Abstract
The odd-chain fatty acids (OCFAs) pentadecanoic acid (15:0) and heptadecanoic acid (17:0), which account for only a small proportion of total saturated fatty acids in milk fat and ruminant meat, are accepted biomarkers of dairy fat intake. However, they can also be synthesized endogenously, for example, from gut-derived propionic acid (3:0). A number of studies have shown an inverse association between OCFA concentrations in human plasma phospholipids or RBCs and risk of type 2 diabetes and cardiovascular disease. We propose a possible involvement in metabolic regulation from the assumption that there is a link between 15:0 and 17:0 and the metabolism of other short-chain, medium-chain, and longer-chain OCFAs. The OCFAs 15:0 and 17:0 can be elongated to very-long-chain FAs (VLCFAs) such as tricosanoic acid (23:0) and pentacosanoic acid (25:0) in glycosphingolipids, particularly found in brain tissue, or can be derived from these VLCFAs. Their chains can be shortened, yielding propionyl-coenzyme A (CoA). Propionyl-CoA, by succinyl-CoA, can replenish the citric acid cycle (CAC) with anaplerotic intermediates and, thus, improve mitochondrial energy metabolism. Mitochondrial function is compromised in a number of disorders and may be impaired with increasing age. Optimizing anaplerotic intermediate availability for the CAC may help to cope with demands in times of increased metabolic stress and with aging. OCFAs may serve as substrates for synthesis of both odd-numbered VLCFAs and propionyl-CoA or store away excess propionic acid.
Keywords: biomarkers, cardiovascular disease, diabetes, dietary intake, fatty acids
Introduction
Both pentadecanoic acid (15:0) and heptadecanoic acid (17:0), which originate from rumen microbial fermentation, are minor SFAs in ruminant fat (1). Their concentrations in conventionally produced cow milk are on average 1.2% and 0.54% of total FAs, respectively. Concentrations in organically produced milk are somewhat higher (2). Both 15:0 and 17:0 are accepted biomarkers for dairy fat intake (3), because their concentrations in human plasma and RBCs increase with higher intake of dairy fat (4–7). For instance, in the EPIC (European Prospective Investigation into Cancer and Nutrition)-InterAct case-cohort study, concentrations of 15:0 and 17:0 in plasma phospholipids were on average 0.21% and 0.41% of total FAs, respectively (5). Interestingly, although 17:0 is present in plasma at approximately twice the concentration of 15:0 [reviewed in (8)] or even more in RBCs (4), the association with dairy fat intake is stronger for 15:0 than for 17:0 (4, 6, 7).
Because odd-chain FAs (OCFAs)3 were detected not only in human plasma and RBCs but also in liver, buccal, and adipose tissues (9), it is conceivable that 15:0 and 17:0 are incorporated into most if not all human tissues. Data from animal studies are supportive of this hypothesis (10). Interestingly, OCFAs are also found in plasma phospholipids of humans on a vegan diet, albeit less than in omnivores and vegetarians (7). Of note, concentrations of 17:0 in RBCs of vegans were as high as in dairy fat consumers (11).
Interest in 15:0 and 17:0 grew as cohort studies (12–14) and case-control studies (4, 15, 16) found an inverse association between OCFA concentration (15:0, 17:0, or both combined) in plasma phospholipids or RBCs and the risk of cardiovascular disease (CVD). Likewise, an inverse association was found with risk of type 2 diabetes (T2D) in cohort studies (17–20) and case-control studies (5, 21, 22), which was significant in all cases except one (19). Thus, there is reason to consider a physiologic function of these OCFAs beyond their role as dietary constituents and specific biomarkers of dairy intake. The purpose of this review is to list potential sources and to present somewhat provocative new ideas about their potential function and metabolic fate to stimulate future research.
Possible Routes of Endogenous Synthesis
The observations that RBCs of vegans, vegetarians, and omnivores contain comparable concentrations of OCFAs (11) and that 17:0 is present in higher concentration than 15:0 in human plasma (8) suggest that there must be other sources of these OCFAs than ruminant fat.
Synthesis by elongation of shorter-chain OCFAs
The first possibility is synthesis from propionic acid (3:0) or other OCFAs shorter than 15:0. Fermentation of dietary fiber by the colonic microbiota is the primary source of SCFAs in humans, that is, acetic acid (2:0), propionic acid, and butyric acid (4:0). Most gut microbial propionic acid is absorbed and mostly metabolized by the liver (23). Data from rodents show that feeding dietary fiber results in a measurable increase of both SCFAs, including propionic acid, and also of 15:0 and 17:0 in plasma phospholipids (24). In the EPIC study, the OCFA concentrations in plasma phospholipids were also significantly associated with the intake of fruits and vegetables naturally rich in fibers (5).
Beyond bacterial synthesis in the gut, endogenous propionic acid can originate from degradation of amino acids such as methionine, valine, isoleucine, and threonine. Shorter-chain OCFAs may also arise from peroxisomal oxidation of the cholesterol side chain during bile acid formation (25) and from peroxisomal α-decarboxylation, followed by successive β-oxidative degradation of the branched-chain FA phytanic acid (3,7,11,15-tetramethyl hexadecanoic acid) (26) (Figure 1). Phytanic acid is derived from phytol, the chlorophyll side-chain that is released during rumen microbial fermentation (27). Dairy fat is its major dietary source (7).
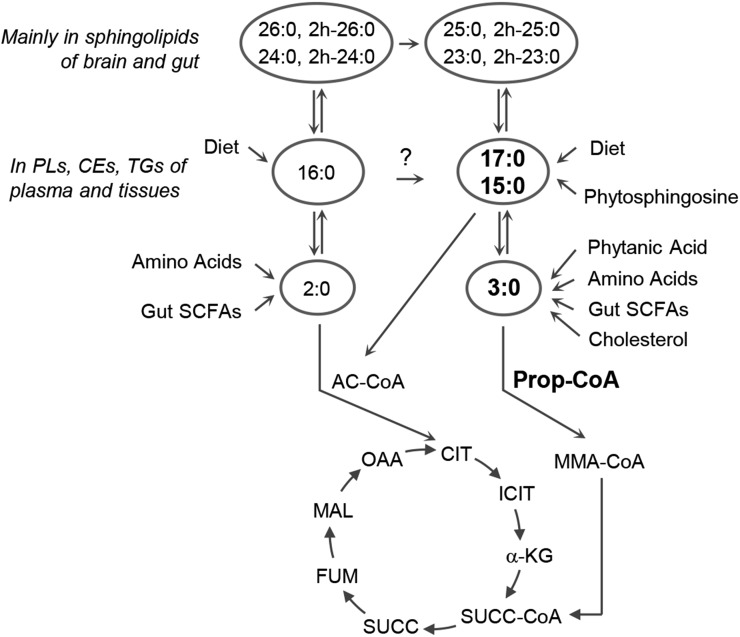
FIGURE 1.
Odd-chain FAs 15:0 and 17:0, their potential exogenous and endogenous origin, and their involvement in metabolic pathways. Only flux into and exchange between pools are depicted. AC-CoA, acetyl-CoA; CE, cholesteryl ester; CIT, citrate; FUM, fumarate; ICIT, isocitrate; MAL, malate; MMA-CoA, methylmalonyl-CoA; OAA, oxaloacetate; PL, phospholipid; Prop-CoA, propionyl-CoA; SUCC, succinate; SUCC-CoA, succinyl-CoA; α-KG, α-ketoglutarate.
The first evidence for an endogenous synthesis of 15:0 and 17:0 from (labeled) propionic acid was provided in subjects with the rare genetic disorders propionic acidemia (PA) and methylmalonic acidemia (28). Normally, propionic acid is converted to propionyl-CoA and enters the citric acid cycle (CAC) at the level of succinyl-CoA. However, deficiencies of propionyl-CoA carboxylase or methylmalonyl-CoA mutase, respectively, block this pathway, leading to unusually high concentrations of 15:0 and 17:0 in a number of tissues (29, 30).
That other OCFAs shorter than 15:0 are both elongated and shortened by β-oxidation was proven in mice; feeding a heptanoic acid (7:0)-enriched diet increased both various longer-chain OCFAs, including 17:0, in liver and skeletal muscle (31) and also shorter-chain OCFAs in plasma and brain, that is, propionic acid and pentanoic acid (5:0) (32). Interestingly, labeled acetic acid also gave rise to OCFAs in the rat brain (33).
Synthesis by chain-shortening of VLCFAs
The OCFAs 15:0 and 17:0 may also be derived from even-numbered hydroxylated very-long-chain FAs (VLCFAs). 2-Hydroxylated FAs (2-hFAs) are SFAs with a hydroxyl group at the C-2 position. Obligatory first step in the degradation of 2-hFAs is the removal of the α-carbon by peroxisomal α-oxidation (34). It may be assumed that partial peroxisomal β-oxidation of the ensuing odd-numbered VLCFAs, for example, 23:0 or 25:0, yields 15:0 and 17:0 (35). The 2-hFAs are important components of mammalian glycosphingolipids, that is, cerebrosides, sulfatides, and gangliosides of nervous tissue, mainly the brain (34, 36, 37). The proportion of 2-hFAs in human brain cerebrosides is ≤70% (36), with 2-hydroxy tetracosanoic acid being the most abundant 2-hFA in humans (36) and other species (37). 2-hFAs are also found in many other tissues such as epidermis and kidney (34) and the small intestine (38).
Other potential sources
Another source of 15:0 may be phytosphingosine, also called dihydrosphingosine, a sphingoid base of glycosphingolipids. Phytosphingosine is degraded to 2-hydroxy hexadecanoic acid, which is finally α-oxidized to produce 15:0 (39). In fact, glycosphingolipids of the rat small intestine mucosa contain far more phytosphingosine than sphingosine (38). However, the concentration in human tissues is not known. 15:0 may also be formed from hexadecanoic acid (16:0) after intermediate hydroxylation. This was observed in cultured differentiating adipocytes (40), as outlined before (8). The relevance of this pathway in vivo is unknown.
Conceivable Biological Functions of the OCFAs 15:0 and 17:0
When different 13C-labeled FAs were administered in equimolar concentrations to mice, appearance of labeled 17:0 in tissues was higher and disappearance was delayed compared with 16:0, octadecanoic acid (18:0), and octanoic acid (8:0) (41). In line with this, 15:0 and 17:0 were less preferred for β-oxidation than even-numbered FAs (42). The metabolic benefit of the OCFAs 15:0 and 17:0 may be 2- or even 3-fold, namely 1) serving as a substrate for the synthesis of odd-numbered VLCFAs for glycosphingolipids in the brain and elsewhere, 2) providing anaplerotic intermediates for the CAC by conversion to propionyl-CoA and further on to succinyl-CoA (Figure 1), and 3) removing excess propionic acid from the circulation.
Synthesis of odd-numbered VLCFAs
As described in the Synthesis by chain-shortening of VLCFAs section, 15:0 and 17:0 may be the result of degradation of odd-numbered VLCFAs. So why not, in turn, be starting material for their synthesis? Propionic acid (33, 43) and, more importantly, OCFAs of intermediate-chain length (43) were used for synthesis of odd-numbered VLCFAs in rat brain. Apparently, the elongation systems act on 16-, 17- and 18-carbon FAs (43). To date, the contribution of 15:0 and 17:0 elongation to overall odd-numbered VLCFA synthesis is not clear. Moreover, it remains elusive which cells, tissues, and organs beside the brain, surely first and foremost the gut, would take advantage of such possibility. The fact that 23:0 and 25:0 account for 10% of total nonhydroxylated and their 2-hFA equivalents even for 25% of total hydroxylated FAs in adult human brain glycosphingolipids (36) indicate considerable physiologic relevance of OCFAs. Human plasma sphingomyelins contain ∼7% 23:0 (44).
Providing anaplerotic intermediates for the CAC
The CAC is the final common pathway for the oxidation of the fuel molecules glucose, FAs, and amino acids for energy production in mitochondria. Intermediates of the CAC are used for biosyntheses (cataplerotic pathways) and need to be replenished (anaplerotic pathways) to keep up CAC function. Anaplerotic substrates may enter the CAC at the level of α-ketoglutarate, succinyl-CoA, and oxaloacetate. β-Oxidation of OCFAs, for example, 7:0 or 17:0, yields 1 propionyl-CoA beside 1 or several acetyl-CoA molecules. Propionyl-CoA can be converted to methylmalonyl-CoA and further to succinyl-CoA for the CAC (Figure 1).
As mentioned, mitochondrial function is compromised in a number of disorders. PA and methylmalonic acidemia, whereby enzyme defects produce a deficit of succinyl-CoA (45), are associated with ultrastructural mitochondrial abnormalities (46) and disturbed mitochondrial energy production (45, 46). LCFA oxidation disorders are also associated with impaired mitochondrial energy production (47, 48). In brain ischemia, large amounts of glutamate are released, and glutamate synthesis depletes stores of α-ketoglutarate (32). If defects in substrate utilization lead to energy deficits, then providing anaplerotic intermediates to the CAC would be a strategy to improve mitochondrial metabolism (31). Treatment with triheptanoin, odd-numbered TGs that contain 7:0, compared with even-numbered medium-chain TGs that contain 8:0 and decanoic acid (10:0), improved symptoms of cardiomyopathy and skeletal muscle damage in patients with LCFA oxidation disorders (47, 48). In a mouse model exposed to ischemic stroke, triheptanoin pretreatment maintained brain mitochondrial function (32). In methyl-CpG binding protein 2 knockout mice, having a disturbed CAC function, triheptanoin treatment improved mitochondria architecture and metabolic variables, including glucose tolerance (31). We herein hypothesize that such beneficial effects are also conceivable for 15:0 and 17:0 when chain-shortened and converted to anaplerotic substrates.
It may be questioned if an increased supply of OCFAs may benefit healthy subjects (47). Indeed, triheptanoin produced little benefit in wild-type compared with methyl-CpG binding protein 2 knockout mice (31). However, the risk of CVD is associated with advancing age (49). Likewise, T2D is more common in older subjects. Aging goes along with impaired mitochondrial function (49, 50). Dysfunctional mitochondria were observed in a wide range of CVDs, and alterations in mitochondrial function are considered to be a major contributing factor (49, 50). It is generally accepted that mitochondrial dysfunction is related to insulin resistance and T2D, but observations also argue against a causal involvement (51–53). No matter if mitochondrial dysfunction is the contributor or the consequence of insulin resistance, targeting it might be an effective way to improve insulin signaling in skeletal muscle and to restore glucose homoeostasis (51, 52).
Removal of excess propionic acid from the circulation
Certain rare gastrointestinal disorders can lead to PA with, however, still poorly understood implications for neurologic disorders (54). With normal propionyl-CoA carboxylase and methylmalonyl-CoA mutase activities, this may provide succinyl-CoA in excess of requirements and shut down the first half of the CAC, thus causing mitochondrial dysfunction as well (54). In healthy subjects too, propionic acid concentrations in plasma and tissues will vary day to day and hour to hour. Increased synthesis of 15:0 and 17:0 as a consequence of increased propionic acid availability, as suggested from an inulin-fed mouse model (24), may help to fine-tune the flow of propionic acid into the CAC and to divert excess propionic acid into neutral pools.
Conclusion
The robust inverse association of 15:0 and/or 17:0 concentrations in plasma phospholipids or RBCs with CVD and T2D risk is quite impressive. The latter is observed in various European populations with different dietary backgrounds (5). This review brings forward hypotheses about the possible sources of 15:0 and 17:0 and their potential involvement in metabolic pathways. They may be used for synthesis of odd-numbered VLCFAs, provide anaplerotic intermediates for the CAC, or store away excess propionic acid. Whether one or all of the suggested metabolic roles apply and whether there is a link to CVD and T2D risk needs to be addressed in appropriate experimental studies. Dairy OCFAs can enlarge the pool of 15:0 and 17:0 in body tissues. The reason why 17:0 is found in tissues at higher concentrations than any other shorter or longer OCFA may be its intermediate-chain length between 16:0 and 18:0. It may thus easily fit into lipid structures of phospholipids, glycosphingolipids, cholesteryl esters, and TGs.
Acknowledgments
We thank Bernhard Watzl for valuable suggestions and for critically reading the manuscript. Both authors read and approved the final manuscript.
Footnotes
Abbreviations used: CAC, citric acid cycle; CVD, cardiovascular disease; LCFA, long-chain fatty acid; OCFA, odd-chain fatty acid; PA, propionic acidemia; T2D, type 2 diabetes; VLCFA, very-long-chain fatty acid; 2-hFA, 2-hydroxylated fatty acid; α-KG, α-ketoglutarate.
References
- 1.Ratnayake WM. Concerns about the use of 15:0, 17:0, and trans-16:1n-7 as biomarkers of dairy fat intake in recent observational studies that suggest beneficial effects of dairy food on incidence of diabetes and stroke. Am J Clin Nutr 2015;101:1102–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kusche D, Kuhnt K, Ruebesam K, Rohrer C, Nierop AF, Jahreis G, Baars T. Fatty acid profiles and antioxidants of organic and conventional milk from low- and high-input systems during outdoor period. J Sci Food Agric 2015;95:529–39. [DOI] [PubMed] [Google Scholar]
- 3.Yakoob MY, Shi P, Hu FB, Campos H, Rexrode KM, Orav EJ, Willett WC, Mozaffarian D. Circulating biomarkers of dairy fat and risk of incident stroke in U.S. men and women in 2 large prospective cohorts. Am J Clin Nutr 2014;100:1437–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Q, Ma J, Campos H, Hu FB. Plasma and erythrocyte biomarkers of dairy fat intake and risk of ischemic heart disease. Am J Clin Nutr 2007;86:929–37. [DOI] [PubMed] [Google Scholar]
- 5.Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB, Crowe FL, Huerta JM, Guevara M, Beulens JW, et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol 2014;2:810–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golley RK, Hendrie GA. Evaluation of the relative concentration of serum fatty acids C14:0, C15:0 and C17:0 as markers of children’s dairy fat intake. Ann Nutr Metab 2014;65:310–6. [DOI] [PubMed] [Google Scholar]
- 7.Allen NE, Grace PB, Ginn A, Travis RC, Roddam AW, Appleby PN, Key T. Phytanic acid: measurement of plasma concentrations by gas-liquid chromatography-mass spectrometry analysis and associations with diet and other plasma fatty acids. Br J Nutr 2008;99:653–9. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins B, West JA, Koulman A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic Acid (c15:0) and heptadecanoic Acid (c17:0) in health and disease. Molecules 2015;20:2425–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hodson L, Eyles HC, McLachlan KJ, Bell ML, Green TJ, Skeaff CM. Plasma and erythrocyte fatty acids reflect intakes of saturated and n-6 PUFA within a similar time frame. J Nutr 2014;144:33–41. [DOI] [PubMed] [Google Scholar]
- 10.Mitchaothai J, Yuangklang C, Wittayakun S, Vasupen K, Wongsutthavas S, Srenanul P, Hovenier R, Everts H, Beynen AC. Effect of dietary fat type on meat quality and fatty acid composition of various tissues in growing-finishing swine. Meat Sci 2007;76:95–101. [DOI] [PubMed] [Google Scholar]
- 11.Kornsteiner M, Singer I, Elmadfa I. Very low n-3 long-chain polyunsaturated fatty acid status in Austrian vegetarians and vegans. Ann Nutr Metab 2008;52:37–47. [DOI] [PubMed] [Google Scholar]
- 12.Yamagishi K, Nettleton JA, Folsom AR. Plasma fatty acid composition and incident heart failure in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am Heart J 2008;156:965–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khaw KT, Friesen MD, Riboli E, Luben R, Wareham N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: the EPIC-Norfolk prospective study. PLoS Med 2012;9:e1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Oliveira Otto MC, Nettleton JA, Lemaitre RN, Steffen LM, Kromhout D, Rich SS, Tsai MY, Jacobs DR, Mozaffarian D. Biomarkers of dairy fatty acids and risk of cardiovascular disease in the Multi-ethnic Study of Atherosclerosis. J Am Heart Assoc 2013;2:e000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warensjö E, Jansson JH, Berglund L, Boman K, Ahrén B, Weinehall L, Lindahl B, Hallmans G, Vessby B. Estimated intake of milk fat is negatively associated with cardiovascular risk factors and does not increase the risk of a first acute myocardial infarction. A prospective case-control study. Br J Nutr 2004;91:635–42. [DOI] [PubMed] [Google Scholar]
- 16.Warensjö E, Jansson JH, Cederholm T, Boman K, Eliasson M, Hallmans G, Johansson I, Sjögren P. Biomarkers of milk fat and the risk of myocardial infarction in men and women: a prospective, matched case-control study. Am J Clin Nutr 2010;92:194–202. [DOI] [PubMed] [Google Scholar]
- 17.Hodge AM, English DR, O’Dea K, Sinclair AJ, Makrides M, Gibson RA, Giles GG. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 2007;86:189–97. [DOI] [PubMed] [Google Scholar]
- 18.Patel PS, Sharp SJ, Jansen E, Luben RN, Khaw KT, Wareham NJ, Forouhi NG. Fatty acids measured in plasma and erythrocyte-membrane phospholipids and derived by food-frequency questionnaire and the risk of new-onset type 2 diabetes: a pilot study in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Norfolk cohort. Am J Clin Nutr 2010;92:1214–22. [DOI] [PubMed] [Google Scholar]
- 19.Mozaffarian D, de Oliveira Otto MC, Lemaitre RN, Fretts AM, Hotamisligil G, Tsai MY, Siscovick DS, Nettleton JA. trans-Palmitoleic acid, other dairy fat biomarkers, and incident diabetes: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 2013;97:854–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santaren ID, Watkins SM, Liese AD, Wagenknecht LE, Rewers MJ, Haffner SM, Lorenzo C, Hanley AJ. Serum pentadecanoic acid (15:0), a short-term marker of dairy food intake, is inversely associated with incident type 2 diabetes and its underlying disorders. Am J Clin Nutr 2014;100:1532–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krachler B, Norberg M, Eriksson JW, Hallmans G, Johansson I, Vessby B, Weinehall L, Lindahl B. Fatty acid profile of the erythrocyte membrane preceding development of Type 2 diabetes mellitus. Nutr Metab Cardiovasc Dis 2008;18:503–10. [DOI] [PubMed] [Google Scholar]
- 22.Kröger J, Zietemann V, Enzenbach C, Weikert C, Jansen EH, Dö F, Joost HG, Boeing H, Schulze MB. Erythrocyte membrane phospholipid fatty acids, desaturase activity, and dietary fatty acids in relation to risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Am J Clin Nutr 2011;93:127–42. [DOI] [PubMed] [Google Scholar]
- 23.Al-Lahham SH, Peppelenbosch MP, Roelofsen H, Vonk RJ, Venema K. Biological effects of propionic acid in humans; metabolism, potential applications and underlying mechanisms. Biochim Biophys Acta 2010;1801:1175–83. [DOI] [PubMed] [Google Scholar]
- 24.Weitkunat K, Schumann S, Petzke KJ, Blaut M, Loh G, Klaus S. Effects of dietary inulin on bacterial growth, short-chain fatty acid production and hepatic lipid metabolism in gnotobiotic mice. J Nutr Biochem 2015;26:929–37. [DOI] [PubMed] [Google Scholar]
- 25.Pedersen JI. Peroxisomal oxidation of the steroid side chain in bile acid formation. Biochimie 1993;75:159–65. [DOI] [PubMed] [Google Scholar]
- 26.Hutton D, Steinberg D. Identification of propionate as a degradation product of phytanic acid oxidation in rat and human tissues. J Biol Chem 1973;248:6871–5. [PubMed] [Google Scholar]
- 27.Hellgren LI. Phytanic acid–an overlooked bioactive fatty acid in dairy fat? Ann N Y Acad Sci 2010;1190:42–9. [DOI] [PubMed] [Google Scholar]
- 28.Oizumi J, Giudici TA, Ng WG, Shaw KN, Donnell GN. Propionate metabolism by cultured skin fibroblasts from normal individuals and patients with methylmalonicaciduria and propionicacidemia. Biochem Med 1981;26:28–40. [DOI] [PubMed] [Google Scholar]
- 29.Sperl W, Murr C, Skladal D, Sass JO, Suormala T, Baumgartner R, Wendel U. Odd-numbered long-chain fatty acids in propionic acidaemia. Eur J Pediatr 2000;159:54–8. [DOI] [PubMed] [Google Scholar]
- 30.Kishimoto Y, Williams M, Moser HW, Hignite C, Biermann K. Branched-chain and odd-numbered fatty acids and aldehydes in the nervous system of a patient with deranged vitamin B 12 metabolism. J Lipid Res 1973;14:69–77. [PubMed] [Google Scholar]
- 31.Park MJ, Aja S, Li Q, Degano AL, Penati J, Zhuo J, Roe CR, Ronnett GV. Anaplerotic triheptanoin diet enhances mitochondrial substrate use to remodel the metabolome and improve lifespan, motor function, and sociability in MeCP2-null mice. PLoS One 2014;9:e109527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwarzkopf TM, Koch K, Klein J. Reduced severity of ischemic stroke and improvement of mitochondrial function after dietary treatment with the anaplerotic substance triheptanoin. Neuroscience 2015;300:201–9. [DOI] [PubMed] [Google Scholar]
- 33.Hajra AK, Radin NS. Biosynthesis of cerebroside odd-numbered fatty acids. J Lipid Res 1962;3:327–32. [Google Scholar]
- 34.Hama H. Fatty acid 2-Hydroxylation in mammalian sphingolipid biology. Biochim Biophys Acta 2010;1801:405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Osmundsen H, Bremer J, Pedersen JI. Metabolic aspects of peroxisomal beta-oxidation. Biochim Biophys Acta 1991;1085:141–58. [DOI] [PubMed] [Google Scholar]
- 36.Svennerholm L, Stallberg-Stenhagen S. Changes in the fatty acid composition of cerebrosides and sulfatides of human nervous tissue with age. J Lipid Res 1968;9:215–25. [PubMed] [Google Scholar]
- 37.Kishimoto Y, Radin NS. Composition of cerebroside acids as a function of age. J Lipid Res 1959;1:79–82. [Google Scholar]
- 38.Dahiya R, Brasitus TA. Distribution of glycosphingolipids and ceramide of rat small intestinal mucosa. Lipids 1986;21:112–6. [DOI] [PubMed] [Google Scholar]
- 39.Kondo N, Ohno Y, Yamagata M, Obara T, Seki N, Kitamura T, Naganuma T, Kihara A. Identification of the phytosphingosine metabolic pathway leading to odd-numbered fatty acids. Nat Commun 2014;5:5338. [DOI] [PubMed] [Google Scholar]
- 40.Roberts LD, Virtue S, Vidal-Puig A, Nicholls AW, Griffin JL. Metabolic phenotyping of a model of adipocyte differentiation. Physiol Genomics 2009;39:109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gotoh N, Moroda K, Watanabe H, Yoshinaga K, Tanaka M, Mizobe H, Ichioka K, Tokairin S, Wada S. Metabolism of odd-numbered fatty acids and even-numbered fatty acids in mouse. J Oleo Sci 2008;57:293–9. [DOI] [PubMed] [Google Scholar]
- 42.Shibata R, Gotoh N, Kubo A, Kanda J, Nagai T, Mizobe H, Yoshinaga K, Kojima K, Watanabe H, Wada S. Comparison of catabolism rate of fatty acids to carbon dioxide in mice. Eur J Lipid Sci Technol 2012;114:1340–4. [Google Scholar]
- 43.Kishimoto Y, Radin NS. Metabolism of brain glycolipid fatty acids. Lipids 1966;1:47–61. [DOI] [PubMed] [Google Scholar]
- 44.Phillips GB, Dodge JT. Composition of phospholipids and of phospholipid fatty acids of human plasma. J Lipid Res 1967;8:676–81. [PubMed] [Google Scholar]
- 45.Wajner M, Goodman SI. Disruption of mitochondrial homeostasis in organic acidurias: insights from human and animal studies. J Bioenerg Biomembr 2011;43:31–8. [DOI] [PubMed] [Google Scholar]
- 46.Schwab MA, Sauer SW, Okun JG, Nijtmans LG, Rodenburg RJ, van den Heuvel LP, Drose S, Brandt U, Hoffmann GF, Ter Laak H, et al. Secondary mitochondrial dysfunction in propionic aciduria: a pathogenic role for endogenous mitochondrial toxins. Biochem J 2006;398:107–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roe CR, Sweetman L, Roe DS, David F, Brunengraber H. Treatment of cardiomyopathy and rhabdomyolysis in long-chain fat oxidation disorders using an anaplerotic odd-chain triglyceride. J Clin Invest 2002;110:259–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vockley J, Marsden D, McCracken E, DeWard S, Barone A, Hsu K, Kakkis E. Long-term major clinical outcomes in patients with long chain fatty acid oxidation disorders before and after transition to triheptanoin treatment–A retrospective chart review. Mol Genet Metab 2015;116:53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marzetti E, Csiszar A, Dutta D, Balagopal G, Calvani R, Leeuwenburgh C. Role of mitochondrial dysfunction and altered autophagy in cardiovascular aging and disease: from mechanisms to therapeutics. Am J Physiol Heart Circ Physiol 2013;305:H459–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wohlgemuth SE, Calvani R, Marzetti E. The interplay between autophagy and mitochondrial dysfunction in oxidative stress-induced cardiac aging and pathology. J Mol Cell Cardiol 2014;71:62–70. [DOI] [PubMed] [Google Scholar]
- 51.Rieusset J. Contribution of mitochondria and endoplasmic reticulum dysfunction in insulin resistance: Distinct or interrelated roles? Diabetes Metab 2015;41:358–68. [DOI] [PubMed] [Google Scholar]
- 52.Montgomery MK, Turner N. Mitochondrial dysfunction and insulin resistance: an update. Endocr Connect 2015;4:R1–R15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crescenzo R, Bianco F, Mazzoli A, Giacco A, Liverini G, Iossa S. Mitochondrial efficiency and insulin resistance. Front Physiol 2015;5:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Frye RE, Rose S, Slattery J, MacFabe DF. Gastrointestinal dysfunction in autism spectrum disorder: the role of the mitochondria and the enteric microbiome. Microb Ecol Health Dis 2015;26:27458. [DOI] [PMC free article] [PubMed] [Google Scholar]