Abstract
Motor vehicle collision (MVC) can trigger chronic widespread pain (CWP) development in vulnerable individuals. Whether such CWP typically develops via the evolution of pain from regional to widespread or via the early development of widespread pain with non-recovery is currently unknown. We evaluated the trajectory of CWP development (American College of Rheumatology criteria) among 948 European-American individuals who presented to the emergency department (ED) for care in the early aftermath of MVC. Pain extent was assessed in the ED and 6 weeks, 6 months, and 1 year after MVC on 100%, 91%, 89%, and 91% of participants, respectively. Individuals who reported prior CWP at the time of ED evaluation (n = 53) were excluded. Trajectory modeling identified a two-group solution as optimal, with the Bayes Factor value (138) indicating strong model selection. Linear solution plots supported a non-recovery model. While the number of body regions with pain in the non-CWP group steadily declined, the number of body regions with pain in the CWP trajectory group (192/895, 22%) remained relatively constant over time. These data support the hypothesis that individuals who develop CWP after MVC develop widespread pain in the early aftermath of MVC which does not remit.
1. Introduction
Population-based studies indicate that 11–13% of adults suffer from chronic widespread pain (CWP) [46], defined as pain on both the left and right side of the body, above and below the waist, and including the axial region [50]. These individuals experience substantial disability and reduced quality of life [11; 27]. The pathogenesis of CWP remains poorly understood [25; 28; 29; 35], and gaining a greater understanding of this disease is an important public health priority [19].
One known trigger of CWP is motor vehicle collision (MVC) [18; 22; 51; 52]; the natural history of CWP development after MVC is currently unknown. One common hypothesis is that chronic regional pain first develops and then progresses to CWP in vulnerable individuals [18; 23; 25; 46]. Another possibility is that in vulnerable individuals CWP develops in the early aftermath of MVC and persists [7; 18]. Accurately identifying the typical trajectory of CWP development after MVC is important both in order to gain insights into potential etiologic mechanisms and also to inform the design of future studies.
In this study, we examined the developmental trajectory of CWP among a cohort of individuals who presented to the emergency department (ED) for care after MVC and were followed prospectively for one year. Trajectory modeling was used to determine if either a progressive onset model of CWP development or an early onset/non-recovery model of CWP development provided a good fit to the data. Because evidence suggests that mechanisms related to stress-induced hyperalgesia play an important role in the pathogenesis of chronic pain after MVC (reviewed in [41]), and because such mechanisms are mediated by central neurobiological systems mechanisms with widespread effects and relatively early onset (reviewed in [41]), we hypothesized that CWP development would be characterized by a non-recovery rather than a progressive accrual model. In addition, we evaluated demographic characteristics of participants with and without CWP after MVC and co-morbid psychological and somatic symptoms in these groups.
2. Methods
2.1 Design and setting
This prospective longitudinal study enrolled patients presenting to the ED within 24 hours of MVC. Data were collected at eight EDs in four no-fault MVC litigation/insurance states, where litigation related to MVC is low, (Michigan, Massachusetts, New York, and Florida) between February 2009 and October 2011. Study participants were enrolled at research network ED sites and received an initial interview evaluation at the time of the ED visit. Follow-up assessments were performed at 6 weeks, 6 months, and one year. The study was approved by the institutional review boards of all participating hospitals, and each participant provided written informed consent. Complete information regarding study design, procedures, and methods has previously been described. [38]
2.2 Participant eligibility criteria and study sites
Patients ages 18 to 65 who presented to the ED within 24 hours after a MVC and were unlikely to require hospitalization were screened for eligibility. Patients who were admitted to the hospital, had fractures other than phalangeal fractures, had more than 4 lacerations requiring sutures or a single laceration more than 20 cm in length, or had intracranial or spinal injuries were excluded. Spinal injury was defined by the presence of a fracture, dislocation, or new neurologic deficit. Enrollment was also limited to non-Hispanic whites (the most common ethnicity at study sites) because the study included the collection of genetic data and genetic analyses are potentially biased by population stratification.[16] Patients who were not alert and oriented were also excluded, as were pregnant patients, prisoners (due to risk of coercion [5]), patients unable to read and understand English, patients taking a β-adrenoreceptor antagonist (due to concern for confounding because β-adrenergic mechanisms have been implicated in chronic pain development [32]), or patients taking opioids above a total daily dose of 20 mg of oral morphine or equivalent.
2.3 Study procedures
Eligible and consenting participants completed ED interview evaluations regarding pre-MVC health status, the details of the MVC, and current symptoms. Interviews were conducted by research staff at the time of the ED visit using a web-based survey with explicit definitions of variables. Before enrolling patients in the ED, each research staff member completed a study training module followed by an interview with a standardized mock ED patient. Comparison of mock ED patient data across research staff demonstrated an error rate of 1.3%. Injury characteristics and medications administered in the ED were obtained by data extraction from the ED medical record. Study personnel extracted participant injury data from the participant’s medical record using a standardized web-based data extraction form with explicit definitions of all variables. All aspects of the emergency medical record, including physician notes, nursing notes, and physical orders were used for completion. Information obtained included the presence and location of any fractures, lacerations, contusions, avulsions, and abrasions. Injury information was used to generate Abbreviated Injury Severity Scores [1]. Six weeks, six months and one year after the MVC, participants completed a follow up interview online, by telephone, or via mail. Regardless of follow up type, survey content was identical. Participants were compensated $80 for completing the ED interview, $60 for completing the 6 week interview, $65 for completing the 6 month interview and $70 for completing the 1 year interview.
2.4 Measures
A number of measures were used to assess health status prior to MVC and symptoms in the ED. Complete study measures are described in full elsewhere.[38]
2.4.1 Participant demographics
Participant demographic characteristics (including age, gender, income, height, weight, and educational attainment) were obtained from the ED medical record and from participant self-report.
2.4.2 Pain assessments and pain outcome definitions
Pain extent during the month prior to the MVC and in the ED were both assessed at the time of ED evaluation; pain extent six weeks, six months and one year after MVC was assessed at the respective timepoint. Pain extent was assessed in 19 discrete body regions evaluated in the regional pain scale[49] and in the head region. Widespread pain (WP) was defined according to the American College of Rheumatology (ACR) 1990 criteria [50]. In each body region in which the participant reported pain, average pain severity was assessed using a verbal 0 to 10 numeric rating scale (NRS). Verbal scores have advantages in acute care settings, and verbally administered NRSs have been validated as highly correlated with visual analogue scale scores. [34] Regional pain was defined by the presence of one or more body regions with a NRS score greater than zero. Number of body regions with pain at each timepoint was defined as the number of body regions with a NRS score greater than zero.
2.4.3 Psychological symptoms
Distress was assessed using the Peritraumatic Distress Inventory (PDI). This measure has high internal consistency (0.75–0.76) and test-retest reliability (0.74).[10] A PDI cut-off score of ≥23 was used to define marked distress symptoms ("distress").[36] Post-traumatic stress disorder symptoms were measured at 6-week, 6-month, and 1-year timepoints using the Impact of Events Scale – Revised (IES-R)[47]. Depressive symptoms during the week prior to the MVC were assessed using the Center for Epidemiologic Studies Depression Scale (CES-D).[40] Based on previous evidence, CES-D scores between 16 and 25 were defined as mild depressive symptoms and scores greater than or equal to 26 were defined as severe depressive symptoms.[48; 53]
2.4.4 Somatic symptoms
Somatic symptoms during the past month were assessed at the time of the ED assessment and via telephone or web-based questionnaire six weeks, six months, and one year after MVC. The somatic symptom assessment consisted of a 21-item questionnaire that evaluated the presence and severity of the following: headache, dizziness, nausea, noise sensitivity, light sensitivity, poor concentration, taking longer to think, blurred vision, double vision, restlessness, upset stomach, fatigue, sensitive or tender skin, ringing in ears, itchy eyes or skin, racing heart, insomnia, hands trembling, feeling faint, abdominal pain, diarrhea and constipation. Participants were asked to report the severity of each symptom on a 0–10 scale, where 0 represented no problem and 10 represented a major problem. Number of somatic symptoms at each timepoint was defined as the number of somatic symptoms greater than zero.
2.5 Statistical analyses
Descriptive and trajectory analyses of extent of pain across time were performed using SAS 9.3 (SAS Institute, Cary, NC). Descriptive analysis of the whole cohort, after excluding previous WP participants, was evaluated. Trajectory analyses were performed using PROC TRAJ. This SAS procedure, developed by Jones, Nagin and Roeder, was designed to estimate clusters of individuals following similar trends over time[21]. Model fit in TRAJ is based on the Bayesian Information Criterion (BIC), a statistic that is similar to adjusted R2 as it incorporates model complexity and overall fit. BIC value is a fit index used to compare competing models that include different numbers or shapes of trajectories. BIC values are negative, and in comparing the different models, the best fit model is the one with the smallest negative number, closest to 0. To test the inclusion of different numbers of trajectories, the estimate of the log Bayes Factor is defined with the following formula: 2loge (B10) ≈ 2(ΔBIC)[21]. The difference is calculated by subtracting the BIC value of the simpler model, with a smaller number of trajectories, from the more complex model. According to the guidelines, values ranging from 0–2 are interpreted as weak evidence for the more complex model, 2 to 6 as moderate evidence, 6–10 as strong and values greater than 10 as very strong evidence. The comparisons are completed in a step-wise manner so that the two-group model is compared to the one-group, and the three-group model is compared to the two-group model. Trajectory membership for each individual was used as an identifier, and further descriptive analyses were conducted.
3. Results
3.1 Cohort characteristics
A total of 10,629 patients were screened, 1,416 were eligible, 969 consented to study participation and 948 completed baseline evaluation. Slightly more than 60% of participants were females, more than three quarters had some education past high school, and more than half worked full time (Table 1). The median age of study participants was 36 (range 18–65). Consistent with eligibility criteria, all participants were discharged to home after ED evaluation. Ninety three percent (710/948) of the study participants had musculoskeletal strain only, and nearly all participants (939/948, 99%) had an Abbreviated Injury Scale score of 1. Fractures were present in 1/948 (<1%, phalanx fracture) participants; a small laceration was present in 53/948 (6%) participants. Six week, six month, and one year follow-up assessments were completed on 859/948 (91%), 840/948(87%), and 861/948 (91%) of enrolled patients, respectively.
Table 1.
Demographic characteristics of all participants, participants with a post-MVC chronic widespread pain trajectory, and participants with a non-chronic widespread pain trajectory.a
| All | Non-widespread pain | Widespread pain | p-value | |
|---|---|---|---|---|
| Age, mean (SD) | 35.4 (13.0) | 34.9 (13.0) | 37.4 (13.0) | 0.02 |
| Female, n (%) | 540 (60.3) | 411 (59.0) | 129 (67.0) | 0.02 |
| BMI, mean (SD) | 27.6 (6.0) | 27.4 (6.0) | 28.5 (7.0) | 0.03 |
| Education, n (%) | 0.02 | |||
| High school or less | 214 (23.9) | 161 (23.0) | 53 (28.0) | |
| Some college or trade school | 348 (38.9) | 272 (39.0) | 76 (39.6) | |
| College/post-graduate degree | 332 (37.1) | 269 (38.3) | 63 (32.8) | |
| Annual income, n (%) | 0.52 | |||
| Below $20,000 | 111 (13.0) | 83 (13.3) | 28 (16.4) | |
| $20,000 to $40,000 | 167 (21.0) | 132 (21.1) | 35 (20.5) | |
| $40,000 to $80,000 | 263 (33.0) | 205 (32.7) | 58 (33.9) | |
| >$80,000 | 256 (32.0) | 206 (32.9) | 50 (29.2) | |
| Works full time, n (%) | 526 (59.0) | 415 (59.0) | 111 (57.8) | 0.41 |
| Damage to vehicle from MVC, n (%) | ||||
| None-minor | 115 (13.0) | 95 (14.0) | 20 (10.9) | 0.28 |
| Moderate | 269 (31.0) | 204 (30.0) | 65 (35.5) | |
| Severe | 479 (56.0) | 381 (56.0) | 98 (53.6) |
MVC, motor vehicle collision; BMI, body mass index; SD, standard deviation
Trajectories assigned using SAS Procedure PROC TRAJ
3.2 WP outcomes in the cohort over time
Widespread pain prior to the MVC was reported by 53/948 (6%) of participants; these patients were excluded from subsequent analyses. MVC-related WP was present in 238/895 (27%) at the ED, 160/808 (20%) participants six weeks after MVC, 97/790 (12%) participants six months after MVC, and 78/809 (10%) participants one year after MVC.
3.3 Identifying trajectories of widespread pain development
Fit indices (Table 2) were used to identify the trajectory model for CWP development that best corresponded to the data. Bayesian information criterion values identified a two-group solution as optimal, with the Bayes Factor value (138) for the two-group linear solution indicating very strong model selection [55]. As shown in Table 221.5% of participants were classified as having a high probability of a CWP trajectory (“CWP” group), and 78.5% participants were classified as having a low probability of a CWP trajectory (“non-CWP” group). Demographic characteristics of participants with and without a CWP trajectory are shown in Table 1. Those developing CWP after MVC were more likely to be older, female, to have a higher BMI, and to have lower educational attainment (Table 1).
Table 2.
Model fit indices for various group solutions for trajectories of chronic widespread pain development after motor vehicle collision from the emergency department, week 6, month 6 and year 1 (n = 895).
| n trajectories | BIC | ΔBIC | BF | Probability to each Group Assignment |
||||
|---|---|---|---|---|---|---|---|---|
| Group 1 | Group 2 | Group 3 |
Group 4 | Group 5 | ||||
| 1 | −1483 | 100% | ||||||
| 2 | −1414 | 72 | 138 | 78.5% | 21.5% | |||
| 3 | −1421 | −7 | −14 | 31.8% | 45.9% | 22.2% | ||
| 4 | −1430 | −9 | −18 | 30.1% | 27.2% | 39.7% | 3.0% | |
| 5 | −1443 | −13 | −26 | 23.1% | 10.6% | 30.1% | 33.2% | 3.0% |
BIC, Bayesian Information Criterion; BF, Bayes Factor
3.4 CWP development after MVC is most consistent with a non-recovery trajectory
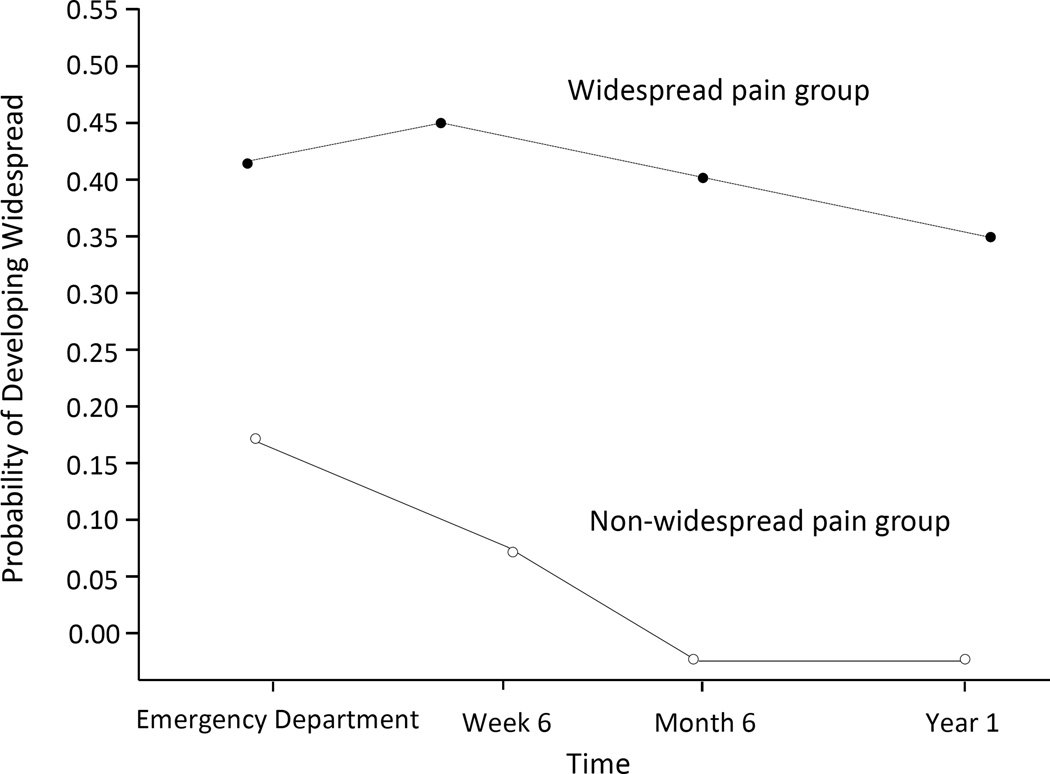
Two-group linear solution plots supported a non-recovery model of CWP development (Figure 1). More than half of individuals (99/192, 52%) in the CWP group met widespread pain criteria in the ED; mean time between MVC and ED presentation among study participants was 1.2 hours. Little change in the probability of widespread pain was observed across follow-up time points within the CWP group, indicating little aggregate improvement or progression over the course of the year (Fig. 2).
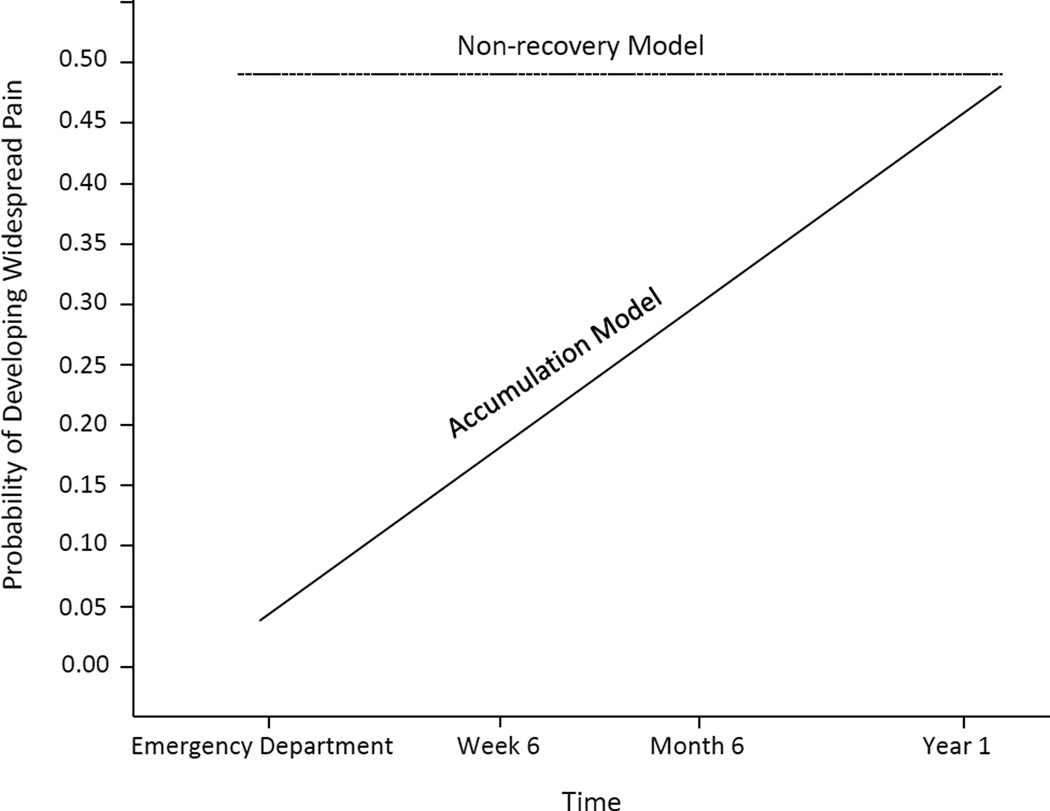
Fig. 1.
Candidate trajectory models of widespread pain development after motor vehicle collision assessed in the study.
Fig. 2.
Trajectory analysis performed using study data indicated that widespread pain after MVC develops via non-recovery.
3.5 Relatively few individuals experienced a substantial increase in number of body regions with pain over time
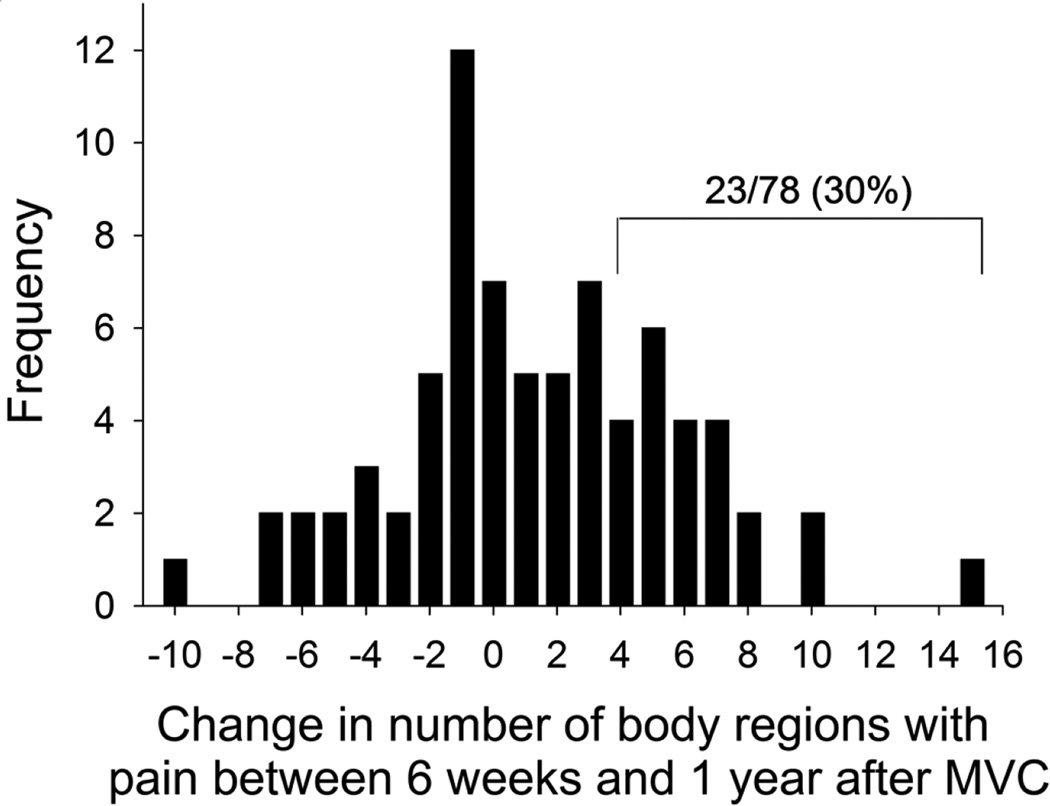
As shown in Figure 3, among individuals with widespread pain one year after MVC, the distribution of change in number of body regions between six weeks and one year is relatively symmetric around zero (47/78 [60%] had experienced an increase in the number of body regions with pain and 31/78 [40%] had experienced no change or a decrease in the number of body regions with pain). As shown in Table 3, individuals with a CWP trajectory already had substantially more regions of pain than those without a CWP trajectory at the time of ED evaluation, and while the number of body regions with pain in the non-CWP group steadily declined over time, the number of body regions with pain in the CWP trajectory group remained relatively constant. Only 23/78 (30%) of participants meeting criteria for CWP at one year had an increase of 4 or more body regions with pain (out of 20 regions assessed) between six weeks and one year (“spread of pain group”, Fig. 3). The pain characteristics in the ED and at week 6 of individuals in the spread of pain group much more closely resembled those who consistently had widespread pain across follow-up time points than those without widespread pain (Table 4).
Fig. 3.
Change in number of body regions with pain between six weeks and one year after MVC among individuals with widespread pain at one year follow-up after MVC. Only 30% of individuals experienced an increase of ≥ 4 body regions with pain between these timepoints (“spread of pain” group).
Table 3.
Number of body regions with pain, number of somatic symptoms, and depressive and posttraumatic stress disorder (PTSD) symptoms in the ED and six weeks, six months, and one year after MVC.
| Non-widespread Pain Trajectory |
Widespread Pain Trajectory |
|||
|---|---|---|---|---|
| mean (SDd) | p-value | |||
| Number of body regions with pain | ||||
| ED | 3.7 (3.1) | 6.2 (4.0) | <0.001 | |
| week 6 | 2.2 (2.6) | 7.4 (4.5) | <0.001 | |
| month 6 | 1.0 (1.7) | 6.2 (4.8) | <0.001 | |
| year 1 | 0.8 (1.5) | 5.2 (4.4) | <0.001 | |
| Depressive symptomsa | ED | 8.6 (0.4) | 9.3 (0.7) | 0.69 |
| week 6 | 9.8 (0.6) | 21.5 (0.9) | <0.001 | |
| month 6 | 9.1 (0.5) | 17.8 (0.9) | <0.001 | |
| year 1 | 8.7 (0.5) | 16.4 (1) | <0.001 | |
| Number of somatic symptomsb | ||||
| ED | 2.6 (0.1) | 2.9 (0.3) | 0.23 | |
| week 6 | 5.0 (0.2) | 9.5 (0.5) | <0.001 | |
| month 6 | 4.5 (0.2) | 8.2 (0.5) | <0.001 | |
| year 1 | 4.7 (0.2) | 8.4 (0.5) | <0.001 | |
| PTSDc symptoms | ||||
| ED | 17.5 (0.5) | 22.5 (0.8) | <0.001 | |
| week 6 | 16.5 (0.9) | 32.4 (1.5) | <0.001 | |
| month 6 | 10.4 (0.8) | 25.4 (1.4) | <0.001 | |
| year 1 | 9.1 (0.8) | 24.3 (1.4) | <0.001 | |
Assessed with Center for Epidemiologic Studies Depression Scale (CES-D).
Assessed using a 21 item somatic symptom scale.
Assessed with the Peritraumatic Distress Inventory in the ED, and with Impact of Events Scale Revised (IES-R) at week 6, month 6 and year 1.
SD, standard deviation
Table 4.
The pain characteristics in the ED and at week 6 of individuals in the spread of pain group ( ≥ 4 increase in body regions with pain between six weeks and one year) much more closely resembled those who consistently had widespread pain across follow-up time points than those without widespread pain.
| ED |
Week 6 |
|||||
|---|---|---|---|---|---|---|
| No WP | Spread of Pain | WP | No WP | Spread of Pain | WP | |
| Maximum pain severity, mean | 6.0 | 7.7 | 7.7 | 4.0 | 6.1 | 7.3 |
| Body regions with pain | 4.1 | 4.9 | 7.0 | 2.9 | 3.4 | 7.3 |
| Pain inference | - | - | - | 14.2 | 34.3 | 35.3 |
3.6 Somatic symptoms, depressive symptoms, and posttraumatic stress symptoms among those in the low vs. high probability of widespread pain groups
Somatic symptoms, depressive symptoms, and posttraumatic stress symptoms among those who did and did not develop CWP after MVC are shown in Table 3. Reported depressive symptoms during the month prior to MVC were similar in the two groups, but depressive symptoms in the widespread pain group at follow up time points were markedly higher. Similarly, the number of reported somatic symptoms in the month prior to MVC was similar among those who did and did not develop CWP after MVC, but the number of somatic symptoms increased markedly among those developing CWP at 6 weeks, 6 months and 1 year. Individuals developing CWP had greater peritraumatic distress symptoms in the ED and greater posttraumatic stress disorder (PTSD) symptoms across follow-up timepoints. At six months, 49/170 (29%) of individuals in the CWP group vs. 58/614 (9%) in the non-CWP group had substantial PTSD symptoms (χ = 42.42, p < 0.001). At one year, 51/174 (30%) of individuals in the CWP group vs. 55/633 (9%) in the non-CWP group had substantial PTSD symptoms (χ = 50.87, p < 0.001).
4. Discussion
The results of this study suggest that the pathogenic trajectory of CWP after MVC is characterized by the immediate development of widespread pain which persists, rather than the gradual progression of pain from regional to widespread. Approximately 1 in 5 individuals presenting to the ED after MVC had a CWP trajectory, and there was little change in the probability of widespread pain across follow-up time points within this group. Similarly, individuals with a CWP trajectory had a high number of body regions with pain in the ED which subsequently changed little over time. In addition, among individuals with a CWP trajectory the change in number of body regions with pain between six weeks and one year after MVC was relatively evenly distributed around zero. A subset of individuals within the CWP trajectory group experienced a substantial progression in the number of body regions with pain, reporting four or more new body regions with pain at one year compared to the six-week timepoint (47/78, [60%]). This data could seem to support a progressive onset model of CWP development, however such individuals had pain characteristics in the early aftermath of MVC (in the ED in the hours after MVC and at six weeks) that much more closely resembled those with CWP than those without CWP.
To our knowledge, the only other study to examine the natural history of CWP after MVC was conducted by Holm et al, who examined widespread pain outcomes over time among 266 individuals who filed an MVC-related insurance claim in the Canadian province of Saskatchewan [18]. The design of this study differed markedly from the present study, making comparison of study findings difficult. Holm et al designed their study based on the accumulation model of CWP development[18], and only individuals completing initial insurance claim evaluation with pain limited to a portion of the head, neck, and/or back regions were eligible for study participation. Those with more extensive neck or back pain (> 5 of 11 areas of pain in these regions) or any pain in a location outside these regions were not enrolled[18]. Even among this subset of individuals with limited neck and/or back pain in the early aftermath of injury, the investigators still found that CWP most often developed before the first follow-up assessment [18]. Of note, only 845/7462 (11%) of those who reported locations of body pain on their initial insurance claim had the limited distribution of pain required for potential study eligibility. “The others either had different pain patterns or already had more widespread pain when making their insurance claim.”[18] We believe that these other patterns of pain or more widespread pain among the great majority of individuals experiencing MVC are also consistent with a non-recovery model.
Other studies examining the natural history of pain over time in the general population have found that the extent of pain exhibits natural variation [4; 37]. Thus, an individual who meets criteria for CWP at a follow-up timepoint might not have exceeded the CWP threshold if assessed on a different day. However, results of both previous studies and the present study indicate that this natural variation occurs across a relatively narrow range. For example, in the study of Papgeorgiou et al [37], only 2% of participants without initial pain developed CWP over a seven-year follow-up period, and in the present study within-group mean scores varied over time but remained distinctly different between groups (Table 3).
Findings of the present study supporting a non-recovery model of CWP development are consistent with increasing evidence that pain outcomes after stress exposures such as MVC are mediated by central neurobiological mechanisms [2; 6; 8; 30; 32; 33; 43; 44]. This evidence includes data that pain in the immediate aftermath of other stressful events such as sexual assault is located in many body regions, most of which experienced no tissue trauma, and that the distribution of body regions with pain continues to expand in the initial week after stress exposure[33]. These data are consistent with evidence that stress exposure can result in widespread changes in sensory processing due to mechanisms such as the sensitization of peripheral afferents by persistently elevated levels of catecholamines and glucocorticoids[24] and by endogenous opioid-induced hyperalgesia[2; 26].
Results of the present study indicates that older age, female sex, increased BMI, and lower educational attainment are associated with vulnerability to CWP are consistent with previous studies [3; 15; 20; 35; 45]. Our finding that crash-related factors such as amount of vehicle damage showed little association with CWP outcomes is also consistent with previous studies[12; 14; 17], as are results of the present study that individuals developing CWP after MVC are much more likely to develop substantial co-morbid somatic symptoms, PTSD symptoms, and depressive symptoms [7; 13; 31].
CWP is a syndrome defined by the extent/distribution rather than severity of pain, and as noted above, has been shown to result in substantial disability and reduced quality of life [11; 27]. Extent and severity of pain, both in the present study and in other studies [42], have been shown to be only moderately correlated (e.g., correlation between overall pain severity and number of body regions with pain in the present study was 0.38 in the ED, 0.53 at week 6, 0.53 at month 6, and 0.52 at year 1). Biopsychosocial factors contributing to extent vs. severity of pain remain poorly understood, and are an important target for future study.
4.1 Limitations
A number of limitations should be considered when interpreting our results. First, our study was limited to individuals who were discharged to home from the ED after evaluation. CWP development may differ substantially in those with severe injuries who are admitted to the hospital. However, because more than 90% of individuals evaluated in the ED after MVC are discharged to home after evaluation[39], and because many individuals who experience an MVC do not present to the ED for care[38], we believe the spectrum of injury examined in the present study accounts for the great majority of individuals developing CWP. Another limitation of the present study is that only European Americans aged 18 to 65 were enrolled. Further studies are needed which evaluate the trajectory of CWP development in other ethnic groups and in older individuals. Finally, self-report information obtained from study participants might have been inaccurate or incomplete. However, baseline data were obtained at the time of initial evaluation, 6 weeks prior to the first assessment of persistent pain outcomes.
4.2 Conclusions
Our study results indicate that a non-recovery model most accurately describes the pathogenesis of CWP after MVC. Individuals developing CWP after MVC generally have an extensive burden of body pain beginning in the immediate aftermath of MVC, which does not remit, rather than experiencing a gradual extension of pain from regional to widespread over time. As Professor George E. P. Box famously said, “Essentially, all models are wrong, but some are useful [9].” We do not believe that a non-recovery model applies to each and every individual who ever has or ever will suffer from CWP after MVC. However, our data suggest that a non-recovery model is an accurate characterization of CWP in most individuals. Future studies evaluating CWP development after stress exposure should begin in the early aftermath of the event, so that both non-recovery and accumulation models of pathogenesis can be evaluated. In addition, the results of the present study suggest that future preventive intervention studies for this common and morbid outcome might best be initiated in the early aftermath of stress exposure, with the goal helping a greater proportion of individuals with widespread pain or a high burden of overall body pain transition to recovery.
Acknowledgments
We would like to thank the participants for taking part in this study. We would also like to thank Dr. Bobby Jones for his advice and support on Proc Traj. Research reported in this publication was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR056328. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Scientific Meeting Presentation: American Pain Society Poster Presentation 2014, Tampa, FL
References
- 1.Baker SP, o'Neill B, Haddon W, Jr, Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. Journal of Trauma and Acute Care Surgery. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 2.Ballina LE, Ulirsch JC, Soward AC, Rossi C, Rotolo S, Linnstaedt SD, Heafner T, Foley KA, Batts J, Collette R, Holbrook D, Zelman S, McLean SA. mu-Opioid Receptor Gene A118G Polymorphism Predicts Pain Recovery After Sexual Assault. J Pain. 2012 doi: 10.1016/j.jpain.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Bergman S. Psychosocial aspects of chronic widespread pain and fibromyalgia. Disability & Rehabilitation. 2005;27(12):675–683. doi: 10.1080/09638280400009030. [DOI] [PubMed] [Google Scholar]
- 4.Bergman S, Herrström P, Jacobsson LT, Petersson IF. Chronic widespread pain: a three year followup of pain distribution and risk factors. The Journal of rheumatology. 2002;29(4):818–825. [PubMed] [Google Scholar]
- 5.Biomedical NCftPoHSo, Behavioral Research B, MD. The Belmont report: Ethical principles and guidelines for the protection of human subjects of research: ERIC Clearinghouse. 1978 [Google Scholar]
- 6.Bortsov AV, Diatchenko L, McLean SA. Complex multilocus effects of catechol-O-methyltransferase haplotypes predict pain and pain interference 6 weeks after motor vehicle collision. Neuromolecular Med. 2014;16(1):83–93. doi: 10.1007/s12017-013-8255-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bortsov AV, Platts-Mills TF, Peak DA, Jones JS, Swor RA, Domeier RM, Lee DC, Rathlev NK, Hendry PL, Fillingim RB. Pain distribution and predictors of widespread pain in the immediate aftermath of motor vehicle collision. European Journal of Pain. 2013;17(8):1243–1251. doi: 10.1002/j.1532-2149.2013.00285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bortsov AV, Smith JE, Diatchenko L, Soward AC, Ulirsch JC, Rossi C, Swor RA, Hauda WE, Peak DA, Jones JS, Holbrook D, Rathlev NK, Foley KA, Lee DC, Collette R, Domeier RM, Hendry PL, McLean SA. Polymorphisms in the glucocorticoid receptor co-chaperone FKBP5 predict persistent musculoskeletal pain after traumatic stress exposure. Pain. 2013;154(8):1419–1426. doi: 10.1016/j.pain.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Box GE. Essentially, all models are wrong, but some are useful. Statistician. 1919;3(28):2013. [Google Scholar]
- 10.Brunet A, Weiss DS, Metzler TJ, Best SR, Neylan TC, Rogers C, Fagan J, Marmar CR. The Peritraumatic Distress Inventory: a proposed measure of PTSD criterion A2. Am J Psychiatry. 2001;158(9):1480–1485. doi: 10.1176/appi.ajp.158.9.1480. [DOI] [PubMed] [Google Scholar]
- 11.Buskila D, Neumann L, Vaisberg G, Alkalay D, Wolfe F. Increased rates of fibromyalgia following cervical spine injury. A controlled study of 161 cases of traumatic injury. Arthritis & Rheumatism. 1997;40(3):446–452. doi: 10.1002/art.1780400310. [DOI] [PubMed] [Google Scholar]
- 12.Carroll LJ, Holm LW, Hogg-Johnson S, Côtè P, Cassidy JD, Haldeman S, Nordin M, Hurwitz EL, Carragee EJ, van der Velde G. Course and prognostic factors for neck pain in whiplash-associated disorders (WAD): results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Journal of manipulative and physiological therapeutics. 2009;32(2):S97–S107. doi: 10.1016/j.jmpt.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Castro W, Meyer S, Becke M, Nentwig C, Hein M, Ercan B, Thomann S, Wessels U, Du Chesne A. No stress–no whiplash? International journal of legal medicine. 2001;114(6):316–322. doi: 10.1007/s004140000193. [DOI] [PubMed] [Google Scholar]
- 14.Côté P, Cassidy JD, Carroll L, Frank JW, Bombardier C. A systematic review of the prognosis of acute whiplash and a new conceptual framework to synthesize the literature. Spine. 2001;26(19):E445–E458. doi: 10.1097/00007632-200110010-00020. [DOI] [PubMed] [Google Scholar]
- 15.Croft P, Rigby A, Boswell R, Schollum J, Silman A. The prevalence of chronic widespread pain in the general population. The Journal of rheumatology. 1993;20(4):710–713. [PubMed] [Google Scholar]
- 16.Diatchenko L, Slade GD, Nackley AG, Maixner W. Responses to Drs. Kim and Dionne regarding comments on Diatchenko, et al. Catechol-O-methyltransferase gene polymorphisms are associated with multiple pain-evoking stimuli. Pain 2006; 125: 216-24. Pain. 2007;129(3):366–370. doi: 10.1016/j.pain.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holm LW, Carroll LJ, Cassidy JD, Hogg-Johnson S, Côté P, Guzman J, Peloso P, Nordin M, Hurwitz E, van der Velde G. The burden and determinants of neck pain in whiplash-associated disorders after traffic collisions: results of the Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Journal of manipulative and physiological therapeutics. 2009;32(2):S61–S69. doi: 10.1016/j.jmpt.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Holm LW, Carroll LJ, Cassidy JD, Skillgate E, Ahlbom A. Widespread pain following whiplash-associated disorders: incidence, course, and risk factors. The Journal of rheumatology. 2007;34(1):193–200. [PubMed] [Google Scholar]
- 19.Institute of Medicine. Committee on Advancing Pain Research C, Education. Relieving pain in America: A blueprint for transforming prevention, care, education, and research. National Academies Press; 2011. [PubMed] [Google Scholar]
- 20.Janke EA, Collins A, Kozak AT. Overview of the relationship between pain and obesity: What do we know? Where do we go next? Journal of rehabilitation research and development. 2007;44(2):245. doi: 10.1682/jrrd.2006.06.0060. [DOI] [PubMed] [Google Scholar]
- 21.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Methods & Research. 2001;29(3):374–393. [Google Scholar]
- 22.Jones GT, Nicholl BI, McBeth J, Davies KA, Morriss RK, Dickens C, Macfarlane GJ. Role of road traffic accidents and other traumatic events in the onset of chronic widespread pain: Results from a population-based prospective study. Arthritis care & research. 2011;63(5):696–701. doi: 10.1002/acr.20417. [DOI] [PubMed] [Google Scholar]
- 23.Kamaleri Y, Natvig B, Ihlebaek CM, Bruusgaard D. Does the number of musculoskeletal pain sites predict work disability? A 14-year prospective study. European Journal of Pain. 2009;13(4):426–430. doi: 10.1016/j.ejpain.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 24.Khasar SG, Burkham J, Dina OA, Brown AS, Bogen O, Alessandri-Haber N, Green PG, Reichling DB, Levine JD. Stress induces a switch of intracellular signaling in sensory neurons in a model of generalized pain. J Neurosci. 2008;28(22):5721–5730. doi: 10.1523/JNEUROSCI.0256-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsson B, Björk J, Börsbo B, Gerdle B. A systematic review of risk factors associated with transitioning from regional musculoskeletal pain to chronic widespread pain. European Journal of Pain. 2012;16(8):1084–1093. doi: 10.1002/j.1532-2149.2012.00117.x. [DOI] [PubMed] [Google Scholar]
- 26.Le Roy C, Laboureyras E, Gavello-Baudy S, Chateauraynaud J, Laulin JP, Simonnet G. Endogenous opioids released during non-nociceptive environmental stress induce latent pain sensitization Via a NMDA-dependent process. J Pain. 2011;12(10):1069–1079. doi: 10.1016/j.jpain.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 27.Macfarlane GJ, Crombie I, McBeth J, Silman AJ. Widespread body pain and mortality: prospective population based studyCommentary: An interesting finding, but what does it mean? Bmj. 2001;323(7314):662. doi: 10.1136/bmj.323.7314.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McBeth J, Jones K. Epidemiology of chronic musculoskeletal pain. Best Practice & Research Clinical Rheumatology. 2007;21(3):403–425. doi: 10.1016/j.berh.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 29.McBeth J, Silman A, Gupta A, Chiu Y, Ray D, Morriss R, Dickens C, King Y, Macfarlane G. Moderation of psychosocial risk factors through dysfunction of the hypothalamic–pituitary–adrenal stress axis in the onset of chronic widespread musculoskeletal pain: findings of a population-based prospective cohort study. Arthritis & Rheumatism. 2007;56(1):360–371. doi: 10.1002/art.22336. [DOI] [PubMed] [Google Scholar]
- 30.McLean SA. The potential contribution of stress systems to the transition to chronic whiplash-associated disorders. Spine. 2011;36(25 Suppl):S226–S232. doi: 10.1097/BRS.0b013e3182387fb4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLean SA, Clauw DJ, Abelson JL, Liberzon I. The development of persistent pain and psychological morbidity after motor vehicle collision: integrating the potential role of stress response systems into a biopsychosocial model. Psychosomatic medicine. 2005;67(5):783–790. doi: 10.1097/01.psy.0000181276.49204.bb. [DOI] [PubMed] [Google Scholar]
- 32.McLean SA, Diatchenko L, Lee YM, Swor RA, Domeier RM, Jones JS, Jones CW, Reed C, Harris RE, Maixner W, Clauw DJ, Liberzon I. Catechol O-methyltransferase haplotype predicts immediate musculoskeletal neck pain and psychological symptoms after motor vehicle collision. J Pain. 2011;12(1):101–107. doi: 10.1016/j.jpain.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McLean SA, Soward AC, Ballina LE, Rossi C, Rotolo S, Wheeler R, Foley KA, Batts J, Casto T, Collette R, Holbrook D, Goodman E, Rauch SA, Liberzon I. Acute severe pain is a common consequence of sexual assault. J Pain. 2012;13(8):736–741. doi: 10.1016/j.jpain.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mohan H, Ryan J, Whelan B, Wakai A. The end of the line? The Visual Analogue Scale and Verbal Numerical Rating Scale as pain assessment tools in the emergency department. Emergency Medicine Journal. 2010;27(5):372–375. doi: 10.1136/emj.2007.048611. [DOI] [PubMed] [Google Scholar]
- 35.Mundal I, Gråwe RW, Bjørngaard JH, Linaker OM, Fors EA. Psychosocial factors and risk of chronic widespread pain: An 11-year follow-up study—The HUNT study. PAIN®. 2014;155(8):1555–1561. doi: 10.1016/j.pain.2014.04.033. [DOI] [PubMed] [Google Scholar]
- 36.Nishi D, Matsuoka Y, Yonemoto N, Noguchi H, Kim Y, Kanba S. Peritraumatic Distress Inventory as a predictor of post-traumatic stress disorder after a severe motor vehicle accident. Psychiatry Clin Neurosci. 2010;64(2):149–156. doi: 10.1111/j.1440-1819.2010.02065.x. [DOI] [PubMed] [Google Scholar]
- 37.Papageorgiou A, Silman A, Macfarlane G. Chronic widespread pain in the population: a seven year follow up study. Annals of the rheumatic diseases. 2002;61(12):1071–1074. doi: 10.1136/ard.61.12.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Platts-Mills TF, Ballina L, Bortsov AV, Soward A, Swor RA, Jones JS, Lee DC, Peak DA, Domeier RM, Rathlev NK, Hendry PL, McLean SA. Using emergency department-based inception cohorts to determine genetic characteristics associated with long term patient outcomes after motor vehicle collision: methodology of the CRASH study. BMC emergency medicine. 2011;11:14. doi: 10.1186/1471-227X-11-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Platts-Mills TF, Hunold KM, Esserman DA, Sloane PD, McLean SA. Motor vehicle collision–related emergency department visits by older adults in the United States. Academic emergency medicine. 2012;19(7):821–827. doi: 10.1111/j.1553-2712.2012.01383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Radloff L. The CES-D Scale. A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- 41.SA M. Neurobiologic mechanisms of whiplash. In: Jensen Troels S, Turk Dennis, Kasch Helge., editors. Whiplash injury: a model for the development of chronic pain. 2015. [Google Scholar]
- 42.Tait RC, Chibnall JT, Margolis RB. Pain extent: relations with psychological state, pain severity, pain history, and disability. Pain. 1990;41(3):295–301. doi: 10.1016/0304-3959(90)90006-Y. [DOI] [PubMed] [Google Scholar]
- 43.Ulirsch JC, Ballina LE, Soward AC, Rossi C, Hauda W, Holbrook D, Wheeler R, Foley KA, Batts J, Collette R, Goodman E, McLean SA. Pain and somatic symptoms are sequelae of sexual assault: results of a prospective longitudinal study. European journal of pain. 2014;18(4):559–566. doi: 10.1002/j.1532-2149.2013.00395.x. [DOI] [PubMed] [Google Scholar]
- 44.Ulirsch JC, Weaver MA, Bortsov AV, Soward AC, Swor RA, Peak DA, Jones JS, Rathlev NK, Lee DC, Domeier RM, Hendry PL, McLean SA. No man is an island: Living in a disadvantaged neighborhood influences chronic pain development after motor vehicle collision, and this effect is moderated by common genetic variation influencing HPA axis function. Pain. 2014 doi: 10.1016/j.pain.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Versteegen G, Kingma J, Meijler W, Ten Duis H. Neck sprain after motor vehicle accidents in drivers and passengers. European Spine Journal. 2000;9(6):547–552. doi: 10.1007/s005860000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wallace DJ, Wallace JB, Wallace JB. Fibromyalgia: An essential guide for patients and their families. New York: Oxford University Press; 2003. [Google Scholar]
- 47.Weiss D. Psychometric review of the impact of events scale-revised. Measurement of stress, trauma, and adaptation. 1996:186–187. [Google Scholar]
- 48.Weissman MMSD, Pottenger M, et al. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 49.Wolfe F. Pain extent and diagnosis: development and validation of the regional pain scale in 12,799 patients with rheumatic disease. The Journal of rheumatology. 2003;30(2):369–378. [PubMed] [Google Scholar]
- 50.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis & Rheumatism. 1990;33(2):160–172. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 51.Wynne-Jones G, Jones GT, Wiles NJ, Silman AJ, Macfarlane GJ. Predicting new onset of widespread pain following a motor vehicle collision. The Journal of rheumatology. 2006;33(5):968–974. [PubMed] [Google Scholar]
- 52.Wynne-Jones G, Macfarlane GJ, Silman AJ, Jones GT. Does physical trauma lead to an increase in the risk of new onset widespread pain? Annals of the rheumatic diseases. 2006;65(3):391–393. doi: 10.1136/ard.2005.037531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zich JM, Attkisson CC, et al. Screening for depression in primary care clinics: the CES-D and the BDI. Int J Psychiatry Med. 1990;20(3):259–277. doi: 10.2190/LYKR-7VHP-YJEM-MKM2. [DOI] [PubMed] [Google Scholar]