Abstract
Zinc is an essential nutrient for humans; however, a sensitive biomarker to assess zinc status has not been identified. The objective of this systematic review was to compile and assess studies that determined zinc transporter and/or metallothionein expression in various blood cell types and to determine their reliability and sensitivity to changes in dietary zinc. Sixteen studies were identified that determined the expression of zrt-, irt-like protein (ZIP) 1 [solute carrier family (SLC) 39A1], ZIP3 (SLC39A3), ZIP5 (SLC39A5), ZIP6 (SLC39A6), ZIP7 (SLC39A7), ZIP8 (SLC39A8), ZIP10 (SLC39A10), ZIP14 (SLC39A14), zinc transporter (ZnT)1 (SLC30A1), ZnT2 (SLC30A2), ZnT4 (SLC30A4), ZnT5 (SLC30A5), ZnT6 (SLC30A6), ZnT7 (SLC30A7), ZnT9 (SLC30A9), and/or metallothionein in various blood cells isolated from healthy adult men and women in response to zinc supplementation or depletion. Cell types included leukocytes, peripheral blood mononuclear cells, T lymphocytes, monocytes, and erythrocytes. ZIP1, ZnT1, and metallothionein were the most commonly measured proteins. Changes in ZIP1 and ZnT1 in response to zinc supplementation or depletion were not consistent across studies. Leukocyte metallothionein decreased with zinc depletion (−39% change from baseline, <5 mg Zn/d, n = 2 studies) and increased with zinc supplementation in a dose-dependent manner (35%, 15–22 mg Zn/d, n = 7 studies; 267%, 50 mg Zn/d, n = 2 studies) and at the earliest time points measured; however, no change or delayed response was observed in metallothionein in erythrocytes. A greater percentage of studies demonstrated that metallothionein in leukocyte subtypes was a more reliable (100%, n = 12; 69%, n = 16) and responsive (92%, n = 12; 82%, n = 11) indicator of zinc exposure than was plasma zinc, respectively. In conclusion, current evidence indicates that metallothionein in leukocyte subtypes may be a component in determining zinc status.
Keywords: metallothionein, zinc transporter, leukocytes, biomarker, zinc status
Introduction
Zinc is an essential trace mineral required for growth, development, DNA synthesis, immunity, and many other critical biological processes. Although less common in those living in developed nations, severe zinc deficiency has been described in individuals consuming diets with low zinc bioavailability and those with extensive burns, chronic diseases, and genetic disorders such as acrodermatitis enteropathica (1). In contrast to severe zinc deficiency, marginal or moderate zinc deficiency may be widespread. However, subclinical zinc deficiency is difficult to diagnose because of the lack of a specific, responsive, and reliable indicator of zinc status. Experimental zinc deficiency in laboratory animals demonstrates that even mild and moderate zinc deficiency negatively affects health. The severity of dietary zinc restriction (adequate zinc and mild, moderate, and severe deficiency) is linked to the severity of disease outcomes, including alopecia, dermatitis, stunted growth and development, and death (2). In humans, mild or moderate zinc deficiency can lead to stunted growth and delayed puberty in adolescents, hypogonadism in males, dermatitis, reduced appetite, mental lethargy, and delayed wound healing (3). Thus, the lack of a biomarker to assess zinc status is a substantial barrier to the development of targeted nutritional interventions and human health.
Optimal zinc status can be defined as the concentration of dietary zinc intake required to saturate tissue concentrations and, as a result, prevent functional declines associated with deficiency. Zinc status is currently assessed by measuring plasma or serum zinc concentration (referred to as “plasma zinc” hereafter). Despite their convenience for assessing zinc status at the population level, circulating concentrations of zinc are considered to be a poor indicator of zinc status (4-6). Because zinc is required for multiple aspects of general metabolism, complex systems tightly regulate zinc homeostasis. When zinc intake is low, there is a reduction in endogenous losses to conserve zinc (7) and zinc is mobilized from small, rapidly exchangeable pools in the plasma, liver, and possibly bone (8, 9). Thus, circulating concentrations of zinc are relatively insensitive to changes in dietary zinc. Moreover, plasma zinc responds to factors unrelated to zinc status. Factors such as pregnancy, oral contraceptive use, inflammation/infection, fasting compared with postprandial state, and time of day all are known to influence circulating concentrations of zinc (4). Because of the lack of a specific, responsive, and reliable indicator of zinc status, various alternative biomarkers have been considered [reviewed in (10)].
One potential method is assessment of the expression of zinc-transporting and zinc-binding proteins, such as metallothionein, in circulating blood cells, such as peripheral blood mononuclear cells (PBMCs)4. These cell populations are readily obtained by venipuncture and may have utility in clinical or field studies. Since the discovery of the first zinc transporter in 1995, 24 zinc transporters have been described in mammals and can be divided into 2 distinct families: the zrt-, irt-like protein (ZIP) [solute carrier family (SLC) 39A] family of zinc importers and the zinc transporter (ZnT, SLC30A) family of zinc exporters. Whereas ZIPs import zinc into the cell or transport zinc from within a subcellular compartment into the cytoplasm, ZnTs export zinc out of the cell or transport zinc from the cytoplasm into subcellular compartments. Metallothioneins are 61–amino acid proteins characterized by their high cysteine (30%) and metal content. Metallothionein binds cellular zinc with high affinity, binding up to 7 zinc atoms (3 zinc atoms in the β domain and 4 zinc atoms in the α domain), and may serve as a small zinc reserve for cells (5). It is important to note that although metallothioneins are capable of binding other essential (e.g., copper) and nonessential (e.g., cadmium) metals, metallothionein-bound zinc is thought to be the predominant form of metallothionein in human tissue. For example, metallothionein is not present in the livers of zinc-deficient rats, even when liver copper concentrations are high (11). Metallothioneins are localized intracellularly. Metallothionein-1 and -2 are the major isoforms and are expressed ubiquitously; metallothionein-3 and -4 are the minor isoforms and are found in specialized cells, such as neurons and stratified squamous epithelium, respectively. Metallothionein expression is induced by zinc through the binding of zinc to metal-regulatory transcription factor 1, which binds to metal-responsive elements in the promoter of the metallothionein gene (12). Some zinc transporters, such as ZnT1 (13), ZnT2 (14), and ZIP10 (15), also contain metal-responsive elements that respond to zinc. Zinc-responsive elements in the promoter region of zinc transporters (16), such as ZnT5 (SLC30A5) (17), suggest additional mechanisms by which zinc may regulate zinc transporter expression. Thus, a number of studies have sought to determine whether the protein or mRNA abundance of zinc transporters and/or metallothionein in available tissues/cells may serve as reliable biomarkers for zinc status. Because of the current lack of comprehensive evidence detailing the use of PBMCs and related cell types as a measure of zinc status, the objectives of the present systematic review were to determine 1) the relation between zinc transporter and metallothionein expression in various circulating blood cell types and zinc exposure in healthy adults and 2) the sensitivity of these measures compared with the responsiveness of plasma zinc concentrations.
Methods
This review was written with the use of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guidelines (18).
Search methods for identification of studies.
Manuscripts were obtained with the use of PubMed and Web of Science. Zinc supplementation and depletion studies were included in the search. The search terms used were “(erythrocyte OR RBC OR peripheral blood mononuclear cell OR PBMC OR leukocyte OR lymphocyte OR monocyte) AND (zinc transporter OR ZIP OR ZnT OR SLC39 OR SLC30 OR metallothionein) AND (supplement OR supplementation OR depletion OR deficiency).” Manuscripts were compiled into one database and duplicates were removed. Initial screening of manuscripts was performed by assessing the title and/or abstract. Full-text manuscripts were retrieved for those that remained after the initial screening and the citations of the full-text manuscripts were searched for additional sources. All manuscripts then were evaluated in their entirety by 2 independent reviewers (SRH and AMK). The last search was performed on 15 December 2015.
Study selection.
A summary of zinc transporters, the participants, interventions, comparisons, outcomes, and study design criteria used for the inclusion and exclusion of studies is included in Table 1. Eligible studies were in the English language and included healthy adults (≥18 y of age) who received a zinc intervention (supplementation and/or depletion) with known concentrations of zinc accompanied by the separation of a blood cell type from human whole blood and a measure of zinc transporter and/or metallothionein abundance in those cells. Additional criteria included the following: 1) reported zinc transporter and/or metallothionein abundance at baseline and after zinc supplementation or depletion or the use of a placebo group, 2) reported daily dose of elemental zinc administered as a supplement or as dietary zinc, and 3) participants did not consume mineral or vitamin supplements. There was no restriction of study design, which included randomized controlled trials, controlled clinical trials, and before–after studies. Exclusion criteria included the following: 1) studies involving pregnant or breastfeeding women or individuals with existing disease and 2) studies in commercial cell lines or studies in which human blood cells were obtained and treated subsequently with zinc and/or a zinc chelator such as N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine (TPEN) in vitro.
TABLE 1.
Summary of participants, interventions, comparisons, outcomes, and study design criteria used for the inclusion and exclusion of studies
| Inclusion | Exclusion | |
| Participants | Healthy | Existing disease |
| Adults (≥18 y) | Children (<18 y) | |
| Female and/or male participants | Pregnant | |
| Interventions | Isolation of blood cell type from zinc supplemented/depleted subjects | No intervention |
| Commercial cell line or isolation of blood cell type and treatment/depletion of zinc in vitro | ||
| Comparisons | Baseline | |
| Placebo | ||
| Outcomes | Expression of metallothionein | |
| Expression of zinc transporter(s) | ||
| Study design | No restriction |
Data extraction.
Data extraction was conducted by one reviewer (AMK) and verified by a second reviewer (SRH). A third reviewer (JPM) was consulted in cases of disagreement. Extracted data included sample size, age, study design, form and quantity of zinc, cell type isolated, proteins measured, collection day(s), significant findings, and plasma zinc response. To approximate the effect of varying concentrations of supplemental zinc on leukocyte metallothionein, the percentage change from baseline was calculated for all studies that examined metallothionein expression in leukocytes. In studies that determined metallothionein expression at multiple time points (19–21), the day with the greatest percentage change was used. Data were grouped on the basis of the amount of dietary zinc consumed per day (<5, 15–22, and 50 mg Zn/d) and are presented as percentage change from baseline ± SE
Results
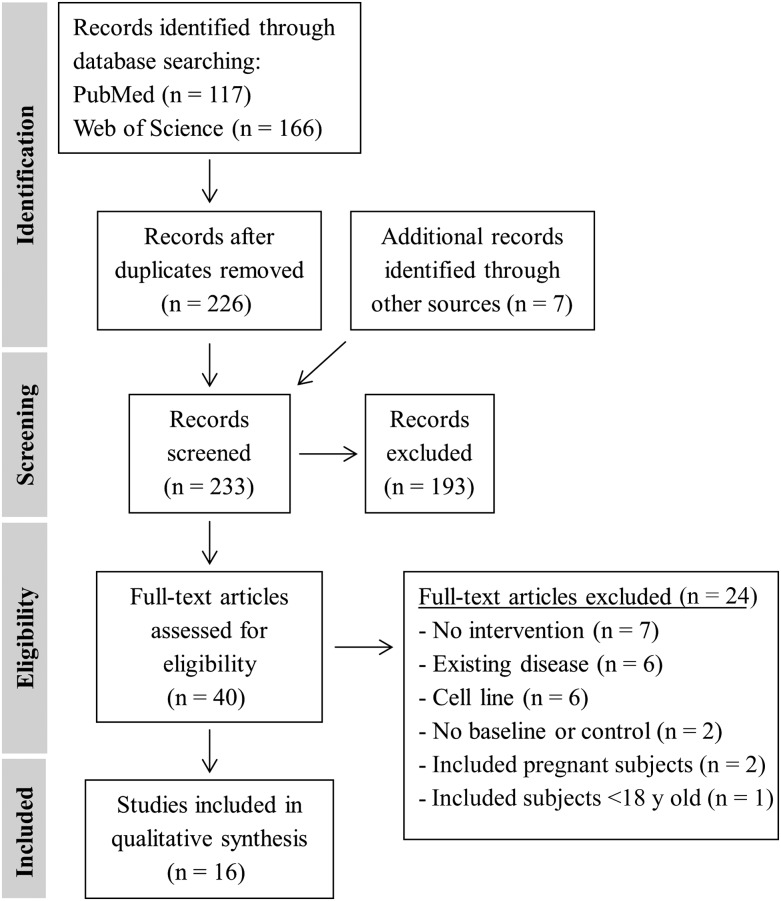
A total of 233 records were identified through database searching (Figure 1). After screening the abstracts, 193 records were excluded and 40 full-text manuscripts were accessed and assessed for eligibility. Common reasons for exclusion included lack of intervention or baseline measurement or control group, pre-existing health condition (e.g., diabetes), <18 y of age, or the study exclusively used animals or commercialized cell lines. One study was not included because high-quality RNA was isolated from only 6–9 subjects despite samples having been obtained from 48 subjects (22). Sixteen studies were included in the qualitative synthesis.
FIGURE 1.
Flow diagram of preferred reporting items for systematic reviews and meta-analyses.
The characteristics and findings of the 16 studies included in this systematic review are summarized in Table 2 (19–21, 23–35). Ten studies enrolled male subjects, 3 studies enrolled female subjects, and 3 studies enrolled both male and female subjects. Thirteen studies enrolled healthy subjects with ages ranging from 18 to 65 y, with one study including a subset of healthy subjects aged 64–75 y (24); one study included only healthy subjects aged 59–78 y (25); and one study included only subjects aged 65–85 y with low plasma zinc (<0.77 mg/L or 11.8 μM) (33). One study only included overweight/obese subjects [defined as BMI (in kg/m2) ≥25] (30). This study was not excluded because not all studies included in this systematic review included the body weight of study participants. All studies included either a depletion phase followed by a repletion phase or a supplementation phase; in some instances this included a post-supplementation phase during which the subjects received a placebo pill. Six studies included controlled feeding for a select period or the entire duration of the study. In the remaining studies, participants consumed a self-selected diet, or the diet was not specified. Zinc supplementation studies ranged from 10 to 84 d and included supplementation with ∼15–50 mg Zn/d. Zinc depletion studies were ∼10 d and consisted of an egg white–based liquid diet containing ∼0.3–0.5 mg Zn/d with added sodium phytate to limit the bioavailability of zinc; one depletion study provided a marginal zinc diet (4.6 mg Zn/d) for 70 d (23).
TABLE 2.
Characteristics of the studies included in the systematic review1
| Study (reference); country | Subjects, n | Sex | Age range, y | Study design | Form/quantity of zinc (elemental Zn/d) | Cell type | Transporter(s)/MT measured | Collection day | Significant findings (P < 0.05)2 | Plasma/serum zinc response |
| Allan et al., 2000 (23); United States | 7 | M | 27–47 | 35-d acclimation | Controlled feeding throughout the study (4.6 mg Zn/d). Zinc gluconate supplement (9.1 mg Zn/d) during acclimation and repletion phase (13.7 mg Zn/d total). A placebo pill during marginal zinc phase. | T lymphocytes | MT-2 | Day 20 of acclimation (baseline) | T lymphocyte MT decreased with zinc depletion vs. baseline and repletion. | Plasma zinc was not affected by zinc depletion or repletion. |
| 70-d depletion (marginal) | Day 49 of depletion | |||||||||
| 35-d repletion | Day 14 of repletion | |||||||||
| Andree et al., 2004 (24); South Korea | 15 | F | 20–24 | 27-d supplementation | Self-selected diet throughout the study. Zinc gluconate supplement (22 mg Zn/d). Estimated dietary zinc intake was 9.0 ± 4.0 and 9.1 ± 5.5 for young and elderly, respectively (∼31 mg Zn/d total). | Leukocytes | ZnT1 | Day 0 of supplementation | Leukocyte ZIP1 decreased with zinc supplementation vs. baseline in both young and elderly. | NA |
| 10 | 64–75 | ZIP1 | Day 27 of supplementation | |||||||
| Aydemir et al., 2006 (19); United States | NA | M | 19–31 | 10-d supplementation | Zinc sulfate supplement (15 mg Zn/d) for 10 d and placebo for 6 d. | Granulocytes | MT-1/-2 | Days 0, 2, 4, 6, 8, and 10 of supplementation | MT increased in all cell types (granulocytes, days 4, 6, 8, and 10; monocytes, days 2, 4, 6, and 8; T lymphocytes, days 2, 4, 6, 8, and 10) and DBSs (days 2, 4, 6, 8, and 10) with zinc supplementation vs. placebo and returned to baseline with placebo (days 2, 4, and 6). DBS ZnT1 and ZIP3 increased and decreased, respectively, at all time points with zinc supplementation; both returned to baseline with placebo (day 2–6). | NA |
| 6-d placebo | Monocytes | ZnT1 (DBS) | Days 2, 4, and 6 of placebo | |||||||
| T lymphocytes | ZIP3 (DBS) | |||||||||
| DBSs | ||||||||||
| Bales et al., 1994 (25); United States | 15 | F/M | 59–78 | 10- to 14-d acclimation | Self-selected diet with adequate zinc (16.4 mg Zn/d) during acclimation phase.§ Controlled feeding with low-zinc diet (3.97 ± 0.21 mg Zn/d) during depletion phase, followed by repletion diet (14.19 ± 0.71 mg Zn/d) plus a zinc gluconate supplement (28.19 mg Zn/d total) during repletion phase. | Erythrocytes | MT | Final day (day 10–14) of acclimation | Erythrocyte MT protein (determined by RIA) decreased post-repletion phase vs. the end of the depletion phase. | Plasma zinc was not affected by zinc depletion or repletion. |
| 15-d depletion | Day 15 of depletion | |||||||||
| 6-d repletion | Day 6 of repletion | |||||||||
| Cao and Cousins, 2000 (20); United States | 8/group | M | 19–31 | 10-d supplementation | Self-selected diet throughout the study. Zinc sulfate/sucrose supplement (15 mg Zn/d) or placebo during supplementation phase. | Monocytes | MT | Days 0, 2, 4, 6, 8, and 10 of supplementation | Monocyte and PBMC MT increased with zinc supplementation at all time points and decreased to baseline post-supplementation. DBS MT increased with zinc supplementation at all time points, but did not decrease to baseline until day 4 of post-supplementation phase. Erythrocyte MT protein (measured by ELISA) increased with zinc supplementation on days 8–10 and stayed elevated post-supplementation. | Plasma zinc increased with zinc supplementation on days 2, 4, and 6. |
| 4-d post-supplementation | PBMCs | Days 2 and 4 of post-supplementation | ||||||||
| DBSs | ||||||||||
| Erythrocytes | ||||||||||
| Cao et al., 2001 (26); United States | 8/group | M | 19–31 | 10-d supplementation | Zinc supplement (type NA; 15 mg Zn/d) or placebo. | PBMCs | MT | Days 0, 4, and 10 of supplementation | PBMC MT increased with zinc supplementation on days 4 and 10. | Plasma zinc increased with zinc supplementation on day 4. |
| Chu et al., 2015 (27); Australia | 39 | F/M | 18–65 | 21-d supplementation | Self-selected diet throughout the study. Supplement of elemental zinc chelated with amino acid (22 mg Zn/d) or no treatment. | PBMCs | MT-1 | Days 0, 2, 7, 14, and 21 of supplementation | PBMC MT-2A increased with zinc supplementation on day 2, but returned to baseline on days 7–21. | Plasma zinc was not affected by zinc supplementation and did not differ between groups. |
| MT-2 | ||||||||||
| ZnT1 | ||||||||||
| ZnT5 | ||||||||||
| ZnT6 | ||||||||||
| ZnT7 | ||||||||||
| ZIP1 | ||||||||||
| ZIP3 | ||||||||||
| ZIP7 | ||||||||||
| ZIP8 | ||||||||||
| ZIP10 | ||||||||||
| ZIP14 | ||||||||||
| Grider et al., 1990 (28); United States | 13 | M | 25–32 | 3-d acclimation (n = 7) | Controlled feeding throughout the study (0.46 mg Zn ⋅ d−1 ⋅ 70 kg body weight−1). Zinc carbonate supplement (4 mg Zn/d) on the first day of acclimation phase. Zinc carbonate supplement (50 mg Zn/d) during repletion phase. | Erythrocytes | MT | Days 1, 2, 3, 4, 5, and 6 of depletion | Erythrocyte MT protein (determined by ELISA) decreased with depletion on days 1 and 2 of repletion phase vs. baseline. | Plasma zinc was not affected by zinc depletion. |
| 6-d depletion | Days 1 and 2 of repletion | |||||||||
| 2-d repletion | ||||||||||
| 63-d supplementation (n = 6) | Self-selected diet throughout the study. Zinc gluconate supplement (50 mg Zn/d) during the supplementation phase. | Days 0, 7, 14, 28, 35, 56, and 63 of supplementation | Erythrocyte MT increased with zinc supplementation on day 7 and stayed elevated throughout the supplementation phase; MT decreased 7 d post-supplementation.‡ | |||||||
| 21-d post-supplementation | Days 7, 14, and 21 of post-supplementation | |||||||||
| Hunt et al., 2008 (29); United States | 26 | F | 21–51 | 56-d controlled feeding with varying amounts of dietary zinc | 4.3-mg or 7.2-mg zinc diet (n = 6) | Leukocytes | ZnT1 | Day 0 of controlled feeding | Leukocyte ZnT1 and ZIP1 were not affected by controlled feeding. | Plasma zinc was not affected by controlled feeding. |
| 10.1-mg zinc diet (n = 4) | ZIP1 | Day 56 of controlled feeding | ||||||||
| 14.0-mg zinc diet (n = 4) | ||||||||||
| 17.0-mg zinc diet (n-4) | ||||||||||
| Noh et al., 2014 (30); South Korea | 35* | F | 18–28 | 56-d supplementation | Self-selected diet throughout the study. Zinc gluconate supplement (30 mg Zn/d) or placebo. | Leukocytes | ZnT1 | Day 0 of supplementation | Leukocyte ZnT1, ZnT5, and ZnT9 increased with zinc supplementation vs. baseline. | Plasma zinc was not affected by zinc supplementation. |
| ZnT2 | Day 56 of supplementation | |||||||||
| ZnT5 | ||||||||||
| ZnT6 | ||||||||||
| ZnT9 | ||||||||||
| Ryu et al., 2011 (31); United States | 9 | M | 21–35 | 7-d acclimation | Controlled feeding during acclimation phase (∼10.4 mg dietary Zn/d). Liquid diet during depletion phase (0.3 mg Zn/d). Self-selected diet with zinc monomethionate supplement (15 mg Zn/d) during repletion phase (28.9 mg Zn/d total). | PBMCs | MT (P, R) | Day 0 of acclimation (all) | PBMC MT, ZnT1, ZnT4, and ZnT5 decreased with zinc depletion on day 10. PBMC ZnT1 decreased on day 6 of depletion (data not shown for others). Reticulocyte ZnT1 and ZIP3 decreased with zinc depletion on days 6 and 10. Whole-blood ZnT1 decreased with zinc depletion on days 6 and 10. | Serum zinc decreased with depletion on days 8 and 10 of depletion phase and increased with diet and supplementation on days 3 and 7 of repletion phase. |
| 10-d depletion | Reticulocytes | ZnT1(P, R, W) | Days 6 (MT, ZnT1, ZIP3) and 10 (all) of depletion | |||||||
| 7-d repletion | Whole blood | ZnT4 (P) | Serum zinc measures at baseline; day 7 of acclimation; days 3, 6, 8, and 10 of depletion; and days 3 and 7 of repletion | |||||||
| ZnT5 (P, R) | ||||||||||
| ZnT6 (P, R) | ||||||||||
| ZnT7 (P) | ||||||||||
| ZIP1 (P) | ||||||||||
| ZIP3 (P, R) | ||||||||||
| ZIP5 (P) | ||||||||||
| ZIP6 (P) | ||||||||||
| ZIP8 (P, R) | ||||||||||
| ZIP10 (P, R) | ||||||||||
| ZIP14 (P) | ||||||||||
| ZnT1 | ||||||||||
| ZIP8 | ||||||||||
| ZIP10 | ||||||||||
| Ryu et al., 2012 (32); United States | 9 | M | 21–35 | 7-d acclimation | Controlled feeding during acclimation phase (∼10.4 mg dietary Zn/d). Liquid diet during depletion phase (0.3 mg Zn/d). Self-selected diet with zinc monomethionate supplement (15 mg Zn/d) during repletion phase (29.5 mg Zn/d total). | Erythrocytes | Day 7 of acclimation | Erythrocyte ZnT1, ZIP8, and ZIP10 protein (determined by Western blot) were not affected during zinc depletion phase. | Serum zinc decreased from baseline with zinc depletion on day 10 of depletion phase. | |
| 10-d depletion | Day 10 of depletion | |||||||||
| 7-d repletion | ||||||||||
| Sharif et al., 2015 (33); Australia | 42/group† | F/M | 65–85 | 84-d supplementation | Self-selected diet throughout the study. Zinc carnosine supplement (20 mg Zn/d) or placebo. | T lymphocytes | MT-1 | Day 0 of supplementation | Lymphocyte MT and ZIP1 increased with zinc supplementation vs. placebo. | Plasma zinc increased with zinc supplementation vs. baseline. |
| ZIP1 | Day 84 of supplementation | |||||||||
| Sullivan and Cousins, 1997 (34); United States | 10/group | M | 19–35 | 15-d supplementation | Self-selected diet throughout the study. Zinc gluconate supplement (50 mg Zn/d) or placebo. Dietary zinc data NA. | Monocytes | MT-2 | Days 0, 6, and 15 of supplementation | Monocyte MT cDNA increased with zinc supplementation on days 6 and 15 vs. placebo. | Plasma zinc increased with zinc supplementation on days 6 and 15 vs. placebo. |
| Sullivan et al., 1998 (21); United States | 25 | M | 19–35 | 7-d acclimation | Self-selected diet throughout the study. Zinc gluconate supplement (50 mg Zn/d) or placebo during supplementation. | Monocytes | MT-1/-2 | Day 7 of acclimation | Monocyte MT cDNA increased with zinc supplementation on days 2–18 vs. placebo and declined to baseline post-supplementation phase. Erythrocyte MT protein (determined by ELISA) increased with zinc supplementation on days 8, 10, and 15 (ns, day 18) vs. placebo and remained elevated 4 d post-supplementation. | Plasma zinc increased with zinc supplementation on days 6 and 15 of supplementation phase. |
| 18-d supplementation | Erythrocytes | Days 0, 2, 4, 6, 8, 10, 15, and 18 of supplementation | ||||||||
| 12-d post-supplementation | Days 4, 8, and 12 of post-supplementation | |||||||||
| Plasma zinc measures on day 7 of acclimation; days 0, 6, and 15 of supplementation; days 4 and 12 of post-supplementation | ||||||||||
| Thomas et al., 1992 (35); United States | 15 | M | 22–35 | 7-d acclimation | Controlled feeding during acclimation phase (15 mg Zn/d) and treatment phase (3.2, 7.2, or 15.2 mg Zn/d; n = 5/group) with added zinc as zinc sulfate. Liquid diet (0.55 mg Zn/d) during depletion phase. Self-selected diet with zinc supplement (form NA; 50 mg Zn/d) during supplementation phase. | Erythrocytes | MT | Day 7 of acclimation | Erythrocyte MT protein (determined by ELISA) decreased in the group receiving 3.2 mg Zn/d during the treatment phase vs. the acclimation phase, but did not change in the other groups. Erythrocyte MT decreased with depletion with all diets and increased with supplementation with all diets vs. the preceding phase. | Plasma zinc decreased in the group receiving 3.2 mg Zn/d during the treatment phase, but did not change in the other groups. Plasma zinc was not affected during depletion or supplementation phases. |
| 42-d treatment | Day 42 of treatment | |||||||||
| 12-d depletion | Day 12 of depletion | |||||||||
| 30-d supplementation | Day 30 of supplementation |
*Subjects were overweight/obese [BMI (in kg/m2) ≥25]. †Subjects were healthy with low plasma zinc (<0.77 mg/L or 11.8 μM). §Individuals with low zinc intake (<7 mg/d) received a daily supplement (7 mg). ‡Statistics for the 63-d supplementation study not shown. DBS, dried spot of whole blood; MT, metallothionein; NA, not available; P, PBMC; PBMC, peripheral blood mononuclear cell; R, reticulocyte; RIA, radioimmunoassay; W, whole blood; ZIP, zrt-, irt-like protein; ZnT, zinc transporter.
All measures are gene amounts unless noted otherwise.
Three studies isolated leukocytes (24, 29, 30); 1 study isolated granulocytes (19), which include neutrophils, eosinophils, and basophils; 4 studies isolated monocytes (19–21, 34); 3 studies isolated T lymphocytes (19, 23, 33); 4 studies isolated PBMCs (20, 26, 27, 31), which include T lymphocytes and monocytes; 1 study isolated reticulocytes (31); 5 studies isolated erythrocytes (20, 21, 25, 28, 35); and 3 studies used whole blood or dried spots of whole blood (DBSs) (19, 20, 31).
With the exception of 6 studies that determined the protein expression of metallothionein (20, 21, 25, 28, 35) and ZnT1, ZIP8, and ZIP10 (32) in erythrocytes, all studies determined the RNA expression of metallothionein or zinc transporters. Expression of ZnT1 was determined in 7 studies (19, 24, 27, 29–32). Four studies (2 in leukocytes, 1 in PBMCs, and 1 in erythrocytes) demonstrated no change in ZnT1 expression in response to zinc supplementation or depletion (24, 27, 29, 32). Two studies, one in DBSs and one in leukocytes, identified an increase with zinc supplementation (19, 30), and one study that isolated PBMCs, reticulocytes, and whole blood found a decrease in ZnT1 in all cell types with zinc depletion (31). Of those studies reporting a change, DBS ZnT1 responded within 48 h to zinc supplementation (earliest time point studied) (19). PBMC, reticulocyte, and whole-blood ZnT1 responded within 6 d of zinc depletion, which was the earliest time point studied (31).
Expression of ZIP1 was determined in 5 studies. Three studies found that ZIP1 did not respond to zinc supplementation or depletion in leukocytes or PBMCs (27, 29, 31). One 27-d zinc supplementation study found a decrease in ZIP1 expression in leukocytes (24), whereas one longer-term supplementation study (84 d) that used lymphocytes found an increase in ZIP1 (33). Expression of ZIP3 was determined in 3 studies (19, 27, 31). DBS ZIP3 decreased with 48-h zinc supplementation (19), and reticulocyte ZIP3 decreased with 6 d of zinc depletion (no change was observed in PBMC ZIP3) (27, 31). ZIP8 and ZIP10 were measured in 3 studies (27, 31, 32). Neither transporter responded to zinc in PBMCs, reticulocytes, or erythrocytes. Expression of ZnT5 and ZnT6 were determined in 3 studies (27, 30, 31); expression of ZnT7 and ZIP14 were determined in 2 studies (27, 31); and expression of ZnT2, ZnT4, ZnT9, ZIP5, and ZIP6 were determined in one study (30, 31). PBMC ZnT5 and ZnT4 decreased with zinc depletion and leukocyte ZnT5 and ZnT9 increased with zinc supplementation (27, 30, 31); no other transporters responded to zinc.
Metallothionein was the most frequently investigated marker of zinc status; 12 studies determined its expression in various blood cell types. T lymphocyte metallothionein increased and decreased with zinc supplementation (19, 33) and depletion (23), respectively. The increase in metallothionein was observed within 48 h of supplementation (earliest time point measured) (19). Monocyte metallothionein increased within 48 h of zinc supplementation (19–21, 34), whereas granulocyte metallothionein did not increase significantly until day 4 of supplementation (19). In both cell types, metallothionein returned to baseline 48 h after supplementation ceased. PBMC metallothionein increased (20, 26, 27) and decreased (31) with zinc supplementation and depletion, respectively. These changes were observed at the earliest time point measured (48 h and day 10, respectively). Erythrocyte metallothionein protein did not increase until day 8 of zinc supplementation and remained elevated after supplementation ceased (20, 21). Similarly, erythrocyte metallothionein protein did not decline until day 7 of zinc depletion, when the volunteers were on the repletion phase (28, 35). DBS metallothionein increased within 48 h of zinc supplementation (19, 20). One study reported that metallothionein returned to baseline 48 h after supplementation ceased (19), whereas another found that metallothionein protein did not return to baseline until 4 d after supplementation ceased (20). Reticulocyte metallothionein did not change with zinc depletion (31).
The plasma zinc response to supplementation and/or depletion was determined in 14 studies. Six studies found no change in plasma zinc with zinc depletion or supplementation (23, 25, 27–30). Two studies that provided 15 mg Zn/d for 10 d indicated an increase in plasma zinc after 2 d and 4 d of zinc supplementation (20, 26). These were the earliest time points of data collection. One study found a decrease in plasma zinc by day 8 of a 10-d depletion (31). In the same study, plasma zinc increased by day 3 of zinc repletion (31). Two studies that supplemented with 50 mg Zn/d for 15–18 d reported an increase in plasma zinc on day 6 (earliest time point measured) (21, 34). One longer-term study documented an increase in plasma zinc on day 56 of an 84-d supplementation trial (20 mg Zn/d) (33).
Eleven studies determined both metallothionein and plasma zinc in response to zinc supplementation and/or depletion. A comparison of the findings for both measures is presented in Table 3. To assess the reliability of these measures to changes in dietary zinc, the percentage of studies that responded to zinc supplementation and/or depletion was determined for metallothionein and plasma zinc. Metallothionein responded to zinc supplementation or depletion in 100% of the studies that used leukocytes (n = 12) and 89% of studies that used erythrocytes, reticulocytes, or DBSs (n = 9). Plasma zinc responded to zinc supplementation or depletion in 69% of studies (n = 16). The sensitivity of changes in metallothionein and plasma zinc was determined by calculating the percentage of studies that detected a change at the earliest time point measured. Of those studies that reported a change, leukocyte metallothionein responded at the earliest time point measured in 92% of studies (n = 12), the exception being granulocyte metallothionein, which responded on day 4 of supplementation (day 2 was the earliest) (19). Erythrocyte, reticulocyte, and DBS metallothionein responded at the earliest time point measured in 63% of studies reporting a change (n = 8) and failed to return to baseline post-supplementation in 2 studies. Plasma zinc responded at the earliest time point measured in 82% of studies reporting a change (n = 11) and did not return to baseline post-supplementation.
TABLE 3.
Comparison of metallothionein and plasma zinc response to zinc supplementation and depletion1
| Metallothionein response |
Plasma zinc response |
|||||
| Response | Day | Response | Day | Notes | Study (reference) | |
| T lymphocytes | ▼ (D) | 49 (earliest) | — | — | Allan et al., 2000 (23) | |
| ▲ (S) | 2 (earliest) | NA | — | Metallothionein returned to baseline with placebo (2 d, earliest) | Aydemir et al., 2006 (19) | |
| ▲ (S) | 84 (earliest) | ▲ (S) | 84 (earliest) | Sharif et al., 2015 (33) | ||
| Granulocytes | ▲ (S) | 4 (2 earliest) | NA | — | Aydemir et al., 2006 (19) | |
| Monocytes | ▲ (S) | 2 (earliest) | NA | — | Metallothionein returned to baseline with placebo (2 d, earliest) | Aydemir et al., 2006 (19) |
| ▲ (S) | 2 (earliest) | ▲ (S) | 2 (earliest) | Metallothionein returned to baseline with placebo (2 d, earliest) | Cao and Cousins, 2000 (20) | |
| ▲ (S) | 6 (earliest) | ▲ (S) | 6 (earliest) | Sullivan and Cousins, 1997 (34) | ||
| ▲ (S) | 2 (earliest) | ▲ (S) | 6 (earliest) | Metallothionein returned to baseline with placebo (4 d, earliest) | Sullivan et al., 1998 (21) | |
| PBMCs | ▼ (D) | 6 (earliest) | ▼ (D) | 8 (3 earliest) | Ryu et al., 2011 (31) | |
| ▲ (S) | 2 (earliest) | ▲ (S) | 2 (earliest) | Metallothionein returned to baseline with placebo (2 d, earliest) | Cao and Cousins, 2000 (20) | |
| ▲ (S) | 4 (earliest) | ▲ (S) | 4 (earliest) | Cao et al., 2001 (26) | ||
| ▲ (S) | 2 (earliest) | — | — | Metallothionein returned to baseline by day 4 of supplementation | Chu et al., 2015 (27) | |
| Erythrocytes | ▼ (D) | 21* (15 earliest) | — | — | *Metallothionein decreased on day 6 of repletion (no change on day 15 of depletion) | Bales et al., 1994 (25) |
| ▼ (D) | 12 (earliest) | — | — | Thomas et al., 1992 (35) | ||
| ▲ (S) | 30 (earliest) | |||||
| ▼ (D) | 7* (1 earliest) | — | — | *Metallothionein decreased on day 1 of repletion (no change on days 1–6 of depletion) | Grider et al., 1990 (28) | |
| ▲ (S) | 7 (earliest) | Metallothionein decreased to baseline after supplementation ceased (7 d, earliest) | ||||
| ▲ (S) | 8 (2 earliest) | ▲ (S) | 2 (earliest) | Metallothionein remained elevated after supplementation ceased (2–4 d) | Cao and Cousins, 2000 (20) | |
| ▲ (S) | 8 (2 earliest) | ▲ (S) | 6 (earliest) | Metallothionein remained elevated after supplementation ceased (4 d) | Sullivan et al., 1998 (21) | |
| Reticulocytes | — (D) | — | ▼ (D) | 8 (3 earliest) | Serum zinc increased by day 3 of repletion (earliest) | Ryu et al., 2011 (31) |
| DBSs | ▲ (S) | 2 (earliest) | NA | — | Metallothionein returned to baseline with placebo (2 d, earliest) | Aydemir et al., 2006 (19) |
| ▲ (S) | 2 (earliest) | ▲ (S) | 2 (earliest) | Metallothionein returned to baseline by day 4 of placebo (2 d, earliest) | Cao and Cousins, 2000 (20) | |
*Response was observed during repletion phase. D, depletion; DBS, dried spot of whole blood; NA, not available; PBMC, peripheral blood mononuclear cell; S, supplementation; ▼, decrease; ▲, increase; —, no change.
To determine whether expression of leukocyte metallothionein reflected the amount of dietary zinc consumed, percentage change from baseline was calculated for all studies that determined metallothionein expression in leukocytes (Figure 2). Data were organized on the basis of the amount of dietary zinc consumed (<5 mg Zn/d, n = 2; 15–22 mg Zn/d, n = 7; 50 mg Zn/d, n = 2). Metallothionein expression decreased 39% from baseline in subjects consuming <5 mg Zn/d (RDA = 8 mg/d and 11 mg/d for women and men >19 y) and increased with elevated concentrations of dietary zinc (135%, 15–22 mg Zn/d and 267%, 50 mg Zn/d).
FIGURE 2.
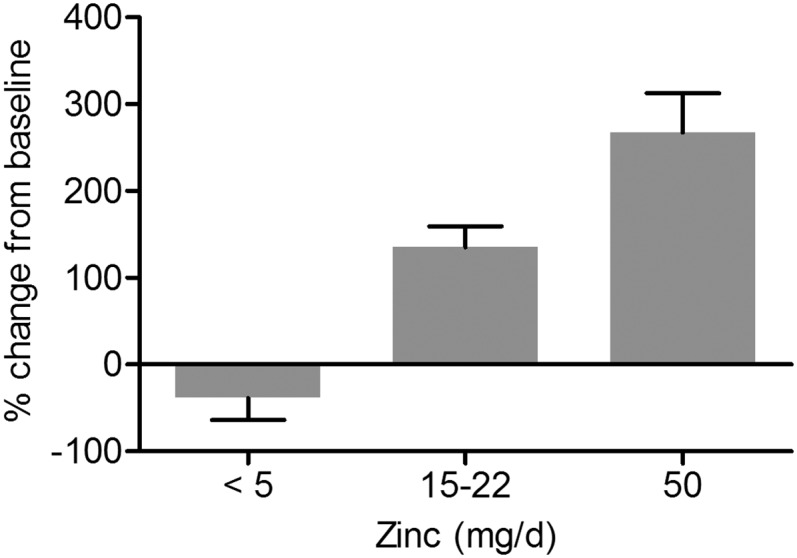
Metallothionein expression in leukocytes. Values are percentage changes from baseline ± SEs for all studies that examined metallothionein expression in leukocytes. In studies that determined metallothionein expression at multiple time points (19–21), the day with the greatest percentage change was used. Data are grouped based on the amount of dietary zinc consumed per day [<5 mg Zn/d, n = 2 (23, 31); 15–22 mg Zn/d, n = 7 (19, 20, 26, 27, 33); 50 mg Zn/d, n = 2 (21, 34)].
Discussion
The major finding from this systematic review was that metallothionein expression in leukocytes was more sensitive to changes in dietary zinc than other indicators. Findings also suggest that metallothionein expression in leukocytes may be a more reliable and sensitive measure than plasma zinc for determining zinc exposure. As such, of currently available methods, leukocyte metallothionein expression may be an important component in determining zinc status. Finally, this systematic review highlights the need for more studies that examine the utility of zinc transporter expression in leukocytes, perhaps in combination with another biomarker, as a clinical indicator of zinc status in human blood.
This systematic review highlights several attributes that suggest metallothionein expression in leukocytes may have potential as a preferred biomarker to assess zinc status. The studies included in this review demonstrate that metallothionein expression is sensitive to changes in dietary zinc. Metallothionein expression in leukocytes decreased in response to zinc depletion and increased in response to zinc supplementation in a dose-dependent manner and at the earliest time point measured, from decreased expression with zinc intake less than the RDA to a corresponding increase with elevated amounts of dietary zinc. This is in agreement with previous work that demonstrated that metallothionein increases in response to extracellular zinc and is degraded when metallothionein-bound zinc is released when zinc is low (13). Interestingly, metallothionein expression in cells isolated from the erythroblast lineage (e.g., reticulocytes and erythrocytes) demonstrated a reduced or lack of sensitivity to changes in dietary zinc. This likely reflects the absence of nuclei and longer lifespan of red blood cells (120 d) compared with leukocytes (4–39 d). Metallothionein, which is concentrated in the reticulocyte fraction, can also change in response to factors influencing the rate of erythropoiesis (e.g., poor iron status) (23), further suggesting that erythrocytes may not be a sensitive cell type for assessing zinc status.
In contrast to leukocyte metallothionein, plasma zinc did not respond consistently to changes in dietary zinc. Some studies did not detect a change in plasma zinc, and, in others, the response was not sensitive (i.e., failed to return to baseline when supplementation ceased). Changes in plasma and tissue zinc are relatively insensitive to changes in dietary zinc because of mechanisms that tightly control zinc homeostasis. Thus, plasma zinc only falls when dietary intake is so low that homeostasis cannot be established without the use of zinc from the exchangeable zinc pools (when dietary zinc falls below 5–6 mg Zn/d) (4). One study included in this systematic review only included subjects with low plasma zinc (<0.77 mg/L or 11.8 μM) and found increases in metallothionein expression and plasma zinc with increased zinc supplementation (33). However, this study only included 2 time points (baseline and day 84), so it is not possible to determine the sensitivity of the measures in individuals who start with low plasma zinc concentrations. Collectively, these findings suggest that, compared with plasma zinc, zinc status may be more appropriately determined by considering assessment of the mechanisms used to control zinc homeostasis in response to fluctuations in zinc intake (i.e., zinc-binding proteins and/or zinc transporters).
Lastly, this review highlights the lack of consistency in the zinc transporter expression data. Because of the large number of zinc transporters and the general lack of studies that examined zinc transporter expression in response to zinc supplementation and/or depletion, more comprehensive studies are required to determine whether zinc transporter expression in leukocytes can be used to assess zinc status. Before conducting these studies, it is important to consider that zinc transporters are expressed in a tissue and cell type–specific manner (36). Thus, it is essential first to characterize the zinc transporters expressed in different human leukocyte populations and to determine which of these transporters responds to dietary zinc. Overbeck et al. (37) assessed the expression of ZnT1–9 in PBMCs and found that ZnT1 was most highly expressed, whereas ZnT2 was not expressed and ZnT3 and ZnT9 were expressed at low levels. Importantly, the authors reported that ZnT8 was not expressed uniformly, suggesting that ZnT8 expression is not a reliable indicator of zinc status. The authors also isolated and cultured PBMCs in vitro in response to supplemental zinc or TPEN and found that ZnT1 expression increased incrementally in cells cultured with 15 and 30 μM zinc and decreased slightly with TPEN. Expression of other zinc transporters has been assessed in commercialized cell lines, such as THP-1 monocytes (38). However, these studies may not represent accurately changes observed in humans, because the expression pattern between commercialized cell lines and primary cells varies (37). Finally, zinc transporters respond to zinc through transcriptional and post-translational mechanisms (39, 40). Thus, no changes in expression, but differences in localization/function, may provide important information regarding their use in assessing status.
The current study is not without limitations. Because of the number of zinc transporters and different cell types included in this systematic review, it was not possible to compare the results statistically. It should also be noted that 9 of the 16 studies included in this systematic review came from one laboratory. Moreover, the majority of studies determined the RNA expression of metallothionein, which precludes comparison of the relative concentrations of metallothionein across studies and clinically. Recent advances allow for the detection of metallothionein with the use of commercially available immunoassays; however, reference material for metallothionein concentrations needs to be developed in order to compare metallothionein concentrations reliably across different immunoassays.
Moving forward, future research should further characterize the use of metallothionein as a biomarker of zinc status, such as whether metallothionein can be used in adolescents and children or individuals with disease. Although the authors did not determine metallothionein expression, one study not included in this systematic review examined ZnT1 and ZIP1 expression in leukocytes isolated from 12- to 16-y-old female patients (41). In agreement with Andree et al. (24), the authors reported a decrease in ZIP1 with zinc supplementation; however, no change was observed in ZnT1 or plasma zinc concentrations. Other questions, such as whether the different proportion of blood cell subtypes in peripheral blood, which vary across individuals, affects the sensitivity to zinc supplementation/depletion, should also be assessed. For example, metallothionein transcript abundance in monocytes is 3 times that found in granulocytes and 2 times that found in T lymphocytes (19). Moreover, factors that affect metallothionein and zinc transporter expression [e.g., inflammatory agents, free radicals, glucocorticoids, and pharmacologic agents (42, 43)] must be considered when assessing zinc status. To circumvent the impact of confounders, other nutrients use multiple indicators and a multivariable model. Following this paradigm, it is likely that the search for a zinc biomarker will not yield a sole indicator to assess zinc status. Thus, the combination of metallothionein and zinc transporter expression in leukocytes (perhaps used in conjunction with plasma zinc) may provide a more comprehensive view of zinc status while minimizing the influence of confounders on any single variable.
Overall, evidence from the current systematic review suggests that metallothionein in leukocytes is sensitive to changes in dietary zinc. This indicates that the detection of metallothionein in leukocytes, which can be obtained readily by venipuncture, may be a reliable marker to assist in the detection of zinc status in a clinical or field setting.
Acknowledgments
We thank Andrew J Young for his critical review of the manuscript. All authors read and approved the final manuscript.
Footnotes
Abbreviations used: DBS, dried spot of whole blood; PBMC, peripheral blood mononuclear cell; SLC, solute carrier family; TPEN, N,N,N′,N′-tetrakis(2-pyridylmethyl)ethylenediamine; ZIP, zrt-, irt-like protein; ZnT, zinc transporter.
References
- 1.Vallee BL, Falchuk KH. The biochemical basis of zinc physiology. Physiol Rev 1993;73:79–118. [DOI] [PubMed] [Google Scholar]
- 2.Beach RS, Gershwin ME, Hurley LS. Growth and development in postnatally zinc-deprived mice. J Nutr 1980;110:201–11. [DOI] [PubMed] [Google Scholar]
- 3.Prasad AS. Clinical, endocrinological and biochemical effects of zinc deficiency. Clin Endocrinol Metab 1985;14:567–89. [DOI] [PubMed] [Google Scholar]
- 4.King JC. Assessment of zinc status. J Nutr 1990;120 Suppl 11:1474–9. [DOI] [PubMed] [Google Scholar]
- 5.King JC. Zinc: an essential but elusive nutrient. Am J Clin Nutr 2011;94:679S–84S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wood RJ. Assessment of marginal zinc status in humans. J Nutr 2000;130:1350S–4S. [DOI] [PubMed] [Google Scholar]
- 7.Baer MT, King JC. Tissue zinc levels and zinc excretion during experimental zinc depletion in young men. Am J Clin Nutr 1984;39:556–70. [DOI] [PubMed] [Google Scholar]
- 8.Miller LV, Hambidge KM, Naake VL, Hong Z, Westcott JL, Fennessey PV. Size of the zinc pools that exchange rapidly with plasma zinc in humans: alternative techniques for measuring and relation to dietary zinc intake. J Nutr 1994;124:268–76. [DOI] [PubMed] [Google Scholar]
- 9.Zhou JR, Canar MM, Erdman JW Jr. Bone zinc is poorly released in young, growing rats fed marginally zinc-restricted diet. J Nutr 1993;123:1383–8. [DOI] [PubMed] [Google Scholar]
- 10.Lowe NM, Fekete K, Decsi T. Methods of assessment of zinc status in humans: a systematic review. Am J Clin Nutr 2009;89:2040S–51S. [DOI] [PubMed] [Google Scholar]
- 11.Sato M, Mehra RK, Bremner I. Measurement of plasma metallothionein-I in the assessment of the zinc status of zinc-deficient and stressed rats. J Nutr 1984;114:1683–9. [DOI] [PubMed] [Google Scholar]
- 12.Heuchel R, Radtke F, Georgiev O, Stark G, Aguet M, Schaffner W. The transcription factor MTF-1 is essential for basal and heavy metal-induced metallothionein gene expression. EMBO J 1994;13:2870–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Langmade SJ, Ravindra R, Daniels PJ, Andrews GK. The transcription factor MTF-1 mediates metal regulation of the mouse ZnT1 gene. J Biol Chem 2000;275:34803–9. [DOI] [PubMed] [Google Scholar]
- 14.Guo L, Lichten LA, Ryu MS, Liuzzi JP, Wang F, Cousins RJ. STAT5-glucocorticoid receptor interaction and MTF-1 regulate the expression of ZnT2 (Slc30a2) in pancreatic acinar cells. Proc Natl Acad Sci USA 2010;107:2818–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lichten LA, Ryu MS, Guo L, Embury J, Cousins RJ. MTF-1-mediated repression of the zinc transporter Zip10 is alleviated by zinc restriction. PLoS One 2011;6:e21526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao H, Butler E, Rodgers J, Spizzo T, Duesterhoeft S, Eide D. Regulation of zinc homeostasis in yeast by binding of the ZAP1 transcriptional activator to zinc-responsive promoter elements. J Biol Chem 1998;273:28713–20. [DOI] [PubMed] [Google Scholar]
- 17.Coneyworth LJ, Jackson KA, Tyson J, Bosomworth HJ, van der Hagen E, Hann GM, Ogo OA, Swann DC, Mathers JC, Valentine RA, et al. Identification of the human zinc transcriptional regulatory element (ZTRE): a palindromic protein-binding DNA sequence responsible for zinc-induced transcriptional repression. J Biol Chem 2012;287:36567–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9, W64. [DOI] [PubMed] [Google Scholar]
- 19.Aydemir TB, Blanchard RK, Cousins RJ. Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations. Proc Natl Acad Sci USA 2006;103:1699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao J, Cousins RJ. Metallothionein mRNA in monocytes and peripheral blood mononuclear cells and in cells from dried blood spots increases after zinc supplementation of men. J Nutr 2000;130:2180–7. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan VK, Burnett FR, Cousins RJ. Metallothionein expression is increased in monocytes and erythrocytes of young men during zinc supplementation. J Nutr 1998;128:707–13. [DOI] [PubMed] [Google Scholar]
- 22.Bogale A, Clarke SL, Fiddler J, Hambidge KM, Stoecker BJ. Zinc supplementation in a randomized controlled trial decreased ZIP4 and ZIP8 mRNA abundance in peripheral blood mononuclear cells of adult women. Nutr Metab Insights 2015;8:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allan AK, Hawksworth GM, Woodhouse LR, Sutherland B, King JC, Beattie JH. Lymphocyte metallothionein mRNA responds to marginal zinc intake in human volunteers. Br J Nutr 2000;84:747–56. [PubMed] [Google Scholar]
- 24.Andree KB, Kim J, Kirschke CP, Gregg JP, Paik H, Joung H, Woodhouse L, King JC, Huang L. Investigation of lymphocyte gene expression for use as biomarkers for zinc status in humans. J Nutr 2004;134:1716–23. [DOI] [PubMed] [Google Scholar]
- 25.Bales CW, DiSilvestro RA, Currie KL, Plaisted CS, Joung H, Galanos AN, Lin PH. Marginal zinc deficiency in older adults: responsiveness of zinc status indicators. J Am Coll Nutr 1994;13:455–62. [DOI] [PubMed] [Google Scholar]
- 26.Cao J, Bobo JA, Liuzzi JP, Cousins RJ. Effects of intracellular zinc depletion on metallothionein and ZIP2 transporter expression and apoptosis. J Leukoc Biol 2001;70:559–66. [PubMed] [Google Scholar]
- 27.Chu A, Foster M, Ward S, Zaman K, Hancock D, Petocz P, Samman S. Zinc-induced upregulation of metallothionein (MT)-2A is predicted by gene expression of zinc transporters in healthy adults. Genes Nutr 2015;10:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grider A, Bailey LB, Cousins RJ. Erythrocyte metallothionein as an index of zinc status in humans. Proc Natl Acad Sci USA 1990;87:1259–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunt JR, Beiseigel JM, Johnson LK. Adaptation in human zinc absorption as influenced by dietary zinc and bioavailability. Am J Clin Nutr 2008;87:1336–45. [DOI] [PubMed] [Google Scholar]
- 30.Noh H, Paik HY, Kim J, Chung J. The changes of zinc transporter ZnT gene expression in response to zinc supplementation in obese women. Biol Trace Elem Res 2014;162:38–45. [DOI] [PubMed] [Google Scholar]
- 31.Ryu MS, Langkamp-Henken B, Chang SM, Shankar MN, Cousins RJ. Genomic analysis, cytokine expression, and microRNA profiling reveal biomarkers of human dietary zinc depletion and homeostasis. Proc Natl Acad Sci USA 2011;108:20970–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ryu MS, Guthrie GJ, Maki AB, Aydemir TB, Cousins RJ. Proteomic analysis shows the upregulation of erythrocyte dematin in zinc-restricted human subjects. Am J Clin Nutr 2012;95:1096–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharif R, Thomas P, Zalewski P, Fenech M. Zinc supplementation influences genomic stability biomarkers, antioxidant activity, and zinc transporter genes in an elderly Australian population with low zinc status. Mol Nutr Food Res 2015;59:1200–12. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan VK, Cousins RJ. Competitive reverse transcriptase-polymerase chain reaction shows that dietary zinc supplementation in humans increases monocyte metallothionein mRNA levels. J Nutr 1997;127:694–8. [DOI] [PubMed] [Google Scholar]
- 35.Thomas EA, Bailey LB, Kauwell GA, Lee DY, Cousins RJ. Erythrocyte metallothionein response to dietary zinc in humans. J Nutr 1992;122:2408–14. [DOI] [PubMed] [Google Scholar]
- 36.Hennigar SR, Kelleher SL. Zinc networks: the cell-specific compartmentalization of zinc for specialized functions. Biol Chem 2012;393:565–78. [DOI] [PubMed] [Google Scholar]
- 37.Overbeck S, Uciechowski P, Ackland ML, Ford D, Rink L. Intracellular zinc homeostasis in leukocyte subsets is regulated by different expression of zinc exporters ZnT-1 to ZnT-9. J Leukoc Biol 2008;83:368–80. [DOI] [PubMed] [Google Scholar]
- 38.Cousins RJ, Blanchard RK, Popp MP, Liu L, Cao J, Moore JB, Green CL. A global view of the selectivity of zinc deprivation and excess on genes expressed in human THP-1 mononuclear cells. Proc Natl Acad Sci USA 2003;100:6952–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gaither LA, Eide DJ. Eukaryotic zinc transporters and their regulation. Biometals 2001;14:251–70. [DOI] [PubMed] [Google Scholar]
- 40.Hennigar SR, McClung JP. Homeostatic regulation of trace mineral transport by ubiquitination of membrane transporters. Nutr Rev 2016;74:59–67. [DOI] [PubMed] [Google Scholar]
- 41.Méndez RO, Santiago A, Yepiz-Plascencia G, Peregrino-Uriarte AB, de la Barca AM, Garcia HS. Zinc fortification decreases ZIP1 gene expression of some adolescent females with appropriate plasma zinc levels. Nutrients 2014;6:2229–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cousins RJ. Absorption, transport, and hepatic metabolism of copper and zinc: special reference to metallothionein and ceruloplasmin. Physiol Rev 1985;65:238–309. [DOI] [PubMed] [Google Scholar]
- 43.Lichten LA, Cousins RJ. Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 2009;29:153–76. [DOI] [PubMed] [Google Scholar]