Abstract
Background
Previous studies indicated that patients undergoing coronary artery bypass graft (CABG) surgery are less likely to receive guideline‐based secondary prevention therapy than are those undergoing percutaneous coronary intervention (PCI) after an acute myocardial infarction. We aimed to evaluate whether these differences have persisted after the implementation of public reporting of hospital metrics.
Methods and Results
The Clinical Outcomes Assessment Program (COAP) database was analyzed retrospectively to evaluate adherence to secondary prevention guidelines at discharge in patients who underwent coronary revascularization after an acute ST‐elevation myocardial infarction in Washington State. From 2004 to 2007, 9260 patients received PCI and 692 underwent CABG for this indication. Measures evaluated included prescription of aspirin, β‐blockers, angiotensin‐converting enzyme inhibitors or angiotensin II receptor blockers, or lipid‐lowering medications; cardiac rehabilitation referral; and smoking‐cessation counseling. Composite adherence was lower for CABG than for PCI patients during the period studied (79.6% versus 89.7%, P<0.01). Compared to patients who underwent CABG, patients who underwent PCI were more likely to receive each of the pharmacological therapies. There was no statistical difference in smoking‐cessation counseling (91.7% versus 90.3%, P=0.63), and CABG patients were more likely to receive referral for cardiac rehabilitation (70.9% versus 48.3%, P<0.01). Adherence rates improved over time among both groups, with no significant difference in composite adherence in 2006 (85.6% versus 87.6%, P=0.36).
Conclusions
Rates of guideline‐based secondary prevention adherence in patients with ST‐elevation myocardial infarction who underwent CABG surgery have been improving steadily in Washington State. The improvement possibly is associated with the implementation of public reporting of quality measures.
Keywords: angioplasty, coronary artery bypass, guideline adherence, myocardial infarction, prevention, registries, secondary prevention
Subject Categories: ,
Introduction
Previous studies have shown that patients undergoing coronary artery bypass graft (CABG) surgery after an acute myocardial infarction (MI) are less likely to receive secondary prevention measures at discharge than are similar patients undergoing percutaneous coronary intervention (PCI).1, 2 Although adherence to guideline‐based therapy has been imperfect for both procedures, secondary prevention adherence rates at discharge have been improving over time, particularly as public reporting of hospital performance has been implemented.2, 3, 4, 5 The Clinical Outcomes Assessment Program (COAP) is a quality improvement initiative developed for hospitals that perform coronary revascularization in Washington State.6 Our aim was to use data from COAP to evaluate whether prescription of guideline‐based secondary prevention therapy at discharge improved after the institution of public reporting among patients with acute ST‐elevation MI (STEMI) undergoing CABG surgery versus PCI.
Methods
Study Design and Population
We performed an observational analysis of data from COAP, which is a quality improvement initiative of the Foundation for Health Care Quality. The registry prospectively collects demographic, procedural, and in‐hospital outcomes data on all patients who undergo coronary revascularization in Washington State. This process has been in place since 1999, and public reporting began in 2005; details on the data collection process have been reported previously.6, 7
We identified all patients with documented STEMI who underwent coronary revascularization (PCI or CABG surgery) during their index hospitalization from January 2004 through December 2007. In all, 9952 patients met these criteria, of whom 9260 (93.0%) received PCI and 692 (7.0%) underwent CABG surgery. Baseline characteristics, presentation variables, clinical management, and procedural complications were determined for each group. The clinical management interventions investigated were 6 of the Class I recommendations from the 2006 ACC/AHA secondary prevention guidelines, including: prescription of aspirin, β‐blockers, lipid‐lowering therapy, and angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers (ACE‐I/ARBs); smoking‐cessation counseling; and referral for cardiac rehabilitation at the time of discharge.8 Notably, the recommendations from the 2006 guidelines with regard to these interventions do not differ significantly from the current 2011 AHA/ACCF secondary prevention guidelines.9 Unless a contraindication was documented, all patients were assumed to be candidates for aspirin, β‐blockers, lipid‐lowering therapy, and cardiac rehabilitation referral. Patients were considered to be candidates for ACE‐I/ARBs if their left ventricular ejection fraction (LVEF) was documented to be ≤40% or if they had a history of diabetes mellitus, hypertension, or chronic kidney disease. Patients were considered candidates for smoking‐cessation counseling if they indicated that they were current smokers on admission. The percent adherence for each discharge medication was determined by dividing all patients who were given the intervention by all those eligible for the intervention, excluding those with documented contraindications. A composite medication adherence score was calculated for each patient by dividing the number of prescribed medications (aspirin, β‐blockers, lipid‐lowering therapy, and ACE‐I/ARBs) by the number for which the patient was eligible. Data were available for all 4 medications during the entire study period, and temporal analyses were performed. Data were available for cardiac rehabilitation referral from 2005 to 2007 and for smoking cessation from 2006 to 2007. Given the shorter time periods during which these 2 interventions were collected, temporal analyses were not performed for these 2 measures. For temporal analyses, date of admission was used to categorize patients into quarters from the first quarter of 2004 through the fourth quarter of 2007.
Statistical Analysis
Unadjusted and adjusted relative risks (RRs) of nonadherence for each intervention were calculated for patients undergoing CABG surgery compared to PCI. Dichotomous variables were compared by the χ2 test, and continuous variables were compared by 2‐sided Student t test. Temporal trends were evaluated with χ2 test of trend. Effect modification of the association of revascularization type and discharge medications by clinical variables was evaluated with the Mantel–Haenzsel method. To account for within‐hospital clustering, a hierarchical model that incorporated Poisson risk regression with a generalized estimating equations modeling method with exchangeable working correlation matrix was used. The factors associated with lower guideline adherence in the unadjusted analyses were included in the adjusted model, except for revascularization type and year, which were included a priori. Acute kidney injury (defined as an increase in hospital creatinine by 50% above admission creatinine level for analytic purposes) was included a priori in the model evaluating ACE‐I/ARB prescription as well. Patients with acute kidney injury were not excluded from the analysis because acute kidney injury is not by itself a contraindication for ACE‐I/ARBs. Variables that were found to significantly affect adherence were included in the adjusted model and included: age, history of diabetes, peripheral vascular disease, hypertension, prior MI, congestive heart failure, procedure priority, LVEF, and the presence of shock during admission. Interactions between variables were evaluated by the Wald test and by a likelihood ratio test when an interaction term was included in the multivariable analysis.
As seen with most large dataset analyses, there were missing data. All variables used in the adjusted risk models had rates <1.0%. For the interventions investigated, rates of missing data were as follows: 1.7% for aspirin, β‐blockers, and lipid‐lowering therapy and 1.8% for ACE‐I/ARB therapy. Given that the frequency of missing data was relatively low, missing data were excluded from analyses.
All analyses were performed in STATA 10 (College Station, Texas). Because this analysis used de‐identified data, it met criteria for exemption from the University of Washington Institutional Review Board review.
Results
Patients who underwent CABG surgery were older, more likely to be male, and generally had more comorbidities and cardiovascular risk factors than patients undergoing PCI, aside from tobacco use and history of prior MI (Table 1). Patients who underwent CABG surgery also were more likely to have lower LVEF, to present in cardiogenic shock or need an intra‐aortic balloon pump during hospitalization, and to require postprocedural dialysis than were patients undergoing PCI.
Table 1.
Baseline Characteristics and Presentation Variables for Patients With STEMI Undergoing Coronary Revascularization in Washington State From 2004 to 2007
| Variable | CABG (n=692) | PCI (n=9260) | P |
|---|---|---|---|
| Age, y, mean±SD | 64.5±11.0 | 61.4±12.6 | <0.01 |
| Sex, male | 542 (78.4) | 6826 (73.8) | 0.01 |
| Race, white | 617 (94.1) | 7591 (94.8) | 0.44 |
| Smoker | 417 (60.4) | 5707 (61.7) | 0.50 |
| Diabetes | 202 (29.2) | 1783 (19.3) | <0.01 |
| Congestive heart failure | 121 (17.6) | 518 (5.6) | <0.01 |
| Chronic obstructive pulmonary disease | 140 (20.2) | 963 (10.4) | <0.01 |
| Peripheral vascular disease | 87 (12.6) | 488 (5.3) | <0.01 |
| Hypertension | 485 (70.1) | 5288 (57.1) | <0.01 |
| Prior coronary revascularization | 218 (31.5) | 1899 (20.5) | <0.01 |
| Dialysis | 13 (1.9) | 70 (0.8) | <0.01 |
| Prior MI | 372 (53.9) | 5264 (57.0) | 0.12 |
| Three‐vessel coronary disease | 157 (22.7) | 838 (9.1) | <0.01 |
| LVEF, %, mean±SD | 46.6±14.7 | 49.7±13.1 | <0.01 |
| LVEF ≤40% | 247 (35.7) | 1857 (20.1) | <0.01 |
| Pre‐procedural creatinine, mean±SD | 1.16±0.82 | 1.07±2.87 | 0.39 |
| Procedure priority | <0.01 | ||
| Elective | 102 (14.7) | 315 (3.4) | |
| Urgent | 408 (59.0) | 1147 (12.4) | |
| Emergent | 117 (25.6) | 7727 (83.5) | |
| Salvage | 5 (0.7) | 66 (0.7) | |
| Cardiogenic shock on presentation | 101 (14.6) | 679 (7.3) | <0.01 |
| IABP used during hospitalization | 171 (24.8) | 175 (1.9) | <0.01 |
| Intra‐procedural or post‐procedural MI | 5 (1.3) | 36 (0.9) | 0.49 |
| Post‐procedural cerebrovascular accident | 7 (1.0) | 53 (0.6) | 0.15 |
| Acute kidney injury | 112 (16.2) | 702 (7.6) | <0.01 |
| Post‐procedural dialysis required | 10 (1.5) | 30 (0.3) | <0.01 |
| Length of stay, d, mean±SD | 9.15±6.2 | 4.2±27.4 | <0.01 |
Values are given as n (%) or mean±SD. SD indicates standard deviation; IABP, intra‐aortic balloon pump; LVEF, left ventricular ejection fraction.
Patients undergoing PCI had statistically significantly higher adherence rates of discharge with prescriptions for aspirin, β‐blockers, ACE‐I/ARBs, and lipid‐lowering therapy than the rates of those undergoing CABG during the 4‐year time period studied (Table 2). The composite measure of discharge medication adherence was also higher for PCI throughout this period (89.7% versus 79.6%, P<0.01). However, there was a significant increase in adherence rates for all 6 interventions over the time period studied for both PCI and CABG (P for trend ≤0.01 within each treatment group during the study period for all 6 measures). Composite adherence rates for all 4 medications showed a strong improvement over time among CABG patients, nearing the rates of PCI patients (Figure 1). In 2006, the composite measure for discharge with all medications for CABG patients was not statistically different from PCI patients, although this metric fell again for both groups in 2007 (Table 2).
Table 2.
Adherence Rates to Guideline‐Based Secondary Prevention Measures at Hospital Discharge After STEMI Stratified by Revascularization Method and Year
| Intervention/Procedure | 2004 | 2005 | 2006 | 2007 | 2004–2007 | P for Trenda |
|---|---|---|---|---|---|---|
| Aspirin (n=9787) | ||||||
| CABG, % | 87.4 | 93.7 | 96.0 | 93.4 | 92.5 | <0.01 |
| PCI, % | 95.2 | 95.9 | 98.1 | 98.1 | 96.8 | <0.01 |
| P b | <0.01 | 0.13 | 0.08 | <0.01 | <0.01 | |
| β‐Blocker (n=9782) | ||||||
| CABG, % | 82.0 | 89.8 | 94.0 | 89.4 | 88.6 | <0.01 |
| PCI, % | 88.3 | 91.4 | 94.2 | 95.5 | 92.3 | <0.01 |
| P | 0.01 | 0.44 | 0.93 | <0.01 | <0.01 | |
| ACE‐I/ARB (n=1903) | ||||||
| CABG, % | 48.9 | 50.5 | 65.7 | 67.6 | 56.8 | <0.01 |
| PCI, % | 81.5 | 83.9 | 89.1 | 88.5 | 85.8 | <0.01 |
| P | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | |
| Lipid‐lowering therapy (n=9787) | ||||||
| CABG, % | 70.5 | 83.5 | 92.8 | 90.1 | 83.5 | <0.01 |
| PCI, % | 90.5 | 91.7 | 94.0 | 93.5 | 92.4 | <0.01 |
| P | <0.01 | <0.01 | 0.52 | 0.11 | <0.01 | |
| Smoking‐cessation counseling (n=1990) | ||||||
| CABG, % | N/A | N/A | 87.3 | 96.1 | 91.7 | 0.01 |
| PCI, % | N/A | N/A | 88.3 | 92.1 | 90.3 | 0.01 |
| P | 0.82 | 0.30 | 0.63 | |||
| Referral to cardiac rehabilitation (n=5007) | ||||||
| CABG, % | N/A | 33.3 | 70.4 | 72.2 | 70.9 | <0.01 |
| PCI, % | N/A | 50.0 | 45.4 | 51.1 | 48.3 | <0.01 |
| P | 0.71 | <0.01 | <0.01 | <0.01 | ||
| Composite adherence | ||||||
| CABG, % | 70.9 | 78.9 | 85.6 | 84.8 | 79.6 | <0.01 |
| PCI, % | 88.1 | 90.2 | 87.6 | 92.8 | 89.7 | <0.01 |
| P | <0.01 | <0.01 | 0.36 | <0.01 | <0.01 |
CABG indicates coronary artery bypass graft; PCI, percutaneous coronary intervention; ACE‐I/ARB, angiotens in‐converting enzyme inhibitor/angiotensin II receptor blocker.
P values for trend compare year‐to‐year trends for a specific procedure group during the study period.
These P values compare annual percentages between procedure groups.
Figure 1.
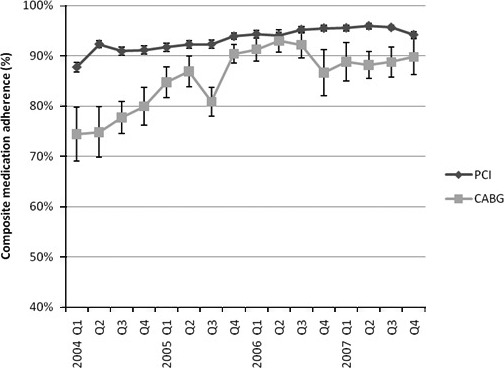
Composite guideline‐based secondary prevention medication prescription adherence at discharge for STEMI patients undergoing coronary revascularization by quarter. Error bars represent standard errors. PCI indicates percutaneous coronary intervention; CABG, coronary artery bypass graft. *P for trend <0.01 for both groups.
Although the unadjusted RRs for discharge without guideline‐based medical therapy were significantly higher for patients who underwent CABG than for patients who had PCI for each of the medications investigated, after adjustment for baseline comorbidities, presentation variables, and temporal trends, the RRs for many of the interventions were similar between the 2 groups (Table 3). After adjustments, CABG patients still had a higher RR of being discharged without aspirin, lipid‐lowering medication, and ACE‐I/ARB than that of PCI patients, though the effects for aspirin (RR: 1.03; 95% confidence interval [CI]: 1.01–1.05) and lipid‐lowering therapy (RR: 1.08; 95% CI: 1.01–1.15) were not large. Conversely, CABG patients had a lower RR for cardiac rehabilitation prescription nonadherence (RR: 0.60; 95% CI: 0.46–0.78) than that of PCI patients.
Table 3.
Unadjusted and Adjusteda RRs for Nonadherence to Prescription of Guideline‐Based Secondary Prevention Therapies at Discharge for Patients Undergoing CABG Versus PCI
| Intervention | Unadjusted RR | 95% CI | Adjusted RR | 95% CI |
|---|---|---|---|---|
| Aspirin | 2.36 | (1.78–3.14) | 1.03 | (1.01–1.05) |
| β‐Blocker | 1.49 | (1.20–1.86) | 1.03 | (0.99–1.06) |
| Lipid‐lowering medication | 2.17 | (1.81–2.61) | 1.08 | (1.01–1.15) |
| ACE‐I/ARB | 2.49 | (2.10–2.95) | 1.43 | (1.20–1.71) |
| Smoking‐cessation counseling | 0.86 | (0.45–1.63) | 0.98 | (0.92–1.04) |
| Referral to cardiac rehabilitation | 0.56 | (0.47–0.67) | 0.60 | (0.46–0.78) |
RR indicates relative risk; CI, confidence interval; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; ACE‐I/ARB, angiotens in‐converting enzyme inhibitor/angiotensin II receptor blocker.
Adjusted for age, diabetes, peripheral vascular disease, hypertension, prior MI, congestive heart failure, procedure priority, year procedure performed, LVEF, and shock. ACE‐I/ARB refers only to patients who were considered eligible and was adjusted for acute kidney injury during hospitalization, in addition to the variables above.
Cardiogenic shock on admission was an independent predictor of patients who did not receive β‐blockers at discharge (RR: 1.66; 95% CI: 1.07–2.60) or smoking‐cessation counseling (RR: 2.14; 95% CI: 1.27–3.60). Older age, diabetes, congestive heart failure, peripheral vascular disease, LVEF, prior MI, hypertension, and need for urgent revascularization were associated with nonadherence to performance measures, but only shock and lower LVEF were independently associated with nonadherence in the adjusted model. Time was an independent predictor of failure to discharge with lipid‐lowering agents among CABG patients (RR: 1.09; 95% CI: 1.03–1.16 for each quarter). Hospital site was not a significant predictor for any of the therapies investigated and did not significantly change RR estimates or explain the variance in prescribing patterns when added into the regression models.
Discussion
In an observational analysis of a statewide registry of patients undergoing coronary revascularization after STEMI, we found that patients who underwent CABG had a lower rate of guideline‐based therapies prescribed at discharge than that of patients undergoing PCI over the 4‐year period studied. We also demonstrated that these differences diminished over time and were explained in large part by patient‐level differences.
Previous Studies on Secondary Prevention Guidelines Adherence
Foody et al2 evaluated 37 376 patients included in the Centers for Medicare and Medicaid Services' National Heart Care Program from April 1998 to March 1999. Among CABG patients in that 1‐year sample, aspirin, β‐blockers, ACE‐Is, and lipid‐lowering therapy were 10% to 20% less frequently prescribed than among patients who underwent PCI. Similar results were published by Fox et al10 in a retrospective analysis in 2002. Hiratzka et al1 evaluated hospitals participating in the Get With The Guidelines program from 2000 to 2005 and reported an increase in the discharge medication performance measures after CABG, although the rates were still significantly lower than for PCI. Publication of these and similar studies led to widespread efforts to educate practitioners, improve hospital processes, and implement public reporting of hospital quality measures. A study conducted from 2002 to 2005 of the Society of Thoracic Surgeons' National Cardiac Database showed that low‐intensity educational efforts, including both provider and patient instruction, along with site‐specific feedback, led to improved adoption of secondary prevention measures in post‐CABG patients.11 In 2006, the Society of Thoracic Surgeons launched the Quality Measurement Task Force to measure and improve adherence to quality metrics among surgical programs, which was implemented by hospitals in Washington State.12, 13 Our data show a significant improvement in all measures during this time period, subsequent to the time period in late 2005 in which the Centers for Medicare and Medicaid Services began requesting public reporting and COAP began publishing hospital‐level quality data, with substantial improvement in discharge prescriptions among patients who underwent CABG during their hospitalizations. Our results are very similar to those presented in the Synergy between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery (SYNTAX) trial,14 which was conducted over a similar time period. The cause of the changes in prescribing practices noted in our study is likely multifactoral, however, and includes the institution of public reporting, efforts made by the Society of Thoracic Surgeons, and dissemination of the guidelines for secondary prevention released in 2006.
Exploring Differences in Secondary Prevention Adherence
Given the data supporting the use of the medications evaluated in this study for reducing peri‐CABG morbidity and mortality rates, it is encouraging to see that the use of these medications after CABG has been trending toward rates closer to those for post‐PCI patients. That patient comorbidities seem to explain a significant proportion of the variation in these performance measures between these 2 groups implies that adherence to quality measures is not simply a problem of differences in hospital processes for CABG versus PCI patients.1 Patients undergoing CABG tend to be older, have an increased number of baseline comorbidities, and can be more prone to postprocedural anemia, hypotension, or renal insufficiency, which could affect providers' inclination to prescribe secondary prevention measures at discharge. This so‐called “treatment‐risk paradox” might be playing a major role in the lack of adherence to guideline‐based therapy, and interventions aimed at increasing adherence should be targeted at improving documentation of contraindications and educating practitioners about evidence‐based therapies in patients with severe, comorbid illnesses, regardless of the procedure received.15
One of the largest discrepancies observed in the present study between CABG and PCI patients was the prescription of ACE‐I/ARBs at discharge, even after adjustment for acute kidney injury, which potentially reflects issues related to hypotension and impairment of renal function in the postoperative period. The COAP database did not capture whether patients had a history of chronic kidney disease before admission, so we could have underrepresented the number of patients eligible for ACE‐I/ARBs at discharge, which would affect adherence estimates. However, the data supporting a benefit for ACE‐I/ARBs pertain primarily to patients with impaired left ventricular function, and some controversy exists as to the absolute long‐term benefit of this medication for all patients.16 Although it might not be necessary to start an ACE‐I/ARB immediately at discharge in such patients, these data underscore the importance of early interaction with a combined multidisciplinary team to ensure that providers caring for postsurgical patients prioritize the implementation of secondary prevention measures as soon as patients' conditions permit in the follow‐up period.
Limitations
Because this is a registry‐based study, we could not differentiate between failure to document a contraindication and oversight by the discharging provider in patients who did not receive a certain therapy. Similarly, metrics in COAP are limited to in‐hospital measures, so patients in whom surgeons and cardiologists chose to start therapy in the first or second week after discharge to ensure that postoperative renal insufficiency or anemia had stabilized would not be reflected by this data. COAP had limited data on cardiac rehabilitation referral and smoking cessation, as these were not collected in COAP until 2005 and 2006, respectively, which limited our analyses for these 2 variables. We also cannot account for the drop in adherence among CABG patients between 2006 and 2007, though the largest drop was observed in β‐blocker prescription, which could have been secondary to the controversy surrounding preoperative β‐blocker use.17 We also restricted our analysis to patients with STEMI because of potential inconsistencies and limited specificity of the diagnoses of non‐STEMI and unstable angina, though guideline‐based therapy could benefit all patients with acute myocardial ischemia. Finally, we are presenting an association between public reporting and improved guideline adherence, but the retrospective nature of this analysis restricts our ability to speculate on the temporal association between these trends.
Conclusions
Trends in guideline‐based secondary prevention adherence from 2004 to 2007 for CABG patients after STEMI in Washington State are encouraging after the institution of public reporting, with secondary prevention performance measures catching up to those for patients undergoing PCI and even surpassing PCI for cardiac rehabilitation referral. The reduction in hospital deaths and major adverse cardiac events in long‐term follow‐up associated with secondary prevention at discharge underscores the importance of continuing to improve adherence to these guideline‐based measures.3, 18, 19
Source of Funding
This work was funded under National Center for Research Resources (NCRR) Grant KL2 RR025015 and National Heart, Lung, and Blood Institute of the National Institutes of Health (NHLBI) Grant T32 HL076132. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
None.
(J Am Heart Assoc. 2012;1:e002733 doi: 10.1161/JAHA.112.002733)
Drs Riley and Don contributed equally to this work and are co‐first authors of this article.
References
- 1. Hiratzka LF, Eagle KA, Liang L, Fonarow GC, LaBresh KA, Peterson ED for the Get With The Guidelines Steering Committee . Atherosclerosis secondary prevention performance measures after coronary bypass graft surgery compared with percutaneous catheter intervention and nonintervention patients in the Get With The Guidelines database. Circulation. 2007;116:207–212. [DOI] [PubMed] [Google Scholar]
- 2. Foody JM, Ferdinand FD, Galusha D, Rathore SS, Masoudi FA, Havranek EP, Nilasena D, Radford MJ, Krumholz HM. Patterns of secondary prevention in older patients undergoing coronary artery bypass grafting during hospitalization for acute myocardial infarction. Circulation. 2003;108:24–28. [DOI] [PubMed] [Google Scholar]
- 3. Peterson ED, Roe MT, Mulgund J, DeLong ER, Lytle BL, Brindis RG, Smith SC, Pollack CV, Newby LK, Harrington RA, Gibler WB, Ohman EM. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA. 2006;295:1912–1920. [DOI] [PubMed] [Google Scholar]
- 4. Pearson TA, Laurora I, Chu H, Kafonek S. The lipid treatment assessment project (L‐TAP): a multicenter survey to evaluate the percentages of dyslipidemic patients receiving lipid‐lowering therapy and achieving low‐density lipoprotein cholesterol goals. Arch Intern Med. 2000;160:459–467. [DOI] [PubMed] [Google Scholar]
- 5. Bauer T, Gitt AK, Jünger C, Zahn R, Koeth O, Towae F, Schwarz AK, Bestehorn K, Senges J, Zeymer U for the ACOS Investigators . Guideline‐recommended secondary prevention drug therapy after acute myocardial infarction: predictors and outcomes of nonadherence. Eur J Cardiovasc Prev Rehabil. 2010;17:576–581. [DOI] [PubMed] [Google Scholar]
- 6. Goss JR, Maynard C, Aldea GS, Marcus‐Smith M, Whitten RW, Johnston G, Phillips RC, Reisman M, Kelley A, Anderson RP. Effects of a statewide physician‐led quality‐improvement program on the quality of cardiac care. Am Heart J. 2006;151:1033–1042. [DOI] [PubMed] [Google Scholar]
- 7. Maynard C, Goss JR, Malenka DJ, Reisman M for the Clinical Outcomes Assessment Program . Adjusting for patient differences in predicting hospital mortality for percutaneous coronary interventions in the Clinical Outcomes Assessment Program. Am Heart J. 2003;145:658–664. [DOI] [PubMed] [Google Scholar]
- 8. Smith SC, Allen J, Blair SN, Bonow RO, Brass LM, Fonarow GC, Grundy SM, Hiratzka L, Jones D, Krumholz HM, Mosca L, Pasternak RC, Pearson T, Pfeffer MA, Taubert KA. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update. Circulation. 2006;113:2363–2372. [DOI] [PubMed] [Google Scholar]
- 9. Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd‐Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. [DOI] [PubMed] [Google Scholar]
- 10. Fox DJ, Kibiro M, Eichhofer J, Curzen NP. Patients undergoing coronary revascularization: a missed opportunity for secondary prevention? Postgrad Med J. 2005;81:401–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams JB, Delong ER, Peterson ED, Dokholyan RS, Ou FS, Ferguson TB. Secondary prevention after coronary artery bypass graft surgery: findings of a national randomized controlled trial and sustained society‐led incorporation into practice. Circulation. 2011;123:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shahian DM, Edwards FH, Ferraris VA, Haan CK, Rich JB, Normand SLT, DeLong ER, O'Brien SM, Shewan CM, Dokholyan RS, Peterson ED. Quality measurement in adult cardiac surgery: part 1: conceptual framework and measure selection. Ann Thorac Surg. 2007;83:S3–S12. [DOI] [PubMed] [Google Scholar]
- 13. O'Brien SM, Shahian DM, DeLong ER, Normand SL, Edwards FH, Ferraris VA, Haan CK, Rich JB, Shewan CM, Dokholyan RS, Anderson RP, Peterson ED. Quality measurement in adult cardiac surgery: part 2: statistical considerations in composite measure scoring and provider rating. Ann Thorac Surg. 2007;83:S13–S26. [DOI] [PubMed] [Google Scholar]
- 14. Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, van Dyck N, Leadley K, Dawkins KD, Mohr FW for the SYNTAX investigators . Percutaneous coronary intervention versus coronary‐artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. [DOI] [PubMed] [Google Scholar]
- 15. van Straten AH, Bekker MW, Soliman Hamad MA, van Zundert AA, Martens EJ, Schönberger JP, de Wolf AM. Transfusion of red blood cells: the impact on short‐term and long‐term survival after coronary artery bypass grafting: a ten‐year follow‐up. Interact Cardiovasc Thorac Surg. 2010;10:37–42. [DOI] [PubMed] [Google Scholar]
- 16. Braunwald E, Domanski MJ, Fowler SE, Geller NL, Gersh BJ, Hsia J, Pfeffer MA, Rice MM, Rosenberg YD, Rouleau JL for the PEACE Trial Investigators . Angiotensin‐converting‐enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351:2058–2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta‐blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349. [DOI] [PubMed] [Google Scholar]
- 18. Jaber WA, Lennon RJ, Mathew V, Holmes DR Jr, Lerman A, Rihal CS. Application of evidence‐based medical therapy is associated with improved outcomes after percutaneous coronary intervention and is a valid quality indicator. J Am Coll Cardiol. 2005;46:1473–1478. [DOI] [PubMed] [Google Scholar]
- 19. Fonarow GC, Gawlinski A, Moughrabi S, Tillisch JH. Improved treatment of coronary heart disease by implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP). Am J Cardiol. 2001;87:819–822. [DOI] [PubMed] [Google Scholar]


