Abstract
In taste buds, glutamate plays a double role as a gustatory stimulus and neuromodulator. The detection of glutamate as a tastant involves several G protein–coupled receptors, including the heterodimer taste receptor type 1, member 1 and 3 as well as metabotropic glutamate receptors (mGluR1 and mGluR4). Both receptor types participate in the detection of glutamate as shown with knockout animals and selective antagonists. At the basal part of taste buds, ionotropic glutamate receptors [N-methyl-d-aspartate (NMDA) and non-NMDA] are expressed and participate in the modulation of the taste signal before its transmission to the brain. Evidence suggests that glutamate has an efferent function on taste cells and modulates the release of other neurotransmitters such as serotonin and ATP. This short article reviews the recent developments in the field with regard to glutamate receptors involved in both functions as well as the influence of glutamate on the taste signal.
Keywords: taste, taste buds, glutamate, umami, T1Rs, neuromodulator
Introduction
l-Glutamate plays a particular role among the various l-amino acids because it elicits a taste distinct from sweet, salty, sour, and bitter. This fifth taste is called “umami” (1), which means savory or delicious in Japanese. Glutamate occurs naturally in many foods and is often present at high concentrations, as in aged cheeses, seafood, and some vegetables. Although umami does not have a pleasant taste by itself (2), it increases the palatability and acceptance of many foods and hence increases food intake (3, 4). For instance, human subjects prefer soups or new food when they contain monosodium glutamate (4, 5). By increasing the palatability of food, researchers have predicted numerous applications for glutamate, such as the reduction in sodium (6) or fat (7) in meals.
The mechanism of glutamate detection involves multiple G protein–coupled receptors, although some of them are not yet completely characterized. In addition to playing a role as a taste stimulus, glutamate may also be a neuromodulator in taste buds. This assumption relies on work from our laboratory and others that showed that glutamate can depolarize taste cells at concentrations below those that are required for its detection as a taste stimulus, and these effects can be mimicked by ionotropic glutamate receptor agonists (8–11). Moreover, different populations of taste cells respond to the different concentrations of glutamate: type II cells express the metabotropic umami taste receptors, whereas the type III cells express a variety of ionotropic glutamate receptors that appear to be activated not by the glutamate in foods but by release of glutamate as a transmitter due to modulatory nerve fiber activity (12–14). This review will focus on these different but important roles of glutamate in taste function.
Current Status of Knowledge
Anatomy and physiology of taste buds.
Taste buds are onion-shaped aggregates of 50–100 elongated taste cells that extend from the basal lamina to the surface of the tongue, where their apical microvilli containing taste receptor proteins protrude through an opening in the epithelium to contact sapid stimuli in the oral cavity. The basolateral membrane forms contacts with afferent nerve fibers, which signal taste-evoked activity to the brain. Taste buds are scattered across the anterior tongue in fungiform papillae innervated by the chorda tympani nerve. Circumvallate and foliate papillae house taste buds on the posterior tongue, which are innervated by the glossopharyngeal nerve. Regardless of location, there are 3 types of taste cells, distinguished by morphologic, molecular, and functional criteria [for review (15)]. Type I cells are generally considered to be support cells, which have a function similar to that of glial cells in the nervous system. These cells have membranes that wrap around the other cell types and contain enzymes for the uptake and degradation of neurotransmitters, including ATP and glutamate. Type II cells contain the taste receptors and signaling effectors for umami, sweet, and bitter compounds. These cells release ATP as a transmitter via a nonvesicular mechanism (16–18) to activate purinergic receptors on afferent nerve fibers. Type III cells are responsive to sour (acidic) and salty stimuli, but the receptors that mediate these responses have not been molecularly identified. Type III cells release a variety of transmitters via vesicular exocytosis (19) to activate afferent fibers, but the receptors involved are still not clear. In addition to the activation of afferent fibers, neurotransmitters released from type II and type III cells can modulate the activity of adjacent taste cells in a paracrine and autocrine fashion to modulate the output of the taste bud (20).
Glutamate as a tastant.
The identity of the glutamate receptors involved in the detection of umami compounds in taste buds has been controversial for many years. However, it is now clear that it is a complex mechanism that involves >1 receptor. The heterodimer taste receptor type 1, member 1 (T1R1) and 3 (T1R3) is the best understood of the glutamate taste receptors. T1R1-T1R3 is broadly tuned and binds all l-amino acids in mice (21), but in humans is narrowly tuned to l-glutamate (22). The receptor also allosterically binds 5′-ribonucleotides, such as inosine 5′-monophosphate (IMP)4 and GMP, which, when present, strongly potentiate the umami taste, particularly in the anterior tongue. Interestingly, in humans, the synergy only occurs between IMP and glutamate, whereas in mice the synergy occurs between IMP and glutamate as well as many other amino acids (21, 22). Binding of both glutamate and nucleotides occurs in the large N-terminal binding domain of the T1R1 monomer (23), which exhibits a venus flytrap binding module similar to other “C type” receptors, such as the metabotropic glutamate receptors. Knockout of either T1R1 or T1R3 decreases taste nerve responses to glutamate and nucleotides as expected, but the results differ according to which laboratory generated the knockout. Zhao et al. (24) showed a complete elimination of all responses to umami compounds for either T1R1 or T1R3 knockout mice. In other studies, knockout of either T1R3 (25) or T1R1 (26) eliminated all or most of the nucleotide potentiation, but considerable responses to glutamate remained, suggesting that additional receptors contribute to umami taste (25, 27). A recent study that used calcium imaging of isolated taste cells in these receptor knockouts confirmed the existence of multiple glutamate receptors and also suggested that some taste cells can even respond to nucleotides in the absence of glutamate (28). However, in isolated taste cells, the stimuli are not restricted to the apical membrane as they are in vivo, and this may account for some of the differences observed.
As originally suggested by Faurion (29), these additional glutamate receptors are likely to be closely related to glutamate receptors found in the brain. Indeed, a metabotropic glutamate receptor (mGluR), mGluR4, and its truncated form called taste-mGluR4 have been identified in taste buds and their expression is restricted to the taste buds (30–32). The use of a behavioral approach, l-2-amino-4-phosphonobutyric acid, a group III mGluR4 agonist, showed responses similar to monosodium glutamate (33, 34). However, the truncated form of mGluR4 requires higher concentrations of glutamate (1–3 mmol/L in adult rats) for activation compared with the brain form of mGluR4 (a few micromolar). This difference may be explained by the 50% truncated N-terminal binding domain in taste-mGluR4, which makes the receptor less sensitive to glutamate (30). The involvement of mGluR4 was confirmed pharmacologically by specific mGluR4 antagonists (26) and was recently validated in vivo by an mGluR4 knockout mouse that showed smaller nerve responses to umami compounds (35). These data confirm that mGluR4 plays a small but relevant role in glutamate taste detection.
mGluR1, a group I metabotropic glutamate receptor, also has an N-terminal truncated form called taste-mGluR1. Both mGluR1 and its truncated form are reported to be expressed in taste buds with the use of RT-PCR and immunohistochemistry (36–38). Although a knockout of mGluR1 has not been tested in taste experiments, a selective antagonist, 1-aminoindan-1,5-dicarboxylic acid, reduces responses to glutamate in chorda tympani and glossopharyngeal nerve recordings (35). Selective agonists for mGluRs from group III (including mGluR4) and group I (including mGluR1) also have been tested in conditioned taste aversion assays, and both groups of agonists caused a conditioned aversion to glutamate at very low concentrations, lower than what would be expected for the truncated versions of the 2 receptors (39). These data suggest that the brain (untruncated) form of the receptors may also be involved in the taste detection of glutamate in mice.
Although the knockout of T1R1 and T1R3 appears to eliminate potentiation with nucleotides in whole-nerve recordings, single-fiber recordings from glutamate-sensitive chorda tympani fibers suggest that at least mGluR1 can show nucleotide potentiation (40). The nucleotide binding sites have not been identified. Interestingly, single-fiber recordings also showed that some fibers with a best sensitivity to sucrose (sweet-best fibers) also respond to glutamate and that the glutamate response is potentiated by nucleotides (40). This may explain why rodents have difficulty distinguishing sweet- and umami-tasting compounds in behavioral studies (41, 42).
In summary, multiple receptors appear to be involved in the detection of glutamate as a taste stimulus. These include the heterodimer T1R1-T1R3, as well as both truncated and brain forms of mGluR4 and mGluR1. The different receptors seem to have different functions in the detection of umami compounds. On one hand, mGluRs are primarily activated by glutamate and not by nucleotides such as IMP or GMP. On the other hand, T1R1-T1R3 receptors are activated by many l-amino acids and by nucleotides. Moreover, each receptor activates a different population of nerve fibers (40) that may be conducting different information to the brain.
Glutamate as a neuromodulator.
Several neurotransmitters have been proposed to play a role in the transmission of the taste signal from taste buds to the brain, including ATP, serotonin, noradrenaline, acetylcholine, or γ-aminobutyric acid [for review (43)]. It is now evident that ATP is necessary for the transmission of the signal (44, 45), whereas the other candidates have a modulatory role. In contrast, glutamate is not likely to be involved in transmitting the taste signal to the nerve fibers, because vesicular transporters for glutamate (VGLUTs) are not expressed in taste cells (12, 46). Instead, the afferent nerve fibers express both VGLUT1 and 2, suggesting that glutamate may be released from the afferent nerve fibers to modulate the taste cells (12, 14, 46). However, to date, glutamate itself has been identified only in the nerve fibers innervating taste buds in the mudpuppy (47, 48); its presence still needs to be shown in the fibers innervating mammalian taste buds.
Physiologic and molecular evidence for a role of glutamate as an “efferent” transmitter is the expression of both N-methyl-d-aspartate (NMDA) and non-NMDA ionotropic glutamate receptors in taste cells (49–51) as well as the kainate receptors GluR6 and KA1 (52) and GluR7 (12). Moreover, glutamate applied at low concentrations, typical of neurotransmitters in the brain, evokes increases in intracellular calcium in taste cells that occur in the cell bodies and basal processes of the taste buds, where the synapses between taste cells and nerve fibers occur. Furthermore, the basolateral application of glutamate, where topical glutamate cannot reach due to the tight junctions, increases the action potential firing rate in some taste cells (13). Finally, a mechanism for glutamate reuptake, a requirement of all neurotransmitter systems, was shown by identifying a glutamate transporter [glutamate-aspartate transporter (GLAST)] in type I cells (53). Taken together, these findings provide strong evidence for a role of glutamate in modulating the taste signal before transmission to the brain. The glutamate receptors identified on taste cells could serve as postsynaptic targets for a potential efferent mechanism or as an axon reflex in response to taste activation of the afferent fibers. Indeed, Huang et al. (14) showed that glutamate at low concentrations elicits release of serotonin from type III cells and consequently causes a decrease in the ATP released from type II cells.
Conclusions
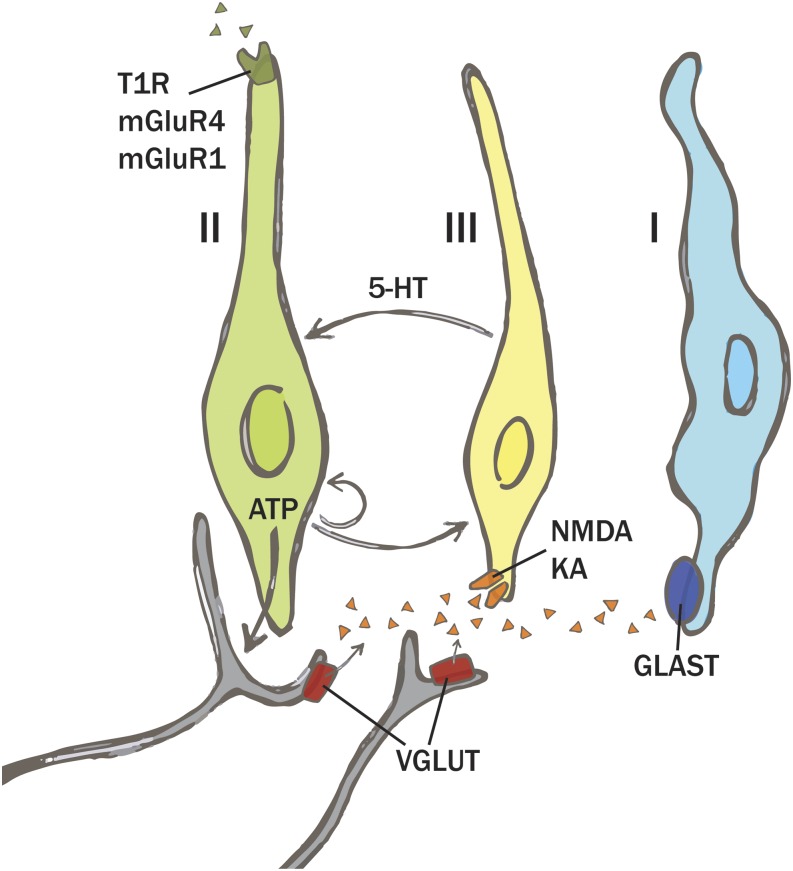
In conclusion, glutamate appears to play a role as both an important taste stimulus and a neuromodulator of taste function, as shown in Figure 1. As a taste stimulus, it activates metabotropic receptors on the apical membrane of type II cells and only at concentrations commonly found in foods that elicit the umami taste quality. In contrast, glutamate functions as a neuromodulator by acting on several ionotropic glutamate receptors located on the basolateral membrane, likely on the type III cells. Several unanswered questions remain for the dual role of glutamate receptors in taste buds. First, are the mGluRs found in the same taste cells as the heterodimer T1R3-T1R1 and do they utilize the same signaling mechanisms? Are there additional, as yet unidentified, umami taste receptors? Concerning the role of glutamate as a taste modulator, what triggers its release from the taste nerve fibers and what is its role in the elaboration of the taste signal to the brain? Additional work in the field will be required to solve these questions.
FIGURE 1.
Simplified model representing the action of glutamate in taste buds. Type II cells express the apical heterodimer T1R1-T1R3, involved in the detection of glutamate as a gustatory stimulus. Although no clear evidence has been shown, mGluR4 and mGluR1 are also most likely to be expressed in the same cell type. The VGLUTs are expressed in fibers innervating the taste cells, suggesting that glutamate is released from those fibers. When released, glutamate activates ionotropic glutamate receptors (NMDA and KA) localized on the basolateral membrane of type III cells, which elicit the release of serotonin (5-HT) and a decrease in the release of ATP from type II cells. Glutamate is then taken up by another transporter, GLAST, expressed on the membrane of type I cells. GLAST, glutamate-aspartate transporter; KA, kainate; mGluR, metabotropic glutamate receptor; NMDA, N-methyl-d-aspartate; T1R1-T1R3, taste receptor type 1, member 1 and 3; VGLUT, vesicular glutamate transporter; 5-HT, serotonin. Figure courtesy of C Wilson.
Acknowledgments
We thank Courtney Wilson for Figure 1 and Thomas Finger for comments on the manuscript. Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: GLAST, glutamate-aspartate transporter; IMP, inosine 5′-monophosphate; mGluR, metabotropic glutamate receptor; NMDA, N-methyl-d-aspartate; T1R1, taste receptor type 1, member 1; T1R3, taste receptor type 1, member 3; VGLUT, vesicular glutamate transporter.
References
- 1.Ikeda K. New seasonings. J Tokyo Chem Soc 1909;30:820–36. [Google Scholar]
- 2.Yamaguchi S. Fundamental properties of umami in human taste sensations. In: Kawamura Y, Kare MR, editors. Umami: a basic taste. New York: Marcel Dekker; 1987. p. 41–73. [Google Scholar]
- 3.Yamaguchi S. Basic properties of umami and effects in humans. Physiol Behav 1991;49:833–41. [DOI] [PubMed] [Google Scholar]
- 4.Bellisle F, Monneuse MO, Chabert M, Larue-Achagiotis C, Lanteaume MT, Louis-Sylvestre J. Monosodium glutamate as a palatability enhancer in the european diet. Physiol Behav 1991;49:869–73. [DOI] [PubMed] [Google Scholar]
- 5.Beauchamp GK, Pearson P. Human development and umami taste. Physiol Behav 1991;49:1009–12. [DOI] [PubMed] [Google Scholar]
- 6.Yamaguchi S, Takahashi C. Interactions of monosodium glutamate and sodium chloride on saltiness and palatability of a clear soup. J Food Sci 1984;49:82–5. [Google Scholar]
- 7.Bellisle F. Experimental studies of food choices and palatability responses in European subjects exposed to the umami taste. Asia Pac J Clin Nutr 2008;17:376–9. Erratum in: Asia Pac J Clin Nutr 2008;17(2):358. [PubMed] [Google Scholar]
- 8.Hayashi Y, Zviman MM, Brand JG, Teeter JH, Restrepo D. Measurement of membrane potential and [Ca2+]i in cell ensembles: application to the study of glutamate taste in mice. Biophys J 1996;71:1057–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bigiani A, Delay RJ, Chaudhari N, Kinnamon SC, Roper SD. Responses to glutamate in rat taste cells. J Neurophysiol 1997;77:3048–59. [DOI] [PubMed] [Google Scholar]
- 10.Lin W, Kinnamon SC. Physiological evidence for ionotropic and metabotropic glutamate receptors in rat taste cells. J Neurophysiol 1999;82:2061–9. [DOI] [PubMed] [Google Scholar]
- 11.Lin W, Ogura T, Kinnamon SC. Responses to di-sodium guanosine 5′-monophosphate and monosodium L-glutamate in taste receptor cells of rat fungiform papillae. J Neurophysiol 2003;89:1434–9. [DOI] [PubMed] [Google Scholar]
- 12.Vandenbeuch A, Tizzano M, Anderson CB, Stone LM, Goldberg D, Kinnamon SC. Evidence for a role of glutamate as an efferent transmitter in taste buds. BMC Neurosci 2010;11:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niki M, Takai S, Kusuhara Y, Ninomiya Y, Yoshida R. Responses to apical and basolateral application of glutamate in mouse fungiform taste cells with action potentials. Cell Mol Neurobiol 2011;31:1033–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang YA, Grant J, Roper S. Glutamate may be an efferent transmitter that elicits inhibition in mouse taste buds. PLoS One 2012;7:e30662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roper SD. Taste buds as peripheral chemosensory processors. Semin Cell Dev Biol 2013;24:71–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Romanov RA, Rogachevskaja OA, Bystrova MF, Jiang P, Margolskee RF, Kolesnikov SS. Afferent neurotransmission mediated by hemichannels in mammalian taste cells. EMBO J 2007;26:657–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang YJ, Maruyama Y, Dvoryanchikov G, Pereira E, Chaudhari N, Roper SD. The role of pannexin 1 hemichannels in ATP release and cell-cell communication in mouse taste buds. Proc Natl Acad Sci USA 2007;104:6436–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taruno A, Vingtdeux V, Ohmoto M, Ma Z, Dvoryanchikov G, Li A, Adrien L, Zhao H, Leung S, Abernethy M, et al. . CALHM1 ion channel mediates purinergic neurotransmission of sweet, bitter and umami tastes. Nature 2013;495:223–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandenbeuch A, Zorec R, Kinnamon SC. Capacitance measurements of regulated exocytosis in mouse taste cells. J Neurosci 2010;30:14695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang YA, Dando R, Roper SD. Autocrine and paracrine roles for ATP and serotonin in mouse taste buds. J Neurosci 2009;29:13909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson G, Chandrashekar J, Hoon MA, Feng L, Zhao G, Ryba NJ, Zuker CS. An amino-acid taste receptor. Nature 2002;416:199–202. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Staszewski L, Xu H, Durick K, Zoller M, Adler E. Human receptor for sweet and umami taste. Proc Natl Acad Sci USA 2002;99:4692–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang F, Klebansky B, Fine RM, Xu H, Pronin A, Liu H, Tachdjian C, Li X. Molecular mechanism for the umami taste synergism. Proc Natl Acad Sci USA 2008;105:20930–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, Zuker CS. The receptors for mammalian sweet and umami taste. Cell 2003;115:255–66. [DOI] [PubMed] [Google Scholar]
- 25.Damak S, Rong M, Yasumatsu K, Kokrashvili Z, Varadarajan V, Zou S, Jiang P, Ninomiya Y, Margolskee RF. Detection of sweet and umami taste in the absence of taste receptor T1r3. Science 2003;301:850–3. [DOI] [PubMed] [Google Scholar]
- 26.Kusuhara Y, Yoshida R, Ohkuri T, Yasumatsu K, Voigt A, Hubner S, Maeda K, Boehm U, Meyerhof W, Ninomiya Y. Taste responses in mice lacking taste receptor subunit T1R1. J Physiol 2013;591:1967–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delay ER, Hernandez NP, Bromley K, Margolskee RF. Sucrose and monosodium glutamate taste thresholds and discrimination ability of T1R3 knockout mice. Chem Senses 2006;31:351–7. [DOI] [PubMed] [Google Scholar]
- 28.Pal Choudhuri S, Delay RJ, Delay ER. L-Amino acids elicit diverse response patterns in taste sensory cells: a role for multiple receptors. PLoS One 2015;10:e0130088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faurion A. Are umami taste receptor sites structurally related to glutamate CNS receptor sites? Physiol Behav 1991;49:905–12. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhari N, Landin AM, Roper SD. A metabotropic glutamate receptor variant functions as a taste receptor. Nat Neurosci 2000;3:113–9. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhari N, Yang H, Lamp C, Delay E, Cartford C, Than T, Roper S. The taste of monosodium glutamate: membrane receptors in taste buds. J Neurosci 1996;16:3817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toyono T, Seta Y, Kataoka S, Harada H, Morotomi T, Kawano S, Shigemoto R, Toyoshima K. Expression of the metabotropic glutamate receptor, mGluR4a, in the taste hairs of taste buds in rat gustatory papillae. Arch Histol Cytol 2002;65:91–6. [DOI] [PubMed] [Google Scholar]
- 33.Delay ER, Beaver AJ, Wagner KA, Stapleton JR, Harbaugh JO, Catron KD, Roper SD. Taste preference synergy between glutamate receptor agonists and inosine monophosphate in rats. Chem Senses 2000;25:507–15. [DOI] [PubMed] [Google Scholar]
- 34.Nakashima K, Katsukawa H, Sasamoto K, Ninomiya Y. Behavioral taste similarities and differences among monosodium L-glutamate and glutamate receptor agonists in C57BL mice. J Nutr Sci Vitaminol (Tokyo) 2001;47:161–6. [DOI] [PubMed] [Google Scholar]
- 35.Yasumatsu K, Manabe T, Yoshida R, Iwatsuki K, Uneyama H, Takahashi I, Ninomiya Y. Involvement of multiple taste receptors in umami taste: analysis of gustatory nerve responses in metabotropic glutamate receptor 4 knockout mice. J Physiol 2015;593:1021–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toyono T, Seta Y, Kataoka S, Kawano S, Shigemoto R, Toyoshima K. Expression of metabotropic glutamate receptor group I in rat gustatory papillae. Cell Tissue Res 2003;313:29–35. [DOI] [PubMed] [Google Scholar]
- 37.San Gabriel A, Maekawa T, Uneyama H, Torii K. The metabotropic glutamate receptor type I in taste tissue. Am J Clin Nutr 2009;90(Suppl):743S–6S. [DOI] [PubMed] [Google Scholar]
- 38.San Gabriel A, Uneyama H, Yoshie S, Torii K. Cloning and characterization of a novel mGluR1 variant from vallate papillae that functions as a receptor for L-glutamate Stimuli. Chem Senses 2005;30(Suppl 1):i25–6. [DOI] [PubMed] [Google Scholar]
- 39.Nakashima K, Eddy MC, Katsukawa H, Delay ER, Ninomiya Y. Behavioral responses to glutamate receptor agonists and antagonists implicate the involvement of brain-expressed mGluR4 and mGluR1 in taste transduction for umami in mice. Physiol Behav 2012;105:709–19. [DOI] [PubMed] [Google Scholar]
- 40.Yasumatsu K, Ogiwara Y, Takai S, Yoshida R, Iwatsuki K, Torii K, Margolskee RF, Ninomiya Y. Umami taste in mice uses multiple receptors and transduction pathways. J Physiol 2012;590:1155–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heyer BR, Taylor-Burds CC, Mitzelfelt JD, Delay ER. Monosodium glutamate and sweet taste: discrimination between the tastes of sweet stimuli and glutamate in rats. Chem Senses 2004;29:721–9. [DOI] [PubMed] [Google Scholar]
- 42.Heyer BR, Taylor-Burds CC, Tran LH, Delay ER. Monosodium glutamate and sweet taste: generalization of conditioned taste aversion between glutamate and sweet stimuli in rats. Chem Senses 2003;28:631–41. [DOI] [PubMed] [Google Scholar]
- 43.Chaudhari N. Synaptic communication and signal processing among sensory cells in taste buds. J Physiol 2014;592:3387–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Finger TE, Danilova V, Barrows J, Bartel DL, Vigers AJ, Stone L, Hellekant G, Kinnamon SC. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science 2005;310:1495–9. [DOI] [PubMed] [Google Scholar]
- 45.Vandenbeuch A, Larson ED, Anderson CB, Smith SA, Ford AP, Finger TE, Kinnamon SC. Postsynaptic P2X3-containing receptors in gustatory nerve fibres mediate responses to all taste qualities in mice. J Physiol 2015;593:1113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Braud A, Boucher Y, Zerari-Mailly F. Vesicular glutamate transporters localization in the rat lingual papillae. Neuroreport 2010;21:64–7. [DOI] [PubMed] [Google Scholar]
- 47.Lu KS, Roper SD. Electron microscopic immunocytochemistry of glutamate-containing nerve fibers in the taste bud of mudpuppy (Necturus maculosus). Microsc Res Tech 1993;26:225–30. [DOI] [PubMed] [Google Scholar]
- 48.Jain S, Roper SD. Immunocytochemistry of gamma-aminobutyric acid, glutamate, serotonin, and histamine in Necturus taste buds. J Comp Neurol 1991;307:675–82. [DOI] [PubMed] [Google Scholar]
- 49.Caicedo A, Jafri MS, Roper SD. In situ Ca2+ imaging reveals neurotransmitter receptors for glutamate in taste receptor cells. J Neurosci 2000;20:7978–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Caicedo A, Kim KN, Roper SD. Glutamate-induced cobalt uptake reveals non-NMDA receptors in rat taste cells. J Comp Neurol 2000;417:315–24. [PubMed] [Google Scholar]
- 51.Kim KN, Caicedo A, Roper SD. Glutamate-induced cobalt uptake reveals non-NMDA receptors in developing rat taste buds. Neuroreport 2001;12:1715–8. [DOI] [PubMed] [Google Scholar]
- 52.Chung KM, Lee SB, Heur R, Cho YK, Lee CH, Jung HY, Chung SH, Lee SP, Kim KN. Glutamate-induced cobalt uptake elicited by kainate receptors in rat taste bud cells. Chem Senses 2005;30:137–43. [DOI] [PubMed] [Google Scholar]
- 53.Lawton DM, Furness DN, Lindemann B, Hackney CM. Localization of the glutamate-aspartate transporter, GLAST, in rat taste buds. Eur J Neurosci 2000;12:3163–71. [DOI] [PubMed] [Google Scholar]