Abstract
Various studies have examined associations between maternal vitamin D (VD) deficiency and offspring health, including offspring brain health. The purpose of this review was to summarize current evidence concerning the impact of maternal VD deficiency on brain development and function in offspring. A systematic search was conducted within Medline (on Ovid) for studies published through 7 May 2015. Animal and human studies that examined associations between maternal VD status or developmental VD deficiency and offspring brain development and function were included. A total of 26 animal studies and 10 human studies met the inclusion criteria. Several animal studies confirmed the hypothesis that low prenatal VD status may affect brain morphology and physiology as well as behavioral outcomes. In humans, subtle cognitive and psychological impairments in offspring of VD-deficient mothers were observed. However, data obtained from animal and human studies provide inconclusive evidence, and results seem to depend on strain or race and age of offspring. To conclude, prenatal VD status is thought to play an important role in brain development, cognitive function, and psychological function. However, results are inconclusive; validation of these findings and investigation of underlying mechanisms are required. Thus, more investigation is needed before recommending supplementation of VD during pregnancy to promote brain health of offspring.
Keywords: maternal, prenatal, developmental, vitamin D, 25(OH)D, brain, cognition, neuropsychological
Introduction
Over the last decades, the role of maternal nutrient status in fetal development has generated considerable research interest. Many reports worldwide have demonstrated high prevalences of vitamin D (VD)4 deficiency in pregnant women (1). VD diffuses across the placenta from mother to fetus; hence, the mother is the sole source of VD substrate for her developing child. It has been shown that in cases of maternal VD deficiency, defined as serum 25-hydroxyvitamin D [25(OH)D] status <50 nmol/L (2), the fetus is also deficient (3).
Discoveries have revealed that many tissues and cells in the body express VD receptors (VDRs) (4, 5) and that both placenta and embryonic kidneys exhibit an enzymatic machinery, which converts 25(OH)D, the inactive VD metabolite, into 1,25-dihydroxycholecalciferol [1,25(OH)2D3], the metabolically active VD metabolite (6). These discoveries have provided new insights into the function of VD. Low VD status during pregnancy has, for instance, been associated with rickets and growth retardation (1, 2) as well as various adverse extra-skeletal outcomes, including type 2 diabetes mellitus and inflammatory disorders in offspring (1, 7, 8). Furthermore, research has suggested that low maternal VD status may affect neuronal development and could result in the onset of various mental illnesses like schizophrenia and autism (1, 7, 8). Therefore, the purpose of this systematic review is to provide a brief overview of current evidence on the impact of maternal VD deficiency on brain development and function and to identify knowledge gaps warranting further research. Two topics will be discussed: 1) animal studies focusing on biological effects of developmental VD (DVD) deficiency on brain development and function and 2) human studies examining the impact of maternal 25(OH)D status on both offspring neurocognitive function and psychological health.
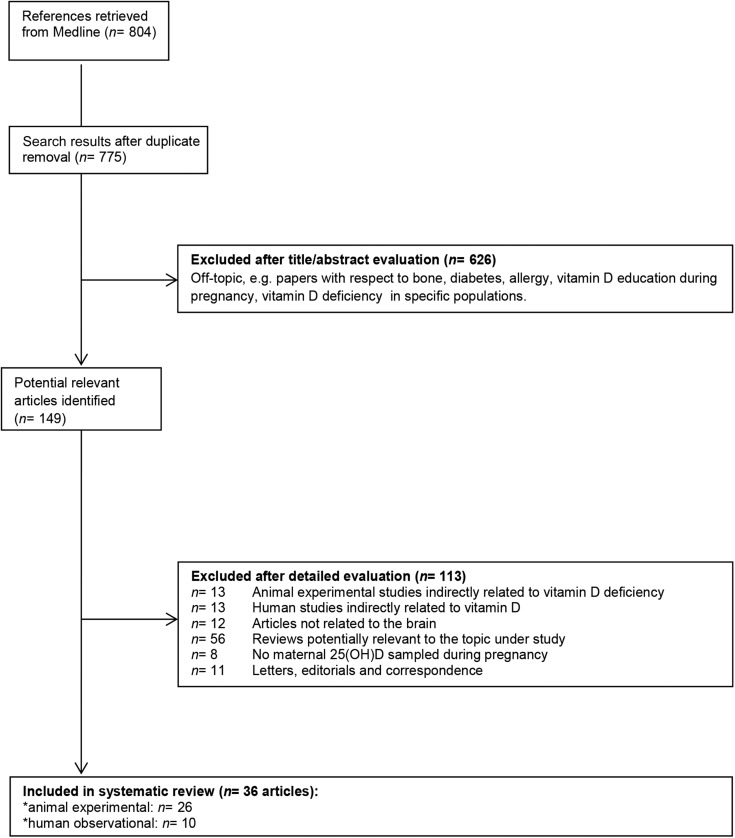
To achieve comprehensive retrieval of relevant articles, a systematic search within Medline (on Ovid) was conducted until 7 May 2015, without time or language limits. Studies investigating potential relations between maternal VD status and offspring brain function and development were systematically reviewed. Gray literature and conference proceedings were not searched. The search string was designed to include search terms on maternal VD intake and status and offspring brain function and development (Table 1). Because no search strategy can guarantee completeness, additional hand searches were conducted to identify studies that were not retrieved by the systematic search in Medline. The selection process started with a title and abstract screening based on inclusion and exclusion criteria; articles identified as potentially relevant were ordered as full text. During full-text screening, articles were included if they met both of the following criteria: 1) providing data on DVD deficiency or maternal 25(OH)D status during gestation obtained before or at delivery and 2) providing data on offspring brain development and/or function. Studies were excluded if 1) they were reviews, case reports, letters, editorials, or correspondence; 2) blood samples to determine maternal 25(OH)D status were obtained after delivery; 3) the exposure was indirectly related to VD such as season of birth or latitude; 4) the associations between prenatal 25(OH)D status and brain development, function, and/or behavior were not explored in the study; and 5) VD mutant or VDR knockout model was used rather than maternal VD–deficient model. In total, 36 articles met the inclusion criteria of this review (Figure 1).
TABLE 1.
Search strategy
| Search no. | Ovid Medline: 7 May 2015 | Search results |
| 1 | exp vitamin d/ or exp vitamin d deficiency/ | 55,674 |
| 2 | (vitamin d or vitamin d2 or vitamin d3 or vitamin-d or vitamin-d2 or vitamin-d3 or vitamin d 2 or vitamin d 3 or ergocalciferol or colecalciferol or cholecalciferol or calciol or calcifediol or calcidiol or calcitriol or 25 hydroxycholecalciferol or 25-hydroxycholecalciferol or 25 hydroxyvitamin D or 25 hydroxyvitamin D2 or 25 hydroxyvitamin D3 or 25-hydroxyvitamin D or 25-hydroxy-vitamin D2 or 25-hydroxy-vitamin D3 or 25OHD or 25-OH-vitamin D or 25-OHD or S-25-OHD or 1,25 dihydroxycholecalciferol or 1,25-dihydroxycholecalciferol or 1,25 dihydroxyvitamin D or 1,25 dihydroxyvitamin D3 or 1,25-dihydroxyvitamin D or 1,25-dihydroxyvitamin D3 or 1,25-dihydroxy-vitamin D or 1,25-dihydroxy-vitamin D3 or 1,25OHD or 1,25-OH-vitamin D or 1,25-OHD or S-1,25-OHD).ti,ab. | 54,154 |
| 3 | Search 1 or 2 | 72,902 |
| 4 | exp maternal nutritional physiological phenomena/ or exp maternal exposure/ or exp pregnancy/ or exp prenatal care/ or exp preconception care/ or exp embryology/ or exp embryonic structures/ or exp embryonic development/ or exp fetal development/ or exp prenatal exposure delayed effects/or exp perinatal care/ | 1,018,871 |
| 5 | (peri-conception or periconception or periconceptional or peri-conceptional or maternal or intrauterine or intra-uterine or gestation or gestational or pregnancy or pregnant or conception or preconception or pre-conception or early life or early-life or fetal or fetus or fetus or fetal or embryonic or embryo or prenatal or perinatal).ti,ab. | 900,584 |
| 6 | Search 4 or 5 | 1,361,051 |
| 7 | Searches 3 and 6 | 4987 |
| 8 | exp brain/ or exp neuroimaging/ or exp mental disorders/ or exp neurologic manifestation/ or exp mental competency/ or exp mental health/ or exp mental processes/ or exp psychomotor performance/ or exp psychophysiology/ or exp behavior control/ or exp behavioral sciences/ or exp psychological tests/ or exp psychological techniques/ or exp psychiatric status rating scales/ or exp psychological adaptation/ or exp attitude/ or exp behavior/ or exp defense mechanisms/ or exp emotions/ or exp mental competency/ or exp motivation/ or exp neurobehavioral manifestations/ | 4,205,228 |
| 9 | (brain or neurocognitive or neurocognition or memory or attention or information processing or executive function or cognition or cognitive or reactivity or emotions or MRI or neuroimaging or mental or neurological or neurologic or behavior or behavior or behavioral or behavioral or neurodevelopment or neurodevelopmental or neuroprotective or neuroprotection or psychosocial or neurologic or psychological or psychologic).ti,ab. | 2,510,335 |
| 10 | Search 8 or 9 | 5,375,024 |
| 11 | Searches 7 and 10 | 804 |
FIGURE 1.
Flowchart of the selection process.
Current Status of Knowledge
Vitamin D and brain development and function: what we know from animal studies
VD has been suggested to affect numerous endocrine functions, such as the regulation of serum calcium and phosphorus concentrations, as well as health outcomes, like bone health, muscle function, and type 2 diabetes (reviewed in 1, 2). Furthermore, VD has been proposed to influence brain processes (9–11). Animal studies and in vitro studies have substantially contributed to our understanding concerning the role of VD in brain development and function. In this section, data resulting from animal studies examining the impact of low maternal 25(OH)D status on fetal brain and offspring brain development, function, and behavior are summarized.
Vitamin D and fetal brain development in animals
Most animal studies investigating the effect of maternal VD depletion on brain development and function used the DVD deficiency model as described by Eyles et al. (12). In this DVD deficiency model, female Sprague-Dawley rats were fed a VD-deficient diet from ~6 wk before conception until birth. As a consequence, the developing fetus was exposed to hypovitaminosis D during gestation. When a DVD deficiency model is discussed in this review, it refers to the model as described by Eyles et al. (12), unless stated otherwise.
DVD and alterations in brain morphology, physiology, and gene expression in rat models.
Data resulting from the DVD model have shown that offspring of VD-deficient mothers exhibit differences in brain morphology, physiology, and gene expression (Table 2). To illustrate this, neonatal offspring of VD-deficient Sprague-Dawley rats were reported to have longer and thinner cerebral hemispheres in comparison to offspring of normal fed rats (12). Furthermore, cell proliferation and the number of mitotic cells were significantly higher and the number of differentiating cells significantly lower throughout the VD-deficient neonatal brain (12, 14, 22). In addition, in a study by Ko et al. (22), a subtle decline in the number of apoptotic cells in both embryos and pups from VD-deficient rats was observed. This decline was most pronounced at birth (embryonic day 23), suggesting an age-dependent alteration in brain apoptotic activity.
TABLE 2.
DVD deficiency and offspring brain development in rodents1
| Article (y) (ref) | Species | Strain | Gestational period of VD deficiency (animals per group) | Age of testing2 | Method | Outcome of DVD-deficient vs. DVD adequate rodents |
| Almeras et al. (2007) (13) | Rats | Sprague-Dawley (♀/♂) | From preconception until birth (n = 4) | 10 | Silver staining; Western blots | Altered expression of 36 genes and proteins involved in mitochondrial, cytoskeletal and synaptic plasticity |
| Cui et al. (2007) (14) | Rats | Sprague-Dawley (♀/♂) | From preconception until birth (n = 6) | Day of birth, ±E23 | Immunohistochemistry | ↑ Neurospheres in SVZ |
| Cui et al. (2010) (15) | Rats | Sprague-Dawley (♀/♂) | From preconception until birth at E12 or E15 (n = 10) | E12 or E15 | Real-time PCR | ↓ Nurr1 at E12,15; ↓ P57Kip2 at E12 |
| Eyles et al. (2003) (12) | Rats | Sprague-Dawley (♀/♂) | From preconception until birth (n = 11–14) | Day of birth | Histology; immunohistochemistry; brain morphology | ↑ Cell proliferation; ↑ mitotic cells; ↓ NGF; ↓ GDNF; ↓ P75ntr expression; cortex longer and thinner; enlarged lateral ventricles |
| Eyles et al. (2007) (16) | Rats | Sprague-Dawley (♀/♂) | From preconception until birth (n = 8) | 10 | Affymetrix gene microarrays; computational analysis | Dysregulation in oxidative phosphorylation, redox balance, cytoskeleton maintenance, calcium homeostasis, chaperoning, post-translational modifications, synaptic plasticity, and neurotransmission |
| Eyles et al. (2014) (5) | Rats | Sprague-Dawley (♀/♂) | From preconception until birth at E18 (n = 3) | E18 | Western blots | No difference in subcellular Vdr distribution in the brain |
| Féron et al. (2005) (17) | Rats | Sprague-Dawley (♀/♂) | From preconception until birth or weaning (n = 10) | 10 | Brain morphology; semiquantitative real-time PCR; Brandfort assay, ELISA | ↑ Lateral ventricle volume; ↓ GABA-Aα4; ↓MAP-2; ↓NF-L and ↓NGF |
| Grecksch et al. (2009) (18) | Rats | Sprague-Dawley (♀/♂) | From preconception until birth (n = 6–13) | ND | Electrophysiology | ↑ LTP both after weak and strong tetanic stimulation |
| Hawes et al. (2015) (19) | Mice | BALB/c (♀/♂) | From preconception until birth at E14.5 or 17.5 (n = 6–8) | E14.5 or E17.5 | Parrafin histology; hemaotoxylin/eosin staining; immunohistohemistry; real-time PCR | ↓ Lateral ventricle volume; Tgf-β1 unchanged at E14.5, increase at E17.5, ♀ 2.2-fold, ♂ 1.5-fold;Bdnf reduced at E14.5, increased at E17.5, ♀ 4.5-fold, ♂ 1.5-fold; Foxp2 reduced at E14.5, increased at E17.5, ♀ 2.4-fold, ♂ 1.5-fold; Th unchanged at E14.5, decreased expression and localization at E17.5 in ♀ |
| Keilhoff et al. (2010) (20) | Rats | Sprague-Dawley (♂) | From preconception until birth (n = 10) | 10 | Immunohistochemistry | ↓ Cell proliferation/neurogenesis in the hippocampal DG |
| Kesby et al. (2009) (21) | Rats | Sprague-Dawley (♀/♂) | From preconception until birth (n = 7–8) | Day of birth, ±E23 | Real-time PCR | ↓ DOPAC; ↓HVA; ↓Comt in the forebrain |
| Ko et al. (2004) (22) | Rats | Sprague-Dawley (♀/♂) | From preconception until birth at E19, E21, or E23 (n = 5) | E19, E21, E23, or P7 | Immunohistochemistry; GEArray | ↓ Apoptotic cells, most pronounced at birth; ↑ mitotic cells, no particular period |
Bdnf, brain-derived neurotrophic factor; Comt, catechol-O-methyltransferase; DG, dentate gyrus; DOPAC, dihydroxyphenylacetic acid; DVD, developmental vitamin D; E, embryonic day; Foxp2, forkhead box protein P2; GABA-Aα4, gamma-aminobutyric acid A; GDNF, glial cell line neurotrophic factor; HVA, homovanillic acid; LTP, long-term potential; MAP-2, microtubule-associated protein 2; ND, not described; NF-L, neurofilament; NGF, nerve growth factor; Nurr1, nuclear receptor related 1 protein; P, postnatal day; P57Kip2, cyclin-dependent kinase inhibitor 1C; P75ntr, p75 neurotrophin receptor; ref, reference; SVZ, subventricular zone; Tgf-β1, transforming growth factor β1; Th, tyrosine hydroxylase; VD, vitamin D; Vdr, vitamin D receptor; ↓, decrease; ↑, increase; ♀, female; ♂, male.
In weeks, unless otherwise noted.
Low prenatal 1,25(OH)2D3 status has also been shown to affect neurotrophin signaling through its effect on the synthesis of nerve growth factor (NGF) and glial cell line neurotrophic factor, and expression of neurotrophin receptor p75 (12). Low fetal 1,25(OH)2D3 status was not related to other neurotrophin receptors, Vdr expression, or the neurons:glia ratio (12). DVD deficiency has also been related to larger ventricles, reduced NGF synthesis, decreased expression of genes involved in neuronal structure (17), and decreased cell proliferation (20).
Experimental studies in rats have also suggested a role for VD in dopaminergic systems, which may be of clinical relevance for certain disorders that are associated with abnormal dopaminergic signaling such as schizophrenia (23, 24), Parkinson disease (25), depression (26), and autism (27). For instance, VD deficiency has been shown to alter gene expression of factors such as nuclear receptor related 1 protein and cyclin-dependent kinase inhibitor 1C, which are involved in the dopaminergic development in the embryonic midbrain (15). Changes in dopaminergic metabolic profile were also observed in offspring forebrain, showing a decreased dihydroxyphenylacetic acid:homovanillic acid ratio as well as catechol-O-methyl transferase expression (21).
With the use of Affymetrix gene microarrays, prenatal hypovitaminosis D has been linked to multiple alterations in gene and protein expression patterns involved in neuronal structure later in life. Specifically, DVD deficiency has been shown to affect the expression of 36 protein molecules that are involved in numerous biological pathways in offspring rat brain, including oxidative phosphorylation, synaptic plasticity, and neurotransmission. With the use of computational analyses these impairments were subsequently associated with the pathogenesis of several neurodevelopmental and psychiatric disorders like schizophrenia and multiple sclerosis (13, 16). DVD deficiency did not affect subcellular Vdr distribution in Sprague-Dawley rats (5).
DVD and alterations in brain morphology and physiology in mice.
A study in prenatal VD-deficient BALB/c mice pointed toward a reduction in lateral ventricle volume and altered expression of genes involved in neuronal survival, specifically brain-derived neurotrophic factor (Bdnf) and transforming growth factor-β1 (Tgf-β1), and speech and language development, specifically forkhead box protein P2 (Foxp2). In DVD-deficient female fetuses dopamine synthesis was also affected, when both brain thyrosine hydroxylase (Th) gene expression and Th protein localization were reduced (19).
Behavior in DVD-deficient animals
The aforementioned studies suggest that prenatal VD deficiency affects brain development; this may affect offspring behavior. Several studies have associated DVD deficiency with offspring behavioral (dys)function, including developmental milestones, locomotion, exploration, anxiety, learning, memory, and sensorimotor gating (Table 3). The influence of prenatal VD deficiency on offspring behavior both in early life and adulthood has predominantly been examined in rats. Most of these animal studies aimed to unravel effects of VD deficiency on developmental disorders such as schizophrenia (30, 33, 38, 39) and autism (12, 32).
TABLE 3.
DVD deficiency and offspring behavior in rodents1
| Article (y) (ref) | Species | Strain | Period of VD deficiency (animals per group) | Age of testing2 | Method | Outcome |
| Becker et al. (2005) (28) | Rats | Sprague-Dawley (♂) | During preconception until birth (n = 8) | 10 | Holeboard test; brightness discrimination in a Y-chamber; avoidance learning in a shuttle-box and radial maze | DVD deficiency was related to reduced habituation in the holeboard test and better maintenance of previously learned rules in a Y-chamber. No effect on radial maze or shuttle-box performance was observed. |
| Burne et al. 2004) (29) | Rats | Sprague-Dawley (♀/♂) | During preconception until birth or until weaning, or remained until 10 wk of age (n = 9–23) | 5, 10 | PPI of ASR | The combination of prenatal and chronic postnatal VD deficiency resulted in an significantly impaired PPI, despite normal ASR, at 10 wk. |
| Burne et al. (2004) (30) | Rats | Sprague-Dawley (♀/♂) | During preconception until birth or until weaning, or remained until 10 wk of age (n = 23–30) | 10 | Holeboard test; elevated plus maze test; ASR test; forced swim test; PPI of ASR; social interaction observation | DVD deficiency increased locomotion in holeboard test and increased activity in the elevated plus maze. No effects on other outcome measures were observed. |
| Burne et al. (2006) (31) | Rats | Sprague-Dawley (♀/♂) | During preconception until birth (n = 14) | 8 | Open field test | DVD deficiency enhanced locomotion in open field test. DVD rats spent significantly less time in corners and more time on the side compared with DVD-adequate rats. |
| Burne et al. (2011) (32) | Rats | Sprague-Dawley (♀/♂) | During preconception until birth (n = 24) | 12–183 | Day of birth pup-retrieval test; isolation-induced USV | VD-deficient dams showed more pup-directed activities and less time was needed to retrieve their pups. Maternal diet did not affect the calling rate of isolation-induced ultrasonic vocalizations by pups. |
| Burne et al. (2014) (33) | Rats | Sprague-Dawley (♀/♂) | During preconception until birth (n = 6–12) | 63 | Injection of 2.5 mg THC or vehicle/kg before PPI of ASR or open field test; injection of 0–2.5 mg THC or vehicle/kg 15 min before DMTS | DVD deficiency enhanced the PPI in DVD-deficient animals after THC injection. DVD- deficiency was not shown to affect other outcome measures under investigation. |
| Eyles et al. (2006) (34) | Rats | Sprague-Dawley (♂) | During preconception until birth (n = 5) | 10 | Open field test | DVD deficiency resulted in a significantly increased activity pattern, more specifically, distance traveled and rearing. |
| Fernandes de Abreu et al. (2010) (35) | Mice | C57BL/6J (♂) | During preconception until birth (n = 8–12) | 30, 60, 70 | MRI; olfactory tubing maze | Compared with DVD-adequate mice, DVD-deficient mice showed significantly impaired learning abilities at 30-wk-old and reduced ventricle volumes. |
| Harms et al. (2008) (36) | Mice | 129/SvJ, C57BL/6J (♀/♂) | During preconception until birth (n = 22–35) | 10 | Holeboard test; open field test; elevated plus maze; forced swim test; PPI of ASR; SHIRPA screening; social interaction test | DVD-deficient 129/SvJ and C57BL/6J mice showed significantly higher levels of exploration than did DVD-adequate mice. In addition, 129/SvJ showed significantly higher levels of spontaneous locomotion than did DVD-adequate mice. No effects with respect to the other outcome measures were observed. |
| Harms et al. (2012) (37) | Mice | 129/X1Sv, C57BL/6J (♀/♂) | During preconception until birth (n = 6–11) | P0, 70 | MRI; open field test, injection of saline, MK-801, or amphetamine | DVD-deficient C57BL/6J females had a reduced hippocampal volume. DVD-deficient C57BL/6J males had a lower lateral ventricle volume and increased striatum volume. No DVD-deficiency effect was shown for any of the other outcome measures under investigation. |
| Kesby et al. (2006) (38) | Rats | Sprague-Dawley (♂) | During preconception until birth (n = 9–124) | 10 | Holeboard test open field test, injection of MK-801 and/or haloperidol; PPI, injection of apomorpine or MK-801 | DVD deficiency was related to an increased baseline locomotion on the holeboard test and in response to MK-801. Haloperidol antagonized this effect. No DVD-deficiency effect on baseline or drug-mediated PPI was observed. |
| O’Loan et al. (2007) (39) | Rats | Sprague-Dawley (♂) | Early, 4 wk before conception VD deplete, thereafter replete; late, conception to birth VD deplete; full, 6 wk before conception until birth VD deplete (n = 123–134) | P7, 14, 21, 70 | Developmental milestones; open field test, injection of MK-801 | No effects of DVD deficiency on developmental milestones were observed. Full and late VD deficiency resulted in MK-801-induced hyperlocomotion. |
| Pan et al. (2014) (40) | Rats | Sprague-Dawley (♀/♂) | From mating to weaning, range 0–10.0 IU VD/g (n = 10–12) | P35–40, 100–105 | Elevated plus maze; locomotor activity box; social behavior and learning | In juveniles, DVD deficiency was related to enhanced anxiety and reduced locomotion. DVD deficiency also affected social behavior and learning in both juveniles and adults. |
| Turner et al. (2013) (41) | Rats | Sprague-Dawley (♀/♂) | During preconception until birth (n = 6–8) | 20 | 5-CPT; 5-CRT | DVD-deficient rats exhibited increased impulsivity and a lack of inhibitory control. DVD deficiency did not affect accuracy. |
ASR, acoustic startle response; DMTS, delay match to sample test; DVD, developmental vitamin D; P, postnatal day; PPI, prepulse inhibition; ref, reference; SHIRPA, SmithKline Beecham, Harwell, Imperial College, and Royal London Hospital phenotype assessment; THC, tetrahydrocannabinol; USV, ultrasonic vocalization; VD, vitamin D; 5-CPT, 5-choice continuous performance test; 5-CRT, 5-choice serial reaction time test; ♀, female; ♂, male.
In weeks, unless otherwise noted.
In months.
Developmental milestones.
O’Loan et al. (39) investigated the effect of prenatal VD deficiency on developmental milestones in Sprague-Dawley rats. The offspring was exposed to 1 of 4 prenatal VD conditions: 1) normal VD status; 2) VD depletion starting 4 to 6 wk preconception up to conception; 3) VD depletion from conception until birth; and 4) VD depletion starting 4 to 6 wk preconception until birth. No significant differences in developmental milestones, including eye/ear opening, ear unfolding, fur growth, upper and lower teeth protrusion, self-righting reflex, posture, and activity, were observed between treatment groups.
Locomotion, exploration, and anxiety in rats.
Other studies did, however, point toward behavioral differences triggered by DVD deficiency. For example, DVD deficiency has been related to novelty-induced hyperlocomotion in adult rats, even when pups were fed a VD-replete diet from birth or weaning onwards (30, 31, 34, 38). O’Loan et al. showed that this novelty-induced hyperlocomotion was most pronounced in rats that were DVD deficient from conception until birth (39), suggesting that there might be a critical window for prenatal VD effects on offspring brain development. DVD deficiency from mating to offspring weaning has also been associated with a more anxious and less social phenotype in juvenile Sprague-Dawley rats. Whereas effects related to social behaviors were still present in adulthood, differences pertaining to anxiety-like behaviors did not persist into adulthood (40).
Learning and memory in rats.
Maternal VD deficiency has also been suggested to play a role in offspring learning capacities and memory function. For example, maternal VD deficiency in Sprague-Dawley offspring has been related to subtle learning and memory effects, as shown by a disruption in latent inhibition, reduced habituation, and superior relearning skills, whereas memory acquisition as well as memory retrieval remained unaffected (28). In addition, offspring of VD-deficient dams showed an increased impulsivity and a lack of control of inhibitory behavior in adulthood compared with control animals, as measured using a 5-choice continuous performance test (41). Unexpectedly, maternal VD deficiency was also associated with improved retention performance in the brightness discrimination in a Y-maze (28) and enhancement of long-term potentiation (18).
Sensorimotor gating.
Burne et al. (29) investigated the impact of both pre- and postnatal VD deficiency on prepulse inhibition (PPI) of the acoustic startle response (ASR) in 5- and 10-wk-old rats. Although 5-wk tests did not suggest differences between treatment groups, impaired PPI—without dysregulation of the ASR itself—was observed in 10-wk-old rats receiving combined pre- and chronic postnatal VD deficiency. No significant impairments were observed in animals exposed to VD depletion during pregnancy or only until weaning. In contrast, another study by Burne et al. (30) did not reveal any significant influence of DVD deficiency on the PPI. Discrepancies between these studies may relate to low calcium levels, which were reported in the former study but not found in the latter approach. Possibly, hypocalcemia instead of pre- and postnatal DVD deficiency impairs PPI.
Behavioral studies in mice.
Harms et al. (36) investigated the effect of hypovitaminosis D on the phenotype of 129/SvJ and C57BL/6J mice in 10-wk-old offspring using a behavioral test battery. DVD-deficient mice of both strains had higher levels of exploratory behavior than did DVD-adequate mice. In 129/SvJ mice locomotion was also increased, but sensorimotor gating and social behavior were unaffected. No differences were observed for simple behavioral and morphological characteristics as assessed by SHIRPA (SmithKline Beecham, Harwell, Imperial College, and Royal London Hospital phenotype assessment) primary screen, nor for anxiety or depressive behavior. These findings were in line with another study of Harms et al. (37) in which brains of both C57BL/6J and 129/X1SvJ mice were imaged using MRI at postnatal day 0 (neonatal) and postnatal day 70 (adulthood), and locomotion sensitivity to psychotomimetic drugs (amphetamine and MK-801) was studied using an open field test. When DVD deficiency was related to reduced hippocampal volume in female neonatal C57BL/6J mice, adult DVD-deficient males had lower lateral ventricle volumes when compared with control animals. No DVD-deficiency effects were observed in 129/X1SvJ mice, nor was there a difference in behavioral phenotype in both strains. Fernandes de Abreu et al. (35) investigated the impact of prenatal VD deficiency on learning abilities in C57BL/6J mice. Offspring of VD-deficient mothers underwent an associative hippocampal dependent memory test at 30 and 60 wk old, and MRI at 30 and 70 wk old. Results showed problems with learning as well as smaller lateral ventricles at 30 wk. However, none of these alternations were observed during the follow-up measurement at 60 and 70 wk.
Vitamin D and brain development and function: what we know from human studies
Based on animal studies, VD deficiency has been proposed to contribute to brain development and function in humans. The aim of this section is to provide an overview of available human data on the potential relation between VD- and brain-related outcomes. The systematic literature search resulted in 10 human observational studies that dealt with maternal 25(OH)D serum status during pregnancy and offspring neurocognitive and psychological outcomes. Characteristics of the included studies are summarized in Table 4. No intervention studies have yet been published.
TABLE 4.
Cohort and case–control studies on low maternal 25(OH)D status and offspring neurocognitive and psychological outcomes1
| Article (y) (ref) | Type of study | Location | Participants | Maternal25(OH)Ddetermination | Mean 25(OH)D | Method | Main findings |
| Allen et al. (2013) (42) | Prospective cohort | Australia (RAINE) | 929 mother-child pairs | 18-wk gestation | Maternal serum: 60 nmol/L | C-EDE and EDE-Q in ♀ offspring (age 20 y) | Female offspring exposed to the lowest prenatal 25(OH)D concentrations (<46 nmol/L) had a significantly higher ED risk at age 20 y than those exposed to the highest prenatal 25(OH)D concentrations [>71 nmol/L, reference; adjusted OR for lowest 25(OH)D quartile: 2.09; 95% CI: 1.03, 5.27]. |
| Gale et al. (2008) (43) | Prospective cohort | United Kingdom | 596 mother-child pairs | 28–42 wk gestation | Maternal serum: 50 nmol/L | WAIS; SDQ (age 9 y) | No associations were observed between maternal 25(OH)D concentrations and child’s intelligence or psychological health. |
| Hanieh et al. (2014) (44) | Prospective cohort | Vietnam | 960 mother-child pairs | 32-wk gestation | Maternal serum: 70 nmol/L | BSID-III (age 6 mo) | Infants exposed to the lowest prenatal 25(OH)D concentrations (<38 nmol/L) had lower language scores than those exposed to 25(OH)D concentrations (≥75 nmol/L, reference; adjusted estimated mean difference: −3.48; 95% CI: −5.52,1.44). Prenatal 25(OH)D status was not associated with cognitive, motor, or socio-emotional scores. |
| Keim et al. (2014) (45) | Prospective cohort | United States | 3896 mother-child pairs | ≤26-wk gestation | Maternal serum: 45 nmol/L Cord serum: 32 nmol/L | WISC (age 7 y); BSID (age 8 mo); behavior (ages 4 and 7 y), SBIC (age 4 y); WRAT (age 7 y) | Lower maternal/cord 25(OH)D concentrations were associated with lower WISC intelligence quotient scores [adjusted β for 5-nmol/L increment in maternal 25(OH)D concentration: 0.10; 95% CI: 0.00, 0.19; adjusted β for cord blood 25(OH)D concentrations: 0.16; 95%CI: 0.03, 0.19]. No associations were observed for the other outcome measures under study. |
| McGrath et al. (2003) (46) | Case-control | United States | 26 cases 51 race, sex, and date of birth matched controls | 2nd trimester | Maternal serum: 47 nmol/L | DSM-IV criteria schizophrenia/schizoaffective disorder | Overall, no association was observed between maternal 25(OH)D status and schizophrenia risk. In a subgroup of black individuals, however, a nonsignificant inverse association between maternal 25(OH)D concentrations and psychosis risk was observed: adjusted OR: 0.78; 95% CI: 0.55,1.08. |
| Morales et al. (2012) (47) | Prospective cohort | Spain | 1820 mother-child pairs | 2nd trimester | Maternal plasma: 75 nmol/L | BSID (age 14 mo) | Offspring exposed to the highest prenatal 25(OH)D concentrations (≥75 nmol/L) had higher mental and psychomotor scores (adjusted β: 2.60; 95% CI: 0.63, 4.56 and β: 2.32; 95% CI: 0.36, 4.28, respectively) than offspring exposed to the lowest prenatal 25(OH)D concentrations (≤50 nmol/L). |
| Strөm et al. (2014) (48) | Prospective cohort | Denmark | 965 mother-child pairs | 30-wk gestation | Maternal serum: 76 nmol/L | First admission diagnosis or prescription of medication for depression and ADHD (age 22 y); scholastic achievement based on the mean grade on standardized written exams (9th grade) | P-trend analysis showed a significant positive association between maternal 25(OH)D concentrations and offspring depression risk (P = 0.02). No association was observed between maternal 25(OH)D status and ADHD. |
| Sullivan et al. (2013) (49) | Prospective cohort | England | 2047 mother-child pairs | Random, adjusted to date corresponding with individuals’ midpoint of 34 wk of gestation | Median maternal serum: 64 nmol/L | PLIKSi (age 18 y) | Maternal 25(OH)D concentration was not associated with psychiatric experiences. |
| Whitehouse et al. (2012) (50) | Prospective cohort | Australia(RAINE) | 929 mother-child pairs | 18-wk gestation | Maternal serum: 60 nmol/L | CBCL (follow-up age 2–17 y); PPVT-R (age 5 and 10 y) | No associations were observed between maternal 25(OH)D concentrations and behavioral/emotional problems at any age. A higher risk of language impairment was observed in offspring of mothers with VD levels ≤46 nmol/L than in offspring of mothers with VD levels >70 nmol/L (adjusted OR: 1.97; 95% CI: 1.00, 3.93). |
| Whitehouse et al. (2013) (51) | Prospective cohort | Australia(RAINE) | 929 mother-child pairs | 18-wk gestation | Maternal serum: 58 nmol/L | ASQ (early adulthood); DSM-IV criteria ASD (ages 5, 8, 10, 14 and 17 y) | Higher Attention Switching subscale scores were observed in offspring of mothers with VD status <49 nmol/L than in offspring of VD-sufficient mothers (≥50 nmol/L) (adjusted OR: 5.46; 95% CI: 1.29, 23.05). No associations were observed between maternal VD concentrations and the other subscales/autism-related outcomes. |
ADHD, attention-deficit hyperactivity disorder; ASD, autism spectrum disorder; ASQ, autism spectrum quotient; BSID, Bayley Scales of Infant Development; CBCL, child behavior checklist; C-EDE, Child Eating Disorder Examination; DSM, Diagnostic and Statistical Manual of Mental Disorders; ED, eating disorder; EDE-Q, Eating Disorder Examination Questionnaire; PLIKSi, psychosis-like symptom interview; PPVT-R, Peabody Picture Vocabulary Test–Revised; RAINE, Western Australian pregnancy cohort; ref, reference; SBIC, Stanford-Binet Intelligence Scale; SDQ, Strengths and Difficulties Questionnaire; VD, vitamin D; WAIS, Wechsler Adult Intelligence Scale; WISC, Wechsler Intelligence Scale for Children; WRAT, Wide Range of Achievement Test; 25(OH)D, 25-hydroxyvitamin D; ♀, female.
Vitamin D, cognition, and behavior
Several studies examined the association between maternal serum 25(OH)D concentrations and offspring neurocognitive development. For instance, Keim et al. (45) did not observe associations of maternal 25(OH)D measured at ≤26 wk of pregnancy or cord blood 25(OH)D with early childhood development among 3308 8-mo-old American boys and girls [Bayley mental score β: 0.02; 95% CI: −0.03, 0.07 per 5 nmol/L increment]. In contrast, after adjustment for child sex and maternal country of origin, significant associations were reported between maternal serum 25(OH)D concentrations and Bayley mental and psychomotor scores in 1820 14-mo-old Spanish offspring. Specifically, mental and psychomotor scores were higher in children of mothers with 25(OH)D status ≥75 nmol/L compared with serum VD concentrations ≤50 nmol/L (reference) (Bayley mental score β: 2.60; 95% CI: 0.63, 4.56; Bayley psychomotor score β: 2.32; 95% CI: 0.36, 4.28) (47).
Studies on maternal VD deficiency and offspring intelligence scores also showed contradictory results. Maternal serum 25(OH)D status during gestation was not associated with total intelligence quotient scores or tests of scholar achievement, reading and spelling, language impairments, or verbal intelligence quotient among Danish, English, and American children (43, 45, 48). However, data of Whitehouse et al. (50) did suggest an association between low prenatal VD status at 18 wk of pregnancy and an increased risk of language impairment, as measured by the Peabody Picture Vocabulary Test-Revised, in 929 Australian boys and girls aged 5 and 10 y [adjusted OR: 1.97; 95% CI: 1.00, 3.93 for maternal 25(OH)D ≤46 nmol/L compared with levels >70 nmol/L]. Previous research has shown that cortical structures critical for language development are formed around the fourteenth week of gestation (52, 53). Therefore, measured 25(OH)D concentrations in studies other than Whitehouse et al. (50) may not have reflected circulating 25(OH)D concentrations during critical phases of neurodevelopment. In a prospective cohort study among 960 Vietnamese mother-offspring pairs, language scores were, however, significantly lower in children who were prenatally exposed to 25(OH)D concentrations <38 nmol/L than in children prenatally exposed to 25(OH)D concentrations >75 nmol/L. In this study, maternal 25(OH)D blood was sampled at 32 wk of gestation (adjusted estimated mean difference: −3.48; 95% CI: −5.52, −1.44) (44).
Vitamin D and neuropsychological disorders
Language impairment is often a prominent feature of the autism spectrum disorder (ASD) phenotype, and previous studies have suggested involvement of maternal VD deficiency in the etiology of ASD. However, to date, only 1 observational study has examined the association between deficient maternal VD status and the risk of the ASD phenotype (51). In the 18th week of gestation, 929 Australian mothers donated blood. During the 17 y of follow-up, parents were asked whether their child ever received a diagnosis of ASD. In early adulthood children were asked to complete the autism spectrum quotient. No significant association was revealed between maternal 25(OH)D status and offspring total score on the autism spectrum quotient. However, offspring of mothers with 25(OH)D concentrations <49 nmol/L did have higher scores on the Attention Switching subscale than offspring of mothers with sufficient VD status (≥67 nmol/L), after adjusting for a range of confounders (adjusted OR: 5.46; 95% CI: 1.29, 23.05). Within that same Australian cohort the link between DVD deficiency and another neuropsychological condition was examined as well, namely, the association between prenatal VD deficiency and the development of eating disorders (EDs) at 14, 17, and 20 y of age. ED risk was assessed with the Child Eating Disorder Examination and Eating Disorder Examination Questionnaire (n = 308). When children were 20 y old, multivariate logistic regression showed an ~2-fold increase in ED risk among female offspring in the lowest 25(OH)D quartile (<46 nmol/L) when compared with those in the highest quartile (>71 nmol/L), after adjusting for BMI (in kg/m2), depressive symptoms, and season of birth (adjusted OR: 2.09; 95% CI: 1.03, 5.27) (42).
Similar to autism, maternal VD deficiency during pregnancy has been proposed as an early life risk factor for the development of psychosis (54, 55). However, a case-control study among American women, including 26 cases of schizophrenia and 51 race, sex, and date of birth matched controls, did not show an association between prenatal 25(OH)D concentrations during the third trimester of pregnancy and psychosis risk. Although not statistically significant, subgroup analysis did suggest that black individuals with schizophrenia were more likely to be prenatally exposed to low 25(OH)D concentrations (OR: 0.78; 95% CI: 0.66, 1.08) (46). The null findings of this study were recently replicated by a British study, which also did not observe associations between maternal 25(OH)D status and psychotic experiences (Psychosis-Like Symptoms Interview) (177 cases) or diagnosis of a psychotic disorder when children were 18 y old (29 cases) (49). Because both studies are limited by their sample sizes, these studies lack power to yield conclusive results.
Finally, no associations have been observed between prenatal VD deficiency and offspring behavior as measured with the Childhood Behavior Checklist and/or Strengths and Difficulties Questionnaire (43–45, 50). These instruments assess internalizing/emotional problems and externalizing/hyperactivity and might therefore reflect key features of depression and attention-deficit hyperactivity disorder (DHD), respectively. Results of a Danish cohort study by Strөm et al. (48) also reported no association between maternal serum levels measured at 30 wk gestation and offspring risk of depression or DHD, as defined as first admission diagnosis or prescribed medicine.
Discussion
As stated previously, the primary goal of this systematic review was to provide an overview of current evidence from animal and human studies investigating the impact of maternal VD status on offspring brain development and function. Results of the systematic search predominantly yielded animal experimental studies, in which DVD-deficient animal model experiments suggest an important role for VD in brain development. More specifically, significant differences were observed between DVD-deficient offspring and offspring exposed to normal prenatal VD concentrations with respect to brain morphology and physiology as well as gene expression. In addition, prenatal VD deficiency was found to alter offspring phenotype. Only a few observational studies have examined the association between maternal 25(OH)D status and offspring psychological health and behavior in humans, providing inconclusive data.
When current scientific evidence is evaluated, there are several aspects that warrant discussion. First, most animal studies were conducted in Sprague-Dawley rats, limiting extrapolation of current data to other animals or humans. Second, not all animal studies reported whether calcium concentrations were measured in offspring animals and whether these levels fell within the normal range. Therefore, some of the observed alterations in offspring behavior may be due to low calcium levels affecting musculoskeletal function and thereby offspring behavior during experimental tasks. Third, even though animal studies offer the opportunity to study more contrasting exposures, e.g., absolute VD deficiency compared with a normal VD status, and to control for many factors, rodent studies do not yet provide conclusive evidence. It may be postulated that the lack of clear differences in offspring health between DVD-deficient and nondeficient animals relates to the presence of lithocholic acid (LCA) (56). Nehring et al. (57) presented data in which LCA acted as a substitute for VD. More specifically, LCA resulted in increased serum calcium levels, replaced VD in the mobilization of calcium from bone, and induced 25-hydroxyvitamin-D-24-hydroxylase (Cyp24 expression in DVD-deficient rats.
The first point of discussion with respect to human studies relates to the fact that most human studies exploring the potential link between prenatal VD exposure and brain development and function in later life studied factors serving as a proxy for maternal VD status, including season of birth, migrant status, urban-rural status, and latitude gradient. Because evidence obtained from these studies may not reflect VD effects but effects caused by other characteristics of sunlight, these studies were not included in this review. Second, most of the included observational studies in this review were conducted in Caucasian populations, limiting extrapolation of these findings to other ethnic populations. Only the study of McGrath et al. (46) reported race-dependent study outcomes. Third, there is some heterogeneity in timing of maternal blood sampling between the included studies. Because there may be a critical window for the impact of maternal VD deficiency during gestation on offspring health, this heterogeneity in timing of maternal blood sampling may explain some of the discrepancies between the different studies. Fourth, differences in outcomes between human studies may also relate to differences with respect to statistical analyses, including use of different cutoffs and potential confounders taken into account (Table 5). Fifth, whereas some studies used validated test batteries, others used data collected using self-reported questionnaires filled in by offspring or their caretakers (Table 5); self-report questionnaires may be more likely to introduce bias. Sixth, several studies experienced difficulties with sample attrition over time. For instance, during the last follow-up round at age 9 y, Gale et al. (43) obtained follow-up data for only 30% of the original study population. Moreover, in the study of Whitehouse et al. (50) only 14% of all participants eventually participated after reaching adolescence (Table 5). Seventh, studies examining the impact of maternal 25(OH)D deficiency on developmental disorders are likely to have lacked power due to the low number of offspring eventually developing a disorder. These limitations pinpoint that the potential link between maternal VD deficiency and offspring brain development and function still warrants further investigation.
TABLE 5.
Study characteristics of included human cohort studies1
| Article (y) (ref) | Potential confounders adjusted for in the analysis | Method of status assessment | Method of outcomeassessment | Number at Start with Complete Exposure Data, Loss to Follow-up | Funder |
| Allen et al. (2013) (42) | Presence of maternal kidney dysfunction at 18 wk gestation, family income, biological father living at home at age 14 y, offspring BMI at age 14 y, and offspring depressive symptoms at age 14 y | EIA | C-EDE; EDE-Q | Baseline: n = 802 Caucasian mothers; follow-up: n = 526 (66%) pairs with available outcome data in adolescence, of which 308 were mother-daughter pairs included in the analyses | Nonprofit |
| Gale et al. (2008) (43) | No adjustment for potential confounders | RIA | WASI; SDQ | Baseline: n = 466; follow-up: n = 178 with available outcome data at 9 y | Nonprofit |
| Hanieh et al. (2014) (44) | Maternal age, maternal education, month of blood sampling, maternal BMI, gravidity, maternal depression, and treatment group during RCT | LC-MS | BSID | Baseline: n = 960; follow-up: n = 886 | Nonprofit |
| Keim et al. (2014) (45) | Maternal education, maternal age, parity, race, maternal BMI, marital status, smoking, gestational age, month of blood sampling, and study site | LC-MS/MS | BSID; SBIC; behavior; WISC; WRAT | Baseline: n = 4444; follow-up: 8 mo, n = 3587; 4 y, n = 3146; 7 y, n = 3237 | Nonprofit |
| Morales et al. (2012) (47) | Study site, offspring sex, birth weight, maternal country of origin, maternal age, parental social class, maternal education, parity, maternal prepregnancy BMI, and maternal smoking and alcohol consumption during pregnancy | HPLC | BSID | Baseline: n = 2389; follow-up: n = 1820 | Nonprofit |
| Strөm et al. (2014) (48) | Parity, maternal age, maternal prepregnancy BMI, maternal smoking during pregnancy, maternal education, offspring sex, and season of blood sampling | LC-MS/MS | Depression and ADHD: first admission diagnosis or medication prescription. Scholastic achievement: mean grade of standardized written exams in grade 9. | Baseline: n = 851; follow-up: n = 798 for scholastic achievement, n = 850 for depression and ADHD | Vitamin D biomarkers in maternal sera were funded by a grant from the Novo Nordisk Foundation. |
| Sullivan et al. (2013) (49) | Maternal age, parity, maternal BMI, maternal smoking, maternal alcohol intake, maternal symptoms of depression, social economic position of head of household, maternal educational level, housing tenure, and season of blood sampling | LC-MS/MS | PLIKSi | Baseline: n = 6780; follow-up: n = 2399; analysis sample: n = 2047, due to missing data on covariables (n = 352) | Nonprofit |
| Whitehouse et al.(2012) (50) | For PPVT-R model: maternal age at conception, family income, maternal smoking during pregnancy, offspring parity, and season of blood sampling. Analyses on CBCL were not adjusted for confounders. | EIA | CBCL; PPVT-R | Baseline: n = 813; follow-up: n = 743 | Nonprofit |
| Whitehouse et al.(2013) (51) | Maternal race, maternal alcohol intake during pregnancy, maternal education, family income, offspring gestational age at birth, offspring parity, offspring Apgar scores 5 min after birth, and season of blood sampling | EIA | AQ | Baseline: n = 929; follow-up: n = 406 | Nonprofit |
Baseline: n, number of participants with available 25(OH)D data. ADHD, attention-deficit hyperactivity disorder; AQ, autism spectrum quotient; BSID, Bayley Scales of Infant Development; CBCL, child behavior checklist; C-EDE, Child Eating Disorder Examination; EDE-Q, Eating Disorder Examination Questionnaire; EIA, enzyme immunoassay; PLIKSi, psychosis-like symptom interview; PPVT-R: Peabody Picture Vocabulary Test-Revised; RCT, randomized controlled trials; ref, reference; SBIC, Stanford-Binet Intelligence Scale; SDQ, Strengths and Difficulties Questionnaire; WASI, Wechsler Abbreviated Scale of Intelligence; WISC, Wechsler Intelligence Scale for Children; WRAT, Wide Range of Achievement Test; 25(OH)D, 25-hydroxyvitamin D.
Future animal studies could add to our understanding if the clinical window during which the brain is particularly vulnerable to VD deficiency is examined. Furthermore, it would be valuable to obtain data on optimal dosage and timing of VD supplementation for offspring brain development and function. Effects on brain development and brain function could be investigated by studying morphological, physiological, and behavioral outcomes from gestation throughout adulthood while at the same time monitoring offspring calcium concentrations. Given the lack of conclusive human data, there is also a critical need for longitudinal cohort studies with maternal 25(OH)D sampling in all 3 trimesters and long-term offspring monitoring by means of regular blood sampling, imaging techniques, and neurocognitive, behavioral, psychiatric, and neurological assessments. In addition to the techniques that have already been applied in studies, serial 3-dimensional ultrasound scans during gestation could be conducted to visualize (ab)normal embryonic brain development (58–60). Cord blood samples, analyzed with microarrays, may also provide insight into the potential impact of DVD deficiency on gene and protein expression patterns already observed in rats (13, 16). Finally, studies in non-Caucasian mother-offspring pairs are warranted in order to obtain a better understanding of ethnic differences in the link between maternal VD status and brain development and function.
Conclusion
As summarized in the body of this review, VD is thought to play an important role in brain development and offspring cognitive and psychological function. Rodent studies suggest deviations in brain morphology and physiology of DVD-deficient offspring. However, observed impairments in behavior are subtle and inconsistent. Furthermore, robust human data supporting the link between 25(OH)D status or maternal VD supplementation and offspring brain development and/or function are lacking. Because the developing fetus is completely dependent on the nutritional status of its mother, the impact of low VD status during gestation needs further clarification by future research efforts.
Acknowledgments
We thank James Dower for proofreading the manuscript. Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: ASD, Autism Spectrum Disorder; Bdnf, brain-derived neurotrophic factor; Comt, catechol-O-methyl transferase; Cyp24a, 25-hydroxyvitamin-D-24-hydroxylase; DVD, developmental vitamin D; Foxp2, forkhead box protein P2; LCA, lithocholic acid; Nurr1, nuclear receptor related 1 protein; P57Kip2, cyclin-dependent kinase inhibitor 1C; P75ntr, pan-neurotrophin receptor; PPI, prepulse inhibition; Tgf-β1, transforming growth factor-β1; Th, thyrosine hydroxylase; VD, vitamin D; Vdr, vitamin D receptor; 1,25(OH)2D3, 1,25-dihydroxyvitamin D3; 25(OH)D, 25-hydroxyvitamin D.
References
- 1.Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc 2013;88:720–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266–81. [DOI] [PubMed] [Google Scholar]
- 3.Shin JS, Choi MY, Longtime MS, Nelson DM. Vitamin D effects on pregnancy and the placenta. Placenta 2010;31:1027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Veenstra TD, Prufer K, Koenigsberger C, Brimijoin SW, Grande JP, Kumar R. 1,25-Dihydroxyvitamin D3 receptors in the central nervous system of the rat embryo. Brain Res 1998;804:193–205. [DOI] [PubMed] [Google Scholar]
- 5.Eyles DW, Liu PY, Josh P, Cui X. Intracellular distribution of the vitamin D receptor in the brain: comparison with classic target tissues and redistribution with development. Neuroscience 2014;268:1–9. [DOI] [PubMed] [Google Scholar]
- 6.Adams JS, Hewison M. Extrarenal expression of the 25-hydroxyvitamin D-1-hydroxylase. Arch Biochem Biophys 2012;523:95–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hollis BW, Wagner CL. Vitamin D and pregnancy: skeletal effects, nonskeletal effects, and birth outcomes. Calcif Tissue Int 2013;92:128–39. [DOI] [PubMed] [Google Scholar]
- 8.Brannon PM. Vitamin D and adverse pregnancy outcomes: beyond bone health and growth. Proc Nutr Soc 2012;71:205–12. [DOI] [PubMed] [Google Scholar]
- 9.Wrzosek M, Lukaszkiewicz J, Wrzosek M, Jakubczyk A, Matsumoto H, Piatkiewicz P, Radziwon-Zaleska M, Wojnar M, Nowicka G. Vitamin D and the central nervous system. Pharmacol Rep 2013;65:271–8. [DOI] [PubMed] [Google Scholar]
- 10.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab 2002;13:100–5. [DOI] [PubMed] [Google Scholar]
- 11.Brouwer-Brolsma EM, de Groot LC. Vitamin D and cognition in older adults: an update of recent findings. Curr Opin Clin Nutr Metab Care 2015;18:11–6. [DOI] [PubMed] [Google Scholar]
- 12.Eyles D, Brown J, Mackay-Sim A, McGrath J, Feron F. Vitamin D3 and brain development. Neuroscience 2003;118:641–53. [DOI] [PubMed] [Google Scholar]
- 13.Almeras L, Eyles D, Benech P, Laffite D, Villard C, Patatian A, Boucraut J, Mackay-Sim A, McGrath J, Feron F. Developmental vitamin D deficiency alters brain protein expression in the adult rat: implications for neuropsychiatric disorders. Proteomics 2007;7:769–80. [DOI] [PubMed] [Google Scholar]
- 14.Cui X, McGrath JJ, Burne TH, Mackay-Sim A, Eyles DW. Maternal vitamin D depletion alters neurogenesis in the developing rat brain. Int J Dev Neurosci 2007;25:227–32. [DOI] [PubMed] [Google Scholar]
- 15.Cui X, Pelekanos M, Burne TH, McGrath JJ, Eyles DW. Maternal vitamin D deficiency alters the expression of genes involved in dopamine specification in the developing rat mesencephalon. Neurosci Lett 2010;486:220–3. [DOI] [PubMed] [Google Scholar]
- 16.Eyles D, Almeras L, Benech P, Patatian A, Mackay-Sim A, McGrath J, Feron F. Developmental vitamin D deficiency alters the expression of genes encoding mitochondrial, cytoskeletal and synaptic proteins in the adult rat brain. J Steroid Biochem Mol Biol 2007;103:538–45. [DOI] [PubMed] [Google Scholar]
- 17.Féron F, Burne TH, Brown J, Smith E, McGrath JJ, Mackay-Sim A, Eyles DW. Developmental Vitamin D3 deficiency alters the adult rat brain. Brain Res Bull 2005;65:141–8. [DOI] [PubMed] [Google Scholar]
- 18.Grecksch G, Ruthrich H, Hollt V, Becker A. Transient prenatal vitamin D deficiency is associated with changes of synaptic plasticity in the dentate gyrus in adult rats. Psychoneuroendocrinology 2009;34 Suppl 1:S258–64. [DOI] [PubMed] [Google Scholar]
- 19.Hawes JE, Tesic D, Whitehouse AJ, Zosky GR, Smith JT, Wyrwoll CS. Maternal vitamin D deficiency alters fetal brain development in the BALB/c mouse. Behav Brain Res 2015;286:192–200. [DOI] [PubMed] [Google Scholar]
- 20.Keilhoff G, Grecksch G, Becker A. Haloperidol normalized prenatal vitamin D depletion-induced reduction of hippocampal cell proliferation in adult rats. Neurosci Lett 2010;476:94–8. [DOI] [PubMed] [Google Scholar]
- 21.Kesby JP, Cui X, Ko P, McGrath JJ, Burne TH, Eyles DW. Developmental vitamin D deficiency alters dopamine turnover in neonatal rat forebrain. Neurosci Lett 2009;461:155–8. [DOI] [PubMed] [Google Scholar]
- 22.Ko P, Burkert R, McGrath J, Eyles D. Maternal vitamin D3 deprivation and the regulation of apoptosis and cell cycle during rat brain development. Brain Res Dev Brain Res 2004;153:61–8. [DOI] [PubMed] [Google Scholar]
- 23.Di Forti M, Lappin JM, Murray RM. Risk factors for schizophrenia - all roads lead to dopamine. Eur Neuropsychopharmol 2007;17 Suppl 2:S101–7. [DOI] [PubMed] [Google Scholar]
- 24.Murray RM, Lappin JM, di Forti LE. Schizophrenia: from developmental deviance to dopamine dysregulation. Eur Neuropsychopharmol 2008;18 Suppl 3:S129–34. [DOI] [PubMed] [Google Scholar]
- 25.Das B, Modi G, Dutta A. Dopamine D3 agonists in the treatment of Parkinson’s disease. Curr Top Med Chem 2015;15:908–26. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery SA. The under-recognized role of dopamine in the treatment of major depressive disorder. Int Clin Psychopharmacol 2008;23:63–9. [DOI] [PubMed] [Google Scholar]
- 27.Bissonette GB, Roesch MR. Development and function of the midbrain dopamine system: what we know and what we need to. Genes Brain Behav 2016;15:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Becker A, Eyles DW, McGrath JJ, Grecksch G. Transient prenatal vitamin D deficiency is associated with subtle alterations in learning and memory functions in adult rats. Behav Brain Res 2005;161:306–12. [DOI] [PubMed] [Google Scholar]
- 29.Burne TH, Feron F, Brown J, Eyles DW, McGrath JJ, Mackay-Sim A. Combined prenatal and chronic postnatal vitamin D deficiency in rats impairs prepulse inhibition of acoustic startle. Physiol Behav 2004;81:651–5. [DOI] [PubMed] [Google Scholar]
- 30.Burne TH, Becker A, Brown J, Eyles DW, Mackay-Sim A, McGrath JJ. Transient prenatal Vitamin D deficiency is associated with hyperlocomotion in adult rats. Behav Brain Res 2004;154:549–55. [DOI] [PubMed] [Google Scholar]
- 31.Burne TH, O’Loan J, McGrath JJ, Eyles DW. Hyperlocomotion associated with transient prenatal vitamin D deficiency is ameliorated by acute restraint. Behav Brain Res 2006;174:119–24. [DOI] [PubMed] [Google Scholar]
- 32.Burne TH, O’Loan J, Splatt K, Alexander S, McGrath JJ, Eyles DW. Developmental vitamin D (DVD) deficiency alters pup-retrieval but not isolation-induced pup ultrasonic vocalizations in the rat. Physiol Behav 2011;102:201–4. [DOI] [PubMed] [Google Scholar]
- 33.Burne TH, Alexander S, Turner KM, Eyles DW, McGrath JJ. Developmentally vitamin D-deficient rats show enhanced prepulse inhibition after acute DELTA9-tetrahydrocannabinol. Behav Pharmacol 2014;25:236–44. [DOI] [PubMed] [Google Scholar]
- 34.Eyles DW, Rogers F, Buller K, McGrath JJ, Ko P, French K, Burne TH. Developmental vitamin D (DVD) deficiency in the rat alters adult behaviour independently of HPA function. Psychoneuroendocrinology 2006;31:958–64. [DOI] [PubMed] [Google Scholar]
- 35.Fernandes de Abreu DA, Nivet E, Baril N, Khrestchatisky M, Roman F, Feron F. Developmental vitamin D deficiency alters learning in C57Bl/6J mice. Behav Brain Res 2010;208:603–8. [DOI] [PubMed] [Google Scholar]
- 36.Harms LR, Eyles DW, McGrath JJ, Mackay-Sim A, Burne TH. Developmental vitamin D deficiency alters adult behaviour in 129/SvJ and C57BL/6J mice. Behav Brain Res 2008;187:343–50. [DOI] [PubMed] [Google Scholar]
- 37.Harms LR, Cowin G, Eyles DW, Kurniawan ND, McGrath JJ, Burne TH. Neuroanatomy and psychomimetic-induced locomotion in C57BL/6J and 129/X1SvJ mice exposed to developmental vitamin D deficiency. Behav Brain Res 2012;230:125–31. [DOI] [PubMed] [Google Scholar]
- 38.Kesby JP, Burne TH, McGrath JJ, Eyles DW. Developmental vitamin D deficiency alters MK 801-induced hyperlocomotion in the adult rat: An animal model of schizophrenia. Biol Psychiatry 2006;60:591–6. [DOI] [PubMed] [Google Scholar]
- 39.O’Loan J, Eyles DW, Kesby J, Ko P, McGrath JJ, Burne TH. Vitamin D deficiency during various stages of pregnancy in the rat; its impact on development and behaviour in adult offspring. Psychoneuroendocrinology 2007;32:227–34. [DOI] [PubMed] [Google Scholar]
- 40.Pan P, Jin DH, Chatterjee-Chakraborty M, Halievski K, Lawson D, Remedios D, Smetka C, Pinto V, Parra E, Fleming AS. The effects of vitamin D3 during pregnancy and lactation on offspring physiology and behavior in Sprague-Dawley rats. Dev Psychobiol 2014;56:12–22. [DOI] [PubMed] [Google Scholar]
- 41.Turner KM, Young JW, McGrath JJ, Eyles DW, Burne TH. Cognitive performance and response inhibition in developmentally vitamin D (DVD)-deficient rats. Behav Brain Res 2013;242:47–53. [DOI] [PubMed] [Google Scholar]
- 42.Allen KL, Byrne SM, Kusel MM, Hart PH, Whitehouse AJ. Maternal vitamin D levels during pregnancy and offspring eating disorder risk in adolescence. Int J Eat Disord 2013;46:669–76. [DOI] [PubMed] [Google Scholar]
- 43.Gale CR, Robinson SM, Harvey NC, Javaid MK, Jiang B, Martyn CN, Godfrey KM, Cooper C. Princess Anne Hospital Study G, Maternal vitamin D status during pregnancy and child outcomes. Eur J Clin Nutr 2008;62:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanieh S, Ha TT, Simpson JA, Thuy TT, Khuong NC, Thoang DD, Tran TD, Tuan T, Fisher J, Biggs BA. Maternal vitamin D status and infant outcomes in rural Vietnam: a prospective cohort study. PLoS One 2014;9:e99005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keim SA, Bodnar LM, Klebanoff MA. Maternal and cord blood 25(OH)-vitamin D concentrations in relation to child development and behaviour. Paediatr Perinat Epidemiol 2014;28:434–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGrath J, Eyles D, Mowry B, Yolken R, Buka S. Low maternal vitamin D as a risk factor for schizophrenia: a pilot study using banked sera. Schizophr Res 2003;63:73–8. [DOI] [PubMed] [Google Scholar]
- 47.Morales E, Guxens M, Llop S, Rodriguez-Bernal CL, Tardon A, Riano I, Ibarluzea J, Lertxundi N, Espada M, Rodriguez A, et al. Circulating 25-hydroxyvitamin D3 in pregnancy and infant neuropsychological development. Pediatrics 2012;130:e913–20. [DOI] [PubMed] [Google Scholar]
- 48.Strøm M, Halldorsson TI, Hansen S, Granstrom C, Maslova E, Petersen SB, Cohen AS, Olsen SF. Vitamin D measured in maternal serum and offspring neurodevelopmental outcomes: a prospective study with long-term follow-up. Ann Nutr Metab 2014;64:254–61. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan S, Wills A, Lawlor D, McGrath J, Zammit S. Prenatal vitamin D status and risk of psychotic experiences at age 18 years-a longitudinal birth cohort. Schizophr Res 2013;148:87–92. [DOI] [PubMed] [Google Scholar]
- 50.Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Kusel MM, Hart PH. Maternal serum vitamin D levels during pregnancy and offspring neurocognitive development. Pediatrics 2012;129:485–93. [DOI] [PubMed] [Google Scholar]
- 51.Whitehouse AJ, Holt BJ, Serralha M, Holt PG, Hart PH, Kusel MM. Maternal vitamin D levels and the autism phenotype among offspring. J Autism Dev Disord 2013;43:1495–504. [DOI] [PubMed] [Google Scholar]
- 52.Quarello E, Stirnemann J, Ville Y, Guibaud L. Assessment of fetal Sylvian fissure operculization between 22 and 32 weeks: a subjective approach. Ultrasound Obstet Gynecol 2008;32:44–9. [DOI] [PubMed] [Google Scholar]
- 53.Chi JG, Dooling EC, Gilles FH. Gyral development of the human brain. Ann Neurol 1977;1:86–93. [DOI] [PubMed] [Google Scholar]
- 54.McGrath J. Does ‘imprinting’ with low prenatal vitamin D contribute to the risk of various adult disorders? Med Hypotheses 2001;56:367–71. [DOI] [PubMed] [Google Scholar]
- 55.McGrath JJ, Burne TH, Feron F, Mackay-Sim A, Eyles DW. Developmental vitamin D deficiency and risk of schizophrenia: a 10-year update. Schizophr Bull 2010;36:1073–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Makishima M, Lu TT, Xie W, Whitfield G, Domoto H, Evans RM, Haussler MR, Mangelsdorf DJ. Vitamin D receptor as an intestinal bile acid sensor. Science 2002;296:1313–6. [DOI] [PubMed] [Google Scholar]
- 57.Nehring JA, Zierold C, deLuca HF. Lithocholic acid can carry out in vivo functions of vitamin D. Proc Natl Acad Sci USA 2007;104:10006–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pooh RK. Neurosonoembryology by three-dimensional ultrasound. Semin Fetal Neonatal Med 2012;17:261–8. [DOI] [PubMed] [Google Scholar]
- 59.Pooh RK. Normal anatomy by three-dimensional ultrasound in the second and third trimesters. Semin Fetal Neonatal Med 2012;17:269–77. [DOI] [PubMed] [Google Scholar]
- 60.Rousian M, Hop WC, Koning AHJ, van der Spek PJ, Steegers EAP, Exalto N. First trimester brain ventricle fluid and embryonic volumes measured by three-dimensional ultrasound with the use of I-Space virtual reality. Hum Reprod 2013;28:1181–9. [DOI] [PubMed] [Google Scholar]