitumor immune responses. European journal of immunology. 1999;29:3867–3876. doi: 10.1002/(SICI)1521-4141(199912)29:12<3867::AID-IMMU3867>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Qu HQ, Bradfield JP, Grant SF, Hakonarson H, Polychronakos C, Type IDGC. Remapping the type I diabetes association of the CTLA4 locus. Genes and immunity. 2009;10(Suppl 1):S27–32. doi: 10.1038/gene.2009.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangachari M, Zhu C, Sakuishi K, Xiao S, Karman J, Chen A, Angin M, Wakeham A, Greenfield EA, Sobel RA, et al. Bat3 protects T cell responses by repressing Tim-3-mediated exhaustion and death. Nat. Med. 2012;18:1394–1400. doi: 10.1038/nm.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Agnellini P, Oxenius A. On the role of the inhibitory receptor LAG-3 in acute and chronic LCMV infection. International immunology. 2010;22:13–23. doi: 10.1093/intimm/dxp107. [DOI] [PubMed] [Google Scholar]
- Robert C, Schachter J, Long GV, Arance A, Grob JJ, Mortier L, Daud A, Carlino MS, McNeil C, Lotem M, et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- Romano E, Michielin O, Voelter V, Laurent J, Bichat H, Stravodimou A, Romero P, Speiser DE, Triebel F, Leyvraz S, Harari A. MART-1 peptide vaccination plus IMP321 (LAG-3Ig fusion protein) in patients receiving autologous PBMCs after lymphodepletion: results of a Phase I trial. Journal of translational medicine. 2014;12:97. doi: 10.1186/1479-5876-12-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatos CA, Chakravarti S, Cha E, Schubart A, Sanchez-Fueyo A, Zheng XX, Coyle AJ, Strom TB, Freeman GJ, Kuchroo VK. Interaction of Tim-3 and Tim-3 ligand regulates T helper type 1 responses and induction of peripheral tolerance. Nat Immunol. 2003;4:1102–1110. doi: 10.1038/ni988. [DOI] [PubMed] [Google Scholar]
- Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. The Journal of experimental medicine. 2010;207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuishi K, Ngiow SF, Sullivan JM, Teng MWL, Kuchroo VK, Smyth MJ, Anderson AC. TIM3+FOXP3+ regulatory Tcells are tissue-specific promoters of T-cell dysfunction in cancer. OncoImmunology. 2013;2:e23849. doi: 10.4161/onci.23849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmond RJ, Filby A, Qureshi I, Caserta S, Zamoyska R. T-cell receptor proximal signaling via the Src-family kinases, Lck and Fyn, influences T-cell activation, differentiation, and tolerance. Immunol Rev. 2009;228:9–22. doi: 10.1111/j.1600-065X.2008.00745.x. [DOI] [PubMed] [Google Scholar]
- Sanchez-Fueyo A, Tian J, Picarella D, Domenig C, Zheng XX, Sabatos CA, Manlongat N, Bender O, Kamradt T, Kuchroo VK, et al. TIM-3 inhibits T helper type 1-mediated auto-and alloimmune responses and promotes immunological tolerance. Nat. Immunol. 2003;4:1093–1101. doi: 10.1038/ni987. [DOI] [PubMed] [Google Scholar]
- Santiago C, Ballestros A, Martinez-Munoz L, Mellado M, Kaplan GG, Freeman GJ, Casanovas JM. Structures of T Cell Immunoglobulin Mucin Protein 4 show a metal-ion-dependent ligand binding site where phosphatidylserine binds. Immunity. 2007a;27:941–945. doi: 10.1016/j.immuni.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago C, Ballestros A, Tami C, Martinez-Munoz L, Kaplan GG, Casanovas JM. Structures of T Cell immunoglobulin mucin receptors 1 and 2 reveal mechanisms for regulation of immune responses by the TIM receptor family. Immunity. 2007b;26:299–310. doi: 10.1016/j.immuni.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smida M, Posevitz-Fejfar A, Horejsi V, Schraven B, Lindquist JA. A novel negative regulatory function of the phosphoprotein associated with glycosphingolipid-enriched microdomains: blocking Ras activation. Blood. 2007;110:596–615. doi: 10.1182/blood-2006-07-038752. [DOI] [PubMed] [Google Scholar]
- Song YW, Im CH, Park JH, Lee YJ, Lee EY, Lee EB, Park K. T-cell immunoglobulin and mucin domain 3 genetic polymorphisms are associated with rheumatoid arthritis independent of a shared epitope status. Human immunology. 2011;72:652–655. doi: 10.1016/j.humimm.2011.04.007. [DOI] [PubMed] [Google Scholar]
- Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, Stahl ML, Seehra J, Somers WS, Mosyak L. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001;410:608–611. doi: 10.1038/35069118. [DOI] [PubMed] [Google Scholar]
- Stanietsky N, Rovis TL, Glasner A, Seidel E, Tsukerman P, Yamin R, Enk J, Jonjic S, Mandelboim O. Mouse TIGIT inhibits NK-cell cytotoxicity upon interaction with PVR. European journal of immunology. 2013;43:2138–2150. doi: 10.1002/eji.201243072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanietsky N, Simic H, Arapovic J, Toporik A, Levy O, Novik A, Levine Z, Beiman M, Dassa L, Achdout H, et al. The interaction of TIGIT with PVR and PVRL2 inhibits human NK cell cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17858–17863. doi: 10.1073/pnas.0903474106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel KF, Harden-Bowles K, Yu X, Rouge L, Yin J, Comps-Agrar L, Wiesmann C, Bazan JF, Eaton DL, Grogan JL. Structure of TIGIT immunoreceptor bound to poliovirus receptor reveals a cell-cell adhesion and signaling mechanism that requires cis-trans receptor clustering. Proceedings of the National Academy of Sciences of the United States of America. 2012 doi: 10.1073/pnas.1120606109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura S, Tsuji-Kawahara S, Yagita H, Akiba H, Sakamoto M, Chikaishi T, Kato M, Miyazawa M. Premature terminal exhaustion of Friend virus-specific effector CD8+ T cells by rapid induction of multiple inhibitory receptors. Journal of immunology. 2010;184:4696–4707. doi: 10.4049/jimmunol.0903478. [DOI] [PubMed] [Google Scholar]
- Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. CTLA-4 deficient mice exhibit massive lymphoproliferation and multi-organ lymphatic infiltration: a critical negative immunoregulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- Triebel F, Jitsukawa S, Baixeras E, Roman-Roman S, Genevee C, Viegas-Pequignot E, Hercend T. LAG-3, a novel lymphocyte activation gene closely related to CD4. The Journal of experimental medicine. 1990;171:1393–1405. doi: 10.1084/jem.171.5.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Weyer PS, Muehlfeit M, Klose C, Bonventre JV, Walz G, Kuehn EW. A highly conserved tyrosine of Tim-3 is phosphorylated upon stimulation by its ligand galectin-9. Biochem Biophys Res Commun. 2006;351:571–576. doi: 10.1016/j.bbrc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- Wang M, Ji B, Wang J, Cheng X, Zhou Q, Zhou J, Cao C, Guo Q. Tim-3 polymorphism downregulates gene expression and is involved in the susceptibility to ankylosing spondylitis. DNA and cell biology. 2014;33:723–728. doi: 10.1089/dna.2014.2456. [DOI] [PubMed] [Google Scholar]
- Wang-Gillam A, Plambeck-Suess S, Goedegebuure P, Simon PO, Mitchem JB, Hornick JR, Sorscher S, Picus J, Suresh R, Lockhart AC, et al. A phase I study of IMP321 and gemcitabine as the front-line therapy in patients with advanced pancreatic adenocarcinoma. Investigational new drugs. 2013;31:707–713. doi: 10.1007/s10637-012-9866-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterhouse PW, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- Welch MJ, Teijaro JR, Lewicki HA, Colonna M, Oldstone MB. CD8 T cell defect of TNF-alpha and IL-2 in DNAM-1 deficient mice delays clearance in vivo of a persistent virus infection. Virology. 2012;429:163–170. doi: 10.1016/j.virol.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wherry EJ, Kurachi M. Molecular and cellular insights into T cell exhaustion. Nature reviews. Immunology. 2015;15:486–499. doi: 10.1038/nri3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo SR, Turnis ME, Goldberg MV, Bankoti J, Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et al. Immune inhibitory molecules LAG-3 and PD-1 synergistically regulate T-cell function to promote tumoral immune escape. Cancer research. 2012;72:917–927. doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. Journal of immunology. 2004;172:5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- Workman CJ, Dugger KJ, Vignali DA. Cutting edge: molecular analysis of the negative regulatory function of lymphocyte activation gene-3. Journal of immunology. 2002;169:5392–5395. doi: 10.4049/jimmunol.169.10.5392. [DOI] [PubMed] [Google Scholar]
- Workman CJ, Vignali DA. The CD4-related molecule, LAG-3 (CD223), regulates the expansion of activated T cells. European journal of immunology. 2003;33:970–979. doi: 10.1002/eji.200323382. [DOI] [PubMed] [Google Scholar]
- Workman CJ, Vignali DA. Negative regulation of T cell homeostasis by lymphocyte activation gene-3 (CD223). Journal of immunology. 2005;174:688–695. doi: 10.4049/jimmunol.174.2.688. [DOI] [PubMed] [Google Scholar]
- Wu W, Shi Y, Li J, Chen F, Chen Z, Zheng M. Tim-3 expression on peripheral T cell subsets correlates with disease progression in hepatitis B infection. Virol J. 2011;8:113. doi: 10.1186/1743-422X-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W, Shi Y, Li S, Zhang Y, Liu Y, Wu Y, Chen Z. Blockade of Tim-3 signaling restores the virus-specific CD8(+) T-cell response in patients with chronic hepatitis B. European journal of immunology. 2012;42:1180–1191. doi: 10.1002/eji.201141852. [DOI] [PubMed] [Google Scholar]
- Xu F, Liu J, Liu D, Liu B, Wang M, Hu Z, Du X, Tang L, He F. LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses. Cancer research. 2014;74:3418–3428. doi: 10.1158/0008-5472.CAN-13-2690. [DOI] [PubMed] [Google Scholar]
- Yan J, Zhang Y, Zhang JP, Liang J, Li L, Zheng L. Tim-3 expression defines regulatory T cells in human tumors. PloS one. 2013;8:e58006. doi: 10.1371/journal.pone.0058006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Anderson DE, Kuchroo J, Hafler DA. Lack of TIM-3 immunoregulation in Multiple Sclerosis. J. Immunol. 2008;180:4409–4414. doi: 10.4049/jimmunol.180.7.4409. [DOI] [PubMed] [Google Scholar]
- Yang X, Jiang X, Chen G, Xiao Y, Geng S, Kang C, Zhou T, Li Y, Guo X, Xiao H, et al. T cell Ig mucin-3 promotes homeostasis of sepsis by negatively regulating the TLR response. Journal of immunology. 2013;190:2068–2079. doi: 10.4049/jimmunol.1202661. [DOI] [PubMed] [Google Scholar]
- Yang ZZ, Grote DM, Ziesmer SC, Niki T, Hirashima M, Novak AJ, Witzig TE, Ansell SM. IL-12 upregulates TIM-3 expression and induces T cell exhaustion in patients with follicular B cell non-Hodgkin lymphoma. The Journal of clinical investigation. 2012;122:1271–1282. doi: 10.1172/JCI59806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Harden K, Gonzalez LC, Francesco M, Chiang E, Irving B, Tom I, Ivelja S, Refino CJ, Clark H, et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat Immunol. 2009;10:48–57. doi: 10.1038/ni.1674. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ma CJ, Wang J, Ji X, Wu XY, Moorman JP, Yao ZQ. Tim-3 regulates pro-and anti-inflammatory cytokine expression in human CD14+ monocytes. J Leukoc Biol. 2012;91:189–196. doi: 10.1189/jlb.1010591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Maksimovic J, Naselli G, Qian J, Chopin M, Blewitt ME, Oshlack A, Harrison LC. Genome-wide DNA methylation analysis identifies hypomethylated genes regulated by FOXP3 in human regulatory T cells. Blood. 2013;122:2823–2836. doi: 10.1182/blood-2013-02-481788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Munger ME, Veenstra RG, Weigel BJ, Hirashima M, Munn DH, Murphy WJ, Azuma M, Anderson AC, Kuchroo VK, Blazar BR. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood. 2011;117:4501–4510. doi: 10.1182/blood-2010-10-310425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat. Immunol. 2005;6:1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- Zhu C, Sakuishi K, Xiao S, Sun Z, Zaghouani S, Gu G, Wang C, Tan DJ, Wu C, Rangachari M, et al. An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nature communications. 2015;6:6072. doi: 10.1038/ncomms7072. [DOI] [PMC free article] [PubMed] [Google Scholar]