Abstract
Accumulation of proteinaceous amyloid β plaques and tau oligomers may occur several years before the onset of Alzheimer disease (AD). Under normal circumstances, misfolded proteins get cleared by proteasome degradation, autophagy, and the recently discovered brain glymphatic system, an astroglial-mediated interstitial fluid bulk flow. It has been shown that the activity of the glymphatic system is higher during sleep and disengaged or low during wakefulness. As a consequence, poor sleep quality, which is associated with dementia, might negatively affect glymphatic system activity, thus contributing to amyloid accumulation. The diet is another important factor to consider in the regulation of this complex network. Diets characterized by high intakes of refined sugars, salt, animal-derived proteins and fats and by low intakes of fruit and vegetables are associated with a higher risk of AD and can perturb the circadian modulation of cortisol secretion, which is associated with poor sleep quality. For this reason, diets and nutritional interventions aimed at restoring cortisol concentrations may ease sleep disorders and may facilitate brain clearance, consequentially reducing the risk of cognitive impairment and dementia. Here, we describe the associations that exist between sleep, cortisol regulation, and diet and their possible implications for the risk of cognitive impairment and AD.
Keywords: Alzheimer disease, glymphatic system, sleep, cortisol, hippocampus, Western diet, acidosis, nutritional interventions, supplements
Introduction
Late-onset Alzheimer disease (AD)8 is a progressive neurodegenerative syndrome, mostly occurring after 65 y of age and characterized by the accumulation of proteinaceous amyloid β (Aβ) plaques and formation of neurofibrillary tangles (1, 2). The disease sequelae often include the development of a progressive cerebral atrophy, cognitive decline, and ultimately death (3).
Formation of both Aβ plaques and neurofibrillary tangles seems to be part of the normal aging process, as revealed by brain immunohistochemical analyses of cognitively normal older people (4).
Aβ and tau oligomers get physiologically cleared by ubiquitination-proteasome degradation, sumoylation, and autophagy (5, 6). If these process results are inefficient, protein aggregates can progressively accumulate, causing neuronal degeneration (6–9).
In addition, the recently discovered glymphatic system, an astroglial-mediated interstitial fluid bulk flow, was shown to play a key role in the regulation of amyloid clearance from the brain by the perivascular space surrounding brain blood vessels (10, 11). This system also plays a role in the brain-wide distribution of growth factors, neuromodulators, glucose, lipids, and amino acids (12). Importantly, the activity of the glymphatic system is higher during sleep and lower (or disengaged) during wakefulness (12). This has special relevance for AD because sleep disorders, such as obstructive sleep apnea (OSA), are associated with the onset of dementia (13–15). In particular, sleep disorders are shown to compromise the normal functioning of the glymphatic system and, as a consequence, contribute to the accumulation of misfolded proteins (16).
Western and/or poorly balanced diets, characterized by high intakes of refined sugars, animal-derived proteins, and saturated fats and a concomitant low intake of plant-based foods, are known to be associated with higher risk of AD (17–20) and can increase the secretion of glucocorticoids (e.g., cortisol), catecholamine, and serotonin, causing oxidative stress (21, 22). Deregulated circadian cortisol concentrations are associated with mild cognitive impairment (MCI) and AD, suggesting that glucocorticoids and cortisol, in particular, may play a role in the onset and/or the progression of AD (23).
Deregulated cortisol concentrations are also correlated with poor sleep quality (24), and even partial acute sleep loss may deregulate cortisol release (25). Nutritional interventions aimed at restoring cortisol concentrations may positively influence sleep quality, thus promoting the regulation of brain clearance systems and a reduction of brain amyloids and, consequentially, reduce the risk of cognitive impairment and dementia. Here, we discuss the role of sleep in the regulation of the glymphatic system and amyloid clearance, the associations between deregulated cortisol concentrations and sleep, and the possible implications for the risk of cognitive impairment and AD, highlighting the role of diet in the modulation of these factors.
Sleep Disorders Are Correlated with the Risk of AD
Lifestyle risk factors associated with AD: a focus on sleep disorders.
It was hypothesized that AD might be much more complex than an amyloidosis- and tauopathy-related syndrome. Although advancing age is considered the main risk factor for developing late-onset AD (26–28), strong evidence suggests that AD is strongly correlated with diabetes and the metabolic syndrome (20, 29–35) and cardiovascular diseases (36–49), suggesting the involvement of systemic, metabolic, and multifactorial mechanisms in the etiopathogenesis of AD. Among the risk factors that were implicated in the pathogenesis of type 2 diabetes, metabolic syndrome, and AD, insulin resistance, imbalance of glucocorticoid concentrations, inflammation, dysfunctions of mitochondrial metabolism, oxidative stress, and hyperhomocysteinemia were reported to play a main role (50–52).
In addition, specific dietary patterns can play a role in the onset of neurodegeneration and dementia (18, 19), such as a low intake of plant-derived foods, which was related to a higher risk of diabetes, metabolic syndrome, and dementia (20).
In addition, sleep disorders seem to play an important role in the onset of AD (13–15). Differences in sleep patterns seem more prominent during the early stages of dementia than during later stages (53). However, a 2012 clinical cross-sectional study that involved 431 patients (204 affected by AD, 138 with MCI, 43 with vascular dementia, 25 with frontotemporal dementia, and 21 with Lewy body or Parkinson disease) measured several types of sleep disturbances (i.e., sleep-disordered breathing, rapid eye movement behavior disorder, restless legs syndrome, and excessive daytime sleepiness) and reported that patients affected by MCI or by AD had the same frequency of sleep disturbances of any type (∼65% prevalence) (54). Analogously, another epidemiologic study of 236 patients affected by different subtypes of dementia showed that insomnia, in particular, was mostly present in persons affected by AD and that persons affected by MCI had the same frequency of any sleep disturbance as AD patients (55).
Among sleep disorders, OSA is characterized by impaired delivery of oxygen (i.e., hypoxia and hypoxemia), which perturbs neuronal homeostasis, and triggers neuronal degeneration and apoptosis (3). Hypoxia and hypoxemia are correlated with sympathetic activation, neuroinflammation, oxidative stress, and several other pathologic perturbations that cause neurodegeneration (3) and late-onset AD (56).
Sleep restriction can also decrease insulin sensitivity, as reported in healthy subjects (57, 58). Further studies that enrolled higher numbers of participants should be conducted to confirm these observations.
Pharmacologic treatments of sleep disturbances (e.g., melatonin, benzodiazepines, non-benzodiazepine hypnotics, trazodone, and ramelteon) in subjects affected by AD were shown inconclusive and were characterized by uncertainty about the balance of benefits and the risks associated with these treatments, as reported by an analysis of all relevant randomized controlled trials that compared the effects of drugs with placebo (59). Only in the case of a low dose of trazodone (i.e., 50 mg administered at night for 2 wk) was some evidence of efficacy, although larger clinical trials will be needed to allow more definitive conclusions and to establish actual risks and benefits (59).
A recent randomized, double-blind, parallel-group study conducted with 80 patients diagnosed with mild-to-moderate AD, with or without insomnia comorbidity, and receiving standard treatments (i.e., acetylcholinesterase inhibitors, with or without memantine) reported that treatment with prolonged-release melatonin (2 mg, administered nightly for 24 wk, followed by 2 wk of placebo) could improve cognitive functioning and sleep quality compared with placebo, and positive effects were particularly evident in those patients who presented with insomnia comorbidity (60).
However, the use of benzodiazepine might increase the risk of AD, as shown in 1796 subjects with a first diagnosis of AD compared with 7184 matched controls, who started using benzodiazepines ≥5 y before the diagnosis of AD (61). For this reason, long-term use of benzodiazepines should be considered as a possible public health concern (61), and their long-term use should not be encouraged (62).
Glymphatic system and role of sleep in the regulation of amyloid clearance.
The glymphatic system, which was first described in 2012, is known to control interstitial solute and fluid clearance from the brain. This system is an interstitial fluid bulk that was shown to regulate brain amyloid clearance by the perivascular space that surrounds cerebral blood vessels (10, 11, 63, 64). As a consequence, dysfunctions of the glymphatic systems might play a role in the onset or consolidation of AD and dementia.
The glymphatic system constitutes perivascular tunnels formed by astroglial cells, and its bulk flow is driven by cerebrovascular pulsation and facilitated by aquaporin-dependent astroglial water flux (65). The glymphatic system plays a role not just in the elimination of soluble proteins and metabolites from the brain but also in the brain-wide distribution of growth factors, neuromodulators, glucose, lipids, and amino acids (12). The activity of the glymphatic system was analyzed in rat models (66, 67); however, to date no clinically approved approaches are developed to evaluate the functionality of the glymphatic system in humans.
Interestingly, dysfunctions of the glymphatic system were recently hypothesized to play a role in glaucoma pathogenesis (68), which is characterized by a progressive degeneration of retinal ganglion cells and accumulation of Aβ, which suggests possible associations with AD (69, 70).
The activity of the glymphatic system is higher during sleep or anesthesia and lower or disengaged during wakefulness (12). Accordingly, sleep plays a critical role in ensuring brain metabolic homeostasis and clearance of potentially neurotoxic waste products, such as amyloids (64). This has special relevance for AD, because sleep disorders, such as OSA and insomnia, are associated with the onset of dementia (13–15, 54, 55), as reported in the previous section. As a consequence, sleep disorders might compromise the normal functioning of the glymphatic system and contribute to the accumulation of misfolded proteins in the brain (16). Accordingly, sleep disorders seem to be associated with early deposition of Aβ plaques (71, 72). In particular, OSA was shown to induce Aβ accumulation, hyperphosphorylation of tau, and synaptic dysfunction (56).
Increased stress and decreased sleep were both linked to accumulations of Aβ in animal models (73, 74), and sleep was shown to regulate several synaptic markers in Drosophila and possibly the metabolism of a number of central nervous system proteins (75, 76).
In addition, body posture during sleep seems to matter. In particular, lateral position, compared with the supine or prone positions, may increase glymphatic transport, as reported in rats (77). Future studies should be conducted to confirm the possible relevance of these observations in humans.
Impact of sleep disorders on hippocampal volume.
Sleep and sleep deprivation exert a bidirectional control on hippocampus-dependent memory consolidation, by modulating signaling pathways that regulate synapsis formation and plasticity (78).
Smaller hippocampal volumes were associated with AD (79–81), neurodegenerative and psychiatric diseases, and mild cognitive impairment (82), and were linked to lower sleep efficiency (82). Accordingly, patients with primary insomnia, compared with control subjects, were characterized by bilateral atrophy of the hippocampus and cognitive impairment (i.e., reduced verbal memory, verbal information processing, and verbal fluency), suggesting that patients affected by chronic sleep disturbances might be at higher risk of cognitive impairment (83).
Both the dentate gyrus of the hippocampus and the subventricular zone of the lateral ventricles are implicated in adult neurogenesis (84–86), which plays a key role, particularly in the case of the hippocampus, in the maintenance of memory processes and the regulation of emotionality (87). In line with this, it is hypothesized that prolonged sleep restriction or disruption may drive a cumulative decrease of hippocampal neuronal cell proliferation, decrease of neuronal cell survival, and neurogenesis (87).
Some observational studies and preliminary clinical trials have suggested that some modifiable factors, such as cognitive stimulation, physical exercise, and the treatment of general medical conditions associated with reduced hippocampal volume and hippocampal atrophy (e.g., obesity, diabetes, hypertension, hypoxic brain injury, OSA, bipolar disorder, cognitive decline, depression, and head trauma) can reverse hippocampal atrophy or even expand hippocampal size (88).
Diet and Specific Nutrients Can Affect Cortisol Regulation, Sleep Quality, and the Risk of AD
Deregulated cortisol concentrations can play a role in the onset of AD: possible associated mechanisms.
Cortisol is a glucocorticoid hormone produced in humans by the adrenal cortex within the adrenal gland in response to stress and low concentration of blood glucose. Its release is mediated by the hypothalamic-pituitary-adrenal (HPA) axis and follows a circadian rhythm characterized by a morning peak [or cortisol awakening response (CAR)], a slow decline throughout the day, and a low or undetectable amount at midnight (89). The hippocampus and other brain structures, such as the amygdala, the prefrontal cortex, and the suprachiasmatic nucleus, play a role in the physiologic regulation of CAR (90).
High diurnal salivary cortisol concentrations were described in subjects affected by amnestic MCI (91, 92), in cognitively normal elderly individuals who experienced subjective memory complaints (93), in nondepressed community-dwelling elderly people (94), especially if homozygous for the apoE ɛ4 allele (95), and in subjects affected by AD and their caregivers (96–98).
Conversely, another study reported that subjects affected by MCI had cortisol concentrations similar to concentrations of the normal elderly group, but lower than young controls (99). Possible conflicting data might be due to differences in study design, such as the time of sampling (i.e., daytime compared with nighttime) and even the season, because cortisol concentrations can change according to seasons (100).
The hippocampus contains a high concentration of corticosteroid receptors and, for this reason, is particularly responsive to cortisol (81), dehydroepiandrosterone sulfate (DHEAS), and other stress hormones that are known to regulate hippocampal plasticity, excitability, long-term potentiation, and depression (101). Opposite to acute glucocorticoid elevations, which were shown to play protective effects (102), chronic release of high glucocorticoid and high nocturnal cortisol concentrations were associated with smaller hippocampal volumes (103, 104). In addition, high cortisol concentrations, in association with reduced hippocampal volumes and cognitive decline, were observed in patients with AD (105, 106), eventually presenting a parallel reduction of DHEAS secretion (101). Accordingly, plasma cortisol and apo A-II, as well as several other cerebrospinal fluid markers (e.g., fibroblast growth factor 4, heart-type FA binding protein, calcitonin, and tumor necrosis factor-related apoptosis-inducing ligand receptor 3) could serve as useful biomarkers to predict midterm progression from MCI to AD, as shown in a recent cohort study that enrolled 928 patients with MCI at baseline (107). Analogously, high cerebrospinal fluid cortisol concentrations were found associated with more rapid clinical worsening and cognitive decline in MCI-AD, suggesting that dysregulation of the HPA axis may occur at early (MCI) stage of AD, possibly accelerating disease progression (23). In addition, high concentrations of morning basal cortisol were associated with lower cognitive functions in postmenopausal women (108).
Activation of plasma macrophages and brain microglia can cause release of proinflammatory cytokines, leading to hypersecretion of cortisol, possibly contributing to the progress from depression to dementia (109). High cortisol concentrations can also downregulate the synthesis of neurotrophic factors and inhibit neuronal repair mechanisms (109).
High concentrations of glucocorticoids and, in particular, cortisol were reported in subjects affected by other hypercortisolemic diseases, such as Cushing disease and depression. Elevated cortisol concentrations in Cushing disease were found associated with cognitive decline, in particular with a reduction of verbal learning, several subtests of learning, delayed recall, and visual–spatial ability, as shown in 48 patients with a first episode of acute, untreated Cushing disease compared with 38 healthy control subjects, suggesting an impairment of both the neocortex and hippocampus (110).
However, although individuals affected by Cushing disease and depression present constantly elevated concentrations of cortisol, mild-to-moderate AD stages are generally characterized by hyperactivity of the HPA axis, a perturbation of the circadian rhythm of cortisol release, and an insufficiency of glucocorticoid receptor signaling (111).
In addition, it was reported that high glucocorticoid and cortisol concentrations may contribute to amyloid formation and may potentiate their toxicity (112, 113). Conversely, DHEA and DHEAS or even inhibition of glucocorticoid release may have neuroprotective effects (113–115).
Glucocorticoids were reported to elevate Aβ production by increasing amyloid precursor protein expression also in primary cultures of astrocytes (116), and activated astrocytes were shown to contribute to Aβ production (117).
Moreover, deregulated cortisol concentrations were shown correlated with diminished sleep quality and insomnia (24), which are associated with AD (55), as we commented in the previous paragraph. However, even partial acute sleep loss can alter the negative glucocorticoid feedback regulation, inducing elevation of cortisol the next evening (25).
Importantly, Western diet and, more in general, low-quality dietary patterns, characterized by high consumption of meat, saturated fat, and refined sugars, are known to be associated with obesity, diabetes, lower cognitive function, reduction of hippocampal volumes, and AD (118). In the next sections we discuss how specific dietary patterns and nutritional interventions can affect cortisol regulation, possibly affecting sleep quality, which might have implications for the risk of cognitive impairment and AD.
Effects of diet in the modulation of circadian cortisol concentrations.
Dietary composition plays an important role in the regulation of glucocorticoid and cortisol release. In particular, Western-like diets, characterized by high intakes of refined carbohydrates, animal proteins, and saturated fats and low intake of plant-based nutrients, fibers, and antioxidants, which are associated with a higher risk of AD (17–20), can upregulate the release of cortisol, catecholamine, and serotonin, causing oxidative stress (21, 22).
It was reported that an isocaloric high-protein diet can increase the amount of cortisol and also of lean body mass, total ghrelin (also known as the “hunger hormone"), growth hormone, and testosterone, as shown in untrained healthy young men (119). In addition, increased intakes of saturated fats were reported to elevate salivary cortisol, as shown in subjects at risk of psychosis (120).
Diets characterized by intakes high in fat and low in fruit and vegetables can decrease CAR and can deregulate cortisol diurnal profile, as reported by an observational study of 24 young adults (aged 18–22 y), who were compared with 48 community-dwelling older adults (aged 65–88 y) (121).
It should be considered that the association between diet and glucocorticoid release is bidirectional; that is, not only the diet can influence glucocorticoid and cortisol release, but also deregulated glucocorticoid and cortisol concentrations can increase the craving for and consumption of low-quality foods, rich in calories, sugar, and fat (102, 122).
Furthermore, Western-like diets, typically rich in animal proteins and salt and deficient in fruit and vegetables, are typically acidogenic; that is, they can cause a subclinical or low-grade state of metabolic acidosis (123, 124). This metabolic acidosis is responsible for increased bone resorption and loss of calcium from bone tissue (22), loss of muscular mass, sarcopenia, negative nitrogen balance (125), and cancer formation (124). In addition, conditions of brain acidosis occur on brain ischemia and hypoxia (126), particularly in association with hyperglycemia and diabetes (127). Under acidic conditions, some AD-related enzymes have shown altered activities, such as the asparaginyl endopeptidase, which results more active in the presence of acidosis, consequentially leading to tau hyperphosphorylation, as shown in histopathologically confirmed AD brain tissues and in SH-SY5Y cells in vitro (127). In addition, brain acidosis can cause endothelial cell and cholinergic neuron degeneration, as shown in an organotypic brain slice model made with brain capillary endothelial cells and cholinergic neurons cultured in medium at pH < 6.6 (128).
Importantly, acid-base balance can also influence adrenal hormone production of cortisol. Indeed, a reduction of bicarbonate concentrations can stimulate the kidneys to upregulate glutaminase activity and to produce cortisol (129). In this regard, a protein-rich diet, low in organic potassium salts, was reported to cause a moderate metabolic acidosis associated with an increase in cortisol production and a consequential increased risk of insulin resistance and type 2 diabetes (130). Accordingly, some studies have reported that a transiently induced metabolic acidosis, as a consequence of a high protein meal intake, can enhance serum and salivary cortisol concentrations in a dose-dependent manner (131).
Considering these bidirectional mechanisms and the important role played by cortisol on sleep regulation, it is conceivable that unbalanced/Western-like diets, by inducing metabolic and brain acidosis, might lead to an alteration of the circadian rhythm of cortisol release, which may affect sleep quality. These effects might be implicated in the onset of AD and cognitive impairment (Figure 1). However, to our knowledge to date no studies directly assess these associations in large cohorts of elderly, MCI, or AD subjects. In the next section we highlight how specific nutritional interventions or supplements can positively modulate cortisol concentrations and ameliorate sleep quality.
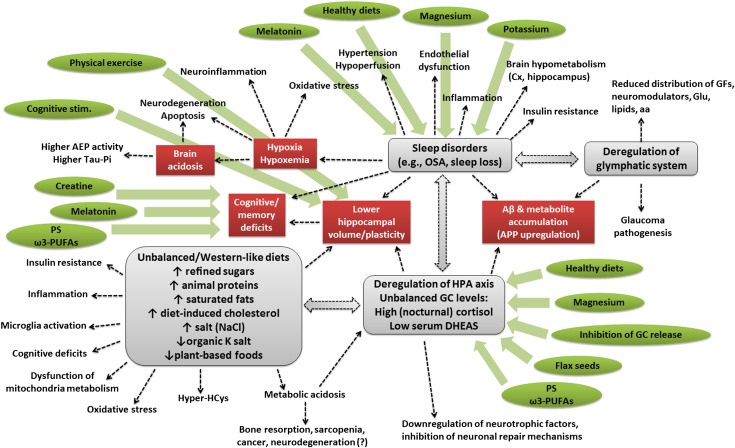
FIGURE 1.
Schematic representation of the complex network underlying the onset of cognitive impairment and eventually AD. Imbalanced, Western-like, high-fat diets elicit deregulation of the HPA axis and of GC (e.g., cortisol) release and lower hippocampal volumes, besides inducing a plethora of other effects (e.g., insulin resistance, inflammation, microglia activation, cognitive impairments, dysfunction of mitochondria metabolism, oxidative stress, hyperhomocysteinemia, and metabolic acidosis), which typically characterize type 2 diabetes, metabolic syndrome, and AD. Deregulation of cortisol release can affect sleep quality, reduce hippocampal volume, promote the accumulation of Aβ plaques and other metabolites, downregulate the synthesis of neurotrophic factors, and inhibit neuronal repair mechanisms. Sleep disorders may themselves compromise cortisol release; reduce hippocampal volume and plasticity; induce cognitive and memory deficits; elicit hypoxia and hypoxemia (which is responsible for increased brain acidosis, neuronal degeneration, inflammation, oxidative stress, induction of asparaginyl endopeptidase activity, and increase of phospho-tau); increase hypertension and hypoperfusion, endothelial dysfunctions, inflammation, cortical and hippocampal hypometabolism, and insulin resistance; and reduce the functionality of the glymphatic system. Deregulations of the glymphatic system can cause accumulation of Aβ plaques and other metabolites; reduce the distribution of growth factors, neuromodulators, glucose, lipids, and amino acids; and can contribute to glaucoma pathogenesis. Some dietary interventions and supplements (indicated in green), such as plant-based and Mediterranean (healthy) diets, PS and ω-3 PUFAs, melatonin, creatine, magnesium, potassium, flax seed cultivars, and inhibitors of GC release, in combination with cognitive stimulation and physical exercise, can soothe ≥1 of these risk factors and, for this reason, might be considered as nonpharmacologic interventions aimed at preventing the risk of AD or reducing its symptoms. Aβ, amyloid β AD, Alzheimer disease; AEP, asparaginyl endopeptidase; APP, amyloid precursor protein; Cx, cortex; DHEAS, dehydroepiandrosterone sulfate; GC, glucocorticoid; GF, growth factor; Glu, glucose; HPA, hypothalamic-pituitary-adrenal; Hyper-HCys, hyperhomocysteinemia; OSA, obstructive sleep anea; PS, phosphatidylserine.
Effects of dietary and nutritional interventions on cortisol regulation, sleep quality, and cognitive functions.
Specific dietary and nutritional interventions might reverse metabolic acidosis, possibly restoring cortisol regulation, sleep quality and, consequentially, may preserve cognitive functions or reduce cognitive impairments.
High adherence to a healthy, well-balanced diet characterized by adequate intakes of fruits, vegetables, whole grains, and fish and moderate/low consumption of saturated fat, trans fat, dietary cholesterol, refined sugars, and salt, as recommended by the American Heart Association, was reported to reduce urinary cortisol concentrations and to elevate serum DHEAS in women, although reducing urinary norepinephrine values in men, as shown in 1318 Puerto Rican adult subjects living in Boston, MA (132).
Analogously, adherence to a Mediterranean diet, with a high intake of MUFAs, seems to positively modulate the HPA axis and cortisol regulation and induce lower abdominal fat distribution, as reported in a cohort of women from a Mediterranean area (Murcia, Spain) (133). In addition, Mediterranean dietary intervention composed of predominantly plant-based foods (i.e., vegetables, fruits, olive oil, legumes, whole-grain cereals, nuts, and seeds) and fish and a low intake of processed foods, dairy products, red meat, and vegetable oils induced an amelioration of cognitive performances, a reduction of inflammation, and oxidative stress and improved psychological well-being factors (e.g., sleep, stress, anxiety, and depression), as shown in elderly healthy Australians (134).
On the contrary, high-fat diets can have a profound impact on microglia activation and the maintenance of cognitive functions. High-fat diets can induce hippocampal inflammatory cytokine production, loss of synaptic protein expression, impairment of hippocampus-dependent memory, and reduction of long-term potentiation in mice (135). Similarly, diet-induced hypercholesterolemia was found to increase both Aβ and phospho-tau and induce microglial activation in Aβ25–35-injected mice, resulting in spatial learning and memory deficits (136). However, because most of these studies were conducted in animal models, it will be important to confirm these results in humans.
Moreover, specific nutrients and supplements were reported to positively affect cortisol regulation and, for this reason, might be suitable to improve sleep quality. For instance, flax seed cultivars, in particular Linola 989, the strain with the highest content of lignan and the lowest content of α-linolenic acid, were reported to reduce responses to stress and plasma cortisol concentrations in 35 postmenopausal women with vascular disease (137). However, the possible effects of flax seed cultivars on sleep regulation and quality were not reported in this study.
Supplementations with the phospholipid phosphatidylserine, together with omega-3 PUFAs 3 times/d for 12 wk were shown to reduce cortisol basal concentrations and to regulate circadian rhythm of salivary cortisol, reducing symptoms in elderly subjects with major depression (138). For this reason, phosphatidylserine alone or in combination with ω-3 PUFAs might positively regulate sleep quality, preserving or increasing brain functions (139). A study reported that bovine cortex-derived phosphatidylserine supplementation for 12 wk induced an improvement of both standard and computerized neuropsychological performance tests in elderly patients with MCI, compared with control subjects who were administered a placebo (140). Similar results were observed with different phosphatidylserine preparations, alternative to the use of bovine cortex-derived phosphatidylserine that might raise concerns of prion transmission. In this regard, a phosphatidylserine preparation that contained ω-3 PUFAs attached to the phosphatidylserine backbone and supplemented for a period of 6 wk (141), or for 7 and 15 wk (142), was proven effective in nondemented elderly subjects with memory complaints. Analogous effects were described with a soybean-derived phosphatidylserine, administered daily for 12 wk to elderly volunteers with memory complaints (143).
In addition, magnesium deficiency is strongly correlated with insomnia, and deficit of magnesium, coupled with excess of calcium, may cause major depression and mental health problems (144). A 2012 clinical trial conducted in 46 elderly subjects showed that daily supplementation of 500 mg Mg compared with placebo for 8 wk significantly increased sleep time, sleep efficiency, the concentration of serum renin, and melatonin and significantly decreased serum cortisol concentration (145).
In addition, potassium is important to guarantee sleep duration, as reported in young men taking oral potassium chloride supplements for 1 wk compared with identical placebo capsules (146). Direct effects of potassium supplementation on cortisol regulation were not reported in this study, although there is evidence that potassium supplementation can elevate serum cortisol (147).
Sleep deprivation can cause a decrease of creatine in the brain, negatively affecting cognitive and psychomotor performance, and mood state. Therefore, creatine supplementations might help reduce these negative effects. In this regard, double-blinded intervention studies assessed the effects elicited by creatine monohydrate (5 g supplemented 4 times/d for 1 wk) compared with placebo and showed that after 24-h sleep deprivation, with mild or moderate exercise, creatine supplementation ameliorated cognitive and psychomotor performance and eventually mood state, although plasma concentrations of catecholamines and cortisol did not differ in the 2 groups (148, 149).
Further Considerations
The risk of dementia and sporadic/late-onset AD is strongly associated with lifestyle factors. In particular, diet, sleep quality, and circadian cortisol regulation, which have been indicated as possible risk factors for AD (13–15, 18–20, 53, 96, 97), are known to be interconnected by regulatory patterns. Another important aspect in this complex network is the recently discovered glymphatic system, which is known to play a role in the clearance of misfolded proteins from the brain and is known to be functional during sleep (10–12, 63–65).
Perturbations of either one of these variables might have an impact on the others and were found to be associated with hippocampal volume reduction (82, 83, 90) and consequentially cognitive impairment and/or AD (79–81).
Considering the pivotal role played by diet in the initiation or consolidation of cognitive impairment and dementia (17–20), it is conceivable that poorly balanced nutritional patterns, with high intakes of refined sugar, animal products, high-calorie foods, and saturated fats, by negatively influencing the circadian rhythm of cortisol release, might perturb sleep quality, thus contributing to the impairment of amyloid clearance pathways (e.g., the glymphatic system), the accumulation of amyloids, and the reduction of hippocampal volumes (Figure 1). However, it should be considered that to date no studies describe a direct effect of deregulated cortisol concentrations on the glymphatic system.
In addition, considering that there are no clinically approved approaches to evaluate the functionality of the glymphatic pathway in humans, current and future research efforts should aim at assessing, by mean of neuroimaging readouts, the effects of dietary interventions in the regulation of the glymphatic system, Aβ clearance, and brain metabolism in MCI and AD patients.
Another aspect to consider that deserves further extensive dissertation is the role of physical activity in the regulation of circadian cortisol rhythm, sleep, cognition, and the prevention of AD, as recently indicated (91).
In addition, intervention strategies aimed at modulating endogenous neurogenesis through the use of neural stem cell-based therapies (150), lifestyle intervention strategies, or pro-neurogenic factors (151, 152) may help promote the regenerative and recovery process in the aging brain (153).
In conclusion, multidisciplinary approaches that involve strategies aimed at promoting healthy lifestyle and that reduce comorbidity-related neurotoxicity and neurodegeneration could be successful in delaying the onset of cognitive impairment and dementia, as recently shown (154). Similar initiatives are currently encouraged and supported both in Europe (155, 156) and in the United States (157). Further research is clearly needed to define intervention strategies aimed at ameliorating diet and sleep quality and to provide recommendations to implement preventative and therapeutic strategies.
Acknowledgments
All authors read and approved the final manuscript.
Footnotes
Abbreviations used: Aβ, amyloid β AD, Alzheimer disease; CAR, cortisol awakening response; DHEAS, dehydroepiandrosterone sulfate; HPA, hypothalamic-pituitary-adrenal; MCI, mild cognitive impairment; OSA, obstructive sleep apnea.
References
- 1.Alzheimer’s Disease International [Internet]. World Alzheimer Report; 2009 [cited 2015 Mar 19]. Available from: http://www.alz.co.uk/research/files/WorldAlzheimerReport.pdf.
- 2.Alzheimer’s Association [Internet]. Alzheimer’s Disease Facts and Figures; 2013 [cited 2015 Mar 19]. Available from: http://www.alz.org/downloads/facts_figures_2013.pdf.
- 3.Daulatzai MA. Quintessential risk factors: their role in promoting cognitive dysfunction and Alzheimer’s disease. Neurochem Res 2012;37:2627–58. [DOI] [PubMed] [Google Scholar]
- 4.Selkoe DJ. Resolving controversies on the path to Alzheimer’s therapeutics. Nat Med 2011;17:1060–5. [DOI] [PubMed] [Google Scholar]
- 5.Kevei É, Hoppe T. Ubiquitin sets the timer: impacts on aging and longevity. Nat Struct Mol Biol 2014;21:290–2. [DOI] [PubMed] [Google Scholar]
- 6.Deger JM, Gerson JE, Kayed R. The interrelationship of proteasome impairment and oligomeric intermediates in neurodegeneration. Aging Cell 2015;14:715–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wolfe DM, Lee JH, Kumar A, Lee S, Orenstein SJ, Nixon RA. Autophagy failure in Alzheimer’s disease and the role of defective lysosomal acidification. Eur J Neurosci 2013;37:1949–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nixon RA, Yang DS. Autophagy failure in Alzheimer’s disease–locating the primary defect. Neurobiol Dis 2011;43:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hol EM, Fischer DF, Ovaa H, Scheper W. Ubiquitin proteasome system as a pharmacological target in neurodegeneration. Expert Rev Neurother 2006;6:1337–47. [DOI] [PubMed] [Google Scholar]
- 10.Kyrtsos CR, Baras JS. Modeling the role of the glymphatic pathway and cerebral blood vessel properties in Alzheimer’s disease pathogenesis. PLoS One 2015;10:e0139574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, et al. Clearance systems in the brain-implications for Alzheimer disease. Nat Rev Neurol 2015;11:457–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jessen NA, Munk AS, Lundgaard I, Nedergaard M. The glymphatic system: a beginner’s guide. Neurochem Res 2015;40:2583–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lim MM, Gerstner JR, Holtzman DM. The sleep-wake cycle and Alzheimer’s disease: what do we know? Neurodegener Dis Manag 2014;4:351–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Videnovic A, Lazar AS, Barker RA, Overeem S. ‘The clocks that time us’-circadian rhythms in neurodegenerative disorders. Nat Rev Neurol 2014;10:683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peter-Derex L, Yammine P, Bastuji H, Croisile B. Sleep and Alzheimer’s disease. Sleep Med Rev 2015;19:29–38. [DOI] [PubMed] [Google Scholar]
- 16.Mendelsohn AR, Larrick JW. Sleep facilitates clearance of metabolites from the brain: glymphatic function in aging and neurodegenerative diseases. Rejuvenation Res 2013;16:518–23. [DOI] [PubMed] [Google Scholar]
- 17.Berrino F. [Western diet and Alzheimer's disease] Epidemiol Prev 2002;26:107–15 (in Italian). [PubMed]
- 18.Oboudiyat C, Glazer H, Seifan A, Greer C, Isaacson RS. Alzheimer’s disease. Semin Neurol 2013;33:313–29. [DOI] [PubMed] [Google Scholar]
- 19.Nicolia V, Lucarelli M, Fuso A. Environment, epigenetics and neurodegeneration: focus on nutrition in Alzheimer’s disease. Exp Gerontol 2015;68:8–12. [DOI] [PubMed] [Google Scholar]
- 20.Pistollato F, Battino M. Role of plant-based diets in the prevention and regression of metabolic syndrome and neurodegenerative diseases. Trends Food Sci Technol 2014;40:62–81. [Google Scholar]
- 21.Singh RB, Gupta S, Dherange P, De Meester F, Wilczynska A, Alam SE, Pella D, Wilson DW. Metabolic syndrome: a brain disease. Can J Physiol Pharmacol 2012;90:1171–83. [DOI] [PubMed] [Google Scholar]
- 22.Maurer M, Riesen W, Muser J, Hulter HN, Krapf R. Neutralization of Western diet inhibits bone resorption independently of K intake and reduces cortisol secretion in humans. Am J Physiol Renal Physiol 2003;284:F32–40. [DOI] [PubMed] [Google Scholar]
- 23.Popp J, Wolfsgruber S, Heuser I, Peters O, Hull M, Schroder J, Moller HJ, Lewczuk P, Schneider A, Jahn H, et al. Cerebrospinal fluid cortisol and clinical disease progression in MCI and dementia of Alzheimer’s type. Neurobiol Aging 2015;36:601–7. [DOI] [PubMed] [Google Scholar]
- 24.Backhaus J, Junghanns K, Hohagen F. Sleep disturbances are correlated with decreased morning awakening salivary cortisol. Psychoneuroendocrinology 2004;29:1184–91. [DOI] [PubMed] [Google Scholar]
- 25.Leproult R, Copinschi G, Buxton O, Van Cauter E. Sleep loss results in an elevation of cortisol levels the next evening. Sleep 1997;20:865–70. [PubMed] [Google Scholar]
- 26.Chai CK. The genetics of Alzheimer’s disease. Am J Alzheimers Dis Other Demen 2007;22:37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gatz M, Reynolds CA, Fratiglioni L, Johansson B, Mortimer JA, Berg S, Fiske A, Pedersen NL. Role of genes and environments for explaining Alzheimer disease. Arch Gen Psychiatry 2006;63:168–74. [DOI] [PubMed] [Google Scholar]
- 28.Rossor MN, Fox NC, Freeborough PA, Harvey RJ. Clinical features of sporadic and familial Alzheimer's disease. Neurodegeneration 1996;5:393–7. [DOI] [PubMed] [Google Scholar]
- 29.Willette AA, Johnson SC, Birdsill AC, Sager MA, Christian B, Baker LD, Craft S, Oh J, Statz E, Hermann BP, et al. Insulin resistance predicts brain amyloid deposition in late middle-aged adults. Alzheimers Dement 2014;11:504–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kenna H, Hoeft F, Kelley R, Wroolie T, DeMuth B, Reiss A, Rasgon N. Fasting plasma insulin and the default mode network in women at risk for Alzheimer’s disease. Neurobiol Aging 2013;34:641–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cherbuin N, Sachdev P, Anstey KJ. Higher normal fasting plasma glucose is associated with hippocampal atrophy: the PATH Study. Neurology 2012;79:1019–26. [DOI] [PubMed] [Google Scholar]
- 32.Blom K, Emmelot-Vonk MH, Koek HL. The influence of vascular risk factors on cognitive decline in patients with dementia: a systematic review. Maturitas 2013;76:113–7. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira ST, Clarke JR, Bomfim TR, De Felice FG. Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer's disease. Alzheimers Dement 2014;10(1 Suppl):S76–83. [DOI] [PubMed] [Google Scholar]
- 34.Morris JK, Vidoni ED, Honea RA, Burns JM; Alzheimer’s Disease Neuroimaging Initiative. Impaired glycemia increases disease progression in mild cognitive impairment. Neurobiol Aging 2014;35:585–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosendorff C, Beeri MS, Silverman JM. Cardiovascular risk factors for Alzheimer’s disease. Am J Geriatr Cardiol 2007;16:143–9. [DOI] [PubMed] [Google Scholar]
- 36.Muqtadar H, Testai FD, Gorelick PB. The dementia of cardiac disease. Curr Cardiol Rep 2012;14:732–40. [DOI] [PubMed] [Google Scholar]
- 37.Román GC. Vascular dementia prevention: a risk factor analysis. Cerebrovasc Dis 2005;20 (Suppl 2):91–100. [DOI] [PubMed] [Google Scholar]
- 38.Polidori MC, Mariani E, Mecocci P, Nelles G. Congestive heart failure and Alzheimer’s disease. Neurol Res 2006;28:588–94. [DOI] [PubMed] [Google Scholar]
- 39.de la Torre JC. How do heart disease and stroke become risk factors for Alzheimer’s disease? Neurol Res 2006;28:637–44. [DOI] [PubMed] [Google Scholar]
- 40.Lee TA, Wolozin B, Weiss KB, Bednar MM. Assessment of the emergence of Alzheimer’s disease following coronary artery bypass graft surgery or percutaneous transluminal coronary angioplasty. J Alzheimers Dis 2005;7:319–24. [DOI] [PubMed] [Google Scholar]
- 41.Zulli R, Nicosia F, Borroni B, Agosti C, Prometti P, Donati P, De Vecchi M, Turini D, Romanelli G, Grassi V, et al. Increased prevalence of silent myocardial ischaemia and severe ventricular arrhythmias in untreated patients with Alzheimer’s disease and mild cognitive impairment without overt coronary artery disease. Clin Neurol Neurosurg 2008;110:791–6. [DOI] [PubMed] [Google Scholar]
- 42.Lima LM, Carvalho M, Ferreira CN, Fernandes AP, Neto CP, Garcia JC, Reis HJ, Janka Z, Palotas A, Sousa M. Atheromatosis extent in coronary artery disease is not correlated with apolipoprotein-E polymorphism and its plasma levels, but associated with cognitive decline. Curr Alzheimer Res 2010;7:556–63. [DOI] [PubMed] [Google Scholar]
- 43.Roberts RO, Knopman DS, Geda YE, Cha RH, Roger VL, Petersen RC. Coronary heart disease is associated with non-amnestic mild cognitive impairment. Neurobiol Aging 2010;31:1894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koschack J, Irle E. Small hippocampal size in cognitively normal subjects with coronary artery disease. Neurobiol Aging 2005;26:865–71. [DOI] [PubMed] [Google Scholar]
- 45.Ikram MA, van Oijen M, de Jong FJ, Kors JA, Koudstaal PJ, Hofman A, Witteman JC, Breteler MM. Unrecognized myocardial infarction in relation to risk of dementia and cerebral small vessel disease. Stroke 2008;39:1421–6. [DOI] [PubMed] [Google Scholar]
- 46.Reitz C, Brickman AM, Luchsinger JA, Wu WE, Small SA, Tang MX. Frequency of subclinical heart disease in elderly persons with dementia. Am J Geriatr Cardiol 2007;16:183–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daulatzai MA. Death by a thousand cuts in Alzheimer’s disease: hypoxia the prodrome. Neurotox Res 2013;24:216–43. [DOI] [PubMed] [Google Scholar]
- 48.Bunch TJ, Weiss JP, Crandall BG, May HT, Bair TL, Osborn JS, Anderson JL, Muhlestein JB, Horne BD, Lappe DL, et al. Atrial fibrillation is independently associated with senile, vascular, and Alzheimer's dementia. Heart Rhythm 2010;7:433–7. [DOI] [PubMed] [Google Scholar]
- 49.Rusanen M, Kivipelto M, Levälahti E, Laatikainen T, Tuomilehto J, Soininen H, Ngandu T. Heart diseases and long-term risk of dementia and Alzheimer’s disease: a population-based CAIDE study. J Alzheimers Dis 2014;42:183–91. [DOI] [PubMed] [Google Scholar]
- 50.Dai Y, Kamal MA. Fighting Alzheimer’s disease and type 2 diabetes: pathological links and treatment strategies. CNS Neurol Disord Drug Targets 2014;13:271–82. [DOI] [PubMed] [Google Scholar]
- 51.de la Monte SM, Tong M. Brain metabolic dysfunction at the core of Alzheimer’s disease. Biochem Pharmacol 2014;88:548–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mattson MP. Will caloric restriction and folate protect against AD and PD? Neurology 2003;60:690–5. [DOI] [PubMed] [Google Scholar]
- 53.Cipriani G, Lucetti C, Danti S, Nuti A. Sleep disturbances and dementia. Psychogeriatrics 2015;15:65–74. [DOI] [PubMed] [Google Scholar]
- 54.Guarnieri B, Adorni F, Musicco M, Appollonio I, Bonanni E, Caffarra P, Caltagirone C, Cerroni G, Concari L, Cosentino FI, et al. Prevalence of sleep disturbances in mild cognitive impairment and dementing disorders: a multicenter Italian clinical cross-sectional study on 431 patients. Dement Geriatr Cogn Disord 2012;33:50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pistacchi M, Gioulis M, Contin F, Sanson F, Marsala SZ. Sleep disturbance and cognitive disorder: epidemiological analysis in a cohort of 263 patients. Neurol Sci 2014;35:1955–62. [DOI] [PubMed] [Google Scholar]
- 56.Daulatzai MA. Evidence of neurodegeneration in obstructive sleep apnea: relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res 2015;93:1778–94. [DOI] [PubMed] [Google Scholar]
- 57.Buxton OM, Pavlova M, Reid EW, Wang W, Simonson DC, Adler GK. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 2010;59:2126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Donga E, van Dijk M, van Dijk JG, Biermasz NR, Lammers GJ, van Kralingen KW, Corssmit EP, Romijn JA. A single night of partial sleep deprivation induces insulin resistance in multiple metabolic pathways in healthy subjects. J Clin Endocrinol Metab 2010;95:2963–8. [DOI] [PubMed] [Google Scholar]
- 59.McCleery J, Cohen DA, Sharpley AL. Pharmacotherapies for sleep disturbances in Alzheimer’s disease. Cochrane Database Syst Rev 2014;3:CD009178. [DOI] [PubMed] [Google Scholar]
- 60.Wade AG, Farmer M, Harari G, Fund N, Laudon M, Nir T, Frydman-Marom A, Zisapel N. Add-on prolonged-release melatonin for cognitive function and sleep in mild to moderate Alzheimer’s disease: a 6-month, randomized, placebo-controlled, multicenter trial. Clin Interv Aging 2014;9:947–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Billioti de Gage S, Moride Y, Ducruet T, Kurth T, Verdoux H, Tournier M, Pariente A, Begaud B. Benzodiazepine use and risk of Alzheimer’s disease: case-control study. BMJ 2014;349:g5205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bocti C, Roy-Desruisseaux J, Hudon C, Roberge P. Benzodiazepine and dementia: a time for reflection. Maturitas 2013;75:105–6. [DOI] [PubMed] [Google Scholar]
- 63.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, Benveniste H, Vates GE, Deane R, Goldman SA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012;4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, O’Donnell J, Christensen DJ, Nicholson C, Iliff JJ, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342:373–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kress BT, Iliff JJ, Xia M, Wang M, Wei HS, Zeppenfeld D, Xie L, Kang H, Xu Q, Liew JA, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol 2014;76:845–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M, Benveniste H. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 2013;123:1299–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yang L, Kress BT, Weber HJ, Thiyagarajan M, Wang B, Deane R, Benveniste H, Iliff JJ, Nedergaard M. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med 2013;11:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wostyn P, Van Dam D, Audenaert K, Killer HE, De Deyn PP, De Groot V. A new glaucoma hypothesis: a role of glymphatic system dysfunction. Fluids Barriers CNS 2015;12:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McKinnon SJ. Glaucoma: ocular Alzheimer's disease? Front Biosci 2003;8:s1140–56. [DOI] [PubMed] [Google Scholar]
- 70.Guo L, Salt TE, Luong V, Wood N, Cheung W, Maass A, Ferrari G, Russo-Marie F, Sillito AM, Cheetham ME, et al. Targeting amyloid-beta in glaucoma treatment. Proc Natl Acad Sci USA 2007;104:13444–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lucey BP, Bateman RJ. Amyloid-beta diurnal pattern: possible role of sleep in Alzheimer’s disease pathogenesis. Neurobiol Aging 2014;35 Suppl 2:S29–34. [DOI] [PubMed] [Google Scholar]
- 72.Ju YE, Lucey BP, Holtzman DM. Sleep and Alzheimer disease pathology–a bidirectional relationship. Nat Rev Neurol 2014;10:115–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kang JE, Cirrito JR, Dong H, Csernansky JG, Holtzman DM. Acute stress increases interstitial fluid amyloid-beta via corticotropin-releasing factor and neuronal activity. Proc Natl Acad Sci USA 2007;104:10673–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, Fujiki N, Nishino S, Holtzman DM. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science 2009;326:1005–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gilestro GF, Tononi G, Cirelli C. Widespread changes in synaptic markers as a function of sleep and wakefulness in Drosophila. Science 2009;324:109–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang Y, Potter R, Sigurdson W, Santacruz A, Shih S, Ju YE, Kasten T, Morris JC, Mintun M, Duntley S, et al. Effects of age and amyloid deposition on Abeta dynamics in the human central nervous system. Arch Neurol 2012;69:51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee H, Xie L, Yu M, Kang H, Feng T, Deane R, Logan J, Nedergaard M, Benveniste H. The effect of body posture on brain glymphatic transport. J Neurosci 2015;35:11034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abel T, Havekes R, Saletin JM, Walker MP. Sleep, plasticity and memory from molecules to whole-brain networks. Curr Biol 2013;23:R774–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Manning EN, Macdonald KE, Leung KK, Young J, Pepple T, Lehmann M, Zuluaga MA, Cardoso MJ, Schott JM, Ourselin S, et al. Differential hippocampal shapes in posterior cortical atrophy patients: a comparison with control and typical AD subjects. Hum Brain Mapp 2015;36:5123–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kotrotsou A, Schneider JA, Bennett DA, Leurgans SE, Dawe RJ, Boyle PA, Golak T, Arfanakis K. Neuropathologic correlates of regional brain volumes in a community cohort of older adults. Neurobiol Aging 2015;36:2798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ricci S, Fuso A, Ippoliti F, Businaro R. Stress-induced cytokines and neuronal dysfunction in Alzheimer’s disease. J Alzheimers Dis 2012;28:11–24. [DOI] [PubMed] [Google Scholar]
- 82.Elcombe EL, Lagopoulos J, Duffy SL, Lewis SJ, Norrie L, Hickie IB, Naismith SL. Hippocampal volume in older adults at risk of cognitive decline: the role of sleep, vascular risk, and depression. J Alzheimers Dis 2015;44:1279–90. [DOI] [PubMed] [Google Scholar]
- 83.Joo EY, Kim H, Suh S, Hong SB. Hippocampal substructural vulnerability to sleep disturbance and cognitive impairment in patients with chronic primary insomnia: magnetic resonance imaging morphometry. Sleep 2014;37:1189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci 2005;28:223–50. [DOI] [PubMed] [Google Scholar]
- 85.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med 1998;4:1313–7. [DOI] [PubMed] [Google Scholar]
- 86.Gould E. How widespread is adult neurogenesis in mammals? Nat Rev Neurosci 2007;8:481–8. [DOI] [PubMed] [Google Scholar]
- 87.Meerlo P, Mistlberger RE, Jacobs BL, Heller HC, McGinty D. New neurons in the adult brain: the role of sleep and consequences of sleep loss. Sleep Med Rev 2009;13:187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol 2012;8:189–202. [DOI] [PubMed] [Google Scholar]
- 89.Martin PA, Crump MH. The adrenal gland: Ames (IA): Iowa State Press, 2003. [Google Scholar]
- 90.Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. Int J Psychophysiol 2009;72:67–73. [DOI] [PubMed] [Google Scholar]
- 91.Tortosa-Martínez J, Clow A, Caus-Pertegaz N, González-Caballero G, Abellán-Miralles I, Saenz MJ. Exercise Increases the Dynamics of Diurnal Cortisol Secretion and Executive Function in People With Amnestic Mild Cognitive Impairment. J Aging Phys Act 2015;23:550–8. [DOI] [PubMed] [Google Scholar]
- 92.Souza-Talarico JN, Chaves EC, Lupien SJ, Nitrini R, Caramelli P. Relationship between cortisol levels and memory performance may be modulated by the presence or absence of cognitive impairment: evidence from healthy elderly, mild cognitive impairment and Alzheimer’s disease subjects. J Alzheimers Dis 2010;19:839–48. [DOI] [PubMed] [Google Scholar]
- 93.Peavy GM, Santiago DP, Edland SD. Subjective memory complaints are associated with diurnal measures of salivary cortisol in cognitively intact older adults. Am J Geriatr Psychiatry 2013;21:925–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beluche I, Carriere I, Ritchie K, Ancelin ML. A prospective study of diurnal cortisol and cognitive function in community-dwelling elderly people. Psychol Med 2010;40:1039–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee BK, Glass TA, Wand GS, McAtee MJ, Bandeen-Roche K, Bolla KI, Schwartz BS. Apolipoprotein e genotype, cortisol, and cognitive function in community-dwelling older adults. Am J Psychiatry 2008;165:1456–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Souza-Talarico JN, Caramelli P, Nitrini R, Chaves EC. Effect of cortisol levels on working memory performance in elderly subjects with Alzheimer’s disease. Arq Neuropsiquiatr 2008;66 3B:619–24. [DOI] [PubMed] [Google Scholar]
- 97.Wahbeh H, Kishiyama SS, Zajdel D, Oken BS. Salivary cortisol awakening response in mild Alzheimer disease, caregivers, and noncaregivers. Alzheimer Dis Assoc Disord 2008;22:181–3. [DOI] [PubMed] [Google Scholar]
- 98.Aboulafia-Brakha T, Suchecki D, Gouveia-Paulino F, Nitrini R, Ptak R. Cognitive-behavioural group therapy improves a psychophysiological marker of stress in caregivers of patients with Alzheimer’s disease. Aging Ment Health 2014;18:801–8. [DOI] [PubMed] [Google Scholar]
- 99.Wolf OT, Convit A, Thorn E, de Leon MJ. Salivary cortisol day profiles in elderly with mild cognitive impairment. Psychoneuroendocrinology 2002;27:777–89. [DOI] [PubMed] [Google Scholar]
- 100.Arsenault-Lapierre G, Chertkow H, Lupien S. Seasonal effects on cortisol secretion in normal aging, mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 2010;31:1051–4. [DOI] [PubMed] [Google Scholar]
- 101.Magri F, Cravello L, Barili L, Sarra S, Cinchetti W, Salmoiraghi F, Micale G, Ferrari E. Stress and dementia: the role of the hypothalamicpituitary-adrenal axis. Aging Clin Exp Res 2006;18:167–70. [DOI] [PubMed] [Google Scholar]
- 102.Duong M, Cohen JI, Convit A. High cortisol levels are associated with low quality food choice in type 2 diabetes. Endocrine 2012;41:76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Knoops AJ, Gerritsen L, van der Graaf Y, Mali WP, Geerlings MI. Basal hypothalamic pituitary adrenal axis activity and hippocampal volumes: the SMART-Medea study. Biol Psychiatry 2010;67:1191–8. [DOI] [PubMed] [Google Scholar]
- 104.McEwen BS. The neurobiology of stress: from serendipity to clinical relevance. Brain Res 2000;886:172–89. [DOI] [PubMed] [Google Scholar]
- 105.Huang CW, Lui CC, Chang WN, Lu CH, Wang YL, Chang CC. Elevated basal cortisol level predicts lower hippocampal volume and cognitive decline in Alzheimer's disease. J Clin Neurosci 2009;16:1283–6. [DOI] [PubMed] [Google Scholar]
- 106.Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NP, Thakur M, McEwen BS, Hauger RL, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nat Neurosci 1998;1:69–73. [DOI] [PubMed] [Google Scholar]
- 107.Lehallier B, Essioux L, Gayan J, Alexandridis R, Nikolcheva T, Wyss-Coray T, Britschgi M; Alzheimer’s Disease Neuroimaging Initiative. Combined Plasma and Cerebrospinal Fluid Signature for the Prediction of Midterm Progression From Mild Cognitive Impairment to Alzheimer Disease. JAMA Neurol 2015;14:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Greendale GA, Kritz-Silverstein D, Seeman T, Barrett-Connor E. Higher basal cortisol predicts verbal memory loss in postmenopausal women: Rancho Bernardo Study. J Am Geriatr Soc 2000;48:1655–8. [DOI] [PubMed] [Google Scholar]
- 109.Leonard BE. Inflammation, depression and dementia: are they connected? Neurochem Res 2007;32:1749–56. [DOI] [PubMed] [Google Scholar]
- 110.Starkman MN, Giordani B, Berent S, Schork MA, Schteingart DE. Elevated cortisol levels in Cushing’s disease are associated with cognitive decrements. Psychosom Med 2001;63:985–93. [DOI] [PubMed] [Google Scholar]
- 111.Notarianni E. Hypercortisolemia and glucocorticoid receptor-signaling insufficiency in Alzheimer’s disease initiation and development. Curr Alzheimer Res 2013;10:714–31. [DOI] [PubMed] [Google Scholar]
- 112.Catania C, Sotiropoulos I, Silva R, Onofri C, Breen KC, Sousa N, Almeida OF. The amyloidogenic potential and behavioral correlates of stress. Mol Psychiatry 2009;14:95–105. [DOI] [PubMed] [Google Scholar]
- 113.Lucassen PJ, Pruessner J, Sousa N, Almeida OF, Van Dam AM, Rajkowska G, Swaab DF, Czeh B. Neuropathology of stress. Acta Neuropathol 2014;127:109–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baglietto-Vargas D, Medeiros R, Martinez-Coria H, LaFerla FM, Green KN. Mifepristone alters amyloid precursor protein processing to preclude amyloid beta and also reduces tau pathology. Biol Psychiatry 2013;74:357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Murialdo G, Barreca A, Nobili F, Rollero A, Timossi G, Gianelli MV, Copello F, Rodriguez G, Polleri A. Dexamethasone effects on cortisol secretion in Alzheimer’s disease: some clinical and hormonal features in suppressor and nonsuppressor patients. J Endocrinol Invest 2000;23:178–86. [DOI] [PubMed] [Google Scholar]
- 116.Wang Y, Li M, Tang J, Song M, Xu X, Xiong J, Li J, Bai Y. Glucocorticoids facilitate astrocytic amyloid-beta peptide deposition by increasing the expression of APP and BACE1 and decreasing the expression of amyloid-beta-degrading proteases. Endocrinology 2011;152:2704–15. [DOI] [PubMed] [Google Scholar]
- 117.Zhao J, O’Connor T, Vassar R. The contribution of activated astrocytes to Abeta production: implications for Alzheimer’s disease pathogenesis. J Neuroinflammation 2011;8:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Kanoski SE, Davidson TL. Western diet consumption and cognitive impairment: links to hippocampal dysfunction and obesity. Physiol Behav 2011;103:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim HH, Kim YJ, Lee SY, Jeong DW, Lee JG, Yi YH, Cho YH, Choi EJ, Kim HJ. Interactive effects of an isocaloric high-protein diet and resistance exercise on body composition, ghrelin, and metabolic and hormonal parameters in untrained young men: A randomized clinical trial. J Diabetes Investig 2014;5:242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Manzanares N, Monseny R, Ortega L, Montalvo I, Franch J, Gutierrez-Zotes A, Reynolds RM, Walker BR, Vilella E, Labad J. Unhealthy lifestyle in early psychoses: the role of life stress and the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology 2014;39:1–10. [DOI] [PubMed] [Google Scholar]
- 121.Heaney JL, Phillips AC, Carroll D. Aging, health behaviors, and the diurnal rhythm and awakening response of salivary cortisol. Exp Aging Res 2012;38:295–314. [DOI] [PubMed] [Google Scholar]
- 122.Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci USA 2003;100:11696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr 2005;81:341–54. [DOI] [PubMed] [Google Scholar]
- 124.Robey IF. Examining the relationship between diet-induced acidosis and cancer. Nutr Metab (Lond) 2012;9:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jehle S, Krapf R. Effects of acidogenic diet forms on musculoskeletal function. J Nephrol 2010;23(Suppl 16):S77–84. [PubMed] [Google Scholar]
- 126.Fang B, Wang D, Huang M, Yu G, Li H. Hypothesis on the relationship between the change in intracellular pH and incidence of sporadic Alzheimer’s disease or vascular dementia. Int J Neurosci 2010;120:591–5. [DOI] [PubMed] [Google Scholar]
- 127.Basurto-Islas G, Grundke-Iqbal I, Tung YC, Liu F, Iqbal K. Activation of asparaginyl endopeptidase leads to Tau hyperphosphorylation in Alzheimer disease. J Biol Chem 2013;288:17495–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Pirchl M, Marksteiner J, Humpel C. Effects of acidosis on brain capillary endothelial cells and cholinergic neurons: relevance to vascular dementia and Alzheimer’s disease. Neurol Res 2006;28:657–64. [DOI] [PubMed] [Google Scholar]
- 129.Karim Z, Attmane-Elakeb A, Bichara M. Renal handling of NH4+ in relation to the control of acid-base balance by the kidney. J Nephrol 2002;15 Suppl 5:S128–34. [PubMed] [Google Scholar]
- 130.McCarty MF. Acid-base balance may influence risk for insulin resistance syndrome by modulating cortisol output. Med Hypotheses 2005;64:380–4. [DOI] [PubMed] [Google Scholar]
- 131.Gibson EL, Checkley S, Papadopoulos A, Poon L, Daley S, Wardle J. Increased salivary cortisol reliably induced by a protein-rich midday meal. Psychosom Med 1999;61:214–24. [DOI] [PubMed] [Google Scholar]
- 132.Mattei J, Bhupathiraju S, Tucker KL. Higher adherence to a diet score based on American Heart Association recommendations is associated with lower odds of allostatic load and metabolic syndrome in Puerto Rican adults. J Nutr 2013;143:1753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.García-Prieto MD, Tébar FJ, Nicolás F, Larqué E, Zamora S, Garaulet M. Cortisol secretary pattern and glucocorticoid feedback sensitivity in women from a Mediterranean area: relationship with anthropometric characteristics, dietary intake and plasma fatty acid profile. Clin Endocrinol (Oxf) 2007;66:185–91. [DOI] [PubMed] [Google Scholar]
- 134.Knight A, Bryan J, Wilson C, Hodgson J, Murphy K. A randomised controlled intervention trial evaluating the efficacy of a Mediterranean dietary pattern on cognitive function and psychological wellbeing in healthy older adults: the MedLey study. BMC Geriatr 2015;15:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hao S, Dey A, Yu X, Stranahan AM. Dietary obesity reversibly induces synaptic stripping by microglia and impairs hippocampal plasticity. Brain Behav Immun 2016;51:230–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Park SH, Kim JH, Choi KH, Jang YJ, Bae SS, Choi BT, Shin HK. Hypercholesterolemia accelerates amyloid beta-induced cognitive deficits. Int J Mol Med 2013;31:577–82. [DOI] [PubMed] [Google Scholar]
- 137.Spence JD, Thornton T, Muir AD, Westcott ND. The effect of flax seed cultivars with differing content of alpha-linolenic acid and lignans on responses to mental stress. J Am Coll Nutr 2003;22:494–501. [DOI] [PubMed] [Google Scholar]
- 138.Komori T. The Effects of Phosphatidylserine and Omega-3 Fatty Acid-Containing Supplement on Late Life Depression. Ment Illn 2015;7:5647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rakel D. Integrative Medicine, 3rd edition: Philadelphia: Saunders Elsevier, 2012.
- 140.Crook TH, Tinklenberg J, Yesavage J, Petrie W, Nunzi MG, Massari DC. Effects of phosphatidylserine in age-associated memory impairment. Neurology 1991;41:644–9. [DOI] [PubMed] [Google Scholar]
- 141.Richter Y, Herzog Y, Cohen T, Steinhart Y. The effect of phosphatidylserine-containing omega-3 fatty acids on memory abilities in subjects with subjective memory complaints: a pilot study. Clin Interv Aging 2010;5:313–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vakhapova V, Cohen T, Richter Y, Herzog Y, Korczyn AD. Phosphatidylserine containing omega-3 fatty acids may improve memory abilities in non-demented elderly with memory complaints: a double-blind placebo-controlled trial. Dement Geriatr Cogn Disord 2010;29:467–74. [DOI] [PubMed] [Google Scholar]
- 143.Richter Y, Herzog Y, Lifshitz Y, Hayun R, Zchut S. The effect of soybean-derived phosphatidylserine on cognitive performance in elderly with subjective memory complaints: a pilot study. Clin Interv Aging 2013;8:557–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Eby GA, Eby KL. Rapid recovery from major depression using magnesium treatment. Med Hypotheses 2006;67:362–70. [DOI] [PubMed] [Google Scholar]
- 145.Abbasi B, Kimiagar M, Sadeghniiat K, Shirazi MM, Hedayati M, Rashidkhani B. The effect of magnesium supplementation on primary insomnia in elderly: a double-blind placebo-controlled clinical trial. J Res Med Sci 2012;17:1161–9. [PMC free article] [PubMed] [Google Scholar]
- 146.Drennan MD, Kripke DF, Klemfuss HA, Moore JD. Potassium affects actigraph-identified sleep. Sleep 1991;14:357–60. [PubMed] [Google Scholar]
- 147.Rastmanesh R. Hypothetical hormonal mechanism by which potassium-rich diets benefit patients with rheumatoid arthritis. Med Hypotheses 2009;73:564–8. [DOI] [PubMed] [Google Scholar]
- 148.McMorris T, Harris RC, Swain J, Corbett J, Collard K, Dyson RJ, Dye L, Hodgson C, Draper N. Effect of creatine supplementation and sleep deprivation, with mild exercise, on cognitive and psychomotor performance, mood state, and plasma concentrations of catecholamines and cortisol. Psychopharmacology (Berl) 2006;185:93–103. [DOI] [PubMed] [Google Scholar]
- 149.McMorris T, Harris RC, Howard AN, Langridge G, Hall B, Corbett J, Dicks M, Hodgson C. Creatine supplementation, sleep deprivation, cortisol, melatonin and behavior. Physiol Behav 2007;90:21–8. [DOI] [PubMed] [Google Scholar]
- 150.Taupin P. Neurogenesis, NSCs, pathogenesis and therapies for Alzheimer’s disease. Front Biosci (Schol Ed) 2011;3:178–90. [DOI] [PubMed] [Google Scholar]
- 151.Irwin RW, Brinton RD. Allopregnanolone as regenerative therapeutic for Alzheimer’s disease: translational development and clinical promise. Prog Neurobiol 2014;113:40–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Brinton RD, Wang JM. Therapeutic potential of neurogenesis for prevention and recovery from Alzheimer’s disease: allopregnanolone as a proof of concept neurogenic agent. Curr Alzheimer Res 2006;3:185–90. [DOI] [PubMed] [Google Scholar]
- 153.Felsenstein KM, Candelario KM, Steindler DA, Borchelt DR. Regenerative medicine in Alzheimer’s disease. Transl Res 2014;163:432–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015;35:2255–63. [DOI] [PubMed] [Google Scholar]
- 155.European Commission [Internet]. Research on Alzheimer’s disease in FP6 and FP7. [cited 2015 Nov 4]. Available from: http://ec.europa.eu/health/major_chronic_diseases/diseases/dementia/index_en.htm (fragment5).
- 156.JPND Research. [Internet] JPco-fuND. [cited 2015 Nov 4]. Available from: http://www.neurodegenerationresearch.eu/initiatives/jpcofund/.
- 157.National Institute on Aging [Internet]. Living Long & Well in the 21st Century: Strategic Directions for Research on Aging. [cited 2015 Nov 4]. Available from: https://www.nia.nih.gov/about/living-long-well-21st-century-strategic-directions-research-aging.