SUMMARY
The intestinal mucosal barrier controlling the resident microbiome is dependent on a protective mucus layer generated by goblet cells, impairment of which is a hallmark of the inflammatory bowel disease Ulcerative Colitis. Here we show that IL-18 is critical in driving the pathologic breakdown of barrier integrity in a model of colitis. Deletion of Il18 or its receptor Il18r1 in intestinal epithelial cells (Δ/EC) conferred protection from colitis and mucosal damage in mice. In contrast, deletion of the IL-18 negative regulator Il18bp resulted in severe colitis associated with loss of mature goblet cells. Colitis and goblet cell loss were rescued in Il18bp−/−;Il18rΔ/EC mice, demonstrating that colitis severity is controlled at the level of IL-18 signaling in intestinal epithelial cells. IL-18 inhibited goblet cell maturation by regulating the transcriptional program instructing goblet cell development. These results inform on the mechanism of goblet cell dysfunction which underlies the pathology of Ulcerative Colitis.
INTRODUCTION
Inflammatory bowel disease (IBD) is a complex and debilitating disorder that can be sub-classified into the distinct multifactorial disorders Crohn’s Disease (CD) and Ulcerative Colitis (UC) (Kaser et al., 2010; Maloy and Powrie, 2011). While both are characterized by chronic relapsing pathogenic inflammation and intestinal epithelial cell injury, they differ substantially in their clinical manifestations. CD patients exhibit discontinuous lesions throughout the entirety of the intestinal tract and disease pathology is closely associated with a dysregulation of the antimicrobial peptide (AMP) response (Fellermann et al., 2003; Neurath, 2014). A genetic basis for CD susceptibility has been linked to genes involved in autophagy and ER stress (e.g. Atg16l1 and Xbp1), as well as microbial recognition (e.g. Nod2), in AMP-producing Paneth cells (Adolph et al., 2013; Cadwell et al., 2008; Hugot et al., 2001; Ogura et al., 2001). Interestingly however, no major defects in AMP production have been observed in UC patients (Nuding et al., 2007; Wehkamp et al., 2007), indicating distinct mechanistic differences in disease etiology. Despite UC having greater worldwide prevalence than CD (Danese and Fiocchi, 2011), surprisingly little is known about the specific underlying host factors that drive susceptibility to disease. One unique and defining feature of human UC pathology is major depletion of mucin producing goblet cells and the mucus layer, which correlates with increased microbiota-induced colonic inflammation and disease pathology (McCormick et al., 1990; Pullan et al., 1994; Strugala et al., 2008; Trabucchi et al., 1986). Intriguingly, the in vivo mechanisms responsible for this important clinical observation during inflammation remain obscure.
Members of the IL-1 family of cytokines play critical roles in intestinal homeostasis and inflammation (Lopetuso et al., 2013; Neurath, 2014; Saleh and Trinchieri, 2011). In particular, IL-18 has emerged as an indispensable factor in governing host-microorganism homeostasis and has been postulated to be a key determining factor in IBD (Dinarello et al., 2013; Elinav et al., 2011; Nakamura et al., 1989). IL-18 is initially synthesized as an inactive precursor molecule that requires coordinated inflammasome activation of the cysteine protease caspase-1 to cleave proIL-18 into a functional mature bioactive cytokine (Fantuzzi et al., 1999; Martinon et al., 2002). Upon activation and release, IL-18 is free to bind the IL-18 receptor alpha chain (IL-18R1) and in cells co-expressing the IL-18R accessory protein (IL-18Rap), ligand binding triggers receptor heterodimerization and the formation of an intracellular Myd88 signaling platform (Adachi et al., 1998; Born et al., 1998; Hoshino et al., 1999). This elicits the recruitment of IRAK and TRAF6, facilitating activation of the inhibitor of κB (IκB) kinases (IKKs), IKKα and IKKβ (Medzhitov et al., 1998; Mercurio et al., 1997). In turn, IKKβ can phosphorylate IκBα, targeting the protein for proteasomal degradation and allowing the NF-κB subunit p65 to translocate to the nucleus to initiate diverse gene expression programs such as proinflammatory cytokine production and NOD-Like Receptor (NLR) upregulation (Bauernfeind et al., 2009). As such, IL-18 signaling requires tight regulation to prevent autoimmunity and this is thought to be directly accomplished by the soluble decoy receptor IL-18 binding protein (IL-18BP), as its transgene overexpression has been shown to neutralize IL-18 activity in vivo to prevent hyper NF-κB activation and inflammation (Fantuzzi et al., 2003).
The use of IL-18- and IL-18R1-deficient mice identified IL-18 as a putative host molecule required to protect intestinal epithelial cells from intestinal inflammation and colitis (Salcedo et al., 2010). In support of a role for IL-18 in promoting intestinal epithelial integrity and protection from acute experimental colitis, mice deficient in the key processing subunits of IL-18, caspase 1 and the NLRP3 inflammasome are also highly susceptible to disease pathology (Dupaul-Chicoine et al., 2010; Zaki et al., 2010). Administration of exogenous recombinant IL-18 rescues colitis in the aforementioned and other inflammasome deficient mice, further supporting a protective role for IL-18 in colitis (Oficjalska et al., 2015). In contrast, however, inhibition of IL-18 has also been shown to instigate protection in experimental colitis, supporting a pro-colitogenic role for IL-18 (Kanai et al., 2001; Siegmund et al., 2001; Ten Hove et al., 2001). Such conflicting findings have led to much controversy and discussion in the field, and the true role of IL-18 in intestinal homeostasis and inflammation is still unresolved (Asquith and Powrie, 2010; Dinarello et al., 2013; Gagliani et al., 2014; Siegmund, 2010).
Underlying this discourse is the fact that most previous work studying the complete IL-18 deletion in mice is confounded by IL-18 effect on colitogenic microbiota (Elinav et al., 2011; Henao-Mejia et al., 2012), while equally important roles of IL-18 during inflammation are masked by dysbiosis. Compound associated phenotypic alterations in Il18−/− mice such as metabolic syndrome may further obscure the direct contribution of IL-18 to intestinal function (Netea et al., 2006). At present, no genetic models exist to specifically dissect the role of IL-18 in colitis risk, and the need for new genetic tools is therefore paramount. To this end, we generated conditional knockout mice for both IL-18 and IL-18R1 to delineate the direct involvement of IL-18 in epithelial and hematopoietic cells to homeostasis and colitis. Here, we show that IL-18 production, irrespective of its cellular source, exacerbated colitis severity after administration of the colitis-inducing agent dextran sodium sulfate (DSS). Deletion of IL-18R in epithelial cells (Il18rΔ/EC) protected mice from developing colitis, suggesting that IL-18 directly disrupts epithelial cell integrity during colitis. By deleting IL-18 binding protein (Il18bp), the IL-18 negative regulator, we asked if increased bioavailability of IL-18 would promote barrier function or rather drive colitis. Remarkably, Il18bp−/− mice developed severe colitis associated with progressive loss of mature goblet cells, which could be reversed by specifically deleting the epithelial IL-18R in these mice. Finally, we show that IL-18-mediated goblet cell dysfunction precedes clinical disease manifestation and is caused by a defect in terminal goblet cell maturation through transcriptional regulation of goblet cell differentiation factors. Taken together, these results uncover the direct role of IL-18 in promoting goblet cell dysfunction during colitis, leading to breakdown of the mucosal barrier. This study may therefore offer a genetic understanding to the pathology of human ulcerative colitis.
RESULTS
Epithelial IL-18/IL-18R signaling promotes DSS-induced colitis
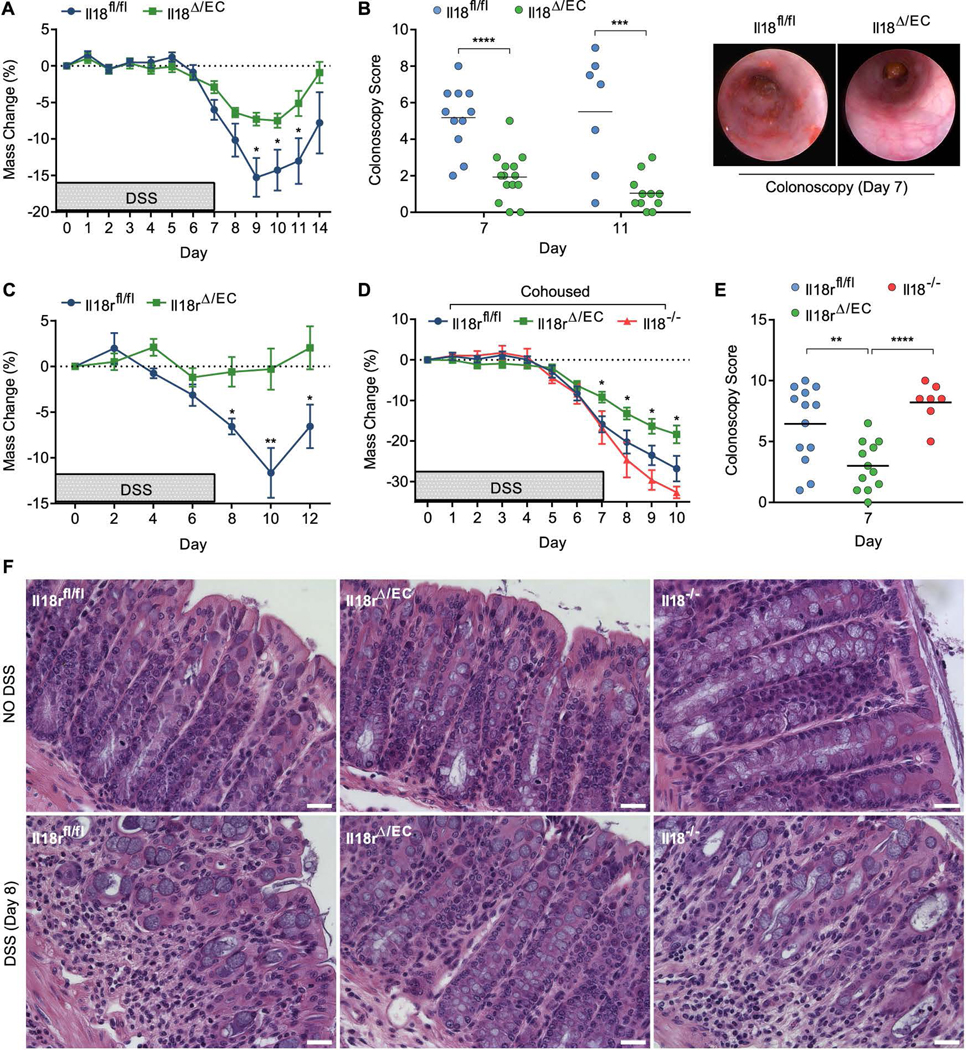
IL-18 is a key mediator of intestinal homeostasis and inflammation, yet the cellular partners and molecular mechanisms driving these effects remain poorly understood. To delineate the compound role of IL-18 in intestinal inflammation, we conditionally deleted Il18 or Il18r1 in intestinal epithelial cells by generating Villin-cre+;Il18fl/fl (hereafter called Il18Δ/EC) and Villin-cre+;Il18rfl/fl (Il18rΔ/EC) mice (Figure S1A–D). To enable mechanistic evaluation of IL-18’s microbiota-independent roles, throughout this study knockout mice were compared to their cohoused floxed (fl/fl) wild-type littermates. Indeed, bacterial 16S ribosomal RNA (rRNA) sequencing confirmed equalized bacterial composition in both Il18Δ/EC and Il18fl/fl littermates (Figure S2A). IL-18 production in Il18Δ/EC total colon explants was markedly reduced (Figure S1B), confirming IECs as the major source of IL-18 under physiological conditions (Takeuchi et al., 1997). Steady state colon sections did not show gross structural or cellular irregularities in Il18Δ/EC or Il18rΔ/EC mice, including goblet cell maturation and tight junction formation, as determined by MUC2, β-catenin and ZO-1 staining (Figure S3). Nevertheless, Il18Δ/EC mice were surprisingly resistant to colonic inflammation following administration of DSS, as reflected by reduced weight loss compared to Il18fl/fl littermates (Figure 1A). Colonoscopy performed on day 7 post DSS showed increased tissue damage in control Il18fl/fl mice, measured by the degree of bleeding, colon wall granularity and translucency, as well as stool consistency (Figure 1B). Similarly to Il18Δ/EC mice, DSS-treated Il18rΔ/EC mice were protected against weight loss, as compared to Il18rfl/fl littermates (Figure 1C). To more rigorously assess these effects in the presence of a ‘colitogenic’ microbiota, Il18rΔ/EC and Il18rfl/fl were cohoused for 8 weeks with dysbiotic Il18−/− mice in order to introduce transmissible dominantly colitogenic bacteria (Elinav et al., 2011) (Figure S2B). Despite an overall higher degree of inflammation, Il18rΔ/EC mice had reduced weight loss and lower colonoscopy score than control Il18rfl/fl mice (Figure 1D, E). Severe colitis and deterioration of tissue integrity in Il18rfl/fl mice, but not in Il18rΔ/EC mice, was corroborated by histological examination of distal colon sections performed on day 8 post DSS (Figure 1F). These results suggest that IL-18 promotes the pathology of DSS-induced colitis through a mechanism dependent on its action on intestinal epithelial cells.
Figure 1. Epithelial IL-18/IL-18R signaling promotes DSS-induced colitis.
(A–F) To induce colitis, mice were administered 2% DSS in drinking water for 7 days. (A) Weight loss of cohoused Il18fl/fl and Il18Δ/EC littermates (n=11–14). (B) Colonoscopy severity score of Il18fl/fl and Il18Δ/EC mice on day 7 and 11 post DSS (left) and representative endoscopic view of the mouse colon on day 7 post DSS (right). (C) Weight loss of cohoused Il18rfl/fl and Il18rΔ/EC littermates (n=8). (D) Littermate Il18rfl/fl and Il18rΔ/EC mice were cohoused with Il18−/− mice for 8 weeks, after which DSS was administered and weight loss recorded (n=7–13). (E) Colonoscopy severity score of cohoused Il18rfl/fl, Il18rΔ/EC and Il18−/− on day 7. (F) Representative H&E staining of distal colon sections obtained from cohoused Il18rfl/fl, Il18rΔ/EC and Il18−/− mice given water (top) or DSS (bottom) and assessed on day 8. Note the disruption of crypt structure and mucosal immune cell infiltration in Il18rfl/fl and Il18−/− mice. Scale bar = 25 μm. Data are represented as mean ± SEM. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001 by unpaired Student’s t-test. Related to Figure S1–3.
Hematopoietic/endothelial IL-18, but not IL-18R, promotes DSS-induced colitis
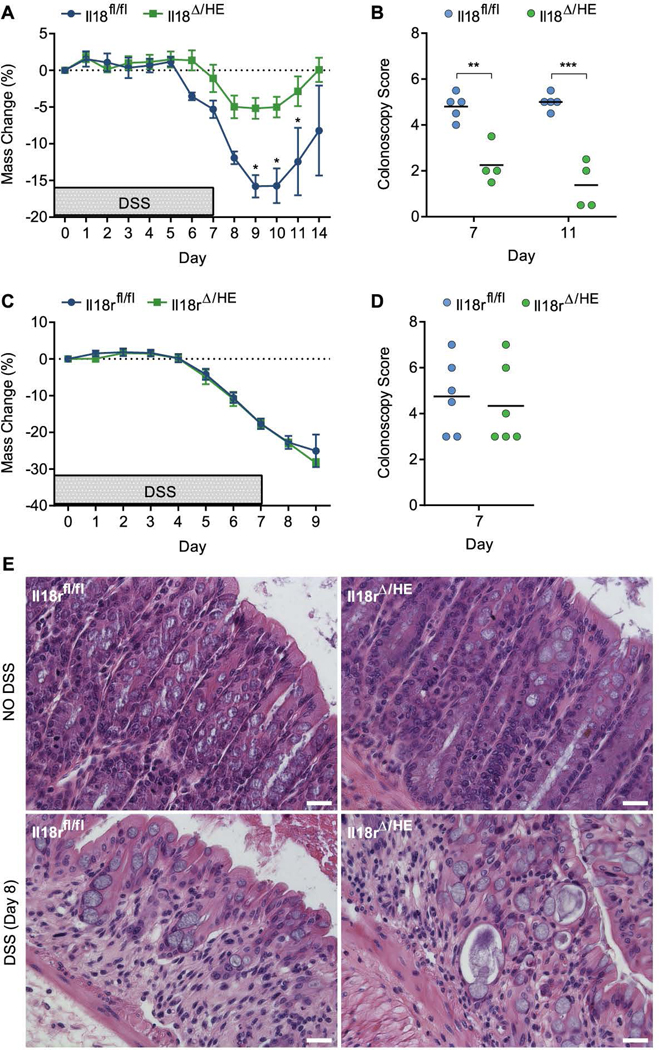
In addition to epithelial cells, IL-18 and IL-18R are also expressed by various hematopoietic and endothelial cells, in particular under inflammatory conditions (Siegmund, 2010). To address the role of the IL-18 axis in these cells during colitis, we generated Flk1-cre+;Il18fl/fl (Il18Δ/HE) and Flk1-cre+;Il18rfl/fl (Il18rΔ/HE) mice in which Il18 or Il18r are specifically deleted in all hematopoietic and endothelial cells (Figure S1B). As above, knockout mice were compared to their cohoused floxed (fl/fl) wild-type littermates, with both featuring similar microbiome configurations (including the colitogenic Prevotellaceae species), thus enabling us to study in detail the microbiome-independent contribution of hematopoietic IL-18 to the intestinal pathology in these mice (Figure S2C, D). Consistent with deletion of IL-18 in epithelial cells, Il18Δ/HE mice were highly protected in DSS-induced colitis, as indicated by reduced weight loss and colonoscopy scores compared to Il18fl/fl littermates (Figure 2A, B). In contrast, Il18rΔ/HE mice were susceptible to extensive weight loss and tissue damage, to a comparable degree as their Il18rfl/fl littermates (Figure 2C, D). Histology performed on day 8 post DSS confirmed similar extent of colitis in both Il18rfl/fl and Il18rΔ/HE mice (Figure 2E). These results further demonstrate that irrespective of its cellular source, IL-18 production during colitis drives disease progression. Colitis severity, however, is not exacerbated by IL-18R signaling in hematopoietic and/or endothelial cells, in contrast to what is observed in epithelial cells. Together these data show that the target of IL-18 mediated pathology is the epithelium.
Figure 2. Hematopoietic/endothelial IL-18, but not IL-18R, promotes DSS-induced colitis.
(A) Weight loss of cohoused Il18fl/fl and Il18Δ/HE littermates treated with 2% DSS for 7 days (n=4). (B) Colonoscopy severity score of Il18fl/fl and Il18Δ/HE mice on day 7 and 11. (C) Weight loss of cohoused Il18rfl/fl and Il18rΔ/HE littermates (n=6). (D) Colonoscopy severity score of Il18rfl/fl and Il18rΔ/HE mice on day 7. (E) Representative H&E staining of distal colon sections obtained from cohoused Il18rfl/fl and Il18rΔ/HE mice given water (top) or DSS (bottom) and assessed on day 8. Scale bar = 25 μm. Data are represented as mean ± SEM. *, p<0.05; **, p<0.01; ***, p<0.001; by unpaired Student’s t-test. Related to Figure S1–3.
Hyperactive IL-18 signaling drives colitis and goblet cell depletion in Il18bp−/− mice
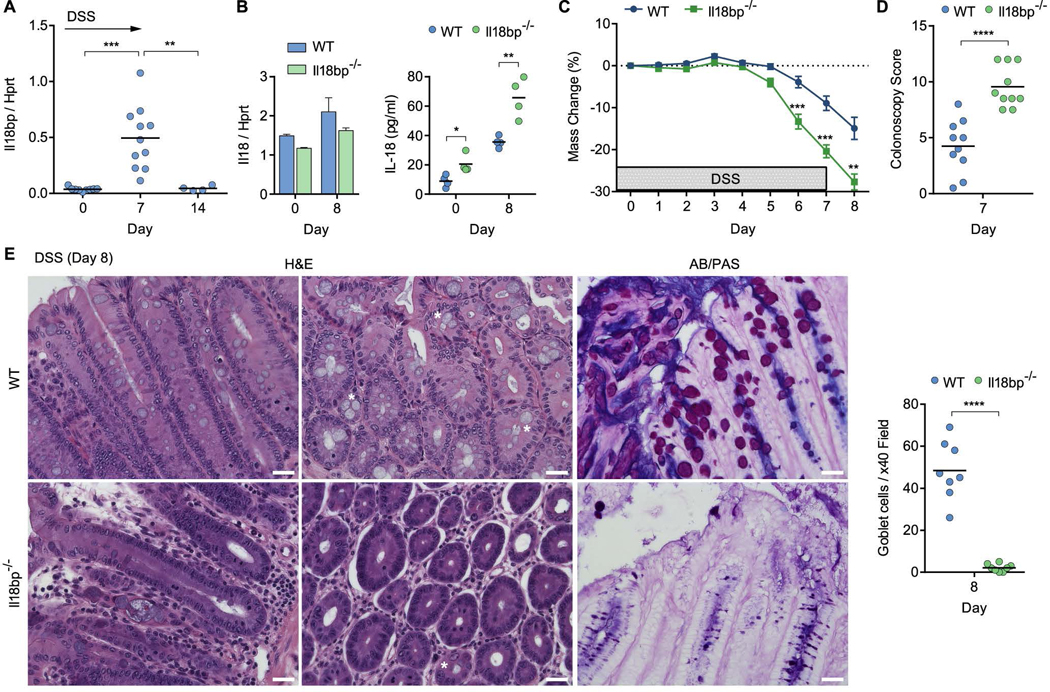
IL-18 is negatively regulated by the IL-18 binding protein (IL-18BP), which serves as a decoy receptor and prevents IL-18 association with IL-18R (Novick et al., 1999). While basal expression levels of Il18bp in the steady state colon were low, it was highly induced during the course of colitis, returning to baseline levels following recovery (Figure 3A). To better understand the mechanism by which IL-18 enhances susceptibility to colitis, we generated mice with hyperactive IL-18 signaling by deleting Il18bp (Figure S1E). Il18bp expression was undetectable in Il18bp−/− mice, whereas the expression of neighboring genes was unaffected (Figure S1F). Furthermore, in the steady state Il18bp−/− mice had equalized flora compared to their wild-type (WT) littermates (Figure S2E) and displayed normal goblet cell development and tight junction structure (Figure S3). Although Il18 mRNA expression was comparable in WT and Il18bp−/− mice, the active secreted form of IL-18 was elevated in Il18bp−/− colon explant supernatants, both in the steady state and following DSS treatment (Figure 3B). During DSS colitis, Il18bp−/− mice developed rapid and severe morbidity associated with extensive bleeding and tissue damage (Figure 3C, D). Extensive tissue deterioration and colitis were also evident in histological sections of Il18bp−/− mice but not of their WT littermate controls (Figure 3E). Remarkably, Il18bp−/− mice suffered an overwhelming loss of mucus-producing goblet cells (Figure 3E). The absence of mature goblet cells and associated mucus layer in Il18bp−/− mice was verified by AB/PAS staining (Figure 3E). Goblet cell enumeration in histological sections showed a dramatic decrease in goblet cell counts in Il18bp−/− mice compared to WT mice at day 8 post DSS (Figure 3E). Decreased goblet cell counts were also noted in Il18fl/fl and Il18rfl/fl mice compared to Il18Δ/EC or Il18rΔ/EC littermates following DSS (Figure S4A). These results suggest that hyper-IL-18 signaling leads to exacerbated colitis associated with mature goblet cell depletion.
Figure 3. Hyperactive IL-18 signaling drives colitis and goblet cell loss in Il18bp−/− mice.
(A) Wild-type (WT) mice were treated with 2% DSS in drinking water and Il18bp mRNA expression in the distal colon was measured over 14 days. (B) IL-18 mRNA expression in distal colon (left) and protein secretion in colonic explants (right) of il18bp−/− and WT littermates. (C) Weight loss following DSS treatment in cohoused WT and Il18bp−/− littermates (n=10). (D) Colonoscopy severity score of WT and Il18bp−/− mice. (E) Representative H&E and AB/PAS staining of distal colon sections obtained from cohoused WT (top) and Il18bp−/− (bottom) littermates on day 8 post DSS treatment. Asterisks mark example goblet cells. Right, enumeration of goblet cells in histological sections from cohoused WT and Il18bp−/− littermates. Scale bar = 25 μm. Data are represented as mean ± SEM. *, p<0.05; **, p<0.01; ***, p<0.001; ****, p<0.0001 by unpaired Student’s t-test. Related to Figure S1–4.
Deletion of epithelial IL-18R rescues goblet cell loss and colitis in Il18bp−/− mice
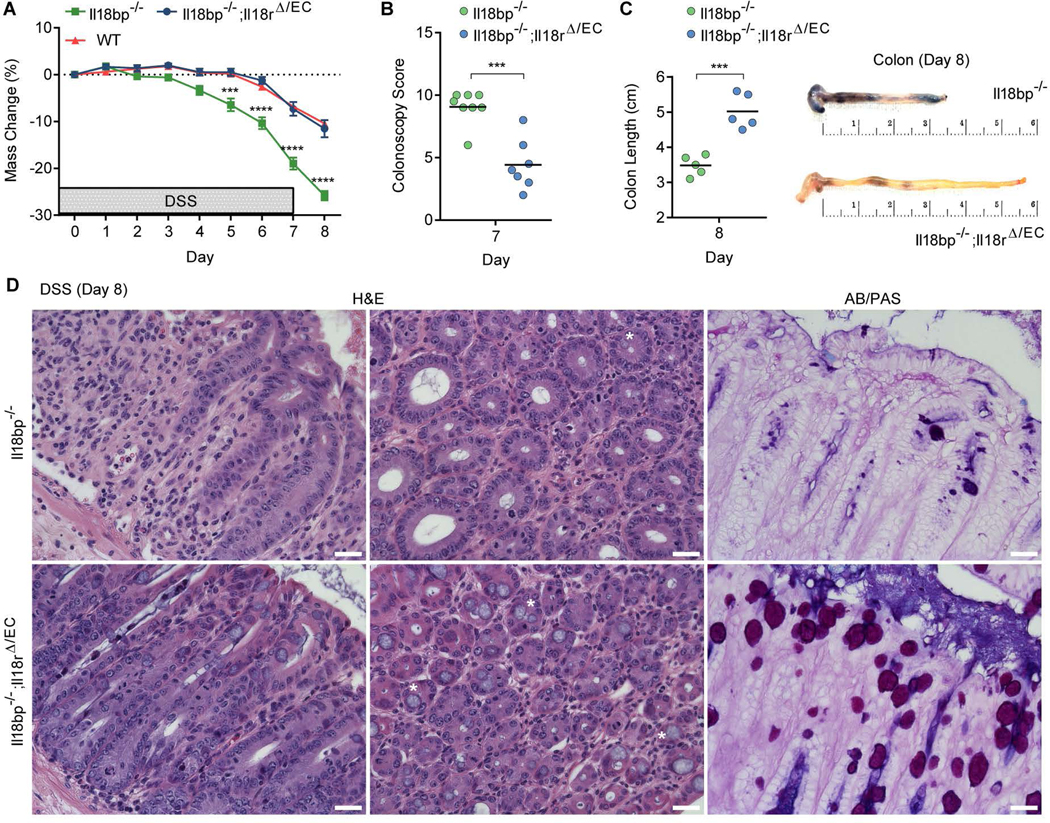
We next asked whether goblet cell loss and the increased colitis severity observed in Il18bp−/− mice arise as a general consequence of immune cell activation and inflammation due to hyperactive IL-18, or is rather directly dependent on IL-18 signaling mediated perturbation of epithelial cell function. To address this question in vivo, we generated Il18bp−/−;Il18rΔ/EC double knockout mice, with the hypothesis that if increased colitis in Il18bp−/− mice is a result of hyper immune activation, loss of IL-18R signaling in epithelial cells will not affect the course of disease. However, if increased colitis in Il18bp−/− mice is a result of epithelial cell dysregulation, loss of IL-18R signaling in epithelial cells may rescue the pathology in Il18bp−/− mice. Following DSS treatment, Il18bp−/−;Il18rΔ/EC mice were dramatically protected from weight loss in comparison to Il18bp−/− littermates, to the same level as Il18bp+/+ WT littermates (Figure 4A). This correlated with decreased intestinal bleeding and tissue damage in Il18bp−/−;Il18rΔ/EC mice, assessed by colonoscopy at day 7 post DSS (Figure 4B). Upon dissection at day 8 post DSS, Il18bp−/− mice exhibited macroscopic indications of severe intestinal inflammation with extensive bleeding throughout the intestine and significant shortening of the colon, while the intestines of Il18bp−/−;Il18rΔ/EC mice appeared normal (Figure 4C). Colon tissue sections obtained at day 8 post DSS revealed reduced immune cell infiltration and maintenance of structural integrity in Il18bp−/−;Il18rΔ/EC mice, while Il18bp−/− mice showed extensive tissue pathology (Figure 4D). Importantly, goblet cells were completely recovered in Il18bp−/−;Il18rΔ/EC mice, representing the baseline levels observed in WT mice, as demonstrated by both H&E and AB/PAS staining (Figure 4D; compare to Figure 3E). These results indicate that goblet cell loss and increased susceptibility to colitis are a direct consequence of increased IL-18 signaling in intestinal epithelial cells.
Figure 4. Deletion of epithelial IL-18R rescues goblet cell loss and colitis in Il18bp−/− mice.
(A) Weight loss of cohoused Il18bp−/−, Il18bp−/−;Il18rΔ/EC and WT littermates treated with 2% DSS for 7 days (n=10–14). (B) Colonoscopy severity score of Il18bp−/− and Il18bp−/−;Il18rΔ/EC littermates on day 7. (C) Gross pathology of colons on day 8 post DSS. Note the extensive shortening, bleeding and diarrhea in Il18bp−/− mice. (D) Representative H&E and AB/PAS staining of distal colon sections obtained from cohoused Il18bp−/− (top) and Il18bp−/−;Il18rΔ/EC mice (bottom) on day 8 post DSS treatment. Asterisks mark example goblet cells. Scale bar = 25 μm. Data are represented as mean ± SEM. **, p<0.01; ***, p<0.001; ****, p<0.0001 by unpaired Student’s t-test.
Hyperactive IL-18 signaling prevents goblet cell maturation prior to colitis
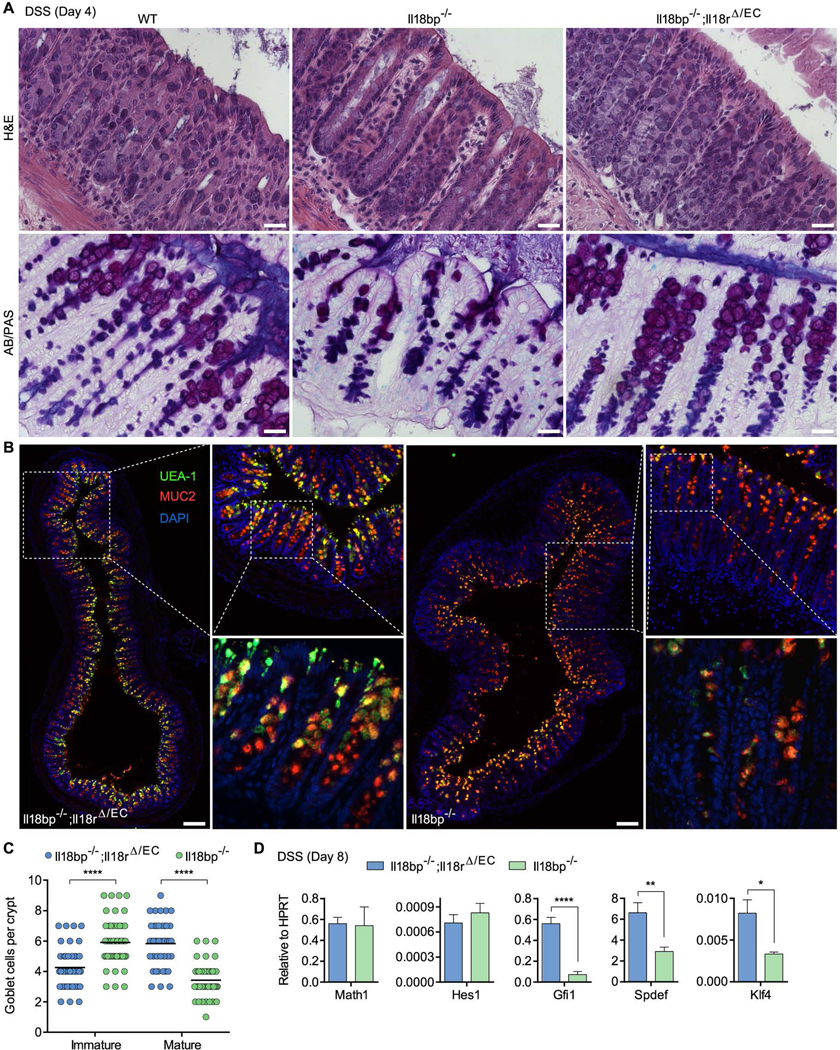
To determine whether the observed goblet cell dysfunction is a cause rather than a consequence of inflammation, we examined histological colon sections from Il18bp−/− mice at day 4 post DSS, preceding significant weight loss and clinical symptoms of colitis. Although goblet cells were readily identified by H&E staining in Il18bp−/− mice, AB/PAS staining indicated decreased abundance of mature PAS+ goblet cells in Il18bp−/− mice compared to WT or Il18bp−/−;Il18rΔ/EC littermates (Figure 5A). Goblet cell maturation was further assessed by staining for fucosylated glycoproteins with the lectin Ulex europaeus agglutinin-1 (UEA-1) and the abundant goblet cell mucin MUC2, both of which accumulate as goblet cells mature. Il18bp−/− mice exhibited an increase of immature goblet cells, determined by low area MUC2 staining (<10 μm in diameter) in UEA-1lo/− cells, and decrease in large mature MUC2+UEA-1bright goblet cells compared to Il18bp−/−;Il18rΔ/EC mice (Figure 5B). The mature/immature goblet cell ratio on day 4 post DSS decreased to 0.58 in Il18bp−/− mice compared to 1.39 in Il18bp−/−;Il18rΔ/EC mice and 1.84 in Il18bp+/+ (WT) mice (Figure 5C and Figure S4B, C). As noted above, mature goblet cells were markedly depleted in Il18bp−/− mice on day 8 post DSS, however small MUC2+UEA-1+/− cells were still highly represented, notably at the lower half of the crypt (Figure S4D). To determine whether dysregulation of goblet cell maturation reflects a transcriptional imbalance, we measured expression of transcription factors involved in goblet cell differentiation and maturation. Whereas no change was noted in the secretory lineage differentiation factors Math1 (Hath1; Atoh1) and Hes1, expression of the goblet cell differentiation/maturation factors Gfi1, Spdef and Klf4 was markedly inhibited in Il18bp−/− mice (Figure 5D). These results suggest that IL-18 promotes colitis by preventing functional goblet cell maturation through regulation of the goblet cell transcriptional maturation program.
Figure 5. Hyperactive IL-18 signaling prevents goblet cell maturation prior to colitis.
(A) Representative H&E and AB/PAS staining of distal colon sections obtained from WT, Il18bp−/− and Il18bp−/−;Il18rΔ/EC littermates on day 4 post DSS treatment prior to onset of colitis. Note reduction in PAS+ mature goblet cells in Il18bp−/− mice. Scale bar = 25 μm. (B) Epifluorescence images (left, top right panels) and confocal stacks (bottom right panels) of distal colon sections obtained on day 4 post DSS and stained with the fucose-binding lectin UEA-1 and anti-MUC2. Scale bar = 150 μm. (C) Enumeration of immature and mature goblet cells in distal colon sections stained as in (B). Immature goblet cells were scored as UEA-1lo/− cells containing low area (<10 μm in diameter) MUC2 staining, and mature goblet cells were scored as cells containing large area (>10 μm in diameter) MUC2+UEA-1bright staining. (D) Distal colon samples were obtained on day 8 post DSS and used for gene expression analysis by qPCR. Data are represented as mean ± SEM. *, p<0.05; ****, p<0.0001 by unpaired Student’s t-test. Related to Figure S4.
IL-18 directly controls goblet cell maturation and colitis
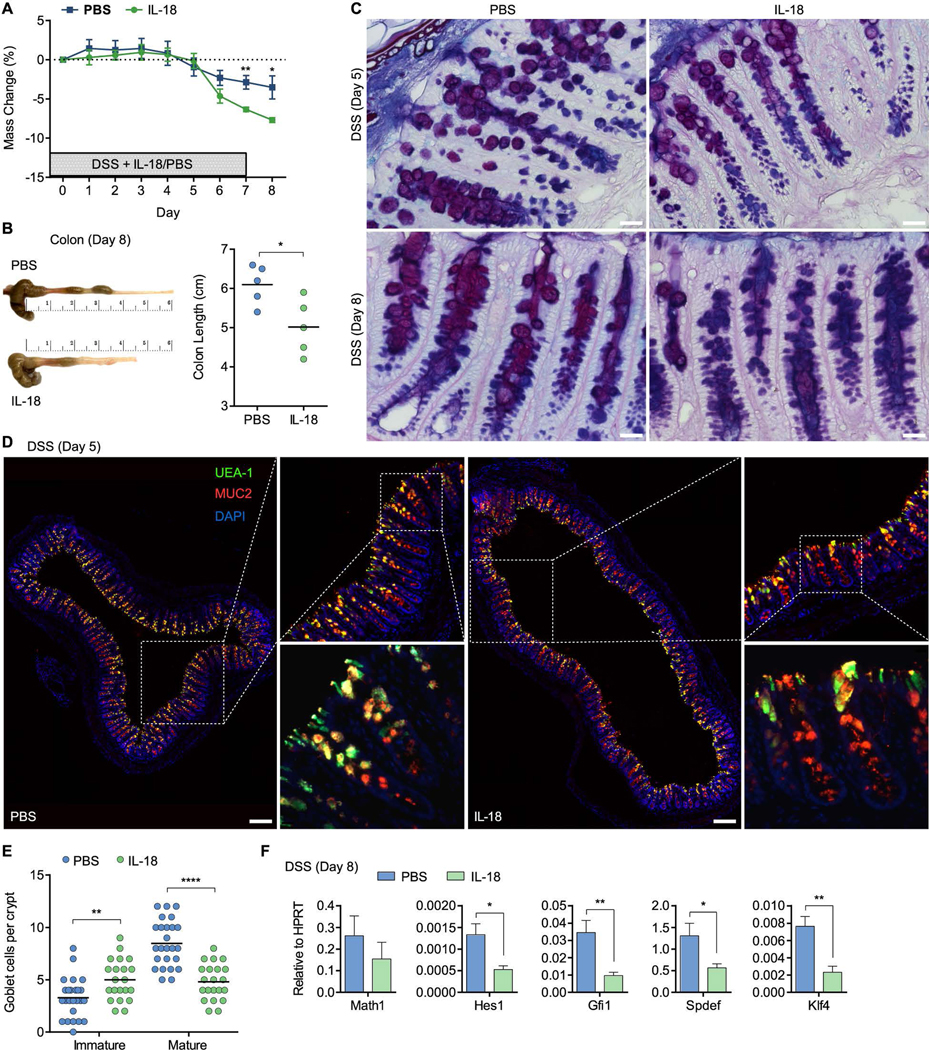
We finally assessed the direct role of IL-18 in goblet cell dysfunction leading to colitis, by injecting recombinant IL-18 protein to WT mice during the course of DSS administration. Disease severity was increased in mice receiving daily IL-18 injections, as determined by weight loss and macroscopic examination of the colon at day 8 post DSS (Figure 6A, B). In line with our observations in Il18bp−/− mice, AB/PAS staining showed gradual decrease in the abundance of mature PAS+ goblet cells in mice receiving IL-18 compared to PBS (Figure 6C). The state of goblet cell maturation was corroborated in colon sections obtained following 5 daily injections prior to weight loss and clinical symptoms of colitis, demonstrating an IL-18-mediated block in goblet cell maturation (Figure 6D, E). The ratio of mature/immature goblet cell decreased further in IL-18-injected mice on day 8 (Figure S4D, E). IL-18 injection was sufficient to reduce Gfi1, Spdef and Klf4 gene expression in isolated IECs, further supporting direct regulation of goblet cell maturation by IL-18 (Figure 6F). These results suggest that elevated IL-18 production during inflammation is responsible for dysregulated goblet cell maturation.
Figure 6. IL-18 directly controls goblet cell maturation and colitis.
(A) WT mice received daily i.p. injections of 1 μg recombinant IL-18 or PBS during the course of 7 day 2% DSS administration and weight loss was recorded (n=5). (B) Gross pathology of colons on day 8 post DSS. Note reduction in colon length following IL-18 treatment. (C) Representative AB/PAS staining of distal colon sections obtained on day 5 or 8 post DSS treatment from WT littermates receiving recombinant IL-18 or PBS. Note reduction in PAS+ mature goblet cells in mice receiving recombinant IL-18. Scale bar = 25 μm. (D) Epifluorescence images (left, top right panels) and confocal stacks (bottom right panels) of distal colon sections obtained on day 5 post DSS and stained with UEA-1 and anti-MUC2. Scale bar = 150 μm. (E) Enumeration of immature and mature goblet cells in distal colon sections stained as in (D). Immature and mature goblet cells were scored as in Figure 5C. (F) Distal colon samples were obtained on day 8 post DSS and used for gene expression analysis by qPCR. Data are represented as mean ± SEM. *, p<0.05; **, p<0.01; ****, p<0.0001 by unpaired Student’s t-test. Related to Figure S4.
DISCUSSION
Despite great strides in our understanding of IL-18 over the past 15 years, its precise contributions to host homeostasis, intestinal inflammation and its overall relevance to IBD still remain controversial and elusive. On one hand, complete loss of IL-18 (or IL-18R) predisposes mice to increased intestinal epithelial damage and fosters an altered inflammatory environment that potentiates intestinal tumor formation (Salcedo et al., 2010; Takagi et al., 2003). This could be explained, at least in part, by the recently identified role of IL-18 in controlling the outgrowth of colitogenic bacterial species (Elinav et al., 2011). On the other hand, IL-18 is a potent proinflammatory cytokine with the ability to promote colitis through the induction of inflammatory mediators such TNFα and chemokines (Sivakumar et al., 2002; Ten Hove et al., 2001). The role of IL-18 in intestinal homeostasis and inflammation and its mechanistic segregation from microbiota-dependent functions therefore remained unresolved.
Previous interpretations of IL-18 functionality have been limited by the lack of precise genetic models required to systematically determine its roles in intestinal biology. Therefore, IL-18 function has been inferred from complete deletion of IL-18, inflammasomes, caspase 1/11 or the multifunctional adapter protein ASC. Such studies have led to the conclusion that epithelial derived IL-18 is required to promote barrier integrity during early inflammation, as acute treatment with recombinant IL-18 during early colitis promotes epithelial proliferation in inflammasome deficient mice, rescuing intestinal pathology (Dupaul-Chicoine et al., 2010; Zaki et al., 2010). However, extrapolation of direct IL-18 functionality from these models should be approached with caution. Firstly, deficiency of NLRP3, which is highly expressed in the myeloid compartment, results in numerous phenotypic alterations beyond IL-18 processing. Most obvious is an inherent defect in processing the closely related and equally important cytokine IL-1β. Like IL-18, IL-1β is also thought to mediate a dichotomous role in intestinal homeostasis and inflammation (Bamias et al., 2012; Lopetuso et al., 2013). Notably, bone marrow chimera experiments have shown that hematopoietic derived IL-1β is also sufficient to rescue epithelial cell damage and promote epithelial restitution during experimental colitis (Bersudsky et al., 2014). Therefore, in NLRP3-deficient mice, which harbor defects in IL-1 family member maturation, IL-18 may compensate for the lack of IL-1β; however, whether this occurs physiologically (or at physiologically relevant levels of IL-18) remains unclear. In addition, caspase 1 plays a key role in the clearance of intracellular intestinal pathogens through the regulated cell death process of pyroptosis (Miao et al., 2010). Although the role of pyroptosis in colitis is still under investigation, the use of pyroptosis-defective mice to examine the specific IL-18 functionality in the intestine proves problematic. The study of direct functions of IL-18 in the intestine is further complicated by NLRP6 regulation of dysbiosis and the outgrowth of pathogenic intestinal microbial communities (Elinav et al., 2011). As demonstrated by Levy et al in this issue, IL-18 processing by the NLRP6 inflammasome shapes the steady state host-microbiome interface by regulating the downstream anti-microbial peptide (AMP) landscape, thereby maintaining intestinal homeostasis. Normally, this axis is controlled by indigenous microbiota-modulated metabolites. However, it can also be directly subverted by inflammasome suppressing metabolites derived from a disease-causing microbiota, which hijacks this pathway, thereby facilitating dysbiosis development and persistence in an invaded host. This highlights the importance of using cohoused littermate control mice, as in the present study, as they harbor near identical bacterial species enabling distinction of the genetic contribution of IL-18 from that of flora driven inflammation.
In this study, we show that during inflammation, not only is IL-18 production in intestinal epithelial and hematopoietic cells not required for protection against experimental colitis, IL-18 signaling in epithelial cells amplifies intestinal damage. This pathogenic role of IL-18 correlates with clinical observations whereby an increase in both epithelial and hematopoietic IL-18 expression and cytokine bioreactivity have been demonstrated in patients with increased severity of IBD (Monteleone et al., 1999; Pizarro et al., 1999). However, the mechanism through which this upregulation of IL-18 in the intestine may contribute to increased disease severity was unknown. An emerging realization in the complexity of IBD is that pathology is not wholly shaped by a dysregulated immune response but highly dependent on an intact mucosal barrier and coordinated cross talk between the intestinal epithelial and immune cells with the microbiota (Kaser et al., 2011; Schreiber et al., 2005; Xavier and Podolsky, 2007). One possible mechanism to explain this association is that increased IL-18 release from epithelial cells acts on resident immune cell to upregulate IL-18 and other proinflammatory mediators, which induce endothelial VCAM-1 expression to enhance immune cell infiltration into the mucosa, and together trigger severe auto-inflammation. In support of this model, we show that deletion of IL-18 production in the hematopoietic compartment results in significant amelioration of intestinal damage during colitis. However, deletion of IL-18R signaling in the hematopoietic compartment fails to rescue mice from DSS-induced inflammation. This suggests that the pathology driven by IL-18 does not occur via signaling in hematopoietic cells, in line with previous reports (Dupaul-Chicoine et al., 2010; Malvin et al., 2012; Saleh and Trinchieri, 2011; Zaki et al., 2010). Rather, we found that deletion of the IL-18R from intestinal epithelial cells dramatically protects mice from DSS induced colitis, suggesting that elevated IL-18 expression during colitis is directly pathogenic to the epithelial cell barrier.
Ulcerative Colitis is characterized by mucosal barrier dysfunction, most notably in epithelial goblet cells and mucus production (Danese and Fiocchi, 2011; Gersemann et al., 2009; McCormick et al., 1990; Pullan et al., 1994; Trabucchi et al., 1986). As goblet cell secretion of protective mucins, trefoil factors and other proteins is essential for barrier integrity and for preventing microflora-driven intestinal inflammation, such dysregulation underlies the pathology exhibited in UC patients. In order to investigate how IL-18 may specifically contribute to intestinal barrier breakdown during DSS colitis, we deleted its decoy receptor inhibitor, IL-18BP. Interestingly, Il18bp−/− mice were characterized by increased colitis severity and lethality associated with major depletion of mature goblet cells, which was reversed in Il18bp−/−;Il18rΔ/EC double knockout mice. Thus, excessive IL-18 signaling on the epithelium leads to progressive depletion of goblet cells and may represent a major risk factor for intestinal inflammation and UC. As severe intestinal inflammation has previously been suggested to result in goblet cell depletion (Bergstrom et al., 2008), we analyzed mice during preclinical manifestation of colitis in order to explore mechanistically if IL-18 was the key determining factor governing goblet cell loss and risk for colitis. Whereas we observed no discernible differences in goblet cell numbers at preclinical time points, we instead discovered that IL-18 promotes disequilibrium in the state of goblet cell development and maturation, decreasing the pool of functional mature goblet cells.
Studies on the transcriptional regulation of intestinal lineage determination have identified the factors controlling goblet cell differentiation and maturation (McCauley and Guasch, 2015). Notch1 signaling drives epithelial progenitor cell specification to absorptive enterocytes and inhibits secretory cell differentiation via the Hes1 transcription factor (Fre et al., 2005; Jensen et al., 2000), whereas Notch1 inhibition results in robust conversion of proliferating crypt progenitors into terminally differentiated goblet cells (van Es et al., 2005). Below a threshold of Notch1 signal, upregulation of Math1 (also known as Hath1, Atoh1) governs commitment of progenitor cells to the epithelial secretory cell lineages, including goblet cells, Paneth cells and neuroendocrine cells (Yang et al., 2001). The transcriptional repressor Gfi1 functions downstream of Math1 to control intestinal secretory cell differentiation, specification and maintenance, and is required for goblet and Paneth cell development (Shroyer et al., 2005). The transcription factors Spdef and Klf4 in turn control goblet cell development and maturation downstream to Gfi1 and are essential for mucosal barrier protection (Gregorieff et al., 2009; Katz et al., 2002; Noah et al., 2010). Analysis of the lineage commitment transcription factors Hes1 and Math1 in the intestinal epithelium of mice with hyperactive IL-18 did not indicate dysregulation at the lineage specification level. Conversely however, the downstream secretory lineage transcription factor Gfi1 and the terminal goblet cell maturation effectors Spdef and Klf4 were markedly downregulated by IL-18. Importantly, all three transcription factors are required to be constitutively expressed in mature goblet cells and loss of either Gfi1, Spdef or Klf4 expression was shown to result in loss of mature goblet cells (Gregorieff et al., 2009; Katz et al., 2002; Shroyer et al., 2005). These data highlight an unexpected role for IL-18 signaling in directly modulating goblet cell maturation and fate downstream to secretory lineage commitment during intestinal inflammation.
In conclusion, we propose that a strict equilibrium of epithelial IL-18 signaling must be maintained in order to preserve its homeostatic functions while preventing progressive loss of mature goblet cells and increased risk of colitis. This work suggests that IL-18 targeting or specific targeting of epithelial IL-18R may represent a novel strategy to prevent the pathologic breakdown of the mucosal barrier in human Ulcerative Colitis.
EXPERIMENTAL PROCEDURES
Mice
Conditional knockout mice with floxed IL-18 alleles or floxed IL-18R alleles were generated using standard homologous gene recombination techniques (Rongvaux et al., 2014). Il18−/− mice have been previously reported (Takeda et al., 1998). Il18bp deletion was generated using Cas9/CrispR technology as described (Wang et al., 2013). All experiments were performed using cohoused littermate control mice. For transmissible dysbiosis cohousing experiments, littermate Il18rfl/fl and Il18rΔ/EC mice and age and gender matched Il18−/− mice were cohoused at ratios of 1:1:1 for 8 weeks. All animal experimentation was performed in compliance with Yale Institutional Animal Care and Use Committee protocols.
Experimental Colitis
For acute experimental colitis induction, mice were administered 2% DSS (M.W. =36,000– 50,000 Da; MP Biomedicals) in their drinking water ad libitum for 7 days, followed by regular drinking water. According to the animal protocol, mice were sacrificed if they lost more than 30% of their initial body weight.
Colonoscopy
Colonoscopy was performed using a high resolution mouse video endoscopic system (‘Coloview’, Carl Storz, Tuttlingen, Germany). The severity of colitis was blindly scored using MEICS (Murine Endoscopic Index of Colitis Severity) based on four parameters: granularity of mucosal surface; vascular pattern; translucency of the colon mucosa; and stool consistency (Becker et al., 2007)
Histology
Colons were fixed in Bouin’s medium and embedded in paraffin. Blocks were serially sectioned along the cephalocaudal axis of the gut to the level of the lumen; the next 5 mm-thick section was stained with hematoxylin and eosin. For goblet cell and mucus layer preservation ex vivo, immediately after excision, colons were submerged in Ethanol–Carnoy’s fixative at 4°C for 2 h and then placed into 100% ethanol. Fixed colon tissues were embedded in paraffin and cut into 5 μm sections. Tissues were stained with Alcian blue/PAS. Images were acquired with Leica DMI6000B inverted microscope and data was analyzed using the LAS-AF software.
Supplementary Material
Acknowledgments
We would like to thank Judith Stein, Jon Alderman, Cindy Hughes and Elizabeth Hughes-Picard for technical assistance. R.N. is supported by the Jane Coffin Childs Fund Postdoctoral Fellowship. N.G. is supported by the Dr. Keith Landesman Memorial Fellowship of the Cancer Research Institute, M.R.d.Z. is supported by a Rubicon Fellowship from the Netherlands Organization of Scientific Research, N.W.P. and W.B. are supported by the Cancer Research Institute Irvington Fellowship Program, C.C.D.H. is supported by a Howard Hughes Medical Institute International Student Research Fellowship. This work was supported in part by the Howard Hughes Medical Institute and The Blavatnik Family Foundation (R.A.F.).
Footnotes
AUTHOR CONTRIBUTIONS
R.N. and R.J. conceived, performed and analyzed the experiments and wrote the manuscript. N.G., M.R.d.Z., N.W.P., W.B., J.S.L., C.C.D.H., M.G. and E.E. provided technical assistance in various experiments. R.A.F. supervised the project and participated in interpreting the results and writing the manuscript.
References
- Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, Sakagami M, Nakanishi K, Akira S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- Adolph TE, Tomczak MF, Niederreiter L, Ko HJ, Bock J, Martinez-Naves E, Glickman JN, Tschurtschenthaler M, Hartwig J, Hosomi S, et al. Paneth cells as a site of origin for intestinal inflammation. Nature. 2013;503:272–276. doi: 10.1038/nature12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asquith M, Powrie F. An innately dangerous balancing act: intestinal homeostasis, inflammation, and colitis-associated cancer. J Exp Med. 2010;207:1573–1577. doi: 10.1084/jem.20101330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamias G, Corridoni D, Pizarro TT, Cominelli F. New Insights into the Dichotomous Role of Innate Cytokines in Gut Homeostasis and Inflammation. Cytokine. 2012;59:451–459. doi: 10.1016/j.cyto.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker C, Fantini MC, Neurath MF. High resolution colonoscopy in live mice. Nat Protocols. 2007;1:2900–2904. doi: 10.1038/nprot.2006.446. [DOI] [PubMed] [Google Scholar]
- Bergstrom KSB, Guttman JA, Rumi M, Ma C, Bouzari S, Khan MA, Gibson DL, Vogl AW, Vallance BA. Modulation of Intestinal Goblet Cell Function during Infection by an Attaching and Effacing Bacterial Pathogen. Infection and Immunity. 2008;76:796–811. doi: 10.1128/IAI.00093-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersudsky M, Luski L, Fishman D, White RM, Ziv-Sokolovskaya N, Dotan S, Rider P, Kaplanov I, Aychek T, Dinarello CA, et al. Non-redundant properties of IL-1α and IL-1β during acute colon inflammation in mice. Gut. 2014;63:598–609. doi: 10.1136/gutjnl-2012-303329. [DOI] [PubMed] [Google Scholar]
- Born TL, Thomassen E, Bird TA, Sims JE. Cloning of a novel receptor subunit, AcPL, required for interleukin-18 signaling. The Journal of biological chemistry. 1998;273:29445–29450. doi: 10.1074/jbc.273.45.29445. [DOI] [PubMed] [Google Scholar]
- Cadwell K, Liu JY, Brown SL, Miyoshi H, Loh J, Lennerz JK, Kishi C, Kc W, Carrero JA, Hunt S, et al. A key role for autophagy and the autophagy gene Atg16l1 in mouse and human intestinal Paneth cells. Nature. 2008;456:259–263. doi: 10.1038/nature07416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese S, Fiocchi C. Ulcerative Colitis. New England Journal of Medicine. 2011;365:1713–1725. doi: 10.1056/NEJMra1102942. [DOI] [PubMed] [Google Scholar]
- Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4:289. doi: 10.3389/fimmu.2013.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupaul-Chicoine J, Yeretssian G, Doiron K, Bergstrom KS, McIntire CR, LeBlanc PM, Meunier C, Turbide C, Gros P, Beauchemin N, et al. Control of intestinal homeostasis, colitis, and colitis-associated colorectal cancer by the inflammatory caspases. Immunity. 2010;32:367–378. doi: 10.1016/j.immuni.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Elinav E, Strowig T, Kau AL, Henao-Mejia J, Thaiss CA, Booth CJ, Peaper DR, Bertin J, Eisenbarth SC, Gordon JI, et al. NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell. 2011;145:745–757. doi: 10.1016/j.cell.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantuzzi G, Banda NK, Guthridge C, Vondracek A, Kim SH, Siegmund B, Azam T, Sennello JA, Dinarello CA, Arend WP. Generation and characterization of mice transgenic for human IL-18-binding protein isoform a. Journal of Leukocyte Biology. 2003;74:889–896. doi: 10.1189/jlb.0503230. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G, Reed DA, Dinarello CA. IL-12-induced IFN-gamma is dependent on caspase-1 processing of the IL-18 precursor. J Clin Invest. 1999;104:761–767. doi: 10.1172/JCI7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellermann K, Wehkamp J, Herrlinger KR, Stange EF. Crohn’s disease: a defensin deficiency syndrome? Eur J Gastroenterol Hepatol. 2003;15:627–634. doi: 10.1097/00042737-200306000-00008. [DOI] [PubMed] [Google Scholar]
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- Gagliani N, Palm NW, de Zoete MR, Flavell RA. Inflammasomes and intestinal homeostasis: regulating and connecting infection, inflammation and the microbiota. International Immunology. 2014;26:495–499. doi: 10.1093/intimm/dxu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gersemann M, Becker S, Kübler I, Koslowski M, Wang G, Herrlinger KR, Griger J, Fritz P, Fellermann K, Schwab M, et al. Differences in goblet cell differentiation between Crohn’s disease and ulcerative colitis. Differentiation. 2009;77:84–94. doi: 10.1016/j.diff.2008.09.008. [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Stange DE, Kujala P, Begthel H, van den Born M, Korving J, Peters PJ, Clevers H. The ets-domain transcription factor Spdef promotes maturation of goblet and paneth cells in the intestinal epithelium. Gastroenterology. 2009;137:1333–1345. e1331–1333. doi: 10.1053/j.gastro.2009.06.044. [DOI] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature. 2012;482:179–185. doi: 10.1038/nature10809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Tsutsui H, Kawai T, Takeda K, Nakanishi K, Takeda Y, Akira S. Cutting edge: generation of IL-18 receptor-deficient mice: evidence for IL-1 receptor-related protein as an essential IL-18 binding receptor. J Immunol. 1999;162:5041–5044. [PubMed] [Google Scholar]
- Hugot JP, Chamaillard M, Zouali H, Lesage S, Cezard JP, Belaiche J, Almer S, Tysk C, O’Morain CA, Gassull M, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411:599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24:36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- Kanai T, Watanabe M, Okazawa A, Sato T, Yamazaki M, Okamoto S, Ishii H, Totsuka T, Iiyama R, Okamoto R, et al. Macrophage-derived IL-18-mediated intestinal inflammation in the murine model of Crohn’s disease. Gastroenterology. 2001;121:875–888. doi: 10.1053/gast.2001.28021. [DOI] [PubMed] [Google Scholar]
- Kaser A, Niederreiter L, Blumberg RS. Genetically determined epithelial dysfunction and its consequences for microflora-host interactions. Cell Mol Life Sci. 2011;68:3643–3649. doi: 10.1007/s00018-011-0827-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaser A, Zeissig S, Blumberg RS. Inflammatory Bowel Disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JP, Perreault N, Goldstein BG, Lee CS, Labosky PA, Yang VW, Kaestner KH. The zinc-finger transcription factor Klf4 is required for terminal differentiation of goblet cells in the colon. Development. 2002;129:2619–2628. doi: 10.1242/dev.129.11.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopetuso LR, Chowdhry S, Pizarro TT. Opposing Functions of Classic and Novel IL-1 Family Members in Gut Health and Disease. Frontiers in Immunology. 2013;4:181. doi: 10.3389/fimmu.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474:298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- Malvin NP, Seno H, Stappenbeck TS. Colonic epithelial response to injury requires Myd88 signaling in myeloid cells. Mucosal immunology. 2012;5:194–206. doi: 10.1038/mi.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Burns K, Tschopp J. The Inflammasome: A Molecular Platform Triggering Activation of Inflammatory Caspases and Processing of proIL-β. Molecular Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- McCauley HA, Guasch G. Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends in Molecular Medicine. 2015;21:492–503. doi: 10.1016/j.molmed.2015.06.003. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Horton LW, Mee AS. Mucin depletion in inflammatory bowel disease. Journal of Clinical Pathology. 1990;43:143–146. doi: 10.1136/jcp.43.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R, Preston-Hurlburt P, Kopp E, Stadlen A, Chen C, Ghosh S, Janeway CA., Jr MyD88 is an adaptor protein in the hToll/IL-1 receptor family signaling pathways. Mol Cell. 1998;2:253–258. doi: 10.1016/s1097-2765(00)80136-7. [DOI] [PubMed] [Google Scholar]
- Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, et al. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, Warren SE, Wewers MD, Aderem A. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleone G, Trapasso F, Parrello T, Biancone L, Stella A, Iuliano R, Luzza F, Fusco A, Pallone F. Bioactive IL-18 expression is up-regulated in Crohn’s disease. J Immunol. 1999;163:143–147. [PubMed] [Google Scholar]
- Nakamura K, Okamura H, Wada M, Nagata K, Tamura T. Endotoxin-induced serum factor that stimulates gamma interferon production. Infect Immun. 1989;57:590–595. doi: 10.1128/iai.57.2.590-595.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netea MG, Joosten LAB, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, Tack CJ, van Krieken H, Kim SH, Stalenhoef AF, et al. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- Noah TK, Kazanjian A, Whitsett J, Shroyer NF. SAM Pointed Domain ETS Factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Experimental cell research. 2010;316:452–465. doi: 10.1016/j.yexcr.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick D, Kim SH, Fantuzzi G, Reznikov LL, Dinarello CA, Rubinstein M. Interleukin-18 binding protein: a novel modulator of the Th1 cytokine response. Immunity. 1999;10:127–136. doi: 10.1016/s1074-7613(00)80013-8. [DOI] [PubMed] [Google Scholar]
- Nuding S, Fellermann K, Wehkamp J, Stange EF. Reduced mucosal antimicrobial activity in Crohn’s disease of the colon. Gut. 2007;56:1240–1247. doi: 10.1136/gut.2006.118646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oficjalska K, Raverdeau M, Aviello G, Wade SC, Hickey A, Sheehan KM, Corr SC, Kay EW, O’Neill LA, Mills KH, et al. Protective role for caspase-11 during acute experimental murine colitis. J Immunol. 2015;194:1252–1260. doi: 10.4049/jimmunol.1400501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, Britton H, Moran T, Karaliuskas R, Duerr RH, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature. 2001;411:603–606. doi: 10.1038/35079114. [DOI] [PubMed] [Google Scholar]
- Pizarro TT, Michie MH, Bentz M, Woraratanadharm J, Smith MF, Jr, Foley E, Moskaluk CA, Bickston SJ, Cominelli F. IL-18, a novel immunoregulatory cytokine, is up-regulated in Crohn’s disease: expression and localization in intestinal mucosal cells. J Immunol. 1999;162:6829–6835. [PubMed] [Google Scholar]
- Pullan RD, Thomas GA, Rhodes M, Newcombe RG, Williams GT, Allen A, Rhodes J. Thickness of adherent mucus gel on colonic mucosa in humans and its relevance to colitis. Gut. 1994;35:353–359. doi: 10.1136/gut.35.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rongvaux A, Jackson R, Harman CC, Li T, West AP, de Zoete MR, Wu Y, Yordy B, Lakhani SA, Kuan CY, et al. Apoptotic caspases prevent the induction of type I interferons by mitochondrial DNA. Cell. 2014;159:1563–1577. doi: 10.1016/j.cell.2014.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcedo R, Worschech A, Cardone M, Jones Y, Gyulai Z, Dai RM, Wang E, Ma W, Haines D, O’hUigin C, et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. The Journal of Experimental Medicine. 2010;207:1625–1636. doi: 10.1084/jem.20100199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh M, Trinchieri G. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol. 2011;11:9–20. doi: 10.1038/nri2891. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Rosenstiel P, Albrecht M, Hampe J, Krawczak M. Genetics of Crohn disease, an archetypal inflammatory barrier disease. Nat Rev Genet. 2005;6:376–388. doi: 10.1038/nrg1607. [DOI] [PubMed] [Google Scholar]
- Shroyer NF, Wallis D, Venken KJ, Bellen HJ, Zoghbi HY. Gfi1 functions downstream of Math1 to control intestinal secretory cell subtype allocation and differentiation. Genes Dev. 2005;19:2412–2417. doi: 10.1101/gad.1353905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegmund B. Interleukin-18 in Intestinal Inflammation: Friend and Foe? Immunity. 2010;32:300–302. doi: 10.1016/j.immuni.2010.03.010. [DOI] [PubMed] [Google Scholar]
- Siegmund B, Fantuzzi G, Rieder F, Gamboni-Robertson F, Lehr HA, Hartmann G, Dinarello CA, Endres S, Eigler A. Neutralization of interleukin-18 reduces severity in murine colitis and intestinal IFN-gamma and TNF-alpha production. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1264–1273. doi: 10.1152/ajpregu.2001.281.4.R1264. [DOI] [PubMed] [Google Scholar]
- Sivakumar PV, Westrich GM, Kanaly S, Garka K, Born TL, Derry JM, Viney JL. Interleukin 18 is a primary mediator of the inflammation associated with dextran sulphate sodium induced colitis: blocking interleukin 18 attenuates intestinal damage. Gut. 2002;50:812–820. doi: 10.1136/gut.50.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strugala V, Dettmar PW, Pearson JP. Thickness and continuity of the adherent colonic mucus barrier in active and quiescent ulcerative colitis and Crohn’s disease. Int J Clin Pract. 2008;62:762–769. doi: 10.1111/j.1742-1241.2007.01665.x. [DOI] [PubMed] [Google Scholar]
- Takagi H, Kanai T, Okazawa A, Kishi Y, Sato T, Takaishi H, Inoue N, Ogata H, Iwao Y, Hoshino K, et al. Contrasting action of IL-12 and IL-18 in the development of dextran sodium sulphate colitis in mice. Scand J Gastroenterol. 2003;38:837–844. doi: 10.1080/00365520310004047. [DOI] [PubMed] [Google Scholar]
- Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- Takeuchi M, Nishizaki Y, Sano O, Ohta T, Ikeda M, Kurimoto M. Immunohistochemical and immuno-electron-microscopic detection of interferon-gamma-inducing factor (“interleukin-18”) in mouse intestinal epithelial cells. Cell and tissue research. 1997;289:499–503. doi: 10.1007/s004410050895. [DOI] [PubMed] [Google Scholar]
- Ten Hove T, Corbaz A, Amitai H, Aloni S, Belzer I, Graber P, Drillenburg P, van Deventer SJ, Chvatchko Y, Te Velde AA. Blockade of endogenous IL-18 ameliorates TNBS-induced colitis by decreasing local TNF-alpha production in mice. Gastroenterology. 2001;121:1372–1379. doi: 10.1053/gast.2001.29579. [DOI] [PubMed] [Google Scholar]
- Trabucchi E, Mukenge S, Baratti C, Colombo R, Fregoni F, Montorsi W. Differential diagnosis of Crohn’s disease of the colon from ulcerative colitis: ultrastructure study with the scanning electron microscope. Int J Tissue React. 1986;8:79–84. [PubMed] [Google Scholar]
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. One-Step Generation of Mice Carrying Mutations in Multiple Genes by CRISPR/Cas-Mediated Genome Engineering. Cell. 2013;153:910–918. doi: 10.1016/j.cell.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehkamp J, Schmid M, Stange EF. Defensins and other antimicrobial peptides in inflammatory bowel disease. Curr Opin Gastroenterol. 2007;23:370–378. doi: 10.1097/MOG.0b013e328136c580. [DOI] [PubMed] [Google Scholar]
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for Secretory Cell Lineage Commitment in the Mouse Intestine. Science. 2001;294:2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- Zaki MH, Boyd KL, Vogel P, Kastan MB, Lamkanfi M, Kanneganti TD. The NLRP3 inflammasome protects against loss of epithelial integrity and mortality during experimental colitis. Immunity. 2010;32:379–391. doi: 10.1016/j.immuni.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.