Abstract
A pandemic of diabetes and obesity has been developing worldwide in close association with excessive nutrient intake and a sedentary lifestyle. Variations in the protein content of the diet have a direct impact on glucose homeostasis because amino acids (AAs) are powerful modulators of insulin action. In this work we review our recent findings on how elevations in the concentration of the circulating AAs leucine and proline activate a metabolic mechanism located in the mediobasal hypothalamus of the brain that sends a signal to the liver via the vagus nerve, which curtails glucose output. This neurogenic signal is strictly dependent on the metabolism of leucine and proline to acetyl-coenzyme A (CoA) and the subsequent production of malonyl-CoA; the signal also requires functional neuronal ATP-sensitive potassium channels. The liver then responds by lowering the rate of gluconeogenesis and glycogenolysis, ultimately leading to a net decrease in glucose production and in concentrations of circulating glucose. Furthermore, we review here how our work with proline suggests a new role of astrocytes in the central regulation of glycemia. Last, we outline how factors such as the consumption of fat-rich diets can interfere with glucoregulatory mechanisms and, in the long term, may contribute to the development of hyperglycemia, a hallmark of type 2 diabetes.
Keywords: amino acids, branched-chain amino acids, glucose homeostasis, hyperglycemia, hypothalamus, insulin action, leucine, liver, nutrient sensing, proline
Introduction
The increasing prevalence of type 2 diabetes and obesity is largely linked to excessive nutrient intake and reduced physical activity (1, 2). Hyperglycemia, a hallmark of type 2 diabetes, stems from the weakening of the regulatory mechanisms that maintain circulating glucose at normal concentrations. In mammals, the maintenance of a narrow range of appropriate and steady concentrations of circulating glucose (euglycemia) is known as glucose homeostasis and is a basic requirement for preserving metabolic health in the face of fluctuating conditions, including the alternation of feeding and fasting periods, physical or mental stress, and other environmental challenges. Fasting concentrations of plasma glucose that are considered normal differ by species, but in humans the accepted normal range is 80–120 mg/dL or 4.4–6.7 mmol/L. It is well known that there are 2 sources of glucose: exogenous (derived from the diet) and endogenous (produced mainly in the liver). In this regard, a key environmental factor is the increasing availability and consumption of calorie-dense and fat-rich diets (overnutrition) in Western societies. Adaptive responses to the increased influx of nutrients include changes in feeding behavior and metabolism designed to prevent deviations in each individual’s normal physiologic state.
Glucose homeostasis depends on complex regulatory systems involving various organs and tissues, including the pancreas, liver, skeletal muscle, intestine, adipose tissue, and the brain. Here we concentrate on the role of the central nervous system (CNS)6 in the maintenance of glucose homeostasis but with an emphasis on hypothalamic metabolic nutrient sensing. First, we distinguish the following 2 primary ways by which the CNS contributes to the regulation of circulating glucose concentrations: 1) adjustment of autonomic activity to prevent hypoglycemia (glucose counterregulation) and 2) hypothalamic sensing of changes in the concentrations of circulating nutrients and the integration of appropriate responses. Selective regions of the brain, including the mediobasal hypothalamus (MBH), are capable of gathering information on the body’s nutritional status in order to implement appropriate behavioral and metabolic responses to changes in fuel availability. Here we focus on one class of nutrients, amino acids (AAs), and discuss the available evidence indicating that the concentrations of circulating AAs can be sensed in the brain to bring about adaptive responses that curtail the production of endogenous glucose by the liver. We also examine the negative impact of diet-induced insulin resistance on these AA-driven glucoregulatory mechanisms.
Current Status of Knowledge
The efforts to understand the effects of AAs on insulin action initially concentrated on their metabolic and signaling actions in the liver, skeletal muscle, and adipose tissue. Postprandial elevations in plasma AAs stimulate endogenous secretion of both insulin and glucagon (3, 4) and thus might modulate hepatic glucose metabolism by changing the portal insulin to glucagon ratio (5). Furthermore, AAs are substrates for gluconeogenesis (6), a pathway that contributes to glucose production. Only in the past 10 y has a meaningful role for the CNS started to emerge supported by a number of studies on the central effects of AAs on food intake and liver glucose metabolism. Among the effects of AAs that were eventually shown to be mediated by the CNS were changes in food intake in rodents reported between 1965 and 1970 (7, 8). Several years ago it was shown that the AA content of the brain, the hypothalamus in particular, reflects the protein composition of the diet (9–12). This means that circulating AAs can directly gain access to the MBH. It is now generally accepted that peptides, AAs, and other circulating metabolites can bypass the blood-brain barrier through a permeable microvasculature with fenestrated endothelial cells in the arcuate nucleus of the hypothalamus (13).
In more recent years a number of studies led to the discovery of so-called hypothalamic metabolic nutrient sensing (14, 15) as a mechanism that regulates glucose metabolism. The paradigm of this form of nutrient sensing requires that, upon gaining access to the MBH, a macronutrient can be metabolically converted to acetyl-CoA and malonyl-CoA in order to elicit a glucoregulatory response (14, 16). Studies of FA oxidation in rats implicated malonyl-CoA in the transition from FAs to glucose oxidation, whereas malonyl-CoA was identified as a potent allosteric inhibitor of carnitine palmitoyltransferase (CPT) 1, the enzyme that controls the entry of long-chain fatty acyl (LCFA)–CoA into the mitochondria (17). It was postulated that the concentrations of malonyl-CoA act as a fuel sensor that can switch substrate oxidation from FAs to glucose (18). Thus, in the presence of high concentrations of glucose and insulin, the accumulation of malonyl-CoA inhibits CPT1 and reduces lipid oxidation, favoring lipid storage into TGs. Moreover, the central administration of oleic acid was shown to inhibit food intake and glucose production in rodents (19). This effect of oleic acid was not reproduced by FAs of medium chain length, suggesting that long-chain fatty acids are a specific signal of nutrient abundance rather than the simple result of FA oxidation. In agreement with this concept, studies in rodents showed that the accumulation of LCFA-CoAs in the hypothalamus acts as a signal of nutrient abundance (20). Under physiologic conditions, the inhibition of CPT1 activity may occur when concentrations of malonyl-CoA increase due to an increased flux of nutrient-derived carbons. Subsequently, the increased availability of LCFA-CoAs may activate a central lipid-sensing signal of negative feedback to prevent the entry of more nutrients in the circulation (14, 20). Because some AAs can be converted to acetyl-CoA, the precursor of malonyl-CoA, we decided to explore the possibility that AAs are involved in the central regulation of circulating glucose concentrations.
In this article we discuss our research, which focuses on 2 AAs, leucine and proline. We considered these AAs as representative of the groups of ketogenic and glucogenic AAs, respectively. This means that, in principle, any other AAs following a similar metabolic fate could also be regulatory, although evidence exists only for a few of them in our studies. Another AA, histidine (21, 22), was also reported to modulate glucose metabolism through a central mechanism that differs from the mechanism found in our own studies with leucine and proline. A brief description of these studies is provided below.
Leucine.
The first AA that we examined was leucine because it has long been known to act as nutrient-signaling molecule (23–25). More important, we picked this BCAA because leucine directly generates acetyl-CoA, which can then be carboxylated to form malonyl-CoA, a precursor for FA synthesis. In this regard, we earlier mentioned that oleic acid in the hypothalamus has been shown to negatively modulate endogenous glucose production and food intake (19). This feature makes leucine a strong candidate for metabolic signaling that can engage hypothalamic biochemical sensor(s) to modulate glucose metabolism. Thus, we first had to show that leucine in the brain can modulate hepatic glucose metabolism. Toward this aim we administered leucine into the MBH of conscious rats under basal conditions. As predicted, leucine rapidly lowered the circulating concentrations of glucose and, consequently, the concentrations of insulin also decreased (26). To eliminate the confounding factor posed by the changes in glucoregulatory hormones that accompany changes in glucose concentrations and gain insight into the glucose-lowering effects of leucine, we performed pancreatic insulin clamp procedures. These clamp experiments are designed to keep the rats under constant, controlled metabolic and hormonal conditions. During the clamp procedure we also performed measurements of whole-body glucose kinetics using isotopic dilution of tritiated glucose to determine whether the decrease in circulating glucose was due to increased peripheral glucose disposal by skeletal muscle and adipose tissue or to a decrease in endogenous glucose production by the liver. The results showed that central leucine markedly inhibited hepatic glucose production (HGP) without changing peripheral glucose disposal (26).
Further in vivo biochemical studies showed that the decrease in HGP was the result of a marked inhibition of glycogenolysis and gluconeogenesis in the liver (26). These pathways are key determinants of the rate of HGP (27, 28). Because leucine is an activator of the mammalian target of rapamycin (mTOR) complex (23, 25, 29), we repeated these studies in the presence of rapamycin, a potent inhibitor of mTOR. Rapamycin robustly inhibited mTOR but did not prevent the effects of central leucine on HPG, indicating that mTOR was not involved (26). Because our hypothesis postulated that the hypothalamic metabolism of leucine to acetyl-CoA and malonyl-CoA (Figure 1) is the mechanism for the regulation of hepatic glucose fluxes, we carried out systematic experiments to examine this idea. Interventions that stimulated or blocked leucine metabolism at its various stages would either have enhanced or attenuated the central glucoregulatory effect (26). Leucine metabolism has been extensively characterized, and excellent reviews of BCAA metabolism have been published (30, 31).
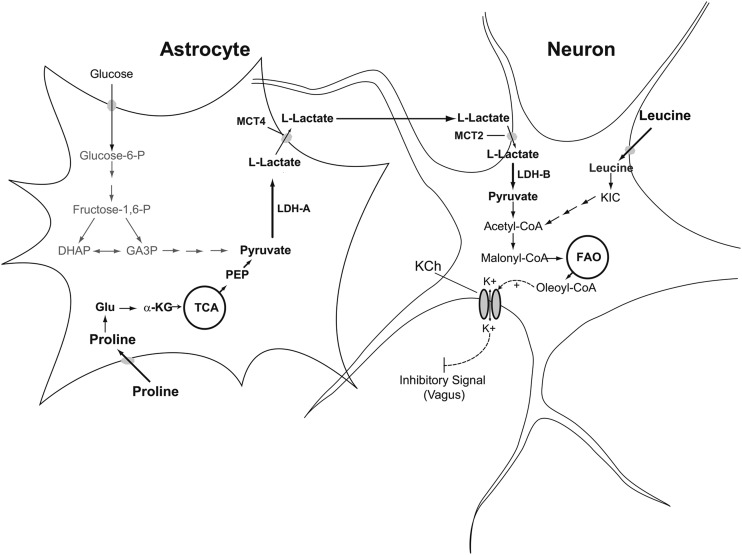
FIGURE 1.
Schematic representation of astrocyte-neuron interaction postulated to regulate glucose production by amino acids. Circulating leucine or proline is metabolized in either the neurons or astrocytes of the MBH and a regulatory signal is generated by neurons. Proline is taken up by astrocytes, converted to glutamate, then to α-KG by glutamate dehydrogenase; α-KG is further metabolized to pyruvate and converted to lactate by LDH-A. Lactate is transferred to neurons via the ANLS. In neurons, lactate is converted back to pyruvate by LDH-B and oxydized to generate acetyl-CoA. In contrast, leucine is directly taken up by neurons and converted to KIC by the enzyme BCAT. KIC is subsequently converted to acetyl-CoA. Carboxylation of acetyl-CoA yields malonyl-CoA, which is used for the synthesis of oleoyl-CoA. ANLS, astrocyte-neuron lactate shuttle; BCAT, branched-chain amino acid aminotransferase; DHAP, dihydroxyacetone phosphate; FAO, fatty acid oxidation; GA3P, glyceraldehyde 3-phosphate; Fructose-1,6-P, fructose-1,6-biphosphate; Glucose-6-P, glucose-6-phosphate; KCh, ATP-sensitive potassium channel; KIC, α-ketoisocaproate; LDH-A, isoenzyme A of lactate dehydrogenase; LDH-B, isoenzyme B of lactate dehydrogenase; MBH, mediobasal hypothalamus; MTC2, monocarboxylate transporter isoform 2; MTC4, monocarboxylate transporter isoform 4; PEP, phosphoenolpyruvate; TCA, tricarboxylic acid cycle; α-KG, α-ketoglutarate.
We first tested the effect of inhibiting the conversion of leucine to α-ketoisocaproic acid (KIC), catalyzed by the enzyme BCAA aminotransferase (BCAT), using the BCAT inhibitor amino-oxyacetic acid (AOAA) (32). Co-infusion of AOAA into the MBH markedly blunted the effect of leucine on liver glucose metabolism. Furthermore, the infusion of KIC alone completely replicated the effects of leucine (Figure 1). We next examined the effect of modulating the activity of branched-chain α-ketoacid dehydrogenase (BCKDH), the enzyme that oxidatively decarboxylates KIC to isovaleryl-CoA. BCKDH is robustly inhibited by phosphorylation catalyzed by the enzyme branched-chain α-ketoacid dehydrogenase kinase (BCKDK). We antagonized this inhibitory action with α-chloro-isocaproic acid (CIC), a potent inhibitor of BCKDK (33). This resulted in the activation of BCKDH, which is predicted to increase the metabolism of endogenous leucine. Indeed, the infusion of CIC into the MBH fully recapitulated the effect of leucine, although in the absence of any exogenous leucine. To obtain further evidence, we also inhibited leucine metabolism overexpression of BCKDK in the MBH. This intervention was designed to inhibit leucine metabolism by enhancing the phosphorylation of BCKDK. As predicted, rats overexpressing BCKDK displayed a weak inhibition of HGP in response to centrally administered leucine, indicating that the metabolism of leucine in the MBH was required for the inhibition of HGP (26).
Our hypothesis postulated that malonyl-CoA is a key intermediate required for the regulation of liver glucose metabolism by leucine (Figure 1). It is produced by the carboxylation of acetyl-CoA catalyzed by the enzyme acetyl-CoA carboxylase (ACC), whose activity is under the control of AMP-activated protein kinase (AMPK). Phosphorylation of ACC by AMPK inhibits its activity and slows down the production of malonyl-CoA. The concentrations of this key metabolite are also lowered by another enzyme, malonyl-CoA decarboxylase (MCD). This enzyme decarboxylates malonyl-CoA to yield acetyl-CoA and carbon dioxide. Taking all this biochemical knowledge into account, we examined the effect of preventing the increase in malonyl-CoA caused by the metabolism of leucine with the use of 2 strategies: 1) pharmacologic inhibition of ACC by using the AMPK activator 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR) and 2) overexpression of MCD. In the first approach, we intended to prevent the synthesis of malonyl-CoA, whereas the second approach was designed to enhance the catabolism of malonyl-CoA in the presence of high concentrations of MCD (34). Both interventions effectively attenuated the central effects of leucine on liver glucose metabolism. Taken together, these results indicated that an increase in malonyl-CoA in the MBH was required for the effects of leucine.
Because studies have shown that ATP-sensitive potassium (KATP) channels are required for the central modulation of glucose metabolism (35), we performed experiments to examine the involvement of these channels in the effects of leucine. KATP channels can be activated by oleoyl-CoA synthesized from leucine-derived malonyl-COA. Briefly, disruption of the functionality of brain KATP channels in rodents pharmacologically (with glibenclamide) or genetically (by using sulfonylurea receptor 1 (Sur1) null mice) resulted in marked attenuation of the effects of central leucine (26). Glibenclamide is a very well-known KATP channel blocker, whereas Sur1 null mice are mice lacking functional KATP channels in the brain (36). Importantly, we also showed that the central delivery of leucine increases the concentrations of oleoyl-CoA in the MBH of rats (26).
Our studies (37) tested other BCAAs to determine if they also regulate HGP in rats. Briefly, we administered valine and isovaline in the MBH of rats at a dose equimolar to the dose of leucine used in our published studies described above. These studies were performed by using protocols analogous to those described for leucine to examine if isoleucine and valine also have central glucoregulatory activity. The unpublished results showed that during pancreatic insulin clamps, the separate administration of these 2 AAs robustly decreased HGP. Interestingly, the potency of the glucose-lowering effect was essentially comparable to that of leucine. This is not surprising because these 2 BCAAs share a common metabolic pathway with leucine and a key downstream metabolite, acetyl-CoA. Additional studies are required to determine in detail to what extent isoleucine and valine are similar to leucine with regard to metabolic sensing in the hypothalamus.
Proline.
Our studies on the hypothalamic sensing of proline (38) were guided not only by our interest in the effects of AAs on liver glucose metabolism but also by our interest in using proline as a probe to test the involvement of astrocytes in the mechanisms of hypothalamic nutrient sensing. Astrocytes produce lactate from various substrates, and lactate is subsequently released from astrocytes, taken up by neurons, and converted back to pyruvate (Figure 1). The latter is then oxidized for energy production in neurons. Lactate transfer from astrocytes to neurons occurs through the astrocyte-neuron lactate shuttle (ANLS) (39, 40). This directional transfer depends on the differential distribution of monocarboxylate transporters (MCTs) and lactate dehydrogenase (LDH) isoenzymes (39, 41): astrocytes express the isoform MCT4 and the isoenzyme LDH-A, whereas neurons are enriched in MCT2 and LDH-B. Lactate is then exported through MCT4, taken up by neurons via MCT2, and converted to pyruvate by LDH-B (Figure 1). Because in previous studies we found that glucose and pyruvate were both LDH-dependent modulators of glucose metabolism (42), we reasoned that this dependency could be due to the involvement of the ANLS. To test this concept we chose the glucogenic AA proline as a probe because astrocytes, not neurons, metabolize glucogenic AAs to pyruvate (43). Proline is first converted to glutamate and subsequently metabolized to α-ketoglutarate (α-KG), which, in turn, enters the tricarboxylic acid cycle to form pyruvate (Figure 1). Next, pyruvate is metabolized to acetyl-CoA and subsequently carboxylated to malonyl-CoA, a key molecule in the central regulation of hepatic glucose metabolism (44).
We first determined whether a primary increase in hypothalamic proline per se was sufficient to modulate HGP by infusing this AA in the MBH of rats. As predicted, central proline decreased the circulating concentrations of both glucose and insulin. To investigate the mechanism by which proline decreases glucose concentrations we examined proline’s effect during the course of pancreatic basal insulin clamps designed to maintain fixed and basal circulating insulin concentrations. Glucose kinetics analysis showed that proline caused a marked inhibition of endogenous glucose production that completely accounted for the effect on whole-body glucose metabolism. To further strengthen our hypothesis that the metabolism of proline is required for its glucoregulatory activity, we performed central infusions of glutamate, a metabolite of proline. As predicted, glutamate completely replicated the effect of proline on liver glucose metabolism (38). To explain what changes in the liver are responsible for the decrease in glucose output, we measured the rates of glucose-producing pathways (45). The livers of rats with central proline showed a marked decrease in the metabolic flux through glucose-6-phosphatase (Glc-6-Pase) as well as a decrease in the rate of glycogenolysis and gluconeogenesis (Figure 2). These changes completely accounted for the observed decrease in HGP.
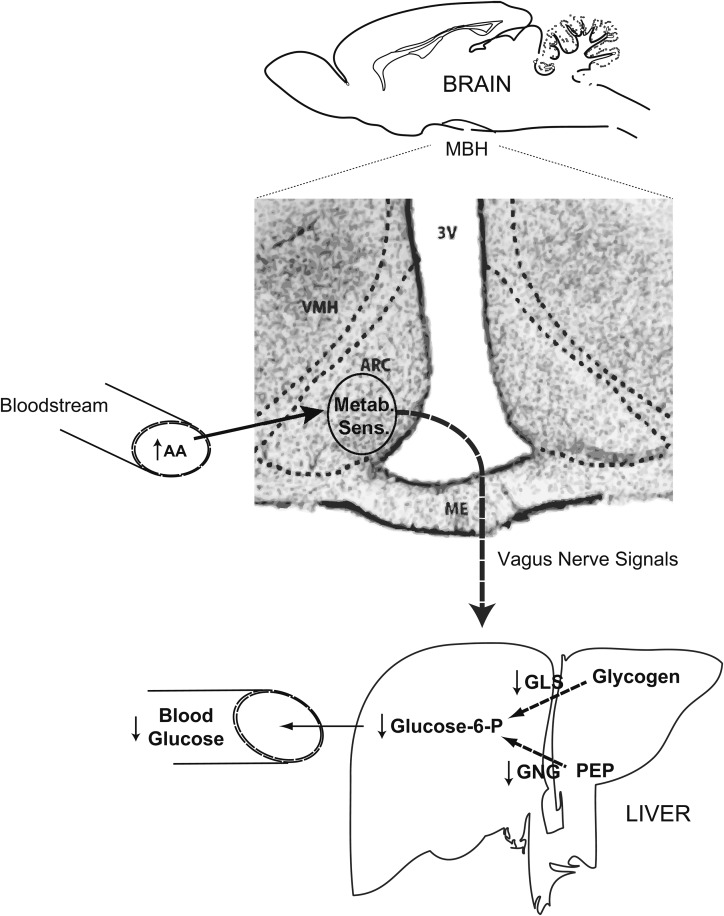
FIGURE 2.
Schematic representation of the brain-liver circuit proposed to regulate liver glucose production by the hypothalamic sensing of AAs. Circulating leucine and proline are metabolized in the ARC of the MBH (shown in the photomicrograph of a rat coronal brain section) to produce acetyl- and malonyl-CoA. The latter is utilized in the synthesis of oleoyl-CoA, which, in turn, activates hypothalamic ATP-sensitive potassium channels. Activation of the channels would be an early event in the generation of the neurogenic signal that is relayed to the liver via the vagus nerve. Vagal input decreases the rate of glucose-yielding fluxes in the liver, leading to inhibition of glucose production and lower concentrations of circulating glucose. AA, amino acid; ARC, arcuate nucleus; GLS, glycogenolysis; Glucose-6-P, glucose-6-phosphate; GNG, gluconeogenesis; MBH, mediobasal hypothalamus; ME, median eminence; Metab. Sens., metabolic sensing; PEP, phosphoenolpyruvate; VMH, ventromedial nucleus; 3V, third ventricle.
The next key experiment to support our hypothesis was to determine if the glucoregulatory activity of proline was LDH dependent. Toward this aim, we co-infused proline and oxamate, a potent competitive inhibitor of LDH (42). The results showed that LDH inhibition in the MBH negated the glucose-lowering effect and the decrease in HGP of central proline (38). Next, to directly examine if perturbing ANLS functionality interferes with the glucoregulatory action of proline, we injected lentiviral particles carrying an LDH-A–specific short-hairpin RNA into the MBH of rats. This viral vector robustly decreased LDH-A in the hypothalamus of the treated rats compared with a control (nonsilencing) vector (38). More important, during pancreatic clamp studies the infusion of proline in the MBH of these LDH-A–deficient rats did not inhibit HGP or decrease circulating glucose (38). Because LDH-A is the isoform expressed in astrocytes and is a component in the mechanism for lactate transfer, these results indicated that the ANLS (Figure 1) was involved in the action of proline. These findings are particularly interesting in light of the identification of newer collaborative roles between astrocytes and neurons involved in the metabolic functions of the ANLS (46).
Histidine.
Although not part of our studies, we would like to briefly discuss the case of histidine, which has been reported to inhibit HGP by a central (hypothalamic) mechanism not involving metabolic conversion (21, 22) but that also exemplifies the role of brain AAs in modulating glucose metabolism. The administration of histidine in the third cerebral ventricle reportedly caused the activation of signal transducer and activator of transcription 3 (STAT3) in the liver, which, in turn, enhanced the suppression of glucose production by insulin. Furthermore, this enhanced suppression of glucose production was apparently a consequence of a robust inhibition of gluconeogenesis. This effect was blocked by the central co-administration of an antagonist of the H1 histamine receptor, suggesting that activation of this receptor in the brain is required. Although the anatomic site of action for histidine was not determined, the MBH is a strong candidate due to its location at the bottom of the third ventricle.
Cellular basis of glucoregulation by AAs.
Although our studies indicate that neurons and astrocytes in the MBH are involved in the glucoregulatory action of AAs, the specific details require further research. Two neuronal populations are known to play a key role in the maintenance of energy metabolism and glucose homeostasis, proopiomelanocortin (POMC)- and Agouti-related peptide (Agrp)–expressing neurons (47–49). Agrp neurons are apparently more involved in the control of glucose metabolism, specifically the inhibition of HGP by insulin (50), and these neurons are probably also involved in the inhibition of glucose production by AAs. Carefully designed experiments are required to confirm or disprove this idea. Additional research is needed to further elucidate the involvement of hypothalamic astrocytes in AA sensing.
Physiologic relevance of glucoregulation by AAs.
The detailed characterization of the biochemical events in the hypothalamus that allow leucine and proline to regulate the metabolism of glucose in the liver was achieved by enhancing the availability of these AAs in the brain. However, it was important to show whether increases in the circulating concentrations of proline and leucine modified hepatic glucose metabolism and whether these effects were dependent on the metabolism of leucine or proline in the brain (Figure 2). Thus, we also performed experiments in conscious rats in which we separately administered systemic infusions of these AAs to produce moderate elevations of their circulating concentrations while monitoring glucose metabolism during the course of the pancreatic clamp procedure (26, 38). In these studies, elevated concentrations of either leucine or proline acutely and robustly decreased circulating glucose and inhibited HGP (Figure 2). More importantly, inhibition of their metabolism in the hypothalamus markedly blunted the glucoregulatory action of circulating proline and leucine. These results clearly pointed toward a physiologic relevance of hypothalamic AA sensing in the control of glucose metabolism. Furthermore, additional experiments in rats in which we conducted molecular interventions (overexpression of BCKDK in the MBH) to incapacitate the mechanism for leucine sensing made the rats prone to developing hyperglycemia when challenged with a protein-rich meal (26). This result suggested that factors that disrupt central leucine sensing may contribute to the development of disease.
On the basis of the work outlined above as well as previous work by our group, we created the schematic shown in Figure 2, which summarizes the metabolic pathways postulated to be involved in the hypothalamic sensing of AAs. Circulating leucine and proline are metabolized in the MBH to produce 2 common metabolites: acetyl-CoA and malonyl-CoA. The latter is utilized to produce oleoyl-CoA, which, in turn, is proposed to activate hypothalamic KATP channels (51). Activation of the channels would represent one of the earliest events in the generation of a neurogenic signal that is relayed to the liver via the hepatic branch of the vagus nerve. The vagal input modifies the partition of glucose fluxes in the liver, resulting in inhibition of glucose production and a decrease in the circulating concentrations of glucose. This loop would be very active postprandially when the influx of nutrients elevates the concentrations of circulating AAs and glucose and decreases the demand for endogenous (hepatic) glucose. This liver-brain circuit would contribute to other organ systems to prevent excessive increases in circulating glucose (Figure 2).
Diet-induced attenuation of central glucoregulation.
Several studies have shown that short-term, diet-induced insulin resistance partially disrupts the hypothalamic glucoregulatory response to FAs (52, 53). This acquired defect was induced by the consumption of a diet enriched in saturated fat. On the basis of these findings, we hypothesized that high-fat-diet feeding would also perturb the glucoregularory response to AAs. In agreement with this prediction, unpublished results from studies in our laboratory showed that short-term feeding of a saturated fat–enriched diet rapidly blunted the response to leucine and KIC in rats. In the case of proline, we have not yet directly examined this question. We know from previous studies that the hypothalamic sensing of glucose and lactate, both of which need to be converted to pyruvate, is attenuated by high-fat-diet feeding (54). Thus, because proline has to be converted to pyruvate, high-fat feeding is predicted to also attenuate the metabolic response to central proline. Few studies have investigated the mechanisms by which high-fat feeding causes the defects that cripple central nutrient sensing. Importantly, previous studies by our group (52) revealed that high-fat feeding caused a marked decrease in the concentrations of hypothalamic LCFA-CoA in rats. More important, restoring these concentrations through a pharmacologic intervention concomitantly restored central glucoregulation by nutrients. Whether the same mechanism also explains the attenuation of the effects of AAs remains to be fully tested.
Conclusions
Changes in the concentrations of circulating AAs modulate metabolic processes in the liver via sensing mechanisms located in the CNS. The sensing mechanisms that modify liver carbohydrate metabolism appear to be localized in the MBH, possibly the arcuate nucleus, and they respond to the increased availability of various AAs such as leucine, proline, histidine, and possibly several others by curtailing HGP through a reduction in gluconeogenesis and glycogenolysis. Central glucoregulation by nutrients is highly sensitive to diet as indicated by rodent studies in which the consumption of a diet rich in saturated fat rendered the animals less responsive to the action of AAs and other macronutrients. Furthermore, chronic experimental attenuation of leucine sensing may facilitate the development of glycemic dysregulation, leading to hyperglycemia. An understanding of the mechanistic basis for this acquired defect of central nutrient sensing is critical to the design of preventative or therapeutic interventions to avoid the onset of disease. Last, future studies are required to shed light on the neurochemical identity of the neurons, astrocytes, and other associated cells that are responsible for hypothalamic glucoregulation.
Acknowledgments
Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: AA, amino acid; ACC, acetyl-CoA carboxylase; Agrp, Agouti-related peptide; AICAR, 5-aminoimidazole-4-carboxamide ribonucleotide; AMPK, AMP-activated protein kinase; ANLS, astrocyte-neuron lactate shuttle; AOAA, amino-oxyacetic acid; BCAT, branched-chain amino acid aminotransferase; BCKDH, branched-chain α-ketoacid dehydrogenase; BCKDK, branched-chain α-ketoacid dehydrogenase kinase; CIC, α-chloro-isocaproic acid; CNS, central nervous system; CPT, carnitine palmitoyltransferase; Glc-6-Pase, glucose-6-phosphatase; HGP, hepatic glucose production; KATP, ATP-sensitive potassium; KIC, α-ketoisocaproate; LCFA, long-chain fatty acyl; LDH, lactate dehydrogenase; MBH, mediobasal hypothalamus; MCD, malonyl-CoA decarboxylase; MCT, monocarboxylate transporter; mTOR, mammalian target of rapamycin; POMC, proopiomelanocortin; STAT3, signal transducer and activator of transcription 3; Sur1, sulfonylurea receptor 1; α-KG, α-ketoglutarate; Sur1, sulfonylurea receptor 1.
References
- 1.Friedman JM. A war on obesity, not the obese. Science 2003;299:856–8. [DOI] [PubMed] [Google Scholar]
- 2.Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science 2003;299:853–5. [DOI] [PubMed] [Google Scholar]
- 3.Floyd JC Jr, Fajans SS, Conn JW, Knopf RF, Rull J. Stimulation of insulin secretion by amino acids. J Clin Invest 1966;45:1487–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohneda A, Parada E, Eisentraut AM, Unger RH. Characterization of response of circulating glucagon to intraduodenal and intravenous administration of amino acids. J Clin Invest 1968;47:2305–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roden M, Perseghin G, Petersen KF, Hwang JH, Cline GW, Gerow K, Rothman DL, Shulman GI. The roles of insulin and glucagon in the regulation of hepatic glycogen synthesis and turnover in humans. J Clin Invest 1996;97:642–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Felig P. Amino acid metabolism in man. Annu Rev Biochem 1975;44:933–55. [DOI] [PubMed] [Google Scholar]
- 7.Krauss RM, Mayer J. Influence of protein and amino acids on food intake in the rat. Am J Physiol 1965;209:479–83. [DOI] [PubMed] [Google Scholar]
- 8.Scharrer E, Baile CA, Mayer J. Effect of amino acids and protein on foot intake of hyperphagic and recovered aphagic rats. Am J Physiol 1970;218:400–4. [DOI] [PubMed] [Google Scholar]
- 9.Peters JC, Harper AE. Adaptation of rats to diets containing different levels of protein: effects on food intake, plasma and brain amino acid concentrations and brain neurotransmitter metabolism. J Nutr 1985;115:382–98. [DOI] [PubMed] [Google Scholar]
- 10.Block KP, Harper AE. High levels of dietary amino and branched-chain alpha-keto acids alter plasma and brain amino acid concentrations in rats. J Nutr 1991;121:663–71. [DOI] [PubMed] [Google Scholar]
- 11.Smith QR. Transport of glutamate and other amino acids at the blood-brain barrier. J Nutr 2000;130(4S, Suppl):1016S–22S. [DOI] [PubMed] [Google Scholar]
- 12.Choi YH, Fletcher PJ, Anderson GH. Extracellular amino acid profiles in the paraventricular nucleus of the rat hypothalamus are influenced by diet composition. Brain Res 2001;892:320–8. [DOI] [PubMed] [Google Scholar]
- 13.Ciofi P, Garret M, Lapirot O, Lafon P, Loyens A, Prevot V, Levine JE. Brain-endocrine interactions: a microvascular route in the mediobasal hypothalamus. Endocrinology 2009;150:5509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obici S, Rossetti L. Minireview: nutrient sensing and the regulation of insulin action and energy balance. Endocrinology 2003;144:5172–8. [DOI] [PubMed] [Google Scholar]
- 15.Lam TK, Schwartz GJ, Rossetti L. Hypothalamic sensing of fatty acids. Nat Neurosci 2005;8:579–84. [DOI] [PubMed] [Google Scholar]
- 16.Hu Z, Cha SH, Chohnan S, Lane MD. Hypothalamic malonyl-CoA as a mediator of feeding behavior. Proc Natl Acad Sci USA 2003;100:12624–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saha AK, Kurowski TG, Ruderman NB. A malonyl-CoA fuel-sensing mechanism in muscle: effects of insulin, glucose, and denervation. Am J Physiol 1995;269:E283–9. [DOI] [PubMed] [Google Scholar]
- 18.Ruderman NB, Saha AK, Kraegen EW. Minireview: malonyl CoA, AMP-activated protein kinase, and adiposity. Endocrinology 2003;144:5166–71. [DOI] [PubMed] [Google Scholar]
- 19.Obici S, Feng Z, Morgan K, Stein D, Karkanias G, Rossetti L. Central administration of oleic acid inhibits glucose production and food intake. Diabetes 2002;51:271–5. [DOI] [PubMed] [Google Scholar]
- 20.Obici S, Feng Z, Arduini A, Conti R, Rossetti L. Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med 2003;9:756–61. [DOI] [PubMed] [Google Scholar]
- 21.Kasaoka S, Tsuboyama-Kasaoka N, Kawahara Y, Inoue S, Tsuji M, Ezaki O, Kato H, Tsuchiya T, Okuda H, Nakajima S. Histidine supplementation suppresses food intake and fat accumulation in rats. Nutrition 2004;20:991–6. [DOI] [PubMed] [Google Scholar]
- 22.Kimura K, Nakamura Y, Inaba Y, Matsumoto M, Kido Y, Asahara S, Matsuda T, Watanabe H, Maeda A, Inagaki F, et al. Histidine augments the suppression of hepatic glucose production by central insulin action. Diabetes 2013;62:2266–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cutler NS, Pan X, Heitman J, Cardenas ME. The TOR signal transduction cascade controls cellular differentiation in response to nutrients. Mol Biol Cell 2001;12:4103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colombani J, Raisin S, Pantalacci S, Radimerski T, Montagne J, Leopold P. A nutrient sensor mechanism controls Drosophila growth. Cell 2003;114:739–49. [DOI] [PubMed] [Google Scholar]
- 25.Nobukuni T, Joaquin M, Roccio M, Dann SG, Kim SY, Gulati P, Byfield MP, Backer JM, Natt F, Bos JL, et al. Amino acids mediate mTOR/raptor signaling through activation of class 3 phosphatidylinositol 3OH-kinase. Proc Natl Acad Sci USA 2005;102:14238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su Y, Lam TK, He W, Pocai A, Bryan J, Aguilar-Bryan L, Gutierrez-Juarez R. Hypothalamic leucine metabolism regulates liver glucose production. Diabetes 2012;61:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giaccari A, Rossetti L. Predominant role of gluconeogenesis in the hepatic glycogen repletion of diabetic rats. J Clin Invest 1992;89:36–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossetti L, Giaccari A, Barzilai N, Howard K, Sebel G, Hu M. Mechanism by which hyperglycemia inhibits hepatic glucose production in conscious rats: implications for the pathophysiology of fasting hyperglycemia in diabetes. J Clin Invest 1993;92:1126–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch CJ, Halle B, Fujii H, Vary TC, Wallin R, Damuni Z, Hutson SM. Potential role of leucine metabolism in the leucine-signaling pathway involving mTOR. Am J Physiol Endocrinol Metab 2003;285:E854–63. [DOI] [PubMed] [Google Scholar]
- 30.Harper AE, Miller RH, Block KP. Branched-chain amino acid metabolism. Annu Rev Nutr 1984;4:409–54. [DOI] [PubMed] [Google Scholar]
- 31.Brosnan JT, Brosnan ME. Branched-chain amino acids: enzyme and substrate regulation. J Nutr 2006;136(1, Suppl)207S–11S. [DOI] [PubMed] [Google Scholar]
- 32.Gao Z, Young RA, Li G, Najafi H, Buettger C, Sukumvanich SS, Wong RK, Wolf BA, Matschinsky FM. Distinguishing features of leucine and alpha-ketoisocaproate sensing in pancreatic beta-cells. Endocrinology 2003;144:1949–57. [DOI] [PubMed] [Google Scholar]
- 33.Harris RA, Paxton R, DePaoli-Roach AA. Inhibition of branched chain alpha-ketoacid dehydrogenase kinase activity by alpha-chloroisocaproate. J Biol Chem 1982;257:13915–8. [PubMed] [Google Scholar]
- 34.He W, Lam TK, Obici S, Rossetti L. Molecular disruption of hypothalamic nutrient sensing induces obesity. Nat Neurosci 2006;9:227–33. [DOI] [PubMed] [Google Scholar]
- 35.Pocai A, Lam TK, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, Aguilar-Bryan L, Rossetti L. Hypothalamic K(ATP channels control hepatic glucose production. Nature 2005;434:1026–31. [DOI] [PubMed] [Google Scholar]
- 36.Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J. Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J Biol Chem 2000;275:9270–7. [DOI] [PubMed] [Google Scholar]
- 37.Arrieta-Cruz I, Su Y, Gutiérrez-Juárez R. Suppression of endogenous glucose production by isoleucine and valine and impact of diet composition. Nutrients 2016;8:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arrieta-Cruz I, Su Y, Knight CM, Lam TK, Gutierrez-Juarez R. Evidence for a role of proline and hypothalamic astrocytes in the regulation of glucose metabolism in rats. Diabetes 2013;62:1152–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellerin L, Pellegri G, Bittar PG, Charnay Y, Bouras C, Martin JL, Stella N, Magistretti PJ. Evidence supporting the existence of an activity-dependent astrocyte-neuron lactate shuttle. Dev Neurosci 1998;20:291–9. [DOI] [PubMed] [Google Scholar]
- 40.Pellerin L, Magistretti PJ. Neuroenergetics: calling upon astrocytes to satisfy hungry neurons. Neuroscientist 2004;10:53–62. [DOI] [PubMed] [Google Scholar]
- 41.Pellerin L, Pellegri G, Martin JL, Magistretti PJ. Expression of monocarboxylate transporter mRNAs in mouse brain: support for a distinct role of lactate as an energy substrate for the neonatal vs. adult brain. Proc Natl Acad Sci USA 1998;95:3990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam TK, Gutierrez-Juarez R, Pocai A, Rossetti L. Regulation of blood glucose by hypothalamic pyruvate metabolism. Science 2005;309:943–7. [DOI] [PubMed] [Google Scholar]
- 43.Bakken IJ, White LR, Unsgard G, Aasly J, Sonnewald U. [U-13C]Glutamate metabolism in astrocytes during hypoglycemia and hypoxia. J Neurosci Res 1998;51:636–45. [DOI] [PubMed] [Google Scholar]
- 44.Pocai A, Obici S, Schwartz GJ, Rossetti L. A brain-liver circuit regulates glucose homeostasis. Cell Metab 2005;1:53–61. [DOI] [PubMed] [Google Scholar]
- 45.Giaccari A, Rossetti L. Isocratic high-performance liquid chromatographic determination of the concentration and specific radioactivity of phosphoenolpyruvate and uridine diphosphate glucose in tissue extracts. J Chromatogr 1989;497:69–78. [DOI] [PubMed] [Google Scholar]
- 46.Suzuki A, Stern SA, Bozdagi O, Huntley GW, Walker RH, Magistretti PJ, Alberini CM. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 2011;144:810–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat Neurosci 2005;8:1289–91. [DOI] [PubMed] [Google Scholar]
- 48.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 2005;310:683–5. [DOI] [PubMed] [Google Scholar]
- 49.Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 2007;5:438–49. [DOI] [PubMed] [Google Scholar]
- 50.Lin HV, Plum L, Ono H, Gutierrez-Juarez R, Shanabrough M, Borok E, Horvath TL, Rossetti L, Accili D. Divergent regulation of energy expenditure and hepatic glucose production by insulin receptor in agouti-related protein and POMC neurons. Diabetes 2010;59:337–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bränström R, Aspinwall CA, Valimaki S, Ostensson CG, Tibell A, Eckhard M, Brandhorst H, Corkey BE, Berggren PO, Larsson O. Long-chain CoA esters activate human pancreatic beta-cell KATP channels: potential role in type 2 diabetes. Diabetologia 2004;47:277–83. [DOI] [PubMed] [Google Scholar]
- 52.Morgan K, Obici S, Rossetti L. Hypothalamic responses to long-chain fatty acids are nutritionally regulated. J Biol Chem 2004;279:31139–48. [DOI] [PubMed] [Google Scholar]
- 53.Pocai A, Lam TK, Obici S, Gutierrez-Juarez R, Muse ED, Arduini A, Rossetti L. Restoration of hypothalamic lipid sensing normalizes energy and glucose homeostasis in overfed rats. J Clin Invest 2006;116:1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lam TK, Gutierrez-Juarez R, Pocai A, Bhanot S, Tso P, Schwartz GJ, Rossetti L. Brain glucose metabolism controls the hepatic secretion of triglyceride-rich lipoproteins. Nat Med 2007;13:171–80. [DOI] [PubMed] [Google Scholar]