Abstract
A variety of modified fats that provide different functionalities are used in processed foods to optimize product characteristics and nutrient composition. Partial hydrogenation results in the formation of trans FAs (TFAs) and was one of the most widely used modification processes of fats and oils. However, the negative effects of commercially produced TFAs on serum lipoproteins and risk for cardiovascular disease resulted in the Institute of Medicine and the 2010 US Dietary Guidelines for Americans both recommending that TFA intake be as low as possible. After its tentative 2013 determination that use of partially hydrogenated oils is not generally regarded as safe, the FDA released its final determination of the same in 2015. Many food technologists have turned to interesterified fat as a replacement. Interesterification rearranges FAs within and between a triglyceride molecule by use of either a chemical catalyst or an enzyme. Although there is clear utility of interesterified fats for retaining functional properties of food, the nutrition and health implications of long-term interesterified fat consumption are less well understood. The Technical Committee on Dietary Lipids of the North American Branch of the International Life Sciences Institute sponsored a workshop to discuss the health effects of interesterified fats, identify research needs, and outline considerations for the design of future studies. The consensus was that although interesterified fat production is a feasible and economically viable solution for replacing dietary TFAs, outstanding questions must be answered regarding the effects of interesterification on modifying certain aspects of lipid and glucose metabolism, inflammatory responses, hemostatic parameters, and satiety.
Keywords: interesterified fats, dietary intake, metabolism, health effects, trans fatty acids
Introduction
A variety of modified fats that provide different functionalities are utilized in processed foods to optimize product characteristics and nutrient composition. Melting behavior, solid fat content, and fat crystal network are important factors in creating shortenings and margarines, whereas oxidative stability is important for frying oil. The desired property of lipids in foods may be achieved by blending, fractionation, hydrogenation, interesterification, or genetic modification.
Until recently, partial hydrogenation was one of the most widely used modification processes of fats and oils. This process uses hydrogen to reduce the concentration of PUFAs while forming positional and geometric FA isomers, including trans FAs (TFAs)9 and SFAs to impart solid function to fats for shortenings and, in some cases, for stability in oils used for deep frying (1). Compared with unsaturated oils, this reduction in PUFAs to modify function also extends the shelf-life for packaged foods. With widespread use as an animal fat replacement in foods, partially hydrogenated oils (PHOs) became the prime source of TFAs in the diet. Industrially produced TFAs are unsaturated FAs (UFAs) with ≥1 carbon–carbon double bond in the trans configuration. In humans, plants, and most mammals, endogenously synthesized UFAs have double bonds that are overwhelmingly in the cis configuration. Only ruminants produce small amounts of TFAs in their rumen (2). Previous studies have described the negative effects of industrially produced TFAs on lipoproteins and cardiovascular disease risk (3). Beginning in 2006, the FDA required declaration of TFA content on nutrition labels (4). Both the Institute of Medicine and the 2010 US Dietary Guidelines for Americans (5) recommended that TFA intake be kept as low as possible. After tentatively determining in 2013 that the use of PHOs is not generally regarded as safe (6), the FDA released its final determination of the same in 2015 (7). Food manufacturers will have a 3-y compliance period to either reformulate their products without PHOs or petition the agency for permission for specific uses (7).
Whereas nutritionists focus primarily on the health effects of specific FAs, food technologists tend to focus on product and ingredient functionality while also keeping health effects in mind. TG composition is an important indicator of the functionality of fats. In the search for alternative fats and oils to replace TFA, food technologists have turned to a process called interesterification (also known as the randomization of fats), which has been used in the edible oils industry for decades. Interesterification rearranges the FAs within and between a TG molecule by use of a chemical catalyst or enzyme. The chemical interesterification process is a random modification tool, whereas enzymatic interesterification can be either random or stereospecific. Depending on the starting fat and/or oils, more SFAs or UFAs can be exchanged into the sn-2 position, changing the original TG structure. This exchange is one way to modify the melting point of the fat without changing its FA composition while providing similar functional qualities as PHOs, without introducing TFAs.
The creation of interesterified fats, also referred to as structured lipids, can also involve the replacement of naturally occurring long-chain FAs at the sn-1 and sn-3 positions with medium-chain FAs (MCFAs), such as caprylic acid (8:0) or capric acid (10:0), to form medium-chain triglycerides (MCTs), or with long-chain ω-3 FAs [DHA (24:6n–3), EPA (22:5n–3), docosapentaenoic acid (22:5n–3)], long-chain ω-6 FAs [arachidonic acid (20:5n–6)], or long-chain SFAs [stearic acid (18:0)], or other “atypical” FAs to produce novel fats. For example, MCTs are easily hydrolyzed, readily absorbed, and directly metabolized for energy, rather than accumulated as depot fat. This group of structured lipids imparts special nutritional or pharmaceutical properties and has historically been used for patients with abnormalities in fat digestion (8). MCTs have also been suggested to facilitate weight loss (9).
Although the utility of interesterified fats for retaining the functional properties of food is clear, the health implications of long-term consumption are less well understood. Based on current understanding from the majority of published studies, it was previously assumed that the triglyceride structure would have little effect on lipid digestion, absorption, and metabolism in adults (10–12). However, limited research suggests that manipulating the natural position of specific FAs on the glycerol backbone may negatively affect lipoprotein metabolism, glycemic control, immune function, and serum liver enzymes (1, 13–16).
The North American Branch of the International Life Sciences Institute’s Technical Committee on Dietary Lipids sponsored a workshop in Washington, DC, in 2012 to review concerns about the effects of interesterified fats on health. This workshop convened representatives from industry, government, professional associations, and academia to better understand the potential acute and chronic health effects of interesterified fats, identify research needs, and outline considerations for the design of future studies. This review summarizes the workshop findings and recommendations.
Current Status of Knowledge
Food science of interesterified fats
Chemical esterification and enzymatic esterification are essential for modifying the physical properties of oils and fats and are utilized extensively in the synthesis of TGs for use as shortenings for baked goods, human milk-fat substitutes, ω-3 FA-enriched TGs, modified digestibility fats, and confectionery fat substitutes. For several decades, interesterified fats have been added in limited amounts as hard stock for products such as margarine. The interesterification reaction can be chemical or enzymatic and is usually carried out between a high-melting-point fat (fully hydrogenated vegetable oil or palm oil fraction) and a liquid oil, leading to a FA exchange within and between TGs and resulting in the formation of new TG molecules with unique properties (desirable plasticity, texture, and mouthfeel). Chemical interesterification is a random reaction, whereas enzymatic interesterification can be specific or random, depending on the selected lipase enzymes. Sodium methoxide is generally used as a catalyst in chemical interesterification. Some commercially available lipase sources used for food processing include Candidancylindracea, Rhizomucor miehei, Mucor miehei, and Penicilliun roquefortii. The newly formed TG molecules have chemical and physical properties that fall between those of the initial starting materials. Because interesterification does not change the FA composition of the oil, the changes can only be analytically detected by a TG structural analysis.
Chemical interesterification has been used since the 1940s to modify the crystallization behavior of lard. This process has disadvantages, such as high oil losses and low oxidative stability of the finished product (17). Enzymatic interesterification is preferable to chemical interesterification because it does not require chemical catalysts, can be carried out at relatively low temperatures, results in less neutral oil loss and industrial effluent, and preserves the oxidative quality of the interesterified oil. However, enzymatic interesterification also has disadvantages such as higher equipment, operating, and catalyst costs (lipase compared with sodium methoxide in chemical esterification). Nonetheless, enzymatic interesterification has replaced chemical interesterification as the method of choice in North America, especially for formulation of low-TFA or TFA-free margarines and shortenings (18).
Estimates of interesterified fats in the diet
The level of intake of interesterified fats in the US population is unclear because of a lack of data on the content of interesterified fats in individual foods. Accurately estimating intake would require input from food companies or extensive analytical work, neither of which is currently available. However, changes in FA intake reported in national food intake surveys can provide some insight into intake of interesterified fats. Because SFAs are incorporated into interesterified fats, intakes of palmitic and stearic acids will likely increase. In support of this, data from 2001–2008 NHANES What We Eat in America suggest a shift in FA intake, including a slight increase in both palmitic and stearic acids (19–22), whereas data from 2003–2006 indicate a decrease in TFA intake (23).
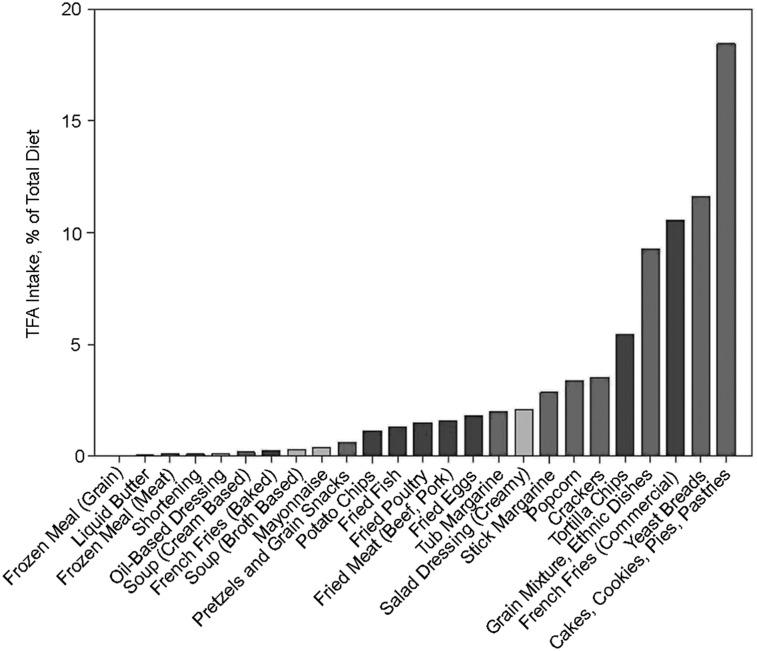
A modeling exercise was performed to predict FA intake subsequent to the replacement of TFA-containing oils in frying processes, with oils containing fewer PUFAs (e.g., low-linolenic, mid-oleic, and low-linolenic soybean oil), and replacement of TFA-containing oils in foods such as baked goods, popcorn, shortening, and stick and tub margarines (with palm-based oils or an interesterified fat made with fully hydrogenated soybean oil) (24, 25). This exercise identified 25 food categories, representing 86% of total soybean oil intake and 79% of total TFA intake from NHANES 1999–2002 intake data. Twelve food categories were identified as those for which TFA-containing oils would most likely be replaced with palm oil (some of which may be interesterified) and an interesterified fat made with fully hydrogenated soybean oil. TFA intake from these 12 categories comprised ∼50% of total TFA intake, with cakes, cookies, and other baked goods being the predominant dietary source of TFAs in the American diet (Figure 1). Based on the replacement of palm-based oils or an interesterified fat made with fully hydrogenated soybean oils in these 12 food categories (25), the predicted mean increases in the intake of palmitic and stearic acids ranged from 1.0% to 2.0% and 0.5% to 1.5% of energy, respectively, for the mean intake of the third quintile and 1.9% to 4.8% of energy and 0.9% to 3.8% of energy, respectively, for the mean intake of the fifth quintile. The amount of total palmitic and stearic acid that currently comes from interesterified fats is unknown. However, according to the modeling exercise, if all palm oil–based products were interesterified with palmitic or stearic acid, the upper limit (the mean of the fifth quintile) of interesterified FA intake would be 4.8% of energy with a mean intake of ∼3.0% of energy.
FIGURE 1.
Potential food categories for interesterified fat applications and the contribution of these categories to total TFA intake. The light bars represent food categories that would likely not be replaced with a functional fat, the darker shaded bars represent food categories that would likely be replaced with a heat-stable oil, and the darkest shaded bars represent food categories that would likely be replaced with an interesterified fat. TFA, trans FA. Data are from reference 25.
In many of the intervention studies investigating the metabolic effect of interesterified fats discussed in the next paragraphs, the interesterified fat content was well above current intakes. Based on the modeling exercise, the amount of interesterified fat (primarily containing stearic or palmitic acids) in clinical studies thus appears to be at least almost twice the amount of interesterified fat that would be consumed at the mean of the fifth quintile of intake. This raises the questions of whether the observed effects in studies are clinically relevant for most of the US population and whether there are enough data on the effects of interesterified fats at projected typical intakes.
If the goal of intervention trials is to cover the mean intake levels of the population, then the third quintile of intake is a reasonable target. However, if the goal is to represent an intake that will cover most of the population, then the fifth quintile might be a better level of intervention. Both levels are significantly less than the amounts used in most intervention studies, some of which suggest that higher intakes can be problematic. It was previously suggested that the interesterified test fat must provide ≥50% of the total fat in the typical diet with 20–40% total energy from fat to detect any possible adverse effects (14). Although understanding the effects at higher-than-usual intake levels has value, one must ensure that the data are not misleading, particularly if such levels are not feasible in the food supply. Trials are needed in which interesterified fats are fed at intervals from 0% to 9% to elucidate their effects at predicted typical mean intakes, as well as at twice the fifth quintile mean intakes. Such studies would realistically address safety concerns and would help determine whether a threshold exists above which adverse effects could occur.
Digestion, absorption, and postprandial metabolism of interesterified fats
The available data indicate that in healthy subjects there is minimal difference in 24-h FA absorption between TG mixtures and interesterified fats of the same composition. Although preferences of the different lipases for FA chain length and saturation can be identified in vitro, the complex system in vivo is difficult to mimic and the overabundance of lipase activity masks these preferences. The exceptions may be saturated long-chain FAs (LCFAs) (16:0, 18:0), which may form FA-calcium soaps and are excreted to a greater degree when in the sn-1,3 position but are better absorbed from the sn-2 position because the monoglyceride is efficiently taken up by enterocytes (26). This has been clearly seen in animal studies, although human data are equivocal (12).
A number of animal studies have compared the digestion and absorption of FAs from physical blends of MCFAs and LCFAs with interesterified TGs derived from the same mixture. Although TG lipolysis and the amount of FAs absorbed are not measurably different between TG mixtures and interesterified fats (except as the modifications affect solubility in mixed micelles), several investigations have shown differing rates of absorption and different lymphatic transport of FAs from interesterified lipids compared with an equivalent mixture of native oils. The position of specific FAs on the glycerol backbone seems to affect the rate of appearance and the route of absorption in these studies. For example, MCFAs are normally transported primarily via the portal circulation; however, when they are primarily present in the sn-2 position by interesterification, a greater proportion is transported as lymph chylomicrons because they are absorbed as monoacylglycerol, which is re-esterified to TGs by enterocytes. In animal studies, both LCFAs and MCFAs are more rapidly transported into lymph from the sn-2 position than the sn-1,3 position (27–29). As a result, interesterified fats may provide a tool to increase absorption of specific FAs in clinical conditions such as malabsorption (30). In addition, greater lymphatic transport of MCFAs in interesterified fats results in better delivery to peripheral tissues of this readily utilized, high energy source, which otherwise is predominately transported to the liver through portal circulation. This is supported by both animal models (31) and clinical studies (32), which suggest that interesterified fats are useful for delivering MCFAs to peripheral tissues for energy utilization. However, there is a lack of thorough, direct analysis of the acute postprandial tissue fate of lipids from interesterified compared with unmodified TGs. For example, there is little information regarding the effects of structured lipids on chylomicron size or lipoprotein composition.
By using interesterified fat, it is possible to improve FA absorption in the lymph of rats following ischemia and reperfusion injury (29). Absorption of fat-soluble vitamins (33) in rat models of malabsorption and bioavailability of lipophilic drugs (34) in dog models have been shown to be enhanced by delivery with certain interesterified fats. Data are mixed regarding targeting of specific FAs with interesterified fats compared with mixtures. Together, these studies suggest that in patients with high physical stress, such as burns or surgical trauma, interesterified fats could be beneficial by diminishing nitrogen wasting and organ damage (31, 32).
Incorporation of DHA and EPA into brain phospholipids of rat dams or pups was not affected by the dietary lipid structure (35). However, EPA (but not DHA) delivery to splenocytes increased when it replaced all 18:3 FAs in the sn-2 position of TGs (36). Studies of mixed compared with interesterified TGs used in parenteral feeding to dogs revealed no differences in the FA composition of tissue fat (37).
Postprandial effects of interesterified fats
Whereas many clinical studies have measured the effects of dietary fats in the fasting state, humans spend most waking hours in the postprandial state. In fact, increased fasting lipoprotein and glucose concentrations, as well as disturbed postprandial TG and glucose metabolism, are all important risk markers for metabolic diseases (38). The postprandial period, when TGs are most elevated (3–6 h), also influences the risk of thrombosis and is considered to be a period of high risk for metabolic disease and cardiovascular events (36, 39–41). Elevated coagulation activation factors, such as factor VII (FVII), in the postprandial period may temporarily increase the risk of severe thrombotic events (42).
Although postprandial data raise interesting questions related to health outcomes, they are best considered within the context of more conventional fasting data after a period of fat intervention, when a new steady-state situation is reached. Dietary fat impacts tissue phospholipid composition, particularly muscle membranes, which can affect insulin sensitivity and glucose uptake, or cell signals affecting insulin secretion (43–45). Therefore, changes in blood glucose and insulin concentrations should theoretically reflect the effect of interesterified fats with sn-2-SFAs such as those incorporated into phospholipids. The importance of the sn position of a FA is further underlined by the trial of Kew et al. (36). Although it was not a postprandial study, the results showed that dietary EPA increased the phagocytic capacity of activated monocytes and neutrophils when supplied in the sn-2 position but not in the sn-1,3 position, possibly as a result of changes to membrane phospholipids of splenocytes.
TG structure and FA composition may be important in affecting the FA destination in the postprandial period. There are a limited number of studies directly comparing postprandial effects of interesterified fats with native fats having identical FA composition. Of the existing studies, almost one-half included a small number of subjects (≤16), and subjects in the smaller studies were mostly healthy, young, and predominately women. However, postprandial studies are fraught with design problems such as sex selection, the amount and kind of test fat incorporated into a meal, the total nutrient composition of the test meal, the length of time the postabsorptive process is followed, and the use of subjects who have not acclimated to the fat being tested. Because each of these factors can affect the postprandial response parameters, it is imperative that design standards be established to more accurately observe the effect of a single fat in a single meal. The lack of a standardized test meal protocol among some studies makes direct comparisons difficult. However, studies conducted by Berry et al. (12, 46) and Sanders et al. (47–49) consistently used the same protocol and 50 g of test fat.
Postprandial inflammation.
Interest in the postprandial inflammatory response has greatly increased in recent years because of its association with diabetes and metabolic disease. Postprandial findings are consistent with the theory that inflammatory responses are exacerbated by meals rich in fat. Decreasing this acute effect in the postprandial plasma compartment may protect against chronic inflammation of adipose and other tissues, which can predispose patients to insulin resistance and diabetes. Results of several in vitro and animal studies suggest that dietary FA composition and availability can modulate inflammatory responsiveness (39, 40, 50). The differing availability of saturated LCFAs from interesterified fats compared with unmodified TGs has the potential to change postprandial inflammation, because palmitate and stearate are known to induce various inflammatory responses in vitro (40).
An in vitro experiment with fasting whole blood from healthy individuals fed specific fat blends showed that the addition of unmodified LCFAs plus an MCFA-TG blend (i.e., soybean plus coconut oil) greatly increased activation and degranulation of monocytes and neutrophils, compared with the addition of interesterified TGs of similar FA composition (39). The in vivo relevance for humans is not currently known. Leukocyte activation markers were reduced in vivo when rats received parenteral MCFAs as structured TGs compared with unmodified TGs after gastrectomy surgery (50). However, monocyte chemotactic protein-1 and macrophage inflammatory protein-2 concentrations in peritoneal lavage fluid were elevated by consumption of the structured TG, leaving the relative risk or benefit open to discussion. In addition to MCFA effects, the differing availability of saturated LCFAs from interesterified fats compared with unmodified TGs also has the potential to change postprandial inflammation, because palmitate and stearate are known to induce various inflammatory responses in vitro (40).
Postprandial lipemia and factor VII.
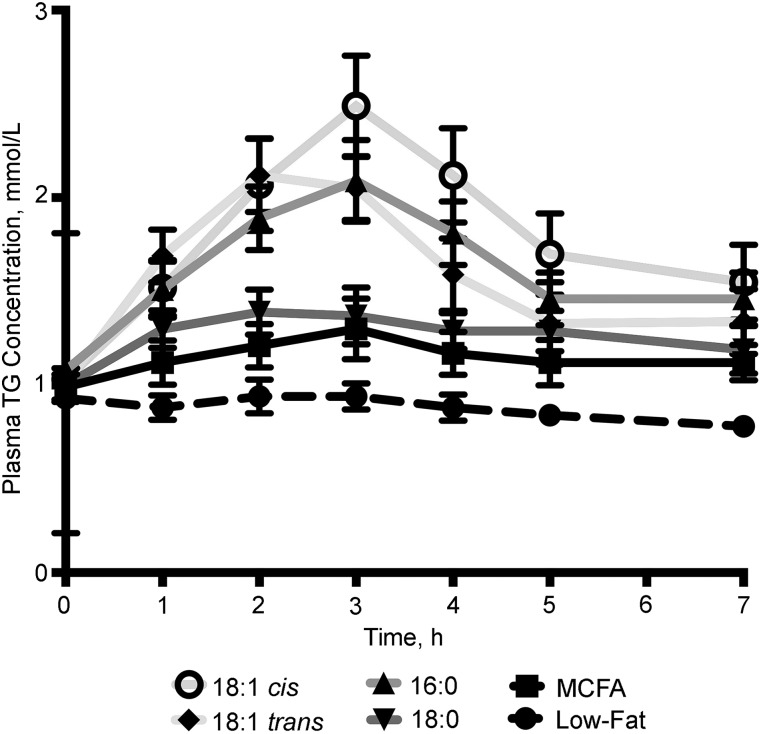
In a randomized crossover study of 11 healthy men and 5 healthy women, the postprandial effects of 5 high-fat meals (90 g) enriched with MCFAs, such as MCTs (8:0 + 10:0), or fats rich in palmitate (16:0), stearate (18:0), elaidate (18:1 trans), and oleate (18:1 cis) were compared with a low-fat meal (51). A slower increase in postprandial plasma TGs and a lower AUC were found for stearate and MCTs than for oleate-, elaidate-, and palmitate-rich fats (Figure 2). The increase in FVIIa at 7 h was greater after the oleate meal than after the stearate and MCT meals. The authors concluded that dietary stearate-rich and MCT fats were not responsible for the postprandial increases in FVII associated with the high-fat meals.
FIGURE 2.
Postprandial changes in plasma TG concentrations after consumption of 6 different test meals. In total, 11 healthy men and 5 healthy women consumed, in random order, a low-fat meal and 5 different high-fat meals (90 g) rich in MCFAs, palmitate (16:0), stearate (18:0), elaidate (18:1 trans), or oleate (18:1 cis). Values are given as means ± SEMs. MCFA, medium-chain FA. Data are from reference 51.
In another study of 16 healthy men, the effects of 6 matching high-fat meals (1 g fat/kg body weight; 43% from the test FA) were examined following breakfast on 6 separate days (42). The fats were interesterified and were rich in stearic, palmitic, palmitic + myristic, oleic, trans-18:1, or linoleic acid, and their effects on the postprandial lipid and hemostatic profiles were investigated. Although all fats increased FVII activation, there was less increase with SFAs, especially the stearic acid–rich fat (randomly interesterified hydrogenated sunflower oil blended with unhydrogenated high-oleic sunflower oil) that corresponded with its reduced postprandial triglyceridemia in comparison with UFAs.
Because interesterification alters the composition of TGs and the position of the FA on the glycerol backbone, this can affect postprandial lipemia. In a randomized crossover study of 35 healthy middle-aged men (n = 17) and women (n = 18), Sanders et al. (47) compared a structured TG (SALATRIM, a synthetic stearic acid–rich TG interesterified with short-chain FA; Danisco Cultor) with high-oleic sunflower oil and cocoa butter after a single meal. Markedly depressed postprandial triglyceridemia occurred with SALATRIM, which is known to be absorbed more slowly (13). The depressed postprandial lipemia was accompanied by decreased activation of FVII as measured by its coagulant activity or activated concentration. There were no effects on indexes of fibrinolysis (plasminogen activator inhibitor type 1 or tissue plasminogen activator). The authors proposed that this was a consequence of SALATRIM’s FA combination and randomness of the FA positional distribution, resulting in a unique asymmetric TG structure (stearic acid combined with short-chain FAs). Some of the differences, however, may have been due to the high proportion of short-chain FAs.
In a randomized crossover study of 17 healthy men, the interesterification of cocoa butter decreased postprandial lipemia and FVII activation compared with native cocoa butter (48). Berry et al. (12) compared native and interesterified shea butter (high sn-1 3-stearic acid content) and found that both native and interesterified shea butter resulted in less postprandial lipemia and decreased FVII activation compared with high-oleic sunflower oil.
Sanders et al. (49) compared test meals containing 50 g of interesterified palm olein [liquid palm oil–enriched sn-2 (16:0) by interesterification] compared to an equal amount of native palm olein [sn-2 (18:1, 18:2)] and 50 g of lard [sn-2 (16:0)], with an equal amount of high-oleic sunflower oil, in a 2-center study in healthy men (n = 25) and women (n = 25). The authors hypothesized that high-fat meals rich in palmitic acid (16:0) in the sn-2 position would decrease lipemia. Sanders et al. (49) found a tendency for the initial postprandial increase in plasma TGs to be lower following consumption of the interesterified fat. Based on changes in postprandial apolipoprotein B-48 concentrations, there was no evidence indicating increased production of apolipoprotein B-48 or increased resistance to chylomicron remnant clearance following the interesterified fat. There were, however, sex differences in response to the test meals for plasma TGs. For example, the postprandial incremental AUC (iAUC) for TGs was 51% lower in women.
Postprandial glycemic control.
Assessing postprandial glycemic responses requires consideration of several parameters. For example, the influence of any dietary fat on glucose and insulin metabolism under experimental conditions may be influenced by study subject characteristics such as age, health status, and genotype, as well as the percentages of daily fat consumed, study length, and prior diet (52). As mentioned above, the interesterified test fat must provide ≥50% of the total fat in the typical diet with 20–40% energy from fat in order to detect any adverse effects, because when only modestly challenged with lower amounts of test fat, the body is able to compensate from UFA in adipose reserves over the short term (14).
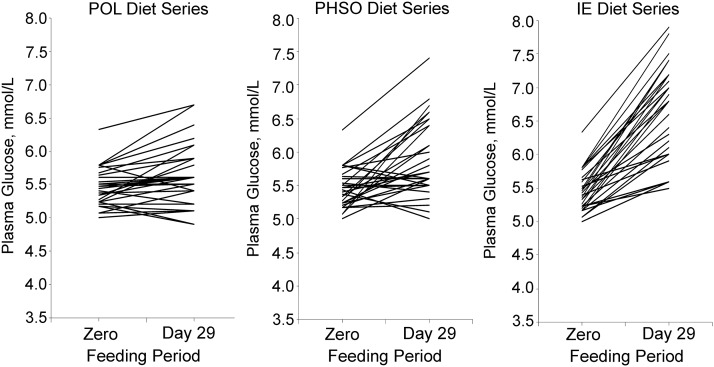
Sundram et al. (1) studied 30 healthy men and women who consumed complete, whole-food diets during 4-wk periods in a crossover design in which total fat (∼31% daily energy, >70% from the test fats) and FA compositions were controlled (Figure 3). One test fat, based on palm olein, provided 12% of energy as palmitic acid (16:0). A second test fat contained trans-rich partially hydrogenated soybean oil and provided 3.2% TFA, plus 6.5% energy as 16:0. The third test fat used an interesterified fat and provided 12.5% of energy as stearic acid. At 2 wk into each diet period, an 8-h postprandial challenge was initiated in a subset of 19 subjects consuming a meal containing 53 g of each test fat. The glucose iAUC following the interesterified meal was 40% greater than after either of the other meals. However, it could also be that slower absorption of interesterified fat postprandially lowered incretin [glucagon-like peptide (GLP), GLP-1] production, leaving carbohydrates as the primary energy absorbed following the meal challenge and thus resulting in the observed glycemia. Future research is needed to explore both of these possibilities. In addition, the unfavorable effects of stearic acid on postprandial glucose metabolism have not been confirmed by others (12). Furthermore, a later comparison of interesterified palm olein with native palm olein in 41 Malaysian men (n = 10) and women (n = 31) showed no difference in insulin secretion or postprandial glucose changes with chronic meals (6 wk of each treatment phase) at two-thirds of the dietary fat intake (53).
FIGURE 3.
Individual fasting glucose values are depicted for 30 healthy men and women at study entry and after 4 wk on diets rich in test fats of POL, PHSO, and IE. Participants consumed complete, whole-food diets for 4-wk periods in a crossover study design. Total fat comprised ∼31% daily energy (>70% from the test fats). Compared with entry values of glucose, increases of 3%, 9%, and 22% were seen after consuming the diets rich in POL, PHSO, and IE, respectively. IE, interesterified fat based on fully hydrogenated soybean oil; PHSO, partially hydrogenated soybean oil; POL, palm olein. Reproduced from reference 1 with permission.
In 2 randomized crossover postprandial trials in 20 healthy men, Berry et al. (46) compared the effects of meals containing 50 g of fat as either interesterified super palm olein (sn-2–16:0, 38%; 18:1, 45%; and 18:2, 11%) or native super palm olein (double fractionated liquid palm oil with sn-2–16:0, 7%; 18:1, 17%; and 18:2, 19%) (study 1) and interesterified palm oil or high-oleic sunflower oil (study 2) on postprandial glucose and insulin concentrations. Although there was a tendency for postprandial plasma insulin to be lower and glucose to be higher with interesterified fats, the authors concluded that interesterified C16 fats did not differ significantly from naturally occurring C16 fats with regard to postprandial effects on glucose homeostasis.
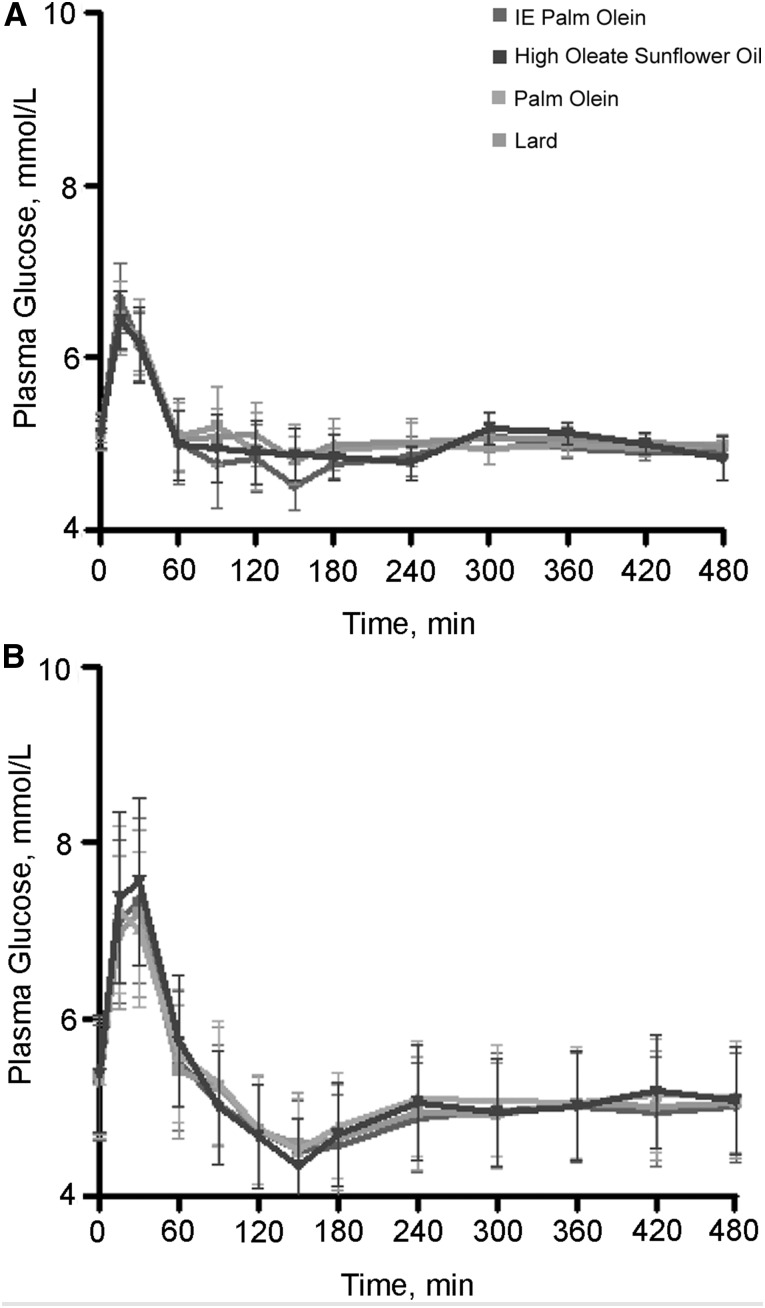
Sanders et al. found no differences in postprandial glucose or insulin when they compared interesterified palm olein, native palm olein, lard, and high-oleic sunflower oils, although the authors observed substantial sex differences (54). As seen with TGs, there were substantial sex differences in response. The increases in plasma insulin and C-peptide were greater, and those of glucose were lower in women than in men. However, plasma glucose–dependent insulinotropic polypeptide was significantly lower following the interesterified palm olein and lard meals than following the native palm olein and high-oleic sunflower meals; there were no differences in response between genders. The postprandial response to glucose in women was relatively flat, suggesting faster removal of glucose from plasma, with an iAUC that was 51% lower than that observed in men. Interesterified C16 fats did not differ from naturally occurring C16 fats with regard to postprandial effects on glucose homeostasis (Figure 4).
FIGURE 4.
Sex differences in postprandial glucose among 25 women and 25 men after high-fat meals rich in interesterified palm olein, native palm olein, lard, and high-oleic sunflower oils. Women (A) and men (B) consumed, in random order, 4 different high-fat meals (50 g) rich in interesterified palm olein, palm olein, lard, or high-oleic acid sunflower oil. The postprandial response to glucose in women was relatively flat, which suggests faster removal of glucose from plasma. Values are geometric means with 95% CIs. For changes from fasting for the meal × times interaction, P = 0.34 for both genders combined, P = 0.38 for women, and P = 0.39 for men. IE, interesterified. Data are from reference 54.
It is known that dietary lipid structure influences gut hormone release. MCTs, for example, suppress cholecystokinin release (55). However, there are no reports of how structured lipids might affect secretion of gut hormones that play important roles in insulin sensitivity and metabolic diseases (e.g., glucagon, insulin, GLP-1).
Longer-term effects of interesterified fats on fasting parameters
Fasting serum lipids and lipoproteins.
Several studies have been carried out to examine the longer-term (i.e., 18–42 d) metabolic effects of interesterified fats. With a few noteworthy exceptions (14), most data suggest only limited differences in the longer-term effects of saturated LCFA from native TG compared with interesterified fats on fasting serum lipids and lipoproteins (56, 57). After a 3-wk intervention period, Zock et al. (57) found no major differences between the effects of palm oil and enzymatically interesterified palm oil (Betapol) on serum lipid and lipoprotein concentrations in a group of 60 healthy men (n = 23) and women (n = 37). Men showed a slight elevation in serum total cholesterol and LDL cholesterol with interesterified palm oil (sn-2) when they consumed diets enriched with either fat (28% of total energy; 38% energy from all fats) for 3 wk in a crossover design. However, in a study in which 20% energy from fat was exchanged, Filippou et al. (53) found no differences between native palm olein and interesterified palm olein but found that LDL cholesterol was 10% higher on both interesterified and native palm olein compared with high-oleic sunflower oil when consumed by 41 Malaysian men (n = 10) and women (n = 31) for 6 wk by use of a crossover design.
Nestel et al. (58) drew a similar conclusion from a double-blind crossover trial of 27 hypercholesterolemic men consuming 3 margarines for 3-wk periods: 1) a high-linoleic acid, moderate TFA margarine; 2) a high-palm oil blend (predominantly lauric, myristic, palmitic, oleic, and linoleic acids, including fully hydrogenated palm kernel oil as hard stock); and 3) an interesterified form of the high-palm oil. Each diet was consumed for 3 wk by use of a crossover design. The study by Nestel et al. (58) in 27 hypercholesterolemic men showed that interesterification did not raise plasma cholesterol more than the high-palm oil margarine’s constituent FA, but the latter control fat was a novel stick margarine that contained as much sn-2–SFA as its interesterified version. Christophe et al. (59) also reported no effects on the serum lipoprotein profile in a 4-wk parallel study among 32 healthy men when the consumption of butter was compared with an enzymatically interesterified butter. This might be expected because the sn-2–SFA content of butterfat is so high that interesterification would not alter the sn-2–FA appreciably. On the basis of these studies, it was suggested that interesterification of 16:0-rich fats and oils does not adversely change fasting serum lipoprotein concentrations. However, evidence to the contrary exists from a trial in infants in which breast milk was compared with 2 formula fats (60): palm oil (sn-1,3–16:0) and enzymatic interesterified palm oil (Betapol; sn-2–16:0). The enzymatic interesterified palm oil resulted in an unbeneficial increased LDL cholesterol/HDL cholesterol ratio compared with palm oil, with unaltered total cholesterol. However, the increase observed with the interesterified formula was not as great as that seen with breast milk. A similar substantial, but less pronounced, shift in the LDL cholesterol/HDL cholesterol ratio was noted after the exchange in sn-2–18:1 from HOSO+ canola oil in trans-18:1 (partially hydrogenated vegetable oil) or interesterified 18:0 in 50 adult men in a carefully conducted crossover study with diet periods of 6 wk (61).
Grande et al. (11) found in 32 middle-aged men that natural cocoa butter (rich in stearic acid) and imitation cocoa butter (interesterified fat with the FA composition of cocoa butter) when consumed for 18 d had similar effects on serum total cholesterol. Effects on LDL cholesterol and HDL cholesterol were not reported at that time. Berry et al. (12) showed in 16 men comparable effects of stearic acid–rich native and interesterified shea butter on plasma total cholesterol and TG concentrations, but the test fats represented only one-third of the daily fat. In a 3-wk study with 30 men and 30 women with relatively low intake of test fats, Meijer and Westrate (10) found no evidence that interesterification of a margarine fat blend (36% coconut oil, 33% palm oil, 22% dry-fractionated palm oil-stearin, and 9% low-trans partially hydrogenated rapeseed oil) changed serum lipid concentrations when either was then blended as a 42:58 ratio with soybean oil, rendering the availability of sn-2 (18:2) extremely high for both margarines. Sundram et al. (1), like Nelson and Innis (60) with interesterified 16:0 and Judd et al. (62) with interesterified 18:0, found that a stearic acid–rich interesterified fat (12% of energy) increased LDL cholesterol, further suggesting that 18:0 may not be neutral when randomized to sn-2 or when it becomes a major SFA in the diet. Many of these details on interesterified fat and lipoproteins were previously reviewed by Hayes and Pronczuk (14).
Fasting inflammatory and hemostatic markers.
Most research on the effects of consuming interesterified fats has focused on postprandial lipids and glucose metabolism and, to a lesser extent, on the chronic effects of interesterification on lipoprotein profiles. Few studies have reported effects on markers of hemostasis and coagulation, either postprandially or over the long term. To date, no studies have reported the effects of interesterified fat intake on fasting inflammatory markers.
Kelly et al. (63) conducted a randomized crossover study with 13 men to determine the effects of diets enriched in either palmitic acid or stearic acid on hemostatic markers. The stearic acid test fats used to alter FA composition of the diet were prepared by interesterification of 100% hydrogenated canola oil and high-oleic sunflower oil, whereas the palmitic acid test fat was prepared by interesterification of palm stearin, palm olein, and high-oleic sunflower oil. Diets were fed over 4 wk (30% of energy from fat; ∼6.6% of energy as stearic acid in the stearic acid diet, and ∼7.8% of energy as palmitic acid in the palmitic acid diet). Fibrinogen, plasminogen, antithrombin III, and activated partial thromboplastin time were not altered by diet. FVII (percent activity) decreased between baseline and the final day of the interesterified stearic acid diet, whereas it was not altered by the palmitic acid interesterified test diet. There were no differences in factor VIII between the 2 interesterified treatments at the end of the test period.
Meijer and Westrate (10) conducted a double-blind crossover study with 30 men and 30 women to determine the effects of random chemical interesterification on blood lipids, enzymes, and hemostasis parameters. A blend of commonly used edible vegetable fats was compared with the same fat blend after random chemical interesterification. Both fat blends were supplied at relatively low energy levels (4% and 8%). At either energy level, the 2 fat blends were consumed for 3-wk periods, without an intermediate washout period. Of the 30 parameters studied, no statistically significant differences between the 2 fat blends were found.
Fasting glycemic control.
Some evidence is available on the effects of interesterified fats on glycemic control. An Australian case cohort study of 3700 women (64) assessed serum phospholipid FAs and dietary fat in relation to type 2 diabetes mellitus and found a 4-fold increase in diabetes risk for women with the highest 18:0 in their serum phospholipids, whereas the highest quintile for phospholipid 18:2 reduced the risk to ∼20% of the amount of the lowest quintile of 18:2. Although interesterified fat consumption was not available, most interesterified fats are interesterified with 18:0 FAs, which might alter the sn-2–FAs in phospholipids to favor stearic acid. In the above-described study by Sundram et al. (1), investigators found that the fasting 4-wk level of insulin was 22% lower after consumption of the interesterified fat than after the palm olein.
Conclusions and Future Directions
Interesterified fats have largely replaced PHOs in processed foods, and interest in and research on their longer-term effects on health continues to grow. The consensus among workshop participants was that interesterified fat production is a feasible and economically viable solution for replacing dietary TFAs. Functionality (melting point, oxidation stability mechanical strength, laminating ability, and shortening ability) is the limiting criterion for developing healthful interesterified fats that are commercially viable. However, a fat that performs well in food applications serves no practical purpose if it cannot readily be incorporated into affordable processed foods or if consumers will not accept it. This review summarizes the current available knowledge on many important issues related to interesterified fats, including the food science of interesterified fats, estimation of interesterified fats in the diet, lipid and glucose metabolism, inflammatory and postprandial responses, longer-term effects on fasting parameters, and inflammatory and hemostatic markers.
Although the above-described studies conducted on interesterified fats have not revealed any health issues, gaps in knowledge exist regarding the metabolic fate and potential health effects of longer-term consumption of interesterified fats. Outstanding questions must be answered regarding the effects of interesterification on modifying certain aspects of lipid and glucose metabolism, inflammatory responses, hemostatic parameters, and satiety. The workshop panel of experts concluded that the following areas warrant further investigation:
Effects of structured lipids on chylomicron size or apolipoprotein composition and metabolism
The fate of lipids from interesterified compared with physical blends of oils in mixed TGs, including the ability of sn-2–SFAs to incorporate in fasting HDL phospholipids and their impact on HDL-phospholipid structure and function
Effects of mixed compared with interesterified lipids on postprandial inflammatory markers in the intestine, plasma leukocytes, vascular endothelium, and adipose tissues in light of their various roles in metabolic diseases
Fate of LCFAs from native compared with interesterified TG presentations by methods to determine whether there are acute postprandial differences in tissues, fate, and/or metabolism that have not been apparent until now
Whether interesterified lipids may have local effects influencing inflammation
Effects of interesterified MCFA/intermediate chain FAs (e.g., C12–C14)
Direct comparisons of stearic-rich compared with palmitic-rich interesterified fats, with careful attention to absolute and relative abundance of the sn-2–FA, particularly 16:0, 18:0, 18:1, and 18:2 over a range of percentage energy intakes
Differential effects of interesterified fats between normal weight, overweight, and obese subjects
Influence of higher-melting-point fats produced by interesterification on the secretion of gut peptides involved in hormonal signaling in the postprandial period that play roles in insulin sensitivity and metabolic diseases (GLP, GLP-1)
Examination of the rate of gastric emptying after consuming fats with a high melt point, by use of ultrasound analysis of stomach volume, postprandially
Long-term effects of interesterified fats on markers of inflammation, hemostatic parameters, and satiety
In addition, because clinical studies with interesterified fats have generally been conducted by use of intake levels that exceed the likely actual intake, future studies should carefully consider interesterified fat load in both study design and interpretation of the results. Addressing these research gaps can shed light on the longer-term effects of interesterification on human health.
Acknowledgments
As Senior Science Program Manager at ILSI North America at the time of this work, P Courtney Gaine contributed significantly to this manuscript by managing the development and editorial stages of this article following the workshop held in 2012. Science Writer Denise Webb contributed to the development of early drafts of this article and received funds from the ILSI North America Technical Committee on Dietary Lipids. All authors read and approved the final version of the paper and take responsibility for all aspects of the paper.
Footnotes
Abbreviations: FVII, factor VII; GLP, glucagon-like peptide; iAUC, incremental AUC; LCFA, long-chain FA; MCFA, medium-chain FA; MCT, medium-chain triglyceride; PHO, partially hydrogenated oil; TFA, trans FA; UFA, unsaturated FA.
References
- 1.Sundram K, Karupaiah T, Hayes KC. Stearic acid-rich interesterified fat and trans-rich fat raise the LDL/HDL ratio and plasma glucose relative to palm olein in humans. Nutr Metab (Lond) 2007;4:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Micha R, Mozaffarian D. Trans fatty acids: effects on cardiometabolic health and implications for policy. Prostaglandins Leukot Essent Fatty Acids 2008;79:147–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Institute of Medicine Panel on Macronutrients, Institute of Medicine Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington (DC): National Academies Press; 2005. [Google Scholar]
- 4.US Food and Drug Administration. Food labeling; trans fatty acids in nutrition labeling; consumer research to consider nutrient content and health claims and possible footnote or disclosure statements; final rule and proposed rule. Fed Regist 2003;68:FR41433. [PubMed] [Google Scholar]
- 5.US Department of Agriculture and Department of Health and Human Services. Dietary Guidelines for Americans, 2010. Washington (DC): US Government Printing Office; 2011. [Google Scholar]
- 6.US Food and Drug Administration. Tentative determination regarding partially hydrogenated oils: request for comments and for scientific data and information. Fed Regist 2013;78:67169–75. [Google Scholar]
- 7.US Food and Drug Administration. Final determination regarding partially hydrogenated oils. Fed Regist 2015;80:34650–70. [PubMed] [Google Scholar]
- 8.Babayan VK. Medium chain triglycerides and structured lipids. Lipids 1987;22:417–20. [DOI] [PubMed] [Google Scholar]
- 9.St-Onge MP, Bosarge A. Weight-loss diet that includes consumption of medium-chain triacylglycerol oil leads to a greater rate of weight and fat mass loss than does olive oil. Am J Clin Nutr 2008;87:621–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meijer GW, Weststrate JA. Interesterification of fats in margarine: effect on blood lipids, blood enzymes, and hemostasis parameters. Eur J Clin Nutr 1997;51:527–34. [DOI] [PubMed] [Google Scholar]
- 11.Grande F, Anderson JT, Keys A. Comparison of effects of palmitic and stearic acids in the diet on serum cholesterol in man. Am J Clin Nutr 1970;23:1184–93. [DOI] [PubMed] [Google Scholar]
- 12.Berry SE, Miller GJ, Sanders TA. The solid fat content of stearic acid-rich fats determines their postprandial effects. Am J Clin Nutr 2007;85:1486–94. [DOI] [PubMed] [Google Scholar]
- 13.Finley JW, Leveille GA, Dixon RM, Walchak CG, Sourby JC, Smith RE, Francis KD, Otterburn MS. Clinical assessment of SALATRIM, a reduced-calorie triacylglycerol. J Agric Food Chem 1994;42:581–96. [Google Scholar]
- 14.Hayes KC, Pronczuk A. Replacing trans fat: the argument for palm oil with a cautionary note on interesterification. J Am Coll Nutr 2010;29:253S–84S. [DOI] [PubMed] [Google Scholar]
- 15.Kritchevsky D, Tepper SA, Chen SC, Meijer GW, Krauss RM. Cholesterol vehicle in experimental atherosclerosis: 23. Effects of specific synthetic triglycerides. Lipids 2000;35:621–5. [DOI] [PubMed] [Google Scholar]
- 16.Hayes KC. Synthetic and modified glycerides: effects on plasma lipids. Curr Opin Lipidol 2001;12:55–60. [DOI] [PubMed] [Google Scholar]
- 17.Rousseau D, Marangoni G. Chemical interesterification of food lipids: theory and practice. In: Akoh C, Min D, editors. Food lipids, chemistry, nutrition and biotechnology. New York: Marcel Dekker Inc.; 2002. p. 319–52. [Google Scholar]
- 18.Gibon V. Enzymatic interesterification of oils. Lipid Technol 2011;23:274–7. [Google Scholar]
- 19.US Department of Agriculture Agricultural Research Service. Nutrient intakes from food: mean amounts consumed per individual, one day, 2001–2002. Beltsville (MD): USDA ARS; 2005. [Google Scholar]
- 20.US Department of Agriculture Agricultural Research Service. Nutrient intakes from food: mean amounts consumed per individual, one day, 2003–2004. Beltsville (MD): USDA ARS; 2007. [Google Scholar]
- 21.US Department of Agriculture Agricultural Research Service. Nutrient intakes from food: mean amounts consumed per individual, one day, 2005–2006. Beltsville (MD): USDA ARS; 2008. [Google Scholar]
- 22.US Department of Agriculture Agricultural Research Service. Energy intakes: percentages of energy from protein, carbohydrate, fat, and alcohol, by gender and age, What We Eat in America, NHANES 2007–2008. Beltsville (MD): USDA ARS; ; 2010. [Google Scholar]
- 23.Doell D, Folmer D, Lee H, Honigfort M, Carberry S. Updated estimate of trans fat intake by the US population. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 2012;29:861–74. [DOI] [PubMed] [Google Scholar]
- 24.Kris-Etherton PM, Lefevre M, Mensink RP, Petersen B, Fleming J, Flickinger BD. Trans fatty acid intakes and food sources in the U.S. population: NHANES 1999–2002. Lipids 2012;47:931–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lefevre M, Mensink RP, Kris-Etherton PM, Petersen B, Smith K, Flickinger BD. Predicted changes in fatty acid intakes, plasma lipids, and cardiovascular disease risk following replacement of trans fatty acid-containing soybean oil with application-appropriate alternatives. Lipids 2012;47:951–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mu H, Porsgaard T. The metabolism of structured triacylglycerols. Prog Lipid Res 2005;44:430–48. [DOI] [PubMed] [Google Scholar]
- 27.Ikeda I, Tomari Y, Sugano M, Watanabe S, Nagata J. Lymphatic absorption of structured glycerolipids containing medium-chain fatty acids and linoleic acid, and their effect on cholesterol absorption in rats. Lipids 1991;26:369–73. [DOI] [PubMed] [Google Scholar]
- 28.Couëdelo L, Vaysse C, Vaique E, Guy A, Gosse I, Durand T, Pinet S, Cansell M, Combe N. The fraction of α-linolenic acid present in the sn-2 position of structured triacylglycerols decreases in lymph chylomicrons and plasma triacylglycerols during the course of lipid absorption in rats. J Nutr 2012;142:70–5. [DOI] [PubMed] [Google Scholar]
- 29.Tso P, Lee T, Demichele SJ. Lymphatic absorption of structured triglycerides vs. physical mix in a rat model of fat malabsorption. Am J Physiol 1999;277:G333–40. [DOI] [PubMed] [Google Scholar]
- 30.McKenna MC, Hubbard VS, Bieri JG. Linoleic acid absorption from lipid supplements in patients with cystic fibrosis with pancreatic insufficiency and in control subjects. J Pediatr Gastroenterol Nutr 1985;4:45–51. [DOI] [PubMed] [Google Scholar]
- 31.Teo TC, DeMichele SJ, Selleck KM, Babayan VK, Blackburn GL, Bistrian BR. Administration of structured lipid composed of MCT and fish oil reduces net protein catabolism in enterally fed burned rats. Ann Surg 1989;210:100–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kenler AS, Swails WS, Driscoll DF, DeMichele SJ, Daley B, Babineau TJ, Peterson MB, Bistrian BR. Early enteral feeding in postsurgical cancer patients: fish oil structured lipid-based polymeric formula versus a standard polymeric formula. Ann Surg 1996;223:316–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tso P, Lee T, DeMichele SJ. Randomized structured triglycerides increase lymphatic absorption of tocopherol and retinol compared with the equivalent physical mixture in a rat model of fat malabsorption. J Nutr 2001;131:2157–63. [DOI] [PubMed] [Google Scholar]
- 34.Holm R, Porter CJ, Edwards GA, Mullertz A, Kristensen HG, Charman WN. Examination of oral absorption and lymphatic transport of halofantrine in a triple-cannulated canine model after administration in self-microemulsifying drug delivery systems (SMEDDS) containing structured triglycerides. Eur J Pharm Sci 2003;20:91–7. [DOI] [PubMed] [Google Scholar]
- 35.Sommer Hartvigsen M, Mu H, Sorig Hougaard K, Lund SP, Xu X, Hoy CE. Influence of dietary triacylglycerol structure and level of n-3 fatty acids administered during development on brain phospholipids and memory and learning ability of rats. Ann Nutr Metab 2004;48:16–27. [DOI] [PubMed] [Google Scholar]
- 36.Kew S, Gibbons ES, Thies F, McNeill GP, Quinlan PT, Calder PC. The effect of feeding structured triacylglycerols enriched in eicosapentaenoic or docosahexaenoic acids on murine splenocyte fatty acid composition and leucocyte phagocytosis. Br J Nutr 2003;90:1071–80. [DOI] [PubMed] [Google Scholar]
- 37.Simoens C, Deckelbaum RJ, Carpentier YA. Metabolism of defined structured triglyceride particles compared to mixtures of medium and long chain triglycerides intravenously infused in dogs. Clin Nutr 2004;23:665–72. [DOI] [PubMed] [Google Scholar]
- 38.O’Keefe JH, Gheewala NM, O’Keefe JO. Dietary strategies for improving post-prandial glucose, lipids, inflammation, and cardiovascular health. J Am Coll Cardiol 2008;51:249–55. [DOI] [PubMed] [Google Scholar]
- 39.Versleijen M, Roelofs H, Preijers F, Roos D, Wanten G. Parenteral lipids modulate leukocyte phenotypes in whole blood, depending on their fatty acid composition. Clin Nutr 2005;24:822–9. [DOI] [PubMed] [Google Scholar]
- 40.Ajuwon KM, Spurlock ME. Palmitate activates the NF-κB transcription factor and induces IL-6 and TNFα expression in 3T3–L1 adipocytes. J Nutr 2005;135:1841–6. [DOI] [PubMed] [Google Scholar]
- 41.Gower RM, Wu H, Foster GA, Devaraj S, Jialal I, Ballantyne CM, Knowlton AA, Simon SI. CD11c/CD18 expression is upregulated on blood monocytes during hypertriglyceridemia and enhances adhesion to vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol 2011;31:160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tholstrup T, Miller GJ, Bysted A, Sandstrom B. Effect of individual dietary fatty acids on postprandial activation of blood coagulation factor VII and fibrinolysis in healthy young men. Am J Clin Nutr 2003;77:1125–32. [DOI] [PubMed] [Google Scholar]
- 43.Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med 1993;328:238–44. [DOI] [PubMed] [Google Scholar]
- 44.Risérus U, Willett WC, Hu FB. Dietary fats and prevention of type 2 diabetes. Prog Lipid Res 2009;48:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee B, Gai W, Laychock SG. Proteasomal activation mediates down-regulation of inositol 1,4,5-trisphosphate receptor and calcium mobilization in rat pancreatic islets. Endocrinology 2001;142:1744–51. [DOI] [PubMed] [Google Scholar]
- 46.Berry SE, Woodward R, Yeoh C, Miller GJ, Sanders TA. Effect of interesterification of palmitic acid-rich triacylglycerol on postprandial lipid and factor VII response. Lipids 2007;42:315–23. [DOI] [PubMed] [Google Scholar]
- 47.Sanders TA, Oakley FR, Cooper JA, Miller GJ. Influence of a stearic acid-rich structured triacylglycerol on postprandial lipemia, factor VII concentrations, and fibrinolytic activity in healthy subjects. Am J Clin Nutr 2001;73:715–21. [DOI] [PubMed] [Google Scholar]
- 48.Sanders TA, Berry SE, Miller GJ. Influence of triacylglycerol structure on the postprandial response of factor VII to stearic acid-rich fats. Am J Clin Nutr 2003;77:777–82. [DOI] [PubMed] [Google Scholar]
- 49.Sanders TA, Filippou A, Berry SE, Baumgartner S, Mensink RP. Palmitic acid in the sn-2 position of triacylglycerols acutely influences postprandial lipid metabolism. Am J Clin Nutr 2011;94:1433–41. [DOI] [PubMed] [Google Scholar]
- 50.Lin MT, Yeh SL, Tsou SS, Wang MY, Chen WJ. Effects of parenteral structured lipid emulsion on modulating the inflammatory response in rats undergoing a total gastrectomy. Nutrition 2009;25:115–21. [DOI] [PubMed] [Google Scholar]
- 51.Sanders TA, de Grassi T, Miller GJ, Morrissey JH. Influence of fatty acid chain length and cis/trans isomerization on postprandial lipemia and factor VII in healthy subjects (postprandial lipids and factor VII). Atherosclerosis 2000;149:413–20. [DOI] [PubMed] [Google Scholar]
- 52.Hall WL, Brito MF, Huang J, Wood LV, Filippou A, Sanders TA, Berry SE. An interesterified palm olein test meal decreases early-phase postprandial lipemia compared to palm olein: a randomized controlled trial. Lipids 2014;49:895–904. [DOI] [PubMed] [Google Scholar]
- 53.Filippou A, Teng KT, Berry SE, Sanders TA. Palmitic acid in the sn-2 position of dietary triacylglycerols does not affect insulin secretion or glucose homeostasis in healthy men and women. Eur J Clin Nutr 2014;68:1036–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Filippou A, Berry SE, Baumgartner S, Mensink RP, Sanders TA. Palmitic acid in the sn-2 position decreases glucose-dependent insulinotropic polypeptide secretion in healthy adults. Eur J Clin Nutr 2014;68:549–54. [DOI] [PubMed] [Google Scholar]
- 55.Costarelli V, Sanders TA. Acute effects of dietary fat composition on postprandial plasma bile acid and cholecystokinin concentrations in healthy premenopausal women. Br J Nutr 2001;86:471–7. [DOI] [PubMed] [Google Scholar]
- 56.Sala-Vila A, Castellote AI, Lopez-Sabater MC. The intramolecular position of docosahexaenoic acid in the triacylglycerol sources used for pediatric nutrition has a minimal effect on its metabolic use. Nutr Res 2008;28:131–6. [DOI] [PubMed] [Google Scholar]
- 57.Zock PL, de Vries JH, de Fouw NJ, Katan MB. Positional distribution of fatty acids in dietary triglycerides: effects on fasting blood lipoprotein concentrations in humans. Am J Clin Nutr 1995;61:48–55. [DOI] [PubMed] [Google Scholar]
- 58.Nestel PJ, Noakes M, Belling GB, McArthur R, Clifton PM. Effect on plasma lipids of interesterifying a mix of edible oils. Am J Clin Nutr 1995;62:950–5. [DOI] [PubMed] [Google Scholar]
- 59.Christophe AB, De Greyt WF, Delanghe JR, Huyghebaert AD. Substituting enzymatically interesterified butter for native butter has no effect on lipemia or lipoproteinemia in Man. Ann Nutr Metab 2000;44:61–7. [DOI] [PubMed] [Google Scholar]
- 60.Nelson CM, Innis SM. Plasma lipoprotein fatty acids are altered by the positional distribution of fatty acids in infant formula triacylglycerols and human milk. Am J Clin Nutr 1999;70:62–9. [DOI] [PubMed] [Google Scholar]
- 61.Judd JT, Baer DJ, Clevidence BA, Kris-Etherton P, Muesing RA, Iwane M. Dietary cis and trans monounsaturated and saturated FA and plasma lipids and lipoproteins in men. Lipids 2002;37:123–31. [DOI] [PubMed] [Google Scholar]
- 62.Judd JT, Baer DJ, Chen SC, Clevidence BA, Muesing RA, Kramer M, Meijer GW. Plant sterol esters lower plasma lipids and most carotenoids in mildly hypercholesterolemic adults. Lipids 2002;37:33–42. [DOI] [PubMed] [Google Scholar]
- 63.Kelly FD, Sinclair AJ, Mann NJ, Turner AH, Abedin L, Li D. A stearic acid-rich diet improves thrombogenic and atherogenic risk factor profiles in healthy males. Eur J Clin Nutr 2001;55:88–96. [DOI] [PubMed] [Google Scholar]
- 64.Hodge AM, English DR, O’Dea K, Sinclair AJ, Makrides M, Gibson RA, Giles GG. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 2007;86:189–97. [DOI] [PubMed] [Google Scholar]