Abstract
OBJECTIVE
We analyzed individuals with epilepsy due to Sturge-Weber syndrome to determine which anticonvulsants provided optimal seizure control and which resulted in the fewest side effects.
METHODS
One-hundred-eight records from a single center were retrospectively analyzed for Sturge-Weber syndrome brain involvement, epilepsy, Sturge-Weber syndrome neuroscores, and currently used anticonvulsants.
RESULTS
Of the fourteen anticonvulsants that had been employed, the most often used agents were oxcarbazepine or carbamazepine, and levetiracetam. Individuals whose seizures at the most recent visit were fully controlled (seizure-free) for 6 months or longer were more likely to have ever tried, or currently used, oxcarbazepine or carbamazepine than those with uncontrolled seizures. Thirty-nine of 69 individuals (56.5%) were seizure-free with oxcarbazepine or carbamazepine history versus 11 of 35 individuals (31.4%) who had not taken these agents (P < 0.05); 38 of 62 patients (61.3%) were seizure-free while currently taking these anticonvulsants versus 12 of 42 (28.6%) not taking them (P < 0.01). Patients with seizure control for 6 months or longer were less likely to have ever tried, or to currently be taking, levetiracetam than those without control. Sixteen of 56 individuals (28.6%) were seizure-free with levetiracetam history versus 34 of 48 (70.8%) without it (P < 0.001); 14 of 43 individuals (32.6%) were seizure-free and currently taking levetiracetam versus 36 of 61 (59.0%) not taking it (P < 0.01). When topiramate was added as second-line medication, five of nine patients (55.6%) experienced decreased seizure severity, and worsening of glaucoma was not reported.
CONCLUSIONS
Carbamazepine and oxcarbazepine were associated with better seizure control than levetiracetam in this Sturge-Weber syndrome cohort and so may be preferred as the initial therapy. When used as adjunctive therapy, topiramate was effective in this limited analysis without a clear increased incidence of glaucoma.
Keywords: anticonvulsant, Sturge-Weber syndrome, epilepsy, side effects, carbamazepine, oxcarbazepine, levetiracetam
Introduction
Sturge-Weber syndrome (SWS) is caused by a somatic mutation in the gene GNAQ and characterized by vascular malformations.1 SWS can present with various characteristics depending on the location of the abnormal blood vessels, including facial port-wine birthmark, glaucoma, and brain involvement. Brain involvement with SWS is characterized by a leptomeningeal vascular malformation that is usually noted on contrast-enhanced magnetic resonance imaging by age 1 year. Brain involvement can lead to complications such as strokes and stroke-like episodes, cognitive impairment, migraines, and epilepsy.2 Epilepsy is present in about 72% of patients with unilateral SWS brain involvement, who make up the majority of SWS cases, but seizures are estimated to occur in 87%–92% of the smaller SWS population who have bilateral brain involvement.3,4 Seizure onset commonly begins during the first year of life.5 About 45% of patients experience seizure clustering, characterized by individual prolonged seizures or many short seizures over a single day, with clusters separated by months or even years of seizure control.5 Intractable or prolonged seizures are associated with worse cognitive impairment and neurological injury.6–9
Strokes and stroke-like episodes, seizures, and migraines can interact and trigger one another, leading to further neurological deterioration.10 Earlier seizure onset and greater extent of brain involvement are the most reliable predictors of poorer outcomes, but the majority of patients respond to anticonvulsant therapy.4 Because of this, anticonvulsants are the first-line treatment for SWS-related epilepsy.5,10 Surgical treatments, such as focal cerebral resection or hemispherectomy, and special diets, such as the ketogenic or modified Atkins diet, are typically considered when anticonvulsant treatment fails.6,11 A better understanding of patterns of anticonvulsant use and efficacy in SWS could help improve targeted treatment of seizures in this population. Currently, there is nearly nothing in the medical literature specific to SWS regarding which anticonvulsants may be particularly useful or conversely unsuitable for this population. Given the comorbidity between seizures and migraines in many patients with SWS, valproate or topiramate may be helpful in treating both, but this dual efficacy has not been studied in depth in these patients.2 We analyzed a large, single-center cohort to determine if any anticonvulsants appeared to be more helpful than others.
Materials and Methods
Participants and data collected
The Johns Hopkins Institutional Review Board approved this study to collect clinical data from consenting subjects for the research database at the Hunter Nelson Sturge-Weber Center at the Kennedy Krieger Institute. This database was retrospectively reviewed for patients with confirmed SWS brain involvement. Brain involvement was confirmed with typical findings on magnetic resonance imaging, including leptomeningeal vascular malformation, choroid plexus glomus, dilated deep draining vessels, and associated venous malformation.
Data collected included age of seizure onset; gender; race; brain, eye, and skin involvement; and history of anticonvulsant use. For each visit in the database, current anticonvulsant use, reports of side effects, and SWS neurological scores (neuroscores) were also collected. The neuroscore is a scale used to evaluate clinical severity by rating seizure frequency, hemiparesis severity, presence or absence and severity of visual field cut, and cognitive function, which are common deficits in SWS.5,12,13 This study examines the seizure score, which is described in Table 1. A lower seizure score represents less frequent seizures, whereas higher scores indicate more severe cases. For example, a seizure score of 1 is given if a subject’s seizures have been controlled for 6 months or more, whereas a seizure score of 4 indicates that seizures are occurring at least weekly.
TABLE 1.
Seizure Neuroscore
| Seizure Score | Definition |
|---|---|
| 0 | No diagnosed history of seizures |
| 1 | ≥1 Prior seizure, but controlled for ≥6 months |
| 2 | Breakthrough seizures |
| 3 | Monthly seizures |
| 4 | ≥ Weekly seizures |
Anticonvulsants studied included carbamazepine, clobazam, felbamate, gabapentin, lamotrigine, levetiracetam, oxcarbazepine, phenobarbital, phenytoin, rufinamide, tiagabine, topiramate, valproate, vigabatrin, and zonisamide. No subjects in the database had a history or current use of rufinamide, and so this anticonvulsant was not analyzed. Oxcarbazepine and carbamazepine were often analyzed together because of the similarity between these two drugs. Where “oxcarbazepine or carbamazepine” is used, this phrase indicates inclusion of subjects using one or both of these drugs.
A smaller pool of subjects was used to examine the effects of adjunctive drug therapies. This group included only subjects with at least one visit where seizure score was at least 1 (seizures that have been fully controlled for 6 months or more) before adding a particular anticonvulsant and at least one follow-up visit after less than 20 months of continuous use.
Statistical analyses
All data analyses were performed in IBM SPSS Statistics Version 22.
Measurement of frequencies was used for descriptive statistics. χ2 and Fisher exact test were used to compare nominal demographics and a two-tailed t test was used to compare the age of seizure onset between those with seizure control for at least 6 months (seizure score = 1) versus those with uncontrolled seizures (seizure score > 1).
The nonparametric median test for k independent samples was used to compare median total seizure scores between those who had or had not ever tried a given anticonvulsant (history of use), and those who were or were not being treated with a given anticonvulsant at a particular visit (current use). The same analysis was also used for each drug to compare median seizure scores of those with history or current use of levetiracetam to those with history or current use of oxcarbazepine or carbamazepine and to compare median seizure scores in those with and without seizure control.
Measurement of frequencies and χ2 and Fisher exact tests were used to examine whether or not specific side effects were reported for those with and without current use of each anticonvulsant at the first and most recent visit. These tests were also used to analyze the use of particular anticonvulsants by those with and without seizure control, i.e., associations between history or current use versus nonuse of each anticonvulsant and seizure control versus lack of seizure control. Measurement of frequencies was also used to determine the distribution of polytherapy, with the χ2 test used to compare polytherapy between those with and without seizure control. Significance for all tests was determined at a level of P < 0.05.
Results
Demographics
One hundred thirty-five patients with confirmed SWS brain involvement who had consented to data collection were seen at the Hunter Nelson Sturge-Weber Center at the Kennedy Krieger Institute and Johns Hopkins Hospital. The earliest visit in the database for each subject took place between January 2006 and September 2013. All follow-up visits from March 2014 or earlier were included.
Of these 135 individuals, 108 subjects (80.0%) met our criteria for this analysis, including current use of one or more anticonvulsants and a history of one or more prior seizures by the time of the most recent visit in the database.
The average age at seizure onset for all subjects analyzed was 2.2 years ± 6.1 years. Although age of seizure onset was slightly lower for individuals with uncontrolled seizures (seizure score > 1, mean 1.9 years ± 3.8 years) than for those whose seizures had been controlled for at least 6 months (seizure score = 1, mean 2.7 years ± 8.1 years), this difference was not significant (P = 0.52). In this cohort, 48.1% were female, 76.9% were Caucasian, 8.3% were black, 5.6% were Hispanic or Latino, 4.6% were Asian, and 4.6% did not fall under any of these categories. A greater percentage of those with uncontrolled seizures were Caucasian (85.2%) compared with the group whose seizures were controlled (66.0%, P = 0.02). There was no significant difference in other race groups, although sample size was much smaller. The majority of subjects had unilateral brain (75.9%) and skin (51.8%) involvement, although eye involvement was more evenly distributed between no involvement (41.7%) and unilateral involvement (41.6%). Bilateral brain involvement was significantly more common in subjects without seizure control (35.2%) than in subjects with seizure control (14.0%, P = 0.01). More complete demographic information is in Table 2.
TABLE 2.
Subject Demographics
| Demographics | Total Analyzed (n = 108) |
Controlled Seizures (n = 50) |
Uncontrolled Seizures (n = 54) |
P Value |
|---|---|---|---|---|
| Age at initial seizure onset (mean years) | 2.22 | 2.70 | 1.88 | 0.52 |
| Sex (female) | 52 (48.1%) | 27 (54.0%) | 24 (44.4%) | 0.33 |
| Race | ||||
| Caucasian | 83 (76.9%) | 33 (66.0%) | 46 (85.2%) | 0.02 |
| Black | 9 (8.3%) | 6 (12.0%) | 3 (5.6%) | 0.31 |
| Hispanic or Latino | 6 (5.6%) | 5 (10.0%) | 1 (1.9%) | 0.10 |
| Asian | 5 (4.6%) | 3 (6.0%) | 2 (3.7%) | 0.67 |
| Other | 5 (4.6%) | 3 (6.0%) | 2 (3.7%) | 0.67 |
| Brain involvement | ||||
| Unilateral | 82 (75.9%) | 43 (86.0%) | 35 (64.8%) | 0.01 |
| Bilateral | 26 (24.1%) | 7 (14.0%) | 19 (35.2%) | |
| Skin involvement | ||||
| None | 14 (13.0%) | 6 (12.0%) | 8 (14.8%) | 0.67 |
| Unilateral | 56 (51.8%) | 29 (58.0%) | 23 (42.6%) | 0.12 |
| Bilateral | 38 (35.2%) | 15 (30.0%) | 23 (42.6%) | 0.18 |
| Eye involvement | ||||
| None | 45 (41.7%) | 18 (36.0%) | 27 (50.0%) | 0.15 |
| Unilateral | 45 (41.6%) | 24 (48.0%) | 17 (31.5%) | 0.09 |
| Bilateral | 18 (16.7%) | 8 (16.0%) | 10 (18.5%) | 0.73 |
One hundred-four of these subjects were compared on the basis of whether they had seizure control for at least 6 months (seizure score = 1, n = 50) or whether they had experienced at least one seizure within the last 6 months (seizure score > 1, n = 54) at their most recent visit in the database. Four subjects who were not included in these analyses were not given any anticonvulsants at the time of their most recent visit. Two of these subjects were excluded because their seizure control was a result of previous hemispherectomy. Another with seizure control was excluded because of having only a remote history of epilepsy and having been weaned off of anticonvulsants many years before the most recent visit. The fourth and final subject without seizure control was excluded because this subject had refused recommended further treatment with anticonvulsants before the most recent visit.
When adjunctive drug therapies were studied, three anticonvulsants had at least five subjects who met criteria. These anticonvulsants were topiramate (n = 9), levetiracetam (n = 11), and valproate (n = 5). Time elapsed between the initiation of the adjunctive therapy and follow-up visit ranged from 1 to 19 months (median 9 months).
Neuroscores and side effects given history of use, current use, or adjunctive therapy
None of the 14 anticonvulsants analyzed was associated with better seizure scores (compared with the group not actively receiving that particular anticonvulsant) as measured at any particular visit (Table 3). In fact, several of these medications were associated with higher seizure scores. A history of clobazam, phenobarbital, or topiramate use was associated with worse seizure scores at the indicated visit compared with the overall group without history of the respective drug (Table 3). Both history and current use of lamotrigine, levetiracetam, and zonisamide were associated with poorer seizure scores.
TABLE 3.
Anticonvulsant Neuroscore and Side Effect Comparisons
| Were Median Neuroscores at This Visit Different for Those With a History of Use? (P Value) |
Were Median Neuroscores Different for Those Currently Using This AED at This Visit? (P Value) |
Were More or Fewer Side Effects Reported for Those on This AED at This Visit? (P Value) |
|
|---|---|---|---|
| Clobazam | Higher: •Recent SZ (0.04) |
None significant | None significant |
| Lamotrigine | Higher: •Visit 1 SZ (0.03) •Visit 1 SZ (0.01) |
Higher: •Visit 6 SZ (0.02) |
None significant |
| Levetiracetam | Higher: •Visit 5 SZ (0.008) •Visit 6 SZ (0.001) •Visit 7 SZ (0.006) •Visit 10 SZ (0.03) •Recent SZ (0.01) |
Higher: •Visit 6 SZ (0.02) |
More reported ≥1 SE: •Visit 1: (0.006) |
| Oxcarbazepine | None significant | None significant | Fewer reported ≥1 SE: •Recent: (<0.05) Fewer reported weight SEs: •Recent: (<0.04) |
| Phenobarbital | Higher: •Visit 10 SZ (0.03) •Visit 11 SZ (0.02) •Recent SZ (0.03) |
None significant | None significant |
| Topiramate | Higher: •Visit 1 SZ (0.006) •Recent SZ (0.04) |
None significant | None significant |
| Valproate | None significant | None significant | More reported ≥1 SE: •Recent: (0.02) More reported weight SEs: •Visit 1: (0.003) •Recent: (0.001) |
| Zonisamide | Higher: •Visit 1 SZ (<0.001) •Visit 3 SZ (<0.05) •Recent SZ (0.003) |
Higher: •Visit 1 SZ (<0.002) •Recent SZ (0.03) |
None significant |
Abbreviations:
AED = Anticonvulsant
SE = Side effects
SZ = Seizure score
With regards to side effects, oxcarbazepine use at the time of the visit was associated with fewer side effects than for those not taking oxcarbazepine (Table 3). Fewer people taking oxcarbazepine reported one or more side effects at recent visit; specifically fewer reported weight side effects. Use of valproate at the time of the visit was associated with more side effects, with more subjects on valproate reporting one or more side effects than those not taking it at the most recent visit. Specifically, more subjects on valproate reported weight-related side effects at the first and most recent visits. Those using levetiracetam at visit one were more likely to report one or more side effects (P = 0.006) with many different side effects being reported. Worsening of glaucoma was not reported with topiramate use. Responses with the adjunctive therapies were similar: five of nine (55.6%) showed decreased seizure scores when adding topiramate, four of 11 (36.4%) with the addition of levetiracetam, and four of five (80.0%) with valproate.
Seizure control versus lack of seizure control
Levetiracetam was the most frequently tried medication and currently used monotherapy among those with uncontrolled seizures (seizure score > 1, n = 54, Table 4). Patients with six or more months of seizure control were less likely to have ever tried, or to currently be taking, levetiracetam than those without control. Sixteen of 56 (28.6%) were seizure-free with levetiracetam history versus 34 of 48 (70.8%) without (P < 0.001). Fourteen of 43 (32.6%) were seizure-free currently taking levetiracetam versus 36 of 61 (59.0%) who were not taking it (P < 0.01). Oxcarbazepine and carbamazepine were the most frequently tried medications and currently used monotherapies among those with controlled seizures for at least 6 months (seizure score = 1, n = 50). Of 69 subjects with oxcarbazepine or carbamazepine history, 39 were seizure-free (56.5%), whereas 11 of 35 (31.4%) were seizure-free without history of either of those drugs (P < 0.05). Thirty-eight were seizure-free of 62 (61.3%) currently taking one or both of these anticonvulsants versus 12 of 42 (28.6%) not taking them (P < 0.01). Fewer subjects had seizure control at their most recent visit when they had a history of phenobarbital (n = 16 of 44 [36.4%], P = 0.04) or lamotrigine (n = 2 of 14 [14.3%], P = 0.008). Fewer subjects had seizure control at their most recent visit with current use of zonisamide (n = 1 of 9 [11.1%], P = 0.03).
TABLE 4.
Drugs Used as Monotherapies by Those With or Without Seizure Control at the Most Recent Visit
| # of Subjects with Uncontrolled Seizures (n = 54) and Monotherapy at Most Recent Visit |
# of Subjects with Controlled Seizures (n = 50) and Monotherapy at Most Recent Visit |
|
|---|---|---|
| Levetiracetam | 10 | 4 |
| Oxcarbazepine | 3 | 12 |
| Carbamazepine | 2 | 10 |
| Zonisamide | 1 | 0 |
| Lamotrigine | 1 | 0 |
| Valproate | 0 | 1 |
| Topiramate | 0 | 1 |
| Phenobarbital | 0 | 2 |
Levetiracetam and oxcarbazepine or carbamazepine comparisons
Seizure scores were also compared directly between levetiracetam and oxcarbazepine or carbamazepine users. In subjects who had tried one of these drugs but not the other, history of oxcarbazepine or carbamazepine (n = 41) was associated with lower (better) median scores than history of levetiracetam (n = 26, P < 0.001). When these two groups were simultaneously compared with those subjects who had tried both drugs (n=31), this association remained, with the lowest (best) median neuroscores for oxcarbazepine or carbamazepine only, the highest (worst) for levetiracetam only, and intermediate for those who had tried both (P = 0.03). In subjects with any history of levetiracetam (including those who may also have had a history of oxcarbazepine or carbamazepine), there was an association with higher (worse) median scores compared with the group of those with oxcarbazepine or carbamazepine history only.
Polypharmacy
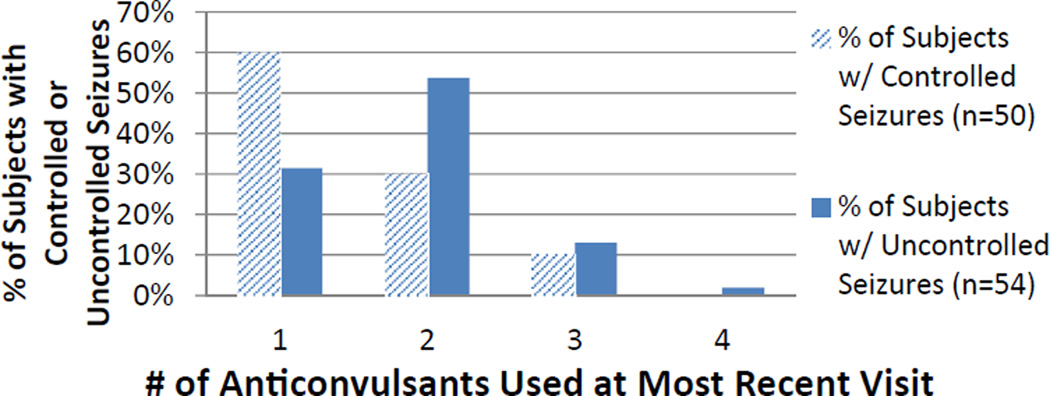
All subjects were taking one to four drugs concurrently at the most recent visit, but polypharmacy was more common in those with uncontrolled seizures (20 of 50 [40.0%] controlled versus 37 of 54 (68.5%) uncontrolled, P = 0.004). The Figure shows the distribution of polypharmacy. The most common two-drug combination was oxcarbazepine or carbamazepine with levetiracetam, with 11 subjects using this combination of 44 subjects (25.0%) taking two anticonvulsants at their most recent visit. A higher proportion of subjects on polytherapy with seizure control were using this combination, either alone or together with an additional anticonvulsant, compared with subjects on polytherapy without seizure control (seven of 21 [33.3%] controlled on polytherapy versus seven of 38 [18.4%] uncontrolled on polytherapy), but there was no significant association (P = 0.18). The next most common drug combination was oxcarbazepine or carbamazepine with topiramate, with eight of 44 subjects (18.2%) on bi-therapy using this combination. There were fewer than five subjects using any other particular two-drug combination.
FIGURE.
Distribution of polypharmacy at the most recent visit. (The color version of this figure is available in the online edition.)
Discussion
Imaging studies have suggested that during seizures, insufficient blood flow to areas of SWS brain involvement can lead to ischemic events that may contribute to deterioration over time.7,8 As the relationship between seizures and neurological deterioration in SWS becomes better understood, seizure control remains a priority for this population. Many individuals with SWS experience focal-onset seizures, and oxcarbazepine has been used as a first-line seizure medication in SWS and in other pediatric patients with focal-onset seizures.14,15 More recently, levetiracetam has become more common as a first-line treatment in SWS, and is sometimes used in conjunction with oxcarbazepine.2,10 Both oxcarbazepine or carbamazepine and levetiracetam were commonly used both alone and in combination in this study population. Oxcarbazepine or carbamazepine was associated with better seizure control in multiple analyses when compared both with subjects not using those drugs and with subjects using levetiracetam. Oxcarbazepine was also associated with fewer reported side effects overall and fewer weight side effects specifically. On the other hand, levetiracetam was associated with more side effects. The combination of either oxcarbazepine or carbamazepine with levetiracetam was not significantly associated with improved seizure control.
Many patients who started taking the more common first-line anticonvulsants such as oxcarbazepine and levetiracetam during the time they were followed began taking these drugs immediately after initial seizure onset, before which the seizure score is 0. Because of this, decreases (improvements) in seizure score could not be determined for those subjects. Adjunctive therapy analysis was limited in this study because of small sample size. Of note, of all the commonly used anticonvulsants, the only one with a mechanism potentially targeting the underlying molecular pathways causing SWS is valproate. As a histone deacetylase inhibitor it has the capacity to impact gene expression dysregulated by the R183Q somatic mutation in GNAQ. The same mutation (R183Q in GNAQ) in melanocytes causes uveal melanoma, and valproate has been studied with some success in the treatment of this condition.16 Further study of valproate for the treatment of SWS is needed.
The reported weight side effects for valproate were similar to those noted in previous studies.17 Multiple previous studies have indicated that topiramate may increase the risk of glaucoma, which is common among patients with SWS.18–20 However, the mechanisms underlying glaucoma in SWS differ from those of glaucoma associated with topiramate use.20,21We examined the data specifically for any evidence that adding topiramate worsened glaucoma issues in these patients. New-onset or exacerbation of existing glaucoma was not reported with topiramate use by any parent or patient in this study, so this drug appears to be appropriate and safe in this limited analysis of SWS patients.
The sizeable number of patients seen at this center affords a larger sample size. The 75.9% of subjects with unilateral brain involvement is similar to the 82.9% reported by Bebin and Gomez.3 The association between bilateral brain involvement and lack of seizure control underscores the current understanding that a greater extent of involvement is associated with poorer prognosis.4 It was not unexpected that the majority of subjects on monotherapy would experience seizure control. However, the finding that over 30% of subjects with higher (worse) seizure scores were also using monotherapy was more surprising given that failure of a single drug would not be a stopping point for anticonvulsant treatment.10 These usage distributions were captured at the most recent visit available, so it is possible that these subjects experiencing seizures on monotherapy were in the middle of a treatment transition at the time of the visit.
Despite its limitations, this study sheds light on how first-line seizure medications are used in this rare syndrome and their associations with seizure control and side effects. A wide variety of classic and newer anticonvulsants are used to treat seizures in this population. Oxcarbazepine is frequently used first, with levetiracetam and others tried next, as is the case in many children with partial-onset seizures.15 No anticonvulsant was clearly superior at reducing seizure frequency, so side effects are an important consideration when choosing an anticonvulsant. Oxcarbazepine was associated with fewer reported side effects than other anticonvulsants, so it may be preferable. In the last few years we have seen increasing numbers of patients with SWS who begin therapy with levetiracetam. In general, however, we recommend starting with oxcarbazepine unless features of generalized seizures are present. Topiramate was not associated with signs concerning for glaucoma, and the side effects reported for valproate were in line with its known effects, so these drugs may be safe alternatives to treat comorbid migraine and epilepsy. Appropriate selection of first-line and adjunctive medications can help to increase the chances of early seizure control.
Acknowledgments
This study was supported by funding from the Celebrate Hope Foundation and by the BVMC (U54NS065705), which is part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS, and the National Institutes of Neurological Diseases and Stroke (NINDS).
References
- 1.Shirley MD, Tang H, Gallione CJ, et al. Sturge-Weber syndrome and port wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971–1979. doi: 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Comi AM. Presentation, diagnosis, pathophysiology, and treatment of the neurological features of Sturge-Weber syndrome. Neurologist. 2011;17:179–184. doi: 10.1097/NRL.0b013e318220c5b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bebin EM, Gomez MR. Prognosis in Sturge-Weber disease: comparison of unihemispheric and bihemispheric involvement. J Child Neurol. 1988;3:181–184. doi: 10.1177/088307388800300306. [DOI] [PubMed] [Google Scholar]
- 4.Sujansky E, Conradi S. Sturge-Weber syndrome: age of onset of seizures and glaucoma and the prognosis for affected children. J Child Neurol. 1995;10:49–58. doi: 10.1177/088307389501000113. [DOI] [PubMed] [Google Scholar]
- 5.Kossoff EH, Ferenc L, Comi AM. An infantile-onset, severe, yet sporadic seizure pattern is common in Sturge-Weber syndrome. Epilepsia. 2009;50:2154–2157. doi: 10.1111/j.1528-1167.2009.02072.x. [DOI] [PubMed] [Google Scholar]
- 6.Arzimanoglou A, Aicardi J. The epilepsy of Sturge-Weber syndrome: clinical features and treatment in 23 patients. Acta Neurol Scand Suppl. 1992;140:18–22. doi: 10.1111/j.1600-0404.1992.tb04465.x. [DOI] [PubMed] [Google Scholar]
- 7.Aylett SE, Neville BG, Cross JH, Boyd S, Chong WK, Kirkham FJ. Sturge-Weber syndrome: cerebral haemodynamics during seizure activity. Dev Med Child Neurol. 1999;41:480–485. [PubMed] [Google Scholar]
- 8.Namer IJ, Battaglia F, Hirsch E, Constantinesco A, Marescaux C. Subtraction ictal SPECT co-registered to MRI (SISCOM) in Sturge-Weber syndrome. Clin Nucl Med. 2005;30:39–40. doi: 10.1097/00003072-200501000-00014. [DOI] [PubMed] [Google Scholar]
- 9.Raches D, Hiscock M, Chapieski L. Behavioral and academic problems in children with Sturge-Weber syndrome: differences between children with and without seizures. Epilepsy Behav. 2012;24:457–463. doi: 10.1016/j.yebeh.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Bachur CD, Comi AM. Sturge-Weber syndrome. Curr Treat Options Neurol. 2013;15:607–617. doi: 10.1007/s11940-013-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kossoff EH, Borsage JL, Comi AM. A pilot study of the modified Atkins diet for Sturge-Weber syndrome. Epilepsy Res. 2010;92:240–243. doi: 10.1016/j.eplepsyres.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Kelley TM, Hatfield LA, Lin DD, Comi AM. Quantitative analysis of cerebral cortical atrophy and correlation with clinical severity in unilateral Sturge-Weber syndrome. J Child Neurol. 2005;20:867–870. doi: 10.1177/08830738050200110201. [DOI] [PubMed] [Google Scholar]
- 13.Lin DD, Barker PB, Hatfield LA, Comi AM. Dynamic MR perfusion and proton MR spectroscopic imaging in Sturge-Weber syndrome: correlation with neurological symptoms. J Magn Reson Imaging. 2006;24:274–281. doi: 10.1002/jmri.20627. [DOI] [PubMed] [Google Scholar]
- 14.Comi AM. Sturge-Weber syndrome and epilepsy: an argument for aggressive seizure management in these patients. Expert Rev Neurother. 2007;7:951–956. doi: 10.1586/14737175.7.8.951. [DOI] [PubMed] [Google Scholar]
- 15.Wheless JW, Clarke DF, Carpenter D. Treatment of pediatric epilepsy: expert opinion, 2005. J Child Neurol. 2005;20(Suppl 1):S1–S56. doi: 10.1177/088307380502000101. [DOI] [PubMed] [Google Scholar]
- 16.Landreville S, Agapova OA, Matatall KA, et al. Histone deacetylase inhibitors induce growth arrest and differentiation in uveal melanoma. Clin Cancer Res. 2012;18:408–416. doi: 10.1158/1078-0432.CCR-11-0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verrotti A, D’Egidio C, Mohn A, Coppola G, Chiarelli F. Weight gain following treatment with valproic acid: pathogenetic mechanisms and clinical implications. Obes Rev. 2011;12:e32–e43. doi: 10.1111/j.1467-789X.2010.00800.x. [DOI] [PubMed] [Google Scholar]
- 18.Awad AH, Mullaney PB, Al-Mesfer S, Zwaan JT. Glaucoma in Sturge-Weber syndrome. J AAPOS. 1999;3:40–45. doi: 10.1016/s1091-8531(99)70093-5. [DOI] [PubMed] [Google Scholar]
- 19.Etminan M, Maberley D, Mikelberg FS. Use of topiramate and risk of glaucoma: a case-control study. Am J Ophthalmol. 2012;153:827–830. doi: 10.1016/j.ajo.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 20.Symes RJ, Etminan M, Mikelberg FS. Risk of angle-closure glaucoma with bupropion and topiramate. JAMA Ophthalmol. 2015;133:1187–1189. doi: 10.1001/jamaophthalmol.2015.2180. [DOI] [PubMed] [Google Scholar]
- 21.Shiau T, Armogan N, Yan DB, Thomson HG, Levin AV. The role of episcleral venous pressure in glaucoma associated with Sturge-Weber syndrome. J AAPOS. 2012;16:61–64. doi: 10.1016/j.jaapos.2011.09.014. [DOI] [PubMed] [Google Scholar]